Abstract
Background and Aims
A 40% risk of disease recurrence post-liver transplantation (LT) for autoimmune hepatitis (AIH) has been previously reported. Risk factors for recurrence and its impact on long-term patient outcome are poorly defined. We aimed to assess prevalence, time to disease recurrence, as well as patient and graft survival in patients with recurrent AIH (rAIH) versus those without recurrence.
Methods
Single-center retrospective study of adult recipients who underwent LT for AIH between January 2007 and December 2017. Patients with AIH overlap syndromes were excluded.
Results
A total of 1436 LTs were performed during the study period, of whom 46 (3%) for AIH. Eight patients had AIH overlap syndromes and were excluded. Patients were followed up for 4.4 ± 3.4 years and mean age at LT was 46.8 years. Average transplant MELD (Model for End-Stage Liver Disease) score was 24.9. About 21% of patients (8 of 38) were transplanted for acute onset of AIH; 66% of patients (n = 25) received a deceased donor liver graft, and 34% a living donor organ. rAIH occurred in 7.8% (n = 3/38) of recipients. Time to recurrence was 1.6, 12.2 and 60.7 months. Patient and graft survival in patients without recurrence was 88.6% and 82.8% in 5 years, whereas in those with rAIH, it was 66.7%, respectively.
Conclusion
Although AIH recurs post-LT, our data indicate a lower recurrence rate when compared to the literature and excellent patient and graft survival.
Keywords: Autoimmune hepatitis, Liver transplantation, Recurrent autoimmune hepatitis
Introduction
Autoimmune hepatitis (AIH) accounts for approximately 4% to 6% of liver transplants in North America and 3% in Europe (1, 2). Clinical indications for liver transplantation (LT) in patients with AIH are similar to patients with other chronic liver diseases that end in acute or semi-acute liver failure, decompensated cirrhosis or hepatocellular carcinoma (3, 4). LT for AIH is associated with good outcomes, reaching survival rates at 1 and 5 years of approximately 90% and 80%, respectively (5, 6). However, AIH is reported to recur in 17% to 42% of transplanted recipients, and its prevalence increases with time following LT (7, 8). Recurrence can be indolent and detected only by surveillance laboratory testing and liver biopsy assessments (7). Diagnosis of recurrent AIH (rAIH) is challenging, as there are no validated histological criteria. It seems reasonable to suspect rAIH in patients with increased serum transaminases, elevated IgG, (re) appearance of typical autoantibodies and characteristic histological findings such as lymphoplasmacytic infiltrate and interface hepatitis, if these occur in the absence of rejection or other transplant complications (6). rAIH can cause graft dysfunction and reduced graft and patient survival, and result in the need for re-transplantation. Risk factors for disease recurrence and its impact on long-term patient outcome are poorly defined. It appears to correlate with severity and level of immune control of the disease (6). The ultimate goal of management is to maximize graft survival by tailoring immunosuppression to prevent graft dysfunction and recurrence of the original disease (5). In this study, we reviewed our experience using LT for the treatment of AIH. We assessed prevalence and time to recurrence of AIH, in addition to patient and graft survival in patients with or without rAIH.
Methods
Study Design
This was a retrospective single-center study that analyzed patients transplanted for AIH and their outcomes, mainly recurrence of AIH and graft and patient survival. Data included baseline demographics, and pre- and post-LT variables such as donor type, explant results, time to AIH recurrence and patient and graft survival. The study was approved by the Research and Ethics Board of University Health Network, University of Toronto.
Study Population
Data were collected retrospectively between January 2007 and December 2017 on a total of 1436 patients who underwent LT at our center.
All adult recipients who underwent LT for AIH were included. Exclusion criteria included age <18 years, LT for other causes than AIH, AIH overlap syndromes and transplantation at another center. Patients that received their first transplant as paediatric patients, then had transitioned to the adult’s clinic and were re-transplanted during the study period, were also included. Re-transplants at our center and multiorgan were included. The minimum follow-up period was defined as 12 months post-transplantation.
Endpoints
The primary endpoints were prevalence and time to recurrence of AIH. The secondary endpoints were graft and patient survival in patients with rAIH versus those without recurrence.
Time to recurrence of AIH was defined as time from LT to rAIH. Patients were censored if no rAIH at last follow-up.
Patient survival was defined as time from LT to the date of death from any cause. Patients were censored if alive in December 2017; and patients who were lost to follow-up, or moved to another transplant center for ongoing care, were censored at the time of their last clinic visit.
Graft survival was defined as time from LT to death from any cause or re-transplantation; when graft failure was caused by recurrence of primary disease it was defined as recurrence-related graft failure.
Immunosuppressive Regimen
All patients received intravenous methylprednisolone followed by an oral course of prednisone and tacrolimus/cyclosporine immediately following LT. The oral prednisone dose followed a standard tapering regimen and terminated at 3 months after LT. Tacrolimus was considered the first-line calcineurin inhibitor (CNI) and was substituted with cyclosporine in case of intolerance or neurotoxicity.
