Abstract
Hemorrhagic stroke is a global health crisis plagued by neuroinflammation in the acute and chronic phases. Neuroinflammation approximates secondary cell death, which in turn robustly contributes to stroke pathology. Both the physiological and behavioral symptoms of stroke correlate with various inflammatory responses in animal and human studies. That slowing the secondary cell death mediated by this inflammation may attenuate stroke pathology presents a novel treatment strategy. To this end, experimental therapies employing stem cell transplants support their potential for neuroprotection and neuroregeneration after hemorrhagic stroke. In this review, we evaluate experiments using different types of stem cell transplants as treatments for stroke-induced neuroinflammation. We also update this emerging area by examining recent preclinical and clinical trials that have deployed these therapies. While further investigations are warranted to solidify their therapeutic profile, the reviewed studies largely posit stem cells as safe and potent biologics for stroke, specifically owing to their mode of action for sequestering neuroinflammation and promoting neuroregenerative processes.
1. Introduction
Treatment with autologous and allogeneic stem cell transplants stands as a valuable tool for regenerative medicine in stroke by harnessing of their capacity for secreting therapeutic molecules and replacing cells.1-4 Endogenous and exogenous stem cells promote amelioration in brain tissues damage by stroke through their enhancement of angiogenesis, neurogenesis, and synaptogenesis.2-8 Stem cell transplants may moderate the secondary cell death caused by stroke-induced neuroinflammation, thus stem cells with anti-inflammatory qualities are especially attractive.2-4,9 Below, we present and evaluate the current state of different stem cell types and their potential in dampening the neuroinflammation caused by stroke.
2. Stem cell therapy improves stroke outcomes
Stroke imposes an immense health and economic burden worldwide.188 Stroke can be classified as ischemic or hemorrhagic, with the ischemic case composing 87 percent of stroke incidences, while the remainder of patients who suffer hemorrhagic are smaller in number, the prognosis is much more devastating with high mortality.10-12,16 Despite these poor clinical outcomes, treatment options for stroke remain scarce. The thrombolytic agent tissue plasminogen activator (tPA) is only effective in the first 4.5 hours post-stroke, limiting its use to the acute phase and thus excludes the majority of stroke patients.3,17 For hemorrhagic stroke, limited treatments are available, which focus on controlling the bleeding and reducing the pressure caused by the bleeding immediately after stroke onset.13-15 Cell therapy has emerged as a compelling biomedical field in the search to identify a treatment that may extend stroke’s currently restricted therapeutic window. Specifically, stem cell therapy has the potential to sequester the neuroinflammation that engenders secondary cell death and is thus indicated as a possible subacute- or chronic-phase treatment.18
A stroke therapy that is effective acutely with long-term functional benefits would improve the outcomes of many stroke patients. Stroke moves from the acute to the subacute phase after a few hours, then progressing to the chronic phase at a few days post-stroke. The emission of chemokines, cytokines, matrix metalloproteinases (MMPs), and cellular adhesion molecules (CAMs) in the stroke penumbra and surrounding tissues creates the neuroinflammation that plagues the subacute phase of stroke.19-22 Dysfunction of the blood brain barrier (BBB) may lead to secondary brain injury, often observed in stroke, and this process may stem from these aforementioned inflammatory elements, most prominently the MMPs.19-21 This deleterious process may be further exacerbated by CAMs, which lure harmful neutrophils, leukocytes, macrophages, and microglia to the damaged cerebral vessels, even though they may also recruit beneficial cells, including stem cells.19-21 Taken together, these inflammatory substances impair BBB integrity and exacerbate lasting inflammation.22-25 These symptoms mediate the gradual secondary cell death that characterizes a large portion of stroke pathology, and thus stem cell transplantation may be ideal due to their distinctive subacute and chronic efficacy.10,23 A multi-pronged approach may be considered in regard to transplantation timing, comprising first a delivery of stem cells in the subacute phase to protect against secondary cell death followed by a second administration of stem cells during the chronic phase to prompt and support native host brain repair processes.3,22,152 Experimental stroke models recapitulate this booster treatment approach, in which stem cells transplants bestow these anticipated early neuroprotective and delayed neuroregenerative effects.9,26-28 Between these two, neuroregeneration may be the favored target because, compared to neuroprotection, it is more dependably beneficial in subacute and chronic stroke.
3. Establishing the ideal stem cell type for transplantation
Preclinical and clinical trials have advanced several types of stem cells as options for transplantation.29,30,32,31,33,25,34,35 The long-standing safety profile of bone-marrow derived stem cells supports their use in the majority of these trials. Concerns regarding the ideal cell transplant timing, delivery route, and dosing for each cell type remain to optimally sequester the neuroinflammation caused by stroke without deleterious side effects.3 Here, we review the preclinical and, where applicable, clinical results of treatments with these stem cell types.
3.1. Embryonic stem cells
Embryonic stem (ES) cells remain the gold standard in cell-based regenerative treatments, due to their differentiation into tissues of all three germ layers and their indeterminate proliferation. These favorable properties notwithstanding, ES cells’ penchant for tumor formation, related to their signature proliferation, and the ethically controversial nature of their tissue source often consign this cell type to preclinical studies. Transplanting ES cells intracerebrally amplifies cerebral angiogenesis and diminishes neuronal damage in experimental models.34,36 In addition, transplanted embryoid body (EB) cells, which are derived from ES cells, induce behavioral benefits in animals seven days post-stroke.36 Likewise, transplanted human embryonic stem cells (hESCs) produce similar results,36 an effect possibly regulated by the differentiation of hESCs into neural phenotypes.37,38 Further, genetically engineering ES cells by overexpressing neuroprotective factors has also proven to be a viable option for functional recovery in stroke animals.8 Moreover, experiments performed with cell cultures reveal that ES cells may emit therapeutic molecules after exposure to a hypoxic microenvironment, which is reminiscent of stroke conditions.39 These therapeutic molecules may, in turn, inhibit the apoptotic cell death normally caused by stroke.34 Additionally, the harsh inflammatory microenvironment achieved through these hypoxic conditions also demonstrates the ability of ES cells to rescue host cells from neuroinflammation. That ES cells may secrete anti-inflammatory signals in response to hypoxic conditions advances a possible mechanism for their sequestration of neuroinflammation. However, in vivo results that reveal an increase in pro-inflammatory cytokines that suppress ES cells conflict with these in vitro findings.40 Thus, further research into the mechanism responsible for these effects is needed to resolve these discordant verdicts and to elucidate ES cells’ potential as a stroke therapy.
When considering the translation of ES cells from the bench to the clinic, their propensity for tumorigenicity mars their otherwise auspicious affinity for cell replacement due to their high proliferative power.3,41 This proliferation actually may be enhanced by stroke conditions.37,41 ES cells’ promising aspects warrant investigation into both implementing a regulator of cell proliferation to check the cells’ tumorigenic tendencies and deriving stem cells that retain the same traits as ES cells from non-embryonic sources.
Taking these tumorigenic issues together with the inherent ethical concerns, ES cells may remain mostly relegated to preclinical studies until the establishment of suitable solutions. Subsequently, due to present lack of federal funding, recent research advancing the use of ES cells as a stroke therapy is severely limited. However, previous inquiries uphold the therapeutic potential of ES cells in treating stroke-induced injury. Namely, ES cells engrafted in the cerebral tissue of stroke mice traveled to the site of injury, alleviated behavioral impairments,42 and restored blemished synaptic connections characteristic of stroke lesions.43,44 While encouraging, these findings clearly require further development in forthcoming investigations. Additionally, transplantation of embryonic neural stem cells in a traumatic brain injury (TBI) model of adult mice evidently attenuated neuroinflammation by reducing microglial activation and astrogliosis, while also enhancing neurogenesis.45 Although not completely overlapping, stroke and TBI possess similar pathologies, particularly with regard to exacerbated neuroinflammation, suggesting that the induction of anti-inflammatory effects by ES cells may be mediated through similar pathways following stroke. To this end, differentiating ES cells towards an anti-inflammation-conferring cell type may enhance their utility for stroke, such as the use of curcumin nanoformulation.46 Nonetheless, the previously discussed ethical concerns and risk of tumorigenesis associated with ES cell transplantation limit the use of these cells, and recent advancement of other stem cell types (i.e., induced pluripotent stem cells) has shifted focus towards these novel options which circumvent many of these logistical issues.
3.2. Adult tissue-derived stem cells
Although use of ES cells in both the laboratory and clinic has been largely discontinued over the past two decades due to the aforementioned lack of federal funding by the United States government, investigation of adult tissue-derived stem cells has progressed markedly in its stead. Lacking the ethical hurdles which had plagued ES cell research, advancement of adult-tissue derived stem cells to clinical trials has gained public support. However, the use of adult stem cells continues to present a number of practical challenges, the most significant being the effective derivation of an abundant and homogeneous stem cell population. Definitively establishing a set of biomarkers to phenotypically characterize adult stem cells is a topic of ongoing debate and is further discussed below.
3.2.1. Hematopoietic stem cells
Hematopoietic stem cells (HSCs) can be identified by their expression of cell surface receptor CD34+ with surrogate markers of CD150+, CD244−, and CD48−.47 Following cerebrovascular insult such as stroke, HSCs begin circulating in the bloodstream and are recruited by cytokines to the injured brain.48,125 Treatment with granulocyte-colony stimulating factor (G-CSF),48 as well as with neurotransmitters that upregulate HSC populations within the bone marrow,49,50 can increase the mobilization of HSCs to blood circulation and, in turn, their availability within the injured brain. Indeed, HSCs may be intimately linked with stroke pathology, as the extent of HSC mobilization not only directly correlates with the severity of cerebral insult,50 but also with the degree of functional recovery.51
Laboratory studies to date have explored HSCs as a therapy for stroke. However, in experimental stroke with supporting evidence from clinical studies of other diseases, HSC transplantation evidently worsened neuroinflammation, inhibited functional recovery,52-54 and may have even caused cerebral infarct.55 While such negative outcomes may indicate that transplanted HSCs lack viability as a stroke therapeutic, one clinical trial found that HSC transplantation in stroke patients was successfully tolerated and even mitigated functional deficits.56 These mixed results clearly warrant further investigation of HSC transplantation for stroke therapy. Elucidating the intrinsic cellular properties of HSCs and the extrinsic microenvironment that influences their fate after transplantation may help to reveal a means of suppressing the pro-inflammatory phenotype of HSCs while optimizing their anti-inflammatory effects against secondary neuronal cell loss following stroke.
