Abstract
Summary: Wide-necked bifurcation aneurysms remain a formidable challenge to the neuroendovascular surgeon. A 36-year-old woman with a wide-necked basilar bifurcation aneurysm was unsuccessfully treated by endovascular methods, despite the use of the balloon-remodeling technique. Successful coiling was ultimately achieved by use of a Y-configuration double stent-assisted technique. This novel method of using self-expanding stents may represent a significant advance in the management of basilar apex and other bifurcation aneurysms.
Recent advances in endovascular techniques have greatly expanded the scope of intracranial aneurysms amenable to endovascular obliteration. The technique of balloon remodeling and the development of a self-expanding stent for intracranial use allow for the safe and effective coiling of wide-necked aneurysms that previously may have been treatable only by microsurgery. We report a novel method of embolizing wide-necked basilar apex aneurysms by employing a Y-configuration, double stent-assisted technique.
Case Report and Description of Technique
The patient was a 36-year-old, right-handed woman with the incidental finding of two broad-based intracranial saccular aneurysms: one at the basilar apex and the other at the anterior communicating artery complex. Because of the low-lying position of the basilar terminus beneath the posterior clinoids, endovascular coiling was recommended for the basilar apex aneurysm. An initial attempt at another major university medical center was made with a Micrus 8-mm framing coil (Micrus Corp., Mountain View, CA), but coil herniation into the parent vessel and an unstable coil position precluded safe deployment.
A second opinion was sought at our institution, and another attempt at endovascular treatment with possible balloon remodeling was recommended. Attempts to safely place a 3D Guglielmi detachable coil (GDC; Boston Scientific/Target Therapeutics, Fremont, CA) without parent vessel protection were unsuccessful. Subsequently, maneuvers to protect the parent vessel with the balloon-remodeling technique and two-catheter technique were attempted. Because a stable coil configuration could not be achieved, no coils were detached and the procedure was aborted. The patient subsequently declined our recommendation for surgical clipping of this aneurysm. She elected to be followed and remained clinically stable for a year.
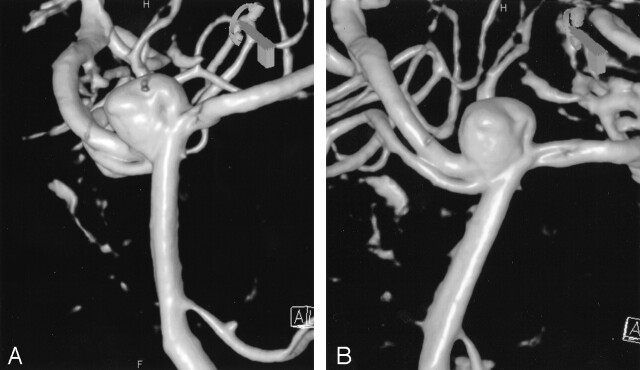
Reconsideration of endovascular treatment was made after the Neuroform self-expanding intracranial stent (Boston Scientific/Target Therapeutics) became available. The patient agreed to undergo another attempt at endovascular therapy. Under general anesthesia, 3D rotational angiography was performed, confirming an 8-mm wide-necked basilar apex aneurysm (Fig 1A and B). The reconstructions demonstrated that the distal basilar artery was dilated in an anteroposterior direction with incorporation of the origins of the posterior cerebral arteries (PCA) bilaterally into the aneurysm neck. To adequately reconstruct the parent vessels, we decided to deploy two Neuroform stents, one in each PCA (Fig 2), to create a bifurcated stent framework. Under roadmap guidance, an SL-10 microcatheter with a Transend EX 0.014-inch microwire (Boston Scientific/Target Therapeutics) was used to select the right PCA. An X-celerator 0.014-inch exchange length wire (Microtherapeutics, Inc., Irvine, CA) was then positioned in the right PCA. The first Neuroform stent (4.0 × 20 mm) was deployed in the right P1 segment extending down to the upper basilar artery distal to the origin of the anterior interior cerebellar arteries (AICA) (Fig 3A). The microwire was then partially withdrawn to the level of the left PCA origin, and, through the interstices of the stent, the left PCA was selected (Fig 3B). A second Neuroform stent (3.5 × 20 mm) was then deployed with half of the stent in the left P1 and the other half within the lumen of the previously placed stent (Fig 4A and B). The microwire was then used to select the dome of the aneurysm, and the SL-10 microcatheter easily catheterized the aneurysm. The SL-10 microcatheter was subsequently used to deliver the following GDC coils: GDC-10–3D 7 mm × 15 cm; GDC-10–2D, 6 mm × 20 cm; GDC-10-soft/2D-SR 5 mm × 10 cm; GDC-10-soft/2D-SR 5 mm × 8 cm; GDC-10-Soft 4 mm × 8 cm; GDC-10-soft/2D-SR 4 mm × 8 cm; GDC-10-Soft/2D-SR 4 mm × 6 cm; GDC-US 4 mm × 8 cm; and a GDC-US 3 mm × 4 cm. A satisfactory result was obtained, with only a minor amount of residual opacification seen in the aneurysm neck (Fig 5A–D). The patient was given oral clopidogrel 425 mg and aspirin 325 mg before the start of the procedure, and she was fully heparinized before deployment of the Neuroform stents. The heparin was not reversed.
