Abstract
A simple strategy for synthesizing supramolecular hybrids was developed for the preparation of bioavailable nanohybrid photosensitizers by assembling visible-light-sensitive Pt(II) meso-tetrakis(4-carboxyphenyl)porphyrinporphyrin (PtTCPP)/tomatine analogues. The hybrids were self-assembled into nanofibrous or nanosheet structures approximately 3–5 nm thick and several micrometers wide. α-Tomatine generated a unique fibrous vesicle nanostructure based on intermolecular interactions, while dehydrotomatine generated nanosheet structures. Nanoassembly of these fibrous vesicles and sheets directly affected the properties of the light-responsive photosensitizer for tumor photodynamic therapy (PDT), depending on the nanostructure of the hybrid PtTCPP/tomatine analogues. The cytotoxicity of PtTCPP to cancer cells under photoirradiation was significantly enhanced by a tomatine assembly with a fibrous vesicle nanostructure, attributable to increased incorporation of the drug into cells.
Introduction
Tomatoes are widely distributed worldwide and used both fresh and in prepared foods such as pasta sauce. The compounds in tomatoes have received much attention in the field of drug system development due to their biological activities, such as antiproliferative, apoptotic, antibacterial, antiherpes, and anti-inflammatory activities, as well as adjuvant and immunostimulant activities.1,2 Here, we employed α-tomatine (Figure 1), a major steroidal alkaloid glycoside (SAG) extracted from the tomato plant (Solanum lycopersicum), which was reported to have antitumor actions, cholesterol-lowering effects, and immune enhancement activities.1,2 SAGs, including tomatine, are also of great interest from the viewpoint of physical chemistry because they directly form self-assembled nanostructured supramolecules in natural environments without the need for chemical derivatization. This unique property is attributable to the amphiphilic structure of SAGs, which consist of hydrophilic sugars and hydrophobic aglycone groups in a single molecule. Similar glycosteroidal compounds reportedly function as organic gelators and liquid crystalline materials.3−5 The dynamic transformation of these assemblies is sensitive to the surrounding environment and can lead to various biofunctionalities. We previously demonstrated6 that different SAG-based nanostructures (tubes or sheets) are generated depending on the SAG structure when complexed with a metal complex anion, [Au(CN2)]−. Further, their photoluminescence changes depending on the intermolecular metallophilic interaction. Ideally, the characteristics of such systems would be tunable by controlling the spatial arrangement of the metal complexes, resulting in electrical interactions between metal complexes without the need for covalent linkage or coordination.
Figure 1.
Chemical structures of α-tomatine (1), dehydrotomatine (2), and Pt(II) tetrakis(4-carboxyphenyl)porphyrin (PtTCPP). The blue double bond of the tomatine analogue indicates the structural difference from α-tomatine (1). The steroid alkaloid core structure of tomatidine is typically composed of three six-member cyclohexane rings (rings A, B, and C in the tomatidine illustration), one cyclopentane ring (D ring), one oxacyclopentane ring (E ring), and one piperidine ring (F ring).
Porphyrin and its complexes with metals such as platinum, zinc, and gold are widely used as photosensitizers in photodynamic therapy (PDT) due to their attractive biological and optical properties. These properties include strong light absorption and efficient energy transfer to surrounding oxygen molecules upon photoirradiation to form toxic singlet oxygen, the predominant cytotoxic agent, thus damaging cancer cells.7−15 However, most porphyrin-based photosensitizers exhibit limited tumor accumulation, poor water solubility, low bioavailability, and high intrinsic cell toxicity under nonphotoirradiation conditions. Various nanocarriers have therefore been developed as vehicles to promote the delivery of safe photosensitizers to tumor sites.16,17 In particular, high-resolution intracellular oxygen concentration imaging using the phosphorescence of Pt(II) meso-tetrakis(4-carboxyphenyl)porphyrinporphyrin (PtTCPP) has been applied to image intracellular oxygen concentration and control cytotoxicity.18,19 In contrast, to date, there are no reports on the biocompatible effects on hierarchical self-assembly of discrete metal porphyrins into one-dimensional (1D) nanofibers, two-dimensional (2D) nanosheets, or three-dimensional (3D) nanoarchitectures, a concept at the very heart of bottom-up nanotechnology and biotechnology.
In this study, we report that two SAGs (Figure 1) extracted from tomato (S. lycopersicum) form differently assembled supramolecular hybrids with PtTCPP.20−22 We show that the cytotoxic effect of the assembly changes depending on the 1D and 2D nanostructures, namely, the cytotoxicity of PtTCPP toward cancer cells under photoirradiation, is significantly enhanced by complexation with SAGs, especially tomatine.
Results and Discussion
SAGs were extracted as previously described (Figure 1),6,23,24 purified, and verified by high-performance liquid chromatography (HPLC) and 1H NMR analyses (see the Supporting Information and Figure S1). The addition of these compounds to deionized (DI) water at a final concentration of 0.1 mM gave cloudy dispersions, indicating that all SAGs formed supramolecular structures in an aqueous solution due to self-assembly based on the steroidal alkaloid and sugar groups in the amphiphilic molecular structures.
