Abstract
Summary: We describe the imaging findings in a dural arteriovenous fistula (AVF) with unilateral subcortical calcification. A 50-year-old woman patient suffered from hypertension and chronic headache. Recently, marked headache and a changed consciousness level were noted. The imaging studies demonstrated left subcortical calcification and cerebral sulcus effacement. MR imaging and angiography revealed multiple abnormal tortuous vessels, mainly from left external carotid artery with left-sided transverse sinus occlusion. The final diagnosis was type II a + b dural AVF (classification of Djindjian and Merland), and the patient underwent endovascular embolization and radiosurgery.
Dural arteriovenous fistulas (AVFs) are thought to be acquired through trauma, sinus occlusion, or chronic venous hypertension (1, 2). Dural AVFs are abnormal arteriovenous connections that are located within the dura mater and involve a dural sinus and/or cortical veins. They constitute 10–15% of all intracranial arteriovenous shunts (3). Transverse/sigmoid sinus dural AVFs are the most common intracranial dural AVFs and have been reported to represent 38% of all dural AVFs (4). We report subcortical calcification in a patient with a dural AVF to demonstrate this finding and explain the pathophysiology of this type of calcification.
Case Report
A 50-year-old woman suffered from headache and long-term hypertension, and recently the headaches worsened with a sudden change in her level of consciousness.
On the initial examination, the Glasgow coma scale was E3V4M6 (eye: open by order; verbal: confusing response; motor: obey), and muscle strength was decreased to about grade 3 throughout. Light reflex was normal. There was no evidence of sensory impairment or cranial nerve deficit. The laboratory examination revealed normal data, including the biochemistry, electrolyte, and complete blood count. The endocrine tests, including thyroid and parathyroid profile, were normal.
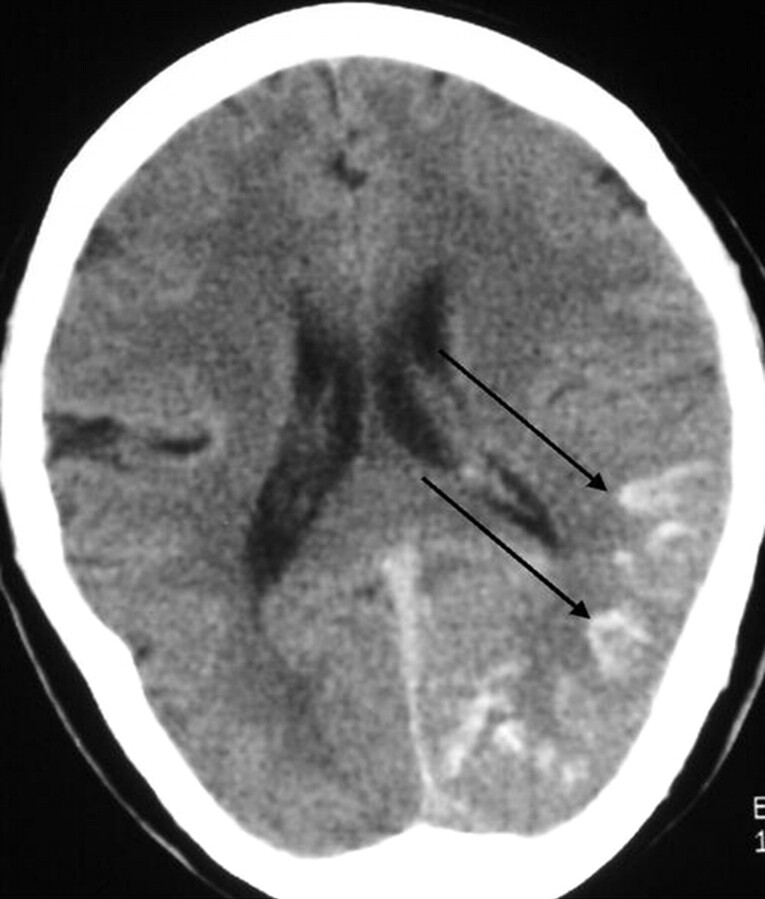
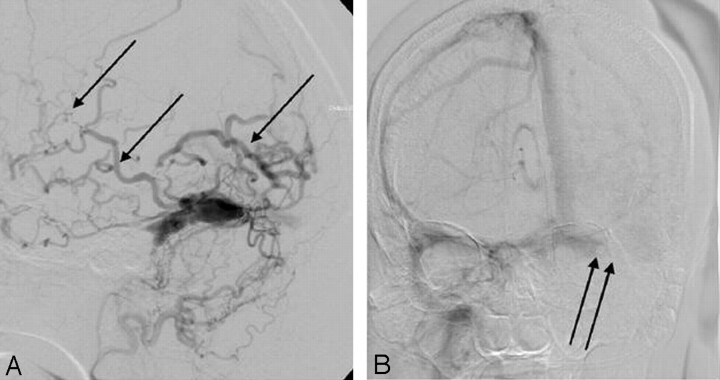
The imaging studies included noncontrast CT scan, 3D time-of-flight MR angiography (MRA) and MR imaging. CT demonstrated subcortical calcification, cerebral sulcus effacement, and a low attenuation area in the subcortical white matter on the left side (Fig 1). MR imaging (Fig 2) revealed multiple enhanced, punctate, and linear signal intensity voids, which indicates the presence of vessels over left cerebrum, and MRA showed multiple abnormal engorged vessels, which arose mainly from the external carotid artery. The left-side transverse sinus and sigmoid sinus were not identified on MR venography (Fig 2D). Digital subtraction angiography (DSA) revealed an AVF of the left transverse and sigmoid sinus (Fig 3) with main feeding vessels from both external carotid arteries. The main venous drainage was through the right transverse and sigmoid sinus, but there was retrograde flow into the straight sinus and superior sagittal sinus, which caused diffuse engorgement of the superifical cortical and the deep intramedullary veins. Occlusion of left transverse and sigmoid sinus was noted. Because of the retrograde flowing pattern of the dural sinus AVF and refluxing into cortical veins, the final diagnosis was type II a + b dural AVF.