Current efforts reveal the advantages and disadvantages of HSC treatment in stroke therapy. Treating mice with hematopoietic stem and progenitor cells (HSPC) after transient MCAO dampens cellular responses, including inflammation, for up to a week post-operation.57 Additionally, HSPCs afford protection against the residual neuroinflammation.57 That metallothionein I may serve as the mechanism behind these effects advances the importance of these findings.57 On the other hand, damage in stroke tissue may prompt HSPC activity among bone marrow-generating myeloid cells. Continuous HSPC activation may incite long-term inflammation and inhibit the tissue recovery process. A murine hindlimb ischemia model demonstrates HSCs’ bidirectional effect on inflammation. Specifically, HSPCs may exacerbate the ischemia-induced inflammation through their monocyte activation.58 Nox 2 may suppress these adverse effects, positing it as a possible method of exploiting the otherwise anti-inflammatory and therapeutic aspects of HSPCs.58 While there is a dearth of recent studies measuring inflammation after HSC transplants in stroke therapies, coupling these two studies still exemplifies the contradictory nature of HSCs warranting further investigations.
3.2.2. Bone marrow-derived stem cells
Bone marrow has been well established as a tissue source of numerous types of stem cells, known in general as bone marrow-derived mesenchymal stem or stromal cells (BM-MSCs). Specific subgroupings of BM-MSCs have been characterized and designated as endothelial progenitor cells (EPCs), notch-transfected mesenchymal stromal cells (SB623), multipotent adult progenitor cells (MAPCs), and multilineage-differentiating stress enduring (Muse) cells. These BM-MSCs have been previously examined in experimental stroke models and are currently being assessed in multiple clinical trials. While peripheral delivery of BM-MSCs is effective due to the cells’ capacity to migrate to the injured brain,59 a number of studies have also tested the direct delivery of BM-MSCs into the stroke-damaged brain.60,61
BM-MSCs can be derived and harvested not only from bone marrow, but also from practically any tissue type, and these stem cells are identified by the phenotypic markers CD29+, CD44+, CD105+, CD73+, CD90+, CD106+, CD166+, CD14−, CD34−, and CD45−.60,93 Further, BM-MSCs display potential to differentiate into osteogenic, chondrogenic, and adipogenic cells,62 as well as the ability to enhance functional recovery following transplantation in stroke animals.63 BM-MSCs have been hypothesized to exert their effects via various mechanisms of action, including the secretion of neurotrophic factors,64-66 stimulation of angiogenesis,93,63 and promotion of endogenous neurogenesis by recruiting host stem cells from the subventricular zone (SVZ) and subgranular zone (SGZ) to the injury site.93
Numerous studies have thoroughly outlined the therapeutic benefits of BM-MSCs in experimental models of stroke. Stroke animals treated with transplanted BM-MSCs exhibited behavioral improvements that concurred with elevated angiogenesis, synaptogenesis, and neurogenesis.64-68 Likewise, the potent anti-inflammatory effects of BM-MSCs have been well described in stroke models and included the modulation of T-cell proliferation rates, activation of regulatory T-cell (Treg) phenotype CD8+CD28−, and the inhibition of CD4+ and CD8+ T-cells.69-71 Moreover, transplanted BM-MSCs upregulated expression of anti-inflammatory cytokines, such as interleukin (IL)-4, IL-10, and transforming growth factor-β1, while downregulating levels of pro-inflammatory cytokines, such as IL-1β and IL-6, and decreasing microglial activation within the stroke brain.69,153,72,73
In vitro studies have provided further evidence supporting the therapeutic benefits of BM-MSCs. Specifically, BM-MSCs protected primary neural cell cultures against stroke damage by limiting apoptosis and necroptosis, decreasing expression of necroptosis-related receptor interacting protein kinase 1 and 3, and deactivating caspase-3, an enzyme which plays a key role in apoptosis.68 Recent data also indicate that BM-MSCs are capable of secreting therapeutic exosomes.64,66,74,75 In this regard, BM-MSCs reduced neuroinflammation by blocking the activation of microglia and lowering levels of astrogliosis in organotypic hippocampal cultures exposed to stroke insult.76 Finally, the activity of T-cells or natural killer cells77 and leukocyte proliferation78,79 were both restricted in the presence of BM-MSCs as well.
Although generally considered safe, use of BM-MSCs in some animal models increases the risk of tumor development.80 which may be linked to a tumor-inducing factor secreted by these cells.81,82 In fact, the tissue substrate from which BM-MSCs are grown and derived may determine their tumorigenicity.83,84 Thoroughly clarifying the therapeutic mechanism mediated by BM-MSCs may illuminate a solution to the tumorigenic risks of BM-MSC transplantation. Furthermore, direct investigation of the host microenvironment, particularly its genetic composition, may provide critical insight into refining BM-MSC transplantation as a stroke therapy. Additionally, preconditioning BM-MSCs in a hypoxic environment may improve their proliferation, angiogenic effects, and migration capacity.85,86 In similar fashion, altering the host microenvironment via hyperbaric oxygen therapy could enhance BM-MSC migration and their anti-inflammatory effects.87,88 Genetically engineering BM-MSCs may improve their post-stroke benefits as well.89-93
Recent laboratory investigations provide further evidence of the anti-inflammatory properties of BM-MSCs in stroke. Intraventricular infusion of BM-MSCs in hemorrhagic stroke rats suppresses inflammation by decreasing mean expression levels of pro-inflammatory cytokines IL-1α, IL-6, and IFN-γ, suggesting that transplanted BM-MSCs attenuate inflammation by altering cytokine expression.94 Furthermore, combined treatment of BM-MSCs and peroxisome proliferator-activated receptor gamma (PPARγ) agonist pioglitazone (PGZ) advances that PGZ-treated rats’ increased PPARγ expression confers anti-inflammatory effects mediated by its mobilization of transplanted allogeneic BM-MSCs, indicating further that BM-MSCs reduce inflammation.95 Additionally, male MCAO rats display decreased numbers of inflammatory M1-like macrophages and significantly downregulated expression of inflammatory IL-6 and caspase-3, though not of inflammatory IL-1β and tumor necrosis factor-α (TNF-α) after combined treatment.95 Echoing this robust anti-inflammatory action of the cells, rats treated with extracellular regulating kinase 1/2 (ERK1/2)-overexpressing BM-MSCs demonstrate better neurological and functional outcomes and reduced levels of CD68, signifying suppression of microglial activation.96 However, the number of reactive astrocytes and levels of TNF-α were elevated in the ERK/BM-MSC treatment group, demonstrating that ERK1/2 overexpression may differentially modulate inflammatory factors.96 Further research concerning the pro- and anti-inflammatory mechanisms of ERK1/2 and BM-MSCs is needed to clarify the connection between increased astrocyte activation and upregulation of TNF-α, as well as the aforementioned unchanged levels of IL-1β and TNF-α. In addition, native populations of Tregs have been identified in BM-MSC samples.97 This finding suggests a possible mechanism of action for BM-MSCs’ capacity to reduce neuroinflammation. Overall, BM-MSCs demonstrate promising therapeutic potential as mediators of anti-inflammation in animal models of stroke.
3.2.3. Endothelial progenitor cells
Endothelial progenitor cells (EPCs) are characterized by their expression of CD34 or CD133 and endothelial cell markers, such as protein vascular endothelial growth factor receptor 2 (VEGRF2), Von Willebrand factor, vascular endothelial cadherin (VE-cadherin or CD144), CD31, Tie2, c-kit/CD117E, and CD62E (E-selectin).98 EPCs can be classified as early EPCs or late EPCs,99,100 with the former being harvested after culturing mononuclear cells from peripheral blood for 4–10 days, and the latter, also known as endothelial colony forming cells (ECFCs), being harvested after culturing mononuclear cells for >14 days. Late EPCs highly express VE-cadherin and kinase insert domain receptors, while early EPCs secrete angiogenic growth factors.99 In the context of stroke, major vascular dysfunction may be treatable via EPC transplantation, as EPCs facilitate neovascularization98,101,102 and thus promote angiogenesis following stroke.101 Additionally, the quantity of circulating EPCs may correlate with the extent of post-stroke recovery for large-artery atherosclerosis and small-vessel disease etiologic subtypes.103
Possibly through mechanisms of vasculogenesis and angiogenesis,98,104-106,114 EPCs may ameliorate cerebral microvascular density, regional cortical blood flow, and behavioral recovery for stroke animals.107,108,109 Although EPCs may be inactive during homeostasis, chemoattractant signaling pathways, such as stromal-derived factor 1110 or granulocyte-stimulating factor, guide EPCs to the stroke-damaged brain.98 Cerebral infrastructure repair, an EPC-mediated process, seems to be linked to anti-inflammatory effects,111,112 as transplanted EPCs preserve mitochondrial morphology in endothelial cells of the stroke brain, reduce perivascular edema, promote therapeutic pinocytotic activity, and reinforce the integrity of microvessels, including those of the BBB.113 When EPCs are supplemented with p38 mitogen-activated protein kinase inhibitor, neuroinflammation decreases as well.114 However, EPCs may paradoxically worsen neuroinflammation through the release of pro-inflammatory molecules.98,99,115,116 Certain in vitro models have suggested mechanisms by which EPCs mediate neuroinflammation, including downregulation of tumor necrosis factor-α-induced apoptosis and caspase-3 activity.117 Nonetheless, further examination is necessary to determine whether EPCs suppress or induce neuroinflammation following stroke insults in vivo.98,99,115,116
Accumulating evidence suggests that EPCs may aid in combating endothelial dysfunction, a major component inherently linked with inflammation in stroke pathology, to preserve vascular homeostasis and enhance neurological and functional recovery.118 Recent studies investigating the effects of EPCs on the inflammatory stroke vasculature reveal an elevation of inflammation-associated genes IκB, Foxf1, ITIH-5, and BRM with OGD, yet co-culture of EPCs impeded this upregulation, suggesting the potential of EPCs to modulate this rampant vascular inflammation in vitro.119 Further, intracerebral administration of EPCs in vivo to the striatum and cortex 4 hours following stroke revealed substantial behavioral improvement and rehabilitation in comparison to vehicle-treated stroke animals.119 Seven days following transplantation, a fluorescent staining procedure uncovered a considerable increase in endothelial marker RECA1 and a declining amount of specific inflammation-related vasculome genes in the ipsilateral cortex and striatum of EPC-administered stroke animals, further suggesting key inflammatory-based mechanisms underlying EPCs effects in stroke.119 Moreover, emerging data document heightened amounts of early EPCs in acute stroke patients.120 The negative impact of inflammatory factors on EPC continuity and differentiation was displayed by the contradictory relationship between inflammatory parameters and EPCs,120 advancing a vital mechanism connecting inflammation and stroke pathology. Moreover, current laboratory investigations report a novel development of regeneration-associated cells (RACs) for transplantation in stroke animals, which not only promote EPC differentiation, but also induce anti-inflammatory monocytes/macrophages,121 thus indicating potential EPC-mediated environmental regulation involving inflammatory mechanisms. Recent efforts aimed to elucidate the effects of EPCs on neuroinflammation in stroke also suggest that levels of alpha-2-macroglobulin (a2MG), a key protein mediating the inflammatory state in stroke, may be correlated with EPC numbers122 Altogether, this emerging data implicate that EPC therapy may largely exert therapeutic effects by modulating the robust inflammatory response to stroke.
