Abstract
Background
Leishmaniasis is a neglected tropical disease caused by parasites of the genus Leishmania and is transmitted by various species of female phlebotomine sand flies. The first report of cutaneous leishmaniasis (CL) in Ghana refer to a cluster of cases in 1999–2003 in the Ho municipality of the Volta Region. We conducted an epidemiological assessment in the Oti Region, encouraged by recent reports of potential cases of CL.
Methodology/Principal findings
Using a cross-sectional study design, the exposure to Leishmania was investigated in three communities of the Oti Region based on the leishmanin skin test (LST). LST results for 3,071 participants comprising 1091, 848, and 1132 persons from the communities of Ashiabre, Keri, and Sibi Hilltop, indicated an overall prevalence of exposure to Leishmania infection of 41.8% and individual community prevalence of 39.4%, 55.1%, and 34.2% respectively. Being male [AOR = 1.27; CI: 1.09, 1.49], and living in Keri [AOR = 1.83; CI: 1.43, 2.34] were associated with an increase in the odds of exposure to Leishmania. Being 5–10 years old [AOR = 1.48; CI: 1.06, 2.05], 11–17 years old [AOR = 2.03; CI: 1.45, 2.85], 18–40 years old [AORR = 2.83; CI: 1.81, 4.43] and 41–65 years old [AOR = 5.08; CI: 2.98, 8.68] were also significantly associated with increased odds of being exposed to Leishmania.
Conclusions/Significance
This study demonstrated exposure to Leishmania in the study communities and also identified associated factors. Future efforts aimed at reducing exposure to Leishmania infection in the study area should take the associated factors into consideration.
Author summary
Leishmaniasis is a neglected vector borne disease caused by parasites of the genus Leishmania and is endemic in over 98 countries globally. Two broad categories of leishmaniasis exist: visceral leishmaniasis (VL) and cutaneous leishmaniasis (CL). The first report of CL in Ghana was from a cluster of cases in 1999–2003 in the Ho municipality of the Volta Region [which has since the year 2019 been divided into Volta and Oti Regions]. However, information on the prevalence of Leishmania infection in the affected communities of Ghana is limited. Using a cross-sectional study design, this study described prevalence of exposure to Leishmania in three communities of the Oti Region based on the leishmanin skin test (LST), following reports of potential cases of CL in that area. In addition, factors associated with an increase in the odds of exposure to Leishmania were also investigated. Demonstration of exposure to, as well as factors associated with Leishmania infection in communities of the Oti Region of Ghana suggests a need for further investigation of other related factors such as prevalence of CL and possible vectors and reservoirs of the disease.
Introduction
Leishmaniasis is a neglected vector borne disease caused by parasites of the genus Leishmania and is endemic in over 98 countries with 350 million people estimated to be at risk of contracting the disease globally [1,2]. Natural transmission of the Leishmania parasites to humans and other mammals occurs through the bite of various species of infected female phlebotomine sand flies belonging to the genus Phlebotomus in the Old World and the following genera in the New World: Lutzomyia, Psychodopygus and Nyssomyia. Majority of the Leishmania infections in humans are transmissible only from animals [zoonotic leishmaniasis] but some can be spread between humans (anthroponotic leishmaniasis) [3–7].
Depending on the area of localization of the parasite in mammalian tissues, two broad categories of leishmaniasis exist: visceral and cutaneous, with cutaneous leishmaniasis (CL) being the most common. Globally, it is estimated that between 0.7 to 1.3 million new cases of CL are reported every year [1–3,8].
Although lesions due to CL are often self-healing, this could take up to six months. Secondary bacterial infections, may increase tissue destruction which can lead to permanent scarring [3,7].
A localized outbreak of skin ulcers suspected to be cases of CL was first reported in Ghana from the Ho municipality of the Volta Region in 1999 based on the identification of Leishmania amastigotes in some skin lesion biopsies obtained from the patients and examined using microscopy [9]. In 2002, molecular typing revealed Leishmania major causing CL in a patient from the Ho municipality, Volta Region [10]. A follow up study involving nine biopsies from five suspected CL cases in 2006 and one biopsy from another suspected CL case in 2007 from Taviefe, a community located about 10 km north of Ho, however, did not confirm L. major. Instead, uncharacterized species of Leishmania were observed [11].
An additional study in some other parts of the Ho municipality successfully cultured and obtained three isolates from individuals suspected with active cutaneous leishmaniasis. Using DNA sequencing and phylogenetic analysis, the isolates were confirmed to be members of the Leishmania enriettii complex, an emerging new subgenus of Leishmania parasites [12]. Also, L. tropica, and L. major DNA have been identified in Sergentomyia sand flies sampled from the Ho municipality [13]. These results suggest a complex epidemiology of CL in the region.
Despite this, information on the prevalence of Leishmania infection in the affected communities of Ghana is limited possibly due to the absence of a surveillance system for leishmaniasis in Ghana [9]. This study was therefore conducted in three communities of the Oti Region [which until December, 2018 was part of the Volta Region] to investigate the prevalence of Leishmania infection following reports of cases of skin ulcers suspected to be CL, using the leishmanin skin test (LST).
