Abstract
This study aimed to compare the effectiveness of 0.12% chlorhexidine alone and 0.12% chlorhexidine in combination with toothbrushing to prevent ventilator-associated pneumonia (VAP) in mechanically ventilated patients.
The Embase, Latin American and Caribbean Health Science Literature, PubMed, Scientific Electronic Library Online, Scopus, LIVIVO, Web of Science, Cochrane Library, OpenThesis, and Open Access Thesis and Dissertations databases were used. Only randomized controlled trials without restrictions on the year or language of publication were included. Two reviewers assessed the risk of bias using the Joanna Briggs Institute Critical Appraisal Tool. A meta-analysis using a random-effects model estimated the combined relative risk (RR). The Grading of Recommendations, Assessment, Development and Evaluations approach was used to assess the certainty of the evidence.
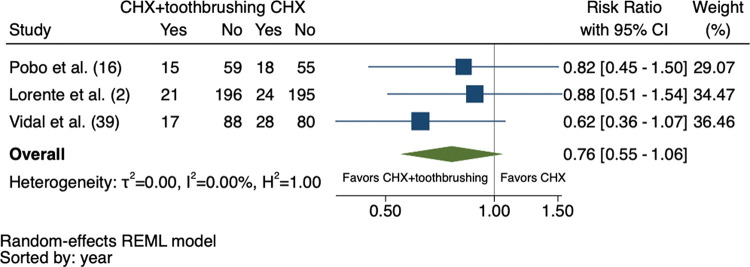
Initially, 2,337 studies were identified, of which 4 were considered in the systematic review and 3 in the meta-analysis (total sample: 796 patients). The studies were published between 2009 and 2017. All eligible studies had a low risk of bias. The meta-analysis revealed that the risk of VAP was 24% lower in patients receiving chlorhexidine combined with toothbrushing than in those receiving chlorhexidine alone (RR: 0.76; 95% confidence interval: 0.55-1.06), with moderate certainty of evidence and without statistical significance.
In conclusion, considering the limitations of this study, a standard protocol for the prevention of VAP is not yet recommended. More studies with larger sample sizes are needed to draw strong conclusions. However, considering that toothbrushing is a simple intervention, it should be a common practice in mechanically ventilated patients, especially among patients with coronavirus disease.
Keywords: Chlorhexidine, COVID-19, Tooth Brushing, Ventilator-Associated Pneumonia
INTRODUCTION
Ventilator-associated pneumonia (VAP) is defined as pneumonia occurring more than 48h after the onset of mechanical ventilation (1). It affects 10%-20% of patients who receive mechanical ventilation for more than 48h. VAP is diagnosed based on the following criteria: presence of purulent sputum, fever (>38°C) or hypothermia (<35.5°C), leukocytosis (>10,000 mm3) or leukopenia (<4,000 mm3), positive bacterial culture of respiratory secretions (>106 cfu/mL), and radiography showing additional or progressive pulmonary infiltrates (2).
Several risk factors are associated with VAP, such as older age, male sex, increased time on mechanical ventilation, sedation, heart and lung disease, regurgitation, aspiration, prior antibiotic therapy, and invasive operations (3). Burns are also a risk factor of VAP through pulmonary inflammation resulting from direct lung injury or systemic immune dysfunction (4). Genetic polymorphisms related to inflammatory mediators may also increase the risk of developing VAP, possibly because of an ineffective response to bacteria (5).
Currently, the global pandemic against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in a higher frequency of patients requiring invasive mechanical ventilation (6). Likewise, prone positioning, heavy sedation, and treatment with neuromuscular blockers, in addition to clear evidence of prolonged immunosuppression, including deep lymphopenia, represent a risk for acquiring secondary infections, including VAP (6,7). VAP is a complication in patients hospitalized for coronavirus disease (COVID-19) (8 -10).
Oral hygiene using a variety of procedures is an important measure to prevent VAP (11). For instance, aspiration of secretions, toothbrushing, or dental and mucosal cleansing with chlorhexidine (CHX) may reduce the risk of VAP (12). CHX is a cationic biguanide that binds to the bacterial cell walls, thus impairing and even perforating phospholipid membranes (13,14). The effect may be bacteriostatic or bactericidal depending on the concentration of the product (15). Its use for oral hygiene in patients under mechanical ventilation reduces the risk of VAP (16-19). As a mouthwash, CHX reduces bacterial colonization in the oral cavity (20,21). However, the presence of a biofilm on the surface of the teeth limits the action of any mouthwash (22). Thus, prior mechanical disruption of dental biofilms through toothbrushing improves the effect of CHX (23-25) and, hence, prevents VAP (25 -27).
High CHX concentrations have been associated with adverse effects (28). Dental discoloration and oral mucosa irritation were attributed to the use of 0.2% and 2% CHX (29). Lesions in the oral mucosa, such as erosive lesions, ulcerations, white/yellow plaque formation, and mucosal bleeding, have been observed in patients admitted in the intensive care units (30). By contrast, when 0.12% CHX was applied, it was effective in preventing VAP in surgical patients (31).
This study aimed to compare the reduction in the risk of VAP between the use of oral 0.12% CHX combined with toothbrushing and use of 0.12% CHX alone in the prevention of VAP through systematic review and meta-analysis.
METHODS
Protocol and registration
This systematic review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) recommendations (32) and Cochrane guidelines (33). It was recorded in the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42020168844) (https://www.crd.york.ac.uk/PROSPERO/).
Study design and eligibility criteria
This systematic review with meta-analysis was conducted based on the patient, intervention, comparison, outcome strategy and aimed at answering the following review question: “Is toothbrushing combined with the use of 0.12% CHX (intervention) more effective in preventing VAP (outcome) among patients under mechanical ventilation (population) than using CHX alone (comparison)?”
Randomized controlled trials that compared oral hygiene using 0.12% CHX with or without toothbrushing in adult patients (aged >18 years) under invasive (tracheal) mechanical ventilation were included in the study. There were no restrictions on the year, language, or publication status (published, accepted/ahead of print articles). Studies not related to the objective of the present study, non-original works (review articles, editorials, and books/book chapters), or papers with insufficient data (letters, personal opinions, and conference abstracts) were excluded.
