Abstract
BACKGROUND AND PURPOSE: Multiple biomarkers are used to quantify the severity of traumatic brain injury (TBI) and to predict outcome. Few are satisfactory. CT and conventional MR imaging underestimate injury and correlate poorly with outcome. New MR imaging techniques, including diffusion tensor imaging (DTI), can provide information about brain ultrastructure by quantifying isotropic and anisotropic water diffusion. Our objective was to determine if changes in anisotropic diffusion in TBI correlate with acute Glasgow coma scale (GCS) and/or Rankin scores at discharge.
METHODS:Twenty patients (15 male, five Female; mean age, 31 years) were evaluated. Apparent diffusion coefficients (ADCs) and fractional anisotropy (FA) values were measured at multiple locations and correlated with clinical scores. Results were compared with those of 15 healthy control subjects.
RESULTS: ADC values were significantly reduced within the splenium (Δ18%, P = .001). FA values were significantly reduced in the internal capsule (Δ14%; P < .001) and splenium (Δ16%; P = .002). FA values were significantly correlated with GCS (r = 0.65–0.74; P < .001) and Rankin (r = 0.68–0.71; P < .001) scores for the internal capsule and splenium. The correlation between FA and clinical markers was better than for the corresponding ADC values. No correlation was found between ADC of the internal capsule and GCS/Rankin scores.
CONCLUSION: DTI reveals changes in the white matter that are correlated with both acute GCS and Rankin scores at discharge. DTI may be a valuable biomarker for the severity of tissue injury and a predictor for outcome.
Diffuse axonal injury (DAI) is identified as one of the most important causes of morbidity and mortality in patients with traumatic brain injury (TBI) (1–3). DAI refers to white matter (WM) injury induced by sudden acceleration-deceleration and/or rotational forces. The resulting tissue injury is characterized by axonal stretching, disruption, and eventual separation of nerve fibers (4, 5).
Currently, no technique is accurate for diagnosing and assessing the distribution and severity of DAI. CT and MR imaging are known to underestimate the extent of DAI and correlate poorly with final outcome (6, 7). This lack of sensitivity is confirmed in studies in which patients had progressive, global cerebral atrophy despite the fact that initial imaging failed to show pathology or revealed only discrete findings (8, 9). Consequently, there is considerable interest in developing more-sensitive diagnostic tools.
Several previous studies have shown that diffusion-weighted imaging (DWI) is valuable in evaluating DAI (10–12). One study showed that DWI depicts shearing injuries that are not visible on conventional T2-/T2*-weighted or fluid attenuated inversion recovery (FLAIR) MR images (12). However, the clinical importance of this extra information was not studied; DWI findings were not correlated with clinical scores. Diffusion tensor imaging (DTI) represents an extension of DWI that allows the quantification of WM architecture in vivo. In DTI, the degree and directionality of water diffusion (anisotropy) is measured. By measuring the degree of water diffusion anisotropy, information about the integrity of WM tracts is obtained (10, 13–16).
In the present study, we hypothesized that the degree of tissue abnormality, as measured with DTI, is correlated with clinical scores. Our overall goal was to extend the results of our previous study (12) by investigating the potential of DTI as a biomarker of tissue injury.
Methods
Institutional review board approval was obtained for our retrospective review of patient records (including MR imaging studies).
Study Patients
Twenty patients (15 male, five female; mean age, 31 years ± 10) were included. Patients were selected by means of an electronic search of all radiologic reports between 1996 and 1999. Specific keywords related to head trauma were used: traumatic brain injury, cerebral trauma, head trauma, diffuse axonal injury, shearing injury, closed head injury, and traumatic cerebral hemorrhage. Initially, 107 patients were identified. Patients were excluded if the time delay between trauma and MR imaging exceeded 7 days (n = 26) to avoid the various changes in anisotropic diffusion related to secondary tissue injury. Patients younger than 18 years were excluded (n = 9) to ensure that the measured changes in anisotropic diffusion were not related to ongoing brain-maturation processes. In addition, patients who had required emergency neurosurgical or surgical interventions or had experienced cardiovascular arrest (n = 7) were excluded. In 11 patients, DWI data were missing. Review of the medical records and final radiologic reports showed that, in 28 patients, the clinical criteria for TBI were not fulfilled or that other nontraumatic cerebral pathologies (eg, cerebral stroke, hemorrhage) had been diagnosed. In six patients, adequate clinical records were not available. Consequently, 87 patients were excluded, leaving 20 patients for our study. The clinical records confirmed that the mechanism of trauma was consistent with DAI. All patients proved to be healthy before trauma, with no known history of other cerebral disease. Fifteen healthy subjects with a matched age distribution served as a control group (mean age, 35 years ± 10 years).