The leishmanin skin test (LST), also known as the Montenegro skin test (MST) is an intradermal test that measures delayed-type hypersensitivity response to Leishmania antigen, and is a useful method for detecting cell-mediated immunity against Leishmania [14,15].
The LST becomes positive after an asymptomatic Leishmania infection, during active CL, and after healing of CL ulcers. A positive LST is defined as skin reaction [induration] ≥5 mm with sensitivity of the LST estimated at between 86.4% to 100%. The LST is therefore a useful tool in epidemiological surveys but has limited value in the diagnosis of CL, as it cannot distinguish between past or active infections. It is more intended as a marker of exposure to Leishmania [14–17]
Materials and methods
Ethics statement
Ethical approval to conduct this study was obtained from the ethics review committee of the Ghana Health Service (GHS-ERC006/08/18). Written informed consent was obtained from all study participants. For participants under 18 years, written consent was obtained from a parent or guardian.
Study design
Using a cross-sectional study design, this study was conducted in three communities of the Oti region of Ghana from October to December, 2018. Prevalence of Leishmania infection as well as factors (individual level, household level, and community of residence) associated with Leishmania infection among study participants were investigated based on the leishmanin skin test and a structured interviewer administered questionnaire [18].
Study area
This study was conducted in the following three communities: Ashiabre, Keri, and Sibi Hilltop. Ashiabre is in the Tutukpene sub-district of the Nkwanta South municipality while Keri is in the Keri sub-district of the municipality. Sibi Hilltop is in the Sibi sub-district of the Nkwanta North district of the region.
The population of Nkwanta South municipality is estimated to be 117,878 with males constituting 49.6% of the population. Covering a land area of approximately 2733 km2, the Nkwanta South municipality is located between latitudes 7o 30’ and 8o 45’ North and longitude 0o 10’ and 0o 45’East [19].
The population of the Nkwanta North district is estimated to be 64,553 with males constituting 50.2% of the population. The district is located between Latitude 7°30’N and 8°45’N and Longitude 0°10’W and 045’E. It shares boundaries with Nkwanta South municipality to the south, Nanumba South to the north, Republic of Togo to the east, and Kpandai District to the west [20].
Inclusion criteria
Eligible study participants were residents in the study community for ≥ 12 months, aged between 2 to 65 years (inclusive).
Sample size consideration
Assuming a 0.05 seropositivity of delayed-type hypersensitivity to Leishmania antigen in the study area, acceptable difference of 0.0175, an alpha error of 0.05, and a design effect of 1.5, a minimum sample of 834 persons was required for LST screening [21].
Sampling method
Of 15 communities in the Oti Region visited by the study team prior to study initiation, at least 3 potential cases of CL were observed in 8 communities.
The three study communities were selected from among the eight using a simple random selection without replacement approach.
Using a sorted list of households in each study community, 200 households [with an average of 5–7 persons per household] were selected for study inclusion using a systematic sampling approach. For this study, a household was defined as a person or a group of persons, who live together in the same house or compound and share the same house-keeping arrangements. The head of each household was defined as a male or female member of the household recognised as such by the other household members. The head of a particular household is generally the person with economic and social responsibility for the household. As a result, household relationships were defined with reference the household head [22]. The community household list was obtained for each study community based on a household census.
The total number of households in Ashiabre, keri, and Sibi Hilltop were 945, 795, and 1184 respectively.
The systematic sampling approach used is described below.
Using the sorted list of households in each study community, a sampling interval I was determined, where I = N/n with N being the number of households in the study community [number of units forming the sample frame] and n was the number of households to be selected [200 for each study community]. The I was rounded to 2 decimal places.
Using Microsoft excel, the RANDBETWEEN command was used to generate a random decimal integer R between 0 and 1 rounded up to two decimal points. The sequence of households that were selected in each study community were R*I, R*I + I, R*I +2*I, R*I +3*I,….R*I + [n-1]*I, each rounded up to the next highest whole number [23].
With the assistance of community-based volunteers, the selected households were identified after which all members of the selected households aged 2 to 65 years were invited to participate in the study, using a door-to-door invitation approach. Because the invitation to participate in the study was extended to households, a household was not included in the study if the household head declined to allow his or her household to participate in the study. However, the agreement of the household head did not make it compulsory for every household member of age 2 to 65 years to participate in the study. Each household member was given the opportunity to go through the informed consent process to decide whether they wish to participate or not.
Pre-study training
Prior to the commencement of field data collection, study team members were taken through a one-week training session comprising in-class training, break out discussion sessions, and field testing of the study questionnaires in a community in the Nkwanta South municipality [the main Nkwanta township].
The training sessions covered all aspects of the study procedures such as informed consent process, questionnaire administration, and administration of the leishmanin skin test. The trainees were also taken through a simulation of all study procedures.
The field team was comprised of health workers (community health nurses often involved in administration of vaccinations such as bacille Calmette-Guérin (BCG), hospital-based nurses and public health officers) and community-based volunteers.
