Abstract
A series of novel 4-aminoquinoline analogues bearing a methyl group at 4-aminoquinoline moiety were synthesized via a new and robust synthetic route comprising in situtert-butoxycarbonyl (Boc) deprotection–methylation cascade resulting in the corresponding N-methylated secondary amine using Red-Al and an efficient microwave-assisted strategy for the fusion of N-methylated secondary amine with 4-chloroquinoline nucleus to access the series of novel 4-N-methylaminoquinoline analogues. The new series of compounds were evaluated for their antimalarial activity in in vitro and in vivo models. Among 21 tested compounds, 9a–i have shown a half-maximal inhibitory concentration (IC50) value less than 0.5 μM (i.e., <500 nM) against both chloroquine-sensitive strain 3D7 and chloroquine-resistant strain K1 of Plasmodium falciparum with acceptable cytotoxicity. Based on the in vitro antimalarial activity, selected compounds were screened for their in vivo antimalarial activity against Plasmodium yoelii nigeriensis (a multidrug-resistant) parasite in Swiss mice. Most of the compounds have shown significant inhibition on day 4 post infection at the oral dose of 100 mg/kg. Compound 9a has shown 100% parasite inhibition on day 4, and out of five treated mice, two were cured till the end of the experiment. The present study suggests that 4-methylamino substitution is well tolerated for the antiplasmodial activity with reduced toxicity and therefore will be highly useful for the discovery of a new antimalarial agent against drug-resistant malaria.
Introduction
Malaria is a vector-borne disease that continues to kill and threaten millions of people prominently in tropical countries, especially in Africa and South Asia. According to World Malaria Report 2019 published by WHO, nearly half of the world’s population are at risk with nearly 228 million worldwide malaria cases and an estimated 405 000 malaria deaths in 2018 alone.1 In the year 2019, India reported 338 494 malaria cases with 77 malaria deaths and still continues to be a major threat to most of the Indian population as 95% of them resides in malaria-endemic areas.2,3 The causative agent of the menacing infectious disease is the protozoa of genus Plasmodium, viz., Plasmodium falciparum, Plasmodium malariae, Plasmodium vivax, and Plasmodium ovale and to some extent by primate malaria parasite Plasmodium knowlesi. Among the five different species of the malaria parasite, P. falciparum is the deadliest species and most widespread in Africa.4P. vivax contributes to nearly 25–40% of total malaria infections especially in South Asia and Central and South America.5
The high morbidity and mortality rates of malarial infection had resulted in intense and focused research all around the world for the development of new antimalarial agents to counter the drug resistance. The commonly used antimalarial agents are grouped into the following chemical classes, viz., (a) 4-aminoquinolines (AQs), e.g., chloroquine (CQ); (b) sulfur drugs, e.g., dapsone and sulfadiazine; (c) antimalarial endoperoxide, e.g., artemisinin and related molecules; (d) dihydrofolate reductase inhibitors, e.g., proguanil and pyrimethamine. The 4-aminoquinoline class of antimalarial agents, e.g., chloroquine, which targets the feeding process of the erythrocyte stage of the parasite, was being extensively used as the drug of choice for the first line of treatment of malaria due to its higher efficacy, selectivity, lower toxicity, and availability at a low cost. However, the emergence of chloroquine resistance in P. falciparum and to a lower extent in P. vivax has been reported from all over the world, which seriously hampered their role in the management of Plasmodium infection and thus in turn opened up a new challenge to develop new molecules active against the resistant strain of the parasite.6 The antimalarial drug resistance continued to be challenging for sustaining the drug pipeline. Further, the single-dose-cure approaches gained momentum recently, and it remains hard to achieve the objective in view of its clinical efficacy. Therefore, the major thrust is to develop a new scaffold toward the discovery of molecules against drug-resistant malaria.
The extensive literature survey related to the antimalarial activity of 4-aminoquinolines and the study of methods by which the parasite acquires resistance suggest that the accumulation of 4-aminoquinolines inside the acidic food vacuole of the parasite is an important factor for the antimalarial activity of these molecules.7 Furthermore, the structure–activity relation (SAR) studies of 4-aminoquinolines ruled out any alteration in the target of these drugs and indicate the involvement of compound-specific efflux mechanism for resistance development. These observations are further supported by the development of reversed chloroquines having nanomolar activity against P. falciparum.8 Recent developments in the synthetic studies on 4-aminoquinoline containing molecules have revealed that the core 4-aminoquinoline moiety is indispensable for the antimalarial activity of the respective molecules. Substitution of 7-chloro group of chloroquine either with electron-donating groups like NH2 and OCH3 or with more electron-withdrawing groups like NO2 has a detrimental effect on antimalarial activity.9 Therefore, only the side chain of aminoquinolines (AQs) is left for the modification and exploration to optimize and modulate the antiplasmodial activity of the 4-aminoquinoline class of antimalarials.10 Earlier reports suggest that the physicochemical properties of the side chain such as pKa, lipophilicity, and length of carbon atoms are the key factor to maintain and retain the antimalarial activity.11
A series of short-chain CQ derivatives have been reported by replacing the terminal diethylamino group with metabolically more stable bulky (tert-butyl) as well as heterocyclic rings (piperidyl, pyrrolidino, morpholino). These modifications in the side chain lead to a substantial increase in the antimalarial activity against CQ-resistant strains.12 In another report, Madrid et al. have replaced the terminal diethylamino group with secondary amine having one bulky or aromatic ring while keeping the other propyl group constant. Some of these analogues are also found active against multidrug-resistant strains of malaria.13 Recently, side-chain-modified 4-aminoquinoline analogues like N′-(7-chloro-quinolin-4-yl)-N,N-diethyl-propane-1,3-diamine also known as AQ-13 have shown potential to be a lead molecule for the development of new antimalarial drugs (Figure 1a). In line with these activities, our group reported a new series of chiral 4-aminoquinoline (Figure 1b) highly active against drug-resistant malaria in both in vitro and in vivo models.14,15 For the present work, a new series of 4-methylaminoquinolines was designed, synthesized, and evaluated for the antimalarial activity to see the impact of alkylation of the 4-amino group of the quinoline moiety. This important substitution has not been explored so far and expected to modulate the biological activity of the molecules (Figure 1).
Figure 1.
Structures of some 4-aminoquinoline-derived compounds.
As evident from the literature, the amino group at the C4-position of 4-aminoquinolines resonates favorably by the delocalization of its lone pair to bind with heme. The introduction of an alkyl group, methyl for the present study, is expected to influence such delocalization and therefore influence the biological activity of the molecules. Among the numerous side chains reported in the case of 4-aminoquinolines, substitution of hydrogen at the 4-amino group remains unexplored. For the present study, we have envisaged to study the impact of 4-methylamino scaffold on the antiplasmodial activity of 4-aminoquinolines. We have speculated that the 4-methylaminoquinoline derivatives may have better activity profile if the altered delocalization favors the biological activity. The delocalization of lone pair electron at the 4-amino group is likely to be more facile compared to its unsubstituted counterpart, and therefore needs further exploration of SAR (Figure 2).
Figure 2.
Unique resonance forms of 4-aminoquinolines and 4-methylaminoquinolines.
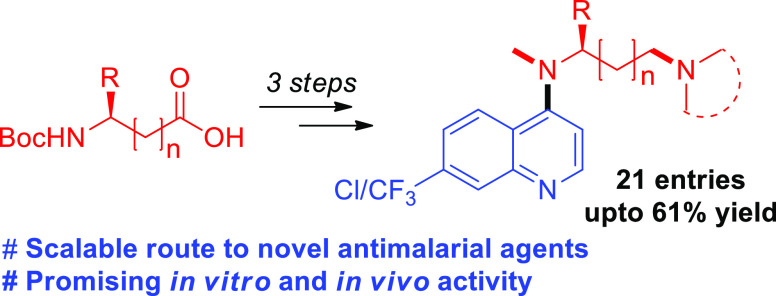
Based on this concept, we attempted the design and synthesis of a novel series of N-methyl-4-aminoquinoline analogues. The side chain and pendent tertiary amine selection was made on the basis of our earlier reports, where a series of 4-aminoquinolines with amino acid-derived modified side chains were synthesized for better activity in CQ-resistant strains.15 In this manuscript, we report the synthesis of a series of 4-methylanino quinolines comprising a chiral side chain derived from the amino acids as shown in Scheme 2 and evaluate the antimalarial activity in vitro and in an animal model against drug-resistant strains.
Scheme 2. Synthesis of 4-N-(Methyl)-4-aminoquinolines from Amino Acid-Derived Side Chain.
Reagents and conditions: (a) 1 (5.0 mmol), dichloromethane (DCM) (0.25 M), N,N′-dicyclohexylcarbodiimide (DCC) (1.5 equiv), hydroxybenzotriazole (HOBt) (1.2 equiv in 2 mL of dimethylformamide (DMF)); (b) 3–5 (5.0 mmol), Vitride (5 equiv), tetrahydrofuran (THF) (0.25 mmol), 0 °C to reflux; (c) 6–8 (1.5 equiv), 4,7-dichloroquinoline or 4-chloro-7-trifluromethylquinoline (1 equiv), phenol (2 equiv) under microwave irradiation at 50 W power and 145 °C temperature for 30 min.
