Abstract
The present study was carried out to investigate the effects of methionine (Met), lysine (Lys), isoleucine (Ile), and threonine (Thr) deficiency in a low-protein diet on laying performance, egg quality, serum biochemical indices, and the gut microbiota in laying hens. A total of 300 Peking Pink laying hens, at 38 weeks of age, were randomly allocated to five dietary treatments, each of which included six replicates of ten hens. Hens were fed an amino acid-balanced diet (Met: 0.46%; Lys: 0.76%; Ile: 0.72%; Thr: 0.56%; positive control, PC), Met deficiency diet (Met-, 0.25%), Lys deficiency diet (Lys-, 0.56%), Ile deficiency diet (Ile-, 0.54%), and Thr deficiency diet (Thr-, 0.46%) for 12 weeks. Hens were housed in pairs in 45 × 45 × 45 cm wire cages with three ladders and three birds per cage. Feed and water were provided ad libitum during the entire experimental period. All data were analyzed using one-way ANOVA with Turkey’s multiple range test. Here, compared to the PC group, final body weight (FBW), average daily gain (ADG), egg production (EP), egg weight (EW), average daily egg mass (EM), average daily feed intake (ADFI), and yield of abdominal fat (AFY) in the Met-group were lower, while EW and EM were higher in the Lys-group. The feed egg ratio (FER) was increased in the Met- and Lys-groups, and EW and AFY were decreased in the Ile-group compared to the controls. Meanwhile, ADG, EP, EM, and ADFI were lower in the Thr group than the PC group. The level of triglycerides (TGs) in the four groups was lower and the concentrations of uric acid (UA) in the Met-group were higher than those in the PC group. The shell color in the Thr group was lower than the PC group. Of note, amino acid deficiency altered the gut microbial structure (e.g., increasing the level of Parabacteroides and decreasing the abundance of Lactobacillus) in hens. The correlation analysis showed that amino acid deficiency-induced gut microbiota alteration is closely associated with the change in key parameters: FER, UA, AFY, EW, EM, TG, FBW, EP, and ADFI. Collectively, our results highlight the role of adequate amino acid ratio supplementation in the low-crude-protein diet structure for laying hens.
Introduction
The increasing consumption of vegetable protein resources and the excessive discharge of nitrogen caused environmental concerns associated with animal husbandry.1 Therefore, the development of new protein utilization strategies to supply the shortage of this nutrient is an urgent need. For the laying hen industry, crude protein is considered as the most important nutrients, which also accounts for about one-third of the total cost of feed production. Therefore, the low crude protein can be used in formulated diets for laying hens and better improve the economic benefits. Recent years have witnessed the great advantages of low-crude-protein dietary with amino acid supplementation for animal industry, especially swine,2 including saving protein resources and reducing nitrogen excretion, feed costs, and the risk of host metabolic dysfunction without impairing performance compared to the normal protein level diet.2,3 However, very few studies have been performed to study about the effects of a low-protein diet supplemented with amino acids on the laying performance, egg quality, and gut microbiota of laying hens.
Soybean meal is the most commonly used protein source because of its highly digestible protein and amino acid profiles, but Met and Lys are the most essential limiting amino acids in corn-soybean meal diets for laying hens, mainly involving in protein synthesis and closely related to laying performance and egg quality.4,5 When fed methionine (Met)-, lysine (Lys)-, and tryptophan (Try)-deficient diets, the feed intakes were lower in the amino acid-deficiency groups than the normal group.6 In addition, the higher level of Met in a low-protein diet significantly improved the daily feed intake, laying percentage, egg mass, and FE of hens,7 and the observed results closely resembled those reported by another study.8 Among the essential amino acids, threonine (Thr) is particularly important for maintenance of gut function; a large proportion of dietary Thr (up to 60%) is retained by the healthy pig or human intestine.9,10 The supplementation of Thr in the low-crude-protein diet affected laying performance, intestinal mucosal barrier, and gut microbial community, suggesting that it is a limiting amino acid of laying hens during the peak production period.11,12 However, although a dietary deficiency of Thr is known to impair the growth of animals (e.g., pigs and rats),13 little information is available concerning its effects on host metabolism and the gut microbial community in a low-protein diet of laying hens. Recent commercialization of isoleucine (Ile) has prompted further interest in optimizing low-protein diets for laying hens.14 Several previous studies revealed that Ile is a limiting amino acid in corn-soybean meal diets for laying hens with low-crude-protein diets.15,16 Peganova and Eder et al. indicated that the feed with Ile-deficient basal ratios supplementing varying amounts of Ile found that the margin between requirement and excess of Ile is narrow in laying hens.15 Recent studies have found that under the conditions which meet the requirements of all other amino acids, a corn-soybean meal-based diet with crude protein reduced by 2% unit points is limiting in Ile and will compromise egg production (EP), whereas excess L-Ile will limit EW.17 These findings suggest that the response to dietary amino acids is likely dependent on a multitude of host, nutrition, gut microbiota, and other important factors, and further research should be conducted on the interaction mechanism of amino acid utilization and host metabolism in the low-crude-protein diet.
The intestinal tract of poultry possesses a complex microbial community consisting primarily of bacteria, which play a key role in host performance and gut health.18 Recently, a study showed that the gut microbiota of laying hens was influenced by multiple factors, such as age, production system, disease, and diets.12,19 In addition, diet is a major factor that influences the microbial community in the gut.20,21 Previous studies have showed that amino acids can regulate protein homeostasis in the body, growth of bacteria, and regulation of immune response.10,22,23 Therefore, it is reasonable to hypothesize that the supplementation of amino acids in low-protein diets can affect the community and structure of the gut microbiota in laying hens.
Collectively, the dietary protein plays an important role in the diet formulation of poultry to maintain and improve growth, feed utilization, intestinal health, gut microbiota, and immune response and reduce environmental risks and pollution by optimizing the use of this nutrient. In this study, we hypothesized that amino acid (Met, Lys, Ile, and Thr) deficiencies mainly affected laying performance, egg quality, serum biochemical parameters, and the gut microbiota in low-protein diets of laying hens.
Results
Effects of Amino Acid Deficiency on Laying Performance in Laying Hens with Low-Protein Diets
During the experiment period (Table 2), the deficiency of the four amino acids in the low-protein diet significantly affected (P < 0.001) the growth and laying performance of hens. For example, main parameters, such as final body weight (FBW), average daily gain (ADG), EP, EW, egg mass (EM), average daily feed intake (ADFI), and yield of abdominal fat (AFY) in the Met-deficiency diet (Met-) group were significantly lower and FER was significantly higher than the positive control (PC) group (Table 2). The EW and EM were lower (P < 0.001) in the Lys-deficiency diet (Lys-) group compared to the PC group. Also, the EW and AFY were lower (P < 0.001) in the Ile-deficiency diet (Ile-) group compared to the PC group (Table 2). Compared to the PC group, the ADG, EP, EM, and ADFI were lower (P < 0.001) in the Thr-deficiency diet (Thr-) group (Table 2).
Table 2. Effects of Amino Acid Deficiency in Low-Protein Diets on Growth and Laying Performance of 38–50 Weeks Laying Hensa.
| groups | IBW (g) | FBW (g) | ADG (g/d) | EP (%) | EW (g) | EM (g/d) | ADFI (g/d) | FER | AFY (%) |
|---|---|---|---|---|---|---|---|---|---|
| PC group | 1512.0 | 1652.9a | 1.72a | 86.9a | 61.7a | 53.5a | 110.7a | 2.07c | 3.08a |
| Met- | 1507.3 | 1509.9b | 0.03c | 68.1c | 56.4c | 38.4c | 97.4c | 2.54a | 1.32c |
| Lys- | 1534.8 | 1626.7a | 1.12ab | 81.3ab | 59.0b | 48.0b | 107.8ab | 2.25b | 2.17abc |
| Ile- | 1533.3 | 1631.7a | 1.20ab | 87.1a | 59.5b | 51.8a | 107.6ab | 2.08c | 2.00bc |
| Thr- | 1545.3 | 1600.3a | 0.67bc | 79.7b | 60.0ab | 47.9b | 103.3b | 2.16bc | 2.46ab |
| SEb | 16.35 | 19.86 | 0.20 | 1.62 | 0.45 | 0.98 | 1.45 | 0.04 | 0.25 |
| P value | 0.327 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
abcMeans within a row with no common superscripts differ (P < 0.05).
Pooled standard error of the mean.
Effects of Amino Acid Deficiency on Egg Quality in Laying Hens with Low-Protein Diets
The effects of diet on shell color and EW were significantly different (P < 0.05) during the experimental period, while there was no difference in shell thickness, Haugh unit, yolk color, albumen, and shell proportion (Table 3). As shown in Table 3, the shell color in the Thr-group was significantly lower (P < 0.05) than the PC group. Meanwhile, the EW in the Met-group was significantly lower (P < 0.05) than the other four groups. The lowest Haugh unit was found in the Ile-group, and the lowest albumen proportion and the highest shell proportion were found in the Met-group (Table 3).
