Abstract
BACKGROUND AND PURPOSE: Intraoperative MR imaging (IMRI) has advantages over conventional framed and frameless techniques. IMRI, however, also has some drawbacks, especially related to interpretation of gadolinium-enhanced intraoperative imaging resulting from surgically induced blood brain barrier injury, vascular changes, and hemorrhage. Ultra-small superparamagnetic iron particles like ferumoxtran-10 have a long plasma half-life and are trapped by reactive cells within the tumor. These trapped particles provide a method to demonstrate enhancing lesions without the artifact of repeat gadolinium administration in the face of blood brain barrier and vascular injury.
METHODS: We present a review of the literature and the cases of two patients who underwent surgery in which IMRI with ferumoxtran-10 was used.
RESULTS: Ultra-small superparamagnetic iron particles represent a method to demonstrate enhancing intrinsic brain tumors without the drawbacks of intraoperative gadolinium enhancement. These lesions appear even on low-field strength IMRI. Ferumoxtran-10, administered preoperatively, provides a stable imaging marker, even after surgical manipulation of the brain.
CONCLUSION: Fermumoxtran-10 provides a way to lessen artifactual enhancement during IMRI related to the administration of gadolinium.
Intraoperative MR imaging (IMRI) provides a unique opportunity for an early look at surgical biopsy and resection results. No other technique currently has the ability to evaluate the location and margins of a biopsy or resection and guide both the approach and further intervention during a single operative setting. Ferumoxtran-10 is an ultra-small superparamagnetic iron oxide (USPIO) particle that can delineate intracranial tumors with peritumoral reactive changes (1, 2). Intraoperative enhancement with gadolinium may reduce the accuracy of IMRI by making surgically disrupted areas of blood brain barrier enhance on the intraoperative images (3–7).
We present two patients who underwent preoperative MR imaging with gadolinium and then with ferumoxtran-10 and then underwent resection for an intrinsic brain tumor with IMRI. These patients demonstrate, along with previously published reports, the efficacy of IMRI without the potential pitfalls of recurrent gadolinium administration, especially in the operative environment (3, 4). In addition, USPIO particles, despite their large size when compared with traditional contrast agents, may provide better imaging of the entire tumor burden, both where the blood brain barrier is grossly and minimally defective. Unlike other contrast agents, ferumoxtran-10 has a 1–2-day plasma half-life and can be histologically identified in the tumor specimen due to CNS cellular trapping (8). IMRI may improve the ability to resect intrinsic brain tumors and even patient outcomes (9, 10).
Methods
Two patients were enrolled into an institutional review board approved study of ferumoxtran-10 (Combidex, Advanced Magnetics, Cambridge, MA). Both patients had a prior diagnosis of an intrinsic brain tumor: one anaplastic oligodendroglioma and one glioblastoma. The patients underwent MR imaging, including T1-weighted (TE 9, TR 400), proton density-weighted (9.3, 2000), T2-weighted (90, 4500), fluid attenuated inversion recovery (FLAIR; 130, 8800), and postgadolinium T1-weighted sequences. They then received a dose of ferumoxtran-10 (2.6 mg Fe/kg, diluted in 50 mL of normal saline at 4 mL/min). Twenty-four hours later, on the morning of surgery, each patient underwent another MR imaging, including T1-weighted, T2-weighted, gradient echo (23, 750), and diffusion-weighted (90, 10 000) sequences. The patients were then taken to the operating room for resection of their lesion guided by a Polestar N-10 0.15T IMRI unit (Odin Medical Technologies, Yokneam Elit, Israel), which allows both imaging and frameless stereotactic guidance from the intraoperative images. Intraoperative images were obtained by using T1-weighted sequences (2.9, 80). Both patients then underwent postoperative MR imaging, including T1-weighted, T2-weighted, proton density-weighted, gradient echo, diffusion-weighted, and postgadolinium T1-weighted sequences, within 72 hours of the operation to assess residual iron enhancement.
Patient 1
Patient 1 is a 41-year-old man with a history of anaplastic oligodendroglioma last resected in April 2000. The patient then underwent chemotherapy with procarbazine, carmustine, and vincristine. Repeat MR imaging showed a stable tumor until Fall 2002. Then he was enrolled in the ferumoxtran-10 study and underwent MR imaging as described above. Twenty-four hours later, the patient went to the operating room for resection of his recurrent tumor by using IMRI. Pathologic examination of the tumor revealed recurrent anaplastic oligodendroglioma.