Sources of information and search
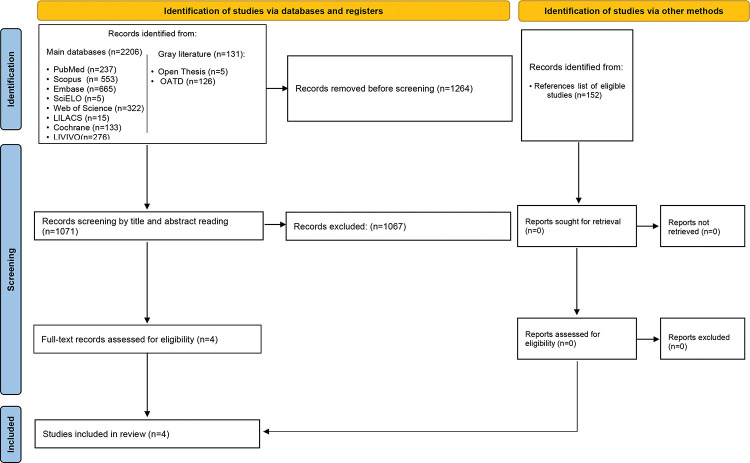
The primary sources of studies were PubMed (including MedLine), Scopus, Embase, Scientific Electronic Library Online, Web of Science, Latin-American and Caribbean Health Sciences Literature, Cochrane, and LIVIVO databases. The Open Access Thesis and Dissertations and OpenGrey databases allowed access to the “gray literature” to avoid bias regarding the lack of published negative results (Figure 1). The sources of search descriptors were the Medical Subject Headings, Health Sciences Descriptors, and Emtree. Several combinations of the Boolean operators “AND” and “OR” enhanced the search strategy, as detailed in Table 1. The search terms were adapted to each database. Bibliographic research was performed until November 2019. The results obtained were imported to the software EndNote Web™ (Thomson Reuters, Toronto, Canada) and then into Microsoft Word™ 2010 (Microsoft™ Ltd., Washington, USA) for the automatic and conventional removal of duplicates, respectively.
Figure 1. Flow chart of the study selection process.
Table 1. Electronic databases and applied search strategy.
| Database | Search strategy (April, 2020) |
|---|---|
|
PubMed
https://www.ncbi.nlm.nih.gov/pubmed |
((“Pneumonia, Ventilator-Associated” OR “Pneumonia, Ventilator-Associated” OR “Ventilator Pneumonia Associated” OR “Ventilator-Associated Pneumonia”) AND (“Chlorhexidine” OR “Chlorhexidine Gluconate”)) |
|
Scopus
http://www.scopus.com/ |
((“Pneumonia, Ventilator Associated” OR “Pneumonia, Ventilator Associated” OR “Ventilator Pneumonia Associated” OR “Ventilator-Associated Pneumonia”) AND (“Chlorhexidine” OR “Chlorhexidine Gluconate”)) |
|
Embase
http://www.embase.com/ |
(“Pneumonia, Ventilator Associated” OR “Pneumonia, Ventilator Associated”/Exp OR “Pneumonia, Ventilator-Associated” OR “Ventilator Pneumonia Associated” OR “Ventilator-Associated Pneumonia”/Exp OR “Ventilator-Associated Pneumonia”) AND (“Chlorhexidine”/Exp OR “Chlorhexidine” OR “Chlorhexidine Gluconate”/Exp OR “Chlorhexidine Gluconate”) |
|
SciELO
www.scielo.org |
“Pneumonia Ventilator Associated” AND “Chlorhexidine” |
|
Web of Science
http://apps.webofknowledge.com/ |
((“Pneumonia, Ventilator Associated” OR “Pneumonia, Ventilator Associated” OR “Ventilator Pneumonia Associated” OR “Ventilator-Associated Pneumonia”) AND (“Chlorhexidine” OR “Chlorhexidine Gluconate”)) |
|
LILACS
lilacs.bvsalud.org |
tw:(tw:(“Pneumonia Ventilator Associated” AND “Chlorhexidine”) AND (db:(“LILACS”))) |
|
Cochrane
https://www.cochranelibrary.com/ |
((“Pneumonia, Ventilator Associated” OR “Pneumonia, Ventilator Associated” OR “Ventilator Pneumonia Associated” OR “Ventilator-Associated Pneumonia”) AND (“Chlorhexidine” OR “Chlorhexidine Gluconate”)) |
|
LIVIVO
https://www.livivo.de/app |
((“Pneumonia, Ventilator Associated” OR “Pneumonia, Ventilator Associated” OR “Ventilator Pneumonia Associated” OR “Ventilator-Associated Pneumonia”) AND (“Chlorhexidine” OR “Chlorhexidine Gluconate”)) |
|
OpenThesis
http://www.openthesis.org/ |
((“Pneumonia, Ventilator Associated” OR “Pneumonia, Ventilator Associated” OR “Ventilator Pneumonia Associated” OR “Ventilator-Associated Pneumonia”) AND (“Chlorhexidine” OR “Chlorhexidine Gluconate”)) |
|
Open Access Thesis and Dissertations
https://oatd.org/ |
((“Pneumonia, Ventilator Associated” OR “Pneumonia, Ventilator Associated” OR “Ventilator Pneumonia Associated” OR “Ventilator-Associated Pneumonia”) AND (“Chlorhexidine” OR “Chlorhexidine Gluconate”)) |
Study selection
Two independent reviewers (PUJS and DMS) previously calibrated 20% of the studies and reached an acceptable inter-examiner agreement (kappa>0.81). Then, these reviewers independently performed the eligibility review, with disagreements resolved by discussion with a third reviewer (LRP) until consensus was reached.
The study selection was performed in two stages. First, the analysis of the titles and abstracts (when available) led to the exclusion of articles not related to the topic of the present review. The second stage involved the evaluation of the full text of the remaining studies to verify their adherence to the eligibility criteria. In both stages, the reviewers had access to the names of the authors and journals. A thorough verification of the references of the eligible articles was performed to identify studies overlooked in the initial search. The excluded studies were registered separately, along with the reasons for exclusion. If any article could not be recovered, other study centers were contacted to retrieve the articles in their libraries. In the case of studies published in languages other than English or Portuguese, the full text was translated.
Data collection
Two reviewers (PUJS and DMS) examined the selected papers to collect the following information: identification (author, year, and country of the research), sample features (number of patients, sex distribution, mean age, and Acute Physiology and Chronic Health Evaluation score) (34,35), and the main results (ventilation time, microbiota assessment, VAP incidence, mortality, and conclusions). The corresponding authors were contacted by email (up to three times over two weeks) to obtain relevant information on missing or unclear data.
To ensure consistency, the reviewers (PUJS and DMS) extracted the information jointly from an eligible study. These reviewers discussed to resolve initial discrepancies, and a third reviewer (LRP) made a final decision in case of persistent disagreement.
