Abstract
Summary: Gorham disease is a rare condition characterized by intraosseous neoplastic proliferation of hemangiomatous tissue with progressive, massive osteolysis. We present a pathologically proved case of Gorham disease that involved the left parietal bone in a 23-year-old man. Imaging studies including conventional radiography of the skull, CT, MR imaging, and Technetium-99 m (Tc-99 m) scintigraphy demonstrated a large skull defect without associated soft tissue mass over the left parietal skull. Contrast enhancement and increased isotope uptake along the margin of the defect were shown at gadolinium-enhanced T1-weighted MR imaging and Tc-99 m methylene diphosphate (Tc-99 m MDP) bone scintigraphy. Pathologic study revealed intraosseous angiomatosis at the periphery of the osteolytic skull lesion.
Gorham disease—also known as massive osteolysis of Gorham, disappearing bone disease, vanishing bone disease, phantom bone disease, and Gorham-Stout syndrome (1–5)—was first reported by Jackson in 1838 in a 12-year-old boy with complete osteolysis of the humerus (6). In 1955, Gorham and Stout further characterized the main pathologic features of this rare disease as nonmalignant intraosseous proliferation of hemangiomatous or lymphangiomatous tissue that caused massive osteolysis (7). Noncranial imaging features of Gorham disease at conventional radiography, CT, MR, and scintigraphy have been reported elsewhere in the literature (1–5, 8, 9), and most of the cases failed to show the vasculature entity of the lesion, an important pathologic feature of the disease (1, 3, 8, 9). Moreover, because of a lack of specific findings on conventional images, including those of conventional radiography and CT, the diagnosis of Gorham disease with skull involvement can be difficult. Other diseases such as Paget disease and metastases may have similar appearance. In this report, we present the unique MR imaging findings of a pathologically proved case of Gorham disease of the cranial vault.
Case Report
A 23-year-old man had an asymptomatic skull defect over the left parietal region that progressively enlarged over a period of 7 years. The defect began as a small left parietal scalp depression that could not link to any previous head trauma. A conventional radiograph of the skull was obtained, and no definite diagnosis was made at that time. He did not undergo further examination, because the soft tissue depression did not bother him. When he was conscripted to the armed forces, he noted that the defect was larger and was then referred to our hospital for further evaluation.
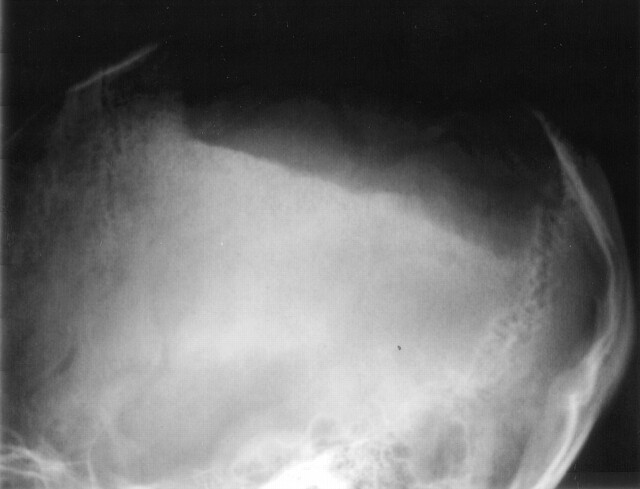
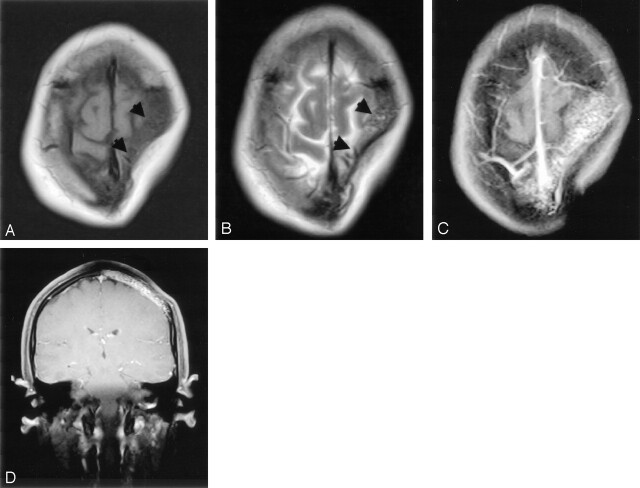