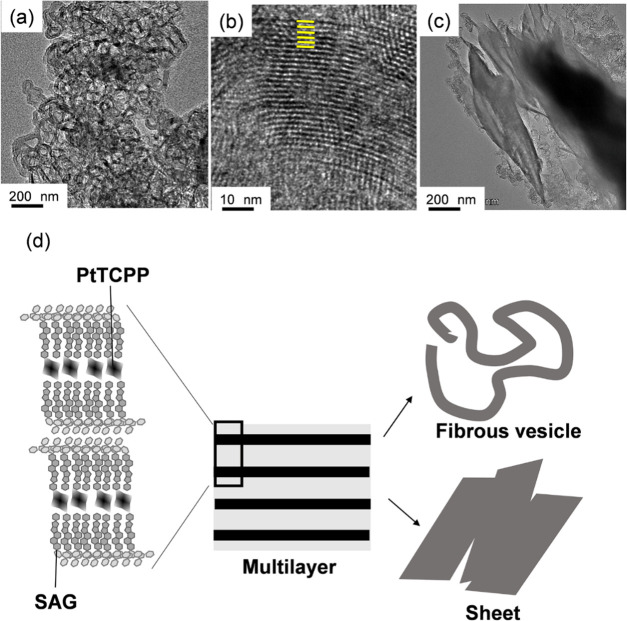
The addition of PtTCPP to aqueous solutions of SAGs 1 and 2 in a 1:4 molar ratio gave transparent dispersions. This stoichiometry of 1:4 (PtTCPP/SAG) molar ratio was determined by titration studies of the luminescence of PtTCPP by SAGs (Figure 1, Supporting Information Figure S5). Aggregation and self-assembly of the PtTCPP ion were investigated by transmission electron microscopy (TEM) of DI water solutions of 1/PtTCPP and 2/PtTCPP (Figure 2). The structures of all hybrids differed from those of 1 and 2 in DI water (Figure S1). The dark regions in the images were ascribed to Pt used to coat the samples because these samples were not stained prior to TEM observation. The 1/PtTCPP composite (Figure 2a) showed fibrous vesicle structures several hundred nanometers long. Observation of 1/PtTCPP by high-resolution TEM (HR-TEM, Figure 2b) showed detailed supramolecular assembly structures approximating a weave pattern ca. 0.8 nm thick consisting of regular sections of assembled layers. Since the observation of pure PtTCPP did not show a specific nanostructure and those of the pure tomatine showed a different nanostructure (Figure S2),6 this result indicated that the multilayered fibrous structures resulted from the intermolecular interaction of 1 with PtTCPP (Figure 2d). This observation was consistent with the results obtained by small-angle X-ray scattering (SAXS), as presented in the Supporting Information figures (Figure S3) and discussed in more detail in the Supporting Information.
Figure 2.
Transmission electron micrographs of samples prepared from 1/PtTCPP (a, b) and 2/PtTCPP (c). The inserted yellow lines in the HR-TEM image of 1/PtTCPP (b) mark structures estimated as weave patterns. Samples were not stained. (PtTCPP = 0.025 mM (1 and 2: PtTCPP = 4:1)). Hierarchical structure estimated by TEM images (d).
In sharp contrast, the 2/PtTCPP composite (Figure 2c) exhibited numerous sheet structures (widths, 200–1200 nm). These nanostructures were more aggregated and more defined than those of 1/PtTCPP, and thus, this regular supramolecular structure, clearly resulting from the use of SAG 2, is attributable to the self-assembly of PtTCPP in conjunction with this SAG.
We also performed small-angle X-ray scattering (SAXS) analysis of these composites in DI water. Although SAXS cannot determine large aggregated structures, it can provide detailed nanoscale information. An upwardly convex curve at 0.1 nm–1 < q < 1 nm–1 and another in the high-q region were observed with 1/PtTCPP (Figure S3) and appear to be due to the form factor. TEM observation of 1/PtTCPP indicated the presence of vesicular structures. The SAXS intensity from a tube should obey a power law of q–1 in the low q region, but instead, it was close to q–2, suggesting the presence of a vesicular structure. For 2/PtTCPP, the scattering intensities approximate a power law of q–2, suggesting the presence of a network. The experimental curves for 1/PtTCPP and 2/PtTCPP were well represented with these models. The obtained parameters are shown in Table S1.
The size distributions of the nanostructures in water were analyzed by dynamic light scattering (DLS) measurements (Figure S4) of 1/PtTCPP and 2/PtTCPP solutions at 25 °C. The results show at least two peaks in the volume-based mean nanostructure size distributions, at 200–500 nm and 5 μm or more, consistent with the TEM images of these samples (Figure 2), although DLS only determines the hydrodynamic radii of dispersed structures in a solvent. PtTCPP clearly interacted electrostatically with the SAGs, promoting the aggregation of PtTCPP ions in water.
SAGs have a hydrophilic part (consisting of two d-glucose units, a d-galactose unit, and a d-xylose unit) and a hydrophobic part (the aglycon derivative in tomatidine) and thus may form one-directionally stacked lamellar structures due to nucleation of the lamellar layers. These lamellar layers would grow anisotropically to yield fibrous vesicles, sheets, and nanonetworks (Figure 2d). In addition, the nanostructure of 1 (with a saturated hydrocarbon) was different from that of 2 (with an unsaturated hydrocarbon), suggesting that 1 generated a unique structure based on intermolecular interactions between specific chemical structures and that fibrous vesicle nanostructures formed, as shown in the schematic model in Figures 2d and S3. This model shows lamellar nanostructures in aqueous conditions, consistent with the fibrous vesicle and sheet structures observed by TEM. Therefore, the SAG and PtTCPP complexes not only interact at the molecular level but also undergo supramolecular self-assembly by intricate intermolecular van der Waals and hydrophobic interactions on the subnanometer scale to form hierarchical structures.