Results and Discussion
The synthesis of the desired compounds of the series 9–11 was carried out by the fusion of 4,7-dichloroquinoline with the amino group of the side chain in the presence of phenol according to the procedure reported previously.15,16 However, the synthesis of N-methylamino side chain remains challenging. We have explored a couple of procedures and reagents to develop a robust route for the synthesis of side chains required for the synthesis of desired compounds. Different possible synthetic routes were explored to access 4-N-(methyl)-4-aminoquinolines. The target compound I could be synthesized either by direct methylation of aromatic NH (route A)17 or by formylation reduction (route B)18 of their nonmethylated 4-aminoquinoline counterparts. In route C, the chirally defined N-methyl group containing side chains could first be obtained from the corresponding amino acid amide followed by fusion of the secondary amine with 4-chloroquinolines (Scheme 1).19
Scheme 1. Possible Synthetic Route for 4-N-(Methyl)-4-aminoquinoline Analogues.
Reagents and conditions: (A) NaH, DMF, 0 °C to room temperature (r.t.); (B) diethoxymethyl acetate, 120 °C; (C) phenol, 11 h, 90 °C.
Initially, we attempted route A, where the alkylation of C-4 NH was attempted using methyl iodide and sodium hydride as base, but N-methylated product could not be isolated in pure form, rather an inseparable mixture of products was obtained. Therefore, we modified our strategy by opting route C and started the synthesis using tert-butoxycarbonyl (Boc)-protected amino acids as the starting material (Scheme 2). Boc-protected amino acids 1a–c were coupled with different secondary amines 2a–e using DCC/HOBt as coupling reagents to get the Boc-protected amino acid amides 3a–e, 4a–d, and 5a–c in quantitative yields. The amide bond reduction was attempted using excess of lithium aluminum hydride (LAH), but high reactivity, moisture sensitivity, and pyrophoric nature of LAH limit the process with its cumbersome operation, tedious purification, and low yield. Therefore, we looked for other mild reagents for the reduction of amide and found Red-Al (Vitride) as the alternative choice to reduce amide bonds mainly due to its mildness and operational ease compared to LAH. Initially, when we treated the Boc-amino acid amide 3a with 10 M excess of Red-Al at 50 °C, it led to the full consumption of the starting material 3a within 1 h. Interestingly, when the reaction was carried out at room temperature under similar conditions, the reaction scenario was changed and the formation of different products was observed. Under both reaction conditions, we noticed complete conversion of starting material and single product formation as indicated by thin-layer chromatography (TLC).
After identification of the products by spectroscopic methods, we concluded that Red-Al reduces both the carbamate protection and the amide group at elevated temperatures to give N-methyl amine 6a, while at room temperature, only the amide group was reduced and the product identified was Boc-protected amine 6a′. The former reaction condition is best suited for our desired synthesis of N-methylated chiral amines, which could then be fused with 4-chloroquinolines according to route C to access the novel series of 4-N-(methyl)-4-aminoquinolines. But before proceeding with that, an optimization of the newly developed step was needed. When 3 or 4 M excess of Red-Al was used, the reaction did not complete even after 5 h of reflux and unreacted starting material was observed on TLC (Supporting Information (SI)). Consequently, we increased the loading and found that 5 M excess of Red-Al was required to complete the reaction within 30 min as indicated by the complete disappearance of the starting material on TLC (SI). Using the optimized procedure for the reduction reaction, Boc-protected amino acid amides 3a–e, 4a–d, and 5a–e were converted to their corresponding N-methyl amines 6a–e, 7a–d, and 8a–c in almost quantitative yields as per TLC.
Having these chiral N-methylated secondary amines in hand, we further proceeded to synthesize our target molecule by the conventional phenol-assisted fusion of amines to 4-chloroquinolines to obtain the 4-aminoquinoline analogues as per the literature procedure. Unfortunately, the conventional fusion reaction did not work well in our case and we obtained a mixture of unresolved products probably because of the sluggish nucleophilic aromatic substitution (SNAr) of phenoxide adduct with N-methylated secondary amine under such a harsh condition. To eliminate the side products, we decreased the reaction time and temperature by performing a microwave-assisted synthesis at atmospheric pressure for phenol-assisted fusion reaction. When amine 6a was reacted with 4,7-dichloroquinoline (4,7-DCQ) using 2 M excess of phenol under microwave irradiation condition at atmospheric pressure and 145 °C temperature for 30 min, we observed the formation of the fusion product 9a. The condition of the microwave-assisted fusion reaction of 4,7-DCQ with N-methylated secondary amine was further optimized to achieve the best yield of the corresponding product (SI).
Under an optimized condition, 1.5 equiv of amine 6a was reacted with 1 equiv of 4,7-dichloroquinoline and 2 equiv of phenol under microwave irradiation at 50 W power and 145 °C temperature for 30 min to give 60% of fusion product 9a (SI). Increasing the amount of amine 6a resulted in the accumulation of a significant amount of unreacted amine 6a in the reaction mixture, which makes purification of the product more difficult (SI). Furthermore, increasing the temperature above 145 °C resulted in significant charring of the reaction mixture (SI). After optimization of the microwave-assisted fusion reaction, a series of novel 4-N-(methyl)-4-amino-7-chloroquinoline derivatives (9a–e, 10a–d, 11a, and 11b) and 4-N-(methyl)-4-amino-7-(trifluromethyl)quinoline derivatives (9f–i, 10e–h, 11c, and 11d) were synthesized by following the method outlined in Scheme 2. All of the synthesized compounds were submitted for their antiplasmodial activity.
Biological Activity
All of the 4-N-(methyl)-4-aminoquinoline compounds were evaluated for in vitro antimalarial activity against chloroquine-sensitive strain (3D7) as well as chloroquine-resistant strain (K1) of P. falciparum, while the cytotoxicity was determined against monkey kidney VERO cell line. The detailed biological activity of compounds synthesized following the above-mentioned method is the subject of discussion for this section.
In Vitro Antimalarial Activity
The in vitro antimalarial activity of the synthesized compounds 9a–i, 10a–h, and 11a–d is given in Table 1. The in vitro antimalarial activity data showed that out of 21 tested compounds, 9a–i have shown a half-maximal inhibitory concentration (IC50) value less than 0.5 μM (i.e., <500 nM) against both chloroquine-sensitive strain 3D7 and in chloroquine-resistant strain K1 of P. falciparum. The IC50 values for compounds 10e, 10f, and 10h were not evaluated in CQ-resistant strain because these compounds were found inactive in CQ-sensitive strain. Two compounds 9a and 9e were found more active in the CQ-sensitive strain (IC50 = 60 and 230 nM, respectively) and less active in the CQ-resistant strain (IC50 = 260 and 540 nM, respectively). Compound 10c (IC50 = 60 and 100 nM, respectively) and 11b (IC50 = 190 and 110 nM, respectively) were found almost equally active against both the strains of P. falciparum. Compounds 11b and 11c showed slightly better in vitro antimalarial activity in the CQ-resistant strain compared to the CQ-sensitive stain. Compound 11a was most active against CQ-sensitive and -resistant strains (IC50 = 40 and 60 nM, respectively). Microscopic examination also showed that these compounds inhibited parasite growth. Giemsa staining revealed that compound treatment resulted in a delay in parasite growth, and in many cases, the parasites were in the stressed trophozoite stage, in contrast to the healthy schizont stage of parasite observed in the untreated sample (Figure 3). In general, compounds having trifluromethyl substitution at the seventh position of the quinoline nucleus were found less active than their chloro- counterpart in both the strains of the parasite.
Table 1. Biological Activity and Cytotoxicity Data of Synthesized Compounds 9a–i, 10a–h, and 11a–da.
| selectivity
index (SI) |
||||||
|---|---|---|---|---|---|---|
| s. no. | compounds code | IC50Pf3D7 (μM) | IC50PfK1 (μM) | cytotoxicity CC50 (μM) in VERO cell line | Pf3D7 | PfK1 |
| 1 | 9a | 0.06 ± 0.0 | 0.26 ± 0 | 36.57 | 610 | 61 |
| 2 | 9b | 1.16 ± 0.26 | 1.19 ± 0.53 | 50.77 | 44 | 43 |
| 3 | 9c | 0.15 ± 0.14 | 0.42 ± 0.23 | 69.52 | 463 | 166 |
| 4 | 9d | 0.08 ± 0.0 | 0.18 ± 0.15 | 47 | 588 | 261 |
| 5 | 9e | 0.23 ± 0.10 | 0.54 ± 0.03 | 60.28 | 262 | 112 |
| 6 | 9f | 2.72 ± 1.44 | 2.25 ± 0.77 | 68.62 | 25 | 30 |
| 7 | 9g | 2.0 ± 0.06 | 2.04 ± 0.40 | 42.63 | 21 | 21 |
| 8 | 9h | 1.24 ± 0.0 | 1.60 ± 0.91 | 51.95 | 42 | 33 |
| 9 | 9i | 3.80 ± 1.05 | 2.5 ± 1.03 | 51.37 | 14 | 21 |
| 10 | 10a | 1.20 ± 0.78 | 1.38 ± 0.07 | 33.50 | 28 | 24 |
| 11 | 10b | 0.28 ± 0.13 | 0.49 ± 0.15 | 69.69 | 348 | 142 |
| 12 | 10c | 0.06 ± 0.16 | 0.10 ± 0.5 | 57.79 | 963 | 577 |
| 13 | 10d | 0.21 ± 0.11 | 0.33 ± 0.06 | 79.05 | 376 | 240 |
| 14 | 10e | 3.89 ± 0.50 | ND | ND | NA | NA |
| 15 | 10f | 1.37 ± 0.52 | ND | ND | NA | NA |
| 16 | 10g | 0.68 ± 0.16 | 1.77 ± 0.0 | 39.21 | 58 | 22 |
| 17 | 10h | 1.75 ± 0.16 | ND | ND | NA | NA |
| 18 | 11a | 0.04 ± 0.0 | 0.06 ± 0.04 | 106.91 | 274 | 1782 |
| 19 | 11b | 0.19 ± 0.0 | 0.11 ± 0.07 | 80.22 | 422 | 729 |
| 20 | 11c | 0.88 ± 0.28 | 0.82 ± 0.06 | >200 | >227 | >243 |
| 21 | 11d | 0.09 ± 0.02 | 0.31 ± 0.15 | 78.22 | 978 | 252 |
| 22 | chloroquine (reference drug) | 0.003 ± 0.25 | 0.795 ± 0.15 | 513 | 171 000 | 645 |
Parasite inhibition experiment was performed in chloroquine-sensitive strain (3D7, MRA-151) and multidrug-resistant strain (K1, MRA-159). Cytotoxicity experiment was performed in VERO cells. IC50 and CC50 are the half-maximal inhibitory and cytotoxic concentrations, respectively. Chloroquine (CQ), was used as a reference compound in the parasite inhibition assay. Podophyllotoxin is used as a reference compound for cytotoxicity in VERO cells (CC50 4 μM). Selectivity index (SI) is calculated as the ratio of CC50/IC50.
