Abstract
A series of aryl N-[ω-(6-fluoroindol-1-yl)alkyl]carbamates with alkyl spacers of varying lengths between the indole and the carbamate group and with differently substituted aryl moieties at the carbamate oxygen were synthesized and tested for inhibition of the pharmacologically interesting serine hydrolases fatty acid amide hydrolase (FAAH), monoacylglycerol lipase (MAGL), butyrylcholinesterase (BuChE), and acetylcholinesterase (AChE). Furthermore, the chemical stability in an aqueous solution and the metabolic stability toward esterases in porcine liver homogenate and porcine blood plasma were determined. While most of the synthesized derivatives were potent inhibitors of FAAH, a considerable inhibition of MAGL and BuChE was elicited only by compounds with a high carbamate reactivity, as evidenced by a significant hydrolysis of these compounds in an aqueous solution. However, the high inhibitory potency of some compounds toward MAGL and BuChE, especially that of the ortho-carboxyphenyl derivative 37, could not be explained by chemical reactivity alone. Several of the carbamates studied possessed varying degrees of stability toward esterases from liver and blood plasma. In some cases, marked inactivation by the pseudo-esterase activity of plasma albumin was observed. Mass spectrometric studies showed that such carbamates formed covalent bonds with albumin at several sites.
Introduction
The endocannabinoid system consists of the G-protein coupled cannabinoid receptors CB1 and CB2, their endogenous ligands, the endocannabinoids, and the enzymes for the synthesis and degradation of these compounds.1−4 The most important endocannabinoids are arachidonoyl ethanolamide (anandamide) and 2-arachidonoyl glycerol (2-AG). They are formed on demand from phospholipid precursors during numerous physiological and pathophysiological processes and mediate analgesic, anti-inflammatory, and neuropharmacological effects by activating the cannabinoid receptors CB1 and CB2. The distribution of these two receptors is different in the body. The CB1 receptor is preferentially found in neuronal tissues, whereas the CB2 receptor is mainly expressed in cells of the immune system such as macrophages.
After stimulation of cannabinoid receptors located on cell surfaces, the endocannabinoids are inactivated by a reuptake process likely facilitated by the putative endocannabinoid membrane transporter (EMT), followed by enzymatic degradation. Anandamide is hydrolyzed by fatty acid amide hydrolase (FAAH) into arachidonic acid and ethanolamine, whereas 2-AG is cleaved by monoacylglycerol lipase (MAGL) and probably also by FAAH into arachidonic acid and glycerol. Both enzymes are members of the serine hydrolase family.5
Inhibition of FAAH and MAGL results in an increase of anandamide and 2-AG levels at the receptor site and a reduction of the production of arachidonic acid, which is a precursor of proinflammatory and algesic eicosanoids like prostaglandin E2 and leukotriene B4.6 While in the periphery, arachidonic acid is mainly released by the serine hydrolase cytosolic phospholipase A2α (cPLA2α), in the brain, MAGL is the primary regulator of arachidonic acid release and controls more than 85% of the 2-AG metabolism.7,8 The important role of FAAH and MAGL in the degradation of the anti-inflammatory and analgesic endocannabinoids as well as in the formation of proinflammatory and algesic eicosanoids demonstrates that inhibitors of these enzymes could be of therapeutic interest.
A large number of potent FAAH inhibitors have been developed in the past decades,9−12 like the carbamate URB597 (1) (Figure 1),13 which is often used as a reference in the development of FAAH inhibitors. Although promising effects of some of these compounds have been observed in various animal models,14−17 Pfizer’s FAAH inhibitor PF-04457845 failed to eliminate pain in a clinical study with patients suffering from knee osteoarthritis.18,19 Vernalis’ FAAH inhibitor V158866 also did not produce the expected effects in a Phase II study of neuropathic pain following spinal cord injury.20 Regardless of these negative results, FAAH inhibitors are still considered new treatment options for some neurological disorders. Thus, the clinical efficacy of PF-04457845 has been demonstrated in patients with cannabis withdrawal symptoms.21 In addition, FAAH inhibitors may be useful in the treatment of anxiety and Parkinson’s disease.22,23 In the last few years, also highly potent MAGL inhibitors have been discovered like ABX-1431 (2),7,24−26 which is currently being evaluated in clinical trials. In contrast, to our knowledge, dual FAAH/MAGL inhibitors have not yet been clinically tested.27
Figure 1.
Structures of known FAAH and MAGL inhibitors.
Recently, we have found that phenyl carbamates with ω-indol-1-yl alkyl groups attached to the nitrogen atom are FAAH inhibitors, with the extent of enzyme inhibition depending on the length of the alkyl spacer (3–7) (Figure 1).28 In addition, it could be shown that in the case of the structurally related (indolylethyl)piperidine carbamate, the FAAH inhibitory potency increases significantly with the introduction of a fluorine atom at the indole-6 position (8).
Here, further structure–activity relationship studies on this type of compounds are presented. In particular, we have investigated whether a fluoro atom in position 6 of the heterocycle also affects the activity of the homologous series of N-(ω-indol-1-ylalkyl)-substituted phenyl carbamates 3–7. Furthermore, the effect of the replacement of their carbamate phenyl residue by differently substituted phenyl and heteroaryl moieties was studied. Carbamate inhibitors of FAAH and other serine hydrolases are known to react covalently with the active site serine of these enzymes, leading to enzyme carbamoylation. Since the reactivity of the carbamate fragment is critical not only for enzyme inhibitory potency but also for hydrolytic stability,29−31 the new target compounds were also tested for their susceptibility against chemical and metabolic hydrolysis. Moreover, their effect on MAGL should also be evaluated, since enhancing the levels of all endocannabinoids in the organism might be beneficial for the treatment of neuroinflammatory disorders. Since it has recently been suggested that effective treatment of Alzheimer’s disease can be achieved by simultaneous inhibition of FAAH and cholinesterases in a multitarget ligand approach,32,33 the inhibition of the serine hydrolases acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) by the new carbamates should be additionally tested.
Results and Discussion
Chemistry
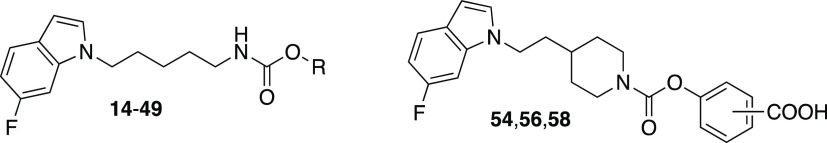
The phenyl N-alkylcarbamates with a 6-fluoroindol-1-yl-substituent at the terminal alkyl carbon and an alkyl spacer length between four and seven carbon atoms were synthesized as shown for the pentyl-substituted derivative 14 in Scheme 1. First, 6-fluoroindole was reacted with N-(5-bromopentyl)phthalimide in dimethylformamide (DMF) with sodium hydride as the base. After cleavage of the phthalimide-protected amino group of the obtained intermediate 12 with hydrazine hydrate, the released amine 13 was treated with phenyl chloroformate in CH2Cl2 in the presence of N,N-diisopropylethylamine (DIPEA) to yield the target compound 14. For the synthesis of the N-octylcarbamate 25, 6-fluoroindole was alkylated with an excess of 1,8-dibromooctane to give the 8-bromooctyl-substituted indole 22 (Scheme 2). By reaction with potassium phthalimide in DMF, the remaining bromine was substituted by a phthalimide group, which was then subjected to hydrazinolysis. The amine 21 formed was converted into the desired carbamate 25 by treatment with phenyl chloroformate.
Scheme 1.
Scheme 2.
The three fluorophenyl-substituted carbamates 26–28 were synthesized by reaction of the amine 13 with the appropriate fluorophenyl chloroformate in tetrahydrofuran (THF) with DIPEA as the base (Scheme 3). The cyanophenyl- and pyridine-substituted compounds 29–31 and 44–48 were obtained in a sequential three-component reaction. Thus, triphosgene was treated with the corresponding hydroxybenzonitrile or 3-hydroxypyridine in THF in the presence of DIPEA, affording a reactive intermediate that after addition of the amine 13 and DIPEA in THF gave the desired product. For the synthesis of the derivatives with carboxyphenyl and carboxymethylphenyl moieties (33, 35, 37, 39, 41, 43), benzyl esters of hydroxybenzoic acids and hydroxyphenylacetic acids were reacted with triphosgene and the amine 13 in the same manner to yield the benzyl esters, whose ester groups were cleaved by catalytic hydrogenation. The carbamate with an indole substituent at the carbamate oxygen (49) was synthesized by reaction of the amine 13 with di-tert-butyl dicarbonate and 4-(dimethylamino)pyridine (DMAP) in acetonitrile and treatment of the formed intermediate with 4-hydroxyindole.
Scheme 3.
For the synthesis of the ortho-, meta-, and para-carboxyphenyl 4-[2-(6-fluoroindol-1-yl)ethyl]piperidine-1-carboxylates 54, 56, and 58, commercially available benzyl 4-(2-hydroxyethyl)piperidine-1-carboxylate was reacted with methanesulfonyl chloride to obtain the methanesulfonate derivative 50 (Scheme 4).34 Nucleophilic substitution of the mesylate by 6-fluoroindole deprotonated with sodium hydride afforded the indolylethylpiperidine-1-carboxylate 51. The benzyloxycarbonyl protecting group of this compound was removed by catalytic hydrogenation. The produced piperidine derivative 52 was converted into the carbamates 53, 55, and 57 by treatment with reactive carbonates obtained by reaction of triphosgene and the appropriate hydroxybenzoic acid benzyl esters in the presence of an amine base as shown for the para-substituted isomer 53. Cleavage of the benzyl esters by hydrogenolysis led to the target compounds 54, 56, and 58.
Scheme 4.
Chemical and Biochemical Evaluation
The inhibitory potency of the target compounds against FAAH, MAGL, BuChE, and AChE was evaluated with high-performance liquid chromatography (HPLC)-based assays applying fluorescence or UV detection of the enzyme product. Inhibition of FAAH was assessed with the fluorogenic substrate N-(2-hydroxyethyl)-4-pyren-1-ylbutanamide and rat brain homogenate as the enzyme source.35,36 The MAGL assay was carried out using the fluorogenic substrate 1,3-dihydroxypropan-2-yl 4-pyren-1-ylbutanoate and the recombinant human enzyme.37 For measuring the inhibition of BuChE, equine serum enzyme was used in combination with the substrate benzoylcholine. The AChE activity was determined with the eel enzyme and the substrate pyridin-2-ylmethyl acetate. The stability against chemical hydrolysis was measured by HPLC/UV adapting the conditions of the FAAH assay (phosphate-buffered saline (PBS) buffer pH 7.4 containing 0.2% Triton X-100; incubation at 37 °C for 60 min). The metabolic and enzymatic stability was determined, respectively, in rat liver and porcine liver homogenate as well as in porcine blood plasma (incubation at 37 °C for 30 min) using HPLC with mass spectrometry (MS) detection.
Homologous Phenyl N-[ω-(6-Fluoroindol-1-yl)alkyl]carbamates
Recently, we have found that the FAAH inhibitory potency of phenyl N-(ω-indol-1-ylalkyl)carbamates depends on the length of the alkyl chain connecting the indole with the carbamate moiety. The most effective of the five compounds studied were those with pentyl (4) and heptyl (6) groups, with no significant difference in potency (Table 1). Elongation or shortening of these two alkyl spacers by one carbon reduced FAAH inhibition by approximately 2- to 10-fold as reflected by the half-maximal inhibitory concentration (IC50) values of carbamates 3, 5, and 7. Since we had also found with the structurally related (indolylethyl)piperidine carbamates that the FAAH inhibitory potency increased significantly after the introduction of a fluorine atom at the indole-6 position, we were interested to see if the same effect could be observed with the phenyl N-[ω-(indol-1-yl)alky]carbamates. Interestingly, the fluorine atom changed the activity differently here (Table 1). In the compounds whose alkyl spacer consisted of an even number of carbon atoms (11, 17, 25), this structural modification did not lead to any change in effect. In contrast, the additional fluorine atom caused an increase in the FAAH inhibitory potency in the compound with the pentyl chain (14) and even a decrease in activity in the case of the heptyl derivative 21. Consequently, in this series of compounds, the pentyl-linked derivative 14 clearly showed the highest FAAH inhibition (IC50: 0.029 μM). All new fluoro-substituted phenyl carbamates did not inhibit MAGL and AChE at 10 μM.
Table 1. Inhibitory Potency against FAAH, MAGL, and BuChE and Metabolic Stability in Biological Environments of Phenyl N-(ω-Indol-1-yl)alkyl- and N-[ω-(6-Fluoroindol-1-yl)alkyl]carbamates.

| inhibition
IC50 (μM)a |
metabolic
stability (%) |
||||||
|---|---|---|---|---|---|---|---|
| comp. | n | R | FAAH | MAGL | BuChE | rat liver S9 fractionb | porcine plasmac |
| 3 | 4 | H | 0.53 | n.t. | n.t. | 53 ± 5 | 82 ± 9 |
| 4 | 5 | H | 0.050 | n.t. | n.t. | 52 ± 2 | 91 ± 7 |
| 5 | 6 | H | 0.085 | n.t. | n.t. | 50 ± 2 | 68 ± 8 |
| 6 | 7 | H | 0.051 | n.t. | n.t. | 59 ± 3 | 39 ± 7 |
| 7 | 8 | H | 0.10 | n.t. | n.t. | 49 ± 1 | 64 ± 4 |
| 11 | 4 | F | 0.47 | n.a. | 7.3 | 75 ± 6 | 74 ± 2 |
| 14 | 5 | F | 0.029 | n.a. | 4.3 | 66 ± 8 | 80 ± 6 |
| 17 | 6 | F | 0.085 | n.a. | 3.3 | 73 ± 2 | 55 ± 2 |
| 21 | 7 | F | 0.084 | n.a. | 3.5 | 74 ± 11 | 34 ± 5 |
| 25 | 8 | F | 0.13 | n.a. | 3.2 | 74 ± 5 | 65 ± 2 |
| URB597 (1) | 0.043 | n.a. | >10d | 82 ± 4 | 77 ± 6 | ||
| ABX-1431 (2) | n.a. | 0.58 | n.a. | 92 ± 2 | 95 ± 2 | ||
| physostigmine | n.a. | n.a. | 2.7 | 76 ± 7 | >95 | ||
Values are the means of at least two independent determinations, errors are within ±20; n.t., not tested; n.a., not active at 10 μM.
Percent of parent remaining after incubation with rat liver S9 fractions for 30 min in the presence of the cofactor reduced nicotinamide adenine dinucleotide phosphate (NADPH); values are means ± standard deviations of independent determinations (n = 3; in the case of 14, n = 5; in the case of 21, n = 4).
Percent of parent remaining after incubation with porcine plasma for 30 min; values are means ± standard deviations of independent determinations (n = 3).
35% inhibition at 10 μM.
In contrast, BuChE was inhibited to some extent. The butyl compound 11 possessed the lowest activity (IC50: 7.3 μM). The derivatives with pentyl to octyl spacers (14, 17, 21, 25) were approximately equipotent. With IC50 values of about 4 μM, they were almost as effective as the reference inhibitor physostigmine. All of the derivatives studied were stable under the experimental conditions in PBS buffer pH 7.4. When treated with NADPH in rat liver S9 fractions, the fluorine-substituted derivatives showed a higher Phase I metabolic stability than their corresponding nonfluorinated derivatives. This could be due to the fact that the fluorine atom makes the indole heterocycle less susceptible to metabolic oxidation reactions. In contrast, sensitivity to cleavage by blood plasma esterases was not altered by the fluorine atom in the indole ring. Also, among the fluorinated compounds, the derivative with the heptyl spacer (21) was the most hydrolyzed.