Patient 2
Patient 2 is a 66-year-old man with a diagnosis of glioblastoma multiforme who had undergone prior resection at another institution in October 2002. The patient was referred to our institution for further care in November 2002. En route to our hospital, the patient had a decrease in his level of consciousness and was treated in another hospital with an increased dose of dexamethasone. On arrival to our hospital, the patient was admitted, underwent repeat MR imaging, and was then enrolled in the ferumoxtran-10 study and underwent MR imaging as described above. The patient was taken to the operating room for resection of residual tumor at the posterior margin of his previous resection as well as mass effect from a fluid collection in his resection cavity by using IMRI. The post-resection IMRI revealed an area of residual tumor at the posterior margin of the resection cavity. This area was then re-explored, and further resection was performed. Pathologic examination of the tumor demonstrated residual, recurrent glioblastoma multiforme, prominent reactive changes and granulation tissue from previous surgery, and features suggestive of seroma. Tumor from this patient was also subjected to iron staining by using Perl’s technique with diaminobenzidine.
Results
Both patients underwent IMRI-guided resection of their tumors. Preoperative MR imaging demonstrated the targeted lesion. Intraoperative MR without gadolinium, following the prior administration of ferumoxtran-10, easily provided images that demonstrated the lesions seen on preoperative MR images obtained in both patient 1 (Fig 1) and patient 2 (Fig 2). In one operation, post-resection imaging revealed a persistently enhancing area that was then resected (Fig 3). Even in the low-field-strength IMRI system, ferumoxtran-10 provided adequate imaging of the lesions in both of these patients. Postoperative MR images obtained in both patients had no significant enhancement with gadolinium, which suggests resection of the enhancing tumor bulk (Figs 4 and 5). Both postoperative MRIs show high T1 signal intensity in the wall of the resection cavity. The walls of the cavities, however, do not appear to enhance significantly with gadolinium. Figure 5 also shows the resection cavity itself filled with T1 hyperintense material. The cause of this residual T1 hyperintensity, either from blood or ferumoxtran, is unclear. Iron staining of one patient’s lesion demonstrated iron accumulation within peritumoral reactive cells (Fig 6).
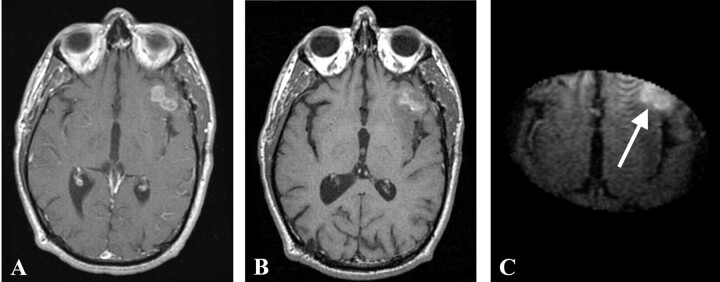
Fig 1.
Preoperative gadolinium-enhanced (A), ferumoxtran-10-enhanced (B), and intraoperative ferumoxtran-10-enhanced T1-weighted MR images (C) with arrow pointing to the enhancing lesion from patient 1. Panels B and C were obtained approximately 24 hours after ferumoxtran-10 administration.
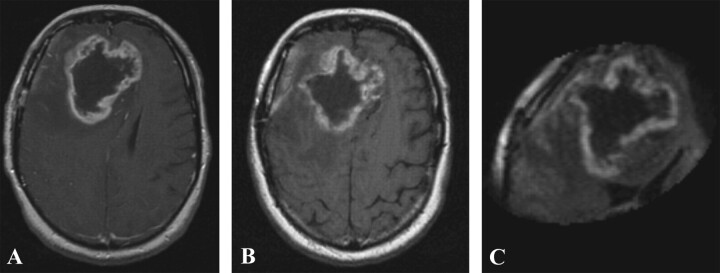
Fig 2.
Preoperative gadolinium-enhanced (A), ferumoxtran-10-enhanced (B), and intraoperative ferumoxtran-10-enhanced T1-weighted MR images (C) from patient 2. Panels B and C were obtained approximately 24 hours after ferumoxtran-10 administration.
