Abstract
Summary: Dural arteriovenous shunts and pial arteriovenous fistulas are uncommonly associated. Their etiology, pathogenesis, and natural history are still unclear and are likely different. We present three cases of high-flow dural arteriovenous shunts associated with pial arteriovenous fistulas and discuss their pathogenesis, anatomic association, and angioarchitecture. We propose that venous steal effect in the dural sinus secondary to the high-flow dural arteriovenous shunt induced the pial arteriovenous fistulas. Treatment of the high-flow dural arteriovenous shunts and the induced pial arteriovenous fistulas are discussed.
Dural arteriovenous shunts (DAVSs) associated with pial arteriovenous fistulas (AVFs) have been rarely reported (1–3). Cerebral pial AVFs induced by high-flow DAVSs are an exceptional subgroup of this uncommon association.
Case 1
A male patient presented with intracerebral hemorrhage at the age of 3 years in September 1998. Angiography showed multifocal DAVSs supplied by dural arteries at both sides. The main supply was from branches of the right middle meningeal artery to the anterior superior sagittal sinus, from the mastoid branch of the right ascending pharyngeal artery to the right transverse sinus, and the ethmoidal branch of the left ophthalmic artery to the anterior superior sagittal sinus. There was cortical venous congestion and reflux. The right transverse sinus showed stenosis and subsequently occluded, resulting in a residual venous pouch. There were bilateral pial AVFs supplied by branches of the anterior cerebral arteries draining via cerebral cortical veins into the superior sagittal sinus. The patient was treated, from the end of 1998, by multiple sessions of transarterial glue embolization to several of his DAVSs, transvenous coiling of the anterior part of the superior sagittal sinus, repeated angioplasty of left jugular bulb stenosis, and endovascular coil occlusion of the residual pouch of the right transverse sinus. Because of recurrent neurologic deterioration, the high-flow pial AVFs at both anterior cerebral artery territories were embolized with glue, resulting in transient improvement. Finally, the patient died from posterior fossa venous congestion and hemorrhagic infarction in November 2003 at the age of 8 years.
Case 2
A male patient presented at the age of 43 years, in 1985, with bilateral bruit. Angiography showed multiple DAVSs on both sides supplied by the meningeal branches of the middle meningeal artery and the superficial temporal artery and a meningeal branch of the right ophthalmic artery. Shunts mainly drained into the superior sagittal sinus. Cortical venous reflux was present. There were pial AVFs supplied by the pericallosal branch of the left anterior cerebral artery and draining via cerebral cortical veins into the superior sagittal sinus. A flow-related aneurysm was present at the A2 segment of left anterior cerebral artery. The patient had been treated with glue embolization of the DAVSs to the superior sagittal sinus with initial improvement. Subsequently, he showed neurologic symptoms due to chronic venous congestion and presented to our institution in 1996. In view of the complex nature of the lesion, operative resection of the superior sagittal sinus dural arteriovenous fistulas was performed following preoperative embolization. The symptoms from the dural arteriovenous fistulas had resolved after surgery.
Case 3
A young male patient presented at age of 17 months with increased head circumference, a bruit over the vertex, and dilated superficial veins near the orbits. Angiography showed bilateral multiple high-flow DAVSs supplied by middle meningeal branches and dural branches of the ophthalmic artery at both sides. These shunts drained into the superior sagittal sinus with cortical venous reflux. Pial AVFs were also demonstrated, supplied by right anterior cerebral branches and draining via cerebral cortical veins into the superior sagittal sinus. This patient underwent three sessions of transarterial embolization with glue through both middle meningeal branches and dural branch to the falx arising from the ophthalmic arteries. This resulted in disappearance of the bruit and stabilization of the head growth and neurologic findings.
Discussion
All of our reported cases had multiple high-flow DAVSs (Fig 1) involving mostly the superior sagittal sinus. DAVSs involving the superior sagittal sinus are relatively uncommon. High-flow multiple DAVSs are more common in the infantile type, but they can also occur in adults, as in our case 2, an adult male 43 years of age at presentation. All three patients had DAVSs from dural branches of the ophthalmic artery to the anterior part of the superior sagittal sinus. All had pial AVFs supplied by branches of the anterior cerebral artery to cortical veins that opened to the superior sagittal sinus, where also most of the DAVSs were draining (Figs 2, 3). All had cortical venous reflux, which accounted for the clinical sequel in two of the three cases. The similarity of the angioarchitecture and the close anatomic relationship of the multiple DAVSs to the pial AVFs in our cases lead us to propose that these lesions are pathogenetically linked.