Aryl N-[ω-(6-Fluoroindol-1-yl)pentyl]carbamates
Next, the influence of changes at the phenyl ring of the carbamate group on the potency and stability of the compounds was investigated. The starting substance here was derivative 14 with a pentyl linker, which had shown the highest activity against FAAH of all synthesized 6-fluoroindolylalkyl-substituted phenyl carbamates. First, we introduced fluorine atoms into the ortho-, meta-, or para-position of the phenyl moiety of the molecule since it was found that the activity of phenyl carbamate inhibitors of FAAH could be enhanced by such a substituent.38 In addition, to reduce the relatively high lipophilicity of the parent compound and thereby improve its druglike properties, polar substituents such as cyano, carboxy, and carboxymethyl were attached analogously or the phenyl ring was replaced by a pyridine heterocycle. The FAAH inhibitory potency was mostly not affected to a greater extent by the substituents introduced in the phenyl ring. With IC50 values from 0.008 to 0.058 μM, nearly all compounds were about as active as the lead 14 (IC50: 0.029 μM) or the reference inhibitor URB597 (IC50: 0.043 μM) (Table 2). The only exception was compound 43 with a carboxymethyl group in the ortho-position, which, with an IC50 of 4.2 μM, was more than 100 times less effective than the starting compound 14. Thus, larger polar residues near the reactive carbamate group do not seem to be well tolerated by the enzyme. The potencies of the 2- and 4-cyano-substituted derivatives 29 and 31 were not measured due to their high chemical lability in the PBS buffer (pH 7.4). For example, 29 was nearly totally cleaved into the amine and 4-hydroxybenzonitrile after 30 min at 37 °C as shown by HPLC/MS.
Table 2. Inhibitory Potency against FAAH, MAGL, and BuChE and Stability in Aqueous PBS Buffer (pH 7.4) of Aryl N-[ω-(6-Fluoroindol-1-yl)pentyl]carbamates and Aryl 4-[2-(6-Fluoroindol-1-yl)ethyl]piperidine-1-carboxylates.
| inhibition
IC50 (μM)a |
stability (%)b | |||||
|---|---|---|---|---|---|---|
| comp. | R | FAAH | MAGL | BuChE | in PBS buffer | log Pc |
| 14 | 4-phenyl | 0.029 | n.a. | 4.3 | >95 | 4.1 |
| 26 | 4-fluorophenyl | 0.049 | n.a. | 7.1 | >95 | 4.2 |
| 27 | 3-fluorophenyl | 0.015 | >10d | 1.5 | >95 | 4.3 |
| 28 | 2-fluorophenyl | 0.017 | 3.7 | 0.74 | 91 ± 3 | 4.1 |
| 29 | 4-cyanophenyl | n.t. | n.t. | n.t. | 12 ± 3 | 3.8 |
| 30 | 3-cyanophenyl | 0.008 | 0.38 | 0.38 | 72 ± 3 | 3.8 |
| 31 | 2-cyanophenyl | n.t. | n.t. | n.t. | 3 ± 2 | 3.9 |
| 33 | 4-carboxyphenyl | 0.012 | 3.3 | 0.97 | >95 | 2.8 |
| 35 | 3-carboxyphenyl | 0.018 | >10e | 5.8 | >95 | 2.8 |
| 37 | 2-carboxyphenyl | 0.038 | 0.038 | 2.3 | 73 ± 6 | 2.7 |
| 39 | 4-(carboxymethyl)phenyl | 0.014 | n.a. | >10f | >95 | 2.7 |
| 41 | 3-(carboxymethyl)phenyl | 0.058 | n.a. | >10g | >95 | 2.8 |
| 43 | 2-(carboxymethyl)phenyl | 4.2 | n.a. | >10h | >95 | 2.8 |
| 44 | pyridin-3-yl | 0.002 | 0.17 | 0.27 | 60 ± 2 | 2.6 |
| 45 | 2-methylpyridin-3-yl | 0.010 | 0.14 | 0.40 | 75 ± 4 | 2.7 |
| 46 | 4-methylpyridin-3-yl | 0.003 | 0.15 | 0.36 | 74 ± 4 | 2.7 |
| 47 | 5-methylpyridin-3-yl | 0.002 | 0.15 | 0.24 | 72 ± 4 | 2.9 |
| 48 | 6-methylpyridin-3-yl | 0.004 | 0.31 | 0.36 | 82 ± 6 | 2.8 |
| 49 | indol-4-yl | 0.18 | n.a. | 0.55 | >95 | 3.5 |
| 54 | 4-carboxy | 0.015 | n.a. | n.a. | >95 | 3.6 |
| 56 | 3-carboxy | 0.027 | n.a. | n.a. | >95 | 3.6 |
| 58 | 2-carboxy | 3.5 | n.a. | n.a. | >95 | 3.4 |
| URB597 (1) | 0.043 | n.a. | >10i | >95 | 2.0 | |
| ABX-1431 (2) | n.a. | 0.58 | n.a. | 90 ± 5 | 7.4 | |
| physostigmine | n.a. | n.a. | 2.7 | >95 | 0.6 | |
Values are the means of at least two independent determinations; errors are within ± 20; n.t., not tested; n.a., not active at 10 μM.
Percent of parent remaining after incubation in PBS buffer (pH 7.4) for 60 min; values are means ± standard deviations of independent determinations (n = 3).
Experimentally determined by reversed-phase HPLC.
39% at 10 μM.
31% at 10 μM.
38% at 10 μM.
42% at 10 μM.
34% at 10 μM.
35% at 10 μM.
A significant increase in activity was achieved by replacing the phenyl ring with a pyridin-3-yl or a methylated pyridin-3-yl heterocycle. With IC50 values of 0.002–0.004 μM, several of these pyridines (44, 46–48) were about 10-fold more active than the corresponding phenyl derivative 14. One reason for this increase in effect could be an enhancement in the carbonyl activity of the carbamate group caused by the electron-withdrawing pyridine residue. Evidence for this assumption is the lower chemical stability of all pyridine-substituted compounds in the aqueous solution (PBS buffer, pH 7.4) compared to that of the phenyl-substituted derivative 14 (60–82 vs >95%). Since it had been described that a particularly high increase in FAAH inhibition could be achieved with an indol-5-yl residue instead of a phenyl ring, a corresponding derivative (49) was also prepared. However, this structural variation led to an about 6-fold decrease in activity.
When looking at the inhibition values toward MAGL, it is noticeable that only the compounds with significant hydrolysis sensitivity in the PBS buffer (3-cyanophenyl, 2-carboxyphenyl, and all pyridine-substituted derivatives) show a significant activity toward the enzyme (IC50 < 1 μM). Accordingly, only very reactive carbamates seem to be able to effectively inhibit the enzyme. However, the 10-fold difference in potency between the 3-cyanophenyl and the 2-carboxyphenyl derivatives 30 and 37, which do not differ in hydrolytic stability, is an indication that reactivity alone cannot explain the high inhibitory potency of the latter compound toward MAGL. It is possible that the carboxylic acid group of the molecule makes specific polar interactions with the part of the enzyme to which the glycerol residue of the natural MAGL substrate 2-AG normally binds. With an IC50 of 0.038 μM, 37 exceeds the activity of the reference inhibitor ABX-1431 by a factor of 15. However, it should be noted that ABX-1431, which is currently in Phase II studies, has a better hydrolysis stability than 37.
All investigated aryl indolylpentylcarbamates were inactive against AChE at the highest test concentration (10 μM), while they showed at least some potency against BuChE. Again, the derivatives with the reactive pyridine groups were the most active ones, possessing about 10-fold lower IC50 values as the lead 14 and the reference physostigmine. In the case of the 3-cyanophenyl- and the 2-carboxyphenyl-derivatives 30 and 37, a reversed order of potency is seen in comparison to MAGL inhibition. Here, the cyano derivative is clearly the more effective of the two.
Some of the synthesized carbamates were also investigated for inhibition of the serine hydrolase cytosolic phospholipase A2α (cPLA2α), which is mainly responsible for the release of proinflammatory arachidonic acid from phospholipids in peripheral cells and tissues. Since most of the known potent inhibitors of this enzyme have an acidic group,39 only carbamate compounds with carboxylic acid functionality were tested. These all showed no inhibition of cPLA2α at 10 μM.40
Hydrolysis of carbamates can take place not only chemically but also enzymatically. In the organism, esterases found in the liver and blood plasma are particularly capable of doing this. To obtain information on the enzymatic stability of the substances, they were incubated in the homogenate of porcine liver and in porcine blood plasma. As ester-cleaving enzymes, carboxylesterase 1 (CES1) is found in a high activity in pig liver,41−43 and butyrylcholinesterase (BuChE), paraoxonase (PON), and a carboxylesterase (CES)-like enzyme are present in pig plasma.44−46 In addition, plasma albumin has a pseudo-esterase activity by reacting with esters via its hydroxy or amino groups, whereby the acid part of the ester is bound to the albumin and the alcohol part is released.47−49
When lead structure 14 was incubated with pig liver homogenate, about 75% of the starting compound was still present at the end of the incubation time (30 min, 37 °C) (Table 3). A much stronger degradation occurred with the 3-fluoro- (27), 3-cyano- (30), 4-carboxy- (33) as well as all pyridine-3-yl-substituted derivatives (44–48). Surprisingly, the 2-carboxyphenyl compound 37 was as stable in the liver homogenate as the parent compound, although it was significantly more labile than the latter in an aqueous solution. Also notable was the relatively strong degradation of the 4-carboxyphenyl carbamate 33, which was largely stable in aqueous solutions. To determine that the observed degradation was caused by esterases (in particular CES1), inhibition experiments were performed on selected carbamates using the CES1 inhibitor bis(4-nitrophenyl)phosphate (BNPP). This compound almost completely blocked the degradation of the reference ester oseltamivir in porcine liver homogenate.50,51 In contrast, breakdown of the carbamate derivatives investigated was only partially inhibited by about 50% (Table 4). This could be due to the fact that other esterases are also involved in their degradation or that chemical ester cleavage or reaction with other proteins occurred. Oxidative or reductive Phase I reactions also cannot be excluded. Although no reduction equivalents in the form of NADPH were supplied during incubation, it could be seen that such coenzymes were still present in some quantity. Thus, the activated carbonyl group of 3-isobutyl-1-[2-oxo-3-(4-phenoxyphenoxy)propyl]indole-5-carboxylic acid52 (Cay10650) used as a molecular probe was significantly reduced to an alcohol. Interestingly, this metabolic reaction was also inhibited by BNPP to about 45%, indicating that this compound is a pure inhibitor not only of esterases but also of carbonyl reductases. This has also been reported in the literature.53
Table 3. Chemical and Metabolic Stability of Aryl N-[ω-(6-Fluoroindol-1-yl)alkyl]carbamates and Aryl 4-[2-(6-Fluoroindol-1-yl)ethyl]piperidine-1-carboxylates.
| stability
(%)a |
|||||
|---|---|---|---|---|---|
| comp. | R | porcine liver S9 fractions | porcine blood plasma | porcine albumin solution | PBS buffer (pH 7.4) |
| 14 | 4-phenyl | 76 ± 2 | 83 ± 9n = 8 | 93 ± 6n = 6 | >95 |
| 26 | 4-fluorophenyl | 69 ± 4 | 59 ± 13n = 6 | 91 ± 6n = 5 | >95 |
| 27 | 3-fluorophenyl | 62 ± 5 | 10 ± 3 | 76 ± 4 | >95 |
| 28 | 2-fluorophenyl | 76 ± 2 | 19 ± 6 | 69 ± 5 | 91 ± 3 |
| 29 | 4-cyanophenyl | n.t. | n.t. | n.t. | 12 ±3 |
| 30 | 3-cyanophenyl | 49 ± 4 | < 5 | <5 | 72 ± 3 |
| 31 | 2-cyanophenyl | n.t. | n.t. | n.t. | 3 ± 2 |
| 33 | 4-carboxyphenyl | 59 ± 6 | 37 ± 2 | 90 ± 9 | >95 |
| 35 | 3-carboxyphenyl | 74 ± 2 | 78 ± 1 | 93 ± 3 | >95 |
| 37 | 2-carboxyphenyl | 77 ± 3 | 28 ± 4 | 74 ± 2 | 73 ± 6 |
| 39 | 4-(carboxymethyl)phenyl | 85 ± 3 | 88 ± 4 | >95 | >95 |
| 41 | 3-(carboxymethyl)phenyl | 73 ± 2 | 83 ± 5 | >95 | >95 |
| 43 | 2-(carboxymethyl)phenyl | 82 ± 5 | 89 ± 1 | 89 ± 3 | >95 |
| 44 | pyridin-3-yl | 53 ± 7 | <5 | 27 ± 3 | 60 ± 2 |
| 45 | 2-methylpyridin-3-yl | 65 ± 9 | 23 ± 6 | 70 ± 8 | 75 ± 4 |
| 46 | 4-methylpyridin-3-yl | 56 ± 8 | 8 ± 2 | 68 ± 6 | 74 ± 4 |
| 47 | 5-methylpyridin-3-yl | 57 ± 5 | <5 | 37 ± 4 | 72 ± 4 |
| 48 | 6-methylpyridin-3-yl | 51 ± 9 | <5 | 62 ± 4 | 82 ± 6 |
| 49 | indol-4-yl | 78 ± 4 | 80 ± 3 | 81 ± 6 | >95 |
| 54 | 4-carboxy | 66 ± 9 | 94 ± 3 | 93 ± 8 | >95 |
| 56 | 3-carboxy | 72 ± 7 | >95 | 94 ± 3 | >95 |
| 58 | 2-carboxy | 72 ± 6 | >95 | 89 ± 6 | >95 |
| URB597 (1) | 80 ± 4 | 77 ± 6 | 95 ± 7 | >95 | |
| ABX-1431 (2) | 81 ± 3 | 95 ± 2 | 87 ± 6 | 90 ± 5 | |
| physostigmine | 57 ± 4 | >95 | >95 | >95 | |
Percent of parent remaining after incubation in porcine liver homogenate, porcine plasma, or porcine albumin solution for 30 min or PBS buffer (pH 7.4) for 60 min; values are means ± standard deviations of independent determinations (n = 3, unless otherwise stated); n.t., not tested.
Table 4. Stability of Representative Carbamates and the Reference Oseltamivir in Porcine Liver Homogenate in the Absence and in the Presence of the Carboxylesterase 1 Inhibitor BNPP (1 mM) and Inhibition of Degradation of These Compounds.
| stability
(%)a |
|||
|---|---|---|---|
| comp. | in the absence of BNNP | in the presence of BNNP | inhibition of degradation (%)b |
| 14 | 76 ± 2 | 87 ± 9 | 46 |
| 27 | 62 ± 5 | 79 ± 9 | 45 |
| 33 | 59 ± 6 | 81 ± 8 | 54 |
| 46 | 56 ± 8 | 76 ± 5 | 45 |
| oseltamivir | 11 ± 2 | 97 ± 1 | 97 |
Percent of parent remaining after incubation in porcine liver homogenate for 30 min; values are means ± standard deviations, n = 3.