Figure 3.
Giemsa-stained Pf3D7 treated with CQ or 9a–e, 10b–d, 10g, and 11a–d compounds. All of the pictures are taken at their respective IC50 concentrations. Treatment was given at the ring stage (1% parasitemia, 1% hematocrit). Microscopic examination was done at 72 h post treatment.
In Vitro Cytotoxicity
The cytotoxicity of target compounds was determined using standard resazurin dye-based fluorescence assay against VERO cell line and is presented in Table 1. The cytotoxicity was not determined for compounds 10e, 10f, and 10h due to lack of activity in both the strains. Compounds having three-carbon-long propyl chain in the pendent amine group (11a–d) showed cytotoxicity at >80 μM concentration. In general, most of the compounds bearing 4-methylamino substitution retained the activity and found active in in vitro analysis and exhibited cytotoxicity at >50 μM concentration except for compounds 9a and 9d, which showed cytotoxicity at 36.5 and 47 μM, respectively.
In Vivo Antimalarial Activity
Based on their in vitro antimalarial activity, newly synthesized compounds 9a, 9d, 10c, 10g, 11b, and 11d were selected and screened for their in vivo antimalarial activity against the Plasmodium yoelii nigeriensis (a multidrug-resistant) parasite in Swiss mice. Promising results were found in the screening, which are depicted below (Table 2).
Table 2. In Vivo Antimalarial Potential of Selected Compounds against P. y. nigeriensis Multidrug-Resistant P. yoelii-MDR in Albino Mice of Swiss Strain.
| compound no. | dose | % suppression on day 4 post infection | survival on day 28 | cure on day 28 | MST (days) |
|---|---|---|---|---|---|
| 9a | 100 mg/kg × 4 days | 100 | 2/5 | 2/5 cured | 21 |
| 9d | 100 mg/kg × 4 days | 99.99 | 0/5 | 0/5 cured | 9.4 |
| 10c | 100 mg/kg × 4 days | 93.99 | 0/5 | 0/5 cured | 9 |
| 10g | 100 mg/kg × 4 days | 62.30 | 0/5 | 0/5 cured | 7.2 |
| 11b | 100 mg/kg × 4 days | 99.8 | 0/5 | 0/5 cured | 8.8 |
| 11d | 100 mg/kg × 4 days | 99.85 | 0/5 | 0/5 cured | 8.8 |
| α/β-arteether (reference drug) | 7.5 mg/kg × 3 days | 100 | 5/5 | 5/5 cured | >28 |
| control | 0/5 | 0/5 cured | 5.4 |
Compound 9a has shown 100% parasite inhibition on day 4, and out of five treated mice, two were cured till the end of the experiment, i.e., day 28. Microscopic examination through giemsa staining corroborated the parasite inhibition upon treatment with compound 9a (Figure 4).
Figure 4.

Giemsa-stained P. yoelii-MDR parasite untreated and treated with compound 9a. Pictures are taken on day 4 post treatment. Treatment was given from day 0 to day 3.
Conclusions
In summary, a series of novel 4-aminoquinoline analogues bearing a methyl group at quinolinic amine were synthesized via a multistep synthetic route for the reduction of carbamate-protected amino acid amides into corresponding N-methylated secondary amine using Red-Al. The method is more advantageous than the earlier reported LAH reduction method of amino acid amide in terms of its ease of operation, mild reaction condition, and high yield with minimal generation of side products. Furthermore, the alkylated hindered secondary amines were successfully fused with the 4-chloroquinoline moiety by an efficient microwave-assisted method in high yield and purity, which is otherwise difficult to synthesize in pure form using the conventional heating method. The compounds synthesized for the present study were subjected to bioevaluation for their antiplasmodial activity by the in vitro model. Most of the compounds exhibited significant antiplasmodial activity against both CQ-sensitive and CQ-resistant strains of P. falciparum. It is worth mentioning that the activity against CQ-resistant strains was more potent compared to activity against susceptible strains. This prompted us to evaluate the activity of the selected compounds in animal model against multidrug-resistant parasite. In line with the in vitro assay results, highly significant activity was exhibited by the compounds. Compound 9a was the most potent compound and 100% inhibition of parasitemia was observed on day 4 post treatment, and also the mice were cured. The promising results of the new series observed in both assays support our hypothesis regarding the activity of the molecules containing 4-N-methylaminoquinoline structures against drug-resistant parasite in particular. We hope that the encouraging results of the present study will be highly beneficial to the researchers working in this field to discover a new, effective, and superior antimalarial drug against drug-resistant parasites.
Experimental Section
General Procedure for the Synthesis of Compounds 3a–e, 4a–d, and 5a–c
To a stirred solution of Boc-protected amino acids 1a–c (5 mmol, 1 equiv) in dry DCM (20 mL) was added DCC (7.5 mmol, 1.5 equiv) dissolved in 5 mL of DCM and HOBt (6 mmol, 1.2 equiv) dissolved in 2 mL of DMF at 0 °C. After 5 min, secondary amines 2a–e was added slowly to the stirred reaction mixture. The reaction mixture was allowed to warm up to room temperature over 30 min and further stirred at room temperature for 1 h. After completion of the reaction as indicated by TLC, the precipitated dicyclohexylurea (DCU) was removed by filtration and the filtrate was washed with 10% aqueous NaHCO3 (3 × 50 mL) and 10% aqueous citric acid (3 × 50 mL) solution followed by a final wash with a brine solution. In the case of amides synthesized from N-methylpiperazine 2a, citric acid wash was omitted to avoid loss of amide as a citrate salt. The organic layer was dried over anhydrous Na2SO4 and evaporated to a gummy residue. The residue was dissolved in a minimum quantity of THF and kept for cooling at 0 °C for 2 h. During this period, the residual DCU was precipitated and filtered. The filtrate was evaporated to get amides 3a–e, 4a–d, and 5a–c as a gummy residue in quantitative yield.
(S)-tert-Butyl 4-Methyl-1-(4-methylpiperazin-1-yl)-1-oxopentan-2-ylcarbamate (3a)
This compound was obtained as a light yellow oil in 89% yield; Rf 0.38 (chloroform/methanol (9.5:0.5)); 1H NMR (500 MHz, CDCl3): δ 5.29 (bs, 1H), 4.65 (t, J = 9.26 Hz, 1H), 3.70–3.50 (m, 4H), 2.46–2.37 (m, 4H), 2.32 (s, 3H), 1.74–1.68 (m, 1H), 1.50–1.35 (m, 11H), 0.99 (d, J = 6.51 Hz, 3H), 0.92 (d, J = 6.66 Hz, 3H); high-resolution mass spectrometry (HRMS) calcd for [C16H32N3O3+] 314.2438, found 314.2431.
(S)-tert-Butyl 4-Methyl-1-morpholino-1-oxopentan-2-ylcarbamate (3b)
This compound was obtained as a colorless oil in 83% yield; Rf 0.42 (chloroform/methanol (9.5:0.5)); 1H NMR (500 MHz, CDCl3): δ 5.26 (bs, 1H), 4.63 (t, J = 9.29 Hz, 1H), 3.73–3.46 (m, 6H), 1.73–1.69 (m, 1H), 1.51–1.37 (m, 11H), 0.98 (d, J = 6.56 Hz, 3H), 0.93 (d, J = 6.66 Hz, 3H); HRMS calcd for [C15H39N2O4+] 301.2122, found 301.2129.
(S)-tert-Butyl 4-Methyl-1-oxo-1-(piperidin-1-yl)pentan-2-ylcarbamate (3c)
This compound was obtained as a colorless oil in 88% yield; Rf 0.45 (chloroform/methanol (9.5:0.5)); 1H NMR (500 MHz, CDCl3): δ 5.35 (bs, 1H), 4.67 (t, J = 9.41 Hz, 1H), 3.56–3.40 (m, 4H), 1.74–1.33 (m, 18H), 0.99 (d, J = 6.52 Hz, 1H), 0.92 (d, J = 6.50 Hz, 1H); HRMS calcd for [C16H31N2O3]+ 299.2329, found 299.2336.