The aggregation and self-assembly of PtTCPP ions were investigated by UV–vis absorption spectroscopy (Figure 3a,b). Mixing 0.2 mM DI water solutions of PtTCPP with SAGs resulted in the appearance of absorption bands in the 350–450 and 480–530 nm regions. Interestingly, the absorbance intensity in these regions decreased and red-shifted in the following order: pH 4.0 > pH 6.3 > pH 7.0 > pH 10.0 > original PtTCPP. Since the pKa’s of the aglycon amine groups and carboxylic acids of PtTCPP are ∼6.625 and ∼6.0,26 respectively, these observations suggest that the intermolecular interactions and J aggregates7−10 formed by PtTCPP are due to hydrophobic interaction and hydrogen bonding between the aglycon amine group of SAGs 1 and 2 and the protonated carboxyl groups of PtTCPP. In addition, the results suggest that Coulomb forces and hydrophobic interactions produced a hypochromic effect based on electrostatic interactions and hydrogen bonding and that both the strength and number of hydrophobic interactions were increased in the complex. Therefore, both the molecular structures and amphiphilic balances of these SAGs, as well as the degree to which they are self-assembled (in particular, to give sheet structures), appear to affect the strong metallophilic interactions of the Pt complex composites.
Figure 3.
UV–vis absorption spectra of 1/PtTCPP (a) and 2/PtTCPP (b) in DI water. Circular dichroism (CD) spectra of 1/PtTCPP (c) and 2/PtTCPP (d) in DI water. Luminescence spectra (excitation wavelength = 405 nm) of 1/PtTCPP (e) and 2/PtTCPP (f) in DI water. [PtTCPP] = 0.2 mM. (SAG/PtTCPP = 4:1). [PtTCPP] = 0.025 mM. (SAG/PtTCPP = 4:1).
Circular dichroism (CD) spectra were used to determine the conformations of the SAGs in water and are presented in Figure 3c,d. In the 1/PtTCPP composite, induced circular dichroism (ICD) appears in the absorbance region of 380–420 nm associated with the Soret band of PtTCPP (positive signal and negative signal: 398, 417 nm (pH 4.0); 401, 413 nm (pH 6.3); 402 and 417 nm (pH 7.0)). The peak at pH 10.0 was not clear. The signals corresponded to an oblique J aggregate and a left-handed helix conformation7−10,27−34 and were especially intense at pH 6.3 (signal order: pH 6.3 > pH 4.0 > pH 7.0 > pH 10.0). In contrast, 2/PtTCPP did not provide an ICD signal. The self-assembly of 1/PtTCPP at pH 6.3 formed from a concurrent arrangement of SAG with PtTCPP via electrostatic interaction between >NH2+ (pKa = 6.6; >NH2+ ⇄ >NH) and −COO– (pKa = 6.0; −COOH ⇄ −COO–) with hydrophobic interactions and hydrogen bonding. In addition, the segments of the amphiphile backbone containing the SAGs depend on slight deference in the chemical structures of the SAGs. Furthermore, the CD spectra of the SAG amphiphiles in DI water solutions indicate a helix conformation due to hydrogen-bonding interactions between the sugars and hydrophobic interactions between aglycon segments. The TEM images (Figure 2) and SAXS analysis results (Figure S3) show that 1/PtTCPP hybrids had fibrous vesicle structures (Figure 2a,b), whereas the 2/PtTCPP hybrid formed numerous sheet structures (Figure 2c). The strong ICD signal from 1/PtTCPP at pH 6.3 therefore suggests that hydrophobic interactions between PtTCPPs, and electrostatic interaction and hydrogen bonding by the amine groups and carboxylic acids between the SAG segments and PtTCPP, induced a helix backbone. The specific conformations of SAG molecules, such as nanotubes and nanosheets, would be closely associated with biological activity and play an important role in the functioning of SAGs. Our general knowledge of SAGs suggests that a SAG sheet structure can be stabilized using hydrophobic metal complexes.