Calculated from the mean values of the stability data.
Compound 14, which is unsubstituted on the phenyl ring of the carbamate group, showed relatively good stability in porcine plasma. After 30 min of incubation at 37 °C, more than 80% of the substance was still present (Table 3). Similar stabilities were exhibited by the 3-carboxy- (35), all three carboxymethyl- (39, 41, 43), and the indolyl-substituted (49) derivatives. For all other compounds, a much stronger degradation was observed. This was particularly pronounced for the pyridinyl-derivatives (44–48), the 3- and 2-fluoro- (27, 28), the 3-cyano- (30), and the 4-carboxy-substituted (33) compounds. It was noticeable that the pyridines 45 and 46 bearing methyl groups at position 2 or 4 were somewhat more stable than the unsubstituted and the other methylated pyridines. In these two compounds, the methyl groups are in close proximity (ortho-position) to the carbamate oxygen, which apparently makes it more difficult for the esterases to attack the carbamate bond. Experiments with inhibitors of esterases present in porcine plasma performed with compounds 27, 33, and 46 showed that these carbamates were degraded by PON- and CES-like esterase, but not by BuChE. Thus, the amount of nonmetabolized parent compound increased significantly after addition of ethylenediaminetetraacetic acid (EDTA) (1.5 mM), which inhibits calcium-dependent PON, and the CES1 inhibitor BNPP (1.0 mM). The BuChE inhibitor tacrine (10 μM) had no effect. However, this is not surprising considering that all three tested compounds inhibit BuChE with IC50 values in the range of 1 μM, and the concentration of carbamates used in the experiments was 20 μM.
The results obtained in the incubation experiments with porcine albumin (30 min, 37 °C) suggested that some of the carbamates studied are also converted by or react with the pseudo-esterase albumin to a greater extent. Thus, significant differences in stability were found after incubation in the albumin solution and in pure phosphate buffer, especially for the 2-fluoro- (27) and the 3-cyano- (30) as well as for some pyridine compounds. To detect such a covalent reaction, both pyridine derivatives 46 and 47 were incubated with albumin (60 min, 37 °C) and then analyzed by mass spectrometry for an increase in the mass of albumin due to binding of the acyl moiety of the carbamates. The mass spectra obtained showed signals of markedly enhanced masses (Figure 2). In the spectrum of 46, still some unreacted albumin could be detected. The signal for a dual carbamoylated albumin had the highest intensity. In the case of 47, unmodified albumin could not be seen any more. Here, the signal caused by albumin molecules that reacted with three molecules of 47 was the most pronounced. In addition, the proportion of albumin molecules with a larger number of bound carbamate molecules (5–7) was higher than in the case of 46. The somewhat higher reactivity of 47 observed in the mass spectrometric experiments compared with 46 was also evident in the stability studies in albumin solution. Here, after 30 min, 68% of 46 but only 37% of 47 were still present (Table 3).
Figure 2.

Maximum entropy (MaxEnt) deconvoluted protein spectra of porcine albumin incubated with compound 46 (A) or compound 47 (B) and spectrum of the unmodified albumin (C) using Biopharma Compass (Compass) software for data processing; a multiple-attribute-method (MAM) protein screening workflow was used for automated drug–protein identification; peaks are labeled with Mr values and number of conjugated drug molecules.
Aryl 4-[2-(6-Fluoroindol-1-yl)ethyl]piperidine-1-carboxylates
A variety of aryl carbamates have been described in the literature as FAAH inhibitors in which the carbamate nitrogen is embedded in a piperidine ring. We had also investigated corresponding compounds, such as 8 (Figure 1).28 The latter had been shown to be a highly potent FAAH inhibitor that did not inhibit MAGL. Finally, we were interested in whether MAGL inhibition could also be achieved by introducing a carboxy group to the phenyl ring of the phenyl piperidine-1-carboxylate 8, particularly in the ortho-position. However, this effect did not occur; no MAGL inhibition was detectable at 10 μM for all three derivatives 54, 56, and 58 (Table 2). FAAH itself was strongly inhibited only by the para- and meta-substituted compounds 54 and 56. Their inhibition strength was approximately the same as that of compound 8 unsubstituted at the phenyl ring. However, these two substances possessed significantly more favorable polarity. Only slightly active toward FAAH was the compound with the ortho-carboxy group, which possessed an IC50 value of 3.5 μM. The strikingly different MAGL inhibitory potency of the pentyl and ethylpiperidinyl carbamates 37 and 58, both bearing a 2-carboxyphenyl substituent, may be due, at least in part, to the different reactivities of their carbamate groups. Thus, it has been described in the literature that N-monosubstituted carbamate structures, as present in 37, are significantly more reactive than compounds with analogous N,N-disubstituted moieties, as present in 58.54
Conclusions
Aryl carbamate inhibitors of serine hydrolases are generally covalently reacting substances that carbamoylate the serine in the active site of the enzyme with loss of the aryl residue. One factor affecting the potency, but also the selectivity and stability, of carbamate-based inhibitors is the extent of their reactivity. This can be particularly high for N-monosubstituted alkyl carbamates, but it is also determined by the nature or substitution pattern of the aryl leaving group. In the present work, potent selective and chemically as well as metabolically stable FAAH inhibitors were obtained with the carboxymethylphenyl-substituted alkyl carbamates 39 and 41 and the carboxyphenyl piperidine-1-carboxylates 54 and 56. Highly potent inhibitors that inhibit all three serine hydrolases, namely, FAAH, MAGL, and BuChE, were the 2-carboxyphenyl and the pyridine-3-yl-substituted 5-(6-fluoroindol-1-yl)pentylcarbamates 37 and 44–48. Unfortunately, the good potency against the three enzymes comes at the price of lower chemical and metabolic stability. Since the high MAGL inhibitory potency of the 2-carboxyphenyl derivative 37 cannot be explained by carbamate reactivity alone, further studies will investigate whether the introduction of other polar residues at the 2-position of the phenyl residue lead to more stable potent MAGL inhibitors.
Due to the particularly high nucleophilic reactivity, the synthesized pyridine carbamates cannot be considered as reasonable drug candidates. However, as the LC/MS experiments with albumin have shown, bifunctional dipyridin-3-ylalkylcarbamate derivatives could be of interest as nucleophilic reagents for protein modifications. In particular, such substances could be used as cross-linkers in the production of drug-loaded albumin nanoparticles.55 Usually, glutaraldehyde is applied for this purpose. It makes nanoparticles more rigid and decreases their free volume space by reaction with ε-amino groups of lysyl residues. However, this reagent is of concern due to its toxicity.56 Furthermore, it is not quite clear yet which kind of products glutaraldehyde forms with albumin. The formation of simple Schiff bases with both aldehyde groups of monomeric glutaraldehyde was excluded as a mechanism.57 Therefore, bifunctional dipyridin-3-ylalkyl carbamates forming clearly defined reaction products could be a useful alternative for glutaraldehyde in albumin cross-linking.
In recent years, the idea of using selective BuChE inhibitors for the treatment of Alzheimer’s disease has emerged, since, among other things, a striking decrease in toxic brain plaques was observed in an Alzheimer’s disease model using BuChE knock-out mice.58−60 Since inhibition of FAAH is also considered a possible approach for this neurodegenerative disease,61 dual inhibitors of these enzymes may be of particular therapeutic interest for its treatment. Indol-4-yl carbamate 49, which has well-balanced inhibitory potency against BuChE and FAAH and a reasonable hydrolytic stability, could be a lead structure for the development of such new agents.
Experimental Section
Chemistry
Column chromatography was carried out on silica gel 60, particle size 0.040–0.063 mm, from Macherey & Nagel (Düren, Germany). Melting points were measured on a Büchi B-540 apparatus (Essen, Germany) and are uncorrected. 1H NMR and 13C NMR spectra were recorded using an Agilent DD2 400 spectrometer (400 MHz) or an Agilent DD2 600 spectrometer (600 MHz) (Agilent Technologies, Richardson). The high-resolution mass spectra (HRMS) were obtained on a Bruker (Bremen, Germany) micrOTOF-Q II spectrometer applying atmospheric pressure chemical ionization (APCI) or electrospray ionization (ESI). Preparative reversed-phase HPLC (RP-HPLC) was performed using a Knauer Azura pump P2.1L equipped with a Knauer RP18 Eurospher II column (20 mm inner diameter × 250 mm, particle size 5 μm) protected with an RP18 Eurospher II guard column (20 mm inner diameter × 30 mm, particle size 5 μm) (Knauer, Berlin, Germany) and eluting at a flow rate of 25 mL/min. Detection was conducted with a Shimazu SPD-6A UV detector at 254 nm (Shimadzu Corporation, Tokyo, Japan). Chromatograms were recorded with MacDAcq32 Control Software from Bischoff (Leonberg, Germany). The compounds were dissolved in dimethyl sulfoxide (DMSO), and the injected sample volume was 0.5–1 mL. The substances were obtained after distilling off the organic solvent and freeze-drying the remaining aqueous phase using a Christ Alpha 1–2 LD plus apparatus (Christ, Osterode am Harz, Germany).
Purity of the target compounds was determined by reversed-phase HPLC with UV detection. The samples were prepared by mixing 20 μL of 5 mM solutions of each compound in DMSO with 180 μL of acetonitrile. Then, 5 μL of the solutions was injected into the HPLC system. Separation was performed using a Nucleosil 100 RP18 column (3 mm inner diameter × 125 mm, particle size 3 μm) (Macherey & Nagel, Düren, Germany) at a flow rate of 0.4 mL/min with a gradient consisting of acetonitrile/water/trifluoroacetic acid (42:58:0.1–86:14:0.1, v/v/v). UV absorbance was measured at 254 nm. Under these conditions, all compounds showed purities of more than 95%.
2-[5-(6-Fluoroindol-1-yl)pentyl]isoindoline-1,3-dione (12)
A solution of 6-fluoroindole (1.28 g, 9.47 mmol) in dry DMF (40 mL) was treated with sodium hydride (60% dispersion in mineral oil) (570 mg, 14.3 mmol) and stirred at room temperature for 30 min until no further formation of hydrogen could be observed. The suspension was added dropwise to a solution of N-(5-bromopentyl)phthalimide (3.38 g, 11.4 mmol) in dry DMF (40 mL), and the resulting mixture was heated at 90 °C for 3.5 h. Subsequently, the organic solvent was removed under reduced pressure. The residue was diluted with saturated aqueous NaCl solution (40 mL) and exhaustively extracted with ethyl acetate. The combined organic layers were dried with anhydrous Na2SO4 and concentrated. Purification of the crude product was carried out by chromatography on silica gel (cyclohexane to cyclohexane/ethyl acetate, 8:2) to obtain 12 as an oil (2.54 g, 77%). C21H19FN2O2 (350.4); 1H NMR (400 MHz, CDCl3): δ (ppm) 1.32–1.41 (m, 2H), 1.66–1.76 (m, 2H), 1.83–1.92 (m, 2H), 3.67 (t, J = 7.2 Hz, 2H), 4.05 (t, J = 7.2 Hz, 2H), 6.43 (dd, J = 3.2 and 0.6 Hz, 1H), 6.84 (ddd, J = 9.4, 8.7, and 2.3 Hz, 1H), 6.99 (dd, J = 10.1 and 2.3 Hz, 1H), 7.05 (d, J = 3.2 Hz, 1H), 7.49 (dd, J = 8.6 and 5.4 Hz, 1H), 7.69–7.74 (m, 2H), 7.81–7.87 (m, 2H); HRMS (APCI, direct probe) m/z [M + H]+ calcd: 351.1503, found: 351.1529.
5-(6-Fluoroindol-1-yl)pentan-1-amine (13)
A solution of compound 12 (2.53 g, 7.22 mmol) in ethanol (35 mL) was treated with hydrazine hydrate (24% in H2O) (12.5 mL) and stirred under reflux for 3 h. The solvent was distilled off. After addition of brine, the aqueous phase was adjusted to pH 10 with dilute aqueous NaOH and the mixture was exhaustively extracted with ethyl acetate. The combined organic layers were dried with anhydrous Na2SO4 and concentrated to give 13 as an oil (1.51 g, 95%). C13H17FN2 (220.3); 1H NMR (400 MHz, CDCl3): δ (ppm) 1.33–1.34 (m, 2H), 1.48–1.57 (m, 2H), 1.79–1.88 (m, 2H), 2.72 (t, J = 7.1 Hz, 2H), 4.06 (t, J = 7.1 Hz, 2H), 6.45 (dd, J = 3.2 and 0.8 Hz, 1H), 6.85 (ddd, J = 9.6, 8.6, and 2.3 Hz, 1H), 7.00 (dd, J = 10.0 and 2.3 Hz, 1H), 7.06 (d, J = 3.2 Hz, 1H), 7.51 (dd, J = 8.6 Hz, J = 5.4 Hz, 1H); HRMS (APCI, direct probe) m/z [M + H]+ calcd: 221.1449, found: 221.1429.
Phenyl N-[5-(6-Fluoroindol-1-yl)pentyl]carbamate (14)
A solution of 13 (0.406 g, 1.84 mmol) in dry CH2Cl2 (20 mL) was treated with DIPEA (376 μL, 2.20 mmol) followed by phenyl chloroformate (276 μL, 2.20 mmol). After stirring the mixture at room temperature for 5 min, silica gel was added and the solvent distilled off. The residue was loaded on the top of a silica gel column. Elution with cyclohexane/ethyl acetate (9:1) gave 14 as a solid (620 mg, 99%). C20H21FN2O2 (340.4); mp 84–85 °C; 1H NMR (400 MHz, DMSO-d6) δ (ppm) 1.22–1.34 (m, 2H), 1.44–1.54 (m, 2H), 1.71–1.80 (m, 2H), 3.03 (q, J = 6.6 Hz, 2H), 4.14 (t, J = 7.1 Hz, 2H), 6.44 (d, J = 3.1 Hz, 1H), 6.83–6.90 (m, 1H), 7.04–7.09 (m, 2H), 7.16–7.23 (m, 1H), 7.32–7.41 (m, 4H), 7.50–7.55 (m, 1H), 7.67–7.74 (m, 1H); 13C NMR (101 MHz, DMSO-d6) δ (ppm) 23.9, 29.3, 29.8, 40.7, 46.0, 96.6 (d, JC–F = 26.1 Hz), 101.2, 107.7 (d, JC–F = 24.4 Hz), 121.8 (d, JC–F = 10.3 Hz), 122.2, 125.1, 125.2, 129.6, 129.7, 136.1 (d, JC–F = 12.4 Hz), 151.6, 154.7, 159.2 (d, JC–F = 234.1 Hz); HRMS (APCI, direct probe) m/z [M + H]+ calcd: 341.1660, found 341.1646.