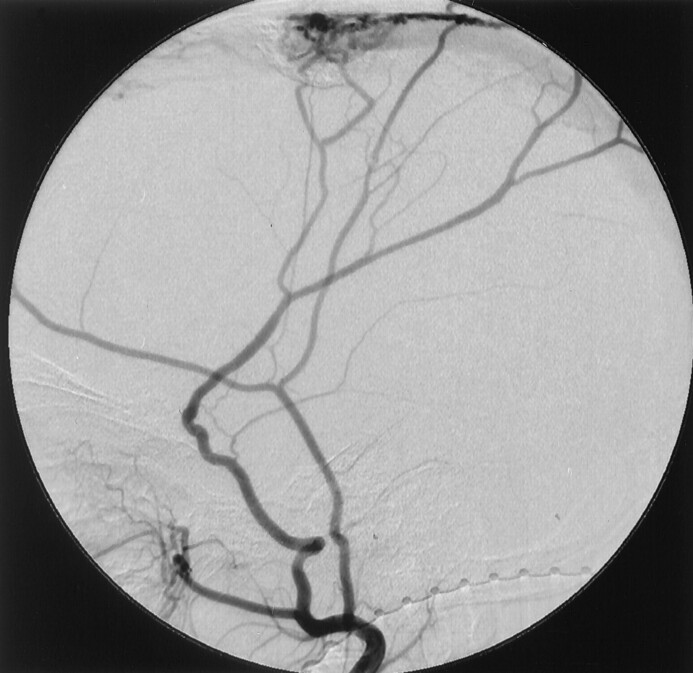
Fig 1.
Right external carotid angiogram of case 3 shows the DAVSs at the superior sagittal sinus.
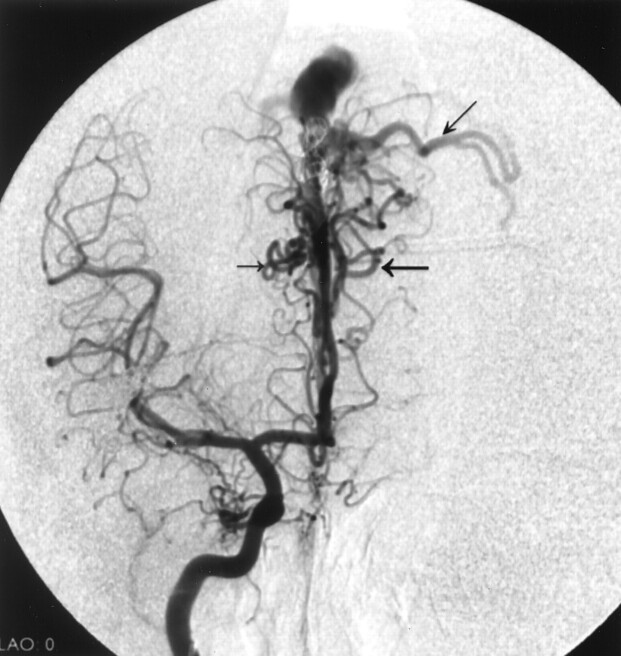
Fig 2.
Frontal-view right internal carotid angiogram of case 1 shows pial AVFs (horizontal arrows) and cortical venous reflux (oblique arrow).