Risk of individual bias of the studies
The Joanna Briggs Institute (JBI) Critical Appraisal Tools for use in JBI Systematic Reviews for randomized controlled trials (36) were utilized to assess the risk of bias and individual quality of the selected studies. Two reviewers (PUJS and DMS) independently judged each domain regarding their potential risk of bias, as recommended by the PRISMA statement (32). The percentage of “yes” answers to the questions on the assessment tool used in each study was rated as follows: the risk of bias was high, moderate, or low when the study obtained 49%, 50%-68%, or more than 69% “yes” answers, respectively.
Summary measures and synthesis of results (meta-analysis)
The statistical analyses included eligible studies that provided sufficient data to calculate the relative risk (RR) of VAP in patients who received 0.12% CHX combined with toothbrushing compared with those who received 0.12% CHX alone. A meta-analysis using a random-effects model estimated the combined RRs. Three measures of heterogeneity were estimated: the τ2 statistic is related to the between-study variance, I2 reflects the percentage of variability caused by heterogeneity excluding sampling error, and H2 indicates the between-study level of heterogeneity (H2=1 indicating homogeneity). The statistical significance level was 5%.
Certainty of evidence
The Grading of Recommendation, Assessment, Development, and Evaluation (GRADE) tool with GRADE Pro GDT software (http://gdt.guidelinedevelopment.org) (37) was used to assess the certainty of evidence and strength of recommendation. The basis for this assessment was the study design, risk of bias, inconsistency, indirect evidence, imprecision, and publication bias. The level of certainty among the identified evidence was characterized as high, moderate, low, or very low (37).
RESULTS
Study selection
The first phase of the study selection revealed a total of 2,337 works. The “gray literature” did not disclose any studies related to the objective of this systematic review. After discarding duplicates, 1,071 papers remained for title and abstract screening. After a detailed analysis, only four studies were eligible for full-text review. The references from these studies did not reveal additional articles of interest; after reading the full text, the qualitative analysis did not exclude any of the four selected studies. Figure 1 presents the process of search, identification, inclusion, and exclusion of studies.
Study characteristics of eligible studies
The studies were published between 2009 and 2017, and the patients from the United States (16), Spain (2,38), and Brazil (39) were included in these studies. Overall, 988 patients were included in the analysis. Sources of information on the demographic and clinical characteristics of the population are presented in Table 2. The Ethics Committee of their respective institution or hospital approved all selected studies, which also provided informed consent before patient recruitment. None of the studies followed the Consolidated Standards of Reporting Trials statement. Half of the selected studies reported calibration among nurses (38) and dentists (39) who performed oral hygiene procedures. Two studies (38,39) presented the registration number of randomized controlled clinical trials.
Table 2. Summary of the main characteristics of the eligible studies (all were randomized clinical trials with previous ethical clearance and application of informed consent, with patients receiving mechanical ventilation for more than 48 hours without pneumonia at baseline).
| Author (year) | Country | Participants | Groups | Sex | Age: Mean (SD) | APACHE, [type]: Mean (SD) [26,27] |
|---|---|---|---|---|---|---|
| Munro et al. (16) | United States | 537 patients | [APACHE III] | |||
| Intervention 1: Toothbrushing (three times daily) | M: 28 F: 21 |
47.9 (17.5) | 76.4 (23.3) | |||
| Intervention 2: Toothbrushing (three times daily) + 0.12% CHX (twice daily) | M: 28 F: 20 |
47.3 (18.8) | 76.2 (25.5) | |||
| Control 1: 0.12% CHX/swab (twice daily) | M: 26 F: 18 |
46.1 (18.2) | 80.4 (28.7) | |||
| Control 2: usual care (NR) | M: 37 F: 14 |
46.8 (16.4) | 76.2 (3.3) | |||
| Pobo et al. (38) | Spain | 147 patients | Intervention: Standard care + toothbrushing (three times daily) | M: 49 F: 25 |
55.3 (17.9) | [APACHE II] 18.8 (7.1) |
| Control: Standard care (gauze containing 20 mL of 0.12% CHX applied to teeth, tongue, and the mucosal surface + 10 mL of 0.12% CHX digluconate was injected into the oral cavity (three times daily) | M: 46 F: 27 |
52.6 (17.2) | 18.7 (7.3) | |||
| Lorente et al. (2) | Spain | 436 patients | Intervention: 0.12% CHX‐impregnated gauze + toothbrushing of the teeth with 0.12% CHX (three times daily) | M: 146 F: 71 |
61 (15.6) | [APACHE II] 17.88 (8.84) |
| Control: 0.12% CHX‐impregnated gauze and oral cavity injection only (three times daily) | M: 145 F: 74 |
60.4 (16.6) | 19.16 (9.88) | |||
| Vidal et al. (39) | Brazil | 213 patients | Intervention: toothbrushing + 0.12% CHX (twice daily) | M: 51 F: 54 |
59.4 (14.5) | [APACHE II] 21.9 (7.5) |
| Control: swab + 0.12% CHX (twice daily) | M: 54 F: 54 |
63.2 (14.5) | 22.2 (7.7) |
SD, standard deviation; APACHE, Acute Physiology and Chronic Health Evaluation; M, male; F, female; CHX, chlorhexidine.
Risk of individual bias
Table 3 shows the risk of bias and individual quality of the selected studies (2,16,38,39). One study (2) did not provide details regarding the randomization procedure. None of study was blinded because the participants were admitted to the intensive care units under invasive mechanical ventilation in an unconscious state. Questions 5 and 6 were answered as “unclear” in three studies (2,16,38) because it was not clear if those applying the treatment were aware of the allocation of the participants. The answer to question 7 was considered “no” in two studies (16,39) because the groups were not treated identically according to the intervention of interest.
Table 3. Risk of bias assessed by the Joanna Briggs Institute Critical Appraisal Tools for use in JBI Systematic Reviews for randomized clinical trial studies.
| Authors | Q.1 | Q.2 | Q.3 | Q.4 | Q.5 | Q.6 | Q.7 | Q.8 | Q.9 | Q.10 | Q.11 | Q.12 | Q.13 | % yes/risk |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Munro et al. (16) | √ | √ | √ | N/A | U | U | -- | √ | √ | √ | √ | √ | √ | 69.2%/low risk of bias |
| Pobo et al. (38) | √ | √ | √ | N/A | U | U | -- | √ | √ | √ | √ | √ | √ | 69.2%/low risk of bias |
| Lorente et al. (2) | √ | U | √ | N/A | U | U | √ | √ | √ | √ | √ | √ | √ | 69.2%/low risk of bias |
| Vidal et al. (39) | √ | √ | √ | N/A | √ | √ | √ | √ | √ | √ | √ | √ | √ | 92.3%/low risk of bias |
Q.1: Was true randomization used for the assignment of participants to treatment groups? Q.2: Was allocation to treatment groups concealed? Q.3: Were treatment groups similar at baseline? Q.4: Were participants blinded to the treatment assignment? Q.5: Were those who delivered treatment blinded to the treatment assignment? Q.6: Were outcome assessors blinded to the treatment assignment? Q.7: Were the treatment groups treated identically other than the intervention of interest? Q.8: Was the follow-up completed, and if not, were the differences between groups in terms of their follow-up adequately described and analyzed? Q.9: Were participants analyzed in the groups to which they were randomized? Q.10: Were outcomes measured in the same way for the treatment groups? Q.11: Were outcomes measured in a reliable way? Q.12: Was appropriate statistical analysis used? Q.13: Was the trial design appropriate, and any deviations from the standard RCT design (individual randomization, parallel groups) accounted for in the conduct and analysis of the trial? √, yes; --, no; U - uncertain; N/A - not applicable.