Table 3. Effects of Amino Acid Deficiency in Low-Protein Diets on Egg Quality of 38–50 Weeks Laying Hensa.
| groups | shell thickness (mm) | shell strength (N) | haugh unit | yolk color | shell color | egg weight (g) | yolk (%) | albumen (%) | shell (%) |
|---|---|---|---|---|---|---|---|---|---|
| PC group | 0.44 | 36.57 | 82.53 | 4.70 | 54.53a | 61.61a | 26.01 | 63.62 | 10.37 |
| Met- | 0.46 | 38.25 | 82.23 | 4.73 | 51.61ab | 56.48b | 27.82 | 61.37 | 10.80 |
| Lys- | 0.46 | 40.63 | 83.90 | 4.83 | 52.12ab | 60.51a | 26.67 | 62.72 | 10.62 |
| Ile- | 0.47 | 38.69 | 80.63 | 4.93 | 53.97ab | 61.01a | 26.34 | 62.97 | 10.68 |
| Thr- | 0.46 | 40.15 | 85.50 | 5.13 | 50.93b | 62.46a | 26.47 | 63.07 | 10.45 |
| SEb | 0.01 | 1.46 | 1.83 | 0.14 | 0.99 | 0.76 | 0.65 | 0.17 | 0.19 |
| P value | 0.08 | 0.30 | 0.41 | 0.21 | 0.047 | <0.001 | 0.35 | 0.66 | 0.48 |
abMeans within a row with no common superscripts differ (P < 0.05).
Pooled standard error of the mean.
Effects of Amino Acid Deficiency on Serum Parameters in Laying Hens with Low-Protein Diets
Amino acid deficiency in low-protein diets significantly affected (P < 0.05) the concentrations of triglycerides (TG) and uric acid (UA) in the serum of laying hens, while no difference was observed in the urea, total protein (TP), albumin (ALB), superoxide dismutase (SOD), glutathione peroxidase (GSH), and immune globulin M (IgM) among the five groups (Table 4). The level of TG in the four amino acid-deficiency groups was lower (P < 0.001) than that in the PC group (Table 4). The content of UA in the Met-group, Ile-group, and Thr-group was higher (P < 0.05) compared to that of the PC group. Additionally, the level of TP and IgM was the lowest (P < 0.05) in the Met-group and the Thr-group had the highest (P < 0.05) urea concentrations in the five groups (Table 4).
Table 4. Effects of Amino Acid Deficiency in Low-Protein Diets on Serum Biochemical Indices of 38–50 Weeks Laying Hensa.
| groups | TG (mmol/L) | UA (μmol/L) | urea (mmol/L) | TP (g/L) | ALB (g/L) | SOD (U/mL) | GSH (U/mL) | IgM (g/L) |
|---|---|---|---|---|---|---|---|---|
| PC group | 11.54a | 64.56b | 0.37 | 39.28 | 13.00 | 163.95 | 687.37 | 0.89 |
| Met- | 7.64b | 82.33a | 0.31 | 37.47 | 12.81 | 169.36 | 695.33 | 0.80 |
| Lys- | 8.40b | 63.33b | 0.31 | 37.86 | 12.37 | 172.15 | 692.38 | 0.78 |
| Ile- | 8.78b | 70.44ab | 0.43 | 39.61 | 12.99 | 170.34 | 720.82 | 0.82 |
| Thr- | 8.41b | 72.78ab | 0.45 | 42.24 | 12.64 | 169.57 | 719.00 | 0.86 |
| SEb | 0.48 | 4.31 | 0.04 | 2.15 | 0.39 | 2.02 | 13.12 | 0.05 |
| P value | <0.001 | 0.025 | 0.070 | 0.551 | 0.765 | 0.074 | 0.239 | 0.570 |
abMeans within a row with no common superscripts differ (P < 0.05).
Pooled standard error of the mean.
Effects of Amino Acid Deficiency on the Cecal Microbiota in Laying Hens with Low-Protein Diets
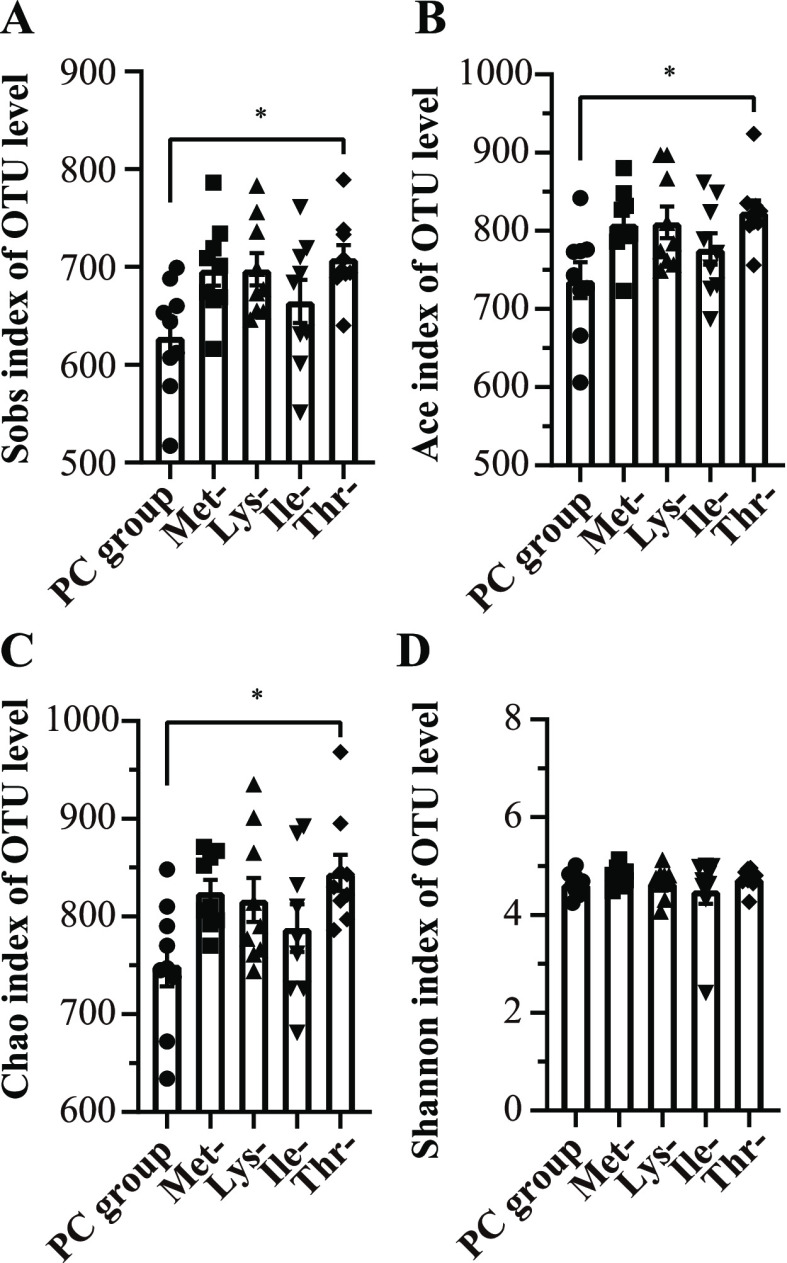
To investigate how four amino acid deficiencies in low-protein diets impacted the gut microbiota composition of laying hens, 16S rDNA sequencing was used. Eventually, after size filtering, quality control, and chimera removal, 2,714,438 valid sequences were obtained with an average of 60,320.8 ± 8579.8 sequences per sample. At the 97% similarity, 1188 distinct OTUS were matched, and among them, 16 phyla, 31 class, and 189 genera of gut microbes were annotated. Based on the alpha diversity of the gut microbiota analysis in five groups, it was found that the Thr-group had higher (P < 0.05) Sobs index, Ace index, and Chao index compared to the PC group (Figure 1A–C). However, no difference was observed in the Shannon index between any two treatments (Figure 1D).
Figure 1.
Effect of amino acid deficiency on the alpha diversity of the cecal microbiota in laying hens with low-protein diets. (a) Sobs index of the community diversity. (b) Ace index of the community richness. (c) Chao index of the community richness. (d) Shannon index of the community diversity. Data were presented as means ± SEM (n = 9 per group). Significant differences were tested by student’s t-test. *P ≤ 0.05.
The relative abundance at phylum and genus levels was studied. At the phylum level, Bacteroidetes and Firmicutes were the two major bacteria in the cecum of laying hens, accounting for more than 90% of the total cecum bacterial community (Figure 2A). Additionally, the Thr-group had lower Firmicute/Bacteroidetes ratios and higher Bacteroidetes than the other groups. At the genus level, main genera in the PC group and four amino acid-deficiency groups were accounted by such as Bacteroids, Rikenellaceae_RC9_gut_group, Lactobacillus, and Parabacteroides (Figure 2B). The Thr-group held the highest Rikenellaceae_RC9_gut_group and the PC group held the lowest Lactobacillus (Figure 2B, Supporting Information Table S1). The five experiment treatments had different proportions of Bacteroides, PC group (20.51%), Ile-group (17.81%), Lys-group (21.72%), Met-group (20.22%), and Thr-group (20.00%). Principal coordinate analysis (PCoA), a multivariate statistical analysis method suitable for high-dimensional data, was performed (Figure 3). The gut microbial community in these groups clustered separately, suggesting that the gut microbiota was significantly altered, caused by the four amino acid deficiencies in low-protein diets. Notably, spots representing the Met-, Lys-, Ile-, and Thr-groups presented more dispersed distribution patterns than those of the PC groups (Figure 3B–E).
