Abstract
Summary: We report a case of recurrent paradoxical brain embolism mediated through a small pulmonary arteriovenous malformation (PAVM) with a 1.8-mm-diameter feeding artery. In this case, the further recurrent stroke was prevented successfully by PAVM embolization. Although embolization therapy is currently recommended only for PAVMs with feeding arteries greater than 3 mm in diameter, the therapy may be needed also in the smaller PAVMs.
Pulmonary arteriovenous malformation (PAVM) is well recognized as a cause of paradoxical brain embolism. Brain infarction associated with PAVMs most likely occurs in patients with feeding arteries of more than 3-mm diameter and not in those of smaller size (1–5). We report the case of a PAVM patient with a 1.8-mm-diameter feeding artery who had recurrent paradoxical brain embolism and was successfully treated with embolotherapy.
Case Report
A 67-year-old right-handed woman was admitted in February 2002 after suddenly developing dizziness, nausea, and speech disturbance. She was admitted to our hospital 7 hours after the onset of symptoms. At the time of admission, her blood pressure was 140/80 mm Hg. She had a regular heart rate of 60 beats per minute and a respiratory rate of 16 breaths per minute. Neither pathologic breath sounds nor heart murmurs were auscultated. No edema was present in her extremities. No cutaneous vascular malformations were observed. She had no episodes of hemoptysis or dyspnea. In neurologic examinations, she had dysarthria and left limb ataxia. Laboratory findings showed normal blood cell counts, normal liver and renal functions, and normal serum cholesterol and blood glucose levels. Plasma protein C, protein S, and antithrombin III levels were within normal ranges. Arterial blood gas analysis showed mild hypoxemia, with PaO2 84.6 mm Hg and PaCO2 43.2 mm Hg in room air. Chest radiographic and electrocardiographic findings were normal. Carotid ultrasonography failed to detect atherosclerotic changes and showed normal blood flow velocities in the common carotid arteries and vertebral arteries. Transthoracic echocardiography failed to find any embolic sources, including valvular pathology, dilated cardiomyopathy, akinetic left ventricular segment, and intracardiac thrombus. Cranial CT demonstrated an equivocal hypoattenuated area in the left cerebellar hemisphere but no abnormality in other areas. Diffusion-weighted MR images disclosed a high-signal-intensity area in the left cerebellar hemisphere (Fig 1A). Twelve hours after the onset of stroke, intraarterial digital subtraction cerebral angiography revealed no stenotic lesion in the extra- or intracranial vertebral arteries or basilar artery. With suspicion of embolic brain infarction, anticoagulation therapy with 10,000 U/day heparin was started 18 hours after the onset of symptoms, although the embolic source could not be identified.
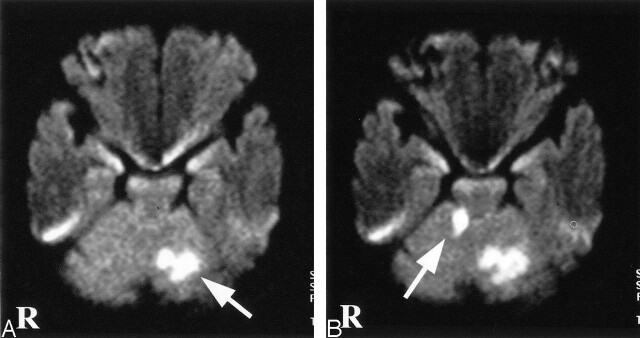
Fig 1.
Diffusion-weighted MR images. A, Diffusion-weighted image on day 1 shows a high-signal-intensity area in the left cerebellar hemisphere (arrow). B, Diffusion-weighted image on day 5 shows a new ischemic lesion in the right cerebellar hemisphere (arrow).