Outcomes of each study
Three studies reported the VAP incidence rate and average number of days of ventilation (2,38,39). None of the studies found a significant difference between CHX+toothbrushing and CHX alone in preventing VAP, except one study (16) that compared groups with inadequate answers to the present question. Table 4 shows other outcomes common to two or more studies. All studies (2,16,38,39) reported positive results on microbiological tests for VAP identification. Moreover, none of the studies reported mortality rates.
Table 4. Summary of the outcomes of the eligible studies.
| Author | VAP incidence | Days ventilated, Mean (SD) | Mortality (VAP) | Microbiology |
|---|---|---|---|---|
| Munro et al. (16) | IG1: NR/49 IG2: NR/48 CG1: NR/44 CG2: NR/51 |
NR | NR | Yes |
| Pobo et al. (38) | IG:15/74 CG:18/73 |
8.9 (5.8) 9.8 (6.1) | NR | Yes |
| Lorente et al. (2) | IG: 21/217 CG: 24/219 |
9.18 (14.13) 9.93 (15.39) | NR | Yes |
| Vidal et al. (39) | IG:17/105 CG: 28/108 |
8.7 (5.0) 11.1 (7.6) | NR | Yes |
IG, intervention group; CG, control group.
Synthesis of meta-analysis
The meta-analysis did not include one of the four eligible studies in the systematic review due to the lack of comparison between the intervention and control groups (16). As shown in Figure 2, there was a 24% reduction in the RR of VAP in patients who underwent CHX + toothbrushing, although this effect was not considered significant (RR: 0.76; 95% confidence interval: 0.55-1.06). The heterogeneity between the studies was low (I2=0%, τ2=0%, H2=1.00).
Figure 2. Forest plot comparing the CHX 0.12% + toothbrushing and CHX 0.12% alone groups.
Certainty of evidence collection
The certainty of evidence from the outcome evaluated by the GRADE approach (37) was assessed as “moderate,” which means that the true effect is likely to be close to the estimated the effect, although there is a possibility that it is substantially different. Table 5 shows more details regarding the evaluation of each GRADE tool domain.
Table 5. Grading of the Recommendations Assessment, Development, and Evaluation (GRADE) Summary of Findings Table for the Outcomes of the Systematic Review and Meta-Analysis.
| Certainty assessment | No. of patients | Effect | Certainty | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Experimental group | Control group | Relative (95% CI) | Absolute (95% CI) | |
| Is toothbrushing combined with the use of 0.12% CHX in patients undergoing mechanical ventilation more effective for preventing VAP than using CHX alone? | ||||||||||
| 3 RCTs (796 patients) | Not seriousa | Not seriousb | Not seriousc | Seriousd | None | 53/396 (13.4%) | 70/400 (17.5%) | RR 0.76 (0.55 to 1.06) | 42 less per 1.000 (from 79 less para 11 more) | ⊕⊕⊕○ Moderate |
CI, confidence interval; RR, risk ratio.
All eligible studies had a low risk of bias.
Low heterogeneity (I2=0%) and overlapping confidence intervals.
Evidence stems from studies with the population suitable for PICO.
Confidence interval suggests no benefit in one extreme and benefit important to patients in other - rated down by one level.
GRADE Working Group grades of evidence.
High certainty: We are very confident that the true effect lies close to that of the estimated effect.
Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimated effect, although there is a possibility that it is substantially different.
Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimated effect.
Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimated effect.
DISCUSSION
This systematic review of the literature compared the performance of 0.12% CHX alone and 0.12% CHX with toothbrushing in the prevention of VAP in adults requiring mechanical ventilation in intensive care units. The results of the meta-analysis revealed a non-significant 24% reduction in the frequency of VAP in the CHX + toothbrushing group as opposed to the group that exclusively used CHX. This reduction in the incidence of VAP suggests the protective effect of toothbrushing associated with CHX, but must be interpreted with caution due to the lack of significant results.
The oral cavity microbiota is highly diverse and dynamic, mainly due to the wide variety of microbial habitats in the mouth and changes that can arise in these environments due to the adjustment in diet, salivary flow, and oral hygiene interventions (40-44). The oral cavity directly connects to the lower airways; therefore, there is an alleged association between oral microbiology and respiratory infections (45). Carrilho-Neto et al. (46) showed a reduction in oral hygiene in most hospitalized patients, and reported a positive correlation between the dental plaque index and gingival inflammation index (46). In intubated patients, gingival inflammation caused by inadequate oral hygiene has also been associated with lung inflammation (46-48).
Dental plaque accumulation and colonization of microorganisms in the mouth were significantly higher from day four of intubation, conferring a higher risk of VAP (49). Sands et al. (45) revealed that in one-third of mechanically ventilated patients, dental plaque is presumed to be a reservoir of certain respiratory pathogens such as Staphylococcus aureus and Pseudomonas aeruginosa (45). In one of the eligible studies, S. aureus and Haemophilus influenzae, organisms connected with respiratory infections, were abundant in dental plaque (50,51).
In 2020, the COVID-19 pandemic scenario contributed to the need for prolonged mechanical ventilation in infected patients, since intubation is frequent in those with more severe cases, also increasing the incidence of COVID-19-related pneumonia (52). The clinical presentation of COVID-19 pneumonia is homogeneous, which greatly overlaps with that of VAP. This situation hinders the use of empiric antibiotics due to the increased risk of multi-drug resistance (6). SARS-CoV-2 infection induces increased cytokine production, causing immune dysregulation and the development of hyperinflammation and defects in lymphoid function (9,10). In addition, the virus has the ability to infect hair cells in the alveoli, decreasing the airway clearance capacity and evolving to respiratory distress syndrome (53). This complication observed in patients hospitalized with COVID-19 is managed by mechanical ventilation (54). There are still serious risks of bacterial infections related to VAP in patients with COVID-19. Coinfection can worsen the clinical picture and increase the mortality of patients with COVID-19, as well as prolong and increase hospitalization costs. When VAP cannot be prevented in patients with COVID-19, this infection must be identified early to increase the chances of successful treatment (55).