Fig 1.
Noncontrast CT scan, axial section of brain. Unilateral subcortical calcification (arrow) and mild adjacent sulcus effacement are seen. Relatively low attenuation appearance was noted in left cerebrum, which indicates the presence of venous ischemia or with infarction.
Fig 2.
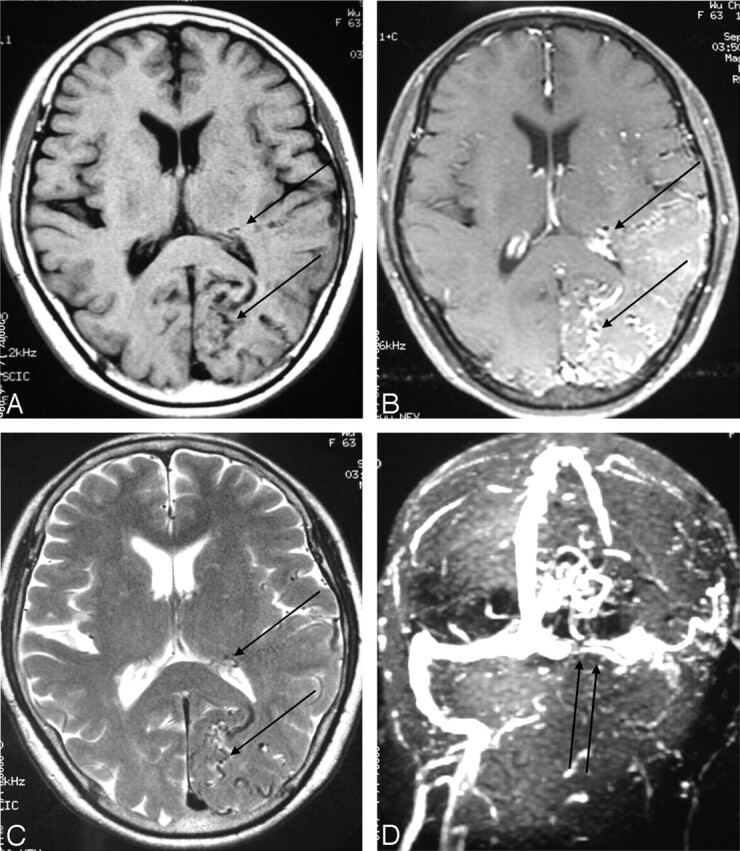
Axial section of brain.
A and B, Pre- and postgadolinium enhancing T1-weighted image (TR/TE/NEX/Matrix, 500/19/2/256 × 256).
C, T2-weighted image (3,200/106/1/256 × 256).
D, MR venography (TR/TE/flip angle, 26/5/20). Multiple tortuous, enhanced cortical and intramedullary vessels (single arrows) were noted over left temporal and occipital region with left transverse sinus occlusion (double arrows).
Fig 3.
DSA.
A, arterial phase of external carotid artery angiogram.
B, Venous phase of internal carotid artery angiogram. Multiple abnormal vessels from external carotid artery feeding a dural AVF at the transverse and sigmoid sinus, the contrast medium retrogradiantly refluxed into cortical and intramedullary veins (single arrows). The main venous drainage was through right-side transverse and sigmoid sinus and occlusive appearance of left-side transverse sinus (double arrows).
Discussion
Dural AVFs are abnormal arteriovenous connections located within the dura mater. Although most dural AVFs have a benign course, they can result in life-threatening hemorrhage (5) and debilitating venous hypertension (6, 7). It is interesting to note that most dural AVFs involve the left-side transverse and sigmoid sinus. Right-side transverse sinus is typically dominant, this may indicate that the smaller sinus generates higher venous pressures and predispose patients to AVF development (8). Our case had an occluded left transverse sinus, but it is difficult to determine whether the occlusion of the left transverse sinus is the result of or the reason for the dural AVFs.