(S)-tert-Butyl 4-Methyl-1-oxo-1-(pyrrolidin-1-yl)pentan-2-ylcarbamate (3d)
This compound was obtained as a colorless oil in 84% yield; Rf 0.43 (chloroform/methanol (9.5:0.5)); 1H NMR (500 MHz, CDCl3): δ 5.23 (d, J = 8.74 Hz, 1H), 4.46 (m, J = 4.55 Hz, 1H), 3.68 (q, J = 7.68 Hz, 1H), 3.52 (m, 1H), 3.41 (m, 2H), 1.97 (m, 2H), 1.87 (m, 2H), 1.73 (m, 1H), 1.51 (m, 1H), 1.43 (s, 9H), 1.38 (m, 1H), 0.99 (d, J = 6.53 Hz, 3H), 0.93 (d, J = 6.70 Hz, 3H).
(S)-tert-Butyl 1-(Diethylamino)-4-methyl-1-oxopentan-2-ylcarbamate (3e)
This compound was obtained as a colorless oil in 86% yield; Rf 0.43 (chloroform/methanol (9.5:0.5)); 1H NMR (500 MHz, CDCl3): δ 5.22 (d, J = 8.81 Hz, 1H), 4.60 (dt, J = 3.23 and 9.57 Hz, 1H), 3.51 (m, 1H), 3.37 (q, J = 7.07 Hz, 2H), 3.24 (m, 1H), 1.72 (m, 1H), 1.53 (m, 1H), 1.49 (s, 9H), 1.34 (m, 1H), 1.25 (t, J = 7.17 Hz, 3H), 1.11 (t, J = 7.13 Hz, 3H), 0.99 (d, J = 6.54 Hz, 3H), 0.93 (d, J = 6.71 Hz, 3H); HRMS calcd for [C15H31N2O3]+ 287.2329; found 287.2336.
(S)-tert-Butyl 1-Morpholino-1-oxo-3-phenylpropan-2-ylcarbamate (4a)
This compound was obtained as a colorless oil in 92% yield; Rf 0.45 (chloroform/methanol (9.5:0.5)); 1H NMR (500 MHz, CDCl3): δ 7.20–7.30 (m, 5H), 5.42 (d, J = 8.33 Hz, 1H), 4.77–4.82 (m, 1H), 3.55–3.66 (m, 2H), 3.39–3.49 (m, 4H), 3.27–3.31 (m, 1H), 3.00–3.04 (m, 1H), 2.89–2.95 (m, 2H), 1.43 (s, 9H); HRMS calcd for [C18H27N2O4]+ 335.1965, found 335.1978.
(S)-tert-Butyl 1-Oxo-3-phenyl-1-(piperidin-1-yl)propan-2-ylcarbamate (4b)
This compound was obtained as a colorless oil in 88% yield; Rf 0.48 (chloroform/methanol (9.5:0.5)); 1H NMR (500 MHz, CDCl3): δ 7.18–7.22 (m, 3H), 7.25–7.28 (m, 2H), 5.44 (d, J = 8.33 Hz, 1H), 4.84 (q, J = 7.54 Hz, 1H), 3.40–3.48 (m, 2H), 3.21–3.26 (m, 1H), 3.01–3.04 (m, 1H), 2.95 (d, J = 7.06 Hz, 2H), 1.48–1.59 (m, 5H), 1.41 (s, 9H), 0.96–1.01 (m, 1H); HRMS calcd for [C18H27N2O4]+ 335.1965, found 335.1978.
(S)-tert-Butyl 1-Oxo-3-phenyl-1-(pyrrolidin-1-yl)propan-2-ylcarbamate (4c)
This compound was obtained as a colorless oil in 79% yield; Rf 0.47 (chloroform/methanol (9.5:0.5)); 1H NMR (500 MHz, CDCl3): δ 7.25–7.28 (m, 2H), 7.20–7.22 (m, 3H), 5.40 (d, J = 8.33 Hz, 1H), 4.56–4.61 (m, 1H), 3.28–3.45 (m, 4H), 2.92–3.01 (m, 2H), 1.71–1.77 (m, 2H), 1.62–1.66 (m, 1H), 1.52–1.55 (m, 1H), 1.42 (s, 9H); HRMS calcd for [C18H27N2O3]+ 319.2016, found 319.2022.
(S)-tert-Butyl 1-(Diethylamino)-1-oxo-3-phenylpropan-2-ylcarbamate (4d)
This compound was obtained as a colorless oil in 87% yield; Rf 0.47 (chloroform/methanol (9.5:0.5)); 1H NMR (500 MHz, CDCl3): δ 7.24–7.26 (m, 2H), 7.19–7.22 (m, 3H), 5.34 (d, J = 8.56 Hz, 1H), 4.71–4.76 (m, 1H), 3.48–3.54 (m, 1H), 2.92–3.12 (m, 5H), 1.41 (s, 9H), 1.04 (t, J = 7.03 Hz, 3H), 0.96 (t, J = 7.09 Hz, 3H); HRMS calcd for [C18H29N2O3]+ 221.2173, found 221.2175.
tert-Butyl 3-Morpholino-3-oxopropylcarbamate (5a)
This compound was obtained as a colorless oil in 90% yield; Rf 0.41 (chloroform/methanol (9.5:0.5)); 1H NMR (500 MHz, CDCl3): δ 5.27 (bs, 1H), 3.66–3.68 (m, 4H), 3.61 (t, J = 4.47 Hz, 2H), 3.41–3.48 (m, 4H), 2.50 (t, J = 5.68 Hz, 2H), 1.42 (m, 9H); HRMS calcd for [C20H29ClN4 + H]+ 259.1652, found 259.1658.
tert-Butyl 3-Oxo-3-(piperidin-1-yl)propylcarbamate (5b)
This compound was obtained as a colorless oil in 84% yield; Rf 0.43 (chloroform/methanol (9.5:0.5)); 1H NMR (500 MHz, CDCl3): δ 5.34 (bs, 1H), 3.54 (t, J = 5.53 Hz, 2H), 3.40–3.43 (m, 2H), 3.36 (t, J = 5.47 Hz, 2H), 2.49 (t, J = 5.66 Hz, 2H), 1.62–1.65 (m, 2H), 1.52–1.57 (m, 4H), 1.43 (s, 9H); HRMS calcd for [C13H25N2O3]+ 257.1807, found 257.1795.
tert-Butyl 3-Oxo-3-(pyrrolidin-1-yl)propylcarbamate (5c)
This compound was obtained as a colorless oil in 81% yield; Rf 0.43 (chloroform/methanol (9.5:0.5)); 1H NMR (500 MHz, CDCl3): δ 5.39 (bs, 1H), 3.46 (t, J = 6.91 Hz, 2H), 3.42 (t, J = 5.94 Hz, 2H), 3.38 (t, J = 6.80 Hz, 2H), 2.45 (t, J = 5.64 Hz, 2H), 1.93–1.98 (m, 2H), 1.83–1.89 (m, 2H), 1.43 (s, 9H); HRMS calcd for [C12H23N2O3]+ 242.1703, found 243.1692.
General Procedure for the Synthesis of Compounds 6a–e, 7a–d, and 8a–c
In an optimized procedure Boc-protected amino acid amide (3a–e, 4a–d and 5a–c) (5 mmol, 1 equiv) in THF 20 mL was slowly added Vitride (Red-Al, 70% in toluene) (25 mmol, 5 equiv) at 0 °C with stirring under nitrogen over 10 min. The reaction mixture was then heated to reflux for 30 min. After completion of the reaction as indicated by TLC, the reaction mixture was cooled to 0 °C in an ice bath and quenched by a slow addition of saturated aqueous solution of potassium sodium tartrate (30 mL). The reaction mixture was extracted with ethyl acetate (30 mL × 3), and the combined organic layer was washed with water (30 mL × 3) and brine (30 mL × 3). The organic layer was dried over anhydrous Na2SO4, filtered, and evaporated to get the N-methylated amines (6a–e, 7a–d, and 8a–c) as a viscous oil, which was used for the next step without further purification.
(S)-N,4-Dimethyl-1-(4-methylpiperazin-1-yl)pentan-2-amine (6a)
This compound was obtained as a colorless viscous oil in 85% yield; Rf 0.15 (chloroform/methanol (9:1)); 1H NMR (400 MHz, CDCl3): δ 2.57–2.25 (m, 17H), 1.72–1.62 (m, 1H), 1.40–1.33 (m, 1H), 1.13–1.06 (m, 1H), 0.91 (dd, J = 6.5 and 1.8 Hz, 6H), 13C NMR (101 MHz, CDCl3): δ 62.7, 55.2, 54.1, 45.9, 41.9, 33.9, 24.8, 23.4, 22.6; electrospray ionization mass spectrometry (ESI MS) calcd for [C12H28N3]+ 214.2, found 214.2.
General Procedure for the Synthesis of Compounds 9a–i, 10a–h and 11a–d
In an optimized procedure, amines 6a–e, 7a–d, and 8a–c (1.5 equiv) were reacted with either 4,7-dichloroquinoline or 4-chloro-7-trifluromethylquinoline (1 equiv) and phenol (2 equiv) under microwave irradiation at 50 W power and 145 °C temperature at atmospheric pressure for 30 min. After completion of the reaction, the reaction mixture was cooled to room temperature and dissolved in 4 N aqueous NaOH solution. The aqueous suspension was extracted with dichloromethane (3 × 50 mL), washed with brine, dried over anhydrous sodium sulfate, filtered, and evaporated under reduced pressure to produce oily residue compounds 9a–i, 10a–h, and 11a–d, which were purified by silica gel column chromatography using methanol–chloroform (0.5:9.5–1:9) as an eluent.