Mycophenolate mofetil was added if CNI dose reduction was required. Sirolimus was considered in those who were intolerant to CNIs or had evidence of impaired renal function or who underwent transplantation for hepatocellular carcinoma. Sirolimus was not started in the first 6 weeks after LT. The immunosuppression protocol was the same for patients transplanted for decompensated AIH cirrhosis and fulminant AIH.
Definition of Autoimmune Hepatitis Recurrence
Diagnosis of recurrence of AIH was made according to the criteria summarized by Faisal et al. (9) and Duclos-Vallee et al. (10), which require: (i) confirmed diagnosis of AIH before LT; (ii) elevated transaminases, hypergammaglobulinemia (elevated IgG) and presence of autoantibodies (antinuclear antibody, anti-smooth muscle antibody and/or anti-liver kidney microsomal antibody); (iii) histological findings of interface hepatitis with portal inflammation and/or lymphoplasmacytic inflammatory infiltrates (Figure 1); (iv) response to corticosteroid and (v) exclusion of differential diagnostic considerations such as acute or late/atypical rejection.
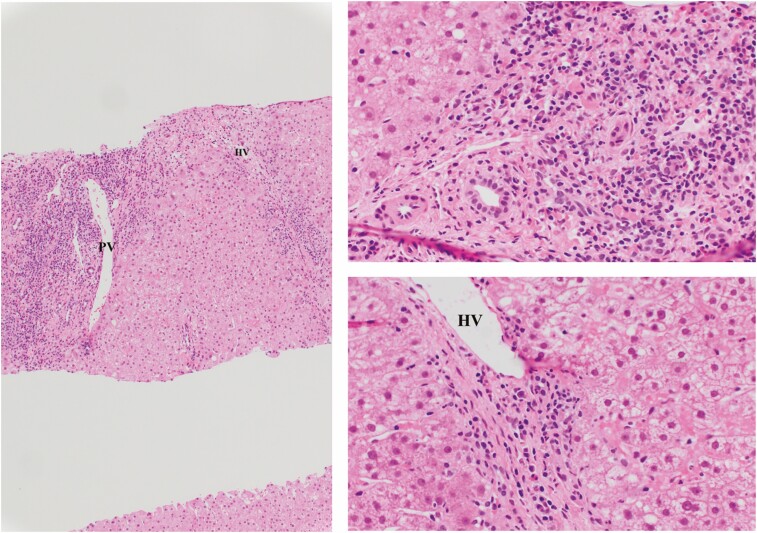
Figure 1.
Hematoxylin and eosin stain showing plasma cell-rich rejection. There is the portal vein (PV) marking inflamed portal tract and with inflammation involving the interface (arrow) as well as perivenular accentuation of inflammation around hepatic vein (HV). Inflammation is rich in plasma cells admixed with other cells (magnification: upper panel ×10; lower panels ×20).
All biopsies performed post-liver transplant were event-driven. Routine screening for disease recurrence with protocol liver biopsy is not undertaken in the Toronto Liver Transplant Program. In our Transplant Centre, all patients are followed for life (>15 to 20 years).
Definition of Acute Cellular Rejection and Antibody-Mediated Rejection
The differential diagnosis with acute cellular rejection (ACR) now referred as T-cell-mediated rejection (11) was made when portal-based mixed lymphocytic and eosinophilic inflammation, the former including different transformed intermediates (e.g., immunoblasts, centroblasts, etc.), and other cell types were identified. Typically, the rejection nature of these infiltrates is highlighted by its target of bile duct epithelium and portal vein endothelium, causing phlebitis (Figure 2) (12). Ductopenic rejection is the commonest outcome of unrecognized, persistent and/or inadequately treated ACR/T-cell-mediated rejection. In such cases, cholestasis predominate due to loss of more than 50% of small/terminal bile ducts and little inflammation (Figure 3).