Administration of leishmanin skin test
All study participants were invited to participate in the LST survey to establish prevalence of Leishmania infection. The leishmanin reagent used for this study was obtained from the Pasteur Institute of Iran and was composed mainly of the following: promastigotes of Leishmania major (MRHO/IR/75/ER strain), phosphate buffered saline, and thimerosal. The leishmanin used was lot number 128, manufactured in February 2016, and expected to expire in February 2021. The sterile aqueous suspension of leishmanin used for this study was stored in a refrigerator at 2–8 degrees Celsius with cold chain maintained during field work using ice packs. Details of the LST procedure are provided below:
A 0.1 mL of LST suspension was injected intradermally into the volar aspect of each study participant’s left forearm using new sterile tuberculin syringes. Between 48 and 72 hours post-LST placement, the delayed-type hypersensitivity (DTH) reaction was assessed by averaging the greatest diameter of induration and the diameter of induration perpendicular to it, measured in millimeters using calipers. A positive LST was defined as induration ≥5 mm (Fig 1) [14].
Fig 1. Induration on skin 48–72 hours after LST placement and measurement.
Data management and analysis
Data were managed using Microsoft Access software version 2013 and analyzed using STATA software version 14. All statistical tests were performed at a 95% confidence level.
Prevalence of LST positivity was described. Logistic regression model [simple and multiple] was used to evaluate association between various factors and Leishmania infection as observed using the LST, in the study communities. Factors with p value less than 0.05 in the bivariate analysis were included in the multiple logistic regression analysis.
The factors compared with LST positivity included age of study participant, sex of participant, number of scars, number of skin ulcers, family history of CL, use of bed nets, being at open field at dawn or sunset, sleeping near forest or farm fields, and sleeping in rooms or places with open windows without screen. Additional factors included were frequency of mosquito bites, having bites from other insects, having contact with dogs, having contact with goats, having contact with other domestic animals, spraying bedroom with insecticide in the last six months, and use of long sleeves, use of mosquito repellents. These variables were obtained from the administration of a structured questionnaire.
Odds ratios for all variables included in the multiple logistic regression analysis were adjusted for all covariates included in the model as well as for clustering at the household level using the vce (cluster clustvar) command in Stata statistical software version 14.
Results
Of 600 households (200 in each study community) invited to participate in this study, a total of 587 households comprising 189 (32.2%), 200 (34.1%), and 198 (33.7%) from Ashiabre, Keri and Sibi Hilltop respectively, agreed to be part of this study and were included. The study households had a total of 3718 members out of which 3,440 (92.5%) consisting of 1,194, 941, 1305 from Ashiabre, Keri, and Sibi Hilltop respectively were enrolled in the study.
The average household size was 6.3 with a range of 1 to 18 household members. Ashiabre and Sibi Hilltop had an average household size of 7 while Keri had an average household size of 5.
A total of 3071 persons comprising 1091, 848, and 1132 participants from Ashiabre, Keri, and Sibi Hilltop respectively were screened using the LST procedure. Of these, 1483 (48.3%) were males and 345 (11.2%) were under five years old (Table 1).
Table 1. Summary of study participants by age, sex, and community of residence.
| Characteristic | Study Community | |||
|---|---|---|---|---|
| Ashiabre | Keri | Sibi Hilltop | Total | |
| Mean age in years [SD] | 18.6 (±15.2) | 19.5 (±15.5) | 17.9 (±14.2) | 18.6 (±14.9) |
| Age group, n [%] | ||||
| <5 years | 137 (12.6) | 86 (10.1) | 122 (10.8) | 345 (11.2) |
| 5–10 years | 289 (26.5) | 249 (29.4) | 330 (29.2) | 868 (28.3) |
| 11–17 years | 277 (25.4) | 189 (22.3) | 297 (26.2) | 763 (24.9) |
| 18–40 years | 251 (23.0) | 210 (24.8) | 268 (23.7) | 729 (23.7) |
| 41–65 years | 137 (12.6) | 114 (13.4) | 115 (10.2) | 366 (11.9) |
| Sex, n [%] | ||||
| Male | 523 (47.9) | 381 (44.9) | 579 (51.2) | 1483 (48.3) |
| Female | 568 (52.1) | 467 (55.1) | 553 (48.9) | 1588 (51.7) |
| Total | 1091 (100) | 848 (100) | 1132 (100) | 3071 (100) |
Prevalence of Leishmania infection observed at Ashiabre and Keri was 39.4% and 55.1% respectively [Table 2]. Prevalence among males at Ashiabre was 43.6% and 35.6% among females in the same community. Among both males and females at Ashiabre, prevalence of Leishmania infection increased with age from 18.8% and 19.1% among males and females under five respectively to 70.8% and 55.6% among males and females 41–65 years old, respectively. Cumulatively, prevalence of Leishmania infection at Ashiabre increased from 19.0% among children under five, to 62.8% among adults 41–65 years old (Table 2).