3.2.4. Very small embryonic-like stem cells
Very small embryonic-like stem cells (VSELs) uniquely express Sca-1+, CD45−, and pluripotent stem cell markers SSEA-1, Oct-4, Nanog, and Rex-1.123,124 Similar to embryonic stem cells, VSELs possess a large nucleus-to-cytoplasm ratio and can be distinguished by the single type of chromatin (euchromatin) within their nuclei.124,125 In the aftermath of an stroke event, VSELs are mobilized from adult tissues to circulation in peripheral blood, mirroring the migratory behavior of endogenous HSCs.125 Considered epiblast-derived pluripotent stem cells, VSELs enter dormancy after embryonic development, yet during aging and pathological conditions, they can function as a reservoir of therapeutic stem cells.125 Furthermore, VSELs may be transplantable for stroke therapy,126 as they exhibit the capacity to differentiate into neurons, oligodendrocytes, and microglia.127 Relative to HSCs, VSELs were highly resilient to radiation damage,125 and further data suggest that VSELs are mobilized to blood circulation after tissue injury, including hypoxia.128,129 Moreover, in one mouse model, exogenous VSELs transplanted after myocardial infarction enhanced ventricular function and cardiac remodeling.130 In an animal model in which cerebral injury was induced by the neurotoxin kainic acid, VSELs were recruited from the bone marrow.126 Notably, upregulation of circulating VSELs has been observed following stroke, indicating their potential as minimally invasive stroke therapeutics via migration from the periphery to the injury site within the brain.131 Additionally, cultured VSELs exhibited a strong chemotactic attraction to HGF, SDF-1, and leukemia inhibitory factor,128 thereby emphasizing the cells’ migratory potential to reach stroke brain sites from their initial peripheral location.124 Evidently, the early mobilization of VSELs into circulation reflects the cells’ prominent function in disease onset and progression, particularly with regard to neuroinflammation. Although VSELs may secrete pro-inflammatory cytokines, such as IL-6, IL-8 and chemokine (C–C motif) ligand 5,132 they may also hold an immunoregulatory role, a discrepancy which requires future studies to clarify VSELs’ true potential as a treatment for stroke-induced neuroinflammation.
Recent laboratory investigations evaluating the transplantation of VSELs to target stroke-induced neuroinflammation remain limited, but current data provide some insights on the relationships between VSELs and inflammation, in that stimulation of inflammasome NIrp3 in an ATP-dependent method coordinates the mobilization of VSELs from the bone marrow into the peripheral blood,133 suggesting an inflammatory-based mechanism that may link stroke-induced tissue damage with VSEL recruitment. Interestingly, recent evidence also reveals that patients with IgA nephropathy possess high levels of circulating VSELs and classic anti-inflammatory M2-like monocytes, further advancing a connection between VSEL mobilization in response to inflammatory activation.134 VSEL transplantation therapy targeting the inflammatory-plagued response may thus represent a novel avenue for stroke therapy, yet the role of this stem cell type in immunoregulation warrants much future investigation before extending this therapy to the clinic.
3.3. Neural stem cells
Neural stem cells (NSCs), whether derived from embryonic, fetal, or adult tissues, are a class of multipotent cells that can give rise to various neural phenotypes.135 Thus, their differentiation capacity makes them an intriguing transplantable cell type for stroke therapy with the disease pathology linked to a defective neurovascular unit. Displayed by their upregulation after stroke insult,136 SGZ and SVZ serve as major brain tissue sources of NSCs.137 Although NSCs are initially grown in culture with basic fibroblast growth factor and epidermal growth factor,137,138 removal of these cytokines allows NSCs to differentiate into neurons, astrocytes, and oligodendrocytes.135 Concerning their therapeutic benefits, NSCs induce angiogenesis139 and neurogenesis138 in stroke models. Based on previous studies, overexpression of miR-381, nuclear factor-kappa B (NF-κB), Nox4-generated superoxide levels,135,140,141 and ephrinB2 and Jagged1142 may influence the activity of NSCs. Also important to note, NSC-associated exosomes can regulate inflammation by releasing neuroprotective compounds such as VEGF, NGF, BDNF, and neurotrophins.143 Assuredly, further study is required to elucidate the interaction between NSC exosomes and secreted growth factors, as well as the mechanism by which this interaction mediates anti-inflammatory effects. While NSC differentiation was evidently altered by pro-inflammatory T-cells,144 intravenous transplantation of NSCs in stroke animals reduced cerebral and splenic expression of TNF-α, IL-6, and NF-κB while also reducing levels of OX-42+microglia and MPO+ infiltration into the injury site.145 Furthermore, an acute anti-inflammatory effect resulted from injection of NSCs into the ipsilesional hippocampus, as this infusion downregulated expression of pro-inflammatory factors (TNF-α, IL-6, IL-1β, MCP-1, MIP-1α), diminished numbers of adhesion molecules (intercellular adhesion molecule-1, vascular cell adhesion molecule-1), attenuated microglial activation, and ameliorated BBB permeability.146 Future research on NSCs is needed to fully illuminate their anti-inflammatory properties in the context of stroke. Moreover, safety concerns regarding the use of NSCs in stroke primarily center upon their possible tumorigenicity.8 Thus, future clarification of the cells’ mechanisms of action will lead the way towards safe and effective use of NSC transplantation as a treatment for stroke.
Emerging evidence provides further support for NSCs’ anti-inflammatory role. Alongside other functional improvements, NSC treatment successfully lowers inflammation by controlling levels of anti-inflammatory cytokine TIMP-1 and pro-inflammatory cytokines leptin, L-selectin, MCP-1, and TNF-α in a permanent MCAO rodent model of stroke.147 Another rat model produced similar results, observing that NSC treatment increases expression of anti-inflammatory cytokines IL-10 and TGF-β1 and decreases expression of pro-inflammatory cytokines IL-1β, IL-6, IFN-γ and TNF-α.148,149 Interestingly, this comes on the heels of the revelation that low doses of IFN-γ can produce greater neuroregenerative effects when co-injected with NSCs than NSCs alone.148 That the combined treatment activated the IFN-γ/Stat1 signaling pathway provides a possible mechanism for these benefits, with IFN-γ enhancing the potency of this “help me” signal.148,148 NSC extracellular vesicles (EV) may also confer similar effects on neuroinflammation. In a middle-aged murine thromboembolic stroke model, NSC EV treatment inhibited pro-inflammatory Th17 cells through its upregulation of Tregs and encouraged macrophages to adopt their anti-inflammatory M2 phenotype.150 While more research is needed, these findings depict a multifaceted effect of NSCs on stroke-induced neuroinflammation.
3.4. Extraembryonic stem cells
Umbilical cord blood-derived MSCs (UCB-MSCs) and placenta-derived MSCs predominantly comprise the extraembryonic stem cells types. Investigated in experimental models of stroke, these extraembryonic MSCs exert therapeutic benefits involving the substitution of damaged cells and alterations of the stroke environment151-154 by improving neurogenesis, enhancing vascular structure, and secreting growth factors.152,155 Moreover, these extraembryonic MSCs may modulate the inflammatory response, owing to their immunosuppressive effects within the innate immune system,151,156 and specifically via secretion of leukemia inhibitory factor (LIF).157
UCB-MSCs have demonstrated remarkable potential thus far in autologous and allogeneic transplantation models, as well as in clinical trials.158 UCB-MSCs remain an attractive option due to their immunological immaturity156 and their capacity to dampen the immune response via reduction of pro-inflammatory factors following transplantation.159,160 Transplantation of umbilical cord blood in stroke animals reduces infarct volume, enhances motor function, and increases neurotrophic factor expression.155,161-164 UCB-MSCs may prevent the mobilization of immune cells to the damaged region.164 Likewise, placenta-derived MSCs may attenuate neuroinflammation by improving microglial survival.136,162 UCB-MSCs may also possess similar anti-inflammatory properties165 as evidenced by reduced proliferation of B-cells and T-cells and modulated monocytes with the co-culture of UCB-MSCs.165,166 Along this line, placenta-derived MSCs dampened allogenic T-cell proliferation.151 Altogether, these findings suggest the potential of extraembryonic stem cells to attenuate the rampant inflammatory response induced by stroke.
The transplantation of placenta-derived MSCs has been explored in experimental models of stroke, yet only placental MSCs derived from the maternal line has exhibited significant improvements.167 Future direction should thus investigate the restrictions of maternally derived placental MSCs effects, and if these findings apply to UCB-MSCs as well. Recognizing the therapeutic benefits of UCB for stroke mediated by estradiol162 also provides grounds for further studies examining combination therapies with extraembryonic stem cells. Moreover, thoroughly investigating the capacity of extraembryonic stem cells to dampen neuroinflammation is necessary to advance their application in stroke.