Figure 2.
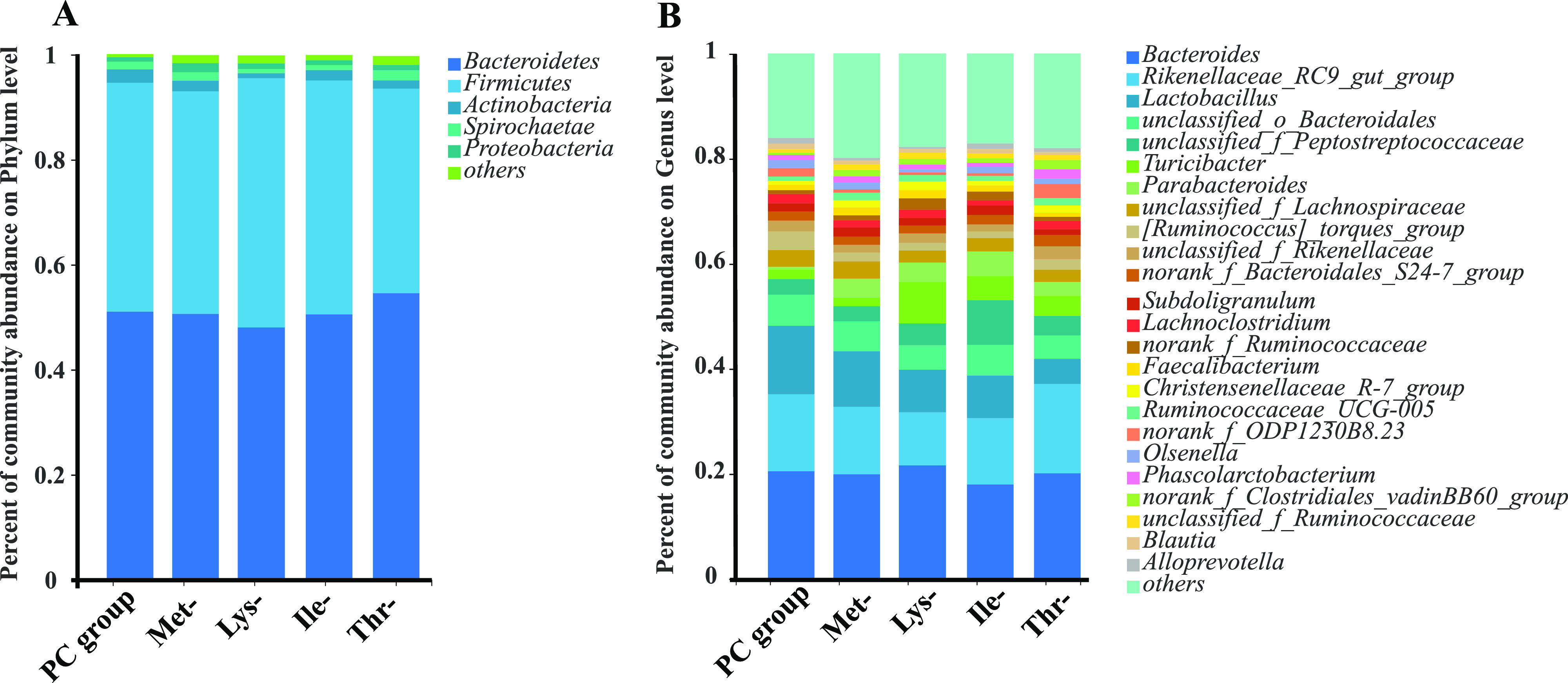
Effect of amino acid deficiency on the relative abundance of the cecal microbiota in laying hens with low-protein diets. (a) Relative abundance of gut microbiota at the phylum level (n = 9 per group). (b) Relative abundance of gut microbiota at the genus level (n = 9 per group).
Figure 3.
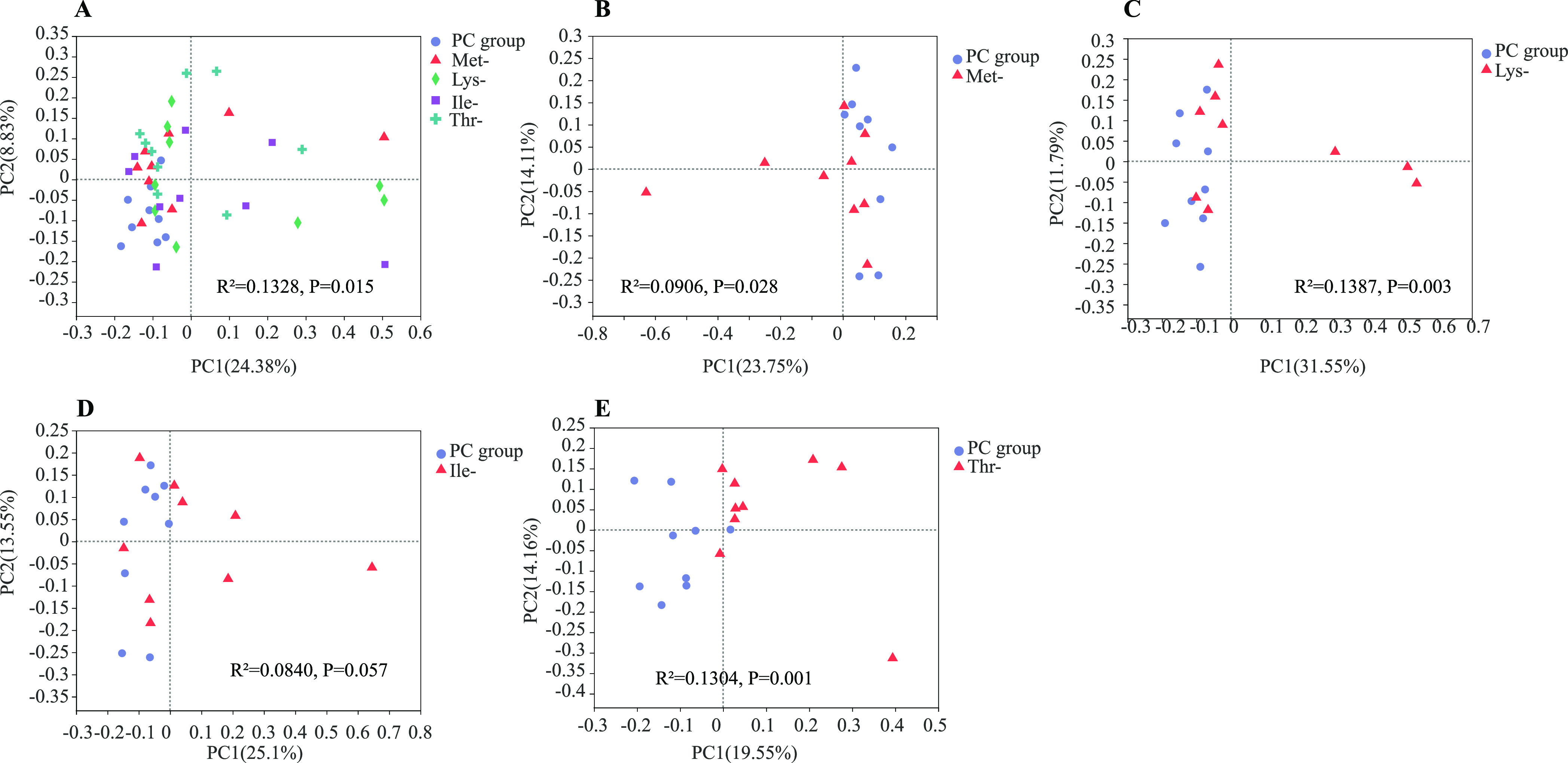
Principal coordinates analysis (PCoA, Bray–Curtis distance) plot of the gut microbial community structure between PC group, Met-group, Lys-group, Ile-group, and Thr-group. n = 9 per group.
In order to further determine which bacterial taxa contributed to the differences both statistically and biologically, we utilized linear discriminant analysis (LDA) effect size (LEfSe) analysis. As shown in Figure 4A, a variety of genera were enriched in the Met-group compared to the PC group including Parabacteroides, Christensenellaceae_R_7_group, Papillibacter, Candidatus_Soleaferrea, Synergistes, and Erysipelotrichaceae_UCG_003. Parabacteroides, Christensenellaceae_R_7_group, Ruminococcaceae_UCG_008, Ruminococcaceae_UCG_013, Ruminococcaceae_UCG_010, Variibacter, and Candidatus_Saccharimonas were enriched in the Lys-group compared to the controls (Figure 4B). LEfSe analysis of the Ile-group further identified genera, including expansion of Parabacteroides, Pseudomonas, Eubacterium_coprostanoligenes_group, Papillibacter, and Ruminococcaceae_UCG_009 in the Ile-group (Figure 4C). The relative abundance of Parabacteroides, Erysipelotrichaceae_UCG_004, Ruminococcaceae_UCG_010, and Acinetobacter in the Thr-group was higher than the PC group (Figure 4D).
