Abstract
Summary: Atypical teratoid/rhabdoid tumor of the CNS is an aggressive infantile neoplasm of uncertain origin. In our two infantile cases, this tumor presented as a bulky cerebellar hemispheric mass with significant mass effect to the fourth ventricle and brain stem. Although the attenuation on CT and signal intensity characteristics at MR imaging of this tumor were similar to those of vermian medulloblastoma, cerebellar hemispheric location and aggressive growth pattern could be considered as different gross morphologic characteristics of this tumor. Despite intensive chemotherapy and radiation therapy, both of our two patients died within 8 months of pathologic diagnosis.
Malignant rhabdoid tumor is initially described as a distinctive renal tumor of children (1–6). It was first reported in 1978 as a rhabdomyosarcomatoid variant of Wilms tumor (1). Since the initial report, several similar, aggressive tumors have been described in the CNS and in the other extrarenal organs (7). In 1995, Rorke et al (2) described a special type of CNS tumor that partially or totally consisted of rhabdoid cells resembling a malignant rhabdoid tumor of the kidney and usually also contained areas resembling primitive neuroectodermal tumor (PNET) and sometimes foci of cells indicating a mesenchymal or epithelial origin. They named it “atypical teratoid/rhabdoid” tumor.
A series of pathology reports of CNS atypical teratoid/rhabdoid tumor have been described in the literature (2, 6–8), but gross morphologic characteristics of this tumor, especially on CT or MR images, have not been described in detail (3–5). We recently treated two cases of pathologically diagnosed atypical teratoid/rhabdoid tumors of the cerebellum. In this report, we will present our cases, with special emphasis on gross morphologic characteristics observed at CT and MR imaging.
Case Reports
Case 1
A 2-month-old girl was admitted to our institution because of vomiting for 2 days. Her mother’s pregnancy was uneventful, and she was delivered at term without complication. The only remarkable abnormality was macrocephaly. A cranial sonogram depicted an intracranial mass of the posterior fossa and obstructive hydrocephalus.
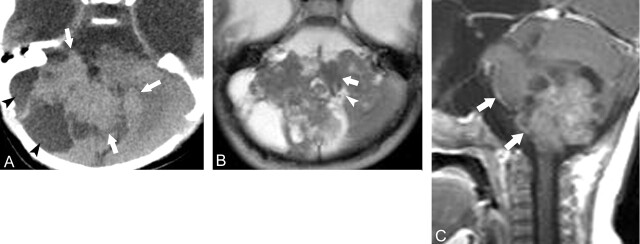
CT showed a 5.2-cm lobulating contoured mass of the left cerebellum growing into the left cerebellomedullary cistern area. The fourth ventricle and medulla oblongata were compressed and displaced to right side by the mass effect of the tumor. The tumor showed slightly higher attenuation than normal cerebellar cortex except some foci of cystic change. Contrast-enhanced CT revealed strong enhancement of the peripheral portion of the tumor (Fig 1A and B). On MR images, the tumor revealed similar imaging features as seen at CT, and the solid portion of the tumor showed nearly isointense signal on both T1- and T2-weighted images as compared with normal cerebellar cortex (Fig 1C). On contrast-enhanced sagittal T1-weighted images, abnormal pial enhancement was observed along the ventral surface of the brain stem, a finding that suggests the possibility of CSF seeding of the tumor.
Fig 1.
Case 1, a 2-month-old girl with atypical teratoid/rhabdoid tumor in left cerebellum.
A, Axial CT scan, showing an ill-defined slightly hyperattenuating mass occupying left cerebellar hemisphere and cerebellopontine cistern area (arrows).
B, Axial postcontrast scan, the same level as in A, showing strong contrast enhancement of the peripheral portion of the tumor.
C, Axial T2-weighted image, showing a large mass of isointense signal with significant mass effect to the fourth ventricle (arrowhead) and adjacent brain stem (arrow)
D, The tumor is composed of diffusely arranged epithelioid cells showing a few glandular components. Hematoxylin and eosin staining, ×100.
E, Rhabdoid cells with eccentric nuclei and abundant eosinophilic cytoplasm (arrow). Necrosis is also noted (arrowhead). Hematoxylin and eosin staining, ×200
Preoperative abdominal CT showed a well-defined 3 × 4.3-cm lobulating contoured mass in the lower pole of the left kidney. After suboccipital craniotomy, near-total resection of the tumor was done. On pathologic examination, the tumor revealed moderate vascularization and high cellularity of undifferentiated polygonal cells with eccentric nuclei (Fig 1D and E). On immunohistochemical staining, the tumor was positive for glial fibrillary acidic protein (GFAP), S-100, epithelial membrane antigen, smooth muscle antigen, cytokeratin (CK), synaptophysin, and vimentin. The final pathologic diagnosis was atypical rhabdoid/teratoid tumor. Postoperative chemotheraphy and radiation therapy were administered. Six months after the operation, the patient experienced active bleeding from the mass in the left lower kidney. Left nephrectomy was performed, and the final pathologic diagnosis was malignant rhabdoid tumor. Despite all these therapeutic efforts, she died at 7 months after her first operation.