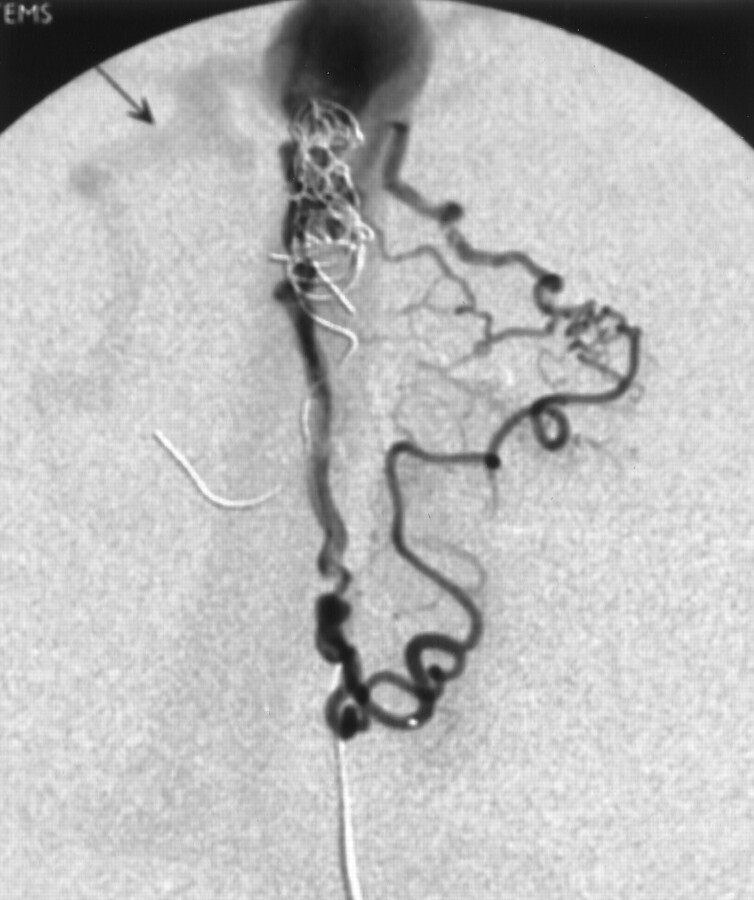
Fig 3.
Selective injection of the left anterior cerebral branch of case 1 shows detailed angioarchitecture of the induced pial AVF. Reflux into the cortical vein in the right side is indicated (arrow).
Steal Phenomenon
An arterial steal phenomenon is often discussed when pial arteriovenous shunts (AVSs) appear to divert flow toward a low-resistance vascular bed, away from adjacent regions. Intraarterial pressure measurements and transcranial velocity studies have shown regional hemodynamic alterations and evidence from single-photon emission CT does suggest a relationship between regional hypoperfusion and neurologic deficits (4). Similar steal effects have been proposed to account for the pial AVFs associated with infantile DAVSs (5).
Pial AVFs
Pial AVFs are considered to be congenital in nature (6). By contrast, there is little evidence that the pial AVF diagnosed in adults are present in the same form at birth. In addition, cases of de novo pial AVF have been reported (7–10). With knowledge of endothelial cell origin (such as the study of genetic vascular diseases including hereditary hemorrhagic telangiectasia and Rendu-Osler-Weber disease) and the biology of the remodeling process of the vascular system, Lasjaunias (6) proposed that the congenital event is primarily involving the vascular modeling and remodeling process at the cellular and structural level. It is likely to affect the endothelial cells at the venous side of the capillaries, resulting in a quiescent dysfunction. The structural manifestation of this dysfunction depends on revealing triggers such as mechanical, hormonal, pharmacologic, hemodynamic, thermal, radiation, viral, infective, and metabolic factors. The diverse nature and timing of the various triggers causes the different time and form of presentation of the abnormal AVS. This postulation can also be extended to explain an acquired lesion to occur as a postnatal acquired event. Under these circumstances, the trigger was applied for a certain length of time and had the same consequence as the postulated congenital event on the target cells related to venous vascular remodeling. The pathogenesis of pial AVF also includes abnormal expression of various angiogenic factors (11–13) such as vascular endothelial growth factor, basic fibroblast growth factor, and alpha transforming growth factor. Suppression of vascular cell growth modulators, including endoglin1, has also been found (14). Cases of acquired pial AVFs in Moya-moya disease and following cerebral vein thrombosis (8,9) suggest ischemia or hypoxia is an important etiological factor. Hypoxia is a powerful trigger for the up-regulation of angiogenic factors expression (15).
DAVSs
DAVSs are a heterogeneous group of lesions that were initially considered to be of congenital origin. They can now be classified as dural sinus malformations, infantile DAVSs, and adult types of DAVSs (16). Only the dural sinus malformation is truly congenital in origin. Many reports have documented the acquired nature of DAVS after dural sinus thrombosis, intracranial infection, trauma, surgery, and occlusion of the dural sinuses by surgery or tumor (17–23). DAVSs are also known to be associated with pregnancy and hypercoagulation states (24). Experiments in a rat model have suggested that chronic venous hypertension is the most important etiologic factor (25). It has been implied that abnormal angiogenic activity is induced directly or indirectly by venous hypertension through decreasing cerebral perfusion and increasing ischemia (26). These activities are likely mediated through abnormal expression of endothelial-cell vascular growth factors (e.g., vascular endothelial growth factor, basic fibroblast growth factor), which are demonstrated in DAVS (27).