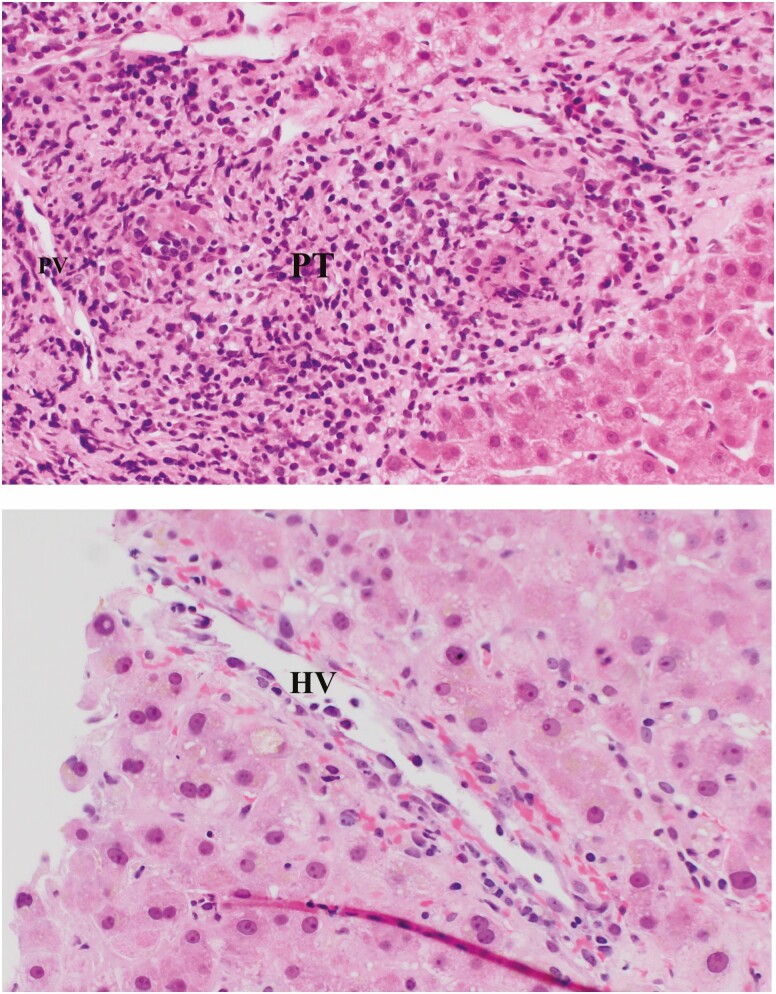
Figure 2.
Hematoxylin and eosin stain showing moderate-to-severe acute cellular rejection/T-cell-mediated rejection. There is dense portal tract (PT) inflammation comprising of mixed cell types, associated with bile duct necrosis (arrow) and endothelial cell injury causing phlebitis of portal vein (PV) and hepatic vein (HV) (magnification: upper panel ×10; lower panels ×20).
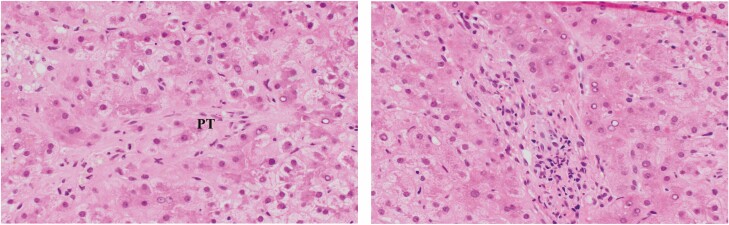
Figure 3.
Hematoxylin and eosin stain showing ductopenic rejection in a patient with poor compliance. There is little inflammation in portal tracts (PT) but the bile duct is missing with an unaccompanied small artery (black arrow) marking its expected location. Earlier in ductopenic rejection senescent bile ducts (blue arrow) could be seen prior to lost of the bile duct (magnification: ×20).
Antibody-mediated rejection was defined as portal inflammation predominantly formed by neutrophils, as well as prominence/distention of usually indistinct portal capillaries. The endothelial cells lining these capillaries usually appear plump and the luminal capillaries contain increased mononuclear cells (‘capillaritis’). Demonstration by immunohistochemistry of C4d deposition in the wall of these capillaries and/or sinusoidal endothelial lining completes the histopathological picture of antibody-mediated rejection (Figure 4).
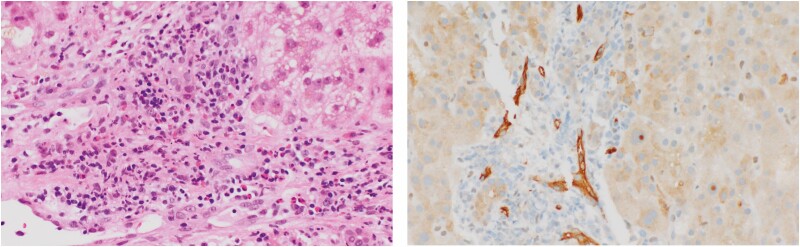
Figure 4.
Hematoxylin and eosin stain (left panel) showing mild (in this case but not always, eosinophil-prominent) inflammation within portal tract (PT), as well as dilated portal capillaries with plump endothelial cells, filled with mononuclear cells (black arrow). Evidence of complement fixation is seen with positive C4d immunostaining of capillary walls (blue arrow) (magnification: ×20).
Statistical Analysis
Continuous data are reported as mean or median values. Categorical data are reported as proportions/percentages. Kaplan–Meier analysis (log-rank test) was undertaken to evaluate patient and graft survival between patients with rAIH versus those without disease recurrence.
Results
Of the total 1436 liver transplants performed during the study period, 46 (3%) of transplants were performed for AIH; of these, 8 patients had AIH overlap syndromes and were excluded.
Patient Demographics
Of 38 AIH transplanted patients, 71% were female, with a mean age at transplant of 46.8 years. The average transplant MELD (Model for End-Stage Liver Disease) score was 24.9. Median follow-up period was 45.4 months (interquartile range 0.1 to 130.7 months). About 64% of patients received a deceased donor liver graft, and 36% a living donor organ. The proportion of patients on steroids alone, steroids in combination with azathioprine and triple therapy (steroid, azathioprine, mycophenolate) was similar between patients with and without recurrence disease (Table 1).