(S)-7-Chloro-N-(4-methyl-1-(4-methylpiperazin-1-yl)pentan-2-yl)quinolin-4-amine (9a)
This compound was obtained as a yellow oil in 60% yield; Rf 0.36 (chloroform/methanol (9:1)); 1H NMR (500 MHz, CDCl3): δ 8.60 (d, J = 5 Hz, 1H), 8.34 (d, J = 8.5 Hz, 1H), 7.99 (d, J = 2 Hz, 1H), 7.34 (dd, J = 9 and 2 Hz, 1H), 6.79 (d, J = 5 Hz, 1H), 4.04–4.09 (m, 1H), 2.89 (s, 3H), 2.73–2.77 (m, 1H), 2.32–2.37 (m, 7H), 2.24 (s, 3H), 1.92 (bs, 2H), 1.59–1.64 (m, 1H), 1.45–1.54 (m, 2H), 0.84 (d, J = 6.5 Hz, 3H), 0.70 (d, J = 6.5 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 157.9, 151.3, 150.5, 134.4, 128.6, 126.4, 124.6, 121.3, 108.6, 59.9, 58.7, 55.1, 53.7, 45.9, 40.9, 32.3, 25.1, 22.8, 22.5; Fourier transform infrared (FTIR) (neat) v = 3583, 2925, 1577 cm–1; HRMS calcd for [C22H31ClN4 + H]+ 375.2310, found 375.2304.
7-Chloro-N-methyl-N-(4-methyl-1-morpholinopentan-2-yl)quinolin-4-amine (9b)
This compound was obtained as a colorless oil in 55% yield; Rf 0.39 (chloroform/methanol (9:1)); 1H NMR (500 MHz, CDCl3): δ 8.50 (d, J = 2.1 Hz, 1H), 8.20 (d, J = 3.5 Hz, 1H), 7.90 (d, J = 2 Hz, 1H), 7.24 (dd, J = 9 and 2.1 Hz, 1H), 6.71 (d, J = 5.25 Hz, 1H), 3.91–4.15 (m, 1H), 3.39–3.48 (m, 4H), 2.80 (s, 3H), 2.61–2.66 (m, 3H), 2.24–2.30 (m, 3H), 2.15–2.19 (m, 2H), 1.53–1.55 (m, 1H), 1.39–1.46 (m, 2H), 0.76 (d, J = 6.35 Hz, 3H), 0.63 (d, J = 6.15 Hz, 3H); 13C NMR (125 MHz, CDCl3): δ 157.9, 151.2, 150.5, 134.5, 128.6, 126.2, 124.7, 121.3, 108.6, 66.9, 60.4, 58.3, 54.1, 40.9, 32.4, 25.1, 22.8, 22.5; FTIR (neat) v = 3583, 1568, 665 cm–1; HRMS calcd for [C20H28ClN3O + H]+ 362.1994, found 362.1990.
(S)-7-Chloro-N-methyl-N-(4-methyl-1-(piperidin-1-yl)pentan-2-yl)quinolin-4-amine (9c)
This compound was obtained as a colorless oil in 61% yield; Rf 0.42 (chloroform/methanol (9:1)); 1H NMR (400 MHz, CDCl3): δ 8.50 (d, J = 4.1 Hz, 1H), 8.39 (d, J = 8.5 Hz, 1H), 7.99 (d, J = 2.8 Hz, 1H), 7.33 (dd, J = 9 and 2.3 Hz, 1H), 6.79 (d, J = 5.25 Hz, 1H), 4.03–4.11 (m, 1H), 2.88 (s, 3H), 2.66–2.71 (m, 1H), 2.24–2.33 (m, 5H), 1.37–1.62 (m, 10H), 0.81 (d, J = 6.3 Hz, 3H), 0.66 (d, J = 6.5 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 157.0, 151.2, 150.5, 134.4, 128.5, 126.6, 124.5, 121.5, 108.5, 60.8, 58.8, 55.3, 40.9, 32.4, 26.1, 25.1, 24.3, 22.8, 22.6; FTIR (neat) v = 3414, 3015, 2949, 1577 cm–1; HRMS calcd for [C21H30ClN3 + H]+ 360.2201, found 360.2193.
(S)-7-Chloro-N-methyl-N-(4-methyl-1-(pyrrolidin-1-yl)pentan-2-yl)quinolin-4-amine (9d)
This compound was obtained as a colorless oil in 65% yield; Rf 0.41 (chloroform/methanol (9:1)); 1H NMR (400 MHz, CDCl3): δ 8.50 (d, J = 5 Hz, 1H), 8.30 (d, J = 9 Hz, 1H), 7.03 (d, J = 2.2 Hz, 1H), 7.35 (dd, J = 9 and 2.3 Hz, 1H), 6.80 (d, J = 5.9 Hz, 1H), 4.05–4.12 (m, 1H), 2.95 (s, 3H), 2.88–2.93 (m, 1H), 2.68–2.73 (m, 1H), 2.60–2.61 (m, 2H), 2.46–2.48 (m, 2H), 1.72–1.77 (m, 4H), 1.63–1.67 (m, 1H), 1.49–1.56 (m, 2H), 0.86 (d, J = 6.5 Hz, 3H), 0.66 (d, J = 6.0 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 157.9, 150.5, 149.9, 134.9, 128.1, 126.5, 124.9, 121.2, 108.1, 57.6, 54.5, 40.5, 33.2, 25.1, 23.5, 22.8, 22.4; FTIR (neat) v = 3583, 2925, 1577 cm–1; HRMS calcd for [C20H28ClN3 + H]+ 346.2045, found 346.2045.
(S)-N2-(7-Chloroquinolin-4-yl)-N1,N1-diethyl-N2,4-dimethylpentane-1,2-diamine (9e)
This compound was obtained as a colorless oil in 54% yield; Rf 0.41 (chloroform/methanol (9:1)); 1H NMR (400 MHz, CDCl3): δ 8.50 (d, J = 5.6 Hz, 1H), 8.40 (d, J = 9.2 Hz, 1H), 8.01 (d, J = 9 Hz, 1H), 7.32 (dd, J = 9 and 2.3 Hz, 1H), 6.70 (d, J = 5.6 Hz, 1H), 4.01–4.08 (m, 1H), 2.90 (s, 3H), 2.78 (dd, J = 13 and 8.5 Hz, 1H), 2.45 (q, J = 7.2 Hz, 4H), 2.41–2.43 (m, 1H), 1.41–1.59 (m, 3H), 0.93 (t, J = 7.2 Hz, 6H), 0.81 (d, J = 6.3 Hz, 3H), 0.64 (d, J = 5.7 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 158.0, 150.6, 150.0, 134.7, 128.0, 126.8, 124.6, 121.2, 108.2, 59.8, 55.3, 47.2, 40.6, 32.7, 25.1, 22.8, 22.5, 11.5; FTIR (neat) v = 3583, 3015, 2949, 1568 cm–1; HRMS calcd for [C20H30ClN3 + H]+ 348.2201, found 348.2205.
(S)-N-Methyl-N-(4-methyl-1-morpholinopentan-2-yl)-7-(trifluoromethyl)quinolin-4-amine (9f)
This compound was obtained as a colorless oil in 58% yield; Rf 0.37 (chloroform/methanol (9:1)); 1H NMR (400 MHz, CDCl3): δ 8.70 (d, J = 5.34 Hz, 1H), 8.47 (d, J = 8.4 Hz, 1H), 8.32 (s, 1H), 7.56 (dd, J = 9 and 1.5 Hz, 1H), 6.90 (d, J = 5 Hz, 1H), 4.08–4.15 (m, 1H), 3.47–3.58 (m, 4H), 2.93 (s, 3H), 2.72 (dd, J = 13 and 9 Hz, 1H), 2.35–2.40 (m, 3H), 2.24–2.29 (m, 2H), 1.65–1.69 (m, 1H), 1.52–1.59 (m, 2H), 0.87 (d, J = 7.0 Hz, 3H), 0.74 (d, J = 6.0 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 157.7, 151.4, 149.0, 130.7, 130.4, 127.6, 126.0, 124.6, 119.4, 109.7, 66.9, 60.4, 58.3, 54.1, 52.6, 40.9, 32.5, 29.7, 28.4, 25.1, 22.8, 22.5; FTIR (neat) v = 3414, 2949, 1577 cm–1; HRMS calcd for [C21H28F3N3O + H]+ 396.2257, found 396.2253.
(S)-N-Methyl-N-(4-methyl-1-(piperidin-1-yl)pentan-2-yl)-7-(trifluoromethyl)quinolin-4-amine (9g)
This compound was obtained as a pale yellow oil in 64% yield; Rf 0.41 (chloroform/methanol (9:1)); 1H NMR (400 MHz, CDCl3): δ 8.67 (d, J = 5.2 Hz, 1H), 8.58 (d, J = 9 Hz, 1H), 8.30 (s, 1H), 7.55 (dd, J = 9 and 1.7 Hz, 1H), 6.80 (d, J = 5.2 Hz, 1H), 4.07–4.13 (m, 1H), 2.91 (s, 3H), 2.67–2.73 (m, 1H), 2.23–2.32 (m, 5H), 1.37–1.62 (m, 9H), 0.83 (d, J = 6.3 Hz, 3H), 0.69 (d, J = 6.5 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 157.9, 151.2, 149.0, 130.2, 127.5, 127.4, 126.4, 125.5, 124.7, 122.8, 119.2, 119.1, 109.6, 60.7, 58.8, 55.3, 40.9, 32.5, 26.1, 25.2, 24.2, 22.8, 22.6; FTIR (neat) v = 3583, 3015, 2925, 1577 cm–1; HRMS calcd for [C22H30F3N3 + H]+ 394.2465, found 394.2455.