Luminescence spectra of the SAG/PtTCPP composites were recorded to assess the aggregation of PtTCPP and intermolecular interactions. The excitation and emission spectra of composites 1 and 2/PtTCPP (SAG/PtTCPP = 4:1) are shown in Figure 3e,f. A 0.025 mM solution of PtTCPP in the absence of SAGs exhibited luminescence at 645 and 715 nm (ex. 405 nm). In contrast, a 0.025 mM PtTCPP solution in the presence of 1 or 2 emitted at approximately 670 and 740 nm (1/PtTCPP: pH 4.0, 669 and 740 nm; pH 6.3, 669 and 740 nm; pH 7.0, 669 and 739 nm; pH 10.0, 669 and 735 nm; 2/PtTCPP: pH 4.0, 669 and 738 nm; pH 6.3, 669 and 737 nm; pH 7.0, 669 and 734 nm; pH 10.0, 667 and 731 nm (ex. 405 nm)). The intensity of the luminescence increased following the addition of SAGs 1 to 2. SAG 1 in particular resulted in a dramatic increase in the luminescence intensity (Figure 3e,f, emission maxima of 1/PtTCPP: 669 nm, 2/PtTCPP: 669 nm). The effect of varying the stoichiometric ratio of hybrid solution components monitored at the emission maxima is described in the Supporting Information, Figure S5. The quantum yields of 1 and 2/PtTCPP in DI water were 0.050 (1/PtTCPP) and 0.044 (2/PtTCPP), respectively. The emission lifetimes of 1 and 2/PtTCPP in DI water were 4.5 μs (1/PtTCPP) and 4.3 μs (2/PtTCPP), indicating that the luminescence originates in phosphorescence from triplet metal–ligand charge transfer (3MLCT) in PtTCPP.18−22 These results demonstrate both greater aggregation in hybrids made with 1 rather than with 2 and that one or more modes of emission quenching were absent. These studies on the self-assembly of PtTCPP with SAGs showed that the red shift and the quantum yields are readily controlled both through hydrophobic interactions between PtTCPPs in the solution and the Coulomb force between amine groups and carboxylic acids at a SAG/PtTCPP molar ratio of 4:1.
Porphyrin derivatives are efficient photosensitizers in photodynamic therapy, and most photosensitizers currently used clinically are based on porphyrin molecules. We therefore studied the photosensitizing ability of SAG/PtTCPP hybrids, including the cytotoxicity of the hybrids to cancer cells, in vitro singlet oxygen (1O2) generation under photoirradiation, and the incorporation of the hybrids into cancer cells. We used 1/PtTCPP and 2/PtTCPP because they have different nanostructures: 1/PtTCPP forms a tubular vesicular structure and 2/PtTCPP forms a sheet structure.
We first examined the photodynamic activity of 1/PtTCPP and 2/PtTCPP hybrids against human lung carcinoma A549 cells and human cervical cancer HeLa cells and compared the results with those obtained using PtTCPP alone (Figure 4). PtTCPP alone dose-dependently significantly reduced cell viability in response to visible light irradiation. The 1/PtTCPP hybrid showed strong photodynamic activity at ptTCPP concentrations above 3 μM and was significantly higher than those for 2/PtTCPP hybrid and PtTCPP alone.
Figure 4.
Photodynamic activity of SAG/PtTCPP hybrids on A549 (a) and HeLa (b) cells. Each point represents the mean ± S.E. of 4–8 experiments. *p < 0.05 versus2/PtTCPP hybrid with light irradiation and †p < 0.05 versus PtTCPP with light irradiation.
To gain insight into the high cytotoxicity of 1/PtTCPP under light irradiation, we evaluated 1O2 generation by the hybrids using X-band electron spin resonance (ESR) spectroscopy (Figure 5). After photoirradiation (400–700 nm), three characteristic signals corresponding to those for the TEMPO-OH adduct were detected in the ESR spectra, indicating that 1O2 was generated by the SAG/PtTCPP hybrids, as confirmed by near-infrared luminescence spectroscopy (Figure S7). Although the baselines of the luminescence spectra (Figure S7) of these hybrids were different, similar luminescence bands were observed at around 1273 nm,20−22 indicating the generation of 1O2. This 1O2 generation increased with increasing PtTCPP concentration (Figure 5), and 1O2 generation by the 1/PtTCPP hybrid was slightly lower than by PtTCPP alone and the 2/PtTCPP hybrid. Since light absorption by these hybrids is similar in the visible range (Figure 3), the mode of energy transfer from PtTCPP to oxygen molecules might differ in these hybrids, i.e., the tightly packed vesicular fibrous structure of the 1/PtTCPP hybrid slightly inhibited the access of oxygen molecules when compared with the simple sheet structure of the 2/PtTCPP hybrid. However, these results suggest that the enhanced cytotoxicity of the 1/PtTCPP hybrid compared with 2/PtTCPP upon irradiation cannot be explained simply by 1O2 generation. We therefore evaluated the cellular uptake of the 1/PtTCPP and 2/PtTCPP hybrids by A549 cells and HeLa cells by measuring the fluorescence of PtTCPP incorporated into the cells (Figure 6). A large amount of PtTCPP was incorporated into the cells within a few hours in the case of 1/PtTCPP and a low amount in the case of PtTCPP alone and 2/PtTCPP. SAG 1 and SAG 2 (Figure 1) have similar chemical structures, i.e., an extended, not bent, structure of SAG 1 and SAG 2 molecules due to the trans A and B ring configuration and the presence of the C5 double bond, respectively. The observed difference in cellular uptake is thus likely due to differences in the nanostructures of these hybrids (fibrous vs sheet assembly) and to the membrane perturbing effect of the fibrous vesicle structure of the 1/PtTCPP hybrid possibly being stronger than that of the 2/PtTCPP hybrid. Indeed, pure 1 showed cell toxicity above 5 μM, whereas pure 2 did not showed cell toxitiy even at 20 μM (Figure S6). SAG 1 likely has a membrane perturbing effect that helps in enhancing drug incorporation into cells, although further studies are required to elucidate its detailed membrane perturbing mechanism.
Figure 5.