1-(8-Bromooctyl)-6-fluoroindole (22)
Sodium hydride (60% dispersion in mineral oil) (222 mg, 5.55 mmol) was carefully added to a solution of 6-fluoroindole (501 mg, 3.71 mmol) in dry DMF (10 mL). The suspension was stirred at room temperature for 30 min until no further formation of hydrogen could be observed. Then, a solution of 1,8-dibromooctane (2.49 mL, 3.68 g, 13.5 mmol) in dry DMF (10 mL) was added and the mixture was stirred at 90 °C for an additional 60 min. The solvent was distilled off and the residue chromatographed on silica gel (cyclohexane to cyclohexane/ethyl acetate, 19:1) to give 22 as an oil (897 mg, 74%). C16H21BrFN (326.3); 1H NMR (400 MHz, CDCl3): δ (ppm) 1.25–1.36 (m, 6H), 1.36–1.45 (m, 2H), 1.77–1.87 (m, 4H), 3.39 (t, J = 6.8 Hz, 2H), 4.05 (t, J = 7.1 Hz, 2H), 6.46 (dd, J = 3.1 and 0.9 Hz, 1H), 6.86 (ddd, J = 9.8, 8.7, and 2.3 Hz, 1H), 7.00 (dd, J = 10.0 and 2.3 Hz, 1H), 7.06 (d, J = 3.1 Hz, 1H), 7.52 (dd, J = 8.6 and 5.4 Hz, 1H); HRMS (APCI, direct probe) m/z [M + H]+ calcd: 326.0914, found: 326.0924.
2-[8-(6-Fluoroindol-1-yl)octyl]isoindoline-1,3-dione (23)
A mixture of 22 (826 mg, 2.53 mmol) and potassium phthalimide (704 mg, 3.80 mmol) in dry DMF was heated at 90 °C for 2.5 h. After addition of saturated NaCl solution (20 mL), the reaction mixture was exhaustively extracted with ethyl acetate. The combined organic layers were dried with anhydrous Na2SO4 and concentrated. The resulting residue was purified by chromatography on silica gel (cyclohexane/ethyl acetate, 9:1) to give 23 as an oil (841 mg, 85%). C24H25FN2O2 (392.5); 1H NMR (400 MHz, CDCl3): δ (ppm) 1.29–1.34 (m, 8H), 1.61–1.70 (m, 2H), 1.76–1.85 (m, 2H), 3.67 (t, J = 7.3 Hz, 2H), 4.03 (t, J = 7.1 Hz, 2H), 6.44 (dd, J = 3.2 and 1.0 Hz, 1H), 6.82–6.88 (m, 1H), 6.99 (dd, J = 10.1 and 2.3 Hz, 1H), 7.06 (d, J = 3.2 Hz, 1H), 7.51 (dd, J = 8.6 and 5.4 Hz, 1H), 7.68–7.73 (m, 2H), 7.81–7.86 (m, 2H); HRMS (APCI, direct probe) m/z [M + H]+ calcd: 393.1973, found: 393.1976.
8-(6-Fluoroindol-1-yl)octan-1-amine (24)
A solution of compound 23 (801 mg, 2.04 mmol) in ethanol (15 mL) was reacted with hydrazine hydrate (24% in H2O) (3.8 mL) in the same manner as described for the preparation of 13 to yield 24 (535 mg, 100%) as an oil. C16H23FN2 (262.4); 1H NMR (400 MHz, CDCl3): δ (ppm) 1.23–1.35 (m, 6H), 1.38–1.48 (m, 2H), 1.76–1.85 (m, 2H), 1.89–2.05 (m, 2H), 2.68 (t, J = 7.0 Hz, 2H), 4.04 (t, J = 7.1 Hz, 2H), 6.45 (dd, J = 3.2 and 0.8 Hz, 1H), 6.85 (ddd, J = 9.6, 8.6, and 2.3 Hz, 1H), 7.00 (dd, J = 10.1 and 2.3 Hz, 1H), 7.06 (d, J = 3.2 Hz, 1H), 7.51 (dd, J = 8.6 Hz, J = 5.4 Hz, 1H); HRMS (APCI, direct probe) m/z [M + H]+ calcd: 263.1918, found: 263.1916.
Phenyl N-[8-(6-Fluoroindol-1-yl)octyl]carbamate (25)
A solution of 24 (100 mg, 0.38 mmol) and DIPEA (77 μL, 58 mg, 0.45 mmol) in dry CH2Cl2 (10 mL) was treated dropwise with phenyl chloroformate (56 μL, 0.45 mmol) in an ice bath. After stirring at room temperature for 1 h, the solvent was distilled off and the crude product purified by chromatography on silica gel (cyclohexane to cyclohexane/ethyl acetate, 8:2) to yield 25 as a solid (99 mg, 68%). C23H27FN2O2 (382.5); mp 47–48 °C. 1H NMR (400 MHz, CDCl3): δ (ppm) 1.29–1.35 (m, 8H), 1.50–1.59 (m, 2H), 1.78–1.86 (m, 2H), 3.24 (q, J = 6.7 Hz, 2H), 4.05 (t, J = 7.1 Hz, 2H), 4.98 (t, J = 5.1 Hz, 1H), 6.46 (dd, J = 3.2 and 0.9 Hz, 1H), 6.86 (ddd, J = 9.6, 8.6, and 2.3 Hz, 1H), 7.00 (dd, J = 10.0 and 2.3 Hz, 1H), 7.07 (d, J = 3.1 Hz, 1H), 7.11–7.14 (m, 2H), 7.19 (tt, J = 7.7 and 1.2 Hz, 1H), 7.33–7.38 (m, 2H), 7.52 (dd, J = 8.7 and 5.4 Hz, 1H); 13C NMR (101 MHz, CDCl3): δ (ppm) 26.7, 27.0, 29.2, 29.2, 29.9, 30.2, 41.3, 46.7, 95.9 (d, JC–F = 26.3 Hz), 101.3, 108.1 (d, JC–F = 24.5 Hz), 121.6, 121.8, 125.1, 125.4, 128.3 (d, JC–F = 3.7 Hz), 129.4, 136.1 (d, JC–F = 11.9 Hz), 151.2, 154.7, 159.8 (d, JC–F = 236.9 Hz); HRMS (APCI, direct probe) m/z [M + H]+ calcd: 383.2129, found: 383.2116.
4-Fluorophenyl N-[5-(6-Fluoroindol-1-yl)pentyl]carbamate (26)
A solution of 13 (120 mg, 0.54 mmol) in dry THF (10 mL) was treated with DIPEA (93 μL, 0.54 mmol) followed by a solution of 4-fluorophenyl chloroformate (95 mg, 0.54 mmol) in dry THF (15 mL). After stirring the mixture at room temperature for 3 h, silica gel was added and the solvent distilled off. The residue was loaded on the top of a silica gel column. Elution with cyclohexane/ethyl acetate (19:1–9:1) gave 26, which was further purified by preparative RP18 chromatography using acetonitrile/water (80:20) as the eluent to give 26 as an oil (9 mg, 5%). C20H20F2N2O2 (358.4); 1H NMR (400 MHz, CDCl3) δ (ppm) 1.31–1.41 (m, 2H), 1.55–1.63 (m, 2H), 1.82–1.91 (m, 2H), 3.19–3.27 (m, 2H), 4.08 (t, J = 7.0 Hz, 2H), 4.96 (s, 1H), 6.47 (d, J = 3.1 Hz, 1H), 6.83–6.90 (m, 1H), 6.98–7.08 (m, 6H), 7.50–7.55 (m, 1H); 13C NMR (101 MHz, CDCl3) δ (ppm) 24.0, 29.5, 29.7, 41.0, 46.3, 95.7 (d, JC–F = 26.2 Hz), 101.3, 108.0 (d, JC–F = 24.5 Hz), 115.8 (d, JC–F = 24.4 Hz), 121.7 (d, JC–F = 10.1 Hz), 122.9 (d, JC–F = 8.5 Hz), 125.0, 128.1, 135.9 (d, JC–F = 12.0 Hz), 146.8, 154.5, 158.6 (d, JC–F = 239.9 Hz), 161.0 (d, JC–F = 244.4 Hz); HRMS (APCI, direct probe) m/z [M + H]+ calcd: 359.1566, found: 359.1571.
4-Cyanophenyl N-[5-(6-Fluoroindol-1-yl)pentyl]carbamate (29)
A solution of triphosgene (152 mg, 0.51 mmol) in dry THF (5 mL) was added at 0 °C to a solution of 4-hydroxybenzonitrile (171 mg, 1.44 mmol) and DIPEA (255 μL, 193 mg, 1.49 mmol) in dry THF (10 mL). After stirring for 2 h at the same temperature, the mixture was warmed up to room temperature. Then, a solution of compound 13 (110 mg, 0.50 mmol) and DIPEA (85 μL, 64 mg, 0.50 mmol) in dry THF (5 mL) was slowly added and stirring was continued for a further 2 h. The reaction mixture was concentrated and directly purified by chromatography on silica gel (cyclohexane to cyclohexane/ethyl acetate, 8:2). Further purification was carried out by preparative RP-HPLC (acetonitrile/H2O/formic acid, 70:30:0.1) to yield compound 29 as a solid (98 mg, 54%). C21H20FN3O2 (365.4); mp 93–94 °C; 1H NMR (400 MHz, DMSO-d6): δ (ppm) 1.24–1.32 (m, 2H), 1.45–1.55 (m, 2H), 1.71–1.80 (m, 2H), 3.05 (td, J = 6.9 and 5.7 Hz, 2H), 4.13 (t, J = 7.1 Hz, 2H), 6.43 (dd, J = 3.2 and 0.9 Hz, 1H), 6.83–6.91 (m, 1H), 7.28–7.31 (m, 2H), 7.32–7.38 (m, 2H), 7.52 (dd, J = 8.7 and 5.5 Hz, 1H), 7.84–7.88 (m, 2H), 7.94 (t, J = 5.7 Hz, 1H); 13C NMR (101 MHz, DMSO-d6): δ (ppm) 23.4, 28.6, 29.3, 40.3, 45.5, 96.1 (d, JC–F = 25.9 Hz), 100.7, 107.2 (d, JC–F = 24.5 Hz), 107.5, 118.5, 121.3 (d, JC–F = 10.3 Hz), 122.7, 124.7, 129.2 (d, JC–F = 3.5 Hz), 133.8, 135.7 (d, JC–F = 12.6 Hz), 153.2, 154.7, 158.8 (d, JC–F = 234.1 Hz); IR (neat): υ̃ [cm–1] = 2230 (cyano group); HRMS (APCI, direct probe) m/z [M + H]+ calcd: 366.1612, found: 366.1613.
Benzyl 4-({[5-(6-Fluoroindol-1-yl)pentyl]carbamoyl}oxy)benzoate (32)
A solution of triphosgene (386 mg, 1.30 mmol) in dry THF (10 mL) was added at 0 °C to a solution of benzyl 4-hydroxybenzoate (594 mg, 2.60 mmol) and DIPEA (442 μL, 334 mg, 2.58 mmol) in dry THF (10 mL). The mixture was stirred for 3.5 h at the same temperature. After warming up to ambient temperature, a solution of compound 13 (291 mg, 1.32 mmol) and DIPEA (221 μL, 167 mg, 1.29 mmol) in dry THF (5 mL) was slowly added and stirring at room temperature was continued overnight. The reaction mixture was diluted with saturated aqueous NaCl solution and exhaustively extracted with ethyl acetate. The combined organic layers were dried with anhydrous Na2SO4 and the solvent was evaporated under reduced pressure. The crude product was purified by chromatography on silica gel (cyclohexane to cyclohexane/ethyl acetate, 8:2) to give 32 as an oil (306 mg, 50%). C28H27FN2O4 (474.5); 1H NMR (400 MHz, CDCl3): δ (ppm) 1.30–1.43 (m, 2H), 1.53–1.65 (m, 2H), 1.81–1.91 (m, 2H), 3.24 (q, J = 6.8 Hz, 2H), 4.08 (t, J = 7.0 Hz, 2H), 5.02 (t, J = 6.1 Hz, 1H), 5.36 (s, 2H), 6.47 (dd, J = 3.1 and 0.9 Hz, 1H), 6.86 (ddd, J = 9.7, 8.6, and 2.3 Hz, 1H), 7.00 (dd, J = 10.0 and 2.5 Hz, 1H), 7.06 (d, J = 3.1 Hz, 1H), 7.15–7.21 (m, 2H), 7.33–7.47 (m, 5H), 7.52 (dd, J = 8.6 and 5.4 Hz, 1H), 8.04–8.11 (m, 2H); HRMS (APCI, direct probe) m/z [M + H]+ calcd: 475.2028, found: 475.2039.
4-({[5-(6-Fluoroindol-1-yl)pentyl]carbamoyl}oxy)benzoic Acid (33)
A catalytic amount of palladium (10%) on activated charcoal (25 mg) was added to a solution of compound 32 (251 mg, 0.53 mmol) in THF/methanol (1:1) (8 mL). The suspension was stirred under a balloon filled with hydrogen at room temperature for 5 h. After addition of Celite 545, the catalyst was filtered off and the filtrate was evaporated. The crude product was purified by silica gel chromatography (cyclohexane/ethyl acetate/formic acid, 9:1:0.1–7:3:0.1) to yield 33 as a solid (99 mg, 49%). C21H21FN2O4 (384.4); mp 163–164 °C; 1H NMR (600 MHz, DMSO-d6): δ (ppm) 1.26–1.31 (m, 2H), 1.47–1.52 (m, 2H), 1.73–1.78 (m, 2H), 3.04 (td, J = 6.9 and 5.7 Hz, 2H), 4.13 (t, J = 7.1 Hz, 2H), 6.43 (dd, J = 3.1 and 0.8 Hz, 1H), 6.86 (ddd, J = 9.7, 8.6, and 2.3 Hz, 1H), 7.17–7.20 (m, 2H), 7.34–7.37 (m, 2H), 7.52 (dd, J = 8.6 and 5.5 Hz, 1H), 7.87 (t, J = 5.8 Hz, 1H), 7.92–7.96 (m, 2H), 12.94 (s, 1H); 13C NMR (151 MHz, DMSO-d6): δ (ppm) 23.4, 28.7, 29.4, 40.3, 45.5, 96.1 (d, JC–F = 26.1 Hz), 100.7, 107.3 (d, JC–F = 24.7 Hz), 121.3 (d, JC–F = 10.3 Hz), 121.6, 124.7, 127.3, 129.3 (d, JC–F = 3.5 Hz), 130.7, 135.7 (d, JC–F = 12.3 Hz), 153.6, 154.7, 158.8 (d, JC–F = 234.1 Hz), 166.7; HRMS (APCI, direct probe) m/z [M + H]+ calcd: 385.1558, found: 385.1565.
Benzyl 2-({[5-(6-Fluoroindol-1-yl)pentyl]carbamoyl}oxy)benzoate (36)
A solution of triphosgene (295 mg, 0.99 mmol) in dry THF (10 mL) was added at 0 °C to a solution of benzyl salicylate (387 μL, 455 mg, 1.99 mmol) and DIPEA (340 μL, 257 mg, 1.99 mmol) in dry THF (10 mL). The mixture was stirred for 2 h at the same temperature and for an additional 17 h at room temperature. Then, a solution of compound 13 (218 mg, 0.99 mmol) and DIPEA (170 μL, 128 mg, 0.99 mmol) in dry THF (5 mL) was slowly added and stirring was continued at room temperature for a further 5.5 h. After workup and purification as described for compound 32, product 36 was yielded as an oil (159 mg, 34%). C28H27FN2O4 (474.5); 1H NMR (400 MHz, CDCl3): δ (ppm) 1.31–1.39 (m, 2H), 1.45–1.56 (m, 2H), 1.79–1.88 (m, 2H), 3.11 (q, J = 6.7 Hz, 2H), 4.05 (t, J = 7.0 Hz, 2H), 4.93 (t, J = 5.5 Hz, 1H), 5.30 (s, 2H), 6.46 (dd, J = 3.2 and 0.9 Hz, 1H), 6.86 (ddd, J = 9.6, 8.6, and 2.3 Hz, 1H), 7.00 (dd, J = 10.1 and 2.3 Hz, 1H), 7.06 (d, J = 3.1 Hz, 1H), 7.16 (dd, J = 8.2 and 0.8 Hz, 1H), 7.27–7.43 (m, 6H), 7.50–7.56 (m, 2H), 8.01 (dd, J = 7.9 and 1.7 Hz, 1H); HRMS (APCI, direct probe) m/z [M + H]+ calcd: 475.2028, found: 475.2015.