Promisingly, a recent Phase 1 safety clinical trial has reported that intravenous administration of unrelated allogeneic UCB cells, matched for blood group antigens but not for human leukocyte antigen, was not only well-tolerated by stroke patients, but also improved their neurological and functional performance at 3 months post-treatment.168 Novel laboratory investigations utilizing extraembryonic stem cells have also expanded upon past rodent models in a variety of ways. Using four animal models of stroke including non-human primates, a preclinical study determined that human amnion epithelial cells, a subset of placenta-derived stem cells, were selectively recruited to the spleen and injured brain, where they promoted functional recovery by reducing apoptosis, inflammation, and infiltration of peripheral immune cells into the brain.169 On another note, brain transcriptome profiling analysis in rats revealed that, of the 275 genes differentially expressed following ischemia, 220 returned to normal expression levels following UCB-MSC treatment and primarily involved downregulation of post-stroke immune response activation.170 Studies using UCB-MSCs in combination therapies have also produced notable results. Although effective as alone treatments, combined administration of UCB-MSCs and erythropoietin in MCAO rats resulted in the most significant behavioral improvements, most elevated levels of neurogenesis and angiogenesis, and most diminished levels astrogliosis.171 Similarly, stroke mice treated with UCB-MSCs in conjunction with nitrogen-doped carbon nanocages exhibit synergistic benefits with regard to the reduction of infarct volume, behavioral improvements, and attenuation of inflammation, as well as downregulation of TNF-α expression, upregulation of IL-10 expression, and elevation of TNF-α stimulated gene/protein 6 and prostaglandin 2 levels in lipopolysaccharide-treated microglia.172 Collectively, these findings suggest that extraembryonic stem cells, whether alone or in combination, may represent an optimal approach to treating stroke due to their potent anti-inflammatory and immunomodulatory effects.
3.5. Induced pluripotent stem cells
Induced pluripotent stem cells (iPSCs) are a type of pluripotent stem cell which can be generated from fibroblasts via retrograde manipulation to be reprogrammed back into an embryonic-like pluripotent state enabling the generation of an unlimited source of any type of human cell needed for therapeutic purposes.173 This property has then been applied to umbilical cord blood cells, placental mesenchymal stromal cells, adipose-derived stem cells, and neural stem cells.174,175
The improved proliferative function of these precursor cells over adult cells marks a key appealing feature of retrograde conversion.173 Transplantation of iPSCs in stroke animals reduces infarction and enhances functional improvements, likely via observed downregulation of pro-inflammatory factors and upregulation of anti-inflammatory factors.176,177 That iPSCs possess anti-inflammatory characteristics is bolstered by findings of reduced activated microglia, decreased inflammatory cytokines (TNF-α, IL-6, IL-1β, MCP-1, MIP-1α), and enhanced BBB integrity in stroke.178 Moreover, cultured iPSCs can differentiate into microglia179 as well as antigen-specific Tregs,180 both key components of immunoregulation in the brain. These findings suggest that iPSCs may exert therapeutic benefits in stroke by differentiating into these anti-inflammatory cells. This utility may be extended to exploit the secretion of anti-inflammatory factors to further enhance stroke recovery. iPSCs thus hold remarkable potential in their ability to dampen inflammation following stroke.
The risk of tumorigenesis remains a valid issue when contemplating the use of iPSCs for stroke. Oncogenic transcription factors are involved in the transfection procedure to generate iPSCs3 indicating a high potential of oncogenesis. High tumorigenicity exists with undifferentiated iPSCs compared to other known cells transplanted to the stroke brain,181 and iPSCs may promote teratoma formation in the stroke tissue.41,181 Another concern involving transplantation of these cells is immunogenicity. iPSCs may activate an immune response, suggesting an increased risk of rejection.182 Elucidating the risk of both tumorigenic and immunogenic effects limiting their transplantation is necessitated to support iPSCs as a viable therapy for stroke.
While direct transplantation of iPSCs has been previously explored,176,177 recent investigations have primarily focused on pre-differentiating iPSCs towards a neuronal phenotype prior to transplantation in experimental models of stroke.183 Current efforts utilizing iPSCs as a source for derivation hence aim to prevent tumorigenesis and maximize neuronal differentiation by exploring adjunctive therapies. Preconditioning human iPSC-derived neural stem cells (hiPSC-NSCs) with FDA-approved drug Metformin prior to transplantation reveals enhanced differentiation, improved graft survival, decreased infarct size, and attenuated motor dysfunction.184 In addition to combination drug therapies, selective excitation of transplanted cells using optochemogenetic fusion protein luminopsin 3 (LMO3) promotes neural network connectivity and functional recovery following stroke.185 While the majority of these studies examining iPSCs for stroke have employed hiPSCs,183 innovative studies recognizing the unique cellular preservation of hibernating mammals support the use of ground squirrel iPSCs (GS-iPSCs). These newly reported GS-iPSCs possess cold-adaptive properties such as microtubule stability to relieve mitochondrial stress and limit reactive oxygen specific (ROS) production,186 which may broaden the application of novel combination therapies for stroke involving stem cells and hypothermia for neuroinflammation. The inflammatory properties of iPSCs have been further explored in recent laboratory investigations implicating a role of apolipoprotein allele epsilon 4 (ApoE4), a prominent genetic risk factor for stroke, with ApoE4-positive iPSCs acquiring a pro-inflammatory state.187 Further elucidating the inflammatory components underlying transplantation of iPSCs, as well as exploration into adjunctive therapies, will likely promote the safety and efficacy of iPSCs for stroke.
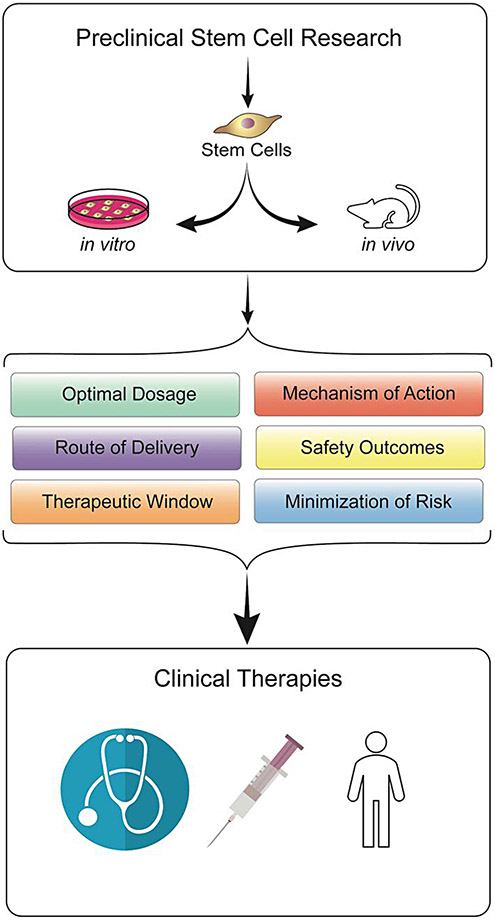
4. Conclusion
While basic science and clinical research have improved our grasp on stroke pathology, this disease possesses limited treatment options and presents a major health and economic burden globally.188 Neuroinflammation represents a key component in the secondary cell death cascade associated with both acute and chronic stroke, but, in turn, marks a potential strategy for therapeutic efforts.3,10,12,18,23 Dampening the rampant inflammatory response to stroke may thus be a key to improving functional outcomes. Stem cells present novel therapeutic potential in this regard due to their regenerative nature, wide administrative window, and anti-inflammatory capabilities demonstrated in in vitro and in vivo models (Fig. 1). Indeed, some stem cell lines have advanced to clinical trials for stroke.56,189-195 Despite their potential to sequester inflammation and promote regeneration, future investigations are warranted to resolve moderate efficacy and safety issues such as tumorigenesis and immunogenicity to advance these stem cells as a viable treatment option in the clinic. Revealing the full potential of stem cell therapy for CNS diseases such as stroke must adhere to the Stem cell Therapeutics as an Emerging Paradigm for Stroke or STEPS guidelines to improve translation from bench to bedsideyy.31,196,197 Altogether, stem cell therapy targeting the neuroinflammatory cascade presents remarkable potential as a stroke therapy.
Fig. 1.
Translation of stem cell therapy from the laboratory to the clinic. Stem cell transplantation has emerged as a potent stroke therapy as evidenced by in vitro and in vivo stroke models. Translational research investigations are necessary to optimize the stem cell transplant regimen in order to enhance the efficacy and safety of its clinical application for stroke patients.
Acknowledgements
We thank the entire staff of Borlongan Neural Transplantation Laboratory for technical assistance and excellent scientific discussion.
Funding
Dr Borlongan is funded by National Institutes of Health (NIH) R01NS090962, NIH R01NS102395, and NIH R21NS109575.
Footnotes
Conflict of interest
Cesar V Borlongan holds patents and patent applications on stem cell biology and their therapeutic applications, and receives royalties and consultancy fees from stem cell companies.