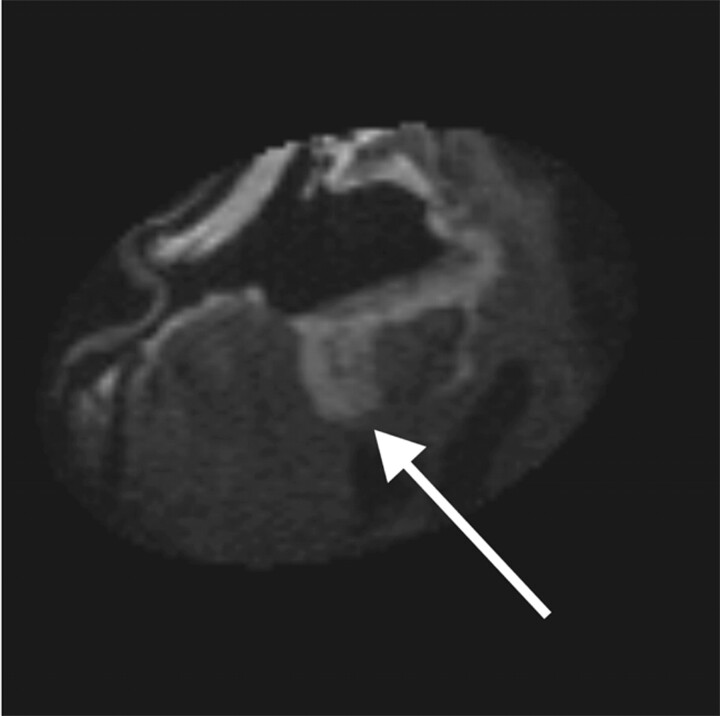
Fig 3.
Intraoperative post-resection T1-weighted MR image from patient 2, demonstrating residual enhancing lesion posteriorly (arrow). This lesion was then localized by using integrated frameless stereotaxy and resected.
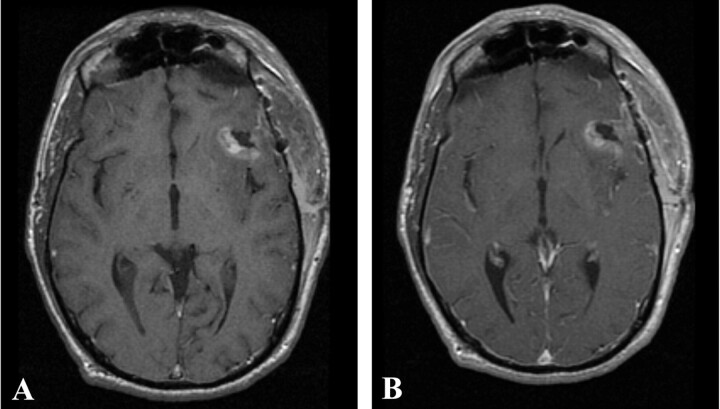
Fig 4.
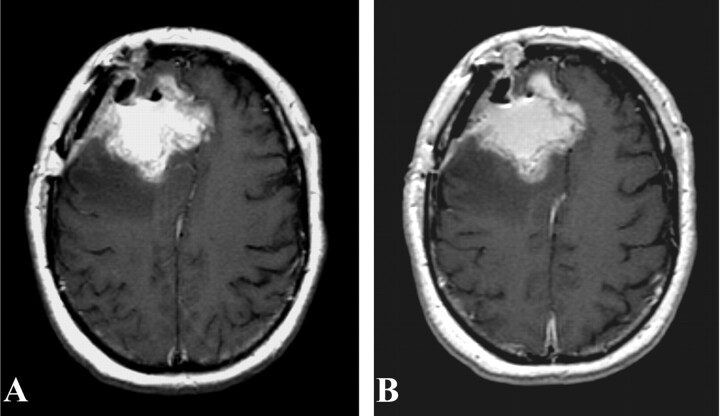
Postoperative T1-weighted MR imaging from patient 1 performed 72 hours after surgery, without (A) and with (B) gadolinium. No residual areas of significant gadolinium enhancement are seen.
Fig 5.
Postoperative T1-weighted MR imaging from patient 2 performed 24 hours after surgery, without (A) and with (B) gadolinium. No significant gadolinium-enhancing areas are seen, although they may be masked by residual T1 signal intensity. These images demonstrate the difficulty with postoperative imaging in the face of blood products and hemostatic agents.
Fig 6.