Fig 1.
Bilateral oblique views (A and B) of the 3D rotational angiogram shows the aneurysm neck incorporating the origins of both PCAs. The aneurysm neck is dilated in the anteroposterior direction.
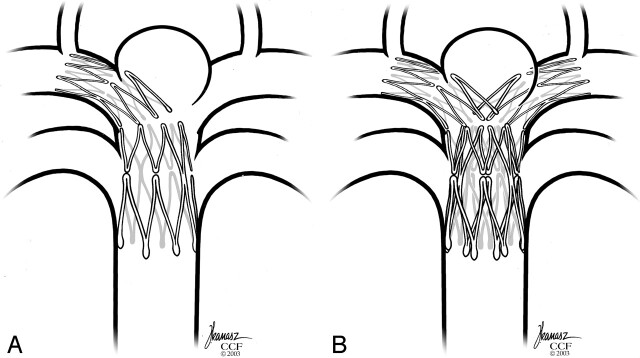
Fig 2.
Final configuration of the dual stents with the distal end of each Neuroform stent in a PCA and the proximal ends telescoped within each other in the midbasilar artery.
A, First stent deployed.
B, Second stent through interstices.
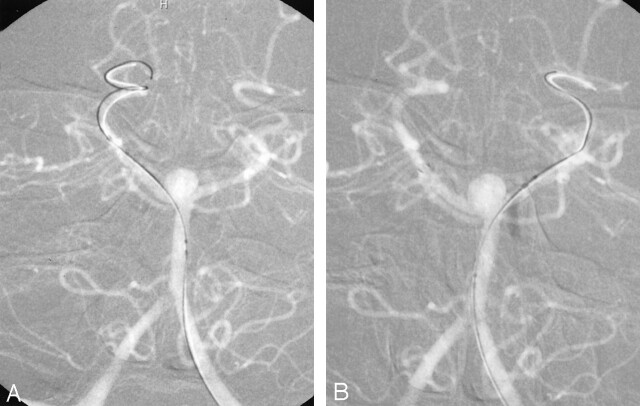
Fig 3.
Intraprocedural roadmap showing sequence of stent deployment.
A, Anteroposterior left vertebral artery angiogram, showing placement of the guidewire in the right PCA before deployment of the initial Neuroform stent.
B, Anteroposterior left vertebral artery angiogram, showing successful deployment of the first Neuroform stent and selection of the left PCA through the interstices of the first stent by the guidewire.
Fig 4.
Anteroposterior left vertebral artery angiogram with (A) and without (B) native anatomy, showing successful deployment of the second Neuroform stent with the distal end in the left P1 segment and the proximal end telescoped within the first stent in the distal basilar artery. Note the position of the microcatheter that has traversed the interstices of the stents to lie within the aneurysm itself.
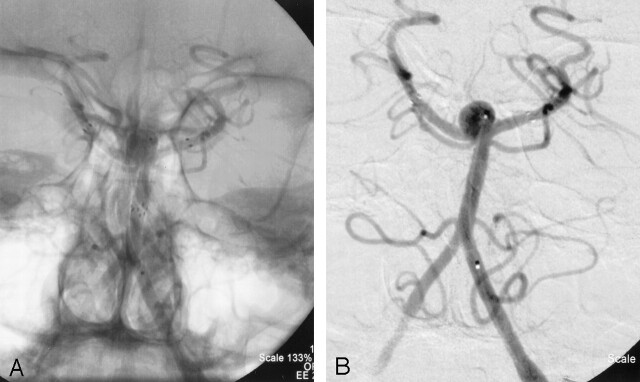
Fig 5.
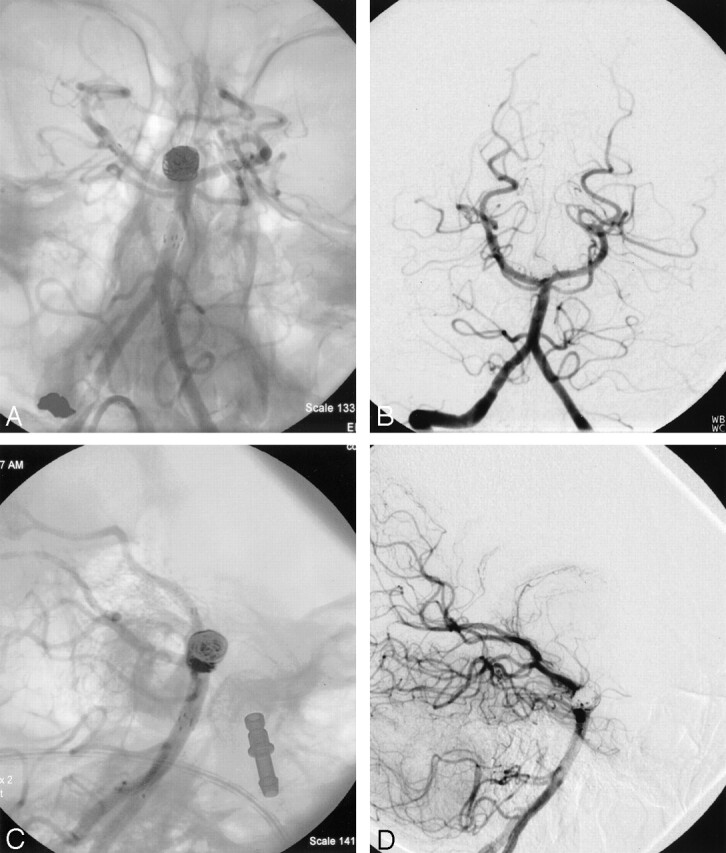
Anteroposterior left vertebral artery angiogram with (A) and without (B) native anatomy, showing complete obliteration of the aneurysm with preservation of bilateral PCAs. Lateral left vertebral artery angiogram with (C) and without (D) native anatomy, showing a small amount of opacification within the coil loops at the neck of the aneurysm. Also note loops of coil excluded from basilar terminus by stents filling the anterior and posterior “pouches” of the aneurysm at the basilar artery terminus.
On emergence from anesthesia, the patient was neurologically normal. Two hours later, she experienced diplopia and occipital headache, and a mild internuclear ophthalmoplegia (INO) was present on examination. She was given dexamethasone 4 mg intravenously, and an emergency head CT demonstrated no abnormalities. A heparin infusion of 900 U/h was initiated. The following morning the diplopia and the INO had resolved. She was discharged later that day, neurologically intact, on a tapering dose of steroids, aspirin, and clopidogrel.
Discussion
The advances in endovascular technology have continued to expand the scope of intracranial aneurysms that are safely treated with interventional techniques. Despite remarkable progress, one of the fundamental problems continues to be the feasibility of coiling wide-necked aneurysms, especially those at vessel bifurcations. To that end, various techniques have been developed to address this problem; in particular, the technique of balloon remodeling (1–4). Another recent innovation has been the development of the TriSpan neck-bridge device (Boston Scientific/Target Therapeutics), which has proved useful in the embolization of some wide-necked bifurcation aneurysms (5). Because the Trispan device currently does not have U.S. Food and Drug Administration approval, it is not available in the United States. After several promising studies performed in animal models, the first report of stent-assisted coiling occurred with a ruptured fusiform aneurysm of the basilar artery (6). Since the initial report, several additional reports in the literature have described the use of intravascular stents to assist in the coil embolization of wide-necked saccular aneurysms (7–9). Nevertheless, proliferation of this technique has been hampered because many of these earlier attempts were performed with relatively stiff, rigid balloon-expandable stents designed for the coronary circulation. With the advent of the more flexible, and self-expanding stents engineered specifically for intracranial use, such as the Neuroform stent, a larger proportion of wide-necked aneurysms have become amenable to endovascular treatment.
The problem posed by a wide-necked basilar bifurcation aneurysm is different from that of a wide-necked, sidewall internal carotid artery (ICA) aneurysm. Prior stent-assisted coiling of basilar apex aneurysms involved deploying a stent into only one PCA, with the proximal end in the basilar thereby “jailing” the contralateral PCA. This technique provides only partial protection from coil herniation into the parent vessel. Another hypothetical concern regarding this asymmetric stent deployment would be the alteration of flow favoring the PCA in which the distal end of the stent was deployed.
To solve the problem of partial protection, we were able to reconstruct the basilar apex by deploying a second stent through the interstices of the first stent. In this fashion, all of the major parent vessels were favorably reconstructed and the entire aneurysm neck was protected from coil herniation. A technical caveat in achieving this configuration involves deploying the more challenging stent first. For instance, depending on the particular anatomy of a basilar bifurcation, one of the PCAs may originate at a more acute angle relative to the basilar trunk and thus be more difficult to catheterize selectively. This PCA should be stented first, if possible. Once a stent is deployed, it would have the effect of slightly straightening the angle of the PCA relative to the basilar artery. The interstices of the stent could make subsequent selection of the contralateral PCA that much more difficult. In addition, we chose to deploy our second stent so that its proximal end coincided with the proximal end of the first stent. Theoretically, this would minimize disruption of laminar blood flow at the bifurcation. For instance, if the second stent was too short and was left abutting the sidewall of the first stent, turbulent flow could potentially lead to thrombus formation with the stent configuration.