Table 2. LST prevalence by age and sex at Ashiabre and Keri.
| Community | Sex | Age | Number of household | LST Prevalence | P value | |
|---|---|---|---|---|---|---|
| members screened | n (%) | 95% CI | ||||
| Ashiabre | Males | < 5 years | 69 | 13 (18.8) | (11.1, 30.1) | <0.001 |
| 5–10 years | 157 | 56 (35.7) | (28.5, 43.5) | |||
| 11–17 years | 147 | 62 (42.2) | (34.4, 50.4) | |||
| 18–40 years | 85 | 51 (60.0) | (49.1, 70.0) | |||
| 41–65 years | 65 | 46 (70.8) | (58.3, 80.7) | |||
| Subtotal | 523 | 228 (43.6) | (39.4, 47.9) | |||
| Females | < 5 years | 68 | 13 (19.1) | (11.3, 30.5) | <0.001 | |
| 5–10 years | 132 | 28 (21.2) | (15.0, 29.1) | |||
| 11–17 years | 130 | 42 (32.3) | (24.7, 40.9) | |||
| 18–40 years | 166 | 79 (47.6) | (40.0, 55.3) | |||
| 41–65 years | 72 | 40 (55.6) | (43.7, 66.8) | |||
| Subtotal | 568 | 202 (35.6) | (31.7, 39.6) | |||
| Total | < 5 years | 137 | 26 (19.0) | (13.2, 26.5) | <0.001 | |
| 5–10 years | 289 | 84 (29.1) | (24.1, 34.6) | |||
| 11–17 years | 277 | 104 (37.5) | (32.0, 43.4) | |||
| 18–40 years | 251 | 130 (51.8) | (45.6, 58.0) | |||
| 41–65 years | 137 | 86 (62.8) | (54.3, 70.5) | |||
| Total | 1091 | 430 (39.4) | (36.5, 42.4) | |||
| Keri | Males | < 5 years | 41 | 10 (24.4) | (13.3, 40.4) | <0.001 |
| 5–10 years | 134 | 62 (46.3) | (37.9, 54.8) | |||
| 11–17 years | 97 | 55 (56.7) | (46.5, 66.3) | |||
| 18–40 years | 61 | 43 (70.5) | (57.6, 80.8) | |||
| 41–65 years | 48 | 41 (85.4) | (71.8, 93.1) | |||
| Subtotal | 381 | 211 (55.4) | (50.3, 60.3) | |||
| Females | < 5 years | 45 | 10 (22.2) | (12.1, 37.2) | <0.001 | |
| 5–10 years | 115 | 38 (33.0) | (25.0, 42.3) | |||
| 11–17 years | 92 | 57 (62.0) | (51.5, 71.4) | |||
| 18–40 years | 149 | 95 (63.8) | (55.7, 71.2) | |||
| 41–65 years | 66 | 56 (84.9) | (73.7, 91.8) | |||
| Subtotal | 467 | 256 (54.8) | (50.3, 59.3) | |||
| Total | < 5 years | 86 | 20 (23.3) | (15.4, 33.5) | <0.001 | |
| 5–10 years | 249 | 100 (40.2) | (34.2, 46.4) | |||
| 11–17 years | 189 | 112 (59.3) | (52.0, 66.1) | |||
| 18–40 years | 210 | 138 (65.7) | (59.0, 71.9) | |||
| 41–65 years | 114 | 97 (85.1) | (70.2, 90.6) | |||
| Total | 848 | 467 (55.1) | (51.7, 58.4) | |||
At Keri, prevalence of Leishmania infection among males was 55.4% while a prevalence of 54.8% was observed among females. Among both males and females at Keri, prevalence of infection increased with age from 24.4% and 22.2% among males and females under five respectively to 85.4% and 84.9% among adult males and females in the 41–65 years old group, respectively. Cumulatively, prevalence of infection at Keri increased from 23.3% among children under five to 85.1% among adults in the 41–65 years group (Table 2).
Table 3 summarizes prevalence of Leishmania infection at Sibi Hilltop (34.2%) as well as the cumulative prevalence of infection for all study sites (41.8%). At Sibi Hilltop, prevalence of infection among males was 35.1% while it was 33.3% among females. This increased from 28.8% and 30.2% among males and females under five respectively to 50.8% and 53.7% among adult males and females in the 41–65 years, respectively. Cumulatively, prevalence of infection at Sibi Hilltop increased from 29.5% among children under five to 52.2% among adults in the 41–65 years group.
Table 3. LST prevalence by age and sex at Sibi Hilltop and cumulatively for all study sites.