MR Image Acquisition
Imaging was performed on a 1.5-T MR imaging unit with echo-planar imaging capabilities. All patients and control subjects underwent DTI averaged over three datasets for a total acquisition time of 126 seconds (TR/TE, 6000/118; field of view, 40 × 20 cm; acquisition matrix, 256 × 128; section thickness, 6 mm section; b values, 3 and 1221 sec/mm2) applied in six directions at 20 section positions. The entire diffusion tensor was sampled by using a T2-weighted, spin-echo, single-shot, echo-planar imaging sequence similar to those previously published (17).
The overall translational water motion, characterized by the apparent diffusion coefficient (ADC), and the anisotropic component of water diffusion, characterized by fractional anisotropy (FA), was calculated on a voxel-by-voxel basis (18, 19). FA, which represents the ratio of the anisotropic component of the diffusion tensor to the whole diffusion tensor, was used because FA has been reported to be the best rotationally invariant scalar metric for measuring diffusion anisotropy (17–19). Rotationally invariant anisotropy metrics are advantageous because they are independent of the frame of reference, of the direction of the applied diffusion gradients, and of the orientation of the tissue microstructure within the voxels (11, 19). In contrast, rotationally variant scalars depend on the direction of the applied diffusion gradients and the orientation of the studied structures within the voxels. FA metrics are scalar indices ranging from 0 to 1, where 0 represents maximal isotropic diffusion as in a perfect sphere and 1 represents maximal anisotropic diffusion, as in the hypothetical case of a long cylinder of minimal diameter. FA maps are displayed as gray-scale maps. Areas with high degrees of FA (eg, corpus callosum) are bright, and areas with low degrees of FA (eg, gray matter) are dark (Fig 1A). In addition, the spatial direction of the mean anisotropic diffusion gradient in each voxel can be displayed graphically by adding a color coding to the FA maps. Red indicated a predominant left-right anisotropic diffusion gradient; green, an anteroposterior gradient; and blue, a superior-inferior gradient (Fig 1B).
Fig 1.
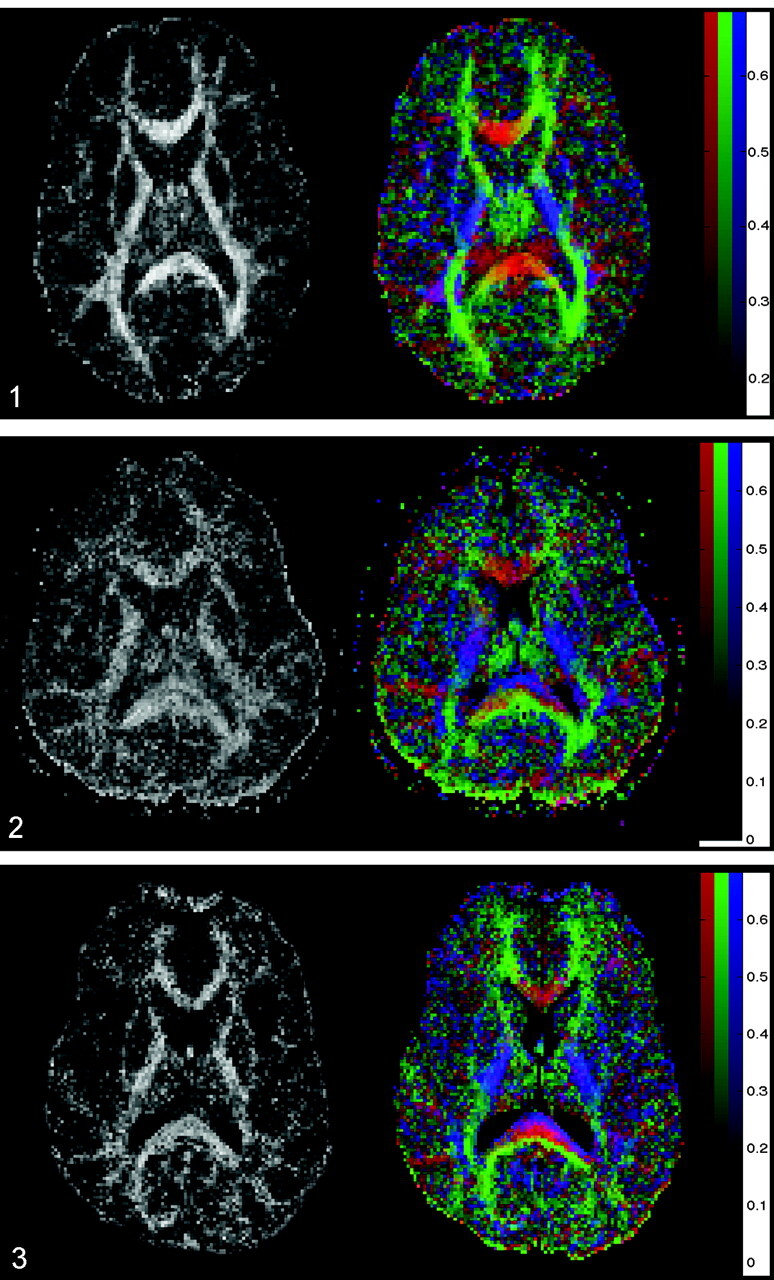
Axial FA map (left) and color coded map of mean diffusion direction (right) at the level of the basal ganglia, thalami and internal capsulae in a healthy control subject. Left, Gray-scale FA map displays a high degree of anisotropic diffusion (bright) within the internal capsule and the splenium of the corpus callosum. The cortex and the central gray matter are dark because of their low degree of anisotropic diffusion. Right, Color-coded image displays a predominant left-right-left mean diffusion direction (red) within the center of the splenium of the corpus callosum, an anteroposterior direction (green) within the optic radiations, and a superior-inferior direction (blue) within the posterior internal capsule.
Data Analysis