2-({[5-(6-Fluoroindol-1-yl)pentyl]carbamoyl}oxy)benzoic Acid (37)
A catalytic amount of palladium (10%) on activated charcoal (14 mg) was added to a solution of compound 36 (135 mg, 0.28 mmol) in THF/methanol (1:1) (5 mL). The suspension was stirred vigorously under a balloon filled with hydrogen at room temperature for 4 h. After addition of Celite 545, the catalyst was filtered off and the filtrate was evaporated. The crude product was first purified by silica gel chromatography (cyclohexane/ethyl acetate/formic acid, 9:1:0.1–7:3:0.1). Further purification was carried out by preparative RP-HPLC (acetonitrile/H2O/formic acid, 50:50:0.1) to yield 37 as a solid (46 mg, 42%). C21H21FN2O4 (384.4); mp 124–125 °C, 1H NMR (400 MHz, DMSO-d6): δ (ppm) 1.23–1.34 (m, 2H), 1.43–1.53 (m, 2H), 1.70–1.80 (m, 2H), 3.01 (q, J = 6.6 Hz, 2H), 4.13 (t, J = 7.1 Hz, 2H), 6.43 (dd, J = 3.1 and 0.9 Hz, 1H), 6.86 (ddd, J = 9.8, 8.6, and 2.3 Hz, 1H), 7.11 (dd, J = 8.1 and 1.1 Hz, 1H), 7.28–7.38 (m, 3H), 7.52 (dd, J = 8.6 and 5.5 Hz, 1H), 7.57 (ddd, J = 8.1, 7.4 and 1.8 Hz, 1H), 7.69 (t, J = 5.8 Hz, 1H), 7.83 (dd, J = 7.7 and 1.8 Hz, 1H), 12.90 (s, 1H); 13C NMR (101 MHz, DMSO-d6): δ (ppm) 23.3, 28.8, 29.3, 40.2, 45.5, 96.1 (d, JC–F = 26.2 Hz), 100.7, 107.2 (d, JC–F = 24.6 Hz), 121.3 (d, JC–F = 10.3 Hz), 123.9, 124.7, 125.1, 125.4, 129.2 (d, JC–F = 3.6 Hz), 130.9, 133.1, 135.6 (d, JC–F = 12.8 Hz), 150.3, 154.1, 158.7 (d, JC–F = 234.1 Hz), 166.1; HRMS (ESI) m/z [M + H]+ calcd: 385.1558, found: 385.1564.
Benzyl 2-[4-({[5-(6-Fluoroindol-1-yl)pentyl]carbamoyl}oxy)phenyl]acetate (38)
A solution of triphosgene (267 mg, 0.90 mmol) in dry THF (10 mL) was added at 0 °C to a solution of benzyl 2-(4-hydroxyphenyl)acetate (433 mg, 1.79 mmol) and DIPEA (306 μL, 231 mg, 1.79 mmol) in dry THF (10 mL). The mixture was stirred for 3 h at the same temperature. After warming up to ambient temperature, a solution of compound 13 (197 mg, 0.89 mmol) and DIPEA (153 μL, 116 mg, 0.90 mmol) in dry THF (5 mL) was slowly added and stirring was continued for an additional 1 h. After workup and purification as described for compound 32, product 38 was yielded as an oil (235 mg, 54%). C29H29FN2O4 (488.6); 1H NMR (600 MHz, CDCl3): δ (ppm) 1.35–1.40 (m, 2H), 1.56–1.61 (m, 2H), 1.85–1.91 (m, 2H), 3.23 (q, J = 6.8 Hz, 2H), 3.65 (s, 2H), 4.07 (t, J = 7.0 Hz, 2H), 4.97 (t, J = 6.1 Hz, 1H), 5.13 (s, 2H), 6.47 (dd, J = 3.1 and 0.9 Hz, 1H), 6.87 (ddd, J = 9.6, 8.6, and 2.3 Hz, 1H), 7.00 (dd, J = 9.9 Hz and 2.4 Hz, 1H), 7.05–7.08 (m, 3H), 7.26–7.29 (m, 2H), 7.30–7.37 (m, 5H), 7.52 (dd, J = 8.6 and 5.4 Hz, 1H); HRMS (APCI, direct probe) m/z [M + H]+ calcd: 489.2184, found: 489.2237.
2-[4-({[5-(6-Fluoroindol-1-yl)pentyl]carbamoyl}oxy)phenyl]acetic Acid (39)
Compound 38 (175 mg, 0.36 mmol) was hydrogenated in THF/methanol (1:1) (5 mL) with palladium (10%) on activated charcoal (18 mg) for 3 h as described for the synthesis of 33. The product obtained after workup and silica gel chromatography was further purified by preparative RP-HPLC (acetonitrile/H2O/formic acid, 55:45:0.1) to yield 39 as a solid (93 mg, 65%). C22H23FN2O4 (398.4); mp 104–105 °C; 1H NMR (400 MHz, CDCl3): δ (ppm) 1.33–1.41 (m, 2H), 1.54–1.62 (m, 2H), 1.82–1.90 (m, 2H), 3.23 (q, J = 6.7 Hz, 2H), 3.62 (s, 2H), 4.07 (t, J = 7.0 Hz, 2H), 4.98 (t, J = 6.1 Hz, 1H), 6.46 (dd, J = 3.2 and 0.8 Hz, 1H), 6.86 (ddd, J = 9.6, 8.6, and 2.3 Hz, 1H), 7.00 (dd, J = 10.0 and 2.3 Hz, 1H), 7.04–7.09 (m, 3H), 7.25–7.30 (m, 2H), 7.52 (dd, J = 8.6 and 5.4 Hz, 1H); 13C NMR (101 MHz, CDCl3): δ (ppm) 24.2, 29.6, 29.8, 40.4, 41.1, 46.5, 95.8 (d, JC–F = 26.3 Hz), 101.4, 108.2 (d, JC–F = 24.6 Hz), 121.8 (d, JC–F = 10.0 Hz), 121.9, 125.1, 128.3 (d, JC–F = 3.5 Hz), 130.4, 130.5, 136.1 (d, JC–F = 12.2 Hz), 150.4, 154.7, 159.9 (d, JC–F = 237.0 Hz), 176.4; HRMS (ESI) m/z [M + H]+ calcd: 399.1715, found: 399.1728.
4-Methylpyridin-3-yl N-[5-(6-fluoroindol-1-yl)pentyl]carbamate (46)
A solution of triphosgene (148 mg, 0.50 mmol) in dry THF (5 mL) was added at 0 °C to a solution of 3-hydroxy-4-methylpyridine (151 mg, 1.38 mmol) and DIPEA (255 μL, 193 mg, 1.49 mmol) in dry THF (10 mL). After stirring for 3 h at the same temperature, the mixture was allowed to warm up to room temperature. Then, a solution of compound 13 (118 mg, 0.54 mmol) and DIPEA (85 μL, 64 mg, 0.50 mmol) in dry THF (5 mL) was slowly added and stirring was continued overnight. The reaction mixture was concentrated and directly purified by chromatography on silica gel (cyclohexane to cyclohexane/ethyl acetate, 1:1). Further purification was carried out by preparative RP-HPLC (acetonitrile/H2O, 45:55) to yield compound 46 as an oil (35 mg, 20%). C20H22FN3O2 (355.4); 1H NMR (600 MHz, CDCl3): δ (ppm) 1.34–1.40 (m, 2H), 1.57–1.63 (m, 2H), 1.85–1.91 (m, 2H), 2.22 (s, 3H), 3.25 (td, J = 7.1 and 6.1 Hz, 2H), 4.08 (t, J = 6.9 Hz, 2H), 5.11 (t, J = 6.2 Hz, 1H), 6.47 (dd, J = 3.2 and 0.9 Hz, 1H), 6.87 (ddd, J = 9.5, 8.6, and 2.3 Hz, 1H), 7.00 (dd, J = 10.0 and 2.4 Hz, 1H), 7.06 (d, J = 3.2 Hz, 1H), 7.17 (d, J = 4.9 Hz, 1H), 7.52 (dd, J = 8.6 and 5.4 Hz, 1H), 8.32–8.34 (m, 2H); 13C NMR (151 MHz, CDCl3): δ (ppm) 15.9, 24.2, 29.6, 29.8, 41.3, 46.5, 95.8 (d, JC–F = 26.4 Hz), 101.5, 108.2 (d, JC–F = 24.4 Hz), 121.8 (d, JC–F = 10.3 Hz), 125.1, 126.0, 128.3 (d, JC–F = 3.5 Hz), 136.1 (d, JC–F = 11.9 Hz), 140.4, 143.8, 146.4, 146.9, 153.8, 159.9 (d, JC–F = 237.5 Hz); HRMS (ESI) m/z [M + H]+ calcd: 356.1769, found: 356.1757.
Indol-4-yl N-[5-(6-Fluoroindol-1-yl)pentyl]carbamate (49)
A solution of di-tert-butyl dicarbonate (138 mg, 0.63 mmol) and DMAP (66 mg, 0.54 mmol) in dry acetonitrile (4 mL) was treated with 13 (102 mg, 0.46 mmol) under a nitrogen atmosphere and anhydrous conditions. After stirring at room temperature for 1 h, 4-hydroxyindole (72 mg, 0.54 mmol) was added and stirring was continued for a further 26 h. The reaction mixture was diluted with saturated aqueous NH4Cl solution and exhaustively extracted with ethyl acetate. The combined organic layers were dried with anhydrous Na2SO4 and concentrated. The resulting residue was first purified by chromatography on silica (cyclohexane to cyclohexane/ethyl acetate, 7:3). Further purification was carried out with preparative RP-HPLC (acetonitrile/H2O, 60:40) to yield 49 as an oil (87 mg, 50%). C22H22FN3O2 (379.4); 1H NMR (400 MHz, DMSO-d6): δ (ppm) 1.25–1.36 (m, 2H,), 1.48–1.57 (m, 2H), 1.73–1.83 (m, 2H), 3.06 (q, J = 6.6 Hz, 2H), 4.15 (t, J = 7.1 Hz, 2H), 6.20–6.22 (m, 1H), 6.44 (dd, J = 3.2 and 0.8 Hz, 1H), 6.68 (m, 1H), 6.86 (ddd, J = 9.7, 8.6, and 2.3 Hz), 7.02 (t, J = 7.9 Hz, 1H), 7.23 (d, J = 8.2 Hz, 1H), 7.29 (t, J = 2.8 Hz, 1H), 7.33–7.40 (m, 2H), 7.52 (dd, J = 8.6 and 5.5 Hz, 1H), 7.72 (t, J = 5.7 Hz, 1H), 11.20 (s, 1H); 13C NMR (101 MHz, DMSO-d6): δ (ppm) 23.5, 28.8, 29.4, 40.2, 45.5, 96.1 (d, JC–F = 26.2 Hz), 97.9, 100.7, 107.2 (d, JC–F = 24.5 Hz), 108.5, 111.0, 121.0, 121.3 (d, JC–F = 10.2 Hz), 121.5, 124.7, 125.1, 129.2 (d, JC–F = 3.5 Hz), 135.6 (d, JC–F = 12.7 Hz), 137.7, 143.8, 154.40, 158.8 (d, JC–F = 234.0 Hz); HRMS (APCI, direct probe) m/z [M + H]+ calcd: 380.1769, found: 380.1731.
Benzyl 4-{2-[(Methylsulfonyl)oxy]ethyl}piperidine-1-carboxylate (50)
Methanesulfonyl chloride (166 μL, 246 mg, 2.15 mmol) was added dropwise to a solution of benzyl 4-(2-hydroxyethyl)piperidine-1-carboxylate (471 mg, 1.79 mmol) and triethylamine (493 μL, 358 mg, 3.54 mmol) in dry CH2Cl2 (25 mL) at 0 °C. After stirring at room temperature for 3 h, the crude product was purified by chromatography on silica gel (cyclohexane to cyclohexane/ethyl acetate, 6:4) to obtain 50 as an oil (474 mg, 78%). C16H23NO5S (341.4); 1H NMR (400 MHz, CDCl3): δ (ppm) 1.11–1.22 (m, 2H), 1.58–1.66 (m, 1H), 1.68–1.74 (m, 4H), 2.73–2.82 (m, 2H), 3.01 (s, 3H), 4.15–4.23 (m, 2H), 4.28 (t, J = 6.2 Hz, 2H), 5.12 (s, 2H), 7.29–7.34 (m, 1H), 7.34–7.37 (m, 4H); HRMS (APCI, direct probe) m/z [M + H]+ calcd: 342.1370, found: 342.1356.
Benzyl 4-[2-(6-Fluoroindol-1-yl)ethyl]piperidine-1-carboxylate (51)
Sodium hydride (60% dispersion in mineral oil) (61 mg, 1.53 mmol) was carefully added to a solution of 6-fluoroindole (201 mg, 1.49 mmol) in dry DMF (15 mL). The suspension was stirred at room temperature for 30 min until no further formation of hydrogen could be observed. Subsequently, the mixture was added dropwise to a solution of 50 (422 mg, 1.24 mmol) in dry DMF (15 mL) and stirred at room temperature overnight. After evaporation of the solvent, the reaction mixture was redissolved in saturated aqueous NaCl solution and exhaustively extracted with ethyl acetate. The combined organic layers were dried with anhydrous Na2SO4 and concentrated. The residue was chromatographed on silica gel (cyclohexane to cyclohexane/ethyl acetate, 8:2) to yield 51 as an oil (392 mg, 83%). C23H25FN2O2 (380.5); 1H NMR (400 MHz, CDCl3): δ (ppm) 1.14–1.26 (m, 2H), 1.38–1.49 (m, 1H), 1.66–1.82 (m, 4H), 2.70–2.79 (m, 2H), 4.07–4.24 (m, 4H), 5.13 (s, 2H), 6.47 (dd, J = 3.2 and 0.9 Hz, 1H), 6.87 (ddd, J = 9.5, 8.6, and 2.3 Hz, 1H), 6.98 (dd, J = 10.0 and 2.3 Hz, 1H), 7.05 (d, J = 3.2 Hz, 1H), 7.29–7.38 (m, 5H), 7.53 (dd, J = 8.6 and 5.4 Hz, 1H); HRMS (APCI, direct probe) m/z [M + H]+ calcd: 381.1973, found: 381.1980.