| Community | Sex | Age | Number of household | LST Prevalence | P value | |
|---|---|---|---|---|---|---|
| members screened | n (%) | 95% CI | ||||
| Sibi Hilltop | Males | < 5 years | 59 | 17 (28.8) | (18.5, 42.0) | 0.007 |
| 5–10 years | 196 | 67 (34.2) | (27.8, 41.2) | |||
| 11–17 years | 165 | 46 (27.9) | (21.5, 35.3) | |||
| 18–40 years | 98 | 42 (42.9) | (33.3, 53.0) | |||
| 41–65 years | 61 | 31 (50.8) | (38.1, 63.4) | |||
| Subtotal | 579 | 203 (35.1) | (31.3, 39.1) | |||
| Females | < 5 years | 63 | 19 (30.2) | (19.9, 42.9) | <0.001 | |
| 5–10 years | 134 | 33 (24.6) | (18.0, 32.7) | |||
| 11–17 years | 132 | 35 (26.5) | (19.6, 34.8) | |||
| 18–40 years | 170 | 68 (40.0) | (32.8, 47.6) | |||
| 41–65 years | 54 | 29 (53.7) | (40.0, 66.8) | |||
| Subtotal | 553 | 184 (33.3) | (29.5, 37.3) | |||
| Total | < 5 years | 122 | 36 (29.5) | (22.0, 38.3) | <0.001 | |
| 5–10 years | 330 | 100 (30.3) | (25.6, 35.5) | |||
| 11–17 years | 297 | 81 (27.3) | (22.5, 32.7) | |||
| 18–40 years | 268 | 110 (41.0) | (35.3, 47.1) | |||
| 41–65 years | 115 | 60 (52.2) | (42.9, 61.3) | |||
| Total | 1132 | 387 (34.2) | (31.5, 37.0) | |||
| Total | Males | < 5 years | 169 | 40 (23.7) | (17.8, 30.7) | <0.001 |
| 5–10 years | 487 | 185 (38.0) | (33.8, 42.4) | |||
| 11–17 years | 409 | 163 (39.9) | (35.2, 44.7) | |||
| 18–40 years | 244 | 136 (55.7) | (49.4, 61.9) | |||
| 41–65 years | 174 | 118 (67.8) | (60.4, 74.4) | |||
| Subtotal | 1483 | 642 (43.3) | (40.8, 45.8) | |||
| Females | < 5 years | 176 | 42 (23.9) | (18.1, 30.8) | <0.001 | |
| 5–10 years | 381 | 99 (26.0) | (21.8, 30.6) | |||
| 11–17 years | 354 | 134 (37.9) | (32.9, 43.0) | |||
| 18–40 years | 485 | 242 (49.9) | (45.4, 54.4) | |||
| 41–65 years | 192 | 125 (65.1) | (58.0, 71.6) | |||
| Subtotal | 1588 | 642 (40.4) | (38.0, 42.9) | |||
| Total | < 5 years | 345 | 82 (23.8) | (19.6, 28.6) | <0.001 | |
| 5–10 years | 868 | 284 (32.7) | (29.7, 35.9) | |||
| 11–17 years | 763 | 297 (38.9) | (35.5, 42.4) | |||
| 18–40 years | 729 | 378 (51.9) | (48.2, 55.5) | |||
| 41–65 years | 366 | 243 (66.4) | (61.4, 71.1) | |||
| Total | 3071 | 1284 (41.8) | (40.1, 43.6) | |||
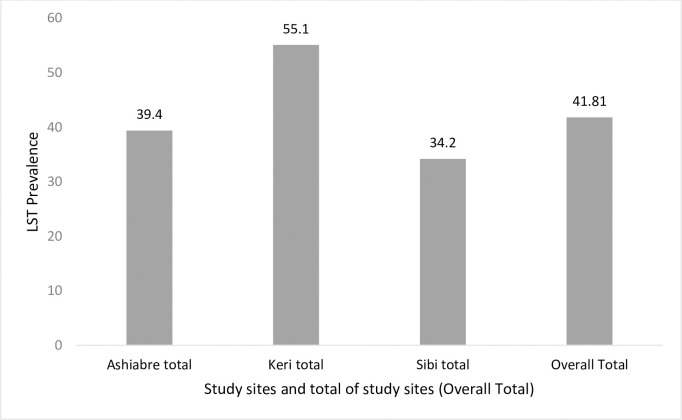
For all the study communities, a significant association was observed between Leishmania infection and age of study participants with the prevalence of infection observed to increase with age. While a cumulative prevalence of 41.8% was observed, prevalence was 23.8%, 32.7%, 38.9%, 51.9%, and 66.4% among those aged <5years, 5–10 years, 11–17 years, 18–40 years, and 41–65 years respectively (Table 3). A summary of the prevalence of Leishmania infection across the study communities and cumulatively is presented in Fig 2.
Fig 2. Prevalence of Leishmania infection at various study sites and cumulatively.
LST positivity was significantly associated with the study community, age of the study participants, and sex of the study participants. Compared to persons in Ashiabre, participants in Keri [AOR = 1.83; 95% CI: 1.43, 2.34] had higher odds of being LST positive. Compared to children under 5 years, participants 5–10 years [AOR = 1.48; 95% CI: 1.06, 2.05], those 11–17 years [AOR = 2.03; 95% CI: 1.45, 2.85], those 18–40 years [AOR = 2.83; 95% CI: 1.81, 4.43] and participants aged 41–65 years [AOR = 5.08; 95% CI: 2.98, 8.68] had higher odds of being LST positive, with the odds increasing with age. Compared to females, males [AOR = 1.27; 95% CI: 1.09, 1.49] had higher odds of being LST positive. Never using insecticide treated bed net (ITN) or use of ITN sometimes was not significantly associated with being LST positive when compared with those who used ITN often (Table 4).