References
- 1.Nishino H, Borlongan CV. Restoration of function by neural transplantation in the ischemic brain. Prog Brain Res. 2000;127:461–476. [DOI] [PubMed] [Google Scholar]
- 2.Yasuhara T, Borlongan CV, Date I. Ex vivo gene therapy: transplantation of neurotrophic factor-secreting cells for cerebral ischemia. Front Biosci. 2006;11:760–775. [DOI] [PubMed] [Google Scholar]
- 3.Dailey T, Metcalf C, Mosley YI, et al. An update on translating stem cell therapy for stroke from bench to bedside. J Clin Med. 2013;2:220–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drago D, Cossetti C, Iraci N, et al. The stem cell secretome and its role in brain repair. Biochimie. 2013;95:2271–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazzini L, Fagioli F, Boccaletti R, et al. Stem cell therapy in amyotrophic lateral sclerosis: a methodological approach in humans. Amyotroph Lateral Sclerosis Other Motor Neuron Disorders. 2003;4:158–161. [DOI] [PubMed] [Google Scholar]
- 6.Lindvall O, Kokaia Z, Martinez-Serrano A. Stem cell therapy for human neurodegenerative disorders–how to make it work. Nat Med. 2004;10:S42–S50. [DOI] [PubMed] [Google Scholar]
- 7.Kim SU, De Vellis J. Stem cell-based cell therapy in neurological diseases: a review. J Neurosci Res. 2009;87:2183–2200. [DOI] [PubMed] [Google Scholar]
- 8.Shinozuka K, Dailey T, Tajiri N, et al. Stem cells for neurovascular repair in stroke. J Stem Cell Res Ther. 2013;4:12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tajiri N, Duncan K, Antoine A, et al. Stem cell-paved biobridge facilitates neural repair in traumatic brain injury. Front Syst Neurosci. 2014;8:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borlongan CV, Glover LE, Sanberg PR, Hess DC. Permeating the blood brain barrier and abrogating the inflammation in stroke: implications for stroke therapy. Curr Pharm Des. 2012;18:3670–3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Truelsen T, Krarup LH, Iversen HK, et al. Causes of death data in the Global Burden of Disease estimates for ischemic and hemorrhagic stroke. Neuroepidemiology. 2015;45:152–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Broderick JP, Brott T, Tomsick T, et al. Intracerebral hemorrhage more than twice as common as subarachnoid hemorrhage. J Neurosurg. 1993;78:188–191. [DOI] [PubMed] [Google Scholar]
- 13.Marcolini E, Stretz C, DeWitt KM. Intracranial hemorrhage and intracranial hypertension. Emerg Med Clin North Am. 2019;37:529–544. [DOI] [PubMed] [Google Scholar]
- 14.Ohtani R, Nakamura M, Nirengi S, et al. Pretreatment blood pressure is a simple predictor of hemorrhagic infarction after intravenous recombinant tissue plasminogen activator (rt-PA) therapy. J Stroke Cerebrovasc Dis. 2019;28:1979–1986. [DOI] [PubMed] [Google Scholar]
- 15.Zhao JL, Du ZY, Sun YR, et al. Intensive blood pressure control reduces the risk of progressive hemorrhage in patients with acute hypertensive intracerebral hemorrhage: a retrospective observational study. Clin Neurol Neurosurg. 2019;180:1–6. [DOI] [PubMed] [Google Scholar]
- 16.Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics–2013 update: a report from the American Heart Association. Circulation. 2013;2013 (127):e6–e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Ying G, Ren C, et al. Administration of human platelet-rich plasma reduces infarction volume and improves motor function in adult rats with focal ischemic stroke. Brain Res. 2015;1594:267–273. [DOI] [PubMed] [Google Scholar]
- 18.Jin R, Liu L, Zhang S, et al. Role of inflammation and its mediators in acute ischemic stroke. J Cardiovasc Transl Res. 2013;6:834–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. 2009;7:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ceulemans AG, Zgavc T, Kooijman R, et al. The dual role of the neuroinflammatory response after ischemic stroke: modulatory effects of hypothermia. J Neuroinflammation. 2010;1:7,74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Acosta SA, Tajiri N, Hoover J, et al. Intravenous bone marrow stem cell grafts preferentially migrate to spleen and abrogate chronic inflammation in stroke. Stroke. 2015;46:2616–2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stonesifer C, Corey S, Ghanekar S, et al. Stem cell therapy for abrogating stroke-induced neuroinflammation and relevant secondary cell death mechanisms. Prog Neurobiol. 2017;S0301-0082(17):30082-30085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park DH, Eve DJ, Musso III J, et al. Inflammation and stem cell migration to the injured brain in higher organisms. Stem Cells Dev. 2009;18:693–702. [DOI] [PubMed] [Google Scholar]
- 25.Tajiri N, Acosta S, Glover LE, et al. Intravenous grafts of amniotic fluid-derived stem cells induce endogenous cell proliferation and attenuate behavioral deficits in ischemic stroke rats. Public Library Sci One. 2012;7 e43779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borlongan CV, Tajima Y, Trojanowski JQ, et al. Transplantation of cryopreserved human embryonal carcinoma-derived neurons (NT2N cells) promotes functional recovery in ischemic rats. Exp Neurol. 1998;149:310–321. [DOI] [PubMed] [Google Scholar]
- 27.Chen J, Chopp M. Neurorestorative treatment of stroke: cell and pharmacological approaches. NeuroRx. 2006;3:466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanchez-Ramos J, Song S, Cardozo-Pelaez F, et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164:247–256. [DOI] [PubMed] [Google Scholar]
- 29.Borlongan CV, Sanberg PR, Freeman TB. Neural transplantation for neurodegenerative disorders. The Lancet. 1999;353:29–30. [DOI] [PubMed] [Google Scholar]
- 30.Borlongan CV, Fournier C, Stahl CE, et al. Gene therapy, cell transplantation and stroke. Front Biosci. 2005;11:1090–1101. [DOI] [PubMed] [Google Scholar]
- 31.Borlongan CV. Cell therapy for stroke. Stroke. 2009;40:146–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Borlongan CV, Kaneko Y, Maki M, et al. Menstrual blood cells display stem cell–like phenotypic markers and exert neuroprotection following transplantation in experimental stroke. Stem Cells Dev. 2010;19:439–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Antonucci I, Stuppia L, Kaneko Y, et al. Amniotic fluid as a rich source of mesenchymal stromal cells for transplantation therapy. Cell Transplant. 2011;20:789–795. [DOI] [PubMed] [Google Scholar]
- 34.Maya-Espinosa G, Collazo-Navarrete O, Millán-Aldaco D, et al. Mouse embryonic stem cell-derived cells reveal niches that support neuronal differentiation in the adult rat brain. Stem Cells. 2015;33:491–502. [DOI] [PubMed] [Google Scholar]
- 35.Stevanato L, Thanabalasundaram L, Vysokov N, et al. Investigation of content, stoichiometry and transfer of miRNA from human neural stem cell line derived exosomes. Public Library Sci One. 2016;11 e0146353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu XY, Wang CP, Liu M, et al. Transplantation of human embryonic neural stem cells protects rats against cerebral ischemic injury. Acta Physiol Sin. 2014;66:691–701. [PubMed] [Google Scholar]
- 37.Seminatore C, Polentes J, Ellman D, et al. The postischemic environment differentially impacts teratoma or tumor formation after transplantation of human embryonic stem cell-derived neural progenitors. Stroke. 2010;41:153–159. [DOI] [PubMed] [Google Scholar]
- 38.Arvidsson A, Collin T, Kirik D, et al. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. [DOI] [PubMed] [Google Scholar]
- 39.Theus MH, Wei L, Cui L, et al. In vitro hypoxic preconditioning of embryonic stem cells as a strategy of promoting cell survival and functional benefits after transplantation into the ischemic rat brain. Exp Neurol. 2008;210:656–670. [DOI] [PubMed] [Google Scholar]
- 40.Ideguchi M, Shinoyama M, Gomi M, et al. Immune or inflammatory response by the host brain suppresses neuronal differentiation of transplanted ES cell–derived neural precursor cells. J Neurosci Res. 2008;86:1936–1943. [DOI] [PubMed] [Google Scholar]
- 41.Kawai H, Yamashita T, Ohta Y, et al. Tridermal tumorigenesis of induced pluripotent stem cells transplanted in ischemic brain. J Cereb Blood Flow Metab. 2010;30:1487–1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagai N, Kawao N, Okada K, et al. Systemic transplantation of embryonic stem cells accelerates brain lesion decrease and angiogenesis. NeuroReport. 2010;21:575. [DOI] [PubMed] [Google Scholar]
- 43.Tae-Hoon L, Yoon-Seok L. Transplantation of mouse embryonic stem cell after middle cerebral artery occlusion. Acta Cirúrgica Brasileira. 2012;27:333. [DOI] [PubMed] [Google Scholar]
- 44.Marei HE, Hasan A, Rizzi R, et al. Potential of stem cell-based therapy for ischemic stroke. Front Neurol. 2018;9:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nasser M, Ballout N, Mantash S, et al. Transplantation of embryonic neural stem cells and differentiated cells in a controlled cortical impact (CCI) model of adult mouse somatosensory cortex. Front Neurol. 2018;9:895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kalani A, Chaturvedi P, Kamat PK, et al. Curcumin-loaded embryonic stem cell exosomes restored neurovascular unit following ischemia-reperfusion injury. Int J Biochem Cell Biol. 2016;79:360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oguro H, Ding L, Morrison SJ. SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors. Cell Stem Cell. 2013;13:102–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shyu WC, Lin SZ, Yang HI, et al. Functional recovery of stroke rats induced by granulocyte colony-stimulating factor–stimulated stem cells. Circulation. 2004;110:1847–1854. [DOI] [PubMed] [Google Scholar]
- 49.Ratajczak MZ, Kim C, Janowska-Wieczorek A, et al. The expanding family of bone marrow homing factors for hematopoietic stem cells: stromal derived factor 1 is not the only player in the game. Sci World J. 2012;2012 758512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sullivan R, Duncan K, Dailey T, et al. A possible new focus for stroke treatment–migrating stem cells. Expert Opin Biol Ther. 2015;15:949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dunac A, Frelin C, Popolo-Blondeau M, et al. Neurological and functional recovery in human stroke are associated with peripheral blood CD34+ cell mobilization. J Neurol. 2007;254:327–332. [DOI] [PubMed] [Google Scholar]
- 52.Bhatt VR, Balasetti V,Jasem JA, et al. Central nervous system complications and outcomes after allogeneic hematopoietic stem cell transplantation. Clin Lymph Myel Leuk. 2015;15:606–611. [DOI] [PubMed] [Google Scholar]
