Abstract
BACKGROUND AND PURPOSE: Simultaneous traumatic carotid-cavernous fistulas(TCCFs) and traumatic cerebral aneurysms (TCAs) of the internal carotid artery (ICA) are rare. We describe the pitfalls of detecting a TCA before TCCF occlusion and the endovascular management of the TCA and TCCF.
METHODS: Over 12 years, 156 patients with TCCFs were treated at our institute. In four men (mean age, 34 years), associated TCAs were detected before (n = 1) or after (n = 3) endovascular occlusion of the TCCFs. Causes for the missed detection of the TCA before TCCF occlusion were masking by a parent artery and fistula drains (n = 1), steal phenomenon (n = 1), and a latent period (n = 1). The TCAs were in the supraclinoid ICA (n = 3) or the paraophthalmic artery (n = 1). Three TCAs were treated with the endosaccular placement of electrodetachable coils.
RESULTS: Two TCCFs and associated TCAs were successfully occluded with preservation of the ICA. The paraophthalmic TCA was treated with coil occlusion of the TCA and TCCF. Spontaneous fatal rupture of the TCA occurred in one patient after subtotal TCCF occlusion. No notable procedure-related complication was observed in the other three patients.
CONCLUSION: TCAs may be difficult to detect before treatment of the TCCF because it may be overlooked, a latent period may occur, flow may be shunted, or they may be masked by a nearby parent artery or fistula drains. As soon as a TCA is found, endovascular management should be initiated promptly.
Traumatic carotid-cavernous fistula (TCCF) is the common cerebrovascular injury requiring endovascular treatment. On the contrary, traumatic cerebral aneurysms (TCAs) of the internal carotid artery (ICA) are rare complications, resulting from both penetrating and nonpenetrating head injuries. The most common sites for TCAs are the cavernous and petrous portions of the ICA; these injuries are often associated with basal skull fractures (1, 2). Other sites, such as the supraclinoid ICA, are seldom affected (3). TCCFs are rarely associated with a concomitant TCA. Clinically, a TCCF manifests as a cranial bruit, chemosis, proptosis, or decreased visual acuity. In contrast, a TCA is frequently asymptomatic until its fatal rupture.
The purpose of this study was to report our experience in the management of four TCAs associated with TCCFs, to describe the pitfalls in diagnosing a TCA in association with a TCCF, and to describe the principles in the endovascular management of these two traumatic cerebrovascular lesions.
Methods
From January 1992 to February 2003, a 156 consecutive patients with TCCFs were treated in our institute. Of them, four male patients (age range, 18–49 years; mean age, 34 years) were found to have associated TCAs. These were detected before (n = 1) or after (n = 3) endovascular occlusion of the TCCFs. The clinical and angiographic findings are summarized in the Table. All four patients were transferred to our institute for endovascular treatment of the TCCF. Their related symptoms and signs on presentation for endovascular embolization were proptosis (n = 4), chemosis (n = 4), bruit (n = 1), decreased visual acuity (n = 3), and diplopia (n = 1). Initial cranial and facial CT findings included facial bone fracture (n = 3), intracerebral hemorrhage (n = 2), and subarachnoid hemorrhage (SAH) (n = 2). The interval between trauma and endovascular treatment varied from 3 to 15 weeks (mean, 10 weeks).