Table 1.
Pre-liver transplantation features in patients with and without AIH recurrence (n = 38)
| Clinical features | AIH recurrence, n = 3 | AIH no recurrence, n = 35 | Total, n = 38 |
|---|---|---|---|
| Age at diagnosis, median (min–max) | 30 (13–50) | 37 (6–71) | 33.5 (6–71) |
| Age at transplant, mean | 42.3 | 47.1 | 46.8 |
| Female sex, n (%) | 2 (83%) | 25 (71%) | 27 (71%) |
| Caucasian: non-Caucasian ratio | 3:0 | 28:7 | 31:7 |
| Concomitant autoimmune conditionsa | 2 (83%) | 7 (21%) | 9 (24%) |
| Associated IBD | 1 (33%) | 3 (9%) | 4 (10%) |
| Listing MELD, mean | 15.3 | 24.2 | 19.7 |
| Transplant MELD, mean | 16 | 25.7 | 24.9 |
| Interval from diagnosis to transplant (years), mean (SD) | 11.3 (±3.9) | 9.7 (±10.4) | 9.8 (±10.1) |
| Time on waiting list (days), mean (SD) | 126 (±35.5) | 137.2 (±263) | 136.3 (±253) |
AIH, Autoimmune hepatitis; IBD, Inflammatory Bowel Disease; MELD, Model for End-Stage Liver Disease score.
aType 1 diabetes, celiac disease and rheumatoid arthritis.
Recurrence of Autoimmune Hepatitis
Disease recurrence occurred in 3 of 38 patients (7.8%) of AIH recipients and time to recurrence was 1.6, 12.2 and 60.7 months, respectively. There was no specific cut-off of liver tests for performing a liver biopsy; however, all patients who had liver biopsy had alanine aminotransferase >150, except one who had alanine aminotransferase 113 and jaundice.
The main pre- and post-LT clinical features of the patients who developed rAIH (n = 3) are described on Table 2. Given the low incidence of rAIH, the statistical power was too low to assess the association of potential risk factors for the development of rAIH.
Table 2.
Post-liver transplantation features of the three patients who developed recurrent AIH
| Clinical features | AIH recurrence, n = 3 |
|---|---|
| Living donor: deceased donor ratio | 3:0 |
| Bile duct anastomosis (%) | |
| Roux-en-Y | 3 (100%) |
| Duct-to-duct | 0 (0%) |
| CMV status | |
| D+/R− | 1 |
| D+/R+ | 1 |
| D−/R+ | 1 |
| D−/R− | 0 |
| Immunosuppression (n) | |
| Tacrolimus | 3 |
| Cyclosporine | 0 |
| Sirolimus | 0 |
| MMF | 3 |
| Azathioprine | 0 |
| Acute cellular rejection, n (%) | 2 (83%) |
| Sepsis, n (%) | 3 (100%) |
AIH, Autoimmune hepatitis; CMV, cytomegalovirus; D, donor; MMF, mycophenolate mofetil; R, recipient.
Pre-transplant Features of the Recurrent Autoimmune Hepatitis Patients
All patients with recurrent disease were Caucasian and had type 1 AIH. Two out of three were female. Two patients had concomitant autoimmune diseases (one of them had scleroderma and lupus, the other had hypothyroidism and ulcerative colitis). Antinuclear antibody (ANA) was positive in two patients and smooth-muscle antibody (SMA) in all patients. All patients received prednisone and azathioprine pre-transplant for their AIH treatment. Liver transplant indications included decompensated liver disease and incomplete response to treatment leading to liver failure and hepatocarcinoma. The decompensated patient had bleeding esophageal varices, ascites and hepatic encephalopathy pre-transplant. The patient with incomplete response to treatment had a history of non-compliance to therapy.
Post-transplant Features of the Recurrent Autoimmune Hepatitis Patients
All patients with rAIH received a living donor organ and all had Roux-en-Y anastomosis. MELD at transplant ranged from 15 to 18. All explants showed cirrhosis (fibrosis grade 4) and no or mild necroinflammatory activity. The explant of the patient transplanted due to AIH and hepatocellular carcinoma showed also cholangiocarcinoma, but no features of primary sclerosing cholangitis overlap. Magnetic resonance imaging was performed for that patient and showed no features of primary sclerosing cholangitis either.
The immunological panel post-transplant was similar to pre-transplant (all three patients remained with SMA positive, and two with ANA positive). Serum IgG was normal in two patients post-transplant and slightly elevated (17.6) in the other. All rAIH patients received the same immunosuppression post-transplant: tacrolimus, mycophenolate and prednisone.
The three rAIH patients had episodes of sepsis post-transplant (liver abscess; urinary tract infection and cholangitis; abdominal abscess, respectively).