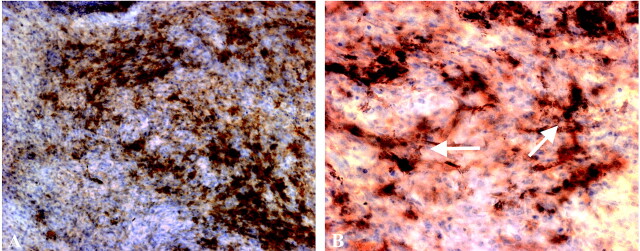
Photomicrograph of 10× (A) and 20× (B) views of patient 2’s tumor. Modified Perl’s stain with diaminobenzidine shows that iron does not stain the tumor cells, but does stain reactive cells, morphologically consistent with astrocytes (arrows).
Discussion
Intraoperative MR imaging provides the most up-to-date data on tumor location, showing any movement of the tissue, the amount of tumor resected, and any residual tumor. Traditional image guidance systems, based on preoperative scanning, do not provide any data about intraoperative brain shift and cannot evaluate for any residual tumor. IMRI updates guidance systems and provides the most current location of intracranial lesions to best guide the surgeon in performing the most complete resection possible (10–13). IMRI does have limitations of limited availability and need for special operating room equipment, increased operating room time, and in some cases a smaller imaging field.
To visualize most tumors, administration of gadolinium has generally been necessary (3, 14–18). This practice may have pitfalls related to surgically induced enhancement of the operative site due to vascular leak, luxury perfusion, and other changes (3, 4, 6, 7, 19–23). Double-dose administration of gadolinium needed for good visualization of the tumor in low-field-strength IMRI environments may worsen the problem (24). If more than one intraoperative assessment of the resection is needed, gadolinium may also need to be dosed more than once. In addition, blood and hemostatic agents can also affect the appearance of MR imaging (25–27). IMRI provides the ability to confirm visually a bloodless operative field before and after imaging and confirm tumor resection without the confounding effects of postoperative brain enhancement (28).
The use of USPIO particles has been suggested as a partial remedy for this problem (8, 29). The USPIO particles have T1 and T2 shortening effects that provide the basis for their use as a contrast agent.(1, 2, 8) These particles have a much longer plasma half-life than gadolinium, which provides them an opportunity to cross the blood brain barrier long after gadolinium has been excreted. Over time, the USPIO particles are taken up and trapped by reactive cells, such as microglia, macrophages, and astrocytes, in the brain parenchyma, which causes persistent enhancement without the need for further contrast medium administration. Although ferumoxtran-10 is not trapped by the tumor cells, it does localize to the same area as the tumor. To date, in 62 patients who have received ferumoxtran and 22 patients who went on to image-guided surgery, iron staining was not found in any area that did not have tumor histologically. In no case was normal brain resected at the lesion margins (authors’ unpublished data). To our knowledge, there is currently no clinically applicable way to confirm the presence of gadolinium in a pathologic specimen. This fixation of the contrast agent within reactive cells in the brain parenchyma, as visualized with histochemistry, reduces the hazards of relying on the flux of a contrast agent back and forth across an incompetent blood brain barrier. It may also demonstrate areas where tumor has caused more subtle disruptions in the blood brain barrier (1).
With a half-life of 24–30 hours for ferumoxtran-10, the question whether this agent could also cause enhancement of surgically traumatized tissues is valid, if surgery occurs between 24–48 hours after administration of the ferumoxtran-10. There is no evidence that enough ferumoxtran-10 accumulates within the interstitial spaces of the brain during the time span of surgery to alter significantly the appearance of MR imaging in surgically traumatized brain (1, 8, 30–32). One animal study demonstrated no increased postoperative signal intensity from USPIO particles given 16 hours preoperatively but did show intense gadolinium enhancement in the surgically disturbed brain (29).
The ability to perform more accurate resection of a brain tumor is the driving force behind IMRI. In early testing, ferumoxtran-10 may provide a way to avoid problems with postsurgical, nontumoral enhancement in the intraoperative setting. This timing allows the evaluation of the tumor resection before hemostatic agents have been placed that may confound attempts to interpret postoperative MR imaging (25). More experience is needed with the use of ferumoxtran-10 and IMRI, but this agent may provide a means to better delineate brain tumors in the intraoperative settings. Even in limited-field-strength magnets used in many IMRI systems, ferumoxtran-10 provides a stable imaging marker that can be confirmed pathologically.