Subcortical calcification is a nonspecific imaging presentation and is commonly bilateral and symmetrical. The frequent causes include Sturge-Weber syndrome, tuberous sclerosis, Fahr disease, postchemoradiotherapy change, and metabolic disorders secondary to parathyroid or thyroid gland abnormalities. Similar calcification changes in corpus striatum and dentate nucleus are also commonly seen in these disorders. In our case, no other calcification foci could be defined except in the left subcortical region. No previous chemoradiotherapy history, hemiartophy of cerebrum, or subependymal nodular lesion was seen. Thus, the previously listed disorders are not likely.
Diffuse engorgement of the superifical cortical and the deep intramedullary veins in our case are identified in the cerebral angiography and MR imaging. High pressure in these cortical and intramedullary veins results in local venous hypertension and impend local venous return. Venous ischemia, venous infarction, and even hemorrhage are likely to happen. The CT showed low attenuation areas in left posterior temporal and occipital lobe (Fig 1), suggestive of a nonhemorrhagic venous ischemia or infarct.
The exact mechanism of the subcortical calcification of dural AVFs is unknown but is proposed to be an arterial steal phenomenon or persistent venous congestion, with calcification occurring in chronic hypoperfused brain parenchyma or secondary to dystrophic changes in the walls of congested veins (9, 10). The cerebral subcortical white matter regions are located in the watershed areas of the arterial supply and therefore are more sensitive to hypoxic or ischemia change. Because of the location of the fistula in our case it would be reasonable to predict that more severe ischemia change would occur in the left cerebrum than on the right side and that would lead to subcortical calcification.
Conclusion
The similar appearance of dystrophic calcification is commonly seen in pial AVM (11, 12). Yu et al (11) and Sheth at el (12) described diffuse intracranial, parenchymal, and dystrophic calcifications due to pial AVMs. They reported intracranial calcification in the watershed areas and away from the pial AVM. Although rare, the reported subcortical calcification in dural AVFs is bilateral and symmetrical (9, 10). To the best of our knowledge, ours is unusual because of the unilateral subcortical calcification in the dural AVFs. In conclusion, dural AVFs should be included in the differential diagnosis of intracranial calcification such as subcortical calcification on an unenhanced CT scan.
References
- 1.Chaudhary MY, Sachdev VP, Cho SH, et al. Dural arteriovenous malformation of the major venous sinuses: an acquired lesion. AJNR Am J Neuroradiol 1982;3:13–19 [PMC free article] [PubMed] [Google Scholar]
- 2.Terada T, Higashida RT, Halbach VV, et al. Development of acquired arteriovenous fistulas in rats due to venous hypertension. J Neurosurg 1994;80:884–889 [DOI] [PubMed] [Google Scholar]
- 3.Newton TH, Cronqvist S. Involvement of dural arteries in intracranial arteriovenous malformations. Radiology 1969;93:1071–1078 [DOI] [PubMed] [Google Scholar]
- 4.Malek AM, Halbach VV, Higashida RT, et al. Treatment of dural arteriovenous malformations and fistulas. Neurosurg Clin North Am 2000;11:147–166 [PubMed] [Google Scholar]
- 5.Brown RD, Wiebers DO, Nichols DA. Intracranial dural arteriovenous fistulae: angiographic predictors of intracranial hemorrhage and clinical outcome in nonsurgical patients. J Neurosurg 1994;81:531–538 [DOI] [PubMed] [Google Scholar]
- 6.Hurst RH, Hackney DB, Goldberg HI, Davis RA. Reversible arteriovenous malformation-induced venous hypertension as a cause of neurological deficits.Neurosurgery 1992;30:422–425 [DOI] [PubMed] [Google Scholar]
- 7.Willinsky R, TerBrugge K, Lasjaunias P, Montanera W. The variable presentations of craniocervical and cervical dural arteriovenous malformations. Surg Neurol 1990;34:118–123 [DOI] [PubMed] [Google Scholar]
- 8.Caragine LP, Halbach VV, Dowd CF, et al. Parallel venous channel as the recipient pouch in transverse/sigmoid sinus dural fistulae. Neurosurgery 2003;53:1261–1267 [DOI] [PubMed] [Google Scholar]
- 9.Lai PH, Chang MH, Liang HL, et al. Unusual signs for dural arteriovenous fistulas with diffuse basal ganglia and cerebral calcification. Zhonghua Yi Xue Za Zhi (Taipei). 2000;63:329–333 [PubMed] [Google Scholar]
- 10.Wang PY, Liu LH, Shen WC. Dural arteriovenous malformation of the transverse sinus with sinus occlusion: report of a case. J Formos Med Assoc 1992;91:102–105 [PubMed] [Google Scholar]
- 11.Yu YL, Chiu EK, Woo E. Dystrophic intracranial calcification: CT evidence of “cerebral steal” from arteriovenous malformation. Neuroradiology 1987;29:519–522 [DOI] [PubMed] [Google Scholar]
- 12.Sheth RD, Bodensteiner JB. Progressive neurological impairment from arteriovenous malformation vascular steals. Pediatr Neurol 1995;13:352–524 [DOI] [PubMed] [Google Scholar]