CHX has been recognized as the gold standard in oral hygiene care and maintenance for over 20 years (56,57). It is an effective ally in the control of plaque and treatment of gum disease, when associated with brushing (57,58), in addition to diseases such as alveolar osteitis and bacteremia after tooth extractions (59). Its use was also considered safe in patients who received implants because it has excellent resistance to titanium corrosion (60).
CHX is a cationic biguanide with lipophilic groups that can bind to bacterial cell walls and alter their osmotic balance (13,14). This effect inhibits bacterial growth and can even prevent the death of patients; the mechanism of action depends on the concentration of the substance (15). In addition to CHX, toothbrushing has shown promising effects on VAP (61,62). Disorganization of plaque or biofilm adherent to the dental surface can be performed mechanically and chemically (63). Brushing assists in the removal of biofilm through the brush bristles, as mechanical contact can break plaque that is adherent to the tooth surface (64,65). Disruption of dental plaque through toothbrushing facilitates the action of CHX on residual biofilms.
Meinberg et al. (17) conducted a clinical trial using CHX (2%) with and without toothbrushing and observed that 55.8% of patients developed VAP (17). All studies included in the present review showed reduced VAP incidence rates (2,38,39). This result supports the use of 0.12% CHX in VAP prevention care in mechanically ventilated patients. Additionally, the use of CHX at high concentrations presumably causes adverse effects, such as oral mucosal irritation (66) and the development of respiratory distress syndrome (RDS) due to the ingestion of CHX (67). RDS is associated with diffuse alveolar and endothelial lesions (68), which can be fatal in fragile patients (69).
Other adverse effects caused by the mechanism of action of CHX, as well as its prolonged use, include changes in taste (70) and pigmentation in the enamel, tongue, and composite resin fillings (71). In an attempt to minimize or to eradicate such effects, researchers have sought changes in the use of this molecule. Guerra et al. (71) demonstrated that the decrease in the concentration of CHX with cetylpyridinium chloride maintains a protective effect without changes in flavor perceived by the patient (71). In a pilot study by Ripari et al. (72), the efficacy of CHX mouthwash and tea tree oil was compared in the treatment of gingivitis; results suggest that tea tree oil may be advantageous in cases where patients spend little time brushing their teeth (72).
VAP increases the period of mechanical ventilation, which has been related to high patient morbidity and mortality rates, as well as increased hospital costs (69,73). In any of the eligible studies in the present systematic review, the comparison between toothbrushing combined with CHX (0.12%) and CHX (0.12%) alone did not reveal a significant reduction in the number of days of mechanical ventilation (2,38). This result may show that the hospital length of stay associated with mechanical ventilation is a risk factor that overlaps with the VAP prevention protocol. One of the eligible studies observed this relationship, in which the majority of VAP cases occurred after day four of mechanical ventilation (39).
Among the included articles, it is possible to recognize that toothbrushing alone is not superior in inhibiting VAP over 0.12% CHX alone (17,38). Manual brushing with CHX does not help prevent VAP among patients receiving intensive mechanical ventilation therapy (2). However, although not significant, the meta-analysis conducted in our study showed a 24% reduction in the incidence of VAP in the CHX (0.12%) + toothbrushing group. This result may demonstrate the protective role of brushing in preventing VAP; however, due to the lack of statistical power, this did not reach the significance level. This also corroborates the study of Yao et al. (74), who assessed the risk of VAP using toothbrushing with purified water and revealed a VAP incidence of 34% (74). Among the eligible studies, the incidence of VAP ranged from 10.3% (2) to 22.4% (38).
VAP has considerable mortality rates, although the cause of death may be associated with previous morbidity (75). The attributable mortality associated with VAP is approximately 10%, ranging from 3% to 22% (76,77). Eligible trials included in the present study did not report the VAP mortality rates, representing an important limitation of the present conclusions.
This systematic review and meta-analysis has other limitations. First, only a small number of studies were included in the review. Second, eligible studies showed lack of relevant information, such as patient mortality and overall length of stay in intensive care units. Thus, our results should be interpreted with caution, and further studies with a standardized design are warranted to examine the use of 0.12% CHX + toothbrushing in reducing the risk of VAP in patients undergoing mechanical ventilation in intensive care units. As a strength, our review had a very comprehensive search strategy, including part of the gray literature; to the best of our knowledge, this is the first meta-analysis of clinical trials to compare the CHX (0.12%) + toothbrushing and CHX (0.12%) protocols.
CONCLUSION
Considering the limitations of this study, a standard protocol for the prevention of VAP is not recommended. Healthcare professionals should be aware of the benefits of oral hygiene in intensive care unit patients, to primarily reduce the incidence of VAP. The adoption of CHX may represent an improvement in mortality rates of patients under mechanical ventilation and, consequently, an improvement in patients’ quality of life, as well as a reduction in hospital expenses. Future research should focus on a single VAP prevention protocol using CHX+toothbrushing, including large sample sizes, aspects related to length of hospital stay, and mortality.
AUTHOR CONTRIBUTIONS
Silva PUJ and Cardoso SV conceived the idea and played full roles in the identification, article review, data extraction, quality assessment, analysis, draft writing, and revision of the manuscript. Meneses-Santos D, Macedo DR, Blumenberg C and Paranhos LR played major roles in the analysis, manuscript draft preparation, and revision. All authors have read and approved the final version of the manuscript for publication. All authors agreed to be equally accountable for all aspects of this study.
ACKNOWLEDGMENTS
This study was funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brazil (CAPES) - Finance code 001 and by the Ministry of Science, Technology, Innovation and Communications, Ministry of Health of Brazil and National Council for Scientific and Technological Development (CNPq; award numbers: 307808/2018-1 and 401612/2020-1).
Footnotes
No potential conflict of interest was reported.