- 53.Hilgendorf I, Greinix H, Halter JP, et al. Long-term follow-up after allogeneic stem cell transplantation. Deutsches Ärzteblatt Int. 2015;112:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kasahara Y, Yamahara K, Soma T, et al. Transplantation of hematopoietic stem cells: intra-arterial versus intravenous administration impacts stroke outcomes in a murine model. Transl Res. 2016;176:69–80. [DOI] [PubMed] [Google Scholar]
- 55.Hsiao HH, Huang HL, Wang HC, et al. Acute cerebral infarct with elevated factor VIII level during the thrombocytopenic stage after hematopoietic stem cell transplant. Exp Clin Transplant. 2014;12:171–172. [PubMed] [Google Scholar]
- 56.Banerjee S, Bentley P, Hamady M, et al. Intra-arterial immunoselected CD34+ stem cells for acute ischemic stroke. Stem Cells Transl Med. 2014;3:1322–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith HK, Omura S, Vital SA, et al. Metallothionein I as a direct link between therapeutic hematopoietic stem/progenitor cells and cerebral protection in stroke. Fed Am Soc Exp Biol J. 2018;32(5):2381–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fang M, Barman PK, Thiruppathi M, et al. Oxidant signaling mediated by Nox2 in neutrophils promotes regenerative myelopoiesis and tissue recovery following ischemic damage. J Immunol. 2018;201(8):2414–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Borlongan CV. Bone marrow stem cell mobilization in stroke: a ‘bonehead’ may be good after all!. Leukemia. 2011;25:1674–1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mafi R, Hindocha S, Mafi P, et al. Sources of adult mesenchymal stem cells applicable for musculoskeletal applications-a systematic review of the literature. Open Orthop J. 2011;5:242–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li G, Yu F, Lei T, et al. Bone marrow mesenchymal stem cell therapy in ischemic stroke: mechanisms of action and treatment optimization strategies. Neural Regener Res. 2016;11:1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang YL, Lin SP, Nelli SR, et al. Self-assembled peptide-based hydrogels as scaffolds for proliferation and multi-differentiation of mesenchymal stem cells. Macromol Biosci. 2016;17:4. [DOI] [PubMed] [Google Scholar]
- 63.Lee JY, Kim E, Choi SM, et al. Microvesicles from brain-extract—treated mesenchymal stem cells improve neurological functions in a rat model of ischemic stroke. Sci Rep. 2016;6:33038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Eckert MA, Vu Q, Xie K, et al. Evidence for high translational potential of mesenchymal stromal cell therapy to improve recovery from ischemic stroke. J Cereb Blood Flow Metab. 2013;33:1322–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chen QH, Liu AR, Qiu HB, et al. Interaction between mesenchymal stem cells and endothelial cells restores endothelial permeability via paracrine hepatocyte growth factor in vitro. Stem Cell Res Ther. 2015;6:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shichinohe H, Ishihara T, Takahashi K, et al. Bone marrow stromal cells rescue ischemic brain by trophic effects and phenotypic change toward neural cells. Neurorehab Neural Repair. 2015;29:80–89. [DOI] [PubMed] [Google Scholar]
- 67.Lai RC, Arslan F, Lee MM, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–222. [DOI] [PubMed] [Google Scholar]
- 68.Kong D, Zhu J, Liu Q, et al. Mesenchymal stem cells protect neurons against hypoxic-ischemic injury via inhibiting parthanatos, necroptosis, and apoptosis, but not autophagy. Cell Mol Neurobiol. 2016;1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Castro-Manrreza ME, Montesinos JJ. Immunoregulation by mesenchymal stem cells: biological aspects and clinical applications. J Immunol Res. 2015;394917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu M, Wang J, Hu X, et al. Study of immunomodulatory function of exosomes derived from human umbilical cord mesenchymal stem cells. Zhonghua Yi Xue Za Zhi. 2015;95:2630–2633. [PubMed] [Google Scholar]
- 71.Chen QQ, Yan L, Wang CZ, et al. Mesenchymal stem cells alleviate TNBS-induced colitis by modulating inflammatory and autoimmune responses. World J Gastroenterol. 2013;19:4702–4717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McGuckin CP, Jurga M, Miller AM, et al. Ischemic brain injury: a consortium analysis of key factors involved in mesenchymal stem cell-mediated inflammatory reduction. Arch Biochem Biophys. 2013;534:88–97. [DOI] [PubMed] [Google Scholar]
- 73.Laranjeira P, Gomes J, Pedreiro S, et al. Human bone marrow-derived mesenchymal stromal cells differentially inhibit cytokine production by peripheral blood monocytes subpopulations and myeloid dendritic cells. Stem Cells Int. 2015;2015 819084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Park HW, Moon HE, Kim HSR, et al. Human umbilical cord blood-derived mesenchymal stem cells improve functional recovery through thrombospondin1, pantraxin3, and vascular endothelial growth factor in the ischemic rat brain. J Neurosci Res. 2015;93:1814–1825. [DOI] [PubMed] [Google Scholar]
- 75.Xin H, Wang F, Li Y, et al. Secondary release of exosomes from astrocytes contributes to the increase in neural plasticity and improvement of functional recovery after stroke in rats treated with exosomes harvested from microRNA 133b-overexpressed multipotent mesenchymal stromal cells. Cell Transplant. 2016;26:243–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhong C, Qin Z, Zhong CJ, et al. Neuroprotective effects of bone marrow stromal cells on rat organotypic hippocampal slice culture model of cerebral ischemia. Neurosci Lett. 2003;342:93–96. [DOI] [PubMed] [Google Scholar]
- 77.Zimmermann JA, Hettiaratchi MH, McDevitt TC. Enhanced immunosuppression of T cells by sustained presentation of bioactive interferon-γ within three-dimensional mesenchymal stem cell constructs. Stem Cells Transl Med. 2017;6:223–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. [DOI] [PubMed] [Google Scholar]
- 79.Duffy MM, Ritter T, Ceredig R, et al. Mesenchymal stem cell effects on T-cell effector pathways. Stem Cell Res Ther. 2011;2:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tolar J, Nauta AJ, Osborn MJ, et al. Sarcoma derived from cultured mesenchymal stem cells. Stem Cells. 2007;25:371–379. [DOI] [PubMed] [Google Scholar]
- 81.McAndrews KM, McGrail DJ, Ravikumar N, et al. Mesenchymal stem cells induce directional migration of invasive breast cancer cells through TGF-β. Sci Rep. 2015;5:16941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Karnoub AE, Dash AB, Vo AP, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–563. [DOI] [PubMed] [Google Scholar]
- 83.Hass R, Kasper C, Böhm S, et al. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Subramanian A, Shu-Uin G, Kae-Siang N, et al. Human umbilical cord Wharton’s jelly mesenchymal stem cells do not transform to tumor-associated fibroblasts in the presence of breast and ovarian cancer cells unlike bone marrow mesenchymal stem cells. J Cell Biochem. 2012;113:1886–1895. [DOI] [PubMed] [Google Scholar]
- 85.Chacko SM, Ahmed S, Selvendiran K, et al. Hypoxic preconditioning induces the expression of prosurvival and proangiogenic markers in mesenchymal stem cells. Am J Physiol Cell Physiol. 2010;299:1562–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wei N, Yu SP, Gu X, et al. Delayed intranasal delivery of hypoxic-preconditioned bone marrow mesenchymal stem cells enhanced cell homing and therapeutic benefits after ischemic stroke in mice. Cell Transplant. 2013;22:977–991. [DOI] [PubMed] [Google Scholar]
- 87.Thom SR, Bhopale VM, Velazquez OC, et al. Stem cell mobilization by hyperbaric oxygen. Am J Physiol Heart Circu Physiol. 2006;290:1378–1386. [DOI] [PubMed] [Google Scholar]
- 88.Pan HC, Chin CS, Yang DY, et al. Human amniotic fluid mesenchymal stem cells in combination with hyperbaric oxygen augment peripheral nerve regeneration. Neurochem Res. 2009;34:1304–1316. [DOI] [PubMed] [Google Scholar]
- 89.Yasuhara T, Matsukawa N, Hara K, et al. Notch-induced rat and human bone marrow stromal cell grafts reduce ischemic cell loss and ameliorate behavioral deficits in chronic stroke animals. Stem Cells Dev. 2009;18:1501–1514. [DOI] [PubMed] [Google Scholar]
- 90.Kurozumi K, Nakamura K, Tamiya T, et al. Mesenchymal stem cells that produce neurotrophic factors reduce ischemic damage in the rat middle cerebral artery occlusion model. Mol Ther. 2005;11:96–104. [DOI] [PubMed] [Google Scholar]
- 91.Horita Y, Honmou O, Harada K, et al. Intravenous administration of glial cell line-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in the adult rat. J Neurosci Res. 2006;84:1495–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Onda T, Honmou O, Harada K, et al. Therapeutic benefits by human mesenchymal stem cells (hMSCs) and Ang-1 gene-modified hMSCs after cerebral ischemia. J Cereb Blood Flow Metab. 2008;28:329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li X, Zheng W, Bai H, et al. Intravenous administration of adipose tissue-derived stem cells enhances nerve healing and promotes BDNF expression via the TrkB signaling in a rat stroke model. Neuropsychiatr Dis Treat. 2016;12:1287–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huang P, Freeman WD, Edenfield BH, Brott TG, Meschia JF, Zubair AC. Safety and efficacy of intraventricular delivery of bone marrow-derived mesenchymal stem cells in hemorrhagic stroke model. Sci Rep. 2019;9:5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kinouchi T, Kitazato KT, Shimada K, et al. Treatment with the PPARc agonist pioglitazone in the early post-ischemia phase inhibits pro-inflammatory responses and promotes neurogenesis via the activation of innate- and bone marrow-derived stem cells in rats. Transl Stroke Res. 2018;9:306–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gao X, Wu D, Dou L, et al. Protective effects of mesenchymal stem cells overexpressing extracellular regulating kinase 1/2 against stroke in rats. Brain Res Bull. 2019;149:42–52. [DOI] [PubMed] [Google Scholar]
- 97.Neal EG, Acosta SA, Kaneko Y, Ji X, Borlongan CV. Regulatory T-cells within bone marrow-derived stem cells actively confer immunomodulatory and neuroprotective effects against stroke. J Cereb Blood Flow Metab. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhao YH, Yuan B, Chen J, et al. Endothelial progenitor cells: therapeutic perspective for ischemic stroke. CNS Neurosci Ther. 2013;19:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hur J, Yoon CH, Kim HS, et al. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288–293. [DOI] [PubMed] [Google Scholar]
- 100.Fadini GP, Losordo D, Dimmeler S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ Res. 2012;110:624–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Takahashi T, Kalka C, Masuda H, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;4:434–438. [DOI] [PubMed] [Google Scholar]