Conclusion
Ferumoxtran-10 provides a solution to the problems related to gadolinium administration during IMRI. Our preliminary data suggest that ferumoxtran-10 provides a stable imaging marker that can be given once preoperatively and provide adequate images for IMRI-guided surgery.
Acknowledgments
Financial support for this work was provided by a Veteran’s Administration merit review grant and by grants NS44687, NS33618 and NS34608 from the National Institute of Neurologic Disorders and Stroke (to E.A.N.). Combidex (IRB #1127) was kindly provided by Advanced Magnetics, Cambridge, MA.
References
- 1.Varallyay P, Nesbit G, Muldoon LL, et al. Comparison of two superparamagnetic viral-sized iron oxide particles ferumoxides and ferumoxtran-10 with a gadolinium chelate in imaging intracranial tumors. AJNR Am J Neuroradiol 2002;23:510–519 [PMC free article] [PubMed] [Google Scholar]
- 2.Enochs WS, Harsh G, Hochberg F, Weissleder R. Improved delineation of human brain tumors on MR images using a long-circulating, superparamagnetic iron oxide agent. J Magn Reson Imaging 1999;9:228–232 [DOI] [PubMed] [Google Scholar]
- 3.Knauth M, Aras N, Wirtz CR, et al. Surgically induced intracranial contrast enhancement: potential source of diagnostic error in intraoperative MR imaging. AJNR Am J Neuroradiol 1999;20:1547–1553 [PMC free article] [PubMed] [Google Scholar]
- 4.Spetzger U, Thron A, Gilsbach JM. Immediate postoperative CT contrast enhancement following surgery of cerebral tumoral lesions. J Comput Assist Tomogr 1998;22:120–125 [DOI] [PubMed] [Google Scholar]
- 5.Cairncross JG, Pexman JH, Rathbone MP, DelMaestro RF. Postoperative contrast enhancement in patients with brain tumor. Ann Neurol 1985;17:570–572 [DOI] [PubMed] [Google Scholar]
- 6.Dina TS, Feaster SH, Laws ER Jr, Davis DO. MR of the pituitary gland postsurgery: serial MR studies following transsphenoidal resection. AJNR Am J Neuroradiol 1993;14:763–769 [PMC free article] [PubMed] [Google Scholar]
- 7.Elster AD, DiPersio DA. Cranial postoperative site: assessment with contrast-enhanced MR imaging. Radiology 1990;174:93–98 [DOI] [PubMed] [Google Scholar]
- 8.Neuwelt EA, Varallyay P, Bago A, et al. Imaging of iron oxide nanoparticles by MR and light microscopy in patients with malignant brain tumors. Neuropathol Appl Neurobiol 2004;30:456–471 [DOI] [PubMed] [Google Scholar]
- 9.Wirtz CR, Knauth M, Staubert A, et al. Clinical evaluation and follow-up results for intraoperative magnetic resonance imaging in neurosurgery. Neurosurgery 2000;46:1112–1120; discussion 1120–1112 [DOI] [PubMed] [Google Scholar]
- 10.Knauth M, Wirtz CR, Tronnier VM, et al. Intraoperative MR imaging increases the extent of tumor resection in patients with high-grade gliomas. AJNR Am J Neuroradiol 1999;20:1642–1646 [PMC free article] [PubMed] [Google Scholar]
- 11.Hall WA, Liu H, Martin AJ, Truwit CL. Intraoperative magnetic resonance imaging. Top Magn Reson Imaging 2000;11:203–212 [DOI] [PubMed] [Google Scholar]
- 12.Jolesz FA, Nabavi A, Kikinis R. Integration of interventional MRI with computer-assisted surgery. J Magn Reson Imaging 2001;13:69–77 [DOI] [PubMed] [Google Scholar]
- 13.Lewin JS, Metzger A, Selman WR. Intraoperative magnetic resonance image guidance in neurosurgery. J Magn Reson Imaging 2000;12:512–524 [DOI] [PubMed] [Google Scholar]
- 14.Alexander E 3rd, Moriarty TM, Kikinis R, et al. The present and future role of intraoperative MRI in neurosurgical procedures. Stereotact Funct Neurosurg 1997;68:10–17 [DOI] [PubMed] [Google Scholar]
- 15.Bohinski RJ, Kokkino AK, Warnick RE, et al. Glioma resection in a shared-resource magnetic resonance operating room after optimal image-guided frameless stereotactic resection. Neurosurgery 2001;48:731–742; discussion 742–734 [DOI] [PubMed] [Google Scholar]