REFERENCES
- 1.Grossman RF, Fein A. Evidence-based assessment of diagnostic tests for ventilator-associated pneumonia. Executive summary. Chest. 2000;117(4 Suppl 2):177S–181S. doi: 10.1378/chest.117.4_suppl_2.177S. [DOI] [PubMed] [Google Scholar]
- 2.Lorente L, Lecuona M, Jiménez A, Palmero S, Pastor E, Lafuente N, et al. Ventilator-associated pneumonia with or without toothbrushing: a randomized controlled trial. Eur J Clin Microbiol Infect Dis. 2012;31(10):2621–9. doi: 10.1007/s10096-012-1605-y. [DOI] [PubMed] [Google Scholar]
- 3.Wu D, Wu C, Zhang S, Zhong Y. Risk Factors of Ventilator-Associated Pneumonia in Critically III Patients. Front Pharmacol. 2019;10:482. doi: 10.3389/fphar.2019.00482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sen S, Johnston C, Greenhalgh D, Palmieri T. Ventilator-Associated Pneumonia Prevention Bundle Significantly Reduces the Risk of Ventilator-Associated Pneumonia in Critically Ill Burn Patients. J Burn Care Res. 2016;37(3):166–71. doi: 10.1097/BCR.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 5.Kotsaki A, Raftogiannis M, Routsi C, Baziaka F, Kotanidou A, Antonopoulou A, et al. Genetic polymorphisms within tumor necrosis factor gene promoter region: a role for susceptibility to ventilator-associated pneumonia. Cytokine. 2012;59(2):358–63. doi: 10.1016/j.cyto.2012.04.040. [DOI] [PubMed] [Google Scholar]
- 6.François B, Laterre PF, Luyt CE, Chastre J. The challenge of ventilator-associated pneumonia diagnosis in COVID-19 patients. Crit Care. 2020;24(1):289. doi: 10.1186/s13054-020-03013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tan L, Wang Q, Zhang D, Ding J, Huang Q, Tang YQ, et al. Lymphopenia predicts disease severity of COVID-19: a descriptive and predictive study. Signal Transduct Target Ther. 2020;5(1):33. doi: 10.1038/s41392-020-0148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–81. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe. 2020;27(6):992–1000.e3. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou Z, Ren L, Zhang L, Zhong J, Xiao Y, Jia Z, et al. Heightened Innate Immune Responses in the Respiratory Tract of COVID-19 Patients. Cell Host Microbe. 2020;27(6):883–90.e2. doi: 10.1016/j.chom.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wise MP, Williams DW. Oral care and pulmonary infection - the importance of plaque scoring. Crit Care. 2013;17(1):101. doi: 10.1186/cc11896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hua F, Xie H, Worthington HV, Furness S, Zhang Q, Li C. Oral hygiene care for critically ill patients to prevent ventilator-associated pneumonia. Cochrane Database Syst Rev. 2016;10(10):CD008367. doi: 10.1002/14651858.CD008367.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Septimus EJ, Schweizer ML. Decolonization in Prevention of Health Care-Associated Infections. Clin Microbiol Rev. 2016;29(2):201–22. doi: 10.1128/CMR.00049-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hugo WB, Longworth AR. Some aspects of the mode of action of chlorhexidine. J Pharm Pharmacol. 1964;16:655–62. doi: 10.1111/j.2042-7158.1964.tb07384.x. [DOI] [PubMed] [Google Scholar]
- 15.Kumar SB. Chlorhexidine Mouthwash- A Review. J Pharm Sci Res. 2017;9(9):1450–2. [Google Scholar]
- 16.Munro CL, Grap MJ, Jones DJ, McClish DK, Sessler CN. Chlorhexidine, toothbrushing, and preventing ventilator-associated pneumonia in critically ill adults. Am J Crit Care. 2009;18(5):428–37. doi: 10.4037/ajcc2009792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meinberg MC, Cheade Mde F, Miranda AL, Fachini MM, Lobo SM. The use of 2% chlorhexidine gel and toothbrushing for oral hygiene of patients receiving mechanical ventilation: effects on ventilator-associated pneumonia. Rev Bras Ter Intensiva. 2012;24(4):369–74. doi: 10.1590/S0103-507X2012000400013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sebastian MR, Lodha R, Kapil A, Kabra SK. Oral mucosal decontamination with chlorhexidine for the prevention of ventilator-associated pneumonia in children - a randomized, controlled trial. Pediatr Crit Care Med. 2012;13(5):e305–10. doi: 10.1097/PCC.0b013e31824ea119. [DOI] [PubMed] [Google Scholar]
- 19.Cabov T, Macan D, Husedzinović I, Skrlin-Subić J, Bosnjak D, Sestan-Crnek S, et al. The impact of oral health and 0.2% chlorhexidine oral gel on the prevalence of nosocomial infections in surgical intensive-care patients: a randomized placebo-controlled study. Wien Klin Wochenschr. 2010;122(13-14):397–404. doi: 10.1007/s00508-010-1397-y. [DOI] [PubMed] [Google Scholar]
- 20.Ellepola AN, Joseph BK, Khan ZU. Changes in the cell surface hydrophobicity of oral Candida albicans from smokers, diabetics, asthmatics, and healthy individuals following limited exposure to chlorhexidine gluconate. Med Princ Pract. 2013;22(3):250–4. doi: 10.1159/000345641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson L, Owens M. Does oral care with chlorhexidine reduce ventilator-associated pneumonia in mechanically ventilated adults? Br J Nurs. 2019;28(11):682–9. doi: 10.12968/bjon.2019.28.11.682. [DOI] [PubMed] [Google Scholar]
- 22.ten Cate JM. Biofilms, a new approach to the microbiology of dental plaque. Odontology. 2006;94(1):1–9. doi: 10.1007/s10266-006-0063-3. [DOI] [PubMed] [Google Scholar]
- 23.Chacko R, Rajan A, Lionel P, Thilagavathi M, Yadav B, Premkumar J. Oral decontamination techniques and ventilator-associated pneumonia. Br J Nurs. 2017;26(11):594–9. doi: 10.12968/bjon.2017.26.11.594. [DOI] [PubMed] [Google Scholar]
- 24.Kishimoto H, Urade M. Mechanical tooth cleaning before chlorhexidine application. Am J Respir Crit Care Med. 2007;175(4):418. doi: 10.1164/ajrccm.175.4.418a. [DOI] [PubMed] [Google Scholar]
- 25.Mori H, Hirasawa H, Oda S, Shiga H, Matsuda K, Nakamura M. Oral care reduces incidence of ventilator-associated pneumonia in ICU populations. Intensive Care Med. 2006;32(2):230–6. doi: 10.1007/s00134-005-0014-4. [DOI] [PubMed] [Google Scholar]