Figure 4.
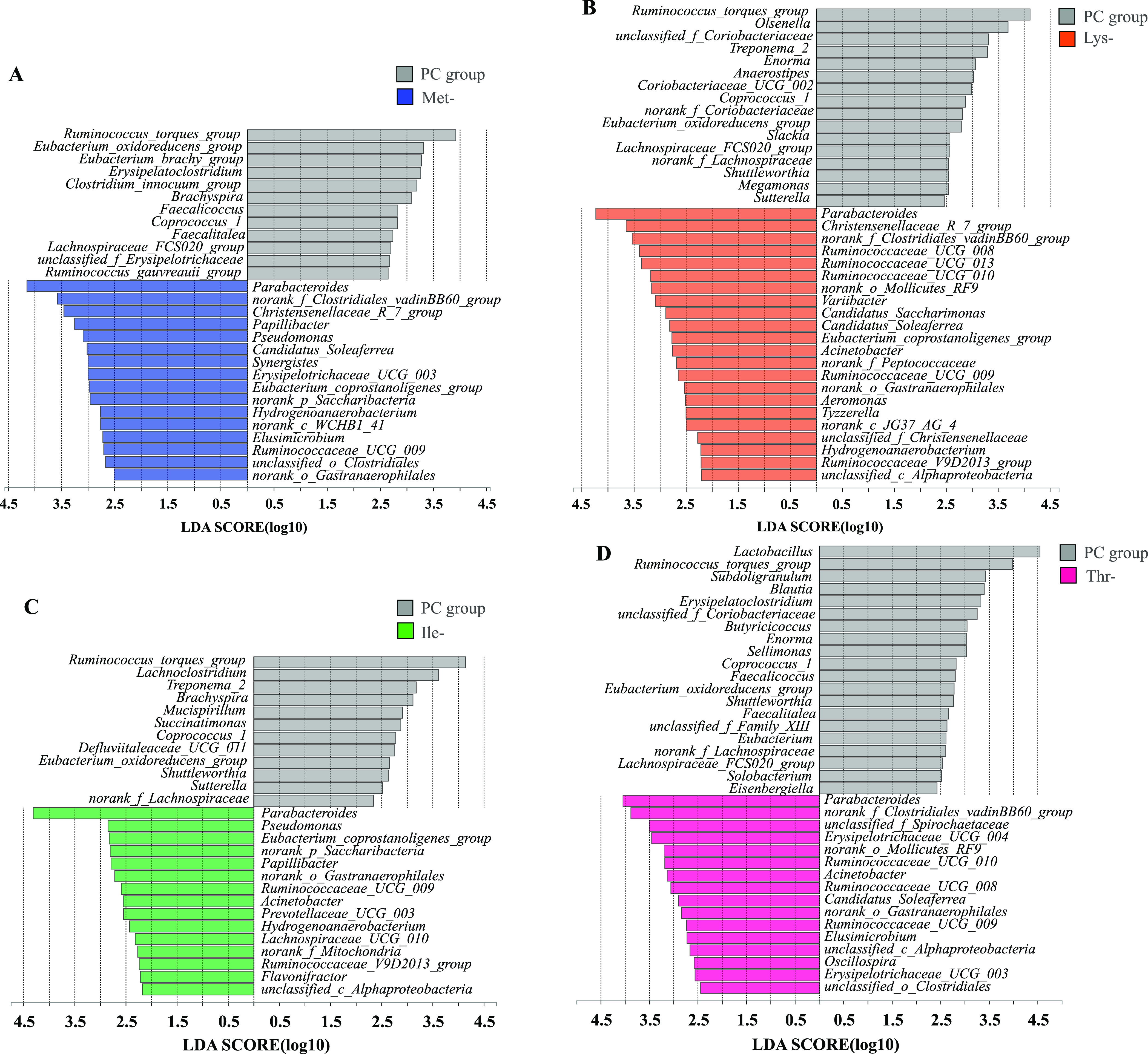
Differentially abundant genera between the PC group and amino acid-deficiency group. Histograms of the linear discriminate analysis (LDA) score (threshold ≥2) in Met- (a), Lys- (b), Ile- (c), and Thr- (d) are plotted. Linear discriminate analysis effect size (LEfSe) was performed to determine the difference in abundance (n = 9 per group).
Relationship between the Differential Bacterial Community and Main Parameters Related to the Amino Acid Deficiency
To predict the correlation between the differential gut microbial community of genera and key parameters, a Spearman correlation matrix was performed. As shown in Figure 5A, the AFY level was positively correlated with Coprococcus_1, Faecalitalea, Erysipelatoclostridium, Brachyspira, and Faecalicoccus; it was negatively correlated with the relative abundance of Parabacteroides. The TG concentration was positively correlated with genera Coprococcus_1, Erysipelatoclostridium, Lachnospiraceae_FCS020_group, and Faecalicoccus; it was negatively correlated with Elusimicrobium, Papillibacter, Parabacteroides, Synergistes, and Candidatus_Soleaferrea. As shown in Figure 5B, the TG level was positively correlated with the relative abundance of Coprococcus_1, Megamonas, Treponema_2, Enorma, Shuttleworthia, Lachnospiraceae_FCS020_group, and Anaerostipes; it was negatively correlated with Variibacter, Candidatus_Saccharimonas, Candidatus_Soleaferrea, and Parabacteroides. As shown in Figure 5C, the ADFI level was positively correlated with Coprococcus_1; it was negatively correlated with the relative abundance of Parabacteroides and Hydrogenoanaerobacterium. The TG level was positively correlated with the relative abundance of Brachyspira, Lachnoclostridium, Sutterella, Succinatimonas, Coprococcus_1, Shuttleworthia, and Faecalicoccus; it was negatively correlated with Flavonifractor, Parabacteroides, Ruminococcaceae_V9D2013_group, Hydrogenoanaerobacterium, and Papillibacter. As shown in Figure 5D, ADFI and TG levels were positively correlated with the relative abundances of Subdoligranulum, Erysipelatoclostridium, Coprococcus_1, Eisenbergiella, Butyricicoccus, Shuttleworthia, Lachnospiraceae_FCS020_group, Enorma, Sellimonas, Blautia, Faecalicoccus, and Eubacterium; it was negatively correlated with Parabacteroides and Elusimicrobium. The TG level was positively correlated with genera Coprococcus_1, Erysipelatoclostridium, Lachnospiraceae_FCS020_group, and Faecalicoccus, it was negatively correlated with Candidatus_Soleaferrea, Parabacteroides, Oscillospira, and Elusimicrobium.
Figure 5.
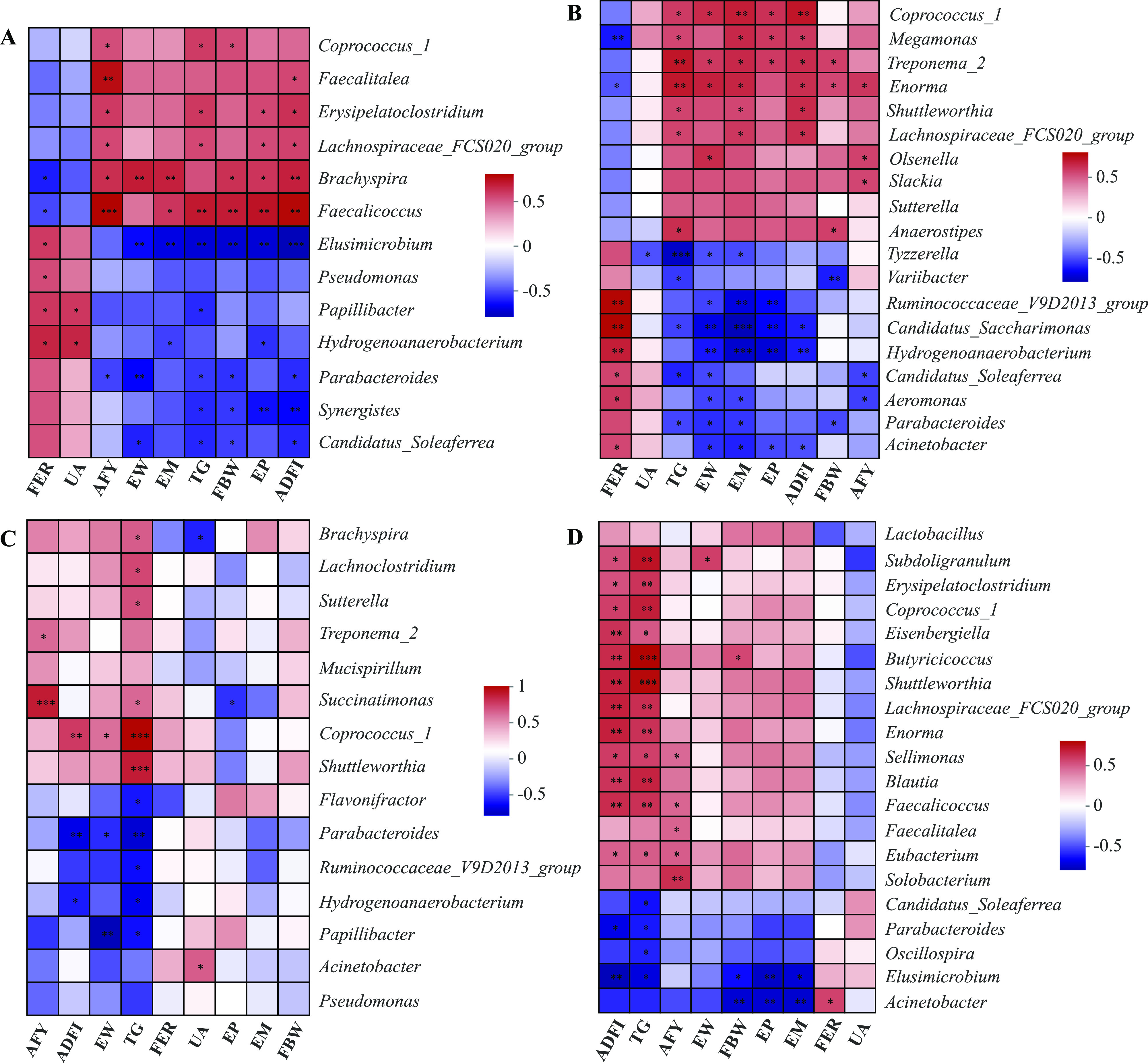
Correlation analysis of key parameters and the differential microbes affected by Met deficiency (a), Lys deficiency (b), Ile deficiency (c), and Thr deficiency (d). Asterisks indicate significant correlations (*P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001). The red represents a significantly positive correlation (P ≤ 0.05), the blue represents a significantly negative correlation (P ≤ 0.05), and the white represents no significant correlation (P > 0.05).