The Association of DAVSs and Pial AVFs
Dural AVSs Inducing Pial AVFs.
Anatomically, both dural and cortical veins open directly into the dural sinus. Abnormal AVSs between dural arteries and veins without intervening capillaries are formed in the wall of the dural sinus triggered by increased venous pressure or thrombosis (28). Increased flow of blood into the low-resistance system of dural sinus can exert a venous steal effect on the upstream cortical veins opening directly into the dural sinus (16). The exact mechanism of the opening up of the pial shunt into these cortical veins remains unknown. The result of steal effect may cause hypoxia in the regions drained by these cortical veins. The hypoxia can result in up-regulation of the angiogenic factors that act on the capillarovenous endothelial cells and other cellular process involved in the remodeling of the vascular system for a significant period of time triggering the formation of the pial AVFs. Because these induced pial AVFs seem to locate preferentially at certain parts of the dural sinus (e.g., at the superior sagittal sinus in our cases) there may be additional regional factors to account for it. The influence on cortical veins by the high flow in the dural sinus is affected by factors such as the orientation of the cortical vein with respect to the dural sinus, the size and shape of the vein, function of the venodural junction, the flow characteristic in the dural sinus near the opening of the cortical veins, and so forth. The multiple anatomic factors and the various hemodynamic situations in the vicinity of the opening of these cortical draining veins dictate whether the steal effect is efficient as a trigger for the pial AVFs and where the location of these fistulas form upstream from the high-flow DAVSs.
Pial AVF Inducing DAVS.
By contrast, the possibility of pial AVFs inducing DAVSs has been proposed particularly in some high-flow pial AVFs associated with DAVSs upstream from their drainage into the dural sinus (29). This could be caused by a similar sump effect created by the high-flow venous drainage of the pial AVF downstream. The venous changes produced by high-flow pial AVS on the venous sinuses, such as increased venous pressure or venous outflow obstruction, can also be triggering factors. But this is not the situation in our cases, because they were high-flow DAVSs anatomically closely related to the pial AVFs that were upstream from the DAVSs. The dominant shunts in our cases were high-flow DAVSs rather than pial AVFs.
Thrombosis of Local Cortical Vein Inducing Pial AVF.
Local thrombosis of cortical veins has been reported in one case to precede the de novo formation of pial AVF (8). It is not possible for us to postulate the same mechanism to account for our cases, because we do not have evidence whether there was thrombosis of the cortical veins before the formation of the pial AVFs. DAVS may rarely have local pial supply, which is more often in the occipito-temporal region (21). This may be related to previous venous thrombosis, hemorrhage, or cortical venous ischemia. Our cases do not seem to fit into this type of association.
The DAVSs of all our cases were draining into the superior sagittal sinus. The induced pial AVFs are from branches of anterior cerebral arteries. This association may indicate that the superior sagittal sinus is a common site for high-flow DAVSs to induce pial AVFs through the venous steal mechanism.
Clinical Course and Management
The clinical course of high-flow multiple infantile-type DAVSs is often progressive, and their prognosis is poor (16). They require multiple endovascular interventions and/or operations, as in our cases. Case 1 required multiple repeated arterial embolizations for the DAVSs, transvenous coil obliteration of the draining venous pouch, venous stent placement, and dilation of sinus strictures. The natural history of the induced pial AVFs is not known, but they can subside after occlusion of the triggering DAVSs (16). It is interesting to note that in case 1 the pial AVFs continued to persist after coiling of that part of the superior sagittal sinus. This may be secondary to the remaining steal effect and the local hemodynamic of the dural sinus near the cortical veins draining these pial AVFs. The triggered dysfunction creating the pial AVFs may persist despite the cessation of the triggering factors. These pial AVFs appear to contribute to the clinical deterioration of the patient in case 1 by contributing to the venous hypertension. Embolization of the pial AVFs in case 1 has led to clinical improvement of the patient. The primary consideration of treatment, however, is of the high-flow DAVSs because they represent the primary lesion mainly responsible for the clinical conditions of the patients. Some of the induced pial AVFs disappear after treatment of the high-flow DAVSs (16). The risk of these induced pial AVFs is unknown. Their treatment decision is based on the knowledge extrapolating from the behavior of pial AVFs in general and on their specific contribution to the clinical symptoms. The induced pial AVFs can cause secondary arterial changes as in case 2, who had an aneurysm (Fig 4) related to the high flow in the shunt, formed in the arterial feeder supplying the fistulas. Case 2 is an example that high-flow DAVSs can also occur in adults and induce pial AVFs similar to those in children or infants.