- 102.Tilling L, Chowienczyk P, Clapp B. Progenitors in motion: mechanisms of mobilization of endothelial progenitor cells. Br J Clin Pharmacol. 2009;68:484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Martí-Fàbregas J, Crespo J, Delgado-Mederos R, et al. Endothelial progenitor cells in acute ischemic stroke. Brain Behav. 2013;6:649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Aicher A, Heeschen C, Mildner-Rihm C, et al. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–1376. [DOI] [PubMed] [Google Scholar]
- 105.Peplow PV. Growth factor-and cytokine-stimulated endothelial progenitor cells in post-ischemic cerebral neovascularization. Neural Regener Res. 2014;9:1425–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Balaji S, Han N, Moles C, et al. Angiopoietin-1 improves endothelial progenitor cell–dependent neovascularization in diabetic wounds. Surgery. 2015;158:846–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bai YY, Peng XG, Wang LS, et al. Bone marrow endothelial progenitor cell transplantation after ischemic stroke: an investigation into its possible mechanism. CNS Neurosci Ther. 2015;21:877–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen ZZ, Jiang XD, Zhang LL, et al. Beneficial effect of autologous transplantation of bone marrow stromal cells and endothelial progenitor cells on cerebral ischemia in rabbits. Neurosci Lett. 2008;445:36–41. [DOI] [PubMed] [Google Scholar]
- 109.Li H, Wang W, Wang G, et al. Interferon-γ and tumor necrosis factor-α promote the ability of human placenta–derived mesenchymal stromal cells to express programmed death ligand-2 and induce the differentiation of CD4+ interleukin-10+ and CD8+ interleukin-10+ Treg subsets. Cytotherapy. 2015;17:1560–1571. [DOI] [PubMed] [Google Scholar]
- 110.Shen L, Gao Y, Qian J, et al. The role of SDF-1a/Rac pathway in the regulation of endothelial progenitor cell polarity; homing and expression of Rac1, Rac2 during endothelial repair. Mol Cell Biochem. 2012;365:1–7. [DOI] [PubMed] [Google Scholar]
- 111.Neuwelt EA, Bauer B, Fahlke C, et al. Engaging neuroscience to advance translational research in brain barrier biology. Nat Rev Neurosci. 2011;12:169–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wong A, Ye M, Levy A, et al. The blood-brain barrier: an engineering perspective. Front Neuroeng. 2013;6:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Garbuzova-Davis S, Haller E, Lin R, et al. Intravenously transplanted human bone marrow endothelial progenitor cells engraft within brain capillaries, preserve mitochondrial morphology, and display pinocytotic activity toward blood-brain barrier repair in ischemic stroke rats. Stem Cells. 2017. [DOI] [PMC free article] [PubMed]
- 114.Bai YY, Wang L, Chang D, et al. Synergistic effects of transplanted endothelial progenitor cells and RWJ 67657 in diabetic ischemic stroke models. Stroke. 2015;46:1938–1946. [DOI] [PubMed] [Google Scholar]
- 115.van der Strate BWA, Popa ER, Schipper M, et al. Circulating human CD34+ progenitor cells modulate neovascularization and inflammation in a nude mouse model. J Mol Cell Cardiol. 2007;42:1086–1097. [DOI] [PubMed] [Google Scholar]
- 116.Moubarik C, Guillet B, Youssef B, et al. Transplanted late outgrowth endothelial progenitor cells as cell therapy product for stroke. Stem Cell Rev Rep. 2011;7:208–220. [DOI] [PubMed] [Google Scholar]
- 117.Gao L, Li P, Zhang J, et al. Novel role of kallistatin in vascular repair by promoting mobility, viability, and function of endothelial progenitor cells. J Am Heart Assoc. 2014;3 e001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bayraktutan U Endothelial progenitor cells: Potential novel therapeutics for ischaemic stroke. Pharmacol Res. 2019;144:181–191. [DOI] [PubMed] [Google Scholar]
- 119.Acosta SA, Lee JY, Nguyen H, Kaneko Y, Borlongan CV. Endothelial progenitor cells modulate inflammation-associated stroke vasculome. Stem Cell Rev. 2019;15:256–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Golab-Janowska M, Paczkowska E, Machalinski B, et al. Elevated inflammatory parameter levels negatively impact populations of circulating stem cells (CD133+), early endothelial progenitor cells (CD133+/VEGFR2+), and fibroblast growth factor in stroke patients. Curr Neurovasc Res. 2019;6:19–26. [DOI] [PubMed] [Google Scholar]
- 121.Nakayama T, Nagata E, Masuda H, Asahara T, Takizawa S. Regeneration-associated cell transplantation contributes to tissue recovery in mice with acute ischemic stroke. Public Library Sci One. 2019;14 e0210198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shimomura R, Nezu T, Hosomi N, et al. Alpha-2-macroglobulin as a promising biological marker of endothelial function. J Atheroscler Thromb. 2018;25:350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kucia M, Halasa M, Wysoczynski M, et al. Morphological and molecular characterization of novel population of CXCR4+ SSEA-4+ Oct-4+ very small embryonic-like cells purified from human cord blood–preliminary report. Leukemia. 2007;21:297–303. [DOI] [PubMed] [Google Scholar]
- 124.Kassmer SH, Krause DS. Very small embryonic-like cells: Biology and function of these potential endogenous pluripotent stem cells in adult tissues. Mol Reprod Dev. 2013;80:677–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ratajczak MZ, Shin DM, Liu R. et al. Very small embryonic/epiblast-like stem cells (VSELs) and their potential role in aging and organ rejuvenation–an update and comparison to other primitive small stem cells isolated from adult tissues. Aging. 2012;4:235–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Grymula K, Tarnowski M, Piotrowska K, et al. Evidence that the population of quiescent bone marrow-residing very small embryonic/epiblast-like stem cells (VSELs) expands in response to neurotoxic treatment. J Cell Mol Med. 2014;18:1797–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Havens AM, Sun H, Shiozawa Y, et al. Human and murine very small embryonic-like cells represent multipotent tissue progenitors, in vitro and in vivo. Stem Cells Dev. 2013;23:689–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kucia M, Reca R, Campbell FR, et al. A population of very small embryonic-like (VSEL) CXCR4+ SSEA-1+ Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006;20:857–869. [DOI] [PubMed] [Google Scholar]
- 129.Bhartiya D, Shaikh A, Anand S, et al. Endogenous, very small embryonic-like stem cells: critical review, therapeutic potential and a look ahead. Hum Reprod. 2016;23:41–76. [DOI] [PubMed] [Google Scholar]
- 130.Zuba-Surma EK, Guo Y, Taher H, et al. Transplantation of expanded bone marrow-derived very small embryonic-like stem cells (VSEL-SCs) improves left ventricular function and remodelling after myocardial infarction. J Cell Mol Med. 2011;15:1319–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Paczkowska E, Kucia M, Koziarska D, et al. Clinical evidence that very small embryonic-like stem cells are mobilized into peripheral blood in patients after stroke. Stroke. 2009;40:1237–1244. [DOI] [PubMed] [Google Scholar]
- 132.Guerin CL, Loyer X, Vilar J, et al. Bone-marrow-derived very small embryonic-like stem cells in patients with critical leg ischaemia: evidence of vasculogenic potential. J Thromb Haemost. 2015;113:1084–1094. [DOI] [PubMed] [Google Scholar]
- 133.Lenkiewicz AM, Adamiak M, Thapa A, et al. The Nlrp3 inflammasome orchestrates mobilization of bone marrow-residing stem cells into peripheral blood. Stem Cell Rev Rep. 2019;15:391–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Eljaszewicz A, Kleina K, Grubczak K, et al. Elevated numbers of circulating very small embryonic-like stem cells (VSELs) and intermediate CD14++CD16+ monocytes in IgA nephropathy. Stem Cell Rev Rep. 2018;14:686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Shi X, Yan C, Liu B, et al. miR-381 regulates neural stem cell proliferation and differentiation via regulating Hes1 expression. Public Library Sci One. 2015;10 e0138973. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 136.Zhang RL, Chopp M, Roberts C, et al. Stroke increases neural stem cells and angiogenesis in the neurogenic niche of the adult mouse. Public Library Sci One. 2014;9 e113972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Santilli G, Lamorte G, Carlessi L, et al. Mild hypoxia enhances proliferation and multipotency of human neural stem cells. Public Library Sci One. 2010;5 e8575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cai M, Zhou Y, Zhou B, et al. Hypoxic conditioned medium from rat cerebral cortical cells enhances the proliferation and differentiation of neural stem cells mainly through PI3-K/Akt pathways. Public Library Sci One. 2014;9 e111938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang P, Li J, Liu Y, et al. Human embryonic neural stem cell transplantation increases subventricular zone cell proliferation and promotes peri-infarct angiogenesis after focal cerebral ischemia. Neuropathology. 2011;31:384–391. [DOI] [PubMed] [Google Scholar]
- 140.Zhang Y, Liu J, Yao S, et al. Nuclear factor kappa B signaling initiates early differentiation of neural stem cells. Stem Cells. 2012;30:510–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Topchiy E, Panzhinskiy E, Griffin WST, et al. Nox4-generated superoxide drives angiotensin II-induced neural stem cell proliferation. Dev Neurosci. 2013;35:293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chou CH, Sinden JD, Couraud PO, et al. In vitro modeling of the neurovascular environment by coculturing adult human brain endothelial cells with human neural stem cells. Public Library Sci One. 2014;9 e106346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lu P, Jones LL, Snyder EY, et al. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003;181:115–129. [DOI] [PubMed] [Google Scholar]
- 144.Takata M, Nakagomi T, Kashiwamura S, et al. Glucocorticoid-induced TNF receptor-triggered T cells are key modulators for survival/death of neural stem/progenitor cells induced by ischemic stroke. Cell Death Differ. 2012;19:756–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lee ST, Chu K, Jung KH, et al. Anti-inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain. 2007;131:616–629. [DOI] [PubMed] [Google Scholar]
- 146.Huang L, Wong S, Snyder EY, et al. Human neural stem cells rapidly ameliorate symptomatic inflammation in early-stage ischemic-reperfusion cerebral injury. Stem Cell Res Ther. 2014;5:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Yu X, Wang X, Zeng S, Tuo X. Protective effects of primary neural stem cell treatment in ischemic stroke models. Exp Ther Med. 2018;16:2219–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang G, Chen L, Chen W, et al. Neural stem cells alleviate inflammation via neutralization of IFN-γ negative effect in ischemic stroke model. J Biomed Nanotechnol. 2018;14:1178–1188. [DOI] [PubMed] [Google Scholar]