Physical examination revealed no palpable mass or tenderness over the scalp, but a large area of softening and depression over the left parietal region about 7 × 4 cm in size was found. The laboratory results were unremarkable. Conventional radiography of the skull showed a large, sharply demarcated bony defect without marginal sclerosis over the left parietal region (Fig 1). An MR study obtained 7 days later disclosed the calvarial defect to be filled with thin soft tissues that were hypointense on both spin-echo T1-weighted (TR/TE, 402 ms/12.1 ms) and fast spin-echo T2-weighted (4275 ms/96 ms) imaging (Fig 2A and B), which suggested fibrous tissue with nonmobile protons. Contrast-enhanced T1-weighted images with fat saturation showed a reticular pattern of contrast enhancement at the periphery of the defect in the presumed marrow space (Fig 2C and D). Noncontrast cranial CT with 3D reconstruction by using a bone algorithm was performed 20 days after MR study and revealed a well-defined skull defect with complete disappearance of the central bone matrix and no evidence of new bone formation (Fig 3). The soft tissue widows showed no associated soft tissue mass in or around the calvarial defect. A chronic but still active destructive process of the calvaria was considered on the basis of the clinical history and imaging findings. To search for other possible similar bony lesion in the body, a Technetium-99 m methylene diphosphate (Tc-99 m MDP) whole-body bone scan was undertaken, which revealed increased uptake over the left parietal area with a translucency in the center (Fig 4). The preoperative differential diagnosis included juvenile Paget disease at destructive stage, eosinophilic granuloma, Brown tumor, and osteolytic metastasis but none of them correlated well with the clinical and radiologic features.
Fig 1.
Lateral-projection conventional radiograph of the skull shows a large, sharply demarcated defect over the left parietal bone.
Fig 2.
A, Axial spin-echo T1-weighted (TR/TE/NEX: 402/12.1/3) and B, T2-weighted (4275/96/3) MR images show a focal scalp depression with loss of subjacent calvarial marrow signal intensity (arrowheads). C and D, Axial and coronal contrast-enhanced fat-saturated T1-weighted images obtained at the margins of the skull defect show reticular-pattern of enhancement.
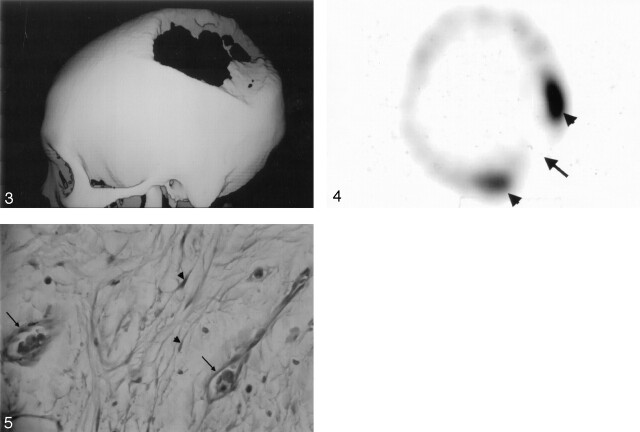
Fig 3.
3D reconstructed CT scan obtained by using a bone algorithm viewed from the left lateral delineates well the large skull defect.
Fig 4. Tc-99 m MDP bone scintigraphy reveals no bone uptake in the resorbed site (arrow) but increased uptake around the margin (arrowheads).