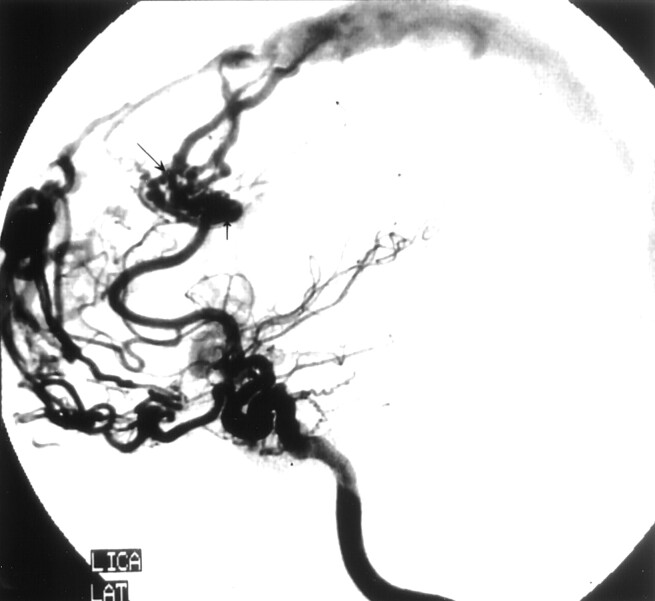
Fig 4.
Left internal carotid angiogram of case two shows induced pial AVFs draining to the superior sagittal sinus (large arrow). A flow-related aneurysm has formed (vertical arrow). Reflux into cortical vein is noted (small arrow).
Conclusion
Our cases were all high-flow DAVSs with anatomically closely related pial AVFs both draining into the superior sagittal sinus. We postulated the high-flow DAVSs cause a venous steal (sump) effect that, in a suitable combination of anatomic and hemodynamic factors, induces pial AVFs. Treatment should focus on treating the DAVS, which is the primary lesion and likely to be the main cause of symptoms. These pial AVFs can subside after successful treatment of the high-flow DAVSs, but they can persist and contribute to the clinical symptoms of the patients. In that case, endovascular embolization of these pial AVFs can be useful to improve the clinical symptoms.
References
- 1.SchlachterL, Fleicher A, Faria M, et al. Multifocal intracranial arteriovenous malformations. Neurosurgery 1980;7:440–444 [DOI] [PubMed] [Google Scholar]
- 2.Willinsky RA, Lasjaunias P, Ter Brugge K, Burrows P. Multiple cerebral arteriovenous malformations: review of our experience from 203 patients with cerebral vascular lesions. Neuroradiology 1990;32:207–210 [DOI] [PubMed] [Google Scholar]
- 3.Vilela P, Ter Brugge K, Willinsky R. Association of distinct intracranial pial and dural arteriovenous shunts. Neuroradiology 2001;43:770–777 [DOI] [PubMed] [Google Scholar]
- 4.Taylor CL, Selman WR, Ratcheson RA. Steal affecting the central nervous system. Neurosurg 2002;4:679–688 [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Monaco R, Rodesch G, ter Brugge K, et al. Multifocal dural arteriovenous shunts in children. Childs Nerv Syst 1991;7:425–431 [DOI] [PubMed] [Google Scholar]
- 6.Lasjaunias P. A revised concept of the congenital nature of cerebral arteriovenous malformations. Intervent Neuroradiol 1997;3:275–281 [DOI] [PubMed] [Google Scholar]
- 7.Ozawa T, Miyasaka Y, Tanaka R, et al. Dural-pial arteriovenous malformation after sinus thrombosis. Stroke 1998;29:1721–1724 [DOI] [PubMed] [Google Scholar]
- 8.Phatouros C, Halbach VV, Dowd C, et al. Acquired pial arteriovenous fistula following cerebral vein thrombosis. Stroke 1999;30:2487–2490 [DOI] [PubMed] [Google Scholar]
- 9.Schmit B, Burrows P, Huban K, et al. Acquired cerebral arteriovenous malformations in a child with moya-moya disease. J Neurosurg 1996;84:677–680 [DOI] [PubMed] [Google Scholar]
- 10.Kader A, Goodrich J, Sonstein W, et al. Recurrent cerebral arteriovenous malformations after negative postoperative angiograms. J Neurosurg 1996;85:14–18 [DOI] [PubMed] [Google Scholar]
- 11.Rothbart D, Awad I, Lee J, et al. Expression of angiogenetic factors and structural proteins in central nervous system vascular malformations. Neurosug 1996;38:915–925 [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto T, Emala C, Hoshi S, et al. Abnormal pattern of tie-2 and vascular endothelial growth factor receptor expression in human cerebral arteriovenous malformations. Neurosurg 2000;47:910–918 [DOI] [PubMed] [Google Scholar]