- 26.Hutchins K, Karras G, Erwin J, Sullivan KL. Ventilator-associated pneumonia and oral care: a successful quality improvement project. Am J Infect Control. 2009;37(7):590–7. doi: 10.1016/j.ajic.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Sona CS, Zack JE, Schallom ME, McSweeney M, McMullen K, Thomas J, et al. The impact of a simple, low-cost oral care protocol on ventilator-associated pneumonia rates in a surgical intensive care unit. J Intensive Care Med. 2009;24(1):54–62. doi: 10.1177/0885066608326972. [DOI] [PubMed] [Google Scholar]
- 28.Ferretti GA, Brown AT, Raybould TP, Lillich TT. Oral antimicrobial agents--chlorhexidine. NCI Monogr. 1990;(9):51–5. [PubMed] [Google Scholar]
- 29.Zand F, Zahed L, Mansouri P, Dehghanrad F, Bahrani M, Ghorbani M. The effects of oral rinse with 0.2% and 2% chlorhexidine on oropharyngeal colonization and ventilator associated pneumonia in adults' intensive care units. J Crit Care. 2017;40:318–22. doi: 10.1016/j.jcrc.2017.02.029. [DOI] [PubMed] [Google Scholar]
- 30.Plantinga NL, Wittekamp BHJ, Leleu K, Depuydt P, Van den Abeele AM, Brun-Buisson C, et al. Oral mucosal adverse events with chlorhexidine 2% mouthwash in ICU. Intensive Care Med. 2016;42(4):620–1. doi: 10.1007/s00134-016-4217-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nicolosi LN, del Carmen Rubio M, Martinez CD, González NN, Cruz ME. Effect of oral hygiene and 0.12% chlorhexidine gluconate oral rinse in preventing ventilator-associated pneumonia after cardiovascular surgery. Respir Care. 2014;59(4):504–9. doi: 10.4187/respcare.02666. [DOI] [PubMed] [Google Scholar]
- 32.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Xiao QM, Qi HN, Li W, Zhu BY, Liu YJ, et al. [Value of APACHE.II score and DIC score in predicting the death of patients with heat stroke] Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2019;37(1):43–5. doi: 10.3760/cma.j.issn.1001-9391.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 35.Fortis S, O'Shea AMJ, Beck BF, Nair R, Goto M, Kaboli PJ, et al. An automated computerized critical illness severity scoring system derived from APACHE III: modified APACHE. J Crit Care. 2018;48:237–42. doi: 10.1016/j.jcrc.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 36.Aromataris E, Munn Z. Joanna Briggs Institute Reviewer‘s Manual [Internet] Adelaide: Joanna Briggs Institute; 2017. [cited 2020 Jun 16] Available from: https://reviewersmanual.joannabriggs.org/ [Google Scholar]
- 37.Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401–6. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 38.Pobo A, Lisboa T, Rodriguez A, Sole R, Magret M, Trefler S, et al. A randomized trial of dental brushing for preventing ventilator-associated pneumonia. Chest. 2009;136(2):433–9. doi: 10.1378/chest.09-0706. [DOI] [PubMed] [Google Scholar]
- 39.de Lacerda Vidal CF, Vidal AK, Monteiro JG, Jr, Cavalcanti A, Henriques APC, Oliveira M, et al. Impact of oral hygiene involving toothbrushing versus chlorhexidine in the prevention of ventilator-associated pneumonia: a randomized study. BMC Infect Dis. 2017;17(1):112. doi: 10.1186/s12879-017-2188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dennesen P, van der Ven A, Vlasveld M, Lokker L, Ramsay G, Kessels A, et al. Inadequate salivary flow and poor oral mucosal status in intubated intensive care unit patients. Crit Care Med. 2003;31(3):781–6. doi: 10.1097/01.CCM.0000053646.04085.29. [DOI] [PubMed] [Google Scholar]
- 41.Marsh PD. Controlling the oral biofilm with antimicrobials. J Dent. 2010;38(Suppl 1):S11–5. doi: 10.1016/S0300-5712(10)70005-1. [DOI] [PubMed] [Google Scholar]
- 42.Tada A, Hanada N. Opportunistic respiratory pathogens in the oral cavity of the elderly. FEMS Immunol Med Microbiol. 2010;60(1):1–17. doi: 10.1111/j.1574-695X.2010.00709.x. [DOI] [PubMed] [Google Scholar]
- 43.Wade WG. The oral microbiome in health and disease. Pharmacol Res. 2013;69(1):137–43. doi: 10.1016/j.phrs.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 44.Wise MP, Cole JM, Williams DW, Lewis MA, Frost PJ. Efficacy of oral chlorhexidine in critical care. Crit Care. 2008;12(3):419. doi: 10.1186/cc6886. author reply 419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sands KM, Wilson MJ, Lewis MAO, Wise MP, Palmer N, Hayes AJ, et al. Respiratory pathogen colonization of dental plaque, the lower airways, and endotracheal tube biofilms during mechanical ventilation. J Crit Care. 2017;37:30–7. doi: 10.1016/j.jcrc.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 46.Carrilho A, Neto, De Paula Ramos S, Sant'ana AC, Passanezi E. Oral health status among hospitalized patients. Int J Dent Hyg. 2011;9(1):21–9. doi: 10.1111/j.1601-5037.2009.00423.x. [DOI] [PubMed] [Google Scholar]
- 47.Eddens T, Kolls JK. Host defenses against bacterial lower respiratory tract infection. Curr Opin Immunol. 2012;24(4):424–30. doi: 10.1016/j.coi.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hunter JD. Ventilator associated pneumonia. Postgrad Med J. 2006;82(965):172–8. doi: 10.1136/pgmj.2005.036905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Munro CL, Grap MJ, Elswick RK Jr, McKinney J, Sessler CN, Hummel RS 3rd. Oral health status and development of ventilator-associated pneumonia: a descriptive study. Am J Crit Care. 2006;15(5):453–60. doi: 10.4037/ajcc2006.15.5.453. [DOI] [PubMed] [Google Scholar]
- 50.Sands KM, Twigg JA, Lewis MAO, Wise MP, Marchesi JR, Smith A, et al. Microbial profiling of dental plaque from mechanically ventilated patients. J Med Microbiol. 2016;65(2):147–59. doi: 10.1099/jmm.0.000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.King P. Haemophilus influenzae and the lung (Haemophilus and the lung) Clin Transl Med. 2012;1(1):10. doi: 10.1186/2001-1326-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it [cited June 16th, 2020]
- 53.Perico L, Benigni A, Remuzzi G. Should COVID-19 Concern Nephrologists? Why and to What Extent? The Emerging Impasse of Angiotensin Blockade. Nephron. 2020;144(5):213–21. doi: 10.1159/000507305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.World Health Organization. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected: interim guidance. Available from: https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratoryinfection-when-novel-coronavirus-(ncov)-infection-is-suspected [cited June 16th, 2020]