Fig 5. Photomicrograph of the bone specimen shows the small capillary-like vessels (arrows) and spindle-shaped fibroblasts (arrowheads) (hematoxylin and eosin stain, × 400)
A left parietal craniectomy was performed, disclosing a large bony defect with a very thin remainder of the inner table. Under microscopy, the bone specimens taken from the edges of the defect showed intraosseous angiomatosis with mixed patterns of bone destruction, fibrous connective tissue replacement, and abnormal small vessel proliferation (Fig 5). The combination of clinical, imaging, and pathologic findings strongly suggested the diagnosis as Gorham disease. The patient had an uneventful postoperative course and was discharged 12 days later without further treatment. A clinical follow-up at 3 months after surgery remained unchanged. Reconstructive surgery with artificial bone graft will be scheduled in the next hospital course.
Discussion
Gorham disease is a nonhereditary disease with no sex predilection. Most patients are younger than 40 years (1–3, 5). More than 150 cases have been documented in the literature (3). Among them, the pelvic and shoulder regions were most frequently involved, although any bone may be affected. The skull is among the least common locations of involvement (1–3, 5, 8, 9). Although the exact etiology of osteolysis is not clearly known, it is considered by most to be due to nonmalignant, neoplastic proliferation of hemangiomatous (or lymphangiomatous) tissue (1–3, 5, 8–10). Some authors have suggested that the slow circulation in the capillary-like vessels produces local hypoxia and lowering of the pH, favoring the activity of various hydrolytic enzymes such as acid phosphatase and leucine aminopeptidase, resulting in bone destruction (2, 5, 8, 11). Some authors have also postulated that local hyperemia and mechanical forces may promote bone resorption and that trauma might trigger the process by stimulating the production of vascular granulation tissue (2). Johnson and McClure suggested that there are two stages of Gorham disease (6). The first stage is characterized by vascular proliferation in connective tissue, which is consistent with hemangiomatosis. This explains some of the pathology reports of Gorham disease as “skeletal hemangioma” (2, 3, 11). In the second stage, fibrous tissue replaces the absorbed bone without regeneration of the bone matrix. Whether osteoclasts are involved in the mechanism of bone destruction remains controversial. Most authors have not observed osteoclasts in the areas of excessive bone resorption by microscopy (1, 2, 5, 11).
Clinical manifestations vary. Some patients have pain, swelling, or a pathologic fracture, whereas others may be asymptomatic or have an insidious onset of soft tissue atrophy or limitation of movement (1–3, 5, 8, 9, 11). Laboratory findings tend to be unremarkable with slightly increased serum alkaline phosphatase level in some cases (1, 2, 9, 11). Gorham disease must be distinguished from osteolysis secondary to other pathologic processes, including the hereditary, metabolic, neoplastic, infectious, and immunologic etiologies (3, 8). Common differential diagnoses include hereditary multicentric osteolysis, essential osteolysis with nephropathy, metastasis, osteomyelitis, and rheumatoid arthritis (1–3). The clinical findings are usually helpful in ruling out these diseases.
The osseous deformity or pathologic fracture in Gorham disease is common, but more serious complications are infrequent (1–3, 8, 11). Probable thoracic duct invasion with chylothorax and respiratory compromise has been reported (2, 3). Paraplegia may occur in cases of vertebral involvement (2). Many different kinds of therapy have been attempted. A complex treatment of hormones with calcium salts and vitamins showed no efficacy (1). Alpha-2b interferon (an antiangiogenic agent) combined with oral clodronate (an antiosteoclastic drug) was reported to be effective in one case, although the authors suggested the clodronate might be less important in the therapy because of the controversial role of the osteoclast in Gorham disease (12). Radiation therapy with a dose of 30–40 Gy has been reported to shift the disease from active to inactive phase or achieve pain relief with varying degrees of success (1, 3, 8, 9). Most authors have suggested that surgical resection with artificial bone replacement has been the most effective treatment for disease control, but the homologous bone grafts may also undergo osteolysis (1, 3, 8, 9).
The initial radiologic feature of Gorham disease may reveal radiolucent foci in the intramedullary or subcortical regions that resemble osteoporosis (1–3). Subsequently, progressive dissolution and disappearance of a portion of the bone may occur. The osteolysis may extend to the contiguous bone and cross the intervening joint (1–4, 8). In the long bones, the tapering of the residual bone may show “licked candy stick” deformity (1, 3, 8). The osseous destruction may persist for a period of years and may eventually stabilize (2, 4, 5). Tc-99 m bone scintigraphy may demonstrate increased uptake of the radiopharmaceutical agents on the initial images and, subsequently, an area of decreased uptake corresponding to the diminished bone region (1, 2, 5).