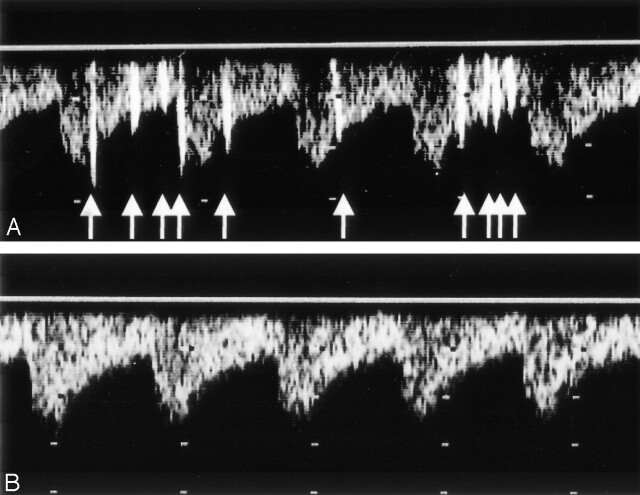
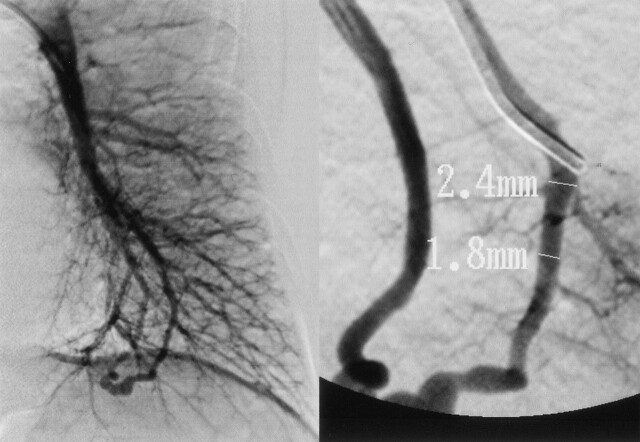
On day 3, the patient also developed right limb ataxia. Activated partial thromboplastin time was 28.0 seconds on admission and was prolonged to 43.1 second on day 3. The second CT scan showed only a hypoattenuated area in the left cerebellar hemisphere. Diffusion-weighted imaging on day 5 confirmed the development of new ischemic lesion in the right cerebellar hemisphere (Fig 1B). On day 6, transcranial Doppler sonography with saline contrast material injection (C-TCD) was performed to examine the presence of the right-to-left shunt. The C-TCD study was performed by using a mixture of saline solution (9 mL) and air (1 mL) agitated between two 10-mL syringes connected by a three-way stopcock. The solution was injected into the left antecubital vein within 2–3 seconds. C-TCD depicted multiple high-intensity transient signals (HITS) at the basilar artery during normal respiration (Fig 2A), which suggested the presence of continuous right-to-left shunt (6). Transesophageal echocardiography with saline contrast material injection into the antecubital vein confirmed a significant entry of microbubbles into the left atrium. Transesophageal echocardiography failed to show any embolic sources, including valvular pathology, intracardiac thrombus, atrial myxoma, spontaneous echo contrast of the left atrium, and complex atheroma of the aortic arch. Holter electrocardiographic monitoring failed to find atrial fibrillation or flutter. Radioisotope venography of the lower extremities displayed a filling defect with collateral pathways in the left popliteal vein, indicating the association of deep vein thrombosis. A contrast-enhanced CT scan of the patient’s chest revealed a small nodular lesion connected with the pulmonary artery and vein branches in the left pulmonary lower lobe. Selective pulmonary angiography on day 13 demonstrated a single PAVM with a 1.8-mm-diamter feeding artery at the corresponding area (Fig 3). The diagnosis of paradoxical brain embolism mediated through the PAVM was, thus, established. Subsequently, the PAVM was obstructed by using an embolization coil (Cook, Bloomington, IN). Following the embolization therapy, C-TCD detected no more HITS (Fig 2B), and the hypoxemia was slightly improved (PaO2 92.4 mm Hg in room air). On day 22, she was discharged without neurologic deficits. C-TCD never detected HITS on day 105. MR imaging on day 126 showed only old brain infarction equivalent to that on day 5. The patient had no recurrent attacks for the following 12 months.
Fig 2.
C-TCD findings before and after the embolization therapy. A, C-TCD shows many HITS (arrows) from the basilar artery before the embolization therapy. B, C-TCD failed to depict any HITS after the embolization therapy.
Fig 3.
Selective pulmonary angiogram shows a single PAVM with a feeding artery diameter of 1.8 mm.
Discussion
PAVMs are commonly associated in hereditary hemorrhagic telangiectasia (HHT): approximately 15–35% of HHT patients have PAVMs, and 50–85% of PAVMs patients have HHT (1). The present patient, however, had no clinical signs suggestive of HHT. PAVMs increase the risk of paradoxical embolism because of continuous right-to-left shunt. Brain infarction is one of the most important complications that may occur in PAVM patients (1, 2). Stroke is often the first manifestation of PAVM and even of HHT (7).