The images were transferred to a personal computer (Apple Computer Inc., Cupertino, CA). ADC and FA values were measured at well-known predilection sites of DAI: the posterior limb of internal capsule (bilaterally) and the splenium of the corpus callosum (1, 10, 11). ADC and FA measurements in the thalamus and putamen, which are not at increased risk for shearing injuries, served as internal references. The anterior limb of the internal capsule was not measured because its small size made region-of-interest (ROI) positioning susceptible to contamination by the adjacent basal ganglia. In addition, no ROIs were measured in the brainstem because magnetic susceptibility artifacts from the adjacent skull base would have made the ADC and FA measurements less reliable. ROIs were positioned by exactly identifying the anatomical structures by using image display software (Alice; Hayden Image Processing Solutions, Boulder, CO). Manually traced outlines were used for all regions, similar to the outlining published by Melhem et al (14). ROIs were positioned by anatomic guidance and not lesion guidance. This ROI positioning makes the technique less investigator-dependant. ROIs included at least nine pixels. The mean ADC and FA values, including the corresponding standard deviations (SDs), were measured. All bilateral measures were averaged for each patient.
The acute GCS score and the modified Rankin score obtained on the day of the patient’s discharge were extracted from the medical records. The GCS score ranges from 3 (worst score) to 15 (best score), combining three categories of evaluation: eye opening, best verbal response, and best motor response. Acute GCS scores were directly extracted from the medical charts. The modified Rankin scale score is the most commonly used outcome classification scale for disabilities and handicaps after cerebral stroke (20). The scale has six grades ranging from 0 (no symptoms) to grade five (severe disability). A board-certified neurologist (L.H.S.) retrospectively assessed the modified Rankin Scale score at the time of discharge by using all information available. In cases in which non-neurologic events caused impairments in function (eg, long-bone fracture that prevented ambulation), a CNS-Rankin score was derived by scoring the impairments based solely on neurologic injury. A conservative approach was taken to calculate this modified CNS-Rankin score so that only factors clearly unrelated to CNS-mediated injury were excluded from scoring.
Statistical Analysis
A Wilcoxon signed-rank test was used to determine whether statistically significant differences existed between the corresponding left- and right-sided ROI measurements in the control subjects. Subsequently, a two-tailed Wilcoxon rank-sum test was performed to evaluate for statistically significant differences between ROI measurements in patients and control subjects. To adjust for multiple comparisons, Bonferroni correction was applied. A P value less than .005 was considered to indicate a statistically significant difference between patients and control subjects. Linear regression analysis was applied to test for statistically significant correlations between the measured ADC and FA values and the GCS and Rankin scores. A statistician performed the analyses by using a SAS software package (version 8; SAS Inc, Cary, NC).
Results
Table 1 lists the results of the left-versus-right comparison of the bilaterally measured ADC and FA values in the control subjects. There was no statistically significant difference between the left- and right-sided measurements (P = .182–.890). Consequently, for further data analysis, all bilateral measurements were averaged.