Table 4. Factors associated with LST positivity.
| Characteristics | Categories | Frequency | Number and proportion LST positive, n(%) | Crude OR (95% CI) | P value | AOR (95% CI) | P value |
|---|---|---|---|---|---|---|---|
| Study community | |||||||
| Ashiabre | 1091 | 430 (39.4) | (Reference) | (Reference) | |||
| Keri | 848 | 467 (55.1) | 1.88 (1.57, 2.26) | <0.001 | 1.83 (1.43, 2.34) | <0.001* | |
| Sibi | 1132 | 387 (34.2) | 0.80 (0.67, 0.95) | 0.011 | 0.65 (0.50, 0.85) | 0.002 | |
| Subtotal | 3071 | 1284 (41.8) | |||||
| Person’s age | |||||||
| < 5 years | 345 | 82 (23.8) | (Reference) | (Reference) | |||
| 5–10 years | 868 | 284 (32.7) | 1.56 (1.17, 2.08) | 0.003 | 1.48 (1.06, 2.05) | 0.020* | |
| 11–17 years | 763 | 297 (38.9) | 2.04 (1.51, 2.76) | <0.001 | 2.03 (1.45, 2.85) | <0.001* | |
| 18–40 years | 729 | 378 (51.9) | 3.45 (2.57, 4.65) | <0.001 | 2.83 (1.81, 4.43) | <0.001* | |
| 41–65 years | 366 | 243 (66.4) | 6.34 (4.51, 8.91) | <0.001 | 5.08 (2.98, 8.68) | <0.001* | |
| Subtotal | 345 | 1284 (41.8) | |||||
| Person’s sex | |||||||
| Female | 1588 | 642 (40.4) | (Reference) | (Reference) | |||
| Male | 1483 | 642 (43.3) | 1.12 (0.97, 1.29) | 0.121 | 1.27 (1.09, 1.49) | 0.002* | |
| Subtotal | 3071 | 1284 (41.8) | |||||
| Number of scars on skin | |||||||
| No scars | 555 | 232 (41.8) | (Reference) | (Reference) | |||
| 1–3 scars | 615 | 224 (36.4) | 0.77 (0.61, 0.98) | 0.035 | 0.99 (0.76, 1.28) | 0.923 | |
| 4–6 scars | 582 | 226 (38.8) | 0.86 (0.67, 1.09) | 0.215 | 1.07 (0.80, 1.42) | 0.665 | |
| 7–9 scars | 482 | 195 (40.5) | 0.90 (0.70, 1.16) | 0.424 | 1.07 (0.78, 1.48) | 0.674 | |
| > = 10 scars | 837 | 407 (48.6) | 1.29 (1.03, 1.61) | 0.028 | 1.28 (0.97, 1.69) | 0.084 | |
| Subtotal | 3071 | 1284 (41.8) | |||||
| Family CL history | |||||||
| No family history | 825 | 341 (41.3) | (Reference) | (Reference) | |||
| 1–3 persons with CL history | 1874 | 772 (41.2) | 0.99 (0.84, 1.17) | 0.946 | 1.01 (0.83, 1.23) | 0.946 | |
| 4–6 persons with CL history | 307 | 139 (45.3) | 1.17 (0.90, 1.53) | 0.233 | 1.40 (0.94, 2.06) | 0.094 | |
| 7–9 persons with CL history | 25 | 13 (52.0) | 1.54 (0.69, 3.41) | 0.29 | 1.39 (0.66, 2.91) | 0.382 | |
| > = 10 persons | 40 | 19 (47.50) | 1.28 (0.68, 2.43) | 0.441 | 1.49 (0.84, 2.65) | 0.170 | |
| Subtotal | 3071 | 1284 (41.8) | |||||
| Use of bed nets | |||||||
| Often | 2128 | 885 (41.6) | (Reference) | (Reference) | |||
| Sometimes | 679 | 306 (45.1) | 1.14 (0.96, 1.36) | 0.137 | 0.85 (0.68, 1.07) | 0.163 | |
| Never | 264 | 93 (35.2) | 0.89 (0.64, 1.24) | 0.490 | 0.80 (0.55, 1.15) | 0.227 | |
| Subtotal | 3071 | 1284 (41.8) | |||||
Discussion
Using the LST, an overall prevalence of 41.8% was determined for Leishmania infection, with individual community Leishmania prevalence of 39.4%, 55.1%, and 34.2% observed in Ashiabre, Keri, and Sibi Hilltop respectively. This result therefore indicates the presence cutaneous leishmaniasis in the region. However, culture or molecular sequencing confirmation from infected ulcers is required to identify the infecting species.
Data on LST positivity obtained from this study compares with that observed in some other African countries such as Mali where community based studies using LST observed Leishmania prevalence as high as 49.9% in certain communities with increase of prevalence associated with age groups such that in a certain community (Diema), Leishmania prevalence increased from 13.8% to 88% for age groups of 2–5 years and 41–56 years respectfully [24].
A systematic review of some studies conducted in Mali further observed an overall prevalence of Leishmania infection using LST to be 22.1% [25]. Country wide estimates for Leishmania infection in Ghana is currently not available. Data obtained from this study on Leishmania infection therefore augments existing data on leishmaniasis in Ghana and Africa as a whole where a general paucity of data on leishmaniasis exists [1].
As has been observed in other studies, this study detected significant association between increase in the prevalence of LST positivity and increase in age across the study communities [26,27]. The observation of a significant association between increase in LST positivity and increasing age suggests a need to put in interventions to protect children in particular from exposure to Leishmania infection while additional studies are carried out to understand the reasons for the increased prevalence observed among the older segments of the study population [26,27].