ESR spectra of the TEMPO-OH adduct generated in the PtTCPP, 1/PtTCPP, and 2/PtTCPP hybrids after visible light irradiation. (a) PtTCPP, (b) 1/PtTCPP, and (c) 2/PtTCPP. (d) Relative intensity increment of the TEMPO-OH adduct as a function of PtTCPP concentration. Each point represents the mean ± S.E. of three experiments (SAG/PtTCPP = 4:1).
Figure 6.
Cellular uptake of SAG/PtTCPP hybrids by A549 (a) and HeLa (b) cells. Each value represents the mean ± S.E. of 3–5 experiments. *p < 0.05 versus2/PtTCPP hybrid and †p < 0.05 versus PtTCPP.
The results of our morphological, spectroscopic, and biochemical investigations provide detailed information regarding the nature of the hybrids self-assembled from SAGs and PtTCPP complexes. Our observations of intermolecular interactions indicate that PtTCPP ions form bilayer-ordered arrays in conjunction with the SAGs. HR-TEM images confirm the generation of molecular-scale strands that depend on the SAG structure and sugar groups. The UV–vis, CD, and luminescence intensity analyses demonstrate that the SAG/PtTCPP complexes contain supramolecular PtTCPP species that undergo intermolecular interactions. In addition, based on the results obtained using a 4:1 molar ratio of SAG and PtTCPP, electrostatic interactions and hydrogen bonding between the amine segments and carboxylic acids, as well as the layered nanostructure, play important roles in enabling these intermolecular interactions. These nanomaterials are useful for preparing unique nanohybrids with high cellular uptake while maintaining adequate 1O2 generation. Thus, we suggest that the nanostructure of the SAG/PtTCPP hybrid improves tumor selectivity by enhancing permeability, the primary focus of supramolecular biomaterials. SAGs are clearly capable of introducing detailed nanostructures and bioactivities based on lamellae in aqueous solutions of the PtTCCP complex.
Distinct geometries such as nanofibers, nanosheets, and nanoparticles affect the cellular uptake, hemorheological dynamics, and in vivo fate of nanostructures. In particular, cylindrical and discoidal shapes show pronounced effects on the pharmacokinetics and biodistribution of the nanoparticles in various organs, including the lungs, liver, spleen, and kidneys.35,36 In addition, nanorods were found to reach the cores of tumors, whereas nanospheres and nanodiscs were only observed on the surfaces of the tumors, suggesting the unique nature of nanorods for cancer treatment.37 Although the chemical structure of 1 is almost the same as that of 2 (differing only in one double bond), the nanostructure of 1 (fibrous vesicle structure) was different from that of 2 (sheet structure), resulting not only in different spectroscopic properties but also in different photodynamic activity against cancer cells. In contrast to the nanosheet structures of 2/PtTCPP, the nanofibrous structure of 1/PtTCPP efficiently induced toxicity in tumor cells. Figures 4 and 6 show the photodynamic activity and pharmacokinetic dynamics of the 1/PtTCPP and 2/PtTCPP nanostructures and indicate that the fibrous vesicle structure of 1/PtTCPP made it lethal to cells due to its supramolecular cell permeability. The results of photodynamic activity and cellular uptake assay indicate that the biodistribution of nanostructures will vary based on the interplay of several of the above parameters, especially the nanosized shape of fibrous vesicle structures.
Conclusions
We have demonstrated that different SAG-based nanostructures differ significantly depending on the structure of the SAG. Hybrid nanomaterials formed by combining these amphiphiles with a Pt complex demonstrate that intermolecular interactions between Pt complexes and SAGs can be controlled to produce specific nanostructures such as fibrous, sheet, and other nanoarchitectures. The technique of combining natural molecules and discrete coordination compounds makes it possible to readily design flexible and responsive supramolecular coordination systems and to conduct photodynamic therapy with high pharmacokinetic dynamics. This general concept of biological composites could be expanded to include useful agricultural compounds and should provide valuable information, leading to further advances in the fields of nanochemistry and biochemistry based on coordination materials and biological units as building blocks.
Materials and Methods
General Materials and Instrumentation
The synthesis and characterization of the hybrids prepared for this study, general materials, and instrumentation are described in the Supporting Information.
Photodynamic Activity of SAG/PtTCPP Hybrids against Cancer Cells
Dulbecco’s modified Eagle’s medium (DMEM) and penicillin–streptomycin were purchased from GIBCO Invitrogen Co. (Tokyo, Japan). Fetal calf serum (FCS) was obtained from Nichirei (Tokyo, Japan). A549 cells and HeLa cells were cultured in DMEM containing 100 U/mL penicillin–streptomycin, supplemented with 10% fetal bovine serum at 37 °C and 5% CO2. The cells were seeded in 96-well plates at a density of 3.0 × 104 cells/well. After growing overnight, the cells were incubated with the samples for 24 h. The cells were washed with phosphate-buffered saline (PBS), and a fresh culture medium was added. The treated cells were exposed to light (35 mW/cm2, 400–700 nm) from a MAX-303 xenon light source (Asahi Spectra Co., Ltd., Tokyo, Japan) for 30 min. A xenon lamp produces homogeneous light in the visible light range, which leads to an efficient photodynamic effect. Cell viability was detected 24 h after photoirradiation using PrestoBlue reagent (GIBCO Invitrogen Company, MO), according to the manufacturer’s protocol. The viability of cells was calculated as the ratio (%) compared with cells not treated with the sample.