(S)-N-Methyl-N-(4-methyl-1-(pyrrolidin-1-yl)pentan-2-yl)-7-(trifluoromethyl)quinolin-4-amine (9h)
This compound was obtained as a colorless oil in 61% yield; Rf 0.39 (chloroform/methanol (9:1)); 1H NMR (400 MHz, CDCl3): δ 8.67 (d, J = 5.3 Hz, 1H), 8.31 (d, J = 9 Hz, 1H), 8.31 (s, 1H), 7.55 (dd, J = 9 and 1.8 Hz, 1H), 6.88 (d, J = 5.3 Hz, 1H), 4.04–4.11 (m, 1H), 2.94 (s, 3H), 2.85 (dd, J = 13 and 8 Hz, 1H), 2.64 (dd, J = 13 and 6 Hz, 1H), 2.52–2.55 (m, 2H), 2.38–2.41 (m, 2H), 1.64–1.74 (m, 5H), 1.48–1.54 (m, 2H), 0.84 (d, J = 6.0 Hz, 3H), 0.63 (d, J = 6.4 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 157.6, 151.5, 149.0, 127.5, 126.2, 124.7, 119.3, 109.3, 59.9, 57.9, 54.5, 40.7, 33.8, 25.1, 23.6, 22.8, 22.4; FTIR (neat) v = 3414, 2949, 1577 cm–1; HRMS calcd for [C21H228F3N3 + H]+ 380.2308, found 380.2305.
(S)-N1,N1-diethyl-N2,4-dimethyl-N2-(7-(trifluoromethyl)quinolin-4-yl)pentane-1,2-diamine (9i)
This compound was obtained as a colorless oil in 54% yield; Rf 0.39 (chloroform/methanol (9:1)); 1H NMR (400 MHz, CDCl3): δ 8.66 (d, J = 6 Hz, 1H), 8.63 (d, J = 9 Hz, 1H), 8.30 (s, 1H), 7.53 (dd, J = 9 and 2 Hz, 1H), 6.88 (d, J = 6 Hz, 1H), 4.03–4.09 (m, 1H), 2.93 (s, 3H), 2.79 (dd, J = 13 and 5 Hz, 1H), 2.44 (q, J = 7 Hz, 4H), 2.41 (dd, J = 13 and 8 Hz, 1H), 1.44–1.61 (m, 3H), 0.93 (t, J = 7 Hz, 6H), 0.82 (d, J = 6 Hz, 3H), 0.64 (d, J = 6 Hz, 3H); 13C NMR (100 MHz, CDCl3): δ 157.7, 151.4, 149.0, 130.5, 130.2, 127.4, 126.6, 124.7, 119.1, 119.0, 109.4, 59.8, 55.4, 47. 2, 40.7, 32.6, 25.1, 22.8, 22.5, 11.6; FTIR (neat) v = 3414, 3015, 2949, 1568 cm–1; HRMS calcd for [C21H30F3N3 + H]+ 382.2465, found 382.2476.
(S)-7-Chloro-N-methyl-N-(1-morpholino-3-phenylpropan-2-yl)quinolin-4-amine (10a)
This compound was obtained as a colorless oil in 55% yield; Rf 0.43 (chloroform/methanol (9:1)); 1H NMR (400 MHz, CDCl3): δ 8.54 (d, J = 3.2 Hz, 1H), 7.97 (d, J = 1.3 Hz, 1H), 7.82 (d, J = 6 Hz, 1H), 7.22–7.28 (m, 4H), 7.18 (dd, J = 5.7 and 1.2 Hz, 1H), 7.11–7.13 (m, 1H), 6.71 (d, J = 3.2 Hz, 1H), 4.21–4.28 (m, 1H), 3.42–3.52 (m, 4H), 3.05 (dd, J = 8.7 and 4.7 Hz, 1H), 3.00 (s, 3H), 2.87 (dd, J = 8.7 and 4.3 Hz, 1H), 2.73 (dd, J = 8.3 and 5.6 Hz, 1H), 2.37 (dd, J = 8.3 and 3.2 Hz, 1H), 2.29 (m, 2H), 2.13–2.16 (m, 2H); 13C NMR (100 MHz, CDCl3): δ 157.7, 151.0, 150.3, 138.4, 134.5, 128.1, 128.6, 128.5, 126.7, 125.9, 124.8, 121.3, 109.5, 66.8, 62.2, 59.8, 53.9, 37.2, 32.3, 29.7; FTIR (neat) v = 3583, 2925, 1577 cm–1 HRMS calcd for [C23H26ClN3O + H]+ 396.1837, found 396.1845.
(S)-7-Chloro-N-methyl-N-(1-phenyl-3-(piperidin-1-yl)propan-2-yl)quinolin-4-amine (10b)
This compound was obtained as a colorless oil in 58% yield; Rf 0.46 (chloroform/methanol (9:1)); 1H NMR (400 MHz, CDCl3): δ 8.49 (d, J = 5.4 Hz, 1H), 7.95 (d, J = 2 Hz, 1H), 7.89 (d, J = 9 Hz, 1H), 7.19–7.25 (m, 4H), 7.17 (dd, J = 9 and 2 Hz, 1H), 7.09–7.10 (d, J = 2 Hz, 1H), 7.07–7.08 (m, 1H), 6.66 (d, J = 5.3 Hz, 1H), 4.21–4.28 (m, 1H), 2.98 (dd, J = 13.9 and 7.8 Hz, 1H), 2.98 (s, 3H), 2.84 (dd, J = 13.9 and 6.7 Hz, 1H), 2.68 (dd, J = 13.3 and 8.8 Hz, 1H), 2.27 (dd, J = 13.3 and 3.1 Hz, 1H), 2.10–2.14 (m, 2H), 1.94 (bs, 2H), 1.27–1.40 (m, 6H); 13C NMR (100 MHz, CDCl3): δ 157.8, 151.9, 150.3, 138.6, 134.3, 129.1, 128.4, 128.3, 126.5, 126.2, 124.6, 124.4, 121.3, 109.4, 62.6, 60.6, 55.1, 37.2, 32.2, 25.9, 24.2; FTIR (neat) v = 3414, 3015, 2949, 1568 cm–1; HRMS calcd for [C24H28ClN3O + H]+ 394.2045, found 394.2051.
(S)-7-Chloro-N-methyl-N-(1-phenyl-3-(pyrrolidin-1-yl)propan-2-yl)quinolin-4-amine (10c)
This compound was obtained as a colorless oil in 53% yield; Rf 0.45 (chloroform/methanol (9:1)); 1H NMR (400 MHz, CDCl3): δ 8.47 (d, J = 5.2 Hz, 1H), 7.95 (d, J = 2 Hz, 1H), 7.79 (d, J = 9 Hz, 1H), 7.20–7.22 (m, 3H), 7.16 (dd, J = 9 and 2 Hz, 1H), 7.08–7.10 (m, 2H), 6.63 (d, J = 5.2 Hz, 1H), 4.17–4.24 (m, 1H), 2.93–3.04 (m, 5H), 2.85 (dd, J = 13 and 8 Hz, 1H), 2.60 (dd, J = 13 and 6 Hz, 1H), 2.41–2.46 (m, 2H), 2.20–2.27 (m, 2H), 1.60–1.67 (m, 4H); 13C NMR (100 MHz, CDCl3): δ 157.7, 150.9, 150.3, 138.7, 134.3, 129.1, 128.4, 126.5, 126.1, 124.7, 121.5, 109.3, 64.0, 57.2, 54.3, 37.0, 32.4, 23.5; HRMS calcd for [C23H26ClN3 + H]+ 380.1888, found 380.1897.
(S)-N2-(7-Chloroquinolin-4-yl)-N1,N1-diethyl-N2-methyl-3-phenylpropane-1,2-diamine (10d)
This compound was obtained as a colorless oil in 51% yield; Rf 0.45 (chloroform/methanol (9:1)); 1H NMR (500 MHz, CDCl3): δ 8.49 (d, J = 5.3 Hz, 1H), 7.96 (d, J = 2 Hz, 1H), 7.91 (d, J = 9 Hz, 1H), 7.18–7.23 (m, 3H), 7.16 (dd, J = 9 and 2 Hz, 1H), 7.07–7.09 (m, 2H), 6.64 (d, J = 5.3 Hz, 1H), 4.15–4.21 (m, 1H), 3.03 (s, 3H), 2.94–2.95 (m, 2H), 2.76 (dd, J = 13 and 9 Hz, 1H), 2.44 (dd, J = 13 and 6 Hz, 1H), 2.34 (q, J = 7 Hz, 4H), 0.84 (t, J = 7 Hz, 6H); 13C NMR (100 MHz, CDCl3): δ 157.7, 150.9, 150.3, 138.8, 134.3, 129.1, 128.4, 128.3, 126.5, 126.3, 124.7, 121.5, 109.3, 63.9, 54.5, 47.2, 36.8, 32.3, 11.6; HRMS calcd for [C23H28ClN3S + H]+ 382.2045, found 382.2054.