The increased uptake over the left parietal area with central translucency seen in our case may indicate ongoing osteolysis in the transitional zone. CT with bone window setting provides delineation of the extent of osteolysis, and 3D imaging is useful for surgical planning for skull reconstruction (1). MR imaging, however, allows superior delineation of bone marrow and soft tissue involvement (1, 3). To our knowledge, the MR imaging appearance of Gorham disease involving the calvaria has not been reported previously in the literature. Yoo et al reviewed noncalvarial MR imaging in seven cases and concluded that the lesion site often showed iso- to hypointense signal at T1-weighted imaging and hyperintense signal at T2-weighted imaging (3). Notwithstanding, their cases often failed to show the vascular component, although one case was found to have a small area with flow-void vessels (5). In our experience, fat-suppressed T1-weighted images with contrast medium injection may improve the visualization of the vascular components of the lesion in which a reticular pattern of contrast enhancement can be seen. Although other pathologic conditions in the skull such as juvenile Paget disease, eosinophilic granuloma, Brown tumor, and osteolytic metastases may have similar imaging findings, the long asymptomatic clinical course and typical CT and MR imaging findings may allow differentiation of Gorham disease.
References
- 1.Vinée P, Tanyü MO, Hauenstein KH, et al. CT and MRI of Gorham syndrome. J Comput Assist Tomogr 1994;18:985–989 [DOI] [PubMed] [Google Scholar]
- 2.Donald Rresnick. Osteolysis and chondrolysis. In: Resnick D, ed. Diagnosis of Bone and Joint Disorders. 4th ed. Philadelphia: WB Saunders;2002. :4928–4931
- 3.Yoo SY, Hong SW, Chung HW, et al. MRI of Gorham’s disease: findings in two cases. Skeletal Radiol 2002;31:301–306 [DOI] [PubMed] [Google Scholar]
- 4.Igel BJ, Shah H, Williamson MR, Sell JJ. Gorham’s syndrome: correlative imaging using nuclear medicine, plain film, and 3-D CT. Clin Nucl Med 1994;11:1017–1019 [PubMed] [Google Scholar]
- 5.Spieth ME, Greenspan A, Forrester DM, et al. Gorham’s disease of the radius: radiographic, scintigraphic, and MRI findings with pathologic correlation: a case report and review of the literature. Skeletal Radiol 1997;26:659–663 [DOI] [PubMed] [Google Scholar]
- 6.Jackson JBS. A boneless arm. Boston Med Surg J 1838;18:368–369 [Google Scholar]
- 7.Gorham LW, Stout AP. Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone): its relation to hemangiomatosis. J Bone Joint Surg Am 1955;37:985–1044 [PubMed] [Google Scholar]
- 8.Sato K, Sugiura H, Yamamura S, et al. Gorham massive osteolysis. Arch Orthop Trauma Surg 1997;116:510–513 [DOI] [PubMed] [Google Scholar]
- 9.Klein HM, Metelmann U-R. Gross massive osteolysis (Gorham-Stout syndrome) in the maxillofacial region: an unusual manifestation. Int J Oral Maxillofac Surg. 1996;25:376–378 [DOI] [PubMed] [Google Scholar]
- 10.Johnson PM, McClure JG. Observations on massive osteolysis. Radiology 1958;71:28–42 [DOI] [PubMed] [Google Scholar]
- 11.Pazzaglia UE, Andrini L, Bonato M, Leutner M. Pathology of disappearing bone disease: a case report with immunohistochemical study. Int Orthop 1997;21:303–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hagberg H, Lamberg K, Astrom G. Alpha-2b interferon and oral clodronate for Gorham’s disease. Lancet 1997;350:1822–1823 [DOI] [PubMed] [Google Scholar]