1O2 Generation Ability of SAGs/PtTCPP
The generation of 1O2 by SAGs/PtTCPP under light irradiation was evaluated using an X-band electron spin resonance (ESR) spectrometer (JES-FA100, JEOL Ltd., Tokyo, Japan). ESR measurements were conducted under the following conditions: microwave frequency, 9.417 GHz; microwave power, 8 mW; field modulation, 0.1 mT at 100 kHz; and sweep time, 30 s. The generation of 1O2 radicals was detected using 4-hydroxy-2,2,6,6-tetramethylpiperidine (TEMP-OH) as a spin-trapping reagent. Sample solution (40 μL), 60 μL of ultrapure water, and 100 μL of TEMP-OH solution (400 mM) were mixed under aerobic conditions and exposed to visible light for 1 min using a xenon light source (400–700 nm) at 25 mW/cm2, and then, ESR measurements were immediately performed.
Cellular Uptake of SAGs/PtTCPP by Cancer Cells
A549 cells and HeLa cells were seeded in 100 mm × 20 mm dishes at a density of 4.0 × 106/dish. After growing to 100% confluence, the cells were incubated with the complexes (PtTCPP = 10 μM) for 1–5 h. The cells were washed with PBS and then removed from the dish by trypsinization. After centrifugation, the supernatant was replaced with 50% DMF to extract PtTCPP from the cells. SAG/PtTCPP complexes were disassembled in 50% DMF, and the amount of PtTCPP in the cells was measured. The cell suspensions were sonicated for 10 min and then centrifuged for 10 min at 15 000 rpm. The concentration of PtTCPP in each supernatant was determined using a microplate reader (TECAN M200PRO, Tokyo, Japan) with an excitation wavelength of 400 nm and a fluorescence wavelength of 670 nm.
Statistical Analysis
Data are presented as the median value from n samples, and the results are reported as the mean ± S.E. Significant differences between the data were calculated using Student’s t-tests. For all analyses, values of p < 0.05 were regarded as statistically significant.
Acknowledgments
This work was financially supported in part by a Grant-in-Aid for Scientific Research on Innovative Areas (New polymeric materials based on element blocks, no. 2401) (Nos. 25102547 and 15H00770). K.K. is also grateful for the financial support of the Canon Foundation (No. K16-0146) and for a research grant from Sojo University (No. RT02000001). The synchrotron radiation experiments were conducted at SPring-8 BL40B2 (2018B1424). The authors thank Dr. Noboru Ohta for help with the experiments at SPring-8.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c01239.
Materials and instrumentation, preparation of SAG/PtTCPP composites, transmission electron microscopy (TEM) of pure SAG, small-angle X-ray scattering (SAXS) measurements of SAG-Pt composites, luminescence spectra of 1/PtTCPP and stoichiometric ratio of the hybrid solution, and near-infrared luminescence spectra for the generation of 1O2 by SAG/PtTCPP complexes (PDF)
Author Contributions
M.F., D.I., S.O., M.M., M.S., M.A., T.I., F.H., and K.K. performed the described experiments and analyzed the data. M.F. D.I., F.H., and K.K. conceived and designed the experiments and co-wrote the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Sinden S.; Schalk J.; Stoner A. Effects of Daylength and Maturity of Tomato Plants on Tomatine Content and Resistance to the Colorado Potato Beetle. J. Am. Soc. Hortic. Sci. 1978, 103, 596–600. [Google Scholar]
- Yang Y.-W.; Sheikh N. A.; Morrow W. J. W. The Ultrastructure of Tomatine Adjuvant. Biomaterials 2002, 23, 4677–4686. 10.1016/S0142-9612(02)00218-1. [DOI] [PubMed] [Google Scholar]
- Amaike M.; Kobayashi H.; Shinkai S. New Organogelators Bearing both Sugar and Cholesterol Units: An Approach toward Molecular Design of Universal Gelators. Bull. Chem. Soc. Jpn. 2000, 73, 2553–2558. 10.1246/bcsj.73.2553. [DOI] [Google Scholar]
- Jiang Q.; Wang Y.; Weng J.; Liu L.; Zhou Z.; Zhang Q.; Chen H.; Yang W. Self-Assembled Nanostructures of a Cholesterol-Saccharide Conjugate which Acts as an Amphiphilic Gelator of Organic Solvents. Curr. Nanosci. 2009, 5, 245–251. 10.2174/157341309788185460. [DOI] [Google Scholar]