ACR was diagnosed in two of three patients with rAIH, one of the patients 12.1 months before the rAIH diagnosis, the other patient, however, had two episodes of ACR after the diagnosis of rAIH.
One of the rAIH patients lost his graft 3.5 months after the diagnosis of recurrence due to liver failure secondary to sepsis and died.
The treatment of rAIH patients was as follows: increase of the prednisone dose to 20 mg in two patients, one of those also had azathioprine dose increased and tacrolimus dose adjusted, the other patient had mycophenolate dose increased in addition to prednisone increase and tacrolimus adjusted; the third and last patient was put on prednisone 50 mg, had the mycophenolate dose decreased, and adjusted the tacrolimus dose.
Graft and Patient Survival
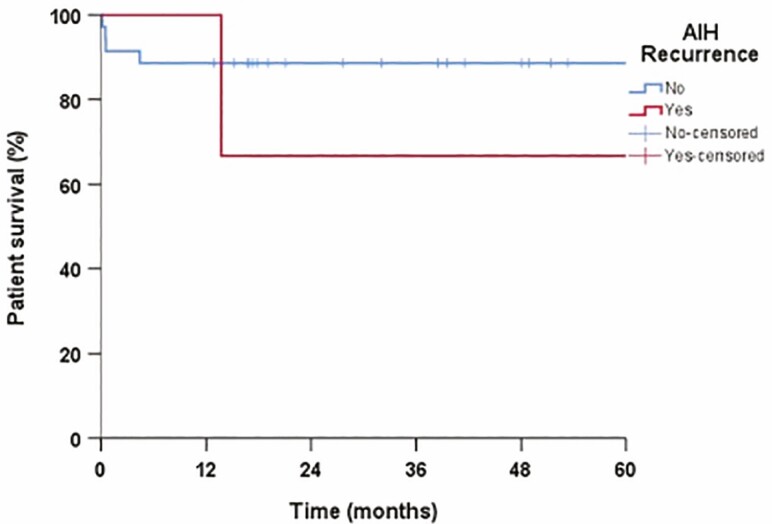
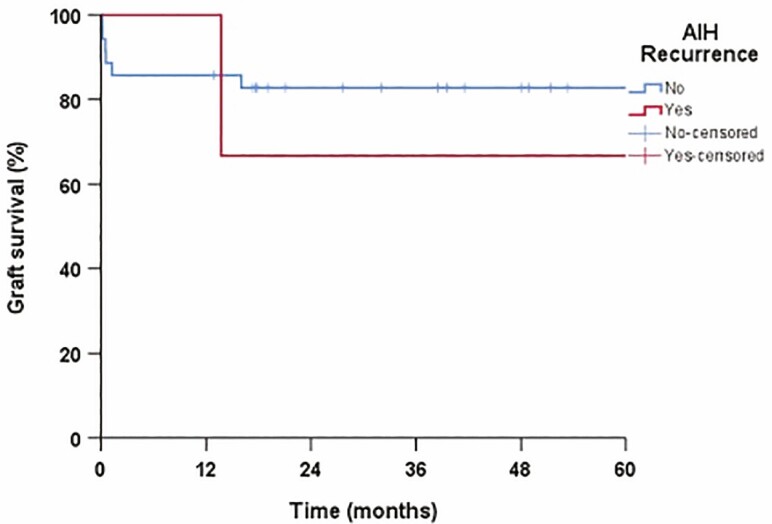
Patient survival was 88.6% in 1 and 5 years in patients without recurrent disease and 66.7% in 1 and 5 years in those with rAIH (P = 0.35) (Figure 5). Graft survival in patients without and with rAIH was 85.7% versus 100% in 1 year, and 82.8% versus 66.7% in 5 years, respectively (Figure 6). Overall patient mortality was 15.7% (6 of 38 patients) and graft loss 21% (8 of 38 patients) at the end of study period. The mortality and graft loss among the three patients with rAIH was 33% (one of three patients) at the end of the follow-up period. The cause of graft loss and death in the rAIH patient was liver failure. There was no re-transplantation in patients with rAIH.
Figure 5.
Patient survival in patients with and without recurrent autoimmune hepatitis (rAIH), no, grey line; yes, black line.
Figure 6.
Graft survival in patients with and without recurrent autoimmune hepatitis (rAIH).
Non-recurrent Autoimmune Hepatitis Patients
The main features of the non-rAIH patients are described in Table 3.
Table 3.
Post liver-transplantation features in patients without AIH recurrence
| Clinical features | AIH no recurrence, n = 35 |
|---|---|
| Living donor: deceased donor ratio | 10:25 |
| Bile duct anastomosis (%) | |
| Roux-en-Y | 4 (12%) |
| Duct-to-duct | 31 (88%) |
| CMV status, n (%) | |
| D+/R− | 4 |
| D+/R+ | 7 |
| D-/R+ | 9 |
| D−/R− | 14 |
| Unavailable | 1 |
| Immunosuppression (n) | |
| Tacrolimus | 35 |
| Cyclosporine | 1a |
| Sirolimus | 1b |
| MMF | 28 |
| Azathioprine | 5 |
| Acute cellular rejection, n (%) | 6 (17%) |
| Sepsis, n (%) | 6 (17%) |
AIH, Autoimmune hepatitis; CMV, cytomegalovirus; D, donor; MMF, mycophenolate mofetil; R, recipient.
aOne patient switched from tacrolimus to cyclosporine.
bOne patient switched from tacrolimus to sirolimus.