Moussouttas et al (1) documented that brain infarction most likely occurs in PAVM patients with feeding arteries more than 3 mm in diameter and not in those with smaller size. Accordingly, they recommended embolization therapy for all PAVMs with feeding artery diameters exceeding 3 mm. To the best of our knowledge, the occurrence of brain infarction in PAVMs with less than 3 mm in feeding artery diameter has not been previously reported. The present patient had a small-sized PAVM, the diameter of which was 1.8 mm. In the present patient, the other embolic sources—including atrial fibrillation or flutter, bacterial endocarditis, valvular pathology, myocardial infarction, dilated cardiomyopathy, akinetic left ventricular segment, intracardiac thrombus, atrial myxoma, spontaneous echo contrast of the left atrium, or complex atheroma of the aortic arch—were ruled out, and other causes of stroke—such as atherosclerotic arterial changes, nonatherosclerotic arteriopathies, coagulopathies, hematologic or systemic disorders, or migranous infarction—were found to be negative (8, 9). The patient had deep vein thrombosis, which may cause arterial embolism under the presence of right-to-left shunt. Thus, paradoxical embolism is only one possible mechanism that can explain recurrent strokes in this patient. After the embolization therapy, this patient had no more recurrent stroke, and C-TCD never detected HITS, indicating successful obstruction of right-to-left shunt (10).
A recent study reported that the stroke recurrence rate in patients with patent foramen ovale (PFO) was unaltered regardless of whether the diameter was more than 2 mm or less (11). Paradoxically, PAVMs and PFO share the similar mechanisms in the development of brain infarction through the right-to-left shunt. The shunt flow goes through PAVMs continuously, whereas it can pass though PFO only intermittently at the time of right atrial pressure increase. In this context, PAVM is regarded to be more risky than PFO as a route of paradoxical embolism. The size of right-to-left shunt may not be a critical factor for stroke occurrence. Embolization therapy appears to be useful for preventing recurrent attacks in stroke patients with a small PAVM in whom other causes of cerebral infarction are definitely ruled out. A randomized controlled study may be necessary to elucidate the requirement of embolization therapy in small-sized PAVMs.
Footnotes
Supported in part by the Research Grants for Cardiovascular Diseases (14–1) from the Ministry of the Health and Welfare of Japan.
References
- 1.Moussouttas M, Fayad P, Rosenblatt M, et al. Pulmonary arteriovenous malformations: cerebral ischemia and neurologic manifestations. Neurology 2000;55:959–964 [DOI] [PubMed] [Google Scholar]
- 2.Shovlin CL, Letarte M. Hereditary hemorrhagic telangiectasia and pulmonary arteriovenous malformations: issues in clinical management and review of pathogenic mechanisms. Thorax 1999;54:714–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hughes JM. Intrapulmonary shunts: coils to transplantation. J R Coll Phys 1994;28:247–253 [PMC free article] [PubMed] [Google Scholar]
- 4.Haitjema TJ, Overtoom TT, Westermann CJ, Lammers JW. Embolisation of pulmonary arteriovenous malformations: results and follow-up in 32 patients. Thorax 1995;50:719–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White RI, Pollack JS. Pulmonary arteriovenous malformations: diagnosis by three-dimensional helical CT, a breakthrough without contrast media. Radiology 1994;191:613–614 [DOI] [PubMed] [Google Scholar]
- 6.Chimowitz MI, Nemec JJ, Marwick TH, et al. Transcranial Doppler ultrasound identifies patients with right-to-left cardiac or pulmonary shunts. Neurology 1991;41:1902–1904 [DOI] [PubMed] [Google Scholar]
- 7.Hewes RC, Auster M, White RI Jr. Cerebral embolism: first manifestation of pulmonary arteriovenous malformation in patients with hereditary hemorrhagic telangiectasia. Cardiovasc Intervent Radiol 1985;8:151–155 [DOI] [PubMed] [Google Scholar]
- 8.Mas JL, Arquizan C, Lamy C, et al. Recurrent cerebrovascular events associated with patent foramen ovale, atrial septal aneurysm, or both. N Engl J Med 2001;345:1740–1746 [DOI] [PubMed] [Google Scholar]
- 9.Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial: TOAST: Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41 [DOI] [PubMed] [Google Scholar]
- 10.Kimura K, Minematsu K, Wada K, et al. Transcranial Doppler of a paradoxical brain embolism associated with a pulmonary arteriovenous fistula. AJNR Am J Neuroradiol 1999;20:1881–1884 [PMC free article] [PubMed] [Google Scholar]
- 11.Homma S, Sacco RL, Di Tullio MR, et al. Effect of medical treatment in stroke patients with patent foramen ovale: Patent Foramen Ovale in Cryptogenic Study. Circulation 2002;105:2625–2631 [DOI] [PubMed] [Google Scholar]