6-Fluoro-1-[2-(piperidin-4-yl)ethyl]indole (52)
A solution of compound 51 (336 mg, 0.88 mmol) in THF/methanol (1:1) (5 mL) was treated with a catalytic amount of palladium (10%) on activated charcoal (34 mg). The suspension was stirred vigorously under a balloon filled with hydrogen at room temperature for 4 h. The catalyst was removed by filtration through Celite 545, and the filtrate was evaporated to dryness to yield 52 as an oil (218 mg, 100%). C15H19FN2 (246.3); 1H NMR (400 MHz, CDCl3): δ (ppm) 1.17–1.28 (m, 2H), 1.36–1.46 (m, 1H), 1.69–1.80 (m, 4H), 2.58 (td, J = 12.1, 12.1, and 2.6 Hz, 2H), 3.09 (dt, J = 12.4, 3.3, and 3.3 Hz, 2H), 4.09 (t, J = 7.3 Hz, 2H), 6.46 (dd, J = 3.1 and 0.7 Hz, 1H), 6.86 (ddd, J = 9.5, 8.6, and 2.3 Hz, 1H), 6.99 (dd, J = 9.9 and 1.7 Hz, 1H), 7.06 (d, J = 3.2 Hz, 1H), 7.52 (dd, J = 8.6 and 5.4 Hz, 1H); HRMS (APCI, direct probe) m/z [M + H]+ calcd: 247.1605, found: 247.1610.
4-[(Benzyloxy)carbonyl]phenyl 4-[2-(6-fluoroindol-1-yl)ethyl]piperidine-1-carboxylate (53)
A solution of triphosgene (246 mg, 0.83 mmol) in dry THF (10 mL) was added to a solution of benzyl 4-hydroxybenzoate (379 mg, 1.66 mmol) and DIPEA (282 μL, 213 mg, 1.65 mmol) in dry THF (10 mL) and stirred at 0 °C for 2 h. The mixture was allowed to warm up to ambient temperature. Then, a solution of compound 52 (205 mg, 0.83 mmol) and DIPEA (141 μL, 106 mg, 0.82 mmol) in dry THF (5 mL) was slowly added and stirring was continued overnight. After workup as described for compound 32 and chromatography on silica gel (cyclohexane to cyclohexane/ethyl acetate, 17:3), product 53 was yielded as an oil (327 mg, 79%). C30H29FN2O4 (500.6); 1H NMR (600 MHz, CDCl3): δ (ppm) 1.27–1.35 (m, 2H), 1.47–1.55 (m, 1H), 1.76–1.86 (m, 4H), 2.81 (t, J = 12.7 and 12.7 Hz, 2 × 0.5H), 2.95 (t, J = 12.7 and 12.7, 2 × 0.5H), 4.13 (t, J = 8.0 Hz, 2H), 4.21–4.31 (m, 2H), 5.36 (s, 2H), 6.49 (dd, J = 3.2 Hz, and 0.9 Hz, 1H), 6.88 (ddd, J = 9.5, 8.6, and 2.3 Hz, 1H), 6.99 (dd, J = 9.9 and 2.2 Hz, 1H), 7.07 (d, J = 3.1 Hz, 1H), 7.16–7.19 (m, 2H), 7.34 (tt, J = 7.2 and 1.4 Hz, 1H), 7.37–7.41 (m, 2H), 7.43–7.45 (m, 2H), 7.54 (dd, J = 8.6 and 5.4 Hz, 1H), 8.07–8.09 (m, 2H); HRMS (APCI, direct probe) m/z [M + H]+ calcd: 501.2184, found: 501.2190.
4-({4-[2-(6-Fluoroindol-1-yl)ethyl]piperidine-1-carbonyl}oxy)benzoic Acid (54)
A solution of compound 53 (295 mg, 0.59 mmol) in THF/methanol (1:1) (5 mL) was treated with a catalytic amount of palladium (10%) on activated charcoal (30 mg). The obtained suspension was stirred vigorously under a balloon filled with hydrogen at room temperature for 4 h. The catalyst was filtered off using Celite 545, and the solvent was removed. The resulting residue was purified by chromatography on silica gel (cyclohexane/formic acid, 100:1 to cyclohexane/ethyl acetate/formic acid, 7:3:0.1) followed by preparative RP-HPLC (acetonitrile/H2O/formic acid, 55:45:0.1) to yield 54 (149 mg, 62%) as a solid. C23H23FN2O4 (410.4); mp 157–158 °C; 1H NMR (400 MHz, DMSO-d6): δ (ppm) 1.14–1.28 (m, 2H), 1.44–1.51 (m, 1H), 1.73 (dt, J = 8.3 and 6.7 Hz, 2H), 1.76–1.81 (m, 2H), 2.83 (t, J = 12.6 and 12.6 Hz, 2 × 0.5H), 2.98 (t, J = 12.9 and 12.9 Hz, 2 × 0.5H), 3.99 (d, J = 13.1 Hz, 2 × 0.5H), 4.12 (d, J = 13.5 Hz, 2 × 0.5H), 4.19 (t, J = 7.3 Hz, 2H), 6.45 (dd, J = 3.2 and 0.8 Hz, 1H), 6.87 (ddd, J = 9.7, 8.6 and 2.3 Hz, 1H), 7.22–7.25 (m, 2H), 7.36 (dd, J = 10.5 and 2.3 Hz, 1H), 7.40 (d, J = 3.1 Hz, 1H), 7.53 (dd, J = 8.6 and 5.5 Hz, 1H), 7.93–7.96 (m, 2H), 12.97 (s, 1H); 13C NMR (101 MHz, DMSO-d6): δ (ppm) 31.1, 31.5, 32.6, 35.8, 43.2, 43.9, 44.3, 96.1 (d, JC–F = 26.3 Hz), 100.8, 107.3 (d, JC–F = 24.4 Hz), 121.4 (d, JC–F = 10.8 Hz), 121.8, 124.8, 127.6, 129.2 (d, JC–F = 3.6 Hz), 130.6, 135.5 (d, JC–F = 12.4 Hz), 152.2, 154.8, 158.8 (d, JC–F = 234.2 Hz), 166.7; HRMS (APCI, direct probe) m/z [M + H]+ calcd: 411.1715, found: 411.1674.
Physicochemical and Biochemical Analyses
Phosphate-buffered saline (PBS) solution prepared from commercially available phosphate-buffered saline tablets (one tablet dissolved in 200 mL of deionized water yields 0.01 M phosphate buffer, 0.0027 M potassium chloride, and 0.137 M sodium chloride, pH 7.4, at 25 °C), dimethyl sulfoxide (DMSO), porcine albumin, bis(p-nitrophenyl)phosphate, oseltamivir phosphate (Sigma-Aldrich, Steinheim, Germany); Triton X-100, methanol HPLC-grade, acetonitrile HPLC-grade, disodium-EDTA (VWR, Darmstadt, Germany); physostigmine, tacrine hydrochloride (Alfa Aesar, Kandel, Germany); URB597, ABX-1431 (Cayman Chemical delivered via Biomol, Hamburg, Germany); heparin (25 000 I.U./5 mL) (Ratiopharm, Ulm, Germany); and Cay10650 was prepared by the published procedure;52 porcine liver and porcine blood (Schlachthof Tummel, Schöppingen or Feinkostfleischerei Hidding, Nordwalde, Germany).
Inhibition of Fatty Acid Amide Hydrolase (FAAH)
Inhibition of FAAH was measured with a slightly modified published method.35,36 The substrate N-(2-hydroxyethyl)-4-pyren-1-ylbutanamide was dissolved in methanol (2.5 mg/mL). An aliquot of this solution was thoroughly dried under a stream of nitrogen. The residue was dissolved in such an amount of DMSO that a 5 mM substrate solution was obtained. The enzyme solution was prepared by diluting 50 μL of rat brain homogenate obtained as recently described36 with 350 μL of potassium phosphate buffer (0.1 M, pH 7.4) containing EDTA (1 mM). Then, 10 parts by volume of this dilution were mixed with 86 parts by volume of Triton X-100 (0.2%, v/v) in PBS. The assay was started by adding 2 μL of the substrate solution in DMSO (5 mM) to 2 μL of a DMSO solution of the inhibitor or to 2 μL of DMSO in the case of the controls. The mixture was preincubated for 5 min at 37 °C. Then, the enzymatic reaction was started by adding 96 μL of the prepared enzyme solution in Triton X-100/PBS. In the final incubation volume of 100 μL, the pyrenylbutanamide substrate concentration was 100 μM. After incubation at 37 °C for 60 min, the enzyme reaction was terminated by addition of 200 μL of acetonitrile/methanol (1:1, v/v) containing the internal standard 6-pyren-1-ylhexanoic acid (0.025 μg/200 μL). After cooling in an ice bath for 10 min, samples were centrifuged at 12 000g at 4 °C for 5 min. In parallel, blank incubations were performed in the absence of the enzyme. FAAH inhibition was determined by measuring the amount of 4-pyren-1-ylbutanoic acid released by the enzyme in the absence and presence of a test compound (corrected for blank) by reversed-phase HPLC with fluorescence detection, as described recently.35,36 IC50 values were calculated using Probit transformation. The changes in the procedure of the assay resulted in somewhat improved inhibitory values compared to previously published data.28 Under the conditions used, an IC50 value of 0.043 μM was measured for the reference FAAH inhibitor URB597.
Inhibition of Monoacylglycerol Lipase (MAGL)
Inhibition of MAGL was studied as previously described37 using the commercial human recombinant MAGL. Briefly, the substrate 1,3-dihydroxypropan-2-yl 4-pyren-1-ylbutanoate was solubilized in N-(2-hydroxyethyl)piperazine-N′-ethanesulfonic acid (HEPES)-buffer containing Triton X-100 (0.2%, v/v) and EDTA (1 mM). The final substrate concentration was 100 μM. The enzyme reaction was terminated after 45 min by addition of a mixture of acetonitrile/methanol (1:1, v/v) with the internal standard 6-pyren-1-ylhexanoic acid. In parallel, blank incubations were performed in the absence of the enzyme. MAGL inhibition was determined by measuring the amount of 4-pyren-1-ylbutanoic acid released by the enzyme in the presence and absence of a test compound (corrected for blank) using reversed-phase HPLC with fluorescence detection. IC50 values were calculated using Probit transformation. Under the conditions applied, an IC50 value of 0.58 μM was measured for the reference MAGL inhibitor ABX-1431.
Inhibition of Butyrylcholinesterase (BuChE)
Inhibition of BuChE was determined by measuring the inhibition of the release of benzoic acid from the substrate benzoylcholine using the commercial enzyme isolated from horse serum.62 Briefly, the substrate benzoylcholine chloride dissolved in PBS (final concentration 250 μM) was incubated at 37 °C for 60 min. The enzyme reaction was terminated by the addition of a solution of the internal standard 3-methylbenzoic acid (50 μM) and formic acid (0.1%, v/v) in acetonitrile. The samples were cooled and centrifuged. The supernatants obtained were subjected to reversed-phase HPLC with UV detection at 273 nm. In parallel, blank incubations were performed in the absence of the enzyme. BuChE inhibition was calculated from the amount of benzoic acid released by the enzyme in the absence and presence of a test compound (corrected for blank). IC50 values were determined using Probit transformation. Under the conditions used, an IC50 value of 2.7 μM was measured for the reference inhibitor physostigmine.
Inhibition of Acetylcholinesterase (AChE)
The inhibition of AChE was determined by measuring the inhibition of the release of pyridin-2-ylmethanol from pyridin-2-ylmethyl acetate using the commercial enzyme isolated from electric eel.62 Briefly, the freshly prepared enzyme solution was preincubated with the test compound in DMSO or DMSO alone in PBS containing Triton X-100 (0.2%, v/v) at 37 °C for 15 min. Then, a solution of the substrate pyridin-2-ylmethyl acetate in DMSO was added (final concentration 250 μM) and incubation was continued at 37 °C for 60 min. The enzyme reaction was terminated by addition of a solution of the internal standard 2-bromopyridine (150 μM) in acetonitrile. The samples were cooled and centrifuged. The supernatants obtained were subjected to reversed-phase HPLC with UV detection at 273 nm using the ion-pair reagent sodium octane-1-sulfonate. In parallel, blank incubations were performed in the absence of the enzyme. AChE inhibition was calculated from the amount of pyridin-2-ylmethanol released by the enzyme in the absence and presence of a test compound (corrected for blank). IC50 values were determined by Probit transformation. Under the conditions used, an IC50 value of 0.18 μM was measured for the reference inhibitor physostigmine.
Inhibition Cytosolic Phospholipase A2α (cPLA2α)
Inhibition of cPLA2α was measured according to a published procedure.40 Briefly, cPLA2α isolated from porcine platelets was incubated with covesicles consisting of the substrate 1-stearoyl-2-arachidonoyl-sn-glycero-3-phosphocholine (200 μM) and 1,2-dioleoyl-sn-glycerol (100 μM). The enzyme reactions were terminated after 60 min, and the cPLA2α activity was determined by measuring the arachidonic acid released by the enzyme in the absence and presence of a test compound using reversed-phase HPLC and UV detection at 200 nm after online solid-phase extraction. In parallel, blank incubations were performed in the absence of the enzyme. Inhibition of cPLA2α was calculated from the amount of arachidonic acid released by the enzyme in the absence and presence of a test compound (corrected for blank). IC50 values were determined by Probit transformation. Under the conditions used, an IC50 value of 0.013 μM was measured for the reference inhibitor Cay10650.52
Determination of log P Values
Partition coefficients (log P) were measured by reversed-phase HPLC according to a published OECD method.63 The following reference compounds were used: dichlorodiphenyltrichloroethane (DDT), triphenylamine, fluoranthene, dibenzyl, phenanthrene, diphenyl, naphthalene, benzophenone, ethyl benzoate, acetophenone, and anisole. To a stock solution of a reference or a test compound in DMSO (5 mM) (2 μL) were added a solution of the dead time marker thiourea in DMSO (5 mM) (2 μL) and the mobile phase (1 mL) consisting of acetonitrile/water/phosphoric acid (85%) (55:45:0.1, v/v/v) in the case of neutral compounds and compounds with carboxylic acid group and acetonitrile/PBS (55:45, v/v) for the basic pyridine derivatives, ABX-1431, and physostigmine. Twenty microliter of each sample was injected into the HPLC system. The analysis was performed on a Kromasil 100–5C18 column (3 mm inner diameter × 60 mm, particle size 5 μm) (AzkoNobel, Bohus, Sweden). The temperature of the column oven was set at 25 °C. The flow rate was 0.6 mL/min, and the eluted compounds were monitored at 254 nm. Linear fitting of the log k values (k: capacity factor) and log P values of the 11 references resulted in coefficients of determination of R2 = 0.9927 and R2 = 0.9925, respectively. The log P for the reference indomethacin (measured under acid conditions) was 2.9.
Chemical Stability in Aqueous Solution
Adapting the conditions of the FAAH assay,36 a solution of the test compound in DMSO (1 mM) (4 μL) was diluted with a solution of Triton X-100 (0.2%, v/v) in PBS containing EDTA (1 mM) (196 μL) and incubated at 37 °C for 60 min. Immediately thereafter, the sample was centrifuged at 12 000g and 20 °C for 5 min and analyzed by HPLC. Separation was achieved using a Synergi Polar-RP 80 Å column (4.6 mm inner diameter × 150 mm, particle size 4 μm) (Phenomenex, Aschaffenburg, Germany) protected with a Phenomenex phenyl guard column (3 mm inside diameter × 4 mm) or an Aqua C18 125 Å column (4.6 mm inner diameter × 150 mm, particle size 3 μm) (Phenomenex, Aschaffenburg, Germany) protected with a Phenomenex C18 guard column (3 mm inside diameter × 4 mm). An aliquot of each sample (30 μL) was injected into the HPLC/UV system. Autosampler and column oven temperatures were set to 20 °C. The mobile phase consisted of acetonitrile/water/H3PO4 conc. (58:42:0.1, v/v/v). The flow rate was 1.0 mL/min, and the absorption wavelength was set to 238 nm. The relative amount of the test compound found in the aqueous sample after 60 min incubation at 37 °C was determined with the aid of a freshly prepared reference solution obtained by dilution of a DMSO solution (1 mM) of the compound (4 μL) with a solution of Triton X-100 (0.2%, v/v) in PBS containing EDTA (1 mM) (196 μL).