- Li Y.; Bai H.; Liu Q.; Bao J.; Han M.; Dai Z. A Nonenzymatic Cholesterol Sensor Constructed by Using Porous Tubular Silver Nanoparticles. Biosens. Bioelectron. 2010, 25, 2356–2360. 10.1016/j.bios.2010.03.036. [DOI] [PubMed] [Google Scholar]
- Toohara S.; Tanaka Y.; Sakurai S.; Ikeda T.; Tanaka K.; Gon M.; Chujo Y.; Kuroiwa K. Self-assembly of [Au(CN)2]− Complexes with Tomato (Solanum lycopersicum) Steroidal Alkaloid Glycosides to Form Sheet or Tubular Structures. Chem. Lett. 2018, 47, 1010–1013. 10.1246/cl.180320. [DOI] [Google Scholar]
- Ethirajan M.; Chen Y.; Joshi P.; Pandey R. K. The Role of Porphyrin Chemistry in Tumor Imaging and Photodynamic Therapy. Chem. Soc. Rev. 2011, 40, 340–362. 10.1039/B915149B. [DOI] [PubMed] [Google Scholar]
- Washington I.; Brooks C.; Turro N. J.; Nakanishi K. Porphyrins as Photosensitizers to Enhance Night Vision. J. Am. Chem. Soc. 2004, 126, 9892–9893. 10.1021/ja0486317. [DOI] [PubMed] [Google Scholar]
- Rajora M. A.; Lou J. W. H.; Zheng G. Advancing Porphyrin’s Biomedical Utility via Supramolecular Chemistry. Chem. Soc. Rev. 2017, 46, 6433–6469. 10.1039/C7CS00525C. [DOI] [PubMed] [Google Scholar]
- Wang D.; Niu L.; Qiao Z.-Y.; Cheng D.-B.; Wang J.; Zhong Y.; Bai F.; Wang H.; Fan H. Synthesis of Self-assembled Porphyrin Nanoparticle Photosensitizers. ACS Nano 2018, 12, 3796–3803. 10.1021/acsnano.8b01010. [DOI] [PubMed] [Google Scholar]
- Feng X.; Shi Y.; Xie L.; Zhang K.; Wang X.; Liu Q.; Wang P. Synthesis, Characterization, and Biological Evaluation of a Porphyrin-based Photosensitizer and its Isomer for Effective Photodynamic Therapy against Breast Cancer. J. Med. Chem. 2018, 61, 7189–7201. 10.1021/acs.jmedchem.8b00547. [DOI] [PubMed] [Google Scholar]
- Maruani A.; Savoie H.; Bryden F.; Caddick S.; Boyle R.; Chudasama V. Site-selective Multi-porphyrin Attachment Enables the Formation of a Next-generation Antibody-based Photodynamic Therapeutic. Chem. Commun. 2015, 51, 15304–15307. 10.1039/C5CC06985H. [DOI] [PubMed] [Google Scholar]
- Yap S. Y.; Price T. W.; Savoie H.; Boyle R. W.; Stasiuk G. J. Selective Radiolabelling with (68)Ga under Mild Conditions: A Route towards a Porphyrin PET/PDT Theranostic Agent. Chem. Commun. 2018, 54, 7952–7954. 10.1039/C8CC03897J. [DOI] [PubMed] [Google Scholar]
- Wang D.; Zhang Z.; Lin Lin.; Liu F.; Wang Y.; Guo Z.; Li Y.; Tian H.; Chen X. Porphyrin-based Covalent Organic Framework Nanoparticles for Photoacoustic Imaging-guided Photodynamic and Photothermal Combination Cancer Therapy. Biomaterials 2019, 223, 119459 10.1016/j.biomaterials.2019.119459. [DOI] [PubMed] [Google Scholar]
- Wang J.; Wang Z.; Zhong Y.; Zou Y.; Wang C.; Wu H.; Lee A.; Yang W.; Wang X.; Liu Y.; et al. Central Metal-derived Co-assembly of Biomimetic GdTPP/ZnTPP Porphyrin Nanocomposites for Enhanced Dual-modal Imaging-guided Photodynamic Therapy. Biomaterials 2020, 229, 119576 10.1016/j.biomaterials.2019.119576. [DOI] [PubMed] [Google Scholar]
- Hou Y.; Zhou J.; Gao Z.; Sun X.; Liu C.; Shangguan D.; Yang W.; Gao M. Protease-activated Ratiometric Fluorescent Probe for pH Mapping of Malignant Tumors. ACS Nano 2015, 9, 3199–3205. 10.1021/acsnano.5b00276. [DOI] [PubMed] [Google Scholar]
- Tang B.; Yu F.; Li P.; Tong L.; Duan X.; Xie T.; Wang X. A Near-infrared Neutral pH Fluorescent Probe for Monitoring Minor pH Changes: Imaging in Living HepG2 and HL-7702 cells. J. Am. Chem. Soc. 2009, 131, 3016–3023. 10.1021/ja809149g. [DOI] [PubMed] [Google Scholar]
- Odai S.; Ito H.; Kamachi T. Dendrimer Porphyrins as the Oxygen Sensor for Intracellular Imaging to Suppress Interaction towards Biological Molecules. J. Clin. Biochem. Nutr. 2019, 65, 178–184. 10.3164/jcbn.19-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa H.; Ito H.; Inoue M.; Tabata K.; Sato Y.; Yamagita K.; Kizaka-Kondoh S.; Kadonosono T.; Yano S.; Inoue M.; Kamachi T. High Resolution Imaging of Intracellular Oxygen Concentration by Phosphorescence Lifetime. Sci. Rep. 2015, 5, 10657 10.1038/srep10657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amao Y.; Asai K.; Okura I. Photoluminescent Oxygen Sensing Using Palladium Tetrakis(4-carboxyphenyl)porphyrin Self-assembled Membrane on Alumina. Anal. Commun. 1999, 36, 179–180. 10.1039/a900721k. [DOI] [Google Scholar]