Discussion
In this single-centre study, we found that over the 10-year study period, only 3% of all the liver transplants was for AIH, a rate that is similar to other cohorts in Europe and the United States (3% to 6%) (1, 2, 13). However, in contrast to other reports, we only had a 7.8% rate of recurrence of AIH on the graft post-LT which is 3-fold lower than the 22% rate of recurrence reported in the literature. Finally, long-term graft and patient survival of AIH recipients was overall excellent with a graft and patient survival rate at the end of the follow-up period of 79% and 84%, respectively.
LT is the treatment of choice in patients with AIH and acute liver failure with poor response to treatment, decompensated liver disease at time of diagnosis or following prolonged immunosuppression therapy (14). In our study, most of our AIH transplanted patients had AIH cirrhosis, only 16% (6 of 38) had acute AIH leading to LT. The diagnosis of rAIH is complex and establishing an accurate frequency of recurrence has been challenging. Autoantibodies, such as ANA, SMA and liver-kidney microsomal antibody can be present in up to 64% of patients transplanted for non-autoimmune liver diseases and are therefore not specific to rAIH (6). In addition, the diagnostic International Auto-immune Hepatitis Group (IAHG) scoring systems for AIH have not been tested nor validated in the post-transplant setting. Thus, different groups have employed variable diagnostic criteria and histological analyses with protocol versus clinically indicated liver biopsies (8, 15, 16). Of note, our patients underwent liver biopsies only when clinically indicated. All these factors together with variable immunosuppression regimens could explain the variability in the recurrence frequency. Hence, is not surprising that a recent systematic review reported a frequency of rAIH ranging from 10% to 68% (15).
We found an incidence of rAIH of 7.8% among patients transplanted due to AIH. This rate is lower to the rate reported by a previous meta-analysis (22%), which pooled all available studies up to 2006 with a total sample size of 414 patients, after median of 43.8 months post-transplantation (17). This higher incidence found on this meta-analysis might be related to the fact that most of the included studies did routine protocol biopsies. Also their sample had a higher percentage of women (84%) at a younger age at transplant (35.8 years) when compared to our cohort. Their histological criteria for recurrence included piece-meal necrosis and bridging necrosis which ours did not include.
Timing of recurrence is also variable. The time to recurrence in our study was 1.6, 12.2 and 60.7 months. Two out of three rAIH patients had a shorter time to recurrence when compared to the time reported in the literature which is 4.6 years (4–6).
Various factors have been reported to be of increased frequency in patients with rAIH compared to those without rAIH and hence are thought to confer predisposition for recurrence (6).
In our study, we could not estimate the pre-transplant parameters predictive of disease recurrence due to the reduced number of rAIH patients.
A previous retrospective study of our group (18) with 263 patients transplanted from 2000 to 2015 for autoimmune diseases found no difference in recurrence rate of AIH according to type of graft (living-donor or deceased-donor LT).
Another retrospective study was published by Montano-Loza et al. (8), which described 46 patients transplanted for AIH with a focus on determining the risk factors for recurrence of AIH. They concluded that patients with concomitant autoimmune disease, high aspartate aminotransferase, alanine aminotransferase and IgG before the transplant, or moderate-to-severe inflammatory activity or plasma cell infiltration in the liver explant have a higher risk of recurrent disease. Additionally, they found that there was no difference in the risk of recurrence of AIH in patients who had and were treated for episodes of acute rejection. This association of occurrence of acute rejection and rAIH is very controversial with some studies demonstrating a higher incidence of rAIH in patients with acute rejection (19), whereas others (like our study) demonstrate a similar incidence and severity of episodes of acute rejection in patients with and without rAIH (20–22).
Furthermore, our three rAIH patients had no major differences in the frequency of pre-transplant administration of prednisone either alone or in combination with azathioprine, and their immunosuppressive regimens were similar. Many previous studies have shown that steroids’ withdrawal plays an important role in recurrence of AIH (23, 24) but direct evidence for this assumption is lacking (6). Krishnamoorthy et al. (25) reported 73 patients transplanted for AIH who were kept on long-term, low-dose corticosteroid therapy and only 5 (7%) of these patients developed recurrence of AIH. The 1-, 5-, and 10-year patient survivals were 92%, 86% and 73%. However, there is a compelling body of evidence that the patients who are withdrawn from corticosteroids benefit from a reduction in serum cholesterol levels, decreased use of antihypertensive medications, reduced use of medications for glucose control and reduced rate of infections (26). Some authors advocate that the use of long-term low-dose steroids does not seem to increase these risks, and it even may reduce the risk of disease recurrence and rejection although the evidence to support this approach is still not robust (27). Others affirm that continuation of steroids after LT does not eliminate rAIH, with the literature supporting steroid withdrawal in LT recipients with underlying AIH (28).