Although Leishmania infection was observed in all three study communities, being a resident of Keri was significantly associated with increase in the likelihood of being exposed to the infection, while staying in Sibi Hill top has a less likelihood of being exposed to the infection, compared with being a resident of Ashiabre. This calls for more studies to better understand the characteristics of the study communities particularly with regards to determining how long members of the study communities may have been living with the health challenge of CL. This is important because of findings from other studies which observed higher prevalence of Leishmania infection in communities considered to be older/endemic foci for CL compared with those considered to be emerging foci [24,26].
Furthermore, observation of a significant association between being male and increased prevalence of Leishmania infection in the study communities calls for more studies to understand the daily activities of males in the study communities to develop appropriate measures to protect them from Leishmania infections. Also, the observation of a lack of statistical association between being LST positive and Never using ITN or use of ITN sometimes, when compared with those who used ITN often, suggests outdoor infection.
The factors associated with LST positivity in this study should therefore be taken into consideration in the development of future measure aimed at reducing exposure to Leishmania infection in the study area.
Conclusions
This study has demonstrated exposure to Leishmania infection in three communities of the Oti region of Ghana using the LST. Being male, living in Keri and being five years or older were associated with an increase in the odds of exposure to Leishmania infection using LST. Future efforts aimed at reducing exposure to Leishmania infection in the study area should take the associated factors into consideration.
Limitations of the study
The leishmanin skin test only indicates exposure to Leishmania and does not differentiate between recent and past exposures. A follow up study involving either culture of the parasite from a wound or molecular detection with sequencing will help to better characterize the infecting strains of Leishmania parasite. The use of questionnaire for this study may introduce some bias such as recall bias on the part of respondents. Furthermore, inclusion of a household in the study depended on the consent of the household head. This may have led to the exclusion of a few households, given that 587 households were included out of 600 households invited.
Supporting information
(DOCX)
(DOCX)
Acknowledgments
The authors wish to thank the Department of Neglected Tropical Diseases of the World Health Organization for supplying the Leishmanin skin test reagents used in this study. We also thank.
Prof. Richard Adanu, Dr. Phyllis Darko-Gyekye, and all the administrators of the WHO/TDR PhD Fellowship for their roles in Coordinating the program.
The assistance of field workers from the Nkwanta South and North District Health directorates is also appreciated. The authors are also grateful for the support from Mr. Emmanuel Agbodogli of the Nkwanta South District hospital and Dr. Laud Boateng, the Nkwanta South District Director during the field component of this study.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This project was funded by the post graduate training scheme fellowship in implementation science by the Special Programme for Research and Training in Tropical Diseases (WHO/TDR) at the School of Public Health, University of Ghana. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Alvar J, Velez DI, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS Negl Trop Dis. 2012;7(5):1–12. 10.1371/journal.pone.0035671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steverding D. The history of leishmaniasis. Parasites {&} Vectors [Internet]. 2017. February;10(1):82. Available from: 10.1186/s13071-017-2028-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Vries HJC, Reedijk SH, Schallig HDFH. Cutaneous Leishmaniasis: Recent Developments in Diagnosis and Management. Am J Clin Dermatol. 2015;16(2):99–109. 10.1007/s40257-015-0114-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maroli M, Feliciangeli MD, Bichaud L, Charrel RN, Gradoni L. Phlebotomine sandflies and the spreading of leishmaniases and other diseases of public health concern. Med Vet Entomol. 2013;27(2):123–47. 10.1111/j.1365-2915.2012.01034.x [DOI] [PubMed] [Google Scholar]
- 5.Maia C, Depaquit J. Can Sergentomyia [Diptera, Psychodidae] play a role in the transmission of mammal-infecting Leishmania? Parasite [Internet]. 2016;23:55. Available from: http://www.parasite-journal.org/articles/parasite/full_html/2016/01/parasite160086/parasite160086.html. 10.1051/parasite/2016062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ready PD. Biology of phlebotomine sand flies as vectors of disease agents. Annu Rev Entomol. 2013;58:227–50. 10.1146/annurev-ento-120811-153557 [DOI] [PubMed] [Google Scholar]