TABLE 1:
Averaged ADC and FA values for the control subjects
| A: ADC values, mm2/sec | |||
|---|---|---|---|
| ROI | Left × 10−6 | Right × 10−6 | P Value |
| Internal capsule | 681 ± 81 | 667 ± 84 | .642 |
| Thalamus | 715 ± 62 | 721 ± 59 | .785 |
| Putamen | 684 ± 80 | 669 ± 73 | .624 |
| B: FA values | |||
|---|---|---|---|
| ROI | Left | Right | P Value |
| Internal capsule | 0.731 ± 0.069 | 0.721 ± 0.077 | .761 |
| Thalamus | 0.371 ± 0.077 | 0.383 ± 0.078 | .182 |
| Putamen | 0.287 ± 0.090 | 0.286 ± 0.074 | .890 |
Note.—Table lists the mean ADC and FA values ± SD for all bilaterally measured ROIs for all control subjects. Results showed no statistically significant difference between the left- and right-sided ADC and FA measurements. A P value <.005 was considered to indicate a statistically significant difference. Analysis was performed by using a Wilcoxon signed-rank test.
Table 2 lists the measured ADC and FA values for patients and control subjects. The measured ADC and FA values in the control group were similar to previously published measurements (18, 19). In the control subjects, the splenium showed the highest level of diffusion anisotropy (mean FA, 0.808 ± 0.060), followed by the internal capsule (mean FA, 0.725 ± 0.066), the thalamus (mean FA, 0.377 ± 0.075), and the putamen (mean FA, 0.286 ± 0.081). There was a statistically significant decrease in ADC for the splenium (Δ16%; P = .001) in patients compared with control subjects. A statistically significant decrease in FA was seen in the internal capsule (Δ14%; P <.001) and splenium (Δ16%, P = 0.002) in patients compared with control subjects (Table 2). No statistically significant differences were observed for the ADC and FA values in the putamen and thalamus. In three patients, visual inspection of the color-coded FA maps showed partial disruption of the fiber tracts oriented left to right in the central area of the splenium (Figs 2, 3).
TABLE 2:
Averaged ADC and FA values for the control subjects and patients
| A: ADC values, mm2/sec | |||
|---|---|---|---|
| ROI | Control Subjects, × 10−6 | Patients, ×10−6 | P Value |
| Internal capsule | 674 ± 81 | 683 ± 59 | .732 |
| Splenium* | 769 ± 61 | 628 ± 74 | .001 |
| Thalamus | 718 ± 60 | 719 ± 62 | .975 |
| Putamen | 677 ± 75 | 707 ± 55 | .184 |
| B: FA values | |||
|---|---|---|---|
| ROI | Control Subjects | Patients | P Value |
| Internal capsule* | 0.725 ± 0.066 | 0.624 ± 0.072 | <.001 |
| Splenium* | 0.808 ± 0.060 | 0.678 ± 0.010 | .002 |
| Thalamus | 0.377 ± 0.075 | 0.401 ± 0.040 | .147 |
| Putamen | 0.286 ± 0.081 | 0.307 ± 0.040 | .257 |
Note.—Table lists the mean ADC and FA values ± SD for all patients and control subjects within the respective ROIs. Left- and right-sided ADC and FA values were averaged. A statistically significant ADC decrease is seen for the splenium, and a statistically significant FA decrease is seen for the internal capsule. A P value of <.005 was considered to indicate a statistically significant difference. Analysis was performed by using a two-tailed Wilcoxon rank sum test.
Statistically significant difference.