Case 2
A 2-month-old male infant was referred to the emergency room because of seizure with eyeball deviation. His mother’s pregnancy was uneventful, and the baby was born at term without complication. The only remarkable abnormality was macrocephaly and subsequent cranial sonography depicted an intracranial mass of the posterior fossa and obstructive hydrocephalus.
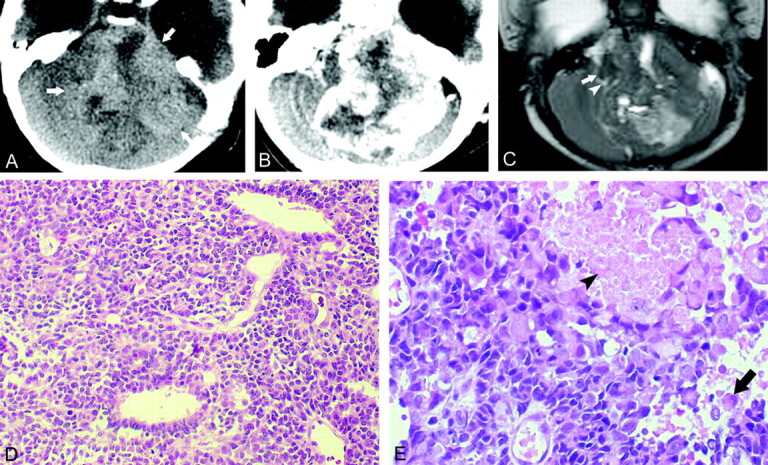
CT showed a 3.4 × 3.1-cm mass in the right inferior cerebellar hemisphere growing into right cerebellopontine cistern The fourth ventricle and medulla oblongata were compressed and displaced to left side by the mass effect of the tumor. Except for the peripheral cystic portion, the tumor showed slightly higher attenuation than normal cerebellar cortex (Fig 2A). At MR imaging, the tumor revealed similar features as those seen on CT scans, and the solid portion of the tumor showed nearly isointense signal on both T1- and T2-weighted images as compared with normal cerebellar cortex. On contrast-enhanced T1-weighted images, the tumor showed mild heterogeneous enhancement. CSF seeding of the tumor was suspected because of abnormal pial enhancement along the ventral surface of the brain stem, and another smaller mass was observed at left cerebellomedullary cistern area (Fig 2B and C).
Fig 2.
Case 2, a 2-month-old boy with atypical teratoid/rhabdoid tumor in right cerebellar hemispere.
A, Axial CT scan, showing an ill-defined mildly hyperattenuating mass (arrows) occupying right cerebellar hemisphere with multiple cystic components (arrowheads) especially along the peripheral portion of the tumor.
B, Axial T2-weighted image, showing a large mass of isointense signal with significant mass effect to the fourth ventricle and adjacent brain stem (arrows)
C, Contrast-enhanced sagittal T1-weighted image shows mild heterogeneous enhancement within the tumor and abnormal pial enhancement along the ventral surface of the brain stem, suggesting CSF seeding of the tumor (arrows).
After suboccipital craniotomy, a subtotal resection of the tumor was performed. Pathologic examination showed the tumor to be composed of rhabdoid cells, large epitheloid cells with papillary growth pattern and fascicles of spindle-shaped mesenchymal cells. On immunohistochemical stain, the tumor was positive for GFAP, CK, synaptophysin, and vimentin, but negative for desmin. The final pathologic diagnosis was atypical rhabdoid/teratoid tumor. Postoperative chemotheraphy and radiation therapy were done. Despite these therapeutic efforts, he died 8 months after surgery.