- 55.Póvoa HCC, Chianca GC, Iorio NLPP. COVID-19: An Alert to Ventilator-Associated Bacterial Pneumonia. Infect Dis Ther. 2020;9(3):417–20. doi: 10.1007/s40121-020-00306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jones CG. Chlorhexidine: is it still the gold standard? Periodontol 2000. 1997;15:55–62. doi: 10.1111/j.1600-0757.1997.tb00105.x. [DOI] [PubMed] [Google Scholar]
- 57.Van der Weijden FA, Van der Sluijs E, Ciancio SG, Slot DE. Can Chemical Mouthwash Agents Achieve Plaque/Gingivitis Control? Dent Clin North Am. 2015;59(4):799–829. doi: 10.1016/j.cden.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 58.Lindhe J, Lang NP, Berglundh T, Giannobile WV, Sanz M. Clinical periodontology and implant dentistry. 6th ed. Hoboken, NJ: Wiley; 2015. [Google Scholar]
- 59.Canullo L, Laino L, Longo F, Filetici P, D'Onofrio I, Troiano G. Does chlorhexidine Prevent Complications in Extractive, Periodontal, and Implant Surgery? A Systematic Review and Meta-analysis with Trial Sequential Analysis. Int J Oral Maxillofac Implants. 2020;35(6):1149–58. doi: 10.11607/jomi.8216. [DOI] [PubMed] [Google Scholar]
- 60.Quaranta A, Ronconi LF, Di Carlo F, Vozza I, Quaranta M. Electrochemical behaviour of titanium in ammine and stannous fluoride and chlorhexidine 0.2 percent mouthwashes. Int J Immunopathol Pharmacol. 2010;23(1):335–43. doi: 10.1177/039463201002300132. [DOI] [PubMed] [Google Scholar]
- 61.Russell AD. Chlorhexidine: antibacterial action and bacterial resistance. Infection. 1986;14(5):212–5. doi: 10.1007/BF01644264. [DOI] [PubMed] [Google Scholar]
- 62.Roberts N, Moule P. Chlorhexidine and tooth-brushing as prevention strategies in reducing ventilator-associated pneumonia rates. Nurs Crit Care. 2011;16(6):295–302. doi: 10.1111/j.1478-5153.2011.00465.x. [DOI] [PubMed] [Google Scholar]
- 63.Teles RP, Teles FR. Antimicrobial agents used in the control of periodontal biofilms: effective adjuncts to mechanical plaque control? Braz Oral Res. 2009;23(Suppl 1):39–48. doi: 10.1590/S1806-83242009000500007. [DOI] [PubMed] [Google Scholar]
- 64.Chandki R, Banthia P, Banthia R. Biofilms: A microbial home. J Indian Soc Periodontol. 2011;15(2):111–4. doi: 10.4103/0972-124X.84377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Verkaik MJ, Busscher HJ, Rustema-Abbing M, Slomp AM, Abbas F, van der Mei HC. Oral biofilm models for mechanical plaque removal. Clin Oral Investig. 2010;14(4):403–9. doi: 10.1007/s00784-009-0309-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tantipong H, Morkchareonpong C, Jaiyindee S, Thamlikitkul V. Randomized controlled trial and meta-analysis of oral decontamination with 2% chlorhexidine solution for the prevention of ventilator-associated pneumonia. Infect Control Hosp Epidemiol. 2008;29(2):131–6. doi: 10.1086/526438. [DOI] [PubMed] [Google Scholar]
- 67.Hirata K, Kurokawa A. Chlorhexidine gluconate ingestion resulting in fatal respiratory distress syndrome. Vet Hum Toxicol. 2002;44(2):89–91. [PubMed] [Google Scholar]
- 68.Cutts S, Talboys R, Paspula C, Prempeh EM, Fanous R, Ail D. Adult respiratory distress syndrome. Ann R Coll Surg Engl. 2017;99(1):12–6. doi: 10.1308/rcsann.2016.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ferreira CR, de Souza DF, Cunha TM, Tavares M, Reis SS, Pedroso RS, et al. The effectiveness of a bundle in the prevention of ventilator-associated pneumonia. Braz J Infect Dis. 2016;20(3):267–71. doi: 10.1016/j.bjid.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cortellini P, Labriola A, Zambelli R, Prato GP, Nieri M, Tonetti MS. Chlorhexidine with an anti discoloration system after periodontal flap surgery: a cross-over, randomized, triple-blind clinical trial. J Clin Periodontol. 2008;35(7):614–20. doi: 10.1111/j.1600-051X.2008.01238.x. [DOI] [PubMed] [Google Scholar]
- 71.Guerra F, Pasqualotto D, Rinaldo F, Mazur M, Corridore D, Nofroni I, et al. Therapeutic efficacy of chlorhexidine-based mouthwashes and its adverse events: Performance-related evaluation of mouthwashes added with Anti-Discoloration System and cetylpyridinium chloride. Int J Dent Hyg. 2019;17(3):229–36. doi: 10.1111/idh.12371. [DOI] [PubMed] [Google Scholar]
- 72.Ripari F, Cera A, Freda M, Zumbo G, Zara F, Vozza I. Tea Tree Oil versus Chlorhexidine Mouthwash in Treatment of Gingivitis: A Pilot Randomized, Double Blinded Clinical Trial. Eur J Dent. 2020;14(1):55–62. doi: 10.1055/s-0040-1703999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Heyland DK, Cook DJ, Griffith L, Keenan SP, Brun-Buisson C. The attributable morbidity and mortality of ventilator-associated pneumonia in the critically ill patient. The Canadian Critical Trials Group. Am J Respir Crit Care Med. 1999;159(4 Pt 1):1249–56. doi: 10.1164/ajrccm.159.4.9807050. [DOI] [PubMed] [Google Scholar]
- 74.Yao LY, Chang CK, Maa SH, Wang C, Chen CC. Brushing teeth with purified water to reduce ventilator-associated pneumonia. J Nurs Res. 2011;19(4):289–97. doi: 10.1097/JNR.0b013e318236d05f. [DOI] [PubMed] [Google Scholar]
- 75.Wu D, Wu C, Zhang S, Zhong Y. Risk Factors of Ventilator-Associated Pneumonia in Critically III Patients. Front Pharmacol. 2019;10:482. doi: 10.3389/fphar.2019.00482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Melsen WG, Rovers MM, Koeman M, Bonten MJ. Estimating the attributable mortality of ventilator-associated pneumonia from randomized prevention studies. Crit Care Med. 2011;39(12):2736–42. doi: 10.1097/CCM.0b013e3182281f33. [DOI] [PubMed] [Google Scholar]
- 77.Corrado RE, Lee D, Lucero DE, Varma JK, Vora NM. Burden of Adult Community-acquired, Health-care-Associated, Hospital-Acquired, and Ventilator-Associated Pneumonia: New York City, 2010 to 2014. Chest. 2017;152(5):930–42. doi: 10.1016/j.chest.2017.04.162. [DOI] [PubMed] [Google Scholar]