(S)-N-Methyl-N-(1-morpholino-3-phenylpropan-2-yl)-7-(trifluoromethyl)quinolin-4-amine (10e)
This compound was obtained as a colorless oil in 58% yield; Rf 0.39 (chloroform/methanol (9:1)); 1H NMR (400 MHz, CDCl3): δ 8.62 (d, J = 5.2 Hz, 1H), 8.28 (s, 1H), 7.99 (d, J = 9 Hz, 1H), 7.39 (dd, J = 9 and 2 Hz, 1H), 7.23–7.28 (m, 3H), 7.12–7.14 (m, 2H), 6.79 (d, J = 5.2 Hz, 1H), 4.25–4.32 (m, 1H), 3.47–3.53 (m, 2H), 3.42–3.43 (m, 2H), 3.06 (dd, J = 14 and 8 Hz, 1H), 3.03 (s, 3H), 2.89 (dd, J = 14 and 7 Hz, 1H), 2.76 (dd, J = 13 and 9 Hz, 1H), 2.36 (dd, J = 13 and 5 Hz, 1H), 2.29 (bs, 2H), 2.11–2.16 (m, 2H); 13C NMR (100 MHz, CDCl3): δ 157.4, 151.2, 148.8, 130.6, 130.2, 129.1, 128.6, 127.6, 127.5, 126.8, 125.7, 124.6, 119.6, 119.5, 110.6, 66.8, 62.1, 59.8, 53.9, 37.2, 32.3; HRMS calcd for [C24H26F3N3O + H]+ 430.2101, found 430.2104.
(S)-N-Methyl-N-(1-phenyl-3-(piperidin-1-yl)propan-2-yl)-7-(trifluoromethyl)quinolin-4-amine (10f)
This compound was obtained as a colorless oil in 58% yield; Rf 0.42 (chloroform/methanol (9:1)); 1H NMR (400 MHz, CDCl3): δ 8.59 (d, J = 5.2 Hz, 1H), 8.27 (s, 1H), 8.06 (d, J = 8.8 Hz, 1H), 7.38 (dd, J = 7 and 1.8 Hz, 1H), 7.20–7.28 (m, 3H), 7.09–7.11 (m, 2H), 6.75 (d, J = 5.2 Hz, 1H), 4.25–4.32 (m, 1H), 3.01 (dd, J = 14 and 6.8 Hz, 1H), 3.01 (s, 3H), 2.80 (dd, J = 14 and 6.6 Hz, 1H), 2.70 (dd, J = 13.3 and 9 Hz, 1H), 2.30 (dd, J = 13.3 and 5 Hz, 1H), 2.25 (bs, 2H), 2.11–2.15 (m, 2H), 1.31–1.40 (m, 6H); 13C NMR (100 MHz, CDCl3): δ 157.7, 151.2, 148.8, 138.5, 129.8, 129.1, 128.5, 127.4, 127.3, 126.6, 126.0, 124.7, 119.4, 119.3, 110.4, 62.6, 60.1, 55.1, 37.2, 32.3, 25.9, 24.2; HRMS calcd for [C25H29F3N3 + H]+ 428.2308, found 428.2327.
(S)-N-Methyl-N-(1-phenyl-3-(pyrrolidin-1-yl)propan-2-yl)-7-(trifluoromethyl)quinolin-4-amine (10g)
This compound was obtained as a colorless oil in 59% yield; Rf 0.41 (chloroform/methanol (9:1)); 1H NMR (400 MHz, CDCl3): δ 8.55 (d, J = 5.2 Hz, 1H), 8.26 (s, 1H), 7.90 (d, J = 8.8 Hz, 1H), 7.38 (dd, J = 8.8 and 1.8 Hz, 1H), 7.19–7.22 (m, 3H), 7.08–7.11 (m, 2H), 6.63 (d, J = 5.2 Hz, 1H), 4.22–4.25 (m, 1H), 3.03 (s, 3H), 2.98–3.03 (m, 2H), 2.88 (dd, J = 12.8 and 5 Hz, 1H), 2.62 (dd, J = 12.8 and 6.1 Hz, 1H), 2.42–2.46 (m, 2H), 2.23–2.27 (m, 2H), 1.61–1.66 (m, 4H); 13C NMR (100 MHz, CDCl3): δ 157.5, 151.2, 148.8, 138.6, 129.1, 128.4, 127.4, 127.3, 126.6, 125.9, 124.8, 119.5, 119.4, 110.3, 63.9, 57.2, 54.3, 37.0, 32.4, 23.5; HRMS calcd for [C24H26F3N3 + H]+ 414.2152, found 414.2154.
(S)-N1,N1-Diethyl-N2-methyl-3-phenyl-N2-(7-(trifluoromethyl)quinolin-4-yl)propane-1,2-diamine (10h)
This compound was obtained as a colorless oil in 51% yield; Rf 0.41 (chloroform/methanol (9:1)); 1H NMR (400 MHz, CDCl3): δ 8.56 (d, J = 5.3 Hz, 1H), 8.27 (s, 1H), 8.10 (d, J = 8.8 Hz, 1H), 7.37 (dd, J = 8.8 and 1.8 Hz, 1H), 7.19–7.24 (m, 3H), 7.08–7.10 (m, 2H), 6.71 (d, J = 5.3 Hz, 1H), 4.21–4.24 (m, 1H), 3.02 (s, 3H), 2.95–2.98 (m, 2H), 2.79 (dd, J = 13.6 and 8 Hz, 1H), 2.44 (dd, J = 13.6 and 5.6 Hz, 1H), 2.34 (q, J = 7 Hz, 4H), 0.84 (t, J = 7 Hz, 6H); 13C NMR (100 MHz, CDCl3): δ 157.5, 151.2, 148.8, 138.7, 130.5, 130.1, 129.1, 128.4, 127.3, 127.2, 126.6, 126.1, 124.7, 119.3, 110.3, 63.9, 54.5, 47.2, 36.8, 32.3, 11.6; HRMS calcd for [C24H28F3N3 + H]+ 416.2308, found 416.2307.
7-Chloro-N-methyl-N-(3-(piperidin-1-yl)propyl)quinolin-4-amine (11a)
This compound was obtained as a colorless oil in 52% yield; Rf 0.48 (chloroform/methanol (9:1)); 1H NMR (400 MHz, CDCl3): δ 8.86 (d, J = 5.2 Hz, 1H), 8.02 (d, J = 2 Hz, 1H), 7.98 (d, J = 9 Hz, 1H), 7.39 (dd, J = 9, 2 Hz, 1H), 6.80 (d, J = 5.2 Hz, 1H), 3.31 (t, J = 7 Hz, 3H), 3.00 (s, 3H), 2.38–2.44 (m, 6H), 1.93–2.01 (m, 2H), 1.60–1.66 (m, 4H), 1.46–1.47 (m, 2H); 13C NMR (100 MHz, CDCl3): δ 157.4, 151.4, 150.4, 134.7, 128.6, 125.7, 125.5, 121.9, 108.6, 56.2, 54.5, 53.9, 40.9, 25.4, 24.4, 24.0; HRMS calcd for [C18H24ClN3 + H]+ 318.1732, found 318.1736.
7-Chloro-N-methyl-N-(3-(pyrrolidin-1-yl)propyl)quinolin-4-amine (11b)
This compound was obtained as a colorless oil in 55% yield; Rf 0.46 (chloroform/methanol (9:1)); 1H NMR (400 MHz, CDCl3): δ 8.63 (d, J = 5.2 Hz, 1H), 8.01 (d, J = 2 Hz, 1H), 7.99 (d, J = 9 Hz, 1H), 7.38 (dd, J = 9 and 2 Hz, 1H), 6.79 (d, J = 5.2 Hz, 1H), 3.32 (t, J = 7 Hz, 3H), 3.00 (s, 3H), 2.51–2.56 (m, 6H), 1.92–2.00 (m, 2H), 1.78–1.81 (m, 4H); 13C NMR (100 MHz, CDCl3): δ 157.4, 151.4, 150.3, 134.7, 128.6, 125.7, 125.5, 121.9, 108.6, 54.1, 54.0, 53.5, 40.8, 29.6, 26.5, 23.4; HRMS calcd for [C17H22ClN3 + H]+ 304.1575, found 304.1576.
N-Methyl-N-(3-morpholinopropyl)-7-(trifluoromethyl)quinolin-4-amine (11c)
This compound was obtained as a colorless oil in 48% yield; Rf 0.41 (chloroform/methanol (9:1)); 1H NMR (400 MHz, CDCl3): δ 8.74 (bs, 1H), 8.34 (s, 1H), 8.17 (d, J = 9 Hz, 1H), 7.61 (d, J = 9 Hz, 1H), 6.89 (d, J = 5.7 Hz, 1H), 3.64 (t, J = 7 Hz, 2H), 3.39 (t, J = 7 Hz, 2H), 3.00 (s, 3H), 2.34–2.39 (m, 6H), 1.87–1.94 (m, 2H); 13C NMR (100 MHz, CDCl3): δ 157.2, 151.7, 148.9, 130.5, 127.6, 125.6, 125.3, 125.1, 122.6, 120.1, 109.7, 66.8, 55.9, 53.8, 53.6, 40.7, 29.6, 24.3; HRMS calcd for [C18H22F3N3 + H]+ 354.1788, found 354.1790.
N-Methyl-N-(3-(pyrrolidin-1-yl)propyl)-7-(trifluoromethyl)quinolin-4-amine (11d)
This compound was obtained as a colorless oil in 52% yield; Rf 0.43 (chloroform/methanol (9:1)); 1H NMR (400 MHz, CDCl3): δ 8.73 (d, J = 5.2 Hz, 1H), 8.33 (bs, 1H), 8.19 (d, J = 9 Hz, 1H), 7.69 (dd, J = 9 and 2 Hz, 1H), 6.89 (d, J = 5.2 Hz, 1H), 3.36 (t, J = 7 Hz, 3H), 3.03 (s, 3H), 2.47–2.51 (m, 6H), 1.92–2.00 (m, 2H), 1.76–1.79 (m, 4H); 13C NMR (100 MHz, CDCl3): δ 157.2, 151.6, 148.9, 130.4, 127.6, 127.4, 125.1, 120.1, 120.0, 109.7, 54.1, 53.5, 40.7, 26.7, 23.4; HRMS calcd for [C18H22F3N3 + H]+ 338.1839, found 338.1837.