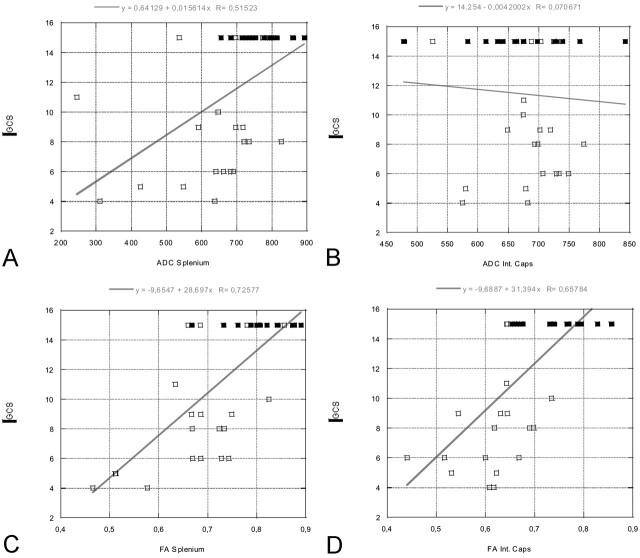
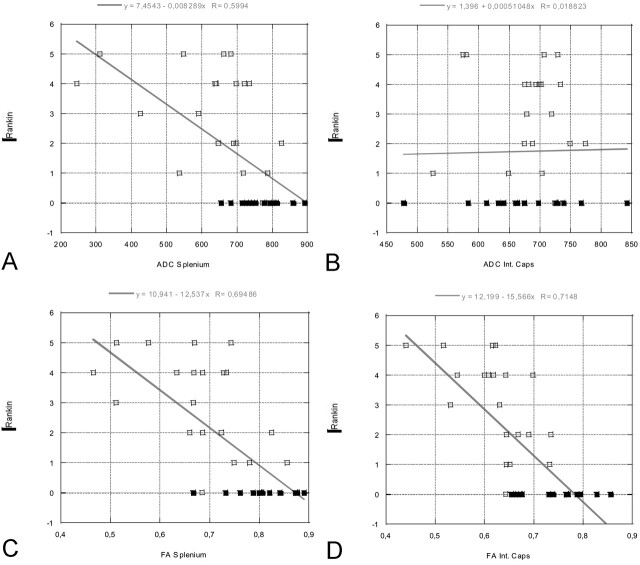
Table 3 lists the correlation coefficients between the ADC and FA values and the GCS and Rankin scores. Our results showed that the ADC and FA values in the splenium were significantly correlated with both the acute GCS and Rankin scores at discharge. In addition, the FA values in the internal capsule were correlated with GCS and Rankin scores, whereas the ADC values did not show a correlation. The strongest correlation was found for the FA values in the splenium. The measured FA values showed a stronger correlation with the clinical scores than did the ADC values. There was no statistically significant correlation between ADC and FA values of the thalamus and putamen and the clinical scores. The data are represented graphically in Figures 4 and 5.
TABLE 3:
Correlation between DTI measurements and clinical scores
| Measurement | GCS Score |
Rankin Score |
||
|---|---|---|---|---|
| r Value | P Value | r Value | P Value | |
| ADC internal capsule | −0.070 | .686 | 0.018 | .914 |
| FA internal capsule* | 0.657 | <.0001 | −0.714 | <.0001 |
| ADC splenium* | 0.515 | .0015 | −0.599 | .0001 |
| FA splenium* | 0.725 | <.0001 | −0.694 | <.0001 |
| ADC thalamus | −0.165 | .342 | 0.147 | .396 |
| FA thalamus | −0.163 | .348 | 0.114 | .513 |
| ADC putamen | −0.314 | .065 | 0.280 | .103 |
| FA putamen | −0.116 | .504 | 0.187 | .281 |
Note.—Table lists the correlation coefficient r between the measured ADC and FA values and GCS or Rankin score for different anatomic locations. There proved to be a statistically significant correlation between the ADC and FA values measured within the splenium and the GCS and Rankin score, as well as between the FA value of the internal capsule and the GCS or Rankin score. The correlation between the FA values and the GCS or Rankin score was stronger than for the corresponding ADC values. A correlation probability of P < .0025 indicates a statistically significantly correlation between the measured ADC- and FA-values and the GCS/Rankin score.
Statistically significant correlation.
Fig 4.
Linear regression plots of ADC and FA values of the splenium and internal capsule versus GCS at the time of acute MR imaging (in patients) or at time of comparison MR imaging (control subjects, all with GCS scores of 15). A statistically significant correlation is seen between the FA values of the splenium/internal capsule and GCS, as well as between the ADC values within the splenium and GCS. GCS scores vary between 3 and 15, where 3 represents the worst score, and 15, the best score. Open rectangles indicate patients; solid rectangles, control subjects.
A, ADC splenium versus GCS.
B, ADC internal capsule versus GCS.
C, FA splenium versus GCS.
D, FA internal capsule versus GCS.
Fig 5.
Linear regression plots of ADC and FA values of the splenium and internal capsule versus Rankin score at the time of discharge (in patients) or at time of comparison MR imaging (control subjects, all with Rankin scores of 0). A statistically significant correlation is seen between the FA values of the splenium/internal capsule and Rankin score, as well as between the ADC values of the splenium and Rankin score. Rankin scores vary between 0 and 5, where 0 represents the best score and 5, the worst score. Open rectangles indicate patients; solid rectangles, control subjects.
A, ADC splenium versus Rankin.
B, ADC internal capsule versus Rankin.
C, FA splenium versus Rankin.
D, FA internal capsule versus Rankin.
The mean GCS score for all patients was 8.7 ± 3.7, and the mean Rankin score was 3.1 ± 1.5. Patients were hospitalized for a mean duration of 18 days.