- 149.Zhang G, Guo X, Chen L, et al. Interferon-γ promotes neuronal repair by transplanted neural stem cells in ischemic rats. Stem Cells Dev. 2018;27:355–366. [DOI] [PubMed] [Google Scholar]
- 150.Webb RL, Kaiser EE, Scoville SL, et al. Human neural stem cell extracellular vesicles improve tissue and functional recovery in the murine thromboembolic stroke model. Transl Stroke Res. 2018;9:530–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Jones BJ, Brooke G, Atkinson K, et al. Immunosuppression by placental indoleamine 2, 3-dioxygenase: a role for mesenchymal stem cells. Placenta. 2007;28:1174–1181. [DOI] [PubMed] [Google Scholar]
- 152.Park DH, Borlongan CV, Willing AE, et al. Human umbilical cord blood cell grafts for brain ischemia. Cell Transplant. 2009;18:985–998. [DOI] [PubMed] [Google Scholar]
- 153.Chen J, Shehadah A, Pal A, et al. Neuroprotective effect of human placenta-derived cell treatment of stroke in rats. Cell Transplant. 2013;22:871–879. [DOI] [PubMed] [Google Scholar]
- 154.Iskander A, Knight RA, Zhang ZG, et al. Intravenous administration of human umbilical cord blood-derived AC133+ endothelial progenitor cells in rat stroke model reduces infarct volume: magnetic resonance imaging and histological findings. Stem Cells Transl Med. 2013;2:703–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hocum Stone LL, Xiao F, Rotschafer J, et al. Amelioration of ischemic brain injury in rats with human umbilical cord blood stem cells: mechanisms of action. Cell Transpl. 2016;8:1473–1488. [DOI] [PubMed] [Google Scholar]
- 156.Wang M, Yang Y, Yang D, et al. The immunomodulatory activity of human umbilical cord blood-derived mesenchymal stem cells in vitro. Immunology. 2009;126:220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Najar M, Raicevic G, Boufker HI, et al. Adipose-tissue-derived and Wharton’s jelly–derived mesenchymal stromal cells suppress lymphocyte responses by secreting leukemia inhibitory factor. Tissue Eng. 2010;16:3537–3546. [DOI] [PubMed] [Google Scholar]
- 158.Kurtzberg J, Lyerly AD, Sugarman J. Untying the Gordian knot: policies, practices, and ethical issues related to banking of umbilical cord blood. J Clin Invest. 2005;115:2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chao K, Zhang S, Qiu Y, et al. Human umbilical cord-derived mesenchymal stem cells protect against experimental colitis via CD5+ B regulatory cells. Stem Cell Res Ther. 2016;7:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Vellasamy S, Tong CK, Azhar NA, et al. Human mesenchymal stromal cells modulate T-cell immune response via transcriptomic regulation. Cytotherapy. 2016;18:1270–1283. [DOI] [PubMed] [Google Scholar]
- 161.Lim JY, Jeong CH, Jun JA, et al. Therapeutic effects of human umbilical cord blood-derived mesenchymal stem cells after intrathecal administration by lumbar puncture in a rat model of cerebral ischemia. Stem Cell Res Ther. 2011;2:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liang L, Li Z, Ma T, et al. Transplantation of human placenta derived mesenchymal stem cell alleviates critical limb ischemia in diabetic nude rat. Cell Transplant. 2016;26:45–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhilai Z, Biling M, Sujun Q et al. Preconditioning in lowered oxygen enhances the therapeutic potential of human umbilical mesenchymal stem cells in a rat model of spinal cord injury. Brain Res. 2016;1642:426–435. [DOI] [PubMed] [Google Scholar]
- 164.Pimentel-Coelho PM, Rosado-de-Castro PH, da Fonseca LMB, et al. Umbilical cord blood mononuclear cell transplantation for neonatal hypoxic-ischemic encephalopathy. Pediatr Res. 2012;71:464–473. [DOI] [PubMed] [Google Scholar]
- 165.Cutler AJ, Limbani V, Girdlestone J, et al. Umbilical cord-derived mesenchymal stromal cells modulate monocyte function to suppress T cell proliferation. J Immunol. 2010;185:6617–6623. [DOI] [PubMed] [Google Scholar]
- 166.Che N, Li X, Zhou S, et al. Umbilical cord mesenchymal stem cells suppress B-cell proliferation and differentiation. Cell Immunol. 2012;274:46–53. [DOI] [PubMed] [Google Scholar]
- 167.Kranz A, Wagner DC, Kamprad M, et al. Transplantation of placenta-derived mesenchymal stromal cells upon experimental stroke in rats. Brain Res. 2010;1315:128–136. [DOI] [PubMed] [Google Scholar]
- 168.Laskowitz DT, Bennett ER, Durham RJ, et al. Allogeneic umbilical cord blood infusion for adults with ischemic stroke: clinical outcomes from a phase I safety study. Stem Cells Transl Med. 2018;7:521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Evans MA, Broughton BRS, Drummond GR, et al. Amnion epithelial cells – a novel therapy for ischemic stroke?. Neural Regener Res. 2018;13:1346–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Shiao ML, Yuan C, Crane AT, et al. Immunomodulation with human umbilical cord blood stem cells ameliorates ischemic brain injury – a brain transcriptome profiling analysis. Cell Transplant. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hwang S, Choi J, Kim M. Combining human umbilical cord blood cells with erythropoietin enhances angiogenesis/neurogenesis and behavioral recovery after stroke. Front Neurol. 2019;10:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhai L, Maimaitiming Z, Cao X, et al. Nitrogen-doped carbon nanocages and human umbilical cord mesenchymal stem cells cooperatively inhibit neuroinflammation and protect against ischemic stroke. Neurosci Lett. 2019;708 134346. [DOI] [PubMed] [Google Scholar]
- 173.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. [DOI] [PubMed] [Google Scholar]
- 174.Cai J, Li W, Su H, et al. Generation of human induced pluripotent stem cells from umbilical cord matrix and amniotic membrane mesenchymal cells. J Biol Chem. 2010;285:11227–11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Tat PA, Sumer H, Jones KL, et al. The efficient generation of induced pluripotent stem (iPS) cells from adult mouse adipose tissue-derived and neural stem cells. Cell Transplant. 2010;19:525–536. [DOI] [PubMed] [Google Scholar]
- 176.Chen SJ, Chang CM, Tsai SK, et al. Functional improvement of focal cerebral ischemia injury by subdural transplantation of induced pluripotent stem cells with fibrin glue. Stem Cells Dev. 2010;19:1757–1767. [DOI] [PubMed] [Google Scholar]
- 177.Jiang M, Lv L, Ji H, et al. Induction of pluripotent stem cells transplantation therapy for ischemic stroke. Mol Cell Biochem. 2011;354:67–75. [DOI] [PubMed] [Google Scholar]
- 178.Eckert A, Huang L, Gonzalez R, et al. Bystander effect fuels human induced pluripotent stem cell-derived neural stem cells to quickly attenuate early stage neurological deficits after stroke. Stem Cells Transl Med. 2015;4:841–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Muffat J, Li Y, Yuan B, et al. Efficient derivation of microglia-like cells from human pluripotent stem cells. Nat Res. 2016;11:1358–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Haque M, Song J, Fino K, et al. Stem cell-derived tissue-associated regulatory T cells ameliorate the development of autoimmunity. Sci Rep. 2016;6:20588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yamashita T, Kawai H, Tian F, et al. Tumorigenic development of induced pluripotent stem cells in ischemic mouse brain. Cell Transplant. 2011;20:883–891. [DOI] [PubMed] [Google Scholar]
- 182.Zhao T, Zhang ZN, Rong Z, et al. Immunogenicity of induced pluripotent stem cells. Nature. 2011;474:212–215. [DOI] [PubMed] [Google Scholar]
- 183.Kokaia Z, Llorente IL, Carmichael ST. Customized brain cells for stroke patients using pluripotent stem cells. Stroke. 2018;49:1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ould-Brahim F, Sarma SN, Syal C, et al. Metformin preconditioning of human induced pluripotent stem cell-derived neural stem cells promotes their engraftment and improves post-stroke regeneration and recovery. Stem Cells Dev. 2018;27:1085–1096. [DOI] [PubMed] [Google Scholar]
- 185.Ping Yu S, Tung JK, Wei ZZ, et al. Optochemogenetics stimulation of transplanted iPS-NPCs enhances neuronal repair and functional recovery after ischemic stroke. J Neurosci. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ou J, Ball JM, Luan Y, et al. IPSCs from a hibernator provide a platform for studying cold adaptation and its potential medical applications. Cell. 2018;17:851–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rieker C, Migliavacca E, Vaucher A, et al. Apolipoprotein E4 expression causes gain of toxic function in isogenic human induced pluripotent stem cell-derived endothelial cells. Arterioscler Thromb Vasc Biol. 2019. [DOI] [PubMed]
- 188.Borlongan CV, Lind JG, Dillon-Carter O, et al. Bone marrow grafts restore cerebral blood flow and blood brain barrier in stroke rats. Brain Res. 2004;1010:108–116. [DOI] [PubMed] [Google Scholar]
- 189.Bang OY, Lee JS, Lee PH, et al. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–882. [DOI] [PubMed] [Google Scholar]
- 190.Savitz SI, Misra V, Kasam M, et al. Intravenous autologous bone marrow mononuclear cells for ischemic stroke. Ann Neurol. 2011;70:59–69. [DOI] [PubMed] [Google Scholar]
- 191.Prasad K, Sharma A, Garg A, et al. Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke. Stroke. 2014;45:3618–3624. [DOI] [PubMed] [Google Scholar]
- 192.Steinberg GK, Kondziolka D, Wechsler LR, et al. Clinical outcomes of transplanted modified bone marrow-derived mesenchymal stem cells in stroke: a phase 1/2a study. Stroke. 2016;47:1817–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Lee JS, Hong JM, Moon GJ, et al. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010;28:1099–1106. [DOI] [PubMed] [Google Scholar]
- 194.Hess DC, Wechsler LR, Clark WM, et al. Safety and efficacy of multipotent adult progenitor cells in acute ischaemic stroke (MASTERS): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol. 2017;16:360–368. [DOI] [PubMed] [Google Scholar]
- 195.Kalladka D, Sinden J, Pollock K, et al. Human neural stem cells in patients with chronic ischaemic stroke (PISCES): A phase 1, first-in-man study. The Lancet. 2016;388:787–796. [DOI] [PubMed] [Google Scholar]
- 196.Borlongan CV, Chopp M, Steinberg GK, et al. Potential of stem/progenitor cells in treating stroke: the missing steps in translating cell therapy from laboratory to clinic. Regen Med. 2008;3:249–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Diamandis T, Borlongan CV. One, two, three steps toward cell therapy for stroke. Stroke. 2015;46:588–591. [DOI] [PMC free article] [PubMed] [Google Scholar]