Demographic and clinical outcomes in patients with TCAs associated with TCCFs
| Patient/Sex/Age, (y) | Clinical Manifestations on Endovascular Treatment | Initial CT Findings | Location and Size of the TCA of the ICA | TCA Detection Prior to TCCF Occlusion | Steal of TCCF | Trauma to Embolization, wk | Outcome | Follow-Up, mo |
|---|---|---|---|---|---|---|---|---|
| 1/M/49 | Chemosis, proptosis, diplopia | Subarachnoid hemorrhage, facial bone fracture | Supraclinoid, 3 × 2 mm | No | Moderate | 8 | Occlusion of TCCF and TCA with ICA preservation | 25 |
| 2/M/40 | Chemosis, proptosis, decreased visual acuity | Brain swelling, facial bone fracture | Supraclinoid, 3 × 3 mm | No | Complete | 15 | Occlusion of TCCF and TCA with ICA preservation | 24 |
| 3/M/29 | Chemosis, proptosis, decreased visual acuity | Subarachnoid and intracerebral hemorrhages, facial bone fracture | Supraclinoid, 3 × 3 mm | No | Moderate | 3 and 5 | Subtotal occlusion of TCCF, fatal rupture of the TCA | 0.25 |
| 4/M/18 | Chemosis, proptosis, decreased visual acuity, bruit | Intracerebral hemorrhage | Paraophthalmic, 5 × 4 mm | Yes | Occlusion of the supraclinoid ICA | 13 | Occlusion of TCA and TCCF | 12 |
Each procedure was performed via the percutaneous transfemoral approach. In three patients, TCAs were detected after occlusion of the TCCFs, and diagnostic angiography and endovascular occlusion of the TCCFs were performed under local anesthesia followed by general anesthesia for the occlusion of the TCAs. Moderate steal with near-equal flow though the fistula and toward the ipsilateral hemisphere was seen in two patients (Fig 1). Complete steal without flow to the distal ICA beyond the fistula was demonstrated in one patient (Fig 2). The causes for missed detection of the TCAs before TCCF occlusion were a small TCA and masking by a nearby parent artery and venous drains (n = 1) (Fig 1), complete steal of the ICA flow and overlooking of the TCA recruited by the vertebrobasilar system (n = 1) (Fig 2), and a latent period in which the TCA was overlooked (n = 1) (Fig 3). In three patients, embolization of the TCCF with a detachable balloon was the first line of treatment. Systemic heparinization was achieved, and the patients’ activated clotting time was maintained at about twice their baseline level. A 7F or 9F guiding catheter was positioned in the ICA. A conventional 2F and 3F coaxial catheter (Nycomed, Ingenor, Paris, France) with a Latex detachable balloon (Goldvalve; Nycomed) was coaxially advanced into the cavernous sinus (CS). Two TCCFs were totally (Fig 1) or subtotally (Fig 2) obliterated. One procedure was attempted twice: The first embolization resulted in partial occlusion with the placement of a single detachable balloon at 3 weeks after trauma (Fig 3A). A second embolization for the residual fistula was undertaken 2 weeks later and involved the deposition of an additional detachable balloon and three microcoils into the CS. The result was subtotal occlusion of the residual fistula (Fig 3B).
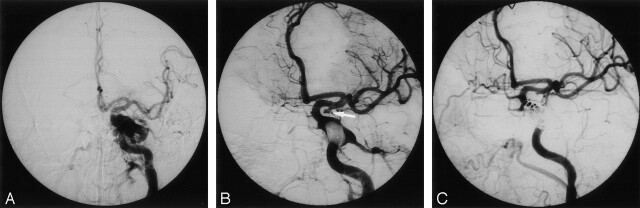
Fig 1.
Images in a 49-year-old man with a left TCCF and a TCA of the supraclinoid ICA.
A, Left frontal carotid angiogram shows a TCCF with moderate steal phenomenon. The aneurysm was overlooked because of its small size and masking by a nearby parent artery and its venous drains.
B, A detachable balloon was placed into the CS with subtotal occlusion of the TCCF. A small TCA about 3 × 2 mm was found at the left supraclinoid ICA (arrow).
C, Occlusion of the TCA was achieved by the use of a Guglielmi detachable coil (GDC). Because of the progressive, increased residual fistula flow, the residual TCCF was subsequently obliterated by using GDCs and liquid adhesive, with preservation of the ICA.
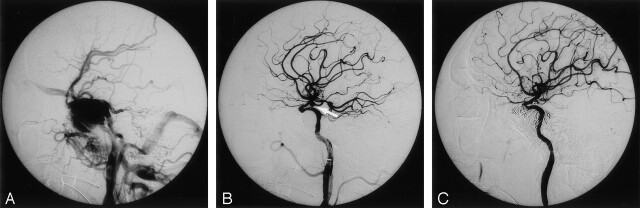
Fig 2.
Images in a 40-year-old man with a TCCF and a TCA of the left supraclinoid ICA.
A, Left lateral carotid angiogram reveals a TCCF with complete steal phenomenon and no opacification of the supraclinoid ICA or TCA.
B, The TCCF was occluded by a detached balloon. A small TCA at the supraclinoid ICA (arrow) was demonstrated and subtotally obliterated with GDCs.
C, The patient had a recurrent TCCF due to puncture of the detached balloon; this was eventually occluded by using a detachable balloon, GDCs, and liquid adhesive, with preservation of the ICA.
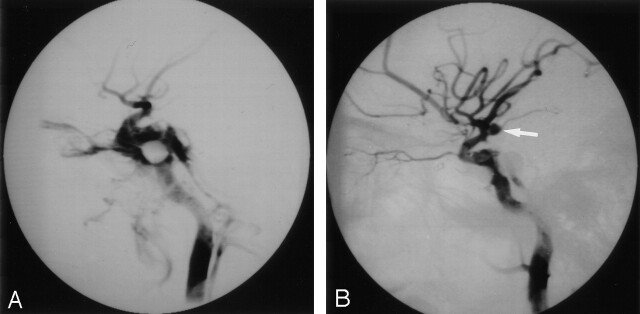
Fig 3.
Images in a 29-year-old man with a left TCCF and a TCA of the supraclinoid ICA.
A, Endovascular occlusion of the TCCF was attempted 3 weeks after trauma. Right lateral postembolization angiogram shows partial occlusion of right TCCF. There is no evidence of the TCA at the supraclinoid ICA because of its latent period.
B, Second embolization was performed 2 weeks after the first attempt. A small TCA was initially ignored (arrow) and found after subtotal occlusion of the residual TCCF. However, fatal rupture occurred before an attempt to treat the TCA was made.
On final postembolization angiograms, small TCAs with a clear neck were found in the supraclinoid ICA in these three patients (Figs 1–3). The aneurysms measured about 3 × 3 mm (n = 2) or 3 × 2 mm (n = 1). Endovascular occlusion of the TCAs was attempted after occlusion of the TCCFs with the patient under general anesthesia. In two patients, a microcatheter catheter (Tracker-10; Target Therapeutics, Fremont, CA) with a 0.010-in flexible-tip wire was navigated into the aneurysm sac by using roadmapping techniques. Then, GDCs (Target Therapeutics) were placed into the aneurysm sac, resulting in its subtotal occlusion (Figs 1, 2). In the other patient, spontaneous rupture of the TCA with a sudden loss of consciousness and an increase in blood pressure to 300/160 mm Hg occurred during preparation for general anesthesia before endosaccular treatment of the TCA. Cerebral angiography revealed increase intracranial pressure without opacification of the intracranial arteries and TCA. Further embolization was impossible, and the patient was returned to the intensive care unit.
In the fourth patient, the TCA was found before occlusion of the TCCF (Fig 4A). General anesthesia was administered for occlusion of both the TCA and the TCCF. Diagnostic angiography showed a paraophthalmic aneurysm measuring about 5 × 4 mm, with rupture into a giant CS varix. This extended into subarachnoid space and drained to the superior ophthalmic vein and inferior petrous sinus. Traumatic occlusion of the supraclinoid ICA was also demonstrated. Endovascular obliteration of the paraophthalmic aneurysm was achieved by using a GDC, which resulted in total occlusion of the TCCF (Fig 4B).
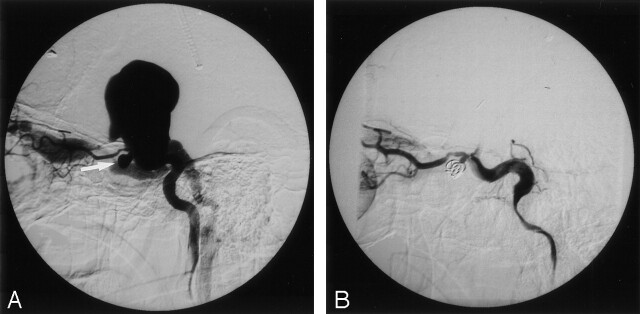
Fig 4.
Images in a 19-year-old man with left paraophthalmic aneurysm associated with a TCCF.
A, Left carotid angiogram reveals a paraophthalmic TCA (arrow) with rupture into a giant CS varix and draining to the superior ophthalmic vein and inferior petrosal sinus.
B, The TCA was subtotally occluded by using GDCs, which also resulted in complete occlusion of the TCCF.
Results
The clinical findings and outcomes of the four patients are summarized in the Table. Two patients who underwent occlusion of the TCA and TCCF with preservation of the ICA flow had a recurrent TCCF or increased residual, shunted flow after the first embolization because of premature balloon deflation. The recurrent or residual TCCFs were eventually treated by using GDCs and 60% N-butyl-2-cyanoacrylate (Melsungen AG, Melsungen, Germany) under the protective balloon in the ICA at the fistula site; the parent arteries were preserved (Figs 1C, 2C). The patient with spontaneous rupture of the TCA and diffuse SAH died 7 days later as a consequence of the SAH; this was considered a procedural outcome. All TCCF-related symptoms were relieved after the last embolization. No notable procedure-related complications or recurrent TCA or TCCF were observed in other three patients. The clinical follow-up period was 12–25 months (mean, 20 months).
Discussion
TCAs of the intracranial carotid artery and its branches are rare, constituting only 0.15–0.04% of all intracranial aneurysms (4, 5). Of the 248 TCAs reported, 48% involved the intracranial ICA. Among them, 69% were situated in the intracavernous and petrous ICA, whereas 31% were located at the supraclinoid portion (6).
Pathoanatomic criteria for TCAs are severely lacking. Several authors report that the following typical angiographic features may be of help in the characterization of TCAs: delayed filling and emptying of the sac, an irregular contour, no visible neck, and an ostium not located at the common arterial branching points (4, 7). However, Fox (6) regards a history of recent trauma as the main criterion for the diagnosis of a TCA. Therefore, the assumption is that the diagnosis of a TCA must be based mainly on clinical evidence, such as the adequacy of injury and the local relationship between injury and the location of the TCA (7). In our small series, all four aneurysms were presumed to be of traumatic origin, largely on the basis of the clinical history of severe head trauma and the location of the TCA adjacent to the TCCF. However, given the 5% natural incidence of cerebral aneurysms in the general population (8), TCCFs may have a coincidence of non-TCA, as in four of 156 patients in the present series.
The associated TCA may be clinically ignored because most TCAs are asymptomatic until their catastrophic rupture, and most patients are referred for treatment of the TCCF and its related visual problems. The consequences of missed diagnoses and treatment may cause devastating SAH or severe disability. There are several angiographic pitfalls involving the early detection of TCAs in the supraclinoid ICA before the occlusion of TCCFs. For example, in the case of a complete and large steal phenomenon of the TCCF, the TCA distal to the fistula may not be demonstrated on ipsilateral carotid angiograms because of insufficient vessel opacification by contrast medium. On occasion, the TCA may be shown by the recruitment of ipsilateral distal ICA flow through the contralateral ICA or vertebrobasilar systems. In addition, the TCAs could be masked by a nearby parent artery or by early opacification of the CS and its dilated venous drains. In these circumstances, associated TCAs may be difficult to assess, and they might be detected only after the fistula is completely or partially occluded. In some instances, TCAs may be missed because of their small size or because of poor image resolution. Therefore, angiography must be performed immediately after occlusion of the TCCFs to check for the possible coexistence of these associated TCAs. If necessary, rotational angiography should be used to clarify the parent artery and nearby TCA. Furthermore, some TCAs may have a latent period and take time to develop. The delay from the initial injury to the manifestation of TCAs varies from several hours to 10 years, but most series report a mean time to presentation of 2–3 weeks (9, 10). Benoit and Wortzman (11) reported a large TCA arising from the supraclinoid ICA in association with a CCF, which was found 14 days following head injury. Waga et al (12) reported a case, similar to our first two cases, in which a TCA was overlooked or not opacified because of a large TCCF shunt proximal to the TCA. In another of our cases, the TCA was not shown on the first cerebral angiogram, which had been obtained during a 3-week latent period. This TCA was detected during the second embolization performed 5 weeks after trauma. In our first two cases, the TCA was first recognized only after a major shunt in the TCCF was occluded, as shown on postembolization angiograms. In our series, the reason why these TCAs escaped detection before the occlusion of the TCCFs was because they were small and overlooked, the flow proximal to the TCA was shunted, or a nearby parent artery and venous drains masked them. TCAs that rupture with the formation of the fistula (Fig 4) and TCAs located before the fistula tract of the TCCF are less difficult to find than TCAs beyond the fistula, as depicted on the initial diagnostic angiogram.
Most TCCFs are essentially benign and involve an impairment of visual acuity. However, there are some hazardous and fatal circumstances in which endovascular embolization should be performed immediately or even on an emergency basis. These include an acute impairment of visual acuity, epistaxis, sphenoid sinus aneurysm, intracerebral hemorrhage, or the presence of a large CS varix; these are found in only 0.9%–9.3% of patients (13, 14). The prognosis of patients with a TCA is generally poor. The mortality rate is almost 50% in cases left untreated (4, 15, 16), and one-half of patients who experience bleeding die after the first bleeding episode (17). Hopefully, earlier recognition and proper management of the TCAs will reduce the current mortality rate of 43% (18). In terms of the priority in which these cerebrovascular injuries are treated, the TCA should be occluded first if it is detected before the TCCF is occluded. However, if it is ignored or overlooked, it should be treated as soon as the TCCF is obliterated.
In our series, we attempted to treat three TCAs at 5, 8, or 15 weeks after trauma. Rupture or re-rupture of the TCAs did not occur, as usually happens 2–3 weeks before TCCF occlusion, largely because of the diversion of ICA blood flow and pressure to the fistula drains. Therefore, in the case of TCCFs with associated TCAs, the shunted flow and pressure of the TCCF probably saved the TCA from rupture or re-rupture. Nevertheless, occlusion of the TCCF with reconstruction of the distal ICA flow can put the associated TCAs in the distal ICA at risk for increased pressure in the intraaneurysmal sac, with further distention and subsequent devastating rupture. In our series, re-rupture of the TCA occurred in one case (but not in two others) after occlusion the TCCF, with rebuilding of the distal flow of the ICA. This was presumed in two cases, which were thought to have a more mature wall at 8 or 15 weeks after trauma, as compared with the third case at 5 weeks.
Regarding to treatment strategy, most TCAs have no formal wall, they are broad based and fragile, and their neck is obscured in the acute phase. Endovascular embolization with the placement of embolic material into the TCA presents an extreme danger of aneurysmal rupture with bleeding. Most reported TCAs in the supraclinoid ICA were treated by means of deconstructive procedures, such as trapping or proximal ICA occlusion with or without distal revascularization. Endovascular occlusion of the proximal parent artery with or without a carotid occlusion test has been preferred in some instances. If a period of time has passed since the initial injury and discovery of the TCA (usually at least 4–6 weeks) the wall of the TCA may have matured enough to permit the safe delivery of an embolic device without fear of rupture. Some reports describe the safe and effective treatment of TCAs by using GDC endosaccular embolization with preservation of the parent artery (19–21). This treatment strategy is obviously favored for TCAs with a mature wall and a clear neck, although this type of aneurysm is less frequently encountered than others.
Conclusion
The occurrence of a TCCF with a TCA is rare. Early detection and treatment of the TCAs before TCCF occlusion may be difficult, largely because of steal phenomenon of the fistula, masking by a nearby parent artery and/or venous drains, overlooking of a small TCA, and a latent period of the TCA. Therefore, in a patient with a skull-base fracture and unexplained brain hemorrhage and neurologic deficits, an exhaustive angiographic workup to search for TCAs is necessary, both before and after occlusion of the TCCF. Prompt occlusion of the TCAs should be performed as soon as they are detected. Endosaccular placement of GDCs is indicated in TCAs with a mature wall and a clear neck.
Footnotes
Supported in part by grants Taipei Veterans General Hospital (VGH-92-A99) and NSC91–2314-B-075–121.
References
- 1.Teal JS, Bergeron RT, Rumbaugh CL, Segall HD. Aneurysm of the petrous or cavernous portions of the internal carotid artery associated with nonpenetrating head trauma. J Neurosurg 1973;38:568–574 [DOI] [PubMed] [Google Scholar]
- 2.Pozzati E, Gaist G, Servadei F. Traumatic aneurysms of the supraclinoid internal carotid artery. J Neurosurg 1982;57:418–422 [DOI] [PubMed] [Google Scholar]
- 3.Yonas H, Dujovny M. True traumatic aneurysm of the intracranial internal carotid artery: case report. Neurosurgery 1980;7:499–502 [DOI] [PubMed] [Google Scholar]
- 4.Parkinson D, West M. Traumatic intracranial aneurysms. J Neurosurg 1980;52:11–20 [DOI] [PubMed] [Google Scholar]
- 5.Uzan M, Cantasdemir M, Seckin MS, et al. Traumatic intracranial carotid tree aneurysm. Neurosurgery 1998;43:1314–1320 [DOI] [PubMed] [Google Scholar]
- 6.Fox JL. Traumatic Intracranial Aneurysms: The Requisites. New York: Springer-Verlag,1983. :1453–1463
- 7.Steinmetz H, Heib E, Mironov A. Traumatic giant aneurysms of the intracranial carotid artery presenting long after head injury. Surg Neurol 1988;30:305–310 [DOI] [PubMed] [Google Scholar]
- 8.Wiebers DO, Whisnant JP, Sundt TM, O’Fallon WM. The significance of unruptured intracranial saccular aneurysms. J Neurosurg 1987;66:23–29 [DOI] [PubMed] [Google Scholar]
- 9.Fleischer AS, Patton JM, Tindall GT. Cerebral aneurysms of traumatic origin. Surg Neurol 1982;4:233–239 [PubMed] [Google Scholar]
- 10.Buckingham MJ, Crone KR, Ball WS, Tomsick TA, Berger TS, Tew JM Jr. Traumatic intracranial aneurysms in childhood: two cases and a review of the literature. Neurosurgery 1988;22:398. [DOI] [PubMed] [Google Scholar]
- 11.Benoit BG, Wortzman G. Traumatic cerebral aneurysms: clinical features and natural history. J Neuro Neurosurg Psychiatr 1973. :36 ;127–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Waga S, Morikawa A, Fujimoto K. Carotid-cavernous fistula associated with traumatic aneurysm of the internal carotid artery. Surg Neurol 1978;9:367–369 [PubMed] [Google Scholar]
- 13.Sattler CH. Pulsierender exophalmus. In: Handbuch der Gesamten Augenheikunde: The Requisites. Berlin: Springer-Verlag,1920. :1–268
- 14.Halbach VV, Hieshima GB, Higashida RT, Reicher M. Carotid cavernous fistulae: indication for urgent treatment. AJNR Am J Neuroradiol 1987;8:627–633 [DOI] [PubMed] [Google Scholar]
- 15.Holmes B, Harbaugh RE. Traumatic intracranial aneurysm: a contemporary review. J Trauma 1993;35:855–860 [PubMed] [Google Scholar]
- 16.Ventureyra ECG, Higgins MJ. Traumatic intracranial aneurysms in childhood and adolescence. Childs Nerv Syst 1994;10:361–379 [DOI] [PubMed] [Google Scholar]
- 17.Aarabi B. Management of traumatic aneurysms caused by high-velocity missile head wounds. Neurosurg Clin N Am 1995;6:775–797 [PubMed] [Google Scholar]
- 18.Ferry DJ, Kempe LG. False aneurysm secondary to penetration of the brain through orbitofacial wounds: report of two cases. J Neurosurg 1972;36:503–506 [DOI] [PubMed] [Google Scholar]
- 19.Lempert T, Halbach VV, Higashida RT, et al. Endovascular treatment of pseudoaneurysms with electrolytically detachable coils. AJNR Am J Neuroradiol 1998;19:907–911 [PMC free article] [PubMed] [Google Scholar]
- 20.Tokunaga K, Kusaka N, Nakashima H, Date I, Ohmoto T. Coil embolization of intradural pseudoaneurysms caused by arterial injury during surgery; report of two cases. AJNR Am J Neuroradiol 2001;22:35–39 [PMC free article] [PubMed] [Google Scholar]
- 21.Oran I, Parildar M, Dalbasti T, Memis A, Ozdamar N. Intradural aneurysm caused by arterial injury during surgery: treatment with coil embolization. Intervent Neuroradiol 2001;7:357–361 [DOI] [PMC free article] [PubMed] [Google Scholar]