Metabolic Stability in Rat Liver S9 Fraction
Metabolic stability was tested with S9 fractions from rat liver homogenate.36 Briefly, the test compounds were incubated in the presence of the cofactor NADPH under aerobic conditions. The metabolic enzyme reactions were stopped after 30 min by addition of acetonitrile. Controls were obtained by adding the test compounds to a mixture of S9 fractions and acetonitrile. In deviation from the published procedure, the extent of metabolism was evaluated by reversed-phase HPLC with MS detection. The HPLC/MS system from Shimadzu (Kyoto, Japan) consisted of two LC-20ADXR HPLC pumps, a SIL-30AC autosampler, and an LCMS-2020 single quad detector. Aliquots of 2 μL were injected onto a HICHROM ACE 3 C18 column (2.1 mm inside diameter × 100 mm, particle size 3 μm) (HiChrom, Berkshire, U.K.) protected with a Phenomenex C18 guard column (3 mm inside diameter × 4 mm). The autosampler temperature was 10 °C, and the column oven temperature was set to 20 °C. The mobile phase consisted of acetonitrile/10 mM aqueous ammonium acetate 10:90 (v/v) adjusted to pH 5 with formic acid (A) and acetonitrile/10 mM aqueous ammonium acetate 90:10 (v/v) adjusted to pH 5 with formic acid. The gradient run was from 10 to 100% of solvent B. The flow rate was 0.3 mL/min. Detection was performed in the ESI+ or ESI– mode. Samples and controls of each test compound were processed and analyzed in rapid succession.
Metabolic Stability in Porcine Liver S9 Fraction
A fresh porcine liver was washed and bled with cooled potassium chloride solution (1.15% m/v). Then, 2 mL of potassium phosphate buffer (0.1 M, pH 7.4) containing 0.5 mM EDTA was added per 1 g of liver, and the tissue was minced with a scalpel and homogenized under cooling in an ice bath using a Potter–Elvehjem-type tissue homogenizer (Braun, Germany) at 600–800 rpm for 3 min. The liver was then centrifuged at 9000g and 4 °C for 20 min. The resulting supernatant was removed and stored at −80 °C until use. The protein concentration of the S9 homogenate, determined by the Bradford method, was 20 mg/mL.
To a mixture of porcine liver S9 fraction (125 μL) and PBS (124 μL) was added a solution of the test compound (5 mM) in DMSO (1 μL). After incubation at 37 °C for 30 min, acetonitrile (500 μL) was added. The mixture was vortexed and allowed to stand in an ice bath for 15 min. After vigorous vortexing, the mixture was centrifuged at 12 000g and 4 °C for 5 min. In parallel, controls were prepared by addition of the DMSO solution (5 mM) of the test compound (1 μL) to a mixture of porcine liver S9 fraction (125 μL), PBS (124 μL), and acetonitrile (500 μL). The controls were allowed to stand at room temperature for 30 min. Then, they were cooled in an ice bath for 15 min, vigorously vortexted, and centrifuged at 12 000g and 4 °C for 5 min. The supernatants of the samples were separated and analyzed by HPLC/MS as described above in the experiments with rat liver S9 fractions. Samples and controls of each test compound were processed and analyzed in rapid succession. The inhibition experiments were performed in the same manner with the following modifications. The amount of PBS was reduced to 123 μL, and 1 μL of a DMSO solution of bis(p-nitrophenyl)phosphate (250 mM) was added additionally.
Metabolic Stability in Porcine Blood Plasma
Immediately after the death of the animal, porcine blood was collected in a 250 mL polypropylene vessel, which contained 1 mL of heparin solution (25 000 I.U./5 mL) to prevent the blood from clotting (final concentration: 20 I.U. heparin/mL). For separating plasma from whole blood, the blood was centrifuged in 50 mL Falcon tubes at 1500g at 20 °C for 5 min. The clear supernatant was retained and stored in aliquots (2 mL) at −20 °C before usage.
The experiments were carried out as described for the determination of metabolic stability in a porcine liver S9 fraction using 125 μL of porcine plasma instead of 125 μL of porcine liver homogenate each. The inhibition experiments were performed in the same manner with the following modifications. In the incubations with tacrine and bis(p-nitrophenyl)phosphate, the amount of PBS was reduced to 123 μL, and 1 μL of a DMSO solution with tacrine (2.5 mM) or bis(p-nitrophenyl)phosphate (250 mM) was added additionally. In the experiments with EDTA, PBS (124 μL) was replaced by a solution of EDTA (3 mM) in PBS (123 μL) and DMSO (1 μL).
Stability in Porcine Albumin Solution
To a solution of porcine albumin (17.5 mg/mL) in PBS (249 μL) was added a solution of the test compound (5 mM) in DMSO (1 μL). After incubation at 37 °C for 30 min, acetonitrile (500 μL) was added. The mixture was vortexed and allowed to stand in an ice bath for 15 min. After vigorous vortexing, the mixture was centrifuged at 12 000g and 4 °C for 5 min. In parallel, controls were prepared by addition of the DMSO solution (5 mM) of the test compound (1 μL) to a solution of porcine albumin (17.5 mg/mL) in PBS (249 μL) and acetonitrile (500 μL). The controls were allowed to stand at room temperature for 30 min. Then, they were cooled in an ice bath for 15 min, vigorously vortexted, and centrifuged at 12 000g and 4 °C for 5 min. The supernatants of the samples were separated and analyzed by HPLC/MS as described in the experiments with rat liver S9 fractions. Samples and controls of each test compound were processed and analyzed in rapid succession.
Investigation of the Reaction of 46 and 47 with Porcine Albumin by MS
A solution of porcine albumin (35 mg/mL) in PBS (25 μL) was diluted with PBS (215 μL). Then, a solution of compound 46 or 47 (5 mM) in DMSO (10 μL) was added, and the mixture was incubated at 37 °C for 60 min (molar ratio of compound 46 or 47/albumin: 4:1). Immediately thereafter, the solutions were frozen in liquid nitrogen and the solvent was removed by freeze-drying. A reference solution was prepared in the same way using pure DMSO instead of the DMSO solution of 46 or 47. Prior to MS analysis, all samples were dissolved and diluted in 0.1% formic acid to a concentration of approximately 1 μg/μL. Then, 2 μL was injected into the HPLC/MS system consisting of a Bruker Elute UPLC with a Waters Protein BEH C4, 300 Å (2.1 mm inside diameter × 100 mm, particle size 1.7 μm) coupled to a Bruker maxis II ETD Ultrahigh Resolution QTOF. Used solvents were 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B), and the gradient had the following conditions: 10% B for 1.5 min, in 1 min to 25% B, after 12.5 min 60% B, in 1 min to 95% B, constant for 2.5 min, back to 10% B in 0.2 min, and constant for 3.3 min at a flow rate of 250 μL/min. Mass spectra were acquired in MS full scan using the Apollo II ESI source in the 600–4300 m/z range at a spectrum rate of 1 Hz. The used capillary voltage was 4500 V with a nebulizer pressure of 1.8 bar, and 8.0 L/min dry gas with a dry gas temperature of 200 °C. All data sets were transferred to Biopharma Compass 2021 for data processing and analysis. The MAM Protein Screening workflow was used to determine the molecular mass of all protein forms and to identify the different drug–protein-conjugate forms.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c01699.
Syntheses of target compounds 11, 17, 21, 27, 28, 30, 31, 35, 41–45, 47, 48, 56, and 58 and 1H NMR spectra of all target compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Fowler C. J.; Doherty P.; Alexander S. P. H. Endocannabinoid Turnover. Adv. Pharmacol. 2017, 80, 31–66. 10.1016/bs.apha.2017.03.006. [DOI] [PubMed] [Google Scholar]
- Iannotti F. A.; Di Marzo V.; Petrosino S. Endocannabinoids and Endocannabinoid-Related Mediators: Targets, Metabolism and Role in Neurological Disorders. Prog. Lipid. Res. 2016, 62, 107–128. 10.1016/j.plipres.2016.02.002. [DOI] [PubMed] [Google Scholar]
- Lu H. C.; Mackie K. An Introduction to the Endogenous Cannabinoid System. Biol. Psychiatry 2016, 79, 516–525. 10.1016/j.biopsych.2015.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur R.; Ambwani S. R.; Singh S. Endocannabinoid System: A Multi-Facet Therapeutic Target. Curr. Clin. Pharmacol. 2016, 11, 110–117. 10.2174/1574884711666160418105339. [DOI] [PubMed] [Google Scholar]
- Bachovchin D. A.; Cravatt B. F. The Pharmacological Landscape and Therapeutic Potential of Serine Hydrolases. Nat. Rev. Drug Discovery 2012, 11, 52–68. 10.1038/nrd3620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura D. K.; Morrison B. E.; Blankman J. L.; Long J. Z.; Kinsey S. G.; Marcondes M. C.; Ward A. M.; Hahn Y. K.; Lichtman A. H.; Conti B.; Cravatt B. F. Endocannabinoid Hydrolysis Generates Brain Prostaglandins That Promote Neuroinflammation. Science 2011, 334, 809–813. 10.1126/science.1209200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J. Z.; Nomura D. K.; Cravatt B. F. Characterization of Monoacylglycerol Lipase Inhibition Reveals Differences in Central and Peripheral Endocannabinoid Metabolism. Chem. Biol. 2009, 16, 744–753. 10.1016/j.chembiol.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister L. A.; Butler C. R.; Mente S.; O’Neil S. V.; Fonseca K. R.; Piro J. R.; Cianfrogna J. A.; Foley T. L.; Gilbert A. M.; Harris A. R.; Helal C. J.; Johnson D. S.; Montgomery J. I.; Nason D. M.; Noell S.; Pandit J.; Rogers B. N.; Samad T. A.; Shaffer C. L.; da Silva R. G.; Uccello D. P.; Webb D.; Brodney M. A. Discovery of Trifluoromethyl Glycol Carbamates as Potent and Selective Covalent Monoacylglycerol Lipase (MAGL) Inhibitors for Treatment of Neuroinflammation. J. Med. Chem. 2018, 61, 3008–3026. 10.1021/acs.jmedchem.8b00070. [DOI] [PubMed] [Google Scholar]
- Otrubova K.; Ezzili C.; Boger D. L. The Discovery and Development of Inhibitors of Fatty Acid Amide Hydrolase (FAAH). Bioorg. Med. Chem. Lett. 2011, 21, 4674–4685. 10.1016/j.bmcl.2011.06.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodola A.; Castelli R.; Mor M.; Rivara S. Fatty Acid Amide Hydrolase Inhibitors: A Patent Review (2009-2014). Expert Opin. Ther. Pat. 2015, 25, 1247–1266. 10.1517/13543776.2015.1067683. [DOI] [PubMed] [Google Scholar]
- Tripathi R. K. P. A Perspective Review on Fatty Acid Amide Hydrolase (FAAH) Inhibitors as Potential Therapeutic Agents. Eur. J. Med. Chem. 2020, 188, 111953 10.1016/j.ejmech.2019.111953. [DOI] [PubMed] [Google Scholar]
- Fazio D.; Criscuolo E.; Piccoli A.; Barboni B.; Fezza F.; Maccarrone M. Advances in the Discovery of Fatty Acid Amide Hydrolase Inhibitors: What Does the Future Hold?. Expert Opin. Drug Discovery 2020, 15, 765–778. 10.1080/17460441.2020.1751118. [DOI] [PubMed] [Google Scholar]
- Kathuria S.; Gaetani S.; Fegley D.; Valiño F.; Duranti A.; Tontini A.; Mor M.; Tarzia G.; La Rana G.; Calignano A.; Giustino A.; Tattoli M.; Palmery M.; Cuomo V.; Piomelli D. Modulation of Anxiety Through Blockade of Anandamide Hydrolysis. Nat. Med. 2003, 9, 76–81. 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- Griebel G.; Stemmelin J.; Lopez-Grancha M.; Fauchey V.; Slowinski F.; Pichat P.; Dargazanli G.; Abouabdellah A.; Cohen C.; Bergis O. E. The Selective Reversible FAAH Inhibitor, SSR411298, Restores the Development of Maladaptive Behaviors to Acute and Chronic Stress in Rodents. Sci. Rep. 2018, 8, 2416 10.1038/s41598-018-20895-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. S.; Stiff C.; Lazerwith S. E.; Kesten S. R.; Fay L. K.; Morris M.; Beidler D.; Liimatta M. B.; Smith S. E.; Dudley D. T.; Sadagopan N.; Bhattachar S. N.; Kesten S. J.; Nomanbhoy T. K.; Cravatt B. F.; Ahn K. Discovery of PF-04457845: A highly potent, orally bioavailable, and selective urea FAAH inhibitor. ACS Med. Chem. Lett. 2011, 2, 91–96. 10.1021/ml100190t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith J. M.; Jones W. M.; Tichenor M.; Liu J.; Seierstad M.; Palmer J. A.; Webb M.; Karbarz M.; Scott B. P.; Wilson S. J.; Luo L.; Wennerholm M. L.; Chang L.; Rizzolio M.; Rynberg R.; Chaplan S. R.; Breitenbucher J. G. Preclinical Characterization of the FAAH Inhibitor JNJ-42165279. ACS Med. Chem. Lett. 2015, 6, 1204–1208. 10.1021/acsmedchemlett.5b00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roques B. P.; Fournié-Zaluski M. C.; Wurm M. Inhibiting the Breakdown of Endogenous Opioids and Cannabinoids to Alleviate Pain. Nat. Rev. Drug Discovery 2012, 11, 292–310. 10.1038/nrd3673. [DOI] [PubMed] [Google Scholar]
- Huggins J. P.; Smart T. S.; Langman S.; Taylor L.; Young T. An Efficient Randomised, Placebo-Controlled Clinical Trial With The Irreversible Fatty Acid Amide Hydrolase-1 Inhibitor PF-04457845, Which Modulates Endocannabinoids but Fails to Induce Effective Analgesia in Patients with Pain Due to Osteoarthritis of the Knee. Pain 2012, 153, 1837–1846. 10.1016/j.pain.2012.04.020. [DOI] [PubMed] [Google Scholar]