Discussion
TBI, and especially DAI, represents a major diagnostic challenge. An early and exact identification of the extent and degree of tissue injury is essential for treatment decisions. Early identification of patients at risk for a poor outcome or severe cognitive and/or behavioral deficits is desirable. Conventional neuroimaging, including CT and MR imaging, demonstrates many trauma-related lesions. The correlation between acute imaging findings and long-tern outcomes is, however, poor. CT and MR imaging are known to underestimate TBI, in particular DAI (6, 7, 21). A quantification of neural injury in the acute phase is essential for making treatment decisions, for developing and monitoring new treatments, and for providing appropriate counseling to patients concerning their long-term prognosis.
Currently, few imaging techniques offer reliable information that correlates with outcome in TBI. Sinson and co-workers evaluated 30 patients with TBI by means of magnetization transfer ratio (MTR) imaging and MR spectroscopy (21). They showed that MTR and MR spectroscopy can be used to quantify damage after TBI and that N-acetylaspartate/creatine ratios may be sensitive indicators of neuronal damage. They correlated their results with clinical outcome scores.
Alternatively, conventional DWI that involves the use of diffusion-weighted images to calculate the ADC can be used. This method can show lesions with decreased ADC in patients with DAI, in both the acute and subacute periods after TBI (10). Furthermore, DWI has been shown to be valuable in evaluating closed-head injury because it can depict additional shearing injuries not visible on conventional T2/FLAIR or T2* MR images (12).
DTI represents a relatively new, noninvasive MR technique that quantifies isotropic and anisotropic water diffusion in tissues (13, 15, 16, 18, 19). The degree of anisotropic diffusion is related to the degree of directionality and the integrity of WM fiber tracts within the brain. Densely packed WM tracts show a high degree of anisotropic diffusion, whereas gray matter has a low degree of anisotropic diffusion (15, 16). WM tracts are typically disrupted in DAI. Consequently, by measuring WM anisotropy, DTI could be a diagnostic tool that quantifies the degree of tissue injury. A previous study in five patients with mild TBI showed that the degree of diffusion anisotropy was reduced in WM regions in the first 24 hours after TBI (11). In the present study, we extended these results by correlating the change in WM tract anisotropic diffusion with clinical scores. ROI positioning was anatomically guided (predilection sites of DAI) instead of lesion guided.
Our results confirm that changes in water diffusion anisotropy occur in TBI. FA was significantly decreased in the posterior limb of the internal capsule (mean decrease in FA, 14%) and splenium of the corpus callosum (mean decrease in FA, 16%). Furthermore, we found a statistically significant correlation between FA values and the severity of head injury, as measured with acute and subacute neurologic assessments (acute GCS and discharge Rankin scores) in predilection sites of DAI.
These results confirm those of previous case reports and studies suggesting that TBI/DAI may be associated with changes in diffusion anisotropy (11, 15, 16, 22–24). Our study extends these earlier reports by quantifying the changes in a larger cohort of patients, by documenting decreases in FA in predilection sites of DAI, and by suggesting that FA changes may be able to serve as predictive parameters for outcome.
While our findings need confirmation in larger, longitudinal studies and correlation with pathologic specimens, they suggest that DTI may be able to provide biologic insight into DAI and that DTI may serve as a marker of disease severity. DTI has a number of advantages as imaging biomarkers of brain injury: 1) DTI can be used to evaluate brain trauma in an unconscious or sedated patient. 2) With further validation, DTI could permit the evaluation of responses to treatment even when the clinical scores are inadequate for assessing the patient. 3) The objective quantitative DTI measurements are unlikely to be tainted by adverse CNS effects of medications or intubation, unlike clinical scores. 4) DTI may be an important alternative marker, as low initial GCS scores are of limited value in predicting the prognosis (25).
The exact mechanisms underlying the changes in diffusion anisotropy are not yet fully understood but most probably reflect changes in the underlying microscopic structure of the tissue being examined. In ordered structures like the myelin sheaths of densely packed WM tracts (as in the internal capsule and splenium of the corpus callosum), a loss of order or structural integrity of the tissue results in a reduction of FA (11, 26). The diffusion along axons decreases, while the diffusion in directions perpendicular increases. Given that TBI is associated with DAI changes in the internal capsule and corpus callosum, we believe that the decrease in FA confined to these WM tracts reflects tissue damage and can be used as a biomarker of TBI severity.
Our data showed that FA is not significantly changed in the deep gray matter (thalamus, putamen). These findings parallel theoretical considerations that the central gray matter is less susceptible to shearing forces because of its mainly cellular contents and fewer fiber tracts.