Discussion
In our limited experience, atypical teratoid/rhabdoid tumor was a distinctive infantile CNS neoplasm in terms of clinicoradiologic features. Our two patients had macrocephaly and clinical symptoms at early infantile period. Subsequently, the tumor was easily detected by use of cranial ultrasonography. At CT or MR imaging, this tumor was characterized by cerebellar hemispheric location, aggressive growth pattern such as significant mass effect to adjacent fourth ventricle and brain stem, and early CSF seeding observed on contrast-enhanced MR images. CT attenuation and signal intensity characteristics of this tumor were similar to those of cerebellar medulloblastoma in childhood. Therefore, CT or MR imaging of this tumor can be easily misinterpreted as the medulloblastoma of unusually young patients. We, however, think that there are some differences between this tumor and medulloblastoma in terms of gross morphologic features at CT or MR imaging. Important differential points of imaging studies are location of the tumor and pattern of tumor growth. In medulloblastoma of childhood, the tumor usually arises from the vermian area and grows into the fourth ventricle, but atypical teratoid/rhabdoid tumor may arise from the cerebellar hemisphere and grow into the adjacent cisternal space instead of filling the fourth ventricle. Compression and displacement of the fourth ventricle by the mass effect of the tumor is very unusual in medulloblastoma. The other clinical differential point is the patient’s age. Medulloblastoma is rare in infants, and the median age for diagnosis is about 6 years (2). By contrast, atypical teratoid/rhabdoid tumor of infancy and childhood presents at a younger age than true medulloblastoma, with median age at diagnosis being 16.5 months (2). The other CT and MR imaging features of atypical teratoid/rhabdoid tumor of brain are nonspecific, showing multiple cystic or necrotic areas, and an inhomogeneous contrast-enhancement pattern (3–6).
In most cases of the atypical teratoid/rhabdoid tumor, the location of the tumor is cerebellar hemisphere (9). This tumor, however, has been seen in the suprasellar, pineal, and temporal regions (9). Leptomeningeal dissemination can be seen from the very beginning, as in the cases presented here. The appearance of the first symptoms was, on average, at the age of 17 months, according to the published accounts (3). This tumor is 50% more common among boys than girls (7). The mean survival time is 3 months after only surgical intervention and 8 months with adjuvant chemo- and radiation therapy (9). Some renal rhabdoid tumors are associated with a synchronous intracranial neoplasm, as our first case (10).
Conclusion
We present clinicoradiologic features of two infantile case of atypical teratoid/rhabdoid tumor. In our two infantile cases, this tumor presented as an aggressive cerebellar hemispheric mass with significant mass effect to the fourth ventricle and brain stem. Although the attenuation characteristics at CT and signal intensity characteristics at MR imaging of this tumor were similar to those of vermian medulloblastoma, cerebellar hemispheric location and aggressive growth pattern could be considered as gross morphologic characteristics of this tumor.
References
- 1.Beckwith JB, Palmer NF. Histopathology and prognosis of Wilms tumors. Cancer 1978;41:1937–1948 [DOI] [PubMed] [Google Scholar]
- 2.Rorke LB, Packer RJ, Biegel JA. Central nervous system atypical teratoid/rhabdoid tumors of infancy and children: definition of an entity. J Neurosurg 1996;85:56–65 [DOI] [PubMed] [Google Scholar]
- 3.Ho PSP, Lee WH, Chen CY, et al. Primary malignant rhabdoid tumor of the brain: CT characteristics. J Comput Assist Tomogr 1990;14:461–463 [DOI] [PubMed] [Google Scholar]
- 4.Hanna SL, Langhston JW, Parham DM, Douglass EC. Primary malignant rhabdoid tumor of the brain: clinical, imaging and pathologic findings. AJNR Am J Neuroradiol 1993;14:107–115 [PMC free article] [PubMed] [Google Scholar]
- 5.Giulio Z, Giancarlo I, Ermanno B, et al. Central nervous system atypical teratoid/rhabdoid tumour of infancy: CT and MR findings. Clin Imaging 1999;23:356–360 [DOI] [PubMed] [Google Scholar]
- 6.Chou SM, Anderson JS. Primary CNS malignant rhabdoid tumor (MRT): report of two cases and review of literature. Clin Neurol Pathol 1991;10:1–10 [PubMed] [Google Scholar]
- 7.Parham DM, Weeks DA, Beckwith JB. The clinicopathologic spectrum of putative extrarenal rhabdoid tumor: an analysis of 42 cases studied with immunohistochemistry or electron microscopy. Am J Surg Pathol 1994;22:231–238 [DOI] [PubMed] [Google Scholar]
- 8.Bhattachrjee M, Hicks J, Langford L, et al. Central nervous system atypical teratoid/ rhabdoid tumors of infancy and children. Ultrastruct Pathol 1997;21:369–378 [DOI] [PubMed] [Google Scholar]
- 9.Hilden JW, Watterson J, Longee DC, et al. Central nervous system atypical teratoid/rhabdoid tumor: response to intensive theraphy and review of the literature. J Neurooncol 1998;40:265–275 [DOI] [PubMed] [Google Scholar]
- 10.Bonnin JM, Rubinstein LJ, Palmer NF, Beckwith JB. The association of embryonal tumors originating in the kidney and in the brain: a report of seven cases. Cancer 1984;54:2137–2146 [DOI] [PubMed] [Google Scholar]