- Di Marzo V. Inhibitors of Endocannabinoid Breakdown for Pain: Not so FA(AH)Cile, after all. Pain 2012, 153, 1785. 10.1016/j.pain.2012.06.016. [DOI] [PubMed] [Google Scholar]
- Pawsey S.; Wood M.; Browne H.; Donaldson K.; Christie M.; Warrington S. Safety, Tolerability and Pharmacokinetics of FAAH Inhibitor V158866: A Double-Blind, Randomised, Placebo-Controlled Phase I Study in Healthy Volunteers. Drugs R&D 2016, 16, 181–191. 10.1007/s40268-016-0127-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza D. FAAH Inhibitor PF-04457845 in the Treatment of Cannabis Dependence. Neuropsychopharmacology 2017, 42, S72. [Google Scholar]
- Marco E. M.; Rapino C.; Caprioli A.; Borsini F.; Laviola G.; Maccarrone M. Potential Therapeutic Value of a Novel FAAH Inhibitor for the Treatment of Anxiety. PLoS One 2015, 10, e0137034 10.1371/journal.pone.0137034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celorrio M.; Fernández-Suárez D.; Rojo-Bustamante E.; Echeverry-Alzate V.; Ramírez M. J.; Hillard C. J.; López-Moreno J. A.; Maldonado R.; Oyarzábal J.; Franco R.; Aymerich M. S. Fatty Acid Amide Hydrolase Inhibition for the Symptomatic Relief of Parkinson’s Disease. Brain, Behav., Immun. 2016, 57, 94–105. 10.1016/j.bbi.2016.06.010. [DOI] [PubMed] [Google Scholar]
- Cisar J. S.; Weber O. D.; Clapper J. R.; Blankman J. L.; Henry C. L.; Simon G. M.; Alexander J. P.; Jones T. K.; Ezekowitz R. A. B.; O’Neill G. P.; Grice C. A. Identification of ABX-1431, a Selective Inhibitor of Monoacylglycerol Lipase and Clinical Candidate for Treatment of Neurological Disorders. J. Med. Chem. 2018, 61, 9062–9084. 10.1021/acs.jmedchem.8b00951. [DOI] [PubMed] [Google Scholar]
- Bononi G.; Poli G.; Rizzolio F.; Tuccinardi T.; Macchia M.; Minutolo F.; Granchi C. An Updated Patent Review of Monoacylglycerol Lipase (MAGL) Inhibitors (2018-Present). Expert Opin. Ther. Pat. 2021, 31, 153–168. 10.1080/13543776.2021.1841166. [DOI] [PubMed] [Google Scholar]
- Granchi C.; Caligiuri I.; Minutolo F.; Rizzolio F.; Tuccinardi T. A Patent Review of Monoacylglycerol Lipase (MAGL) Inhibitors (2013-2017). Expert Opin. Ther. Pat. 2017, 27, 1341–1351. 10.1080/13543776.2018.1389899. [DOI] [PubMed] [Google Scholar]
- van Egmond N.; Straub V. M.; van der Stelt M. Targeting Endocannabinoid Signaling: FAAH and MAG Lipase Inhibitors. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 441–463. 10.1146/annurev-pharmtox-030220-112741. [DOI] [PubMed] [Google Scholar]
- Dahlhaus H.; Hanekamp W.; Lehr M. (Indolylalkyl)Piperidine Carbamates as Inhibitors of Fatty Acid Amide Hydrolase (FAAH). Med. Chem. Commun. 2017, 8, 616–620. 10.1039/C6MD00683C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacondio F.; Silva C.; Lodola A.; Fioni A.; Rivara S.; Duranti A.; Tontini A.; Sanchini S.; Clapper J. R.; Piomelli D.; Mor M.; Tarzia G. Structure-Property Relationships of a Class of Carbamate-Based Fatty Acid Amide Hydrolase (FAAH) Inhibitors: Chemical and Biological Stability. ChemMedChem 2009, 4, 1495–1504. 10.1002/cmdc.200900120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapper J. R.; Vacondio F.; King A. R.; Duranti A.; Tontini A.; Silva C.; Sanchini S.; Tarzia G.; Mor M.; Piomelli D. A Second Generation of Carbamate-Based Fatty Acid Amide Hydrolase Inhibitors with Improved Activity In Vivo. ChemMedChem 2009, 4, 1505–1513. 10.1002/cmdc.200900210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacondio F.; Silva C.; Lodola A.; Carmi C.; Rivara S.; Duranti A.; Tontini A.; Sanchini S.; Clapper J. R.; Piomelli D.; Tarzia G.; Mor M. Biphenyl-3-yl Alkylcarbamates as Fatty Acid Amide Hydrolase (FAAH) Inhibitors: Steric Effects of N-Alkyl Chain on Rat Plasma and Liver Stability. Eur. J. Med. Chem. 2011, 46, 4466–4473. 10.1016/j.ejmech.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampa A.; Bartolini M.; Bisi A.; Belluti F.; Gobbi S.; Andrisano V.; Ligresti A.; Di Marzo V. The First Dual ChE/FAAH Inhibitors: New Perspectives for Alzheimer’s Disease?. ACS Med. Chem. Lett. 2012, 3, 182–186. 10.1021/ml200313p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montanari S.; Scalvini L.; Bartolini M.; Belluti F.; Gobbi S.; Andrisano V.; Ligresti A.; Di Marzo V.; Rivara S.; Mor M.; Bisi A.; Rampa A. Fatty Acid Amide Hydrolase (FAAH), Acetylcholinesterase (AChE), and Butyrylcholinesterase (BuChE): Networked Targets for the Development of Carbamates as Potential Anti-Alzheimer’s Disease Agents. J. Med. Chem. 2016, 59, 6387–6406. 10.1021/acs.jmedchem.6b00609. [DOI] [PubMed] [Google Scholar]
- Mills S. D.Heterocyclic Compounds. US5,753,659, May 19, 1998.
- Forster L.; Schulze Elfringhoff A.; Lehr M. High-Performance Liquid Chromatographic Assay with Fluorescence Detection for the Evaluation of Inhibitors Against Fatty Acid Amide Hydrolase. Anal. Bioanal. Chem. 2009, 394, 1679–1685. 10.1007/s00216-009-2850-5. [DOI] [PubMed] [Google Scholar]
- Holtfrerich A.; Hanekamp W.; Lehr M. (4-Phenoxyphenyl)Tetrazolecarboxamides and Related Compounds as Dual Inhibitors of Fatty Acid Amide Hydrolase (FAAH) and Monoacylglycerol Lipase (MAGL). Eur. J. Med. Chem. 2013, 63, 64–75. 10.1016/j.ejmech.2013.01.050. [DOI] [PubMed] [Google Scholar]
- Holtfrerich A.; Makharadze T.; Lehr M. High-Performance Liquid Chromatography Assay with Fluorescence Detection for the Evaluation of Inhibitors Against Human Recombinant Monoacylglycerol Lipase. Anal. Biochem. 2010, 399, 218–224. 10.1016/j.ab.2009.12.015. [DOI] [PubMed] [Google Scholar]
- Sit S. Y.; Conway C.; Bertekap R.; Xie K.; Bourin C.; Burris K.; Den H. Novel Inhibitors of Fatty Acid Amide Hydrolase. Bioorg. Med. Chem. Lett. 2007, 17, 3287–3291. 10.1016/j.bmcl.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Soubhye J.; van Antwerpen P.; Dufrasne F. Targeting Cytosolic Phospholipase A2α for Novel Anti-Inflammatory Agents. Curr. Med. Chem. 2018, 25, 2418–2447. 10.2174/0929867325666180117103919. [DOI] [PubMed] [Google Scholar]
- Hanekamp W.; Lehr M. Determination of Arachidonic Acid by On-Line Solid-Phase Extraction HPLC with UV Detection for Screening of Cytosolic Phospholipase A2α Inhibitors. J. Chromatogr. B 2012, 900, 79–84. 10.1016/j.jchromb.2012.05.018. [DOI] [PubMed] [Google Scholar]
- Horgan D. J.; Stoops J. K.; Webb E. C.; Zerner B. Carboxylesterases (EC 3.1.1). A Large-Scale Purification of Pig Liver Carboxylesterase. Biochemistry 1969, 8, 2000–2006. 10.1021/bi00833a033. [DOI] [PubMed] [Google Scholar]
- Landowski C. P.; Lorenzi P. L.; Song X.; Amidon G. L. Nucleoside Ester Prodrug Substrate Specificity of Liver Carboxylesterase. J. Pharmacol. Exp. Ther. 2006, 316, 572–580. 10.1124/jpet.105.092726. [DOI] [PubMed] [Google Scholar]
- Hu Z. Y.; Edginton A. N.; Laizure S. C.; Parker R. B. Physiologically Based Pharmacokinetic Modeling of Impaired Carboxylesterase-1 Activity: Effects on Oseltamivir Disposition. Clin. Pharmacokinet. 2014, 53, 825–836. 10.1007/s40262-014-0160-3. [DOI] [PubMed] [Google Scholar]; Erratum in: Clin. Pharmacokinet. 2014, 53, 959.
- Bahar F. G.; Ohura K.; Ogihara T.; Imai T. Species Difference of Esterase Expression and Hydrolase Activity in Plasma. J. Pharm. Sci. 2012, 101, 3979–3988. 10.1002/jps.23258. [DOI] [PubMed] [Google Scholar]
- Rudakova E. V.; Boltneva N. P.; Makhaeva G. F. Comparative Analysis of Esterase Activities of Human, Mouse, and Rat Blood. Bull. Exp. Biol. Med. 2011, 152, 73–75. 10.1007/s10517-011-1457-y. [DOI] [PubMed] [Google Scholar]
- Berry L. M.; Wollenberg L.; Zhao Z. Esterase Activities in the Blood, Liver and Intestine of Several Preclinical Species and Humans. Drug Metab. Lett. 2009, 3, 70–77. 10.2174/187231209788654081. [DOI] [PubMed] [Google Scholar]
- Sakurai Y.; Ma S. F.; Watanabe H.; Yamaotsu N.; Hirono S.; Kurono Y.; Kragh-Hansen U.; Otagiri M. Esterase-Like Activity of Serum Albumin: Characterization of its Structural Chemistry Using P-Nitrophenyl Esters as Substrates. Pharm. Res. 2004, 21, 285–292. 10.1023/B:PHAM.0000016241.84630.06. [DOI] [PubMed] [Google Scholar]
- Lockridge O.; Xue W.; Gaydess A.; Grigoryan H.; Ding S. J.; Schopfer L. M.; Hinrichs S. H.; Masson P. Pseudo-Esterase Activity of Human Albumin: Slow Turnover on Tyrosine 411 and Stable Acetylation of 82 Residues Including 59 Lysines. J. Biol. Chem. 2008, 283, 22582–22590. 10.1074/jbc.M802555200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncharov N. V.; Belinskaia D. A.; Shmurak V. I.; Terpilowski M. A.; Jenkins R. O.; Avdonin P. V. Serum Albumin Binding and Esterase Activity: Mechanistic Interactions with Organophosphates. Molecules 2017, 22, 1201 10.3390/molecules22071201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R. B.; Hu Z. Y.; Meibohm B.; Laizure S. C. Effects of Alcohol on Human Carboxylesterase Drug Metabolism. Clin. Pharmacokinet. 2015, 54, 627–638. 10.1007/s40262-014-0226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi D.; Yang J.; Yang D.; LeCluyse E. L.; Black C.; You L.; Akhlaghi F.; Yan B. Anti-Influenza Prodrug Oseltamivir Is Activated by Carboxylesterase Human Carboxylesterase 1, and the Activation Is Inhibited by Antiplatelet Agent Clopidogrel. J. Pharmacol. Exp. Ther. 2006, 319, 1477–1484. 10.1124/jpet.106.111807. [DOI] [PubMed] [Google Scholar]
- Drews A.; Bovens S.; Roebrock K.; Sunderkötter C.; Reinhardt D.; Schäfers M.; van der Velde A.; Schulze Elfringhoff A.; Fabian J.; Lehr M. 1-(5-Carboxyindol-1-yl)propan-2-one Inhibitors of Human Cytosolic Phospholipase A2α with Reduced Lipophilicity: Synthesis, Biological Activity, Metabolic Stability, Solubility, Bioavailability, and Topical In Vivo Activity. J. Med. Chem. 2010, 53, 5165–5178. 10.1021/jm1001088. [DOI] [PubMed] [Google Scholar]
- Ghosal A.; Yuan Y.; Tong W.; Su A. D.; Gu C.; Chowdhury S. K.; Kishnani N. S.; Alton K. B. Characterization of Human Liver Enzymes Involved in the Biotransformation of Boceprevir, a Hepatitis C Virus Protease Inhibitor. Drug Metab. Dispos. 2011, 39, 510–521. 10.1124/dmd.110.036996. [DOI] [PubMed] [Google Scholar]
- Hegarty A. F.; Frost L. N. Isocyanate Intermediates in E1cb Mechanism of Carbamate Hydrolysis. J. Chem. Soc., Chem. Commun. 1972, 500–501. 10.1039/c39720000500. [DOI] [Google Scholar]
- Kratz F. Albumin as a Drug Carrier: Design of Prodrugs, Drug Conjugates and Nanoparticles. J. Controlled Release 2008, 132, 171–183. 10.1016/j.jconrel.2008.05.010. [DOI] [PubMed] [Google Scholar]
- Tarhini M.; Benlyamani I.; Hamdani S.; Agusti G.; Fessi H.; Greige-Gerges H.; Bentaher A.; Elaissari A. Protein-Based Nanoparticle Preparation via Nanoprecipitation Method. Materials 2018, 11, 394 10.3390/ma11030394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migneault I.; Dartiguenave C.; Bertrand M. J.; Waldron K. C. Glutaraldehyde: Behavior In Aqueous Solution, Reaction With Proteins, And Application To Enzyme Crosslinking. Biotechniques 2004, 37, 790–802. 10.2144/04375RV01. [DOI] [PubMed] [Google Scholar]
- Hoffmann M.; Stiller C.; Endres E.; Scheiner M.; Gunesch S.; Sotriffer C.; Maurice T.; Decker M. Highly Selective Butyrylcholinesterase Inhibitors with Tunable Duration of Action by Chemical Modification of Transferable Carbamate Units Exhibit Pronounced Neuroprotective Effect in an Alzheimer’s Disease Mouse Model. J. Med. Chem. 2019, 62, 9116–9140. 10.1021/acs.jmedchem.9b01012. [DOI] [PubMed] [Google Scholar]
- Jing L.; Wu G.; Kang D.; Zhou Z.; Song Y.; Liu X.; Zhan P. Contemporary Medicinal-Chemistry Strategies for the Discovery of Selective Butyrylcholinesterase Inhibitors. Drug Discovery Today 2019, 24, 629–635. 10.1016/j.drudis.2018.11.012. [DOI] [PubMed] [Google Scholar]
- Darvesh S. Butyrylcholinesterase as a Diagnostic and Therapeutic Target for Alzheimer’s Disease. Curr. Alzheimer Res. 2016, 13, 1173–1177. 10.2174/1567205013666160404120542. [DOI] [PubMed] [Google Scholar]
- Benito C.; Núñez E.; Tolón R. M.; Carrier E. J.; Rábano A.; Hillard C. J.; Romero J. Cannabinoid CB2 Receptors and Fatty Acid Amide Hydrolase Are Selectively Overexpressed in Neuritic Plaque-Associated Glia in Alzheimer’s Disease Brains. J. Neurosci. 2003, 23, 11136–11341. 10.1523/JNEUROSCI.23-35-11136.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels G.; Lehr M.. High Performance Liquid Chromatographic Assays with UV-Detection for Evaluation of Inhibitors of Acetylcholinesterase and Butyrylcholinesterase. J. Liq. Chromatogr. Relat. Technol. 2021, in press, accepted manuscript. [Google Scholar]
- OECD . Guidelines for the Testing of Chemicals. No. 117—Partition Coefficient (n-Octanol/Water), High Performance Liquid Chromatography (HPLC) Method; OECD, 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.