Our data also showed that the degree of FA change was better correlated with the clinical scores in both the acute and subacute states than the ADC values. This finding was especially apparent for the packed, parallel-oriented fiber tracts in the internal capsule. Our data showed a statistically significant correlation between the measured FA values of the internal capsule and the clinical scores, whereas no correlation was observed for the corresponding ADC values.
Finally, our data showed that, in areas where a statistically significant change in ADC values was found (splenium), ADC values were decreased rather than increased. This finding make it unlikely that decreases in FA result from an increased accumulation of water in the interstitial space. Interstitial edema would increase the isotropic diffusion component while reducing anisotropic water diffusion.
Limitations of our study were that only a selection of WM fiber tracts were investigated, that the eigenvalues/eigenvectors of the diffusion tensor were not separately studied, that the results were not correlated with magnetization transfer imaging or MR spectroscopic findings; that no long-term follow-up measurements were performed, and finally, that the number of patients was small.
Conclusion
Our data demonstrate that DTI is feasible in the setting of TBI and that TBI and DAI is associated with changes in diffusion anisotropy along fiber tracts at predilection sites for DAI. Furthermore, the degree of FA change is correlated with the acute GCS score and the discharge Rankin score. The correlation between FA and clinical scores was stronger than that between ADC and clinical scores. If these data are replicated in larger studies, DTI results with measurements of FA at predilection sites of DAI could be used as biomarkers for the severity of head trauma and as predictors of later clinical outcome in the acute setting of injury. Advantages are that DTI can be performed in unconscious or sedated patients and that FA measurements (ROI positioning) can be done with anatomic guidance instead of lesion guidance.
Fig 2.

Images in a 24-year-old man with severe TBI. Acute GCS, 5. Rankin score at discharge, 3. Left, FA map shows a reduced FA index of the splenium of the corpus callosum (FA = 0.511 ± 0.036, mean control FA = 0.808 ± 0.060) and internal capsule (FA = 0.531 ± 0.036, mean control FA = 0.735 ± 0.066). Right, Color-coded map shows that, within the center of the splenium of the corpus callosum, the normally predominant red voxels are missing and replaced by a mixture of blue and green voxels (compare with Fig 1). This finding suggests that fiber tracts that connect both cerebral hemispheres are injured or disrupted within the center of the splenium.
Fig 3.

Images in a 37-year-old man with severe TBI. His GCS score at the time of MR imaging was 3, and his Rankin score at discharge was 4. Left, FA map shows a reduced FA index of the corpus callosum (FA = 0.634 ± 0.036). Right, Color-coded map shows a layered blue, red, and green aspect of the splenium of the corpus callosum. This could indicate a partial, selective injury of the most anterior and posterior left-right-left fiber tracts.
Acknowledgments
The authors thank Dave Tuch, PhD, and Mette Wiegell, PhD, for their help in color coding the FA images presented.
Footnotes
Supported by grants PHS R01NS38477 and P41-RR14075.
Presented at the 40th Annual Meeting of the American Society of Neuroradiology, May 2002, Vancouver, British Columbia, Canada.
References
- 1.Gean AD. White matter shearing injury and brainstem. injury In: Imaging of Head Trauma. New York: Raven,1994. :207–248
- 2.Murray JG, Gean AD, Evans SJ. Imaging of acute head injury. Semin Ultrasound CT MR. 1996;17:185–205 [DOI] [PubMed] [Google Scholar]
- 3.Gentry LR. Head trauma. In: Atlas SW, ed. Magnetic Resonance Imaging of the Brain and Spine. New York: Raven,1996;611–647
- 4.Strich SJ. Shearing of nerve fibres as a cause of brain damage due to head injury: a pathological study of twenty cases. Lancet 1961;2:443–448 [Google Scholar]
- 5.Adams JH, Graham DI, Murray LS, Scott G. Diffuse axonal injury due to nonmissile head injury in humans: an analysis of 45 cases. Ann Neurol 1982;12:557–563 [DOI] [PubMed] [Google Scholar]
