Abstract
Background
While time in targeted blood glucose range (TIR) 70–140 mg/dL is a known factor associated with mortality in critically ill patients, it remains unclear whether TIR is associated with 28-day mortality under the glycemic control with a less tight target glucose range of 70–180 mg/dL. We aimed to examine whether TIR 70–180 mg/dL was associated with 28-day mortality.
Methods
This is a retrospective cohort study using data from a tertiary care center in Japan collected from January 2016 through October 2019. We included adult patients (aged ≥20 years) admitted to the ICU. We excluded patients 1) with diabetic ketoacidosis patients, 2) discharged within 48 hours, 3) with repeated ICU admissions. We calculated TIR 70–180 mg/dL using the measured blood glucose values (≥3 times per day). The primary outcome was 28-day mortality. We examined the association between TIR and 28-day mortality using a logistic regression and Cox proportional hazard models with a stratification by glycosylated hemoglobin (HbA1c) level of 6.5%. Additionally, we repeated the analyses using the TIR category to assess the optimal TIR. For the sensitivity analysis, we repeated the primary analysis using TIR during the first three days from ICU admission.
Results
Of 1,230 patients, the median age was 72 years, 65% were male, and 250 patients (20%) had HbA1c ≥6.5% on admission. In patients with HbA1c <6.5%, TIR <80% was associated with an increased risk of 28-day mortality, with an adjusted odds ratio (OR) of 1.88 (95%CI: 1.36–2.61). Likewise, when using 10% incremental TIR as a categorical variable, lower TIR was associated with a worse 28-day mortality compared with TIR ≥90% (e.g., adjusted OR of TIR <60%, 3.62 [95%CI 2.36–5.53]). Similar associations were found in the analyses using Cox proportional hazards model and using TIR during the first three days. By contrast, in patients with HbA1c ≥6.5%, there was no consistent association of TIR with 28-day mortality.
Conclusions
We found that lower TIR 70–180 mg/dL was associated with a higher 28-day mortality in critically ill patients with HbA1c <6.5%, whereas there was no consistent association in patients with HbA1c ≥6.5%.
Introduction
Glycemic control for critically ill patients remains an important issue related to critical care. Although intensive insulin therapy had been recommended [1, 2], a large randomized controlled trial, "NICE-SUGAR study" demonstrated higher mortality in the intensive insulin therapy group (i.e., interventional arm) [3]. Therefore, the current recommendations for glycemic control management in critically ill patients have been changed from "intensive insulin therapy" to "manage with less tight control with the limit of <180 mg/dL." [4–6].
Recently, the importance of time in targeted blood glucose range (TIR) has been emphasized as a prognostic factor for critically ill patients [7–11]. In previous studies, TIR 70–140 mg/dL <80% was associated with a higher mortality in nondiabetic critically ill patients with HbA1c ≤6.5% [9]. However, current national guidelines recommend the more liberal upper target glucose level of 180 mg/dL [12]. Indeed, the Surviving Sepsis Campaign Guidelines suggest the upper limit of the glucose level at ≤180 mg/dL [6]. Furthermore, a systematic review of 36 randomized controlled trials and the American Diabetes Association also recommend a target glucose range of 140–180 mg/dL for most critically ill patients (grade A) [13]. Despite the clinical consensus on the liberal upper target glucose level of 180 mg/dL, little is known about the association between glycemic control with TIR 70–180 mg/dL and prognosis of critically ill patients. Additionally, it remains unclear whether the optimal TIR for critically ill patients should be maintained at a target upper glucose level of ≤180 mg/dL.
To address the knowledge gap in the literature, we examined the association of TIR 70–180 mg/dL with 28-day mortality among patients admitted to the intensive care unit (ICU). We hypothesized that lower TIR would be associated with a higher risk of 28-day mortality in patients admitted to the ICU.
Materials and methods
Study design and setting
This retrospective cohort study used data from the Hitachi General Hospital from 1 January 2016 through 31 October 2019. Hitachi General Hospital, a tertiary care center in Japan, serves an area with approximately three million residents. Annual emergency department visits are about 27,000 encounters. The Hitachi General Hospital has 18-beds ICU and 6-beds cardiac care unit. Of the 18 ICU beds, eight beds have a 2:1 patient-nurse ratio. The remaining ten beds have a 4:1 patient-nurse ratio. The ICU physicians manage all critically ill patients (both intrinsic [e.g., respiratory failure, heart failure, sepsis, stroke, gastrointestinal perforation] and extrinsic [e.g., suffocation, toxicity, burn, trauma] conditions except for patients after cardiovascular surgery). The ICU physicians input clinical information into the ICU database, which automatically registers medