Abstract
Summary: Four patients with brain tumors were diagnosed with atypical teratoid/rhabdoid tumors and underwent CT and MR imaging. For all tumors, aggressive features were shown by imaging studies and included hydrocephalus, apparent invasion of the adjacent brain and dura, and marked mass effect. The striking heterogeneity of the atypical teratoid/rhabdoid tumor shown by imaging studies reflects the histopathologic complexity of these tumors, and awareness of atypical teratoid/rhabdoid tumor is important in making the correct diagnosis of this uncommon but probably underdiagnosed entity.
Atypical teratoid/rhabdoid tumor, formerly known as malignant rhabdoid tumor, is an uncommon CNS malignancy with a relatively frequent occurrence in very young children (1). Historically, atypical teratoid/rhabdoid tumors were frequently misdiagnosed as primitive neuroectodermal tumors because of their similar histologic features (1, 2). The diagnosis of atypical teratoid/rhabdoid tumor is made based on light microscopy and immunohistochemistry findings. Molecular genetic analysis may provide supportive information (1, 3). Several authors have described the radiologic properties of atypical teratoid/rhabdoid tumor in case reports that shared some common features, such as large tumor size and enhancement with contrast material (4–9). We describe the imaging characteristics of atypical teratoid/rhabdoid tumor with modern imaging modalities used in four cases.
Case Reports
Four patients with brain tumors were diagnosed with atypical teratoid/rhabdoid tumors at our institution between 1996 and 2000. All patients underwent CT of the brain without the use of IV administered contrast material. All patients underwent MR imaging of the brain, performed on 1.5-T units (Signa; GE Medical Systems, Milwaukee, WI) with a standard quadrature head coil and IV administration of contrast material at 0.1 mmol/kg. All patients underwent surgery for removal/debulking of their intracranial masses. Their charts and imaging studies were reviewed. We analyzed the size, location, composition, enhancement, and invasion patterns of the tumors.
One male and three female patients were included in the study. Three patients were younger than 1 year. One patient was 16 years old. Clinical, imaging, and pathologic findings are summarized in Table 1.
Clinical, pathological, and imaging findings of four patients with the diagnosis of atypical teratoid/rhabdoid tumor
| Patient No. | Age/Sex | Symptoms/Findings | CT and MR Imaging Findings |
Resection | Pathologic Findings | Outcome | |||
|---|---|---|---|---|---|---|---|---|---|
| Location/Invasion | Composition | Enhancement | Calcification | ||||||
| 1 | 9 mo/F | R-sided weakness | Cystic L frontoparietal occipital, skull destruction | Solid and cystic, peripheral cyst | Thick irregular nodular rim enhancement | Yes | GTR | + Vimentin | 7 mo no therapy other than surgery |
| + GFAP | |||||||||
| + EMA | |||||||||
| + Cytokeratins | |||||||||
| − Synaptophysin | |||||||||
| − Actin | |||||||||
| 2 | 8 mo/F | Poor feeding, macrocephaly, lethargy | L hemisphere, infiltrative to adjacent parenchyma | Solid and cystic | Thick irregular nodular rim enhancement | Yes | STR | + Vimentin | 5 mo with therapy |
| + GFAP | |||||||||
| + EMA | |||||||||
| 3 | 16 y/F | R-sided weakness, numbness of the feet | Tectal/pineal, brain stem invasion | Solid and cystic, peripheral cyst | Intense enhancement | Yes | STR | + Vimentin | 3 mo with therapy |
| + GFAP | |||||||||
| + EMA | |||||||||
| − Synaptophysin | |||||||||
| − Actin | |||||||||
| − Ki-1 | |||||||||
| 4 | 11 mo/M | Multiple cranial nerve deficits | Brain stem/R CPA, brain stem invasion | Solid and cystic, peripheral cyst | Moderate enhancement | Yes | STR | + Vimentin | 1 mo with therapy |
| + GFAP | |||||||||
| + EMA | |||||||||
| + Cytokeratins | |||||||||
Note.—F indicates Female; M, male; R, right; L, left; CPA, cerebellopontine angle; GTR, gross total resection; STR, subtotal resection; GFAP, glial fibrillary acidic protein; EMA, epithelial membrane antigen.
Presenting symptoms depended on tumor location and included extremity weakness, macrocephaly, lethargy, extraocular muscle weakness, facial paralysis, and decreased gag reflex. All patients underwent surgical excision of the tumor, with gross total resection achieved in only one patient. Three patients were treated with chemotherapy. All patients died, with a mean survival of 5 months (range, 1–12 months) from diagnosis. All tumors stained with vimentin, glial fibrillary acidic protein, and epithelial membrane antigen.
Three of the tumors were supratentorial. One was infratentorial. Two of the three supratentorial tumors were located in the left hemisphere (Figs 1 and 2). The third supratentorial tumor was in the midbrain-tectum/pineal region (Fig 3). The infratentorial tumor involved the pons, cerebellum, and right cerebellopontine angle (Fig 4). The two hemispheric tumors were very large and occupied >60% of the hemisphere. The midbrain tectum and posterior fossa masses measured 3 to 4 cm in greatest dimension.
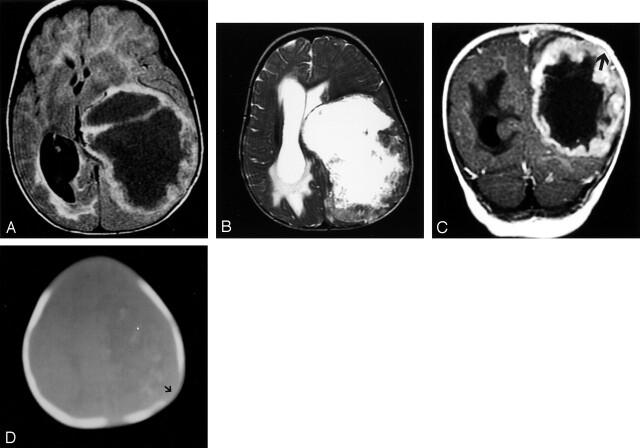
Fig 1.
Patient 1, with lesion in left cerebral hemisphere.
A, Axial view fluid-attenuated inversion recovery image shows a very large cyst mass with thick nodular walls and mass effect but little edema.
B, T2-weighted fast spin-echo image, obtained at the same level, shows a necrotic mass with a thick heterogeneous wall causing midline shift.
C, Coronal view contrast-enhanced image shows a nodular rind of tumor. Remodeling of the calvarium by the mass can be seen (arrow).
D, CT bone window shows erosion of the left parietal bone (arrow) and faint calcification.
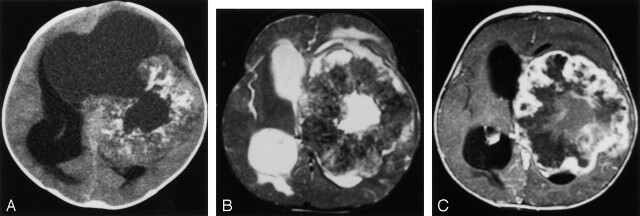
Fig 2.
Patient with left hemispheric mass.
A, Unenhanced CT scan displays a large doughnut-shaped mass with central cyst that opens into an eccentric large cystic component. Lesion has mass effect but little edema. Several calcifications are present in the tumor.
B, T2-weighted fast spin-echo image shows a large thick walled mass with mild surrounding edema. Note that the tumor is hypointense, presumably secondary to the calcifications.
C, Contrast-enhanced T1-weighted axial view image reveals a nodular peripheral enhancement in the thick calcified rind.
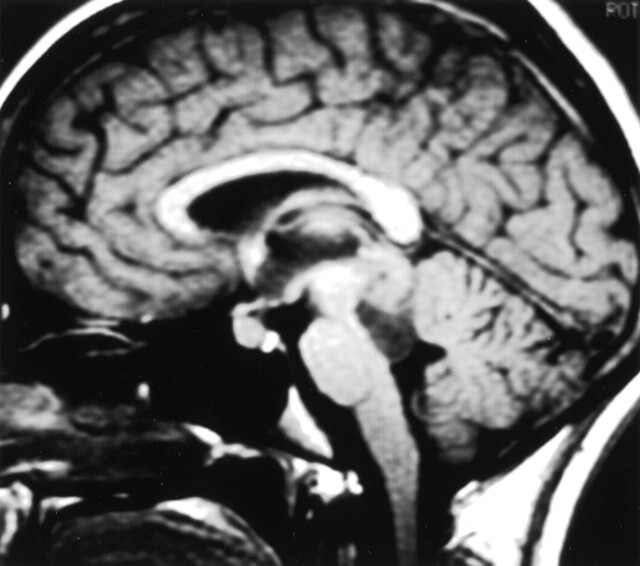
Fig 3.
Tectal mass. Mass with an eccentric cyst distorting the tectum can be seen.
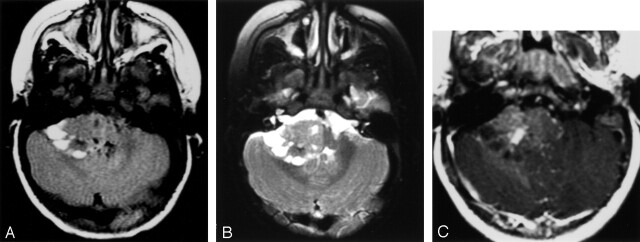
Fig 4.
Cerebellopontile angle mass.
A, Fluid-attenuated inversion recovery image shows a right cerebellopontile angle extra-axial mass with solid and peripheral cystic components. Focal hypointensities correspond to calcifications shown on CT scans (not shown).
B, T2-weighted fast spin-echo image shows a right cerebellopontile angle extra-axial mass with solid and peripheral cystic components. Focal hypointensities correspond to calcifications shown on CT scans (not shown).
C, Contrast-enhanced image shows mild enhancement with a small, relatively more enhancing nodule.
All tumors showed marked heterogeneity on both CT scans and MR images. Readily visible multiple foci of calcifications measuring approximately 1 to 5 mm were present on the CT scans of all patients. No calcification >1 cm was seen. All the tumors had cystic and solid areas. The location of the cysts was remarkable in that in three patients, they were situated eccentrically between the solid portion of the tumor and adjacent brain (Figs 2–4). In one patient with a hemispheric mass, the cystic part was in the center of the tumor. The cysts showed increased attenuation on CT scans and increased T1 signal intensity on MR images, as compared with the CSF. The walls of the eccentric cysts showed varying degrees of enhancement. The solid component of the masses was heterogeneous and showed varying degrees of increased signal intensity on T2-weighted images, decreased signal intensity on T1-weighted images, and mildly increased attenuation on CT scans. Even within the solid portion of the masses, there were small cystic areas. Enhancement was variable: moderate to marked in two patients (thick, irregular, nodular rim enhancement) and mild in others.
Aggressive features of all tumors were shown by the imaging studies. Hydrocephalus, apparent invasion of the adjacent brain and dura, and marked mass effect were present in all patients. One of the hemispheric tumors grossly invaded the skull (Fig 1D). Despite this aggressive appearance, there was little, if any, edema in the surrounding parenchyma.
Discussion
Atypical teratoid/rhabdoid tumor is an uncommon malignant CNS tumor typically seen in young children although it has been reported in adults as well. Atypical teratoid/rhabdoid tumor was included for the first time in the World Health Organization classification of tumors of the CNS in 2000, although it had been recognized during the early 1980s as a rhabdoid tumor of the CNS with an unfavorable prognosis. These tumors can arise at any location in the CNS, with approximately half of all tumors arising in the posterior fossa. The real incidence is difficult to determine, and it is probably underestimated in children younger than 2 years (3). In one study from Taiwan, the ratio of atypical teratoid/rhabdoid tumor to primitive neuroectodermal tumor was found to be 1:3.8 among patients younger than 3 years and 1:11 for all age groups (3). Almost all the posterior fossa atypical teratoid/rhabdoid tumor cases in the largest two series, which included 100 patients, were initially diagnosed as primitive neuroectodermal tumor (1, 2). This confusion occurs because only a minority of atypical teratoid/rhabdoid tumors has predominance of typical rhabdoid cells (the histologic hallmark of atypical teratoid/rhabdoid tumor), whereas >70% of atypical teratoid/rhabdoid tumors contain fields indistinguishable from primitive neuroectodermal tumor. Atypical teratoid/rhabdoid tumor shows characteristic immunohistochemical features, which tend to aid in the differentiation of atypical teratoid/rhabdoid tumor from primitive neuroectodermal tumor. These features include epithelial membrane antigen, vimentin, and smooth muscle actin positivity. More recently, cytogenetic abnormalities have allowed for improved diagnostic classification. Monozomy 22 or deletions of chromosome band 22q11 with alterations of the hSNF5/INI1 gene are shown in patients with atypical teratoid/rhabdoid tumors (1, 2). A priori suspicion of this entity is important, however, in directing the immunohistochemical studies. Yet neuroradiologists rarely mention atypical teratoid/rhabdoid tumor in their differential diagnosis.
Imaging features of atypical teratoid/rhabdoid tumor have been described in multiple case reports (4–10). In larger series, the clinical and histopathologic features of atypical teratoid/rhabdoid tumor have been defined, with some reference to imaging findings (1, 2). In 36 of 53 patients in the study presented by Burger et al (2) and 29 of 52 patients in the study presented by Rorke et al (1), the tumor was located in the posterior fossa. The rest of the tumors were supratentorial. Multifocal tumors at presentation were described, emphasizing the propensity of atypical teratoid/rhabdoid tumor to metastasize via CSF seeding. One patient with a spinal tumor has also been reported. No attempt has been made in the histopathologic studies to assign these tumors into specific subsites in the posterior fossa, but it seems that atypical teratoid/rhabdoid tumor has a tendency to occur off-midline. The tumor was in the cerebellopontine angle in our only patient with a posterior fossa mass. This may be helpful in differentiating atypical teratoid/rhabdoid tumor from primitive neuroectodermal tumor, which more commonly arises at midline, although the location of both entities is variable and may be difficult to clearly identify because of the large size. Ependymomas and pilocytic astrocytomas frequently extend to the cerebellopontine angle, but they are more commonly seen in older children. The location of atypical teratoid/rhabdoid tumor in the supratentorial brain is variable as is primitive neuroectodermal tumors.
The striking heterogeneity of the atypical teratoid/rhabdoid tumor shown by imaging studies, as seen on the images of our patients and as previously reported, apparently reflects the histopathologic complexity of these tumors. All four tumors in the present study had readily visible calcifications. Approximately 15% of primitive neuroectodermal tumors in the posterior fossa contain calcification shown by histologic examination, but visible calcification is very rare, even on CT scans. Ependymomas not infrequently show calcification and are heterogeneous on imaging studies but do not contain large cysts. In the supratentorial compartment, primitive neuroectodermal tumors have visible calcifications on CT scans in half of the cases.
Eccentrically located cysts between the solid tumor and adjacent brain were present in three of the four patients in the present study. The cyst wall showed enhancement in all cases. The frequent presence of cysts associated with atypical teratoid/rhabdoid tumor has been reported, but no specific reference has been made to the location of these cysts relative to the usually bulky mass. When reviewing the images provided in five previous case reports, one can identify similar peripherally located and relatively large cysts (3, 5, 6, 10, 11). This may be an important differentiating point between primitive neuroectodermal tumor and atypical teratoid/rhabdoid tumor, because primitive neuroectodermal tumors are usually more uniform, although in approximately 10% of cases, marked heterogeneity and cyst formation can be seen. Pilocytic astrocytomas typically have eccentric cysts but are not infiltrative in appearance and more commonly occur in older children. Supratentorial primitive neuroectodermal tumor, pilocytic astrocytoma, and desmoplastic infantile ganglioglioma not infrequently have cystic components, making this finding ineffective in the supratentorial compartment.
Skull invasion was readily identified on both CT scans and MR images in one of the hemispheric atypical teratoid/rhabdoid tumors in this study, which reflects the very aggressive nature of these tumors. A similar occurrence has been reported in association with atypical teratoid/rhabdoid tumor and primitive neuroectodermal tumor (12). Skull invasion is not seen with desmoplastic infantile ganglioglioma or pilocytic astrocytoma.
Lack or paucity of vasogenic edema has been described in association with primitive neuroectodermal tumor. No significant edema was observed in any of our patients when transependymal CSF leak due to hydrocephalus is excluded. A variable degree of enhancement was present in all our cases, but this is not a helpful finding in differential diagnosis because most of the other tumors also show enhancement. Leptomeningeal spread, although not present in any of our patients, can be seen at presentation.
The outcome for children diagnosed with atypical teratoid/rhabdoid tumor is very poor. The mean survival in our four patients was only 5 months. In their series of 53 patients, Burger et al (2) reported a mean survival of 11 months.
In conclusion, atypical teratoid/rhabdoid tumor is frequently confused with primitive neuroectodermal tumor based on histopathologic evaluation because of their similar features. Awareness of atypical teratoid/rhabdoid tumor is important in making the correct diagnosis of this uncommon but probably underdiagnosed entity. Bulky, heterogeneous masses with calcifications, eccentric cysts, and off-midline location in the posterior fossa in children younger than 2 years should alert the radiologist to the possibility of atypical teratoid/rhabdoid tumor in the differential diagnosis of the mass.
References
- 1.Rorke LB, Packer RJ, Biegel JA. Central nervous system atypical teratoid/rhabdoid tumors of infancy and childhood: definition of an entity. J Neurosurg 1996;85:56–65 [DOI] [PubMed] [Google Scholar]
- 2.Burger PC, Yu IT, Tihan T, et al. Atypical teratoid/rhabdoid tumor of the central nervous system: a highly malignant tumor of infancy and childhood frequently mistaken for medulloblastoma: a Pediatric Oncology Group study. Am J Surg Pathol 1998;22:1083–1092 [DOI] [PubMed] [Google Scholar]
- 3.Packer RJ, Biegel JA, Blaney S, et al. Atypical teratoid/rhabdoid tumor of the central nervous system: report on workshop. J Pediatr Hematol Oncol 2002;24:337–342 [DOI] [PubMed] [Google Scholar]
- 4.Arrazola J, Pedrosa I, Mendez R, Saldana C, Scheithauer BW, Martinez A. Primary malignant rhabdoid tumour of the brain in an adult. Neuroradiology 2000;42:363–367 [DOI] [PubMed] [Google Scholar]
- 5.Howlett DC, King AP, Jarosz JM, et al. Imaging and pathological features of primary malignant rhabdoid tumours of the brain and spine. Neuroradiology 1997;39:719–723 [DOI] [PubMed] [Google Scholar]
- 6.Proust F, Laquerriere A, Constantin B, Ruchoux MM, Vannier JP, Freger P. Simultaneous presentation of atypical teratoid/rhabdoid tumor in siblings. J Neurooncol 1999;43:63–70 [DOI] [PubMed] [Google Scholar]
- 7.Bambakidis NC, Robinson S, Cohen M, Cohen AR. Atypical teratoid/rhabdoid tumors of the central nervous system: clinical, radiographic and pathologic features. Pediatr Neurosurg 2002;37:64–70 [DOI] [PubMed] [Google Scholar]
- 8.Yoon CS, Chuang S, Jay V. Primary malignant rhabdoid tumor of the brain: CT and MR findings. Yonsei Med J 2000;41:8–16 [DOI] [PubMed] [Google Scholar]
- 9.Lutterbach J, Liegibel J, Koch D, Madlinger A, Frommhold H, Pagenstecher A. Atypical teratoid/rhabdoid tumors in adult patients: case report and review of the literature. J Neurooncol 2001;52:49–56 [DOI] [PubMed] [Google Scholar]
- 10.Zuccoli G, Izzi G, Bacchini E, Tondelli MT, Ferrozzi F, Bellomi M. Central nervous system atypical teratoid/rhabdoid tumour of infancy: CT and MR findings. Clin Imaging 1999;23:356–360 [DOI] [PubMed] [Google Scholar]
- 11.Chang HK, Kim JH. Classical malignant rhabdoid tumor of central nervous system in 9-year-old Korean. Yonsei Med J 2001;42:142–146 [DOI] [PubMed] [Google Scholar]
- 12.Evans A, Ganatra R, Morris SJ. Imaging features of primary malignant rhabdoid tumour of the brain. Pediatr Radiol 2001;31:631–633 [DOI] [PubMed] [Google Scholar]