- 6.Gentry LR, Godersky JC, Thompson B, Dunn VD. Prospective comparative study of intermediate-field MR and CT in the evaluation of closed head trauma. AJR Am J Roentgenol 1988;150:673–682 [DOI] [PubMed] [Google Scholar]
- 7.Kelly AB, Zimmerman RD, Snow RB, et al. Head trauma: comparison of MR and CT-experience in 100 patients. AJNR Am J Neuroradiol 1988;9:699–708 [PMC free article] [PubMed] [Google Scholar]
- 8.Gale SD, Johnson SC, Bigler ED, Blatter DD. Trauma-induced degenerative changes in brain injury: a morphometric analysis of three patients with preinjury and postinjury MR scans. J Neurotrauma 1995;12:151–158 [DOI] [PubMed] [Google Scholar]
- 9.Anderson CV, Wood DM, Bigler ED, Blatter DD. Lesion volume, injury severity, and thalamic integrity following head injury. J Neurotrauma 1996;13:59–65 [DOI] [PubMed] [Google Scholar]
- 10.Liu AY, Maldjian JA, Bagley LJ, Sinson GP, Grossman RI. Traumatic brain injury: diffusion-weighted MR imaging findings. AJNR Am J Neuroradiol 1999;20:1636–1641 [PMC free article] [PubMed] [Google Scholar]
- 11.Arfanakis K, Haughton VM, Carew JD, Rogers BP, Dempsey RJ, Meyerand ME. Diffusion tensor MR imaging in diffuse axonal injury. AJNR Am J Neuroradiol 2002;23:794–802 [PMC free article] [PubMed] [Google Scholar]
- 12.Huisman TAGM, Sorensen AG, Hergan K, Gonzalez RG, Schaefer PW. Diffusion-weighted imaging for the evaluation of diffuse axonal injury in closed head injury. J Comput Assist Tomogr 2003;27:5–11 [DOI] [PubMed] [Google Scholar]
- 13.Pierpaoli C, Jezzard P, Basser PJ, et al. Diffusion tensor MR imaging of the human brain. Radiology 1996;201:637–648 [DOI] [PubMed] [Google Scholar]
- 14.Melhem ER, Itoh R, Jones L, Barker PB. Diffusion tensor MR imaging of the brain: Effect of diffusion weighting on trace and anisotropy measurements. AJNR Am J Neuroradiol 2000;21:1813–1820 [PMC free article] [PubMed] [Google Scholar]
- 15.Pfefferbaum A, Sullivan EV, Hedehus M, Lim KO, Adalsteinsson E, Moseley M. Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magn Reson Med 2000;44:259–268 [DOI] [PubMed] [Google Scholar]
- 16.Papadakis NG, Martin KM, Mustafa MH, et al. Study of the effect of CSF suppression on white matter diffusion anisotropy mapping of healthy human brain. Magn Reson Med 2002;38:394–398 [DOI] [PubMed] [Google Scholar]
- 17.Sorensen AG, Wu O, Copen WA, et al. Human acute cerebral ischemia: detection of changes in water diffusion anisotropy by using MR imaging. Radiology 1999;212:785–792 [DOI] [PubMed] [Google Scholar]
- 18.Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 1996;111:209–219 [DOI] [PubMed] [Google Scholar]
- 19.Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med 1996;36:893–906 [DOI] [PubMed] [Google Scholar]
- 20.Lai SM, Duncan PW. Evaluation of the American Heart Association Stroke Outcome Classification. Stroke 1999;30:1840–1843 [DOI] [PubMed] [Google Scholar]
- 21.Sinson G, Bagley LJ, Cecil KM, et al. Magnetization transfer imaging and proton MR spectroscopy in the evaluation of axonal injury: Correlation with clinical outcome after traumatic brain injury. AJNR Am J Neuroradiol 2001;22:143–151 [PMC free article] [PubMed] [Google Scholar]
- 22.Wieshmann UC, Symms MR, Clark CA, et al. Blunt-head trauma associated with widespread water-diffusion changes. Lancet 1999;10:353:1242–1243 [DOI] [PubMed] [Google Scholar]
- 23.Ulug AM, Moore DF, Bojko AS, Zimmerman RD. Clinical use of diffusion-tensor imaging for diseases causing neuronal and axonal damage. AJNR Am J Neuroradiol 1999;20:1044–1048 [PMC free article] [PubMed] [Google Scholar]
- 24.Wieshmann UC, Clark CA, Symms MR, et al. Reduced anisotropy of water diffusion in structural cerebral abnormalities demonstrated with diffusion tensor imaging. Magn Reson Imaging 1999;17:1269–1274 [DOI] [PubMed] [Google Scholar]
- 25.Brain Trauma Foundation. The American Association of Neurological Surgeons: The joint section on neurotrauma and critical care—Glasgow coma scale score. J Neurotrauma 2000;17:563–571 [DOI] [PubMed] [Google Scholar]
- 26.Yang Q, Tress BM, Barber PA, et al. Serial study of apparent diffusion coefficient and anisotropy in patients with acute stroke. Stroke 1999;30:2382–2390 [DOI] [PubMed] [Google Scholar]