In our center, 50% of patients were on triple immunosuppression therapy at 1 year post-transplant but at 5 years majority were only on dual agents without prednisone.
The presence of lymphocytic or lymphoplasmacytic infiltration with moderate-to-severe inflammatory activity in the explant has also been linked with rAIH (Grade B, level 2b) (3, 8, 19). Most of our patients transplanted with AIH had no or mild inflammatory activity in the explant and there was no difference between the group with rAIH and no rAIH. Other previous studies (29, 30) also reported no difference in graft survival between rAIH and non-rAIH.
In adults, graft failure has been reported in 13% to 50% with rAIH (20) and rAIH has been associated with a higher risk of graft loss and an increased risk of death from liver failure compared to other liver diseases 1 year post-LT (31).
Our study has important limitations. Although it is a relatively large cohort of patients transplanted for AIH, it remains somewhat underpowered, due to its size and short follow-up period, to evaluate predictors of disease recurrence and long-term outcomes post-LT. Additionally, routine protocol liver biopsies were not performed, potentially limiting the early diagnosis of disease recurrence in asymptomatic patients with normal or near normal liver enzymes. Perhaps these limitations could explain the low recurrence rate in our cohort. Another limitation is that we do not routinely test HLA DR3.
Currently, there is no standard approach to reduce the risk of rAIH. Future studies should focus on identification of high-risk recipients using genetic and translational studies. Research into understanding the factors driving late allograft dysfunction could potentially open up new therapeutic options, leading to continued improvement in long-term patient and graft survival and quality of life (7).
Acknowledgments
The authors thank Fiorella Murillo for her statistical support.
Author contributions: F.Q.O.: Study concept and design, acquisition of data, data analysis and interpretation, drafting of the manuscript, and critical revision of the manuscript; E.N., D.A., D.K., O.A.A. and S.F.: study concept and design, acquisition of data, and data analysis and interpretation; B.E.H.: study concept and design, data analysis and interpretation, and critical revision of the manuscript; G.M.H.: study concept and design, data analysis and interpretation, drafting of the manuscript, and critical revision of the manuscript; B.E.H., M.B., Z.G. and L.B.L.: study concept and design, data analysis and interpretation, and critical revision of the manuscript; N.Z.: study concept and design, data analysis and interpretation, drafting of the manuscript, and critical revision of the manuscript.
Conflict of interest: The authors have no conflict of interest to disclose related to this manuscript.
References
- 1. Manns MP, Czaja AJ, Gorham JD, et al. ; American Association for the Study of Liver Diseases . Diagnosis and management of autoimmune hepatitis. Hepatology 2010;51(6):2193–213. [DOI] [PubMed] [Google Scholar]
- 2. Adam R, McMaster P, O’Grady JG, et al. ; European Liver Transplant Association . Evolution of liver transplantation in Europe: Report of the European Liver Transplant Registry. Liver Transpl 2003;9(12):1231–43. [DOI] [PubMed] [Google Scholar]
- 3. Stirnimann G, Ebadi M, Czaja AJ, et al. Recurrent and de novo autoimmune hepatitis. Liver Transpl 2019;25(1):152–66. [DOI] [PubMed] [Google Scholar]
- 4. Carbone M, Neuberger JM. Autoimmune liver disease, autoimmunity and liver transplantation. J Hepatol 2014;60(1):210–23. [DOI] [PubMed] [Google Scholar]
- 5. Kerkar N, Yanni G. ‘De novo’ and ‘recurrent’ autoimmune hepatitis after liver transplantation: A comprehensive review. J Autoimmun 2016;66:17–24. [DOI] [PubMed] [Google Scholar]
- 6. Visseren T, Darwish Murad S. Recurrence of primary sclerosing cholangitis, primary biliary cholangitis and auto-immune hepatitis after liver transplantation. Best Pract Res Clin Gastroenterol 2017;31(2):187–98. [DOI] [PubMed] [Google Scholar]
- 7. Edmunds C, Ekong UD. Autoimmune liver disease post-liver transplantation: A summary and proposed areas for future research. Transplantation 2016;100(3):515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Montano-Loza AJ, Mason AL, Ma M, et al. Risk factors for recurrence of autoimmune hepatitis after liver transplantation. Liver Transpl 2009;15(10):1254–61. [DOI] [PubMed] [Google Scholar]