- 16.Hadani M, Spiegelman R, Feldman Z, et al. Novel, compact, intraoperative magnetic resonance imaging-guided system for conventional neurosurgical operating rooms. Neurosurgery 2001;48:799–807; discussion 807–809 [DOI] [PubMed] [Google Scholar]
- 17.Nimsky C, Ganslandt O, Kober H, et al. Intraoperative magnetic resonance imaging combined with neuronavigation: a new concept. Neurosurgery 2001;48:1082–1089; discussion 1089–1091 [DOI] [PubMed] [Google Scholar]
- 18.Rubino GJ, Farahani K, McGill D, et al. Magnetic resonance imaging-guided neurosurgery in the magnetic fringe fields: the next step in neuronavigation. Neurosurgery 2000;46:643–653; discussion 653–644 [DOI] [PubMed] [Google Scholar]
- 19.Forsting M, Albert FK, Kunze S, et al. Extirpation of glioblastomas: MR and CT follow-up of residual tumor and regrowth patterns. AJNR Am J Neuroradiol 1993;14:77–87 [PMC free article] [PubMed] [Google Scholar]
- 20.Henegar MM, Moran CJ, Silbergeld DL. Early postoperative magnetic resonance imaging following nonneoplastic cortical resection. J Neurosurg 1996;84:174–179 [DOI] [PubMed] [Google Scholar]
- 21.Jeffries BF, Kishore PR, Singh KS, et al. Contrast enhancement in the postoperative brain. Radiology 1981;139:409–413 [DOI] [PubMed] [Google Scholar]
- 22.Anzai Y, Lufkin R, DeSalles A, et al. Preliminary experience with MR-guided thermal ablation of brain tumors. AJNR Am J Neuroradiol 1995;16:39–48; discussion 49–52 [PMC free article] [PubMed] [Google Scholar]
- 23.Penn RD, Walser R, Kurtz D, Ackerman L. Tumor volume, luxury perfusion, and regional blood volume changes in man visualized by subtraction computerized tomography. J Neurosurg 1976;44:449–457 [DOI] [PubMed] [Google Scholar]
- 24.Knauth M, Wirtz CR, Aras N, Sartor K. Low-field interventional MRI in neurosurgery: finding the right dose of contrast medium. Neuroradiology 2001;43:254–258 [DOI] [PubMed] [Google Scholar]
- 25.Spiller M, Tenner MS, Couldwell WT. Effect of absorbable topical hemostatic agents on the relaxation time of blood: an in vitro study with implications for postoperative magnetic resonance imaging. J Neurosurg 2001;95:687–693 [DOI] [PubMed] [Google Scholar]
- 26.Gomori JM, Grossman RI, Goldberg HI, et al. Intracranial hematomas: imaging by high-field MR. Radiology 1985;157:87–93 [DOI] [PubMed] [Google Scholar]
- 27.Gomori JM, Grossman RI, Hackney DB, et al. Variable appearances of subacute intracranial hematomas on high-field spin-echo MR. AJR Am J Roentgenol 1988;150:171–178 [DOI] [PubMed] [Google Scholar]
- 28.Sato N, Bronen RA, Sze G, et al. Postoperative changes in the brain: MR imaging findings in patients without neoplasms. Radiology 1997;204:839–846 [DOI] [PubMed] [Google Scholar]
- 29.Knauth M, Egelhof T, Roth SU, et al. Monocrystalline iron oxide nanoparticles: possible solution to the problem of surgically induced intracranial contrast enhancement in intraoperative MR imaging. AJNR Am J Neuroradiol 2001;22:99–102 [PMC free article] [PubMed] [Google Scholar]
- 30.Zimmer C, Wright SC Jr, Engelhardt RT, et al. Tumor cell endocytosis imaging facilitates delineation of the glioma-brain interface. Exp Neurol 1997;143:61–69 [DOI] [PubMed] [Google Scholar]
- 31.Muldoon LL, Pagel MA, Kroll RA, et al. A physiological barrier distal to the anatomic blood-brain barrier in a model of transvascular delivery. AJNR Am J Neuroradiol 1999;20:217–222 [PMC free article] [PubMed] [Google Scholar]
- 32.Neuwelt EA, Weissleder R, Nilaver G, et al. Delivery of virus-sized iron oxide particles to rodent CNS neurons. Neurosurgery 1994;34:777–784 [DOI] [PubMed] [Google Scholar]