Another potential advantage of redirecting the laminar blood flow pattern is that, theoretically, the incidence of coil compaction might be reduced. Many investigators believe that incompletely coiled basilar terminus aneurysms are particularly prone to re-canalization secondary to the hemodynamics of the parent artery and the resultant high-pressure pulsatile flow directly at the coil mass (10). The placement of both stents may alter the flow pattern at the aneurysm neck, redirecting flow toward the PCAs to potentially reduce the likelihood of recanalization.
Even with the optimal placement of both stents, the threat of thrombus formation and possible embolization is very real. This was clearly illustrated in our patient who developed a transient INO that was felt to be secondary to intraprocedural thrombus formation and subsequent postprocedural embolization into the brain stem. Besides full heparinization during the procedure, it is our practice to treat all of our patients who undergo Neuroform stent placement with 325 mg of aspirin and 425 mg of clopidogrel before the procedure. Alternatively, patients can be started on clopidogrel 75 mg/day 3 days before the procedure. Furthermore, to lessen the incidence of delayed thromboembolism and long-term stenosis within the stent, patients are maintained on clopidogrel for a total of 6 weeks in addition to 6 months of aspirin therapy.
We feel that the implications of this technique are significant. Middle cerebral artery bifurcation aneurysms, which have long been considered by many to be primarily surgical lesions, could potentially be safely treated by endovascular means, by using the Y-configuration double-stent technique. The major current limitation is the size of the parent vessel relative to the diameter of the smallest available stent.
Conclusion
This solitary case needs to bear the scrutiny of long-term clinical follow-up, and we anticipate further limitations of this technique to be discovered with its use in future patients. The technical and clinical result achieved, however, was highly encouraging, and we feel the Y-configuration double-stent technique may significantly contribute to the endovascular treatment of intracranial aneurysms.
References
- 1.Aletich VA, Debrun GM, Misra M, et al. The remodeling technique of balloon-assisted Guglielmi detachable coil placement in wide-necked aneurysms: experience at the University of Illinois at Chicago. J Neurosurg 2000;93:388–396 [DOI] [PubMed] [Google Scholar]
- 2.Lefkowitz MA, Gobin YP, Akiba Y, et al. Balloon-assisted Guglielmi detachable coiling of wide-necked aneurysma. Part II. Clinical results. Neurosurgery 1999;45:531–537; discussion 537–538 [DOI] [PubMed] [Google Scholar]
- 3.Malek AM, Halbach VV, Phatouros CC, et al. Balloon-assist technique for endovascular coil embolization of geometrically difficult intracranial aneurysms. Neurosurgery 2000;46:1397–1406; discussion 1406–1407 [DOI] [PubMed] [Google Scholar]
- 4.Moret J, Cognard C, Weill A, et al. [Reconstruction technique in the treatment of wide-neck intracranial aneurysms. Long-term angiographic and clinical results: apropos of 56 cases]. J Neuroradiol 1997;24:30–44 [PubMed] [Google Scholar]
- 5.Raymond J, Guilbert F, Roy D. Neck-bridge device for endovascular treatment of wide-neck bifurcation aneurysms: initial experience. Radiology 2001;221:318–326 [DOI] [PubMed] [Google Scholar]
- 6.Higashida RT, Smith W, Gress D, et al. Intravascular stent and endovascular coil placement for a ruptured fusiform aneurysm of the basilar artery: case report and review of the literature. J Neurosurg 1997;87:944–949 [DOI] [PubMed] [Google Scholar]
- 7.Lanzino G, Wakhloo AK, Fessler RD, et al. Efficacy and current limitations of intravascular stents for intracranial internal carotid, vertebral, and basilar artery aneurysms. J Neurosurg 1999;91:538–546 [DOI] [PubMed] [Google Scholar]
- 8.Lavine SD, Larsen DW, Giannotta SL, Teitelbaum GP. Parent vessel Guglielmi detachable coil herniation during wide-necked aneurysm embolization: treatment with intracranial stent placement: two technical case reports. Neurosurgery 2000;46:1013–1017 [PubMed] [Google Scholar]
- 9.Mericle RA, Lanzino G, Wakhloo AK, et al. Stenting and secondary coiling of intracranial internal carotid artery aneurysm: technical case report. Neurosurgery 1998;43:1229–1234 [DOI] [PubMed] [Google Scholar]
- 10.Mericle RA, Wakhloo AK, Lopes DK, et al. Delayed aneurysm regrowth and recanalization after Guglielmi detachable coil treatment: case report. J Neurosurg 1998;89:142–145 [DOI] [PubMed] [Google Scholar]