Discussion
Certain exogenic amino acids (e.g., Met, Lys, Thr, and Ile) have been used in animal feeding for a long time as feed additives, which play an essential role in the maintenance, growth, and functioning of farm animals and the human body.24−26 Therefore, the deficiency of essential amino acids in a low-protein diet caused different alterations of laying hens: reduced layer performance and egg quality15 and impaired immune function.27,28 In this study, the Peking Pink strain was used as the model of laying hens, to investigate the effects of four amino acid deficiencies (Met, Lys, Ile, and Thr) in low-protein diets on laying performance, egg quality, serum biochemical indices, and gut microbiota. Here, our results demonstrated that the four amino acid deficiencies (Met, Lys, Ile, and Thr) in low-protein diets had led to varying degrees of negative impacts on laying hens.
Notably, the laying performance was significantly decreased and the level of FER in the Met-group was dramatically increased compared to the PC group. Compared to other groups, the Met-deficiency group held the lowest FBW, ADG, EP, EW, EM, ADFI, and AFY in the experiment period, which was in agreement with the previous studies which showed that Met deficiency had significantly decreased laying performance, especially by the end of the experiment.29,30 Also, a previous study revealed that the Met plays a crucial role in lipid metabolism and fat accumulation.26 Compared to the PC group, Met deficiency produces less fat, similarly, Orentreich et al. and Richie et al. found that Met restriction limited fat accumulation of rats.31,32 However, the underlying mechanisms of fat accumulation between adipose tissue and liver need further investigation in laying hens. Although no statistical difference was observed, the key parameters of FBW, ADG, EP, ADFI, and AFY were slightly lower than those in the PC group, which was similar to the effect of low Lys concentration in low-protein diets on EW and EM.33 Phuoc et al. also found that a severe deficiency of dietary Lys resulted in lower EW compared to normal diets.4 Therefore, our results demonstrated that the deficiency of Met and Lys in low-protein diets was concomitant with poor laying performance and abnormal lipid metabolism, causing substantial economic losses in the animal husbandry industry. Notably, the levels of ADG, EP, EM, and ADFI were lower in the Thr-group compared to the PC group. Our results are in agreement with the results of the previous study, in which the Thr diet decreased the ADG of blunt snout bream.34 Moreover, the low-protein diet supplemented with Thr increased the EP and EM compared to the control diet,12 suggesting that Thr plays an important role in laying performance. Interestingly, the level of EP in the Ile-deficiency group was higher compared to those groups, suggesting that the lower concentration of Ile did not influence the EP in the Peking Pink hens. A previous study showed that at the 0.8–1.0% isoleucine level, the parameter BW and EM was significantly decreased in hens.15 Our study found that no difference in the FBW, ADG, EP, EM, ADFI, and FER was observed in the Ile-group, except the EW and AFY. Dong et al. uncovered that there were no effects observed from the different dietary Ile levels on laying performance and immunomodulation of laying hens.14 Therefore, Ile is not a limiting amino acid in the low-protein diet on laying hens.
In this study, the four amino acid deficiencies had significant differences with shell color and EW, which were in contrast to the previous studies that Met deficiency had negative impacts on egg quality.29,30 Phuoc et al. found that the increasing level of dietary total sulphur amino acids significantly increased the white and yolk index and Haugh unit, whereas eggshell thickness, yolk color, and egg components were not affected.4 Meanwhile, Karunajeewa et al. found that the levels of dietary Lys did not affect the egg quality.35 In line with this study, Abdel-Wareth et al. also found that there were no effects observed between the lower level of Thr (0.47%) and other higher level on shell thickness and albumen of laying hens.36 Peganova and Eder found that supplementation of Ile did not affect the albumen quality (albumen height and Haugh units).15
As we know, TG is associated with obesity, altered lipid metabolism, and intestinal inflammation.37 In the present study, the level of TG in the four amino acid-deficiency groups was lower than the PC group. According to the previous study, the levels of UA in low-protein diets supplemented with Ile or Thr were decreased,38 and we found that the concentrations of serum UA in the Met-, Ile-, and Thr-deficiency diets were higher compared to the PC group. As known to all, as a fecal metabolite of poultry, the higher serum UA level was significantly associated with the presence of host systemic inflammation and insulin resistance.39 Therefore, our results suggested that the Met, Ile, and Thr deficiency in the low-protein diet could decrease the efficiency of protein utilization and cause host metabolic dysfunction in hens.
The gut microbiome is a complex community of hundreds of diverse microorganisms.40 The gut microbiota influences the host, playing a role in the modulation of gut health, nutrient digestion, and regulation of immune function.40,41 Therefore, the gut microbiota plays a vital role in maintaining gut health and influences the overall performance of chickens. In this study, our data showed that as follows: At the phylum level, the Thr-group had lower Firmicute/Bacteroidetes ratios and higher Bacteroidetes levels than other groups. Bacteroidetes is known to be associated with fat accumulation in chickens,42 and the bacteria are present in obese human individuals,43 which is consistent with our results. Meanwhile, in the ceca of mature laying chickens, the representative microbial communities at the phylum level, in order of their typical abundance, are Firmicutes, Bacteroidetes, and so on.12 At the genus level, the relative abundance of Parabacteroides and Ruminococcaceae_UCG_009 was higher in the Met-, Lys-, Ile-, and Thr-groups compared to the PC group. According to the previous study, the genus Parabacteroides was closely related to metabolic disorders.44 Of note, the relative abundance of Lactobacillus was lower in the Thr-group compared to the PC group. Previous studies indicated that the level of Lactobacillus was significantly decreased in colorectal cancer and inflammatory bowel disease (IBD),45−47 which strongly supported our results that a higher proportion of Lactobacillus is present in the controls compared to the Thr-group. Some studies have shown that Clostridia is a potentially harmful bacteria species,48 which strongly support our results that the four amino acid-deficiency groups had a higher abundance of Clostridia compared to the PC group. Moreover, the abundance of Bacilli in the four amino acid-deficiency groups was lower than the PC group, which is in line with the previous study.49 Of note, our results revealed that the relative abundance of Parabacteroides was negatively related to AFY, TG, EW, and ADFI. Meanwhile, the Parabacteroides was significantly consistent with human oral and intestinal diseases and metabolic diseases.44,50 Collectively, the amino acid deficiency causes several disease states and the alteration of performance. Also, the interactions between the gut microbiota and amino acid deficiencies in low-protein diets are still unclear, and more research studies are needed.
Conclusions
The amino acid deficiencies mainly damaged laying performance, egg quality, and serum biochemical parameters and then altered the structure and community of the gut microbiota in low-protein diets. Of note, our results showed that the relative abundance of Parabacteroides was significantly enriched in four amino acid-deficiency groups, and it was negatively correlated with the level of TG in laying hens. The alteration of the gut microbiota by amino acid deficiency is closely related to host performance and lipid metabolism. Our study provides new insights into amino acid deficiency-induced alterations of performance, gut microbiota, and host metabolism and provides a regulation strategy involving modulation of gut microbiota in the low-protein diet.
Materials and Methods
Animals and Experimental Design
All experiments were carried out with the approval of China Agricultural University Animal Care and Use Committee (AW32301202-2-1, Beijing, China).