- Amao Y.; Okura I. An Oxygen Sensing System Based on the Phosphorescence Quenching of Metalloporohyrin Thin Film on Allumina Plates. Analyst 2000, 125, 1601–1604. 10.1039/b004065g. [DOI] [Google Scholar]
- Lo L.-W.; Koch C. J.; Wilson D. F. Calibration of Oxygen Dependent Quenching of the Phosphorescence of Pd-meso-tetra (4-carboxyphenyl)porphine: a Phosphor with General Application for Measuring Oxygen Concentration in Biological Systems. Anal. Biochem. 1996, 236, 153–160. 10.1006/abio.1996.0144. [DOI] [PubMed] [Google Scholar]
- Ikeda T.; Yamauchi K.; Nakano D.; Nakanishi K.; Miyashita H.; Ito S. -i.; Nohara T. Chemical Trans-glycosylation of Bioactive Glycolinkage: Synthesis of an α-Lycotetraosyl Cholesterol. Tetrahedron Lett. 2006, 47, 4355–4359. 10.1016/j.tetlet.2006.04.107. [DOI] [Google Scholar]
- Ikeda T.; Tsumagari H.; Honbu T.; Nohara T. Cytotoxic Activity of Steroidal Glycosides from Solanum Plants. Biol. Pharm. Bull. 2003, 26, 1198–1201. 10.1248/bpb.26.1198. [DOI] [PubMed] [Google Scholar]
- Johns T. Detoxification Function of Geophagy and Domestication of the Potato. J. Chem. Ecol. 1986, 12, 635–645. 10.1007/BF01012098. [DOI] [PubMed] [Google Scholar]
- Organic Chemistry, 5th ed.; McMurry J. E., Ed.; Brooks/Cole: Pacific Grove, 2000; p 607. [Google Scholar]
- Gaeta M.; Raciti D.; Randazzo R.; Gangemi C. M. A.; Raudino A.; D’Urso A.; Fragalá M. E.; Purrello R. Chirality Enhancement of Porphyrin Supramolecular Assembly Driven by a Template Preorganization Effect. Angew. Chem., Int. Ed. 2018, 57, 10656–10660. 10.1002/anie.201806192. [DOI] [PubMed] [Google Scholar]
- Fuhrhop J. H.; Demoulin C.; Boettcher C.; Koning J.; Siggel U. Chiral Micellar Porphyrin Fibers with 2-Aminoglycosamide Head Groups. J. Am. Chem. Soc. 1992, 114, 4159–4165. 10.1021/ja00037a018. [DOI] [Google Scholar]
- Fuhrhop J. H.; Bindig U.; Siggel U. Micellar Rods and Vesicular Tubules Made of 14‴,16‴-Diaminoporphyrins. J. Am. Chem. Soc. 1993, 115, 11036–11037. 10.1021/ja00076a090. [DOI] [Google Scholar]
- Bindig U.; Schulz A.; Fuhrhop J. H. Micellar Fibers made of Porphyrin and Metalloporphyrin Amides. New J. Chem. 1995, 19, 427–435. [Google Scholar]
- Fuhrhop J. H.; Bindig U.; Demoulin C.; Rosengarten B.; Siggel U. Polymeric Porphyrin and Metalloporphyrin Assemblies in Bulk Solution. Macromol. Symp. 1994, 80, 63–82. 10.1002/masy.19940800106. [DOI] [Google Scholar]
- Shirakawa M.; Fujita N.; Shinkai S. A Stable Single Piece of Unimolecularly π-Stacked Porphyrin Aggregate in a Thixotropic Low Molecular Weight Gel: A One-Dimensional Molecular Template for Polydiacetylene Wiring up to Several Tens of Micrometers in Length. J. Am. Chem. Soc. 2005, 127, 4164–4165. 10.1021/ja042869d. [DOI] [PubMed] [Google Scholar]
- Lu G.; Zhang X.; Gai X.; Jiang J. Tuning the Morphology of Self-assembled Nanostructures of Amphiphilic Tetra(p-hydroxyphenyl)porphyrins with Hydrogen Bonding and Metal–ligand Coordination Bonding. J. Mater. Chem. 2009, 19, 2417–2424. 10.1039/b820127g. [DOI] [Google Scholar]
- Engelkamp H.; Middelbeek S.; Nolte R. J. M. Self-Assembly of Disk-Shaped Molecules to Coiled-Coil Aggregates with Tunable Helicity. Science 1999, 284, 785–788. 10.1126/science.284.5415.785. [DOI] [PubMed] [Google Scholar]
- Blanco E.; Shen H.; Ferrari M. Principles of Nanoparticle Design for Overcoming Biological Barriers to Drug Delivery. Nat. Biotechnol. 2015, 33, 941–951. 10.1038/nbt.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan V. P.; Popović Z.; Chen O.; Cui J.; Fukumura D.; Bawendi M. G.; Jain R. K. Fluorescent Nanorods and Nanospheres for Real-time In Vivo Probing of Nanoparticle Shape-Dependent Tumor Penetration. Angew. Chem., Int. Ed. 2011, 50, 11417–11420. 10.1002/anie.201104449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black K. C. L.; Wang Y.; Luehmann H. P.; Cai X.; Xing W.; Pang B.; Zhao Y.; Cutler C. S.; Wang L. V.; Liu Y.; Xia Y. Radioactive 198Au-doped Nanostructures with Different Shapes for In Vivo Analyses of Their Biodistribution, Tumor Uptake, and Intratumoral Distribution. ACS Nano 2014, 8, 4385–4394. 10.1021/nn406258m. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.