- 13.Kilic T, Pamir N, Kullu S, et al. Expression of structural proteins and angiogenetic factors in cerebrovascular anomalies. Neurosurg 2000;5:1179–1191 [DOI] [PubMed] [Google Scholar]
- 14.Rhoto P, Comair Y, Shedid D, et al. Specific repression of the preproendothelin-1 gene in intracranial arteriovenous malformations. J Neurosurg 1997;86:101–108 [DOI] [PubMed] [Google Scholar]
- 15.Schweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature 1992;359:843–884 [DOI] [PubMed] [Google Scholar]
- 16.Lasjaunias P. Vascular diseases in neonates, infants and children. Berlin: Springer-Verlag;1997:321–371
- 17.Houser OW, Campbell JK, Campbell RJ, et al. Arteriovenous malformation affecting the transverse dural sinuses: an acquired lesion. Mayo Clin Proc 1979;54:651–661 [PubMed] [Google Scholar]
- 18.Chaudhary MY, Sachdev VP, Cho SH, et al. Dural arteriovenous malformation of the major dural sinuses: an acquired lesion. AJNR Am J Neuroradiol 1982;3:13–19 [PMC free article] [PubMed] [Google Scholar]
- 19.Graeb DA, Dolman CL. Radiological and pathological aspects of dural arteriovenous fistulas. J Neurosurg 1986;64:962–967 [DOI] [PubMed] [Google Scholar]
- 20.Sakaki T, Morimoto T, Nakase H, et al. Dural arteriovenous fistula of the posterior fossa developing after surgical occlusion of the sigmoid sinus. J Neurosurg 1996;84:113–118 [DOI] [PubMed] [Google Scholar]
- 21.Awad IA, Little JR, Akrawi WP. Intracranial dural arteriovenous malformations: factors predisposing to an aggressive neurological course. J Neurosurg 1990;72:839–850 [DOI] [PubMed] [Google Scholar]
- 22.Bito S, Ohnishi T, Takimoto N, et al. Dural arteriovenous fistulae found after removal of meningioma: a case report. Neurol Surg 1978;6:397–400 [PubMed] [Google Scholar]
- 23.Vilela P, Willinsky R, Ter Brugge K. Dural arterivenousfistula associated with neoplastic dural sinus thrombosis: two cases. Neuroradiology 2001;43:816–820 [DOI] [PubMed] [Google Scholar]
- 24.Berenstein A, Lasjaunias P, eds. Surgical Neuroangiography. Vol 4. Endovascular treatment of cerebral lesions. Berlin: Springer-Verlag;1992. :6–81
- 25.Terada T, Higashida R, Halbach VV, et al. Development of acquired arteriovenous fistulas in rats due to venous hypertension. J Neurosurg 1994;80:884–889 [DOI] [PubMed] [Google Scholar]
- 26.Lawson M, Jacobowitz R, Spetzler R. Redefined role of angiogenesis in the pathogenesis of dural arteriovenous malformations. J Neurosurg 1997;87:267–274 [DOI] [PubMed] [Google Scholar]
- 27.Uranishi R, Nakase H, Sakati T. Expression of angiogenetic growth factors in dural arteriovenous fistulae. J Neurosurg 1999;91:781–786 [DOI] [PubMed] [Google Scholar]
- 28.Hamada Y, Goto K, Inoue T, et al. Histopathological aspects of dural arteriovenous fistulas in the transverse-sigmoid sinus region in nine patients. Neurosurg 1997;40:452–458 [DOI] [PubMed] [Google Scholar]
- 29.Valavanis A, Yasargil MG. The endovascular treatment of brain arteriovenous malformations. Adv Tech Stand Neurosurg 1998;24:131–214 [DOI] [PubMed] [Google Scholar]