- 9. Faisal N, Renner EL. Recurrence of autoimmune liver diseases after liver transplantation. World J Hepatol 2015;7(29):2896–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Duclos-Vallee JC, Sebagh M. Recurrence of autoimmune disease, primary sclerosing cholangitis, primary biliary cirrhosis, and autoimmune hepatitis after liver transplantation. Liver Transpl 2009;15(Suppl. 2):S25–34. [DOI] [PubMed] [Google Scholar]
- 11. Demetris AJ, Bellamy C, Hübscher SG, et al. 2016 Comprehensive update of the Banff Working Group on Liver Allograft Pathology: Introduction of antibody-mediated rejection. Am J Transplant 2016;16(10):2816–35. [DOI] [PubMed] [Google Scholar]
- 12. Adeyi O, Fischer SE, Guindi M. Liver allograft pathology: Approach to interpretation of needle biopsies with clinicopathological correlation. J Clin Pathol 2010;63(1):47–74. [DOI] [PubMed] [Google Scholar]
- 13. Manns MP, Czaja AJ, Gorham JD, et al. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51(6):1–31. [DOI] [PubMed] [Google Scholar]
- 14. Montano-Loza AJ, Carpenter HA, Czaja AJ. Features associated with treatment failure in type 1 autoimmune hepatitis and predictive value of the model of end-stage liver disease. Hepatology 2007;46(4):1138–45. [DOI] [PubMed] [Google Scholar]
- 15. Montano-Loza AJ, Bhanji RA, Wasilenko S, et al. Systematic review: Recurrent autoimmune liver diseases after liver transplantation. Aliment Pharmacol Ther 2017;45(4):485–500. [DOI] [PubMed] [Google Scholar]
- 16. Duclos-Vallée JC, Sebagh M, Rifai K, et al. A 10 year follow up study of patients transplanted for autoimmune hepatitis: Histological recurrence precedes clinical and biochemical recurrence. Gut 2003;52(6):893–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gautam M, Cheruvattath R, Balan V. Recurrence of autoimmune liver disease after liver transplantation: A systematic review. Liver Transpl 2006;12(12):1813–24. [DOI] [PubMed] [Google Scholar]
- 18. Aravinthan AD, Doyle AC, Issachar A, et al. First-degree living-related donor liver transplantation in autoimmune liver diseases. Am J Transplant 2016;16(12):3512–21. [DOI] [PubMed] [Google Scholar]
- 19. Ayata G, Gordon FD, Lewis WD, et al. Liver transplantation for autoimmune hepatitis: A long-term pathologic study. Hepatology 2000;32(2):185–92. [DOI] [PubMed] [Google Scholar]
- 20. Vogel A, Heinrich E, Bahr MJ, et al. Long-term outcome of liver transplantation for autoimmune hepatitis. Clin Transplant 2004;18(1):62–9. [DOI] [PubMed] [Google Scholar]
- 21. Reich DJ, Fiel I, Guarrera JV, et al. Liver transplantation for autoimmune hepatitis. Hepatology 2000;32(4 Pt 1):693–700. [DOI] [PubMed] [Google Scholar]
- 22. Beal EW, Black SM, Michaels A. Autoimmune hepatitis in the liver transplant graft. Clin Liver Dis 2017;21(2):381–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Czaja AJ. Diagnosis, pathogenesis, and treatment of autoimmune hepatitis after liver transplantation. Dig Dis Sci 2012;57(9):2248–66. [DOI] [PubMed] [Google Scholar]
- 24. Hübscher SG. Recurrent autoimmune hepatitis after liver transplantation: Diagnostic criteria, risk factors, and outcome. Liver Transpl 2001;7(4):285–91. [DOI] [PubMed] [Google Scholar]
- 25. Krishnamoorthy TL, Miezynska-Kurtycz J, Hodson J, et al. Long term corticosteroid use after liver transplantation for autoimmune hepatitis is safe and associated with a lower incidence of recurrent disease. Liver Transpl 2016;22(1):34–41. [DOI] [PubMed] [Google Scholar]
- 26. Trouillot TE, Shrestha R, Kam I, et al. Successful withdrawal of prednisone after adult liver transplantation for autoimmune hepatitis. Liver Transpl Surg 1999;5(5):375–80. [DOI] [PubMed] [Google Scholar]
- 27. Theocharidou E, Heneghan MA. Con: Steroids should not be withdrawn in transplant recipients with autoimmune hepatitis. Liver Transpl 2018;24(8):1113–8. [DOI] [PubMed] [Google Scholar]
- 28. Kalra A, Burton JR. Jr, Forman LM. Pro: Steroids can be withdrawn posttransplant in recipients with autoimmune hepatitis. Liver Transpl 2018;24(8):1109–12. [DOI] [PubMed] [Google Scholar]
- 29. González-Koch A, Czaja AJ, Carpenter HA, et al. Recurrent autoimmune hepatitis after orthotopic liver transplantation. Liver Transpl 2001;7(4):302–10. [DOI] [PubMed] [Google Scholar]
- 30. Prados E, Cuervas-Mons V, de la Mata M, et al. Outcome of autoimmune hepatitis after liver transplantation. Transplantation 1998;66(12):1645–50. [DOI] [PubMed] [Google Scholar]
- 31. Gelson W, Hoare M, Dawwas MF, et al. The pattern of late mortality in liver transplant recipients in the United Kingdom. Transplantation 2011;91(11):1240–4. [DOI] [PubMed] [Google Scholar]