- 7.Masmoudi A, Hariz W, Marrekchi S, Amouri M, Turki H. Old World cutaneous leishmaniasis: Diagnosis and treatment. J Dermatol Case Rep. 2013;7(2):31–41. 10.3315/jdcr.2013.1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Savoia D. Recent updates and perspectives on leishmaniasis. J Infect Dev Ctries. 2015;9(6):588–96. 10.3855/jidc.6833 [DOI] [PubMed] [Google Scholar]
- 9.Kweku MA, Odoom S, Puplampu N, Desewu K, Nuako GK, Gyan B, et al. An outbreak of suspected cutaneous leishmaniasis in Ghana: lessons learnt and preparation for future outbreaks. Glob Health Action. 2011;4:1–9. 10.3402/gha.v4i0.5527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fryauff DJ, Hanafi HA, Klena JD, Hoel DF, Appawu M, Rogers W, et al. Short report: ITS-1 DNA sequence confirmation of Leishmania major as a cause of cutaneous leishmaniasis from an outbreak focus in the Ho District, southeastern Ghana. Am J Trop Med Hyg. 2006;75(3):502–4. [PubMed] [Google Scholar]
- 11.Villinski JT, Klena JD, Abbassy M, Hoel DF, Puplampu N, Mechta S, et al. Evidence for a new species of Leishmania associated with a focal disease outbreak in Ghana. Diagn Microbiol Infect Dis. 2008;60(3):323–7. 10.1016/j.diagmicrobio.2007.09.013 [DOI] [PubMed] [Google Scholar]
- 12.Kwakye-Nuako G, Mosore MT, Duplessis C, Bates MD, Puplampu N, Mensah-Attipoe I, et al. First isolation of a new species of Leishmania responsible for human cutaneous leishmaniasis in Ghana and classification in the Leishmania enriettii complex. Int J Parasitol [Internet]. 2015;45(11):679–84. Available from: 10.1016/j.ijpara.2015.05.001 [DOI] [PubMed] [Google Scholar]
- 13.Nzelu CO, Kato H, Puplampu N, Desewu K, Odoom S, Wilson MD, et al. First Detection of Leishmania tropica DNA and Trypanosoma Species in Sergentomyia Sand Flies [Diptera: Psychodidae] from an Outbreak Area of Cutaneous Leishmaniasis in Ghana. PLoS Negl Trop Dis. 2014;(23580424):1–10. 10.1371/journal.pntd.0002630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antonio L de F, Fagundes A, Oliveira RVC, Pinto PG, Bedoya-Pacheco SJ, Vasconcellos Ér de CF e, et al. Montenegro skin test and age of skin lesion as predictors of treatment failure in cutaneous leishmaniasis. Rev Inst Med Trop Sao Paulo. 2014;56(5):375–80. 10.1590/s0036-46652014000500002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krolewiecki AJ, Almazan MC, Quipildor M, Juarez M, Gil JF, Espinosa M, et al. Reappraisal of Leishmanin Skin Test [LST] in the management of American Cutaneous Leishmaniasis: A retrospective analysis from a reference center in Argentina. PLoS Negl Trop Dis. 2017;11(10):1–11. 10.1371/journal.pntd.0005980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.WHO. Control of the leishmaniases: WHO TRS N°949. Report of a meeting of the WHO Expert Committee on the. 2010. [Google Scholar]
- 17.Alimohammadian H, Hakimi H, Nikseresht M. The preparation and evaluation of reference leishmanin from Leishmania Major for use in man for diagnostic and experimental purposes. Med J Islam Repub Iran. 1991;10(2):149–52. [Google Scholar]
- 18.Manzur A, Bari A ul. Sensitivity of leishmanin skin test in patients of acute cutaneous leishmaniasis. Dermatol Online J. 2006. May;12(4):2. [PubMed] [Google Scholar]
- 19.Ghana Statistical Service [GSS]. 2010 POPULATION & HOUSING CENSUS; DISTRICT ANALYTICAL REPORT: Nkwanta south district. 2014. [Google Scholar]
- 20.Ghana Statistical Service. 2010 POPULATION & HOUSING CENSUS; DISTRICT ANALYTICAL REPORT: Nkwanta north district. 2014. [Google Scholar]
- 21.Ranasinghe S, Wickremasinghe R, Bandara S, Sivanantharajah S, Athauda I, Seneviratne K, et al. Cross-Sectional Study to Assess Risk Factors for Leishmaniasis in an Endemic Region in Sri Lanka. Am J Trop Med Hyg [Internet]. 2013;89(4):742–9. Available from: http://www.ajtmh.org/content/journals/10.4269/ajtmh.12-0640. 10.4269/ajtmh.12-0640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghana Statistical Service. Ghana Demographic and Health Survey. Stud Fam Plann. 2014;21(1):1–5. [Google Scholar]
- 23.Demographic and Health Surveys Methodology. Sampling and Household Listing Manual. Demographic and Health Surveys Methodology. 2012. [Google Scholar]
- 24.Oliveira F, Doumbia S, Anderson JM, Faye O, Diarra SS, Traoré P, et al. Discrepant prevalence and incidence of Leishmania infection between two neighboring villages in Central Mali based on leishmanin skin test surveys. PLoS Negl Trop Dis. 2009;3(12). 10.1371/journal.pntd.0000565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kone AK, Sa D, Thera MA, Kayentao K, Djimde A, Delaunay P, et al. Epidemiology of the outbreak, vectors and reservoirs of cutaneous leishmaniasis in Mali: A systematic review and meta-analysis. Asian Pac J Trop Med. 2016;9(10):985–90. 10.1016/j.apjtm.2016.07.025 [DOI] [PubMed] [Google Scholar]
- 26.Traore B, Oliveira F, Faye O, Dicko A, Coulibaly A, Sissoko IM, et al. Prevalence of Cutaneous Leishmaniasis in Districts of High and Low Endemicity in Mali. PLoS Negl Trop Dis. 2016;10(11):1–12. 10.1371/journal.pntd.0005141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bettaieb J, Toumi A, Chlif S, Chelghaf B, Boukthir A, Gharbi A, et al. Prevalence and determinants of Leishmania major infection in emerging and old foci in Tunisia. Parasit Vectors. 2014;7(386):1–8. 10.1186/1756-3305-7-386 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.