In this study, a total of 300 commercial hens of the Peking Pink strain (Yukou Poultry Co., Ltd. of Beijing, China) at the age of 38 weeks with a similar performance were randomly allotted to five treatment groups, comprising the positive control and four experimental groups. Each of the groups consisted of six replicates in five different cages (two birds per cage). Cages (H45 × W45 × D45 cm) were equipped with one nipple drinker and an exterior feed trough that expanded the length of the cage. Hens were raised in an enclosed, ventilated, and conventional house with 16 h-light and 55% relative humidity on average. Feed and water were provided ad libitum during the entire experimental period. The basal corn-soybean meal diet was formulated to meet the requirements of Peking Pink laying hens (NYT33-2004). Birds were adapted to diets for 1 wk before data collection began. The contents of the PC group in Met, Lys, Ile, and Thr were 0.46, 0.76, 0.72, and 0.56%; the other four groups were restricted Met, Lys, Ile, and Thr levels, respectively. The Met-group: Met (0.25%); Lys-group: Lys (0.56%); Ile-group: Ile (0.54%); and Thr-group: Thr (0.46%). Ingredients and nutrient content of the diets are shown in Table 1.
Table 1. Ingredients and Nutrient Content of the Diets (% DM).
| ingredients | (%) | nutrientc | (%) |
|---|---|---|---|
| corn | 67.40 | crude protein | 13.49 |
| soybean meal | 12.40 | ME (Mcal/kg) | 2.70 |
| peanut meal | 3.00 | Met | 0.46 |
| soybean hull | 4.00 | Met + cysteine | 0.66 |
| Met | 0.27 | Lys | 0.76 |
| Lys-HCl | 0.24 | Ile | 0.72 |
| Ile | 0.26 | Thr | 0.56 |
| Thr | 0.10 | tryptophan | 0.16 |
| tryptophan | 0.03 | arginine | 0.84 |
| valine | 0.04 | histidine | 0.34 |
| phenylalanine | 0.03 | leucine | 1.17 |
| limestone | 8.30 | phenylalanine | 0.63 |
| dicalcium phosphate | 1.50 | phenylpropionyl tyrosine | 1.08 |
| NaCl (salt) | 0.30 | valine | 0.60 |
| vitamin premixa | 0.03 | glycine + serine | 1.06 |
| mineral premixb | 0.30 | Na | 0.15 |
| choline chloride | 0.07 | Cl | 0.26 |
| Ssoybean oil | 1.00 | Ca | 3.54 |
| zeolite powder | 0.73 | total P | 0.54 |
| total | 100.00 | non-phytate phosphorus | 0.36 |
Vitamin premix supplied (per kg of diet): vitamin A, 96,000 IU; vitamin D3, 3600 IU; vitamin E, 75 mg; vitamin K3, 4.8 mg; vitamin B1, 4.8 mg; vitamin B2, 9 mg; folic acid, 0.9 mg; calcium pantothenate, 15 mg; niacin 45 mg; vitamin B6, 4.4 mg; vitamin B12, 24 μg; and biotin: 0.15 mg.
Mineral premix provided (per kg of diet): Cu, 6.8 mg; Fe, 66 mg; Zn, 83 mg; Mn, 80 mg; I, 1 mg; and Se, 0.3 mg.
The nutrient levels were calculated values.
Laying Performance and Egg Quality
Feed intake was recorded weekly by calculating the difference between full bucket weights and remaining feed. Hen-day EP and EW were recorded daily, and BW was recorded weekly on a replication basis. Egg mass was calculated. The feed conversion ratio was calculated as grams of feed intake per gram of egg mass produced. At the end of the experiment, 30 eggs from each treatment were randomly collected to assess egg quality parameters. The eggshell strength and eggshell thickness were measured with a digital egg tester (ESTG-01, Orka Technology Ltd). Haugh unit, yolks color, and EW were measured using a multifunctional egg quality tester (EA-01, Orka Technology Ltd). The eggshell was weighed, and yolks were separated using a separator and were then weighed to determine the relative yolk and albumen proportion. Shell color was measured on the blunt; these losses are often related to the poor shell quality of the end of the eggs with a QCR color reflectometer (QCR SPA, TSS England) as previously described.51,52
Blood Sampling and Biochemical Analysis
At the end of the experiment, blood samples were collected from birds via the wing vein on sampling days as previously described.53 The serum was centrifuged at 3000 rpm for 15 min at room temperature. Serum samples were aspirated using a pipette and stored in 1.5 mL tubes at −20 °C until analyzed. Serum concentrations of TG, UA, urea, TP, ALB, and IgM were measured using an automatic biochemical analyzer (7600, Hitachi, Japan) following the manufacturer’s instructions. Serum SOD and GSH were measured using a commercial kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the kit instructions.54,55 Hens were humanely euthanized using an injection of pentobarbital sodium (0.4 mL kg·BW–1; Sile Biological Technology Co. LTD, Guangzhou city, China). Abdominal adipose tissue was weighed to calculate the AFY.
DNA Isolation and 16S rRNA Illumina Sequencing
Total DNA of the caecum contents was extracted using the E.Z.N.A. Stool DNA Kit (Omega Bio-tek, Norcross, GA, USA) according to the manufacturer’s instructions. The extracted DNA was determined using a NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA) to assess the quantity and quality of the DNA. Illumina MiSeq sequencing and general data analyses were performed by a commercial company (Majorbio Bio-Pharm Technology, Shanghai, China).
The 16S rRNA gene V3–V4 hypervariable regions were amplified using the 338F (5′-ACTCCTACGGGAGGCAGCA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) primers. PCR was set in a 20 μL volume, with 10 ng of template DNA, 2.5 Mm dNTPs, 4 μL of fivefold FastPfu buffer (TransGen Biotech, China), 0.4 μL of FastPfu polymerase (TransGen Biotech, China), and 0.4 μL (5 μM) of each primer. The polymerase chain reaction (PCR) was performed on a MyCyclerTM Thermal Cycler (Bio-Rad Laboratories, Hercules, CA, USA) set as follows: 94 °C for 4 min, 25 cycles of 94 °C for 30 s, 55 °C for 30 s, 72 °C for 50 s, and 72 °C for 10 min. The products were extracted from a 2% agarose gel and further purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA) and quantified using QuantiFluor-ST (Promega, USA) according to the manufacturer’s protocol. Purified amplicons were sequenced on the Illumina MiSeq paired-end sequenced (2 × 300) platform (Majorbio Bio-Pharm Technology Co., Ltd., Shanghai, China) and sequencing data were subjected to bioinformatics analysis.
Statistical Analysis
All data were analyzed with one-way ANOVA followed by Turkey’s post hoc test (version 9.2, SAS Institute Inc., Cary, NC, USA) for multiple comparisons. Differences were considered significant at P < 0.05.
The raw paired-end reads were assembled into longer sequences and quality-filtered using PANDAseq (version 2.9) to remove the low-quality reads. The high-quality sequences were clustered into OTUs with a 97% similarity using UPARSE (version 7.0) in QIIME (version 1.17), and the chimeric sequences were removed using UCHIME. Taxonomy was assigned to OTUs using the RDP classifier. The subsequent clean reads were clustered as operational taxonomic units (OTUs) using UPARSE (version 7.0) and annotated with the SILVA 16S rRNA gene database using MOTHUR program (version v.1.30.1). Alpha-diversity (the Chao index, Ace index, and Sob index) was calculated based on the profiles of OUT using the MOTHUR program. Bar plots and heat maps were generated with the “vegan” package in R (version 3.3.1). PCoA was performed based on the Bray–Curtis distance using QIIME (version 1.17). ANOSIM was performed to compare the similarity of bacterial communities among groups using the “vegan” package of R (version 3.3.1). LEfSe analysis was performed to identify the bacterial taxa differentially enriched in different bacterial communities. Finally, correlations between key parameters and bacterial communities were assessed by Spearman’s correlation analysis using the “pheatmap” package in R (version 3.3.1). Data were expressed as mean values.
Acknowledgments
We thank all technicians in the experimental animal facility of China Agricultural University for providing daily care of laying hens. We also thank Shanghai Majorbio Bio-Pharm Technology Co. for their bioinformatic analysis of the data.
Glossary
Abbreviations
- ADG
average daily gain
- ADFI
average daily feed intake
- AFY
yield of abdominal fat
- ALB
albumin
- BW
body weight
- EP
egg production
- EW
egg weight
- EM
average daily egg mass
- FBW
final body weight
- FER
feed egg ratio
- GSH
glutathione
- Ile
isoleucine
- Ile-
Ile deficiency
- IBW
initial body weight
- IgM
immune globulin M
- Lys
lysine
- Lys-
Lys deficiency
- Met
methionine
- PC
positive control
- SOD
superoxide dismutase
- Thr
threonine
- Thr-
Thr deficiency
- TG
triglyceride
- TP
total protein
- UA
uric acid
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c00739.
Genus-level taxonomic composition of the cecal bacterial communities (PDF)
Author Contributions
The authors’ responsibilities were as follows: Q.M., J.Z., and S.G. obtained financial support and designed the study. S.G. and S.H. conducted the experiments and drafted the manuscript. S.H. polished the manuscript and finished the submission. F.L. guided to analyze the experimental data. L.Z. and J.Z. helped with revisiting and reviewing the manuscript. All authors read and approved the final manuscript.
Author Contributions
§ S.G. and S.H. are co-first authors.
This study was supported by the National Science Foundation of China (grant No.31772627), a Special Fund for China Agricultural Research System program (CARS-40-K08), National Key Research and Development Program of China (2017YFD0500500), and the Special Fund from Chinese Universities Scientific Fund (2018TC043).
The authors declare no competing financial interest.
Supplementary Material
References
- Aletor V. A.; Hamid I. I.; Nieß E.; Pfeffer E. Low-protein amino acid-supplemented diets in broiler chickens: effects on performance, carcass characteristics, whole-body composition and efficiencies of nutrient utilisation. J. Sci. Food Agric. 2000, 80, 547–554. . [DOI] [Google Scholar]
- Wang Y.; Zhou J.; Wang G.; Cai S.; Zeng X.; Qiao S. Advances in low-protein diets for swine. J. Anim. Sci. Biotechnol. 2018, 9, 60. 10.1186/s40104-018-0276-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagawany M.; El-Hack M. E. A.; Laudadio V.; Tufarelli V. Effect of low-protein diets with crystalline amino acid supplementation on egg production, blood parameters and nitrogen balance in laying Japanese quails. Avian Biol. Res. 2014, 7, 235–243. 10.3184/175815514x14152945166603. [DOI] [Google Scholar]
- Phuoc T. V.; Dung N. N. X.; Manh L. H. Effects of dietary total sulphur amino acids to lysine ratio on performance, nitrogen utilization of Ac layers (black-boned chicken). S. Afr. J. Anim. Sci. 2019, 49, 156–165. 10.4314/sajas.v49i1.18. [DOI] [Google Scholar]
- Zhan X. A.; Li J. X.; Xu Z. R.; Zhao R. Q. Effects of methionine and betaine supplementation on growth performance, carcase composition and metabolism of lipids in male broilers. Br. Poult. Sci. 2006, 47, 576–580. 10.1080/00071660600963438. [DOI] [PubMed] [Google Scholar]
- Picard M. L.; Uzu G.; Dunnington E. A.; Siegel P. B. Food intake adjustments of chicks: short term reactions to deficiencies in lysine, methionine and tryptophan. Br. Poult. Sci. 1993, 34, 737–746. 10.1080/00071669308417632. [DOI] [PubMed] [Google Scholar]
- Gomez S.; Angeles M. Effect of threonine and methionine levels in the diet of laying hens in the second cycle of production. J. Appl. Poultry Res. 2009, 18, 452–457. 10.3382/japr.2008-00090. [DOI] [Google Scholar]
- Shafer D.; Carey J.; Prochaska J.; Sams A. Dietary methionine intake effects on egg component yield, composition, functionality, and texture profile analysis. Poult. Sci. 1998, 77, 1056–1062. 10.1093/ps/77.7.1056. [DOI] [PubMed] [Google Scholar]
- Li Y.; Yin J.; Han H.; Liu G.; Deng D.; Kim S. W.; Wu G.; Li T.; Yin Y. Metabolic and proteomic responses to long-term protein restriction in a pig model. J. Agric. Food Chem. 2018, 66, 12571. 10.1021/acs.jafc.8b05305. [DOI] [PubMed] [Google Scholar]
- Mao X.; Zeng X.; Qiao S.; Wu G.; Li D. Specific roles of threonine in intestinal mucosal integrity and barrier function. Front. Biosci. 2011, 3, 1192–1200. 10.2741/e322. [DOI] [PubMed] [Google Scholar]
- Azzam M. M. M.; Dong X. Y.; Zou X. T. Effect of dietary threonine on laying performance and intestinal immunity of laying hens fed low-crude-protein diets during the peak production period. J. Anim. Physiol. Anim. Nutr. 2017, 101, e55–e66. 10.1111/jpn.12559. [DOI] [PubMed] [Google Scholar]
- Dong X. Y.; Azzam M. M. M.; Zou X. T. Effects of dietary threonine supplementation on intestinal barrier function and gut microbiota of laying hens. Poult. Sci. 2017, 96, 3654–3663. 10.3382/ps/pex185. [DOI] [PubMed] [Google Scholar]
- Wang X.; Qiao S.; Yin Y.; Yue L.; Wang Z.; Wu G. A deficiency or excess of dietary threonine reduces protein synthesis in jejunum and skeletal muscle of young pigs. J. Nutr. 2007, 137, 1442–1446. 10.1093/jn/137.6.1442. [DOI] [PubMed] [Google Scholar]
- Dong X. Y.; Azzam M. M. M.; Zou X. T. Effects of dietary L-isoleucine on laying performance and immunomodulation of laying hens. Poult. Sci. 2016, 95, 2297–2305. 10.3382/ps/pew163. [DOI] [PubMed] [Google Scholar]
- Peganova S.; Eder K. Studies on requirement and excess of isoleucine in laying hens. Poult. Sci. 2002, 81, 1714–1721. 10.1093/ps/81.11.1714. [DOI] [PubMed] [Google Scholar]
- Shivazad M.; Harms R. H.; Russell G. B.; Faria D. E.; Antar R. S. Re-evaluation of the isoleucine requirement of the commercial layer. Poult. Sci. 2002, 81, 1869–1872. 10.1093/ps/81.12.1869. [DOI] [PubMed] [Google Scholar]
- Parenteau I. A.; Stevenson M.; Kiarie E. G. Egg production and quality responses to increasing isoleucine supplementation in Shaver white hens fed a low crude protein corn-soybean meal diet fortified with synthetic amino acids between 20 and 46 weeks of age. Poult. Sci. 2020, 99, 1444–1453. 10.1016/j.psj.2019.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisbin J. T.; Gong J.; Sharif S. Interactions between commensal bacteria and the gut-associated immune system of the chicken. Anim. Health. Res. Rev. 2008, 9, 101–110. 10.1017/s146625230800145x. [DOI] [PubMed] [Google Scholar]
- Kers J. G.; Velkers F. C.; Fischer E. A. J.; Hermes G. D. A.; Stegeman J. A.; Smidt H. Host and Environmental Factors Affecting the Intestinal Microbiota in Chickens. Front. Microbiol. 2018, 9, 235. 10.3389/fmicb.2018.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott K. P.; Gratz S. W.; Sheridan P. O.; Flint H. J.; Duncan S. H. The influence of diet on the gut microbiota. Pharmacol. Res. 2013, 69, 52–60. 10.1016/j.phrs.2012.10.020. [DOI] [PubMed] [Google Scholar]
- Hussain T.; Murtaza G.; Kalhoro D. H.; Kalhoro M. S.; Metwally E.; Chughtai M. I.; Mazhar M. U.; Khan S. A. Relationship between gut microbiota and host-metabolism: Emphasis on hormones related to reproductive function. Anim. Nutr. 2021, 7, 1. 10.1016/j.aninu.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L.; Tang Z.; Chen H.; Ren Z.; Ding Q.; Liang K.; Sun Z. The mutual interaction between gut microbiota and protein/amino acid metabolism for host mucosal immunity and health. Anim. Nutr. 2021, 7, 11. 10.1016/j.aninu.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.; Yu K.; Zhu W. Amino acid sensing in the gut and its mediation in gut-brain signal transduction. Anim. Nutr. 2016, 2, 69–73. 10.1016/j.aninu.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. 10.1007/s00726-009-0269-0. [DOI] [PubMed] [Google Scholar]
- Yi D.; Li B.; Hou Y.; Wang L.; Zhao D.; Chen H.; Wu T.; Zhou Y.; Ding B.; Wu G. Dietary supplementation with an amino acid blend enhances intestinal function in piglets. Amino Acids 2018, 50, 1089. 10.1007/s00726-018-2586-7. [DOI] [PubMed] [Google Scholar]
- Zhou X.; He L.; Wan D.; Yang H.; Yao K.; Wu G.; Wu X.; Yin Y. Methionine restriction on lipid metabolism and its possible mechanisms. Amino Acids 2016, 48, 1533–1540. 10.1007/s00726-016-2247-7. [DOI] [PubMed] [Google Scholar]
- Konashi S.; Takahashi K.; Akiba Y. Effects of dietary essential amino acid deficiencies on immunological variables in broiler chickens. Br. J. Nutr. 2000, 83, 449–456. 10.1017/S0007114500000556. [DOI] [PubMed] [Google Scholar]
- Wu B.-y.; Cui H.-m.; Peng X.; Fang J.; Cui W.; Liu X.-d. Effect of Methionine Deficiency on the Thymus and the Subsets and Proliferation of Peripheral Blood T-Cell, and Serum IL-2 Contents in Broilers. J. Integr. Agric. 2012, 11, 1009–1019. 10.1016/s2095-3119(12)60093-8. [DOI] [Google Scholar]
- Bodin L.; Sécula A.; Chapuis H.; Cornuez A.; Lessire M.; Cobo E.; Marie-Louise S.; Bonnefont C. M. D.; Barrieu J.; Mercerand F.; Bravo C.; Manse H.; Le Bourhis M. C.; Martin X.; Pitel F.; Brun J. M.; Morisson M. Dietary methionine deficiency reduces laying performances of female common ducks and impacts traits of interest of their mule ducklings. Poult. Sci. 2019, 98, 5590–5600. 10.3382/ps/pez315. [DOI] [PubMed] [Google Scholar]
- Daşkıran M.; Önol A. G.; Cengiz Ö.; Tatlı O.; Sarı M. Effects of dietary methionine levels and L-carnitine supplementation on performance and egg quality parameters of layers. J. Anim. Feed Sci. 2009, 18, 650–661. 10.22358/jafs/66439/2009. [DOI] [Google Scholar]
- Orentreich N.; Matias J. R.; DeFelice A.; Zimmerman J. A. Low methionine ingestion by rats extends life span. J. Nutr. 1993, 123, 269–274. [DOI] [PubMed] [Google Scholar]
- Richie J. P.; Leutzinger Y.; Parthasarathy S.; Maixoy V.; Orentreich N.; Zimmerman J. A. Methionine restriction increases blood glutathione and longevity in F344 rats. FASEB J. 1994, 8, 1302–1307. 10.1096/fasebj.8.15.8001743. [DOI] [PubMed] [Google Scholar]
- Kumari K. N. R.; Reddy V. R.; Preetham V. C.; Kumar D. S.; Sen A. R.; Rao S. V. R. Effect of supplementation of crystalline lysine on the performance of WL layers in tropics during summer. Trop. Anim. Health Prod. 2016, 48, 705–710. 10.1007/s11250-016-1003-z. [DOI] [PubMed] [Google Scholar]
- Habte-Tsion H.-M.; Ge X.; Liu B.; Xie J.; Ren M.; Zhou Q.; Miao L.; Pan L.; Chen R. A deficiency or an excess of dietary threonine level affects weight gain, enzyme activity, immune response and immune-related gene expression in juvenile blunt snout bream (Megalobrama amblycephala). Fish Shellfish Immunol. 2015, 42, 439–446. 10.1016/j.fsi.2014.11.021. [DOI] [PubMed] [Google Scholar]
- Karunajeewa H.; Abu-Serewa S.; Tham S. H.; Eason P. The effects of dietary level of sunflower seeds and lysine on egg quality and laying performance of White Leghorn hens. J. Sci. Food Agric. 1987, 41, 325–333. 10.1002/jsfa.2740410405. [DOI] [Google Scholar]
- Abdel-Wareth A. A. A.; Esmail Z. S. H. Some Productive, Egg Quality and Serum Metabolic Profile Responses Due to L-threonine Supplementation to Laying Hen Diets. Asian J. Poultry Sci. 2014, 8, 75–81. 10.3923/ajpsaj.2014.75.81. [DOI] [Google Scholar]
- Liu C.; Zhang J.; Li M.; Zhao L.; Ji C.; Ma Q. Alterations and structural resilience of the gut microbiota under dietary fat perturbations. J. Nutr. Biochem. 2018, 61, 91–100. 10.1016/j.jnutbio.2018.07.005. [DOI] [PubMed] [Google Scholar]
- Rojas I. C. O.; Murakami A. E.; Fanhani J. C.; Picoli K. P.; Barbosa M. J. B. Tryptophan, threonine and isoleucine supplementation in low-protein diets for commercial laying hens. Semina: Cienc. Agrar. 2015, 36, 1735–1743. 10.5433/1679-0359.2015v36n3p1735. [DOI] [Google Scholar]
- Chao T.-F.; Hung C.-L.; Chen S.-J.; Wang K.-L.; Chen T.-J.; Lin Y.-J.; Chang S.-L.; Lo L.-W.; Hu Y.-F.; Tuan T.-C.; Chen S.-A. The association between hyperuricemia, left atrial size and new-onset atrial fibrillation. Int. J. Cardiol. 2013, 168, 4027–4032. 10.1016/j.ijcard.2013.06.067. [DOI] [PubMed] [Google Scholar]
- Valdes A. M.; Walter J.; Segal E.; Spector T. D. Role of the gut microbiota in nutrition and health. Br. Med. J. 2018, 361, k2179. 10.1136/bmj.k2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan S.; Moore R. J.; Stanley D.; Chousalkar K. K. The Gut Microbiota of Laying Hens and Its Manipulation with Prebiotics and Probiotics To Enhance Gut Health and Food Safety. Appl. Environ. Microbiol. 2020, 86, e00600 10.1128/aem.00600-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torok V. A.; Allison G. E.; Percy N. J.; Ophel-Keller K.; Hughes R. J. Influence of antimicrobial feed additives on broiler commensal posthatch gut microbiota development and performance. Appl. Environ. Microbiol. 2011, 77, 3380–3390. 10.1128/aem.02300-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippo C.; Cavalieri D.; Di Paola M.; Ramazzotti M.; Poullet J. B.; Massart S.; Collini S.; Pieraccini G.; Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. U.S.A. 2010, 107, 14691–14696. 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K.; Liao M.; Zhou N.; Bao L.; Ma K.; Zheng Z.; Wang Y.; Liu C.; Wang W.; Wang J.; Liu S.-J.; Liu H. Parabacteroides distasonis Alleviates Obesity and Metabolic Dysfunctions via Production of Succinate and Secondary Bile Acids. Cell Rep. 2019, 26, 222–235. 10.1016/j.celrep.2018.12.028. [DOI] [PubMed] [Google Scholar]
- Craven M.; Egan C. E.; Dowd S. E.; McDonough S. P.; Dogan B.; Denkers E. Y.; Bowman D.; Scherl E. J.; Simpson K. W. Inflammation drives dysbiosis and bacterial invasion in murine models of ileal Crohn’s disease. PLoS One 2012, 7, e41594 10.1371/journal.pone.0041594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey N.; Soergel D. A.; Repo S.; Brenner S. E. Association of gut microbiota with post-operative clinical course in Crohn’s disease. BMC Gastroenterol. 2013, 13, 131. 10.1186/1471-230x-13-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevers D.; Kugathasan S.; Knights D.; Kostic A. D.; Knight R.; Xavier R. J. A Microbiome Foundation for the Study of Crohn’s Disease. Cell Host Microbe 2017, 21, 301–304. 10.1016/j.chom.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo Y.; Dobashi Y.; Sakai T.; Monma C.; Miyatani H.; Yoshida Y. Epidemiological and pathobiological profiles of Clostridium perfringens infections: review of consecutive series of 33 cases over a 13-year period. Int. J. Clin. Exp. Pathol. 2015, 8, 569–577. [PMC free article] [PubMed] [Google Scholar]
- Cutting S. M. Bacillus probiotics. Food Microbiol. 2011, 28, 214–220. 10.1016/j.fm.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Kverka M.; Zakostelska Z.; Klimesova K.; Sokol D.; Hudcovic T.; Hrncir T.; Rossmann P.; Mrazek J.; Kopecny J.; Verdu E. F.; Tlaskalova-Hogenova H. Oral administration of Parabacteroides distasonis antigens attenuates experimental murine colitis through modulation of immunity and microbiota composition. Clin. Exp. Immunol. 2011, 163, 250–259. 10.1111/j.1365-2249.2010.04286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safaa H. M.; Serrano M. P.; Valencia D. G.; Arbe X.; Jiménez-Moreno E.; Lázaro R.; Mateos G. G. Effects of the levels of methionine, linoleic Acid, and added fat in the diet on productive performance and egg quality of brown laying hens in the late phase of production. Poult. Sci. 2008, 87, 1595–1602. 10.3382/ps.2008-00005. [DOI] [PubMed] [Google Scholar]
- Kim C.-H.; Song J.-H.; Lee J.-C.; Lee K.-W. Age-Related Changes in Egg Quality of Hy-Line Brown Hens. Int. J. Poultry Sci. 2014, 13, 510–514. 10.3923/ijps.2014.510.514. [DOI] [Google Scholar]
- Hamid H.; Zhang J. Y.; Li W. X.; Liu C.; Li M. L.; Zhao L. H.; Ji C.; Ma Q. G. Interactions between the cecal microbiota and non-alcoholic steatohepatitis using laying hens as the model. Poult. Sci. 2019, 98, 2509–2521. 10.3382/ps/pey596. [DOI] [PubMed] [Google Scholar]
- Fan Y.; Zhao L.; Ji C.; Li X.; Jia R.; Xi L.; Zhang J.; Ma Q. Protective Effects of Bacillus subtilis ANSB060 on Serum Biochemistry, Histopathological Changes and Antioxidant Enzyme Activities of Broilers Fed Moldy Peanut Meal Naturally Contaminated with Aflatoxins. Toxins 2015, 7, 3330–3343. 10.3390/toxins7083330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T.; Ma Q.; Zhao L.; Jia R.; Zhang J.; Ji C.; Wang X. Protective Effects of Sporoderm-Broken Spores of Ganderma lucidum on Growth Performance, Antioxidant Capacity and Immune Function of Broiler Chickens Exposed to Low Level of Aflatoxin B(1). Toxins 2016, 8, 278. 10.3390/toxins8100278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.