Acknowledgments
V.S.T. thanks UGC New Delhi for financial support. S.C. thanks DST (SERB wing and INSPIRE division) for providing fellowship and research grants (NPDF-file no. PDF/2016/003851 and INSPIRE Faculty programme-registration no.: IFA17-CH274). The authors also thank the SAIF Division, CSIR-CDRI, for providing the analytical data. The P. falciparum strains PF3D7 (MRA-151) and K1 (MRA-159) were obtained from BEI resources NIAID, NIH. Human Ethics Committee clearance number for the P. falciparum culture in human RBCs is CDRI/IEC/2019/A8 (communication no. 10236).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c06053.
General information about biological and chemical methods used; details of the method optimization; characterization details of the synthesized compounds; and NMR, 1H, and 13C NMR spectra (PDF)
Author Contributions
∥ R.T. and W.H. contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- World Malaria Report 2019; World Health Organization; Geneva, 2019. (accessed April 5, 2021).
- World Malaria Report 2020: 20 Years of Global Progress and Challenges; World Health Organization: Geneva, 2020. (accessed April 5, 2021).
- a Pradhan A.; Anasuya A.; Pradhan M. M.; Kavitha A. K.; Kar P.; Sahoo K. C.; Panigrahi P.; Dutta A. Trends in Malaria in Odisha, India-An Analysis of the 2003-2013 Time-Series Data from the National Vector Borne Disease Control Program. PLoS One 2016, 11, e0149126 10.1371/journal.pone.0149126. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Sharma R. K.; Thakor H. G.; Saha K. B.; Sonal G. S.; Dhariwal A. C.; Singh N. Malaria situation in India with special reference to tribal areas. Indian J. Med. Res. 2015, 141, 537–545. 10.4103/0971-5916.159510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow R. W.; Guerra C. A.; Noor A. M.; Myint H. Y.; Hay S. I. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 2005, 434, 214–217. 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley E.; McGready R.; Proux S.; Nosten F. Malaria. Travel Med. Infect. Dis. 2006, 4, 159–173. 10.1016/j.tmaid.2005.06.009. [DOI] [PubMed] [Google Scholar]
- a Martin S. K.; Oduola A. M.; Milhous W. K. Reversal of chloroquine resistance in Plasmodium falciparum by verapamil. Science 1987, 235, 899–901. 10.1126/science.3544220. [DOI] [PubMed] [Google Scholar]; b Bitonti A. J.; Sjoerdsma A.; McCann P. P.; Kyle D. E.; Oduola A. M.; Rossan R. N.; Milhous W. K.; Davidson D. E. Jr. Reversal of chloroquine resistance in malaria parasite Plasmodium falciparum by desipramine. Science 1988, 242, 1301–1303. 10.1126/science.3057629. [DOI] [PubMed] [Google Scholar]; c Ohsawa K.; Tanabe K.; Kimata I.; Miki A. Ultrastructural changes associated with reversal of chloroquine resistance by verapamil in Plasmodium chabaudi. Parasitology 1991, 103, 185–189. 10.1017/S0031182000059461. [DOI] [PubMed] [Google Scholar]; d Kyle D. E.; Milhous W. K.; Rossan R. N. Reversal of Plasmodium falciparum Resistance to Chloroquine in Panamanian Aotus Monkeys. Am. J. Trop. Med. Hyg. 1993, 48, 126–133. 10.4269/ajtmh.1993.48.126. [DOI] [PubMed] [Google Scholar]
- a Burgess S. J.; Selzer A.; Kelly J.-X.; Smilkstein M. J.; Riscoe M. K.; Peyton D. H. A chloroquine-like molecule designed to reverse resistance in Plasmodium falciparum. J. Med. Chem. 2006, 49, 5623–5625. 10.1021/jm060399n. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Andrews S.; Burgess S. J.; Skaalrud D.; Kelly J.-X.; Peyton D. H. Reversal Agent and Linker Variants of Reversed Chloroquines: Activities against Plasmodium falciparum. J. Med. Chem. 2010, 53, 916–919. 10.1021/jm900972u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Burgess S. J.; Selzer A.; Kelly J.-X.; Smilkstein M. J.; Riscoe M. K.; Peyton D. H. A chloroquine-like molecule designed to reverse resistance in Plasmodium falciparum. J. Med. Chem. 2006, 49, 5623–5625. 10.1021/jm060399n. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Andrews S.; Burgess S. J.; Skaalrud D.; Kelly J.-X.; Peyton D. H. Reversal Agent and Linker Variants of Reversed Chloroquines: Activities against Plasmodium falciparum. J. Med. Chem. 2010, 53, 916–919. 10.1021/jm900972u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan T. J.; Hunter R.; Kaschula C. H.; Marques H. M.; Misplon A.; Walden J. Structure–Function Relationships in Aminoquinolines: Effect of Amino and Chloro Groups on Quinoline–Hematin Complex Formation, Inhibition of β-Hematin Formation, and Antiplasmodial Activity. J. Med. Chem. 2000, 43, 283–291. 10.1021/jm990437l. [DOI] [PubMed] [Google Scholar]
- a Bawa S.; Kumar S.; Drabu S.; Kumar R. Structural modifications of quinoline-based antimalarial agents: Recent developments. J. Pharm. BioAllied Sci. 2010, 2, 64–71. 10.4103/0975-7406.67002. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Lawrenson A. S.; Cooper D. L.; O’Neill P. M.; Berry N. G. Study of the antimalarial activity of 4-aminoquinoline compounds against chloroquine-sensitive and chloroquine-resistant parasite strains. J. Mol. Model. 2018, 24, 237 10.1007/s00894-018-3755-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill P. M.; Ward S. A.; Berry N. G.; Jeyadevan J. P.; Biagini G. A.; Asadollaly E.; Park B. K.; Bray P. G. A medicinal chemistry perspective on 4-aminoquinoline antimalarial drugs. Curr. Top. Med. Chem. 2006, 6, 479–507. 10.2174/156802606776743147. [DOI] [PubMed] [Google Scholar]
- Stocks P. A.; Raynes K. J.; Bray P. G.; Park B. K.; O’Neill P. M.; Ward S. A. Novel Short Chain Chloroquine Analogues Retain Activity Against Chloroquine Resistant K1 Plasmodium falciparum. J. Med. Chem. 2002, 45, 4975–4983. 10.1021/jm0108707. [DOI] [PubMed] [Google Scholar]
- Madrid P. B.; Wilson N. T.; De Risi J. L.; Guy R. K. Parallel Synthesis and Antimalarial Screening of a 4-Aminoquinoline Library. J. Comb. Chem. 2004, 6, 437–442. 10.1021/cc0340473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Rajapakse C. S. K.; Lisai M.; Deregnaucourt C.; Sinou V.; Latour C.; Roy D.; Schrével J.; Sánchez-Delgado R. A. Synthesis of New 4-Aminoquinolines and Evaluation of Their In Vitro Activity against Chloroquine-Sensitive and Chloroquine-Resistant Plasmodium falciparum. PLoS One 2015, 10, e0140878 10.1371/journal.pone.0140878. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Parhizgar A. R.; Tahghighi A. Introducing New Antimalarial Analogues of Chloroquine and Amodiaquine: A Narrative Review. Iran. J. Med. Sci. 2017, 42, 115–128. [PMC free article] [PubMed] [Google Scholar]
- Sinha M.; Dola V. R.; Soni A.; Agarwal P.; Srivastava K.; Haq W.; Puri S. K.; Katti S. B. Synthesis of chiral chloroquine and its analogues as antimalarial agents. Bioorg. Med. Chem. 2014, 22, 5950–5960. 10.1016/j.bmc.2014.09.009. [DOI] [PubMed] [Google Scholar]
- Dola V. R.; Soni A.; Agarwal P.; Ahmad H.; Raju K. S.; Rashid M.; Wahajuddin M.; Srivastava K.; Haq W.; Dwivedi A. K.; Puri S. K.; Katti S. B. Synthesis and Evaluation of Chirally Defined Side Chain Variants of 7-Chloro-4-Aminoquinoline To Overcome Drug Resistance in Malaria Chemotherapy. Antimicrob. Agents Chemother. 2017, 61, e01152-16 10.1128/AAC.01152-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y.; Yan W.; Cao D.; Shao M.; Li D.; Wang F.; Yang Z.; Chen Y.; He L.; Wang T.; Shen M.; Chen L. Design, synthesis and biological evaluation of 4-anilinoquinoline derivatives as novel potent tubulin depolymerization agents. Eur. J. Med. Chem. 2017, 138, 1114–1125. 10.1016/j.ejmech.2017.07.040. [DOI] [PubMed] [Google Scholar]
- Berger D. M.; Dutia M. D.; Demorin F. F.; Boschelli D. H.; Powell D. W.; Tsou H.-R.; Wissner A.; Zhang N.; Ye F.; Wu B.; Zhang N.; Ye F.; Wu B.. Preparation of Substituted Aromatic Tricyclic Compounds Containing Nicotinonitrile Rings as Protein Kinase Inhibitors. WO2001047892A1, Jul 05, 2001.
- Gallo S.; Atifi S.; Mahamoud A.; Santelli-Rouvier C.; Wolfárt K.; Molnar J.; Barbe J. Synthesis of aza mono, bi and tricyclic compounds. Evaluation of their anti MDR activity. Eur. J. Med. Chem. 2003, 38, 19–26. 10.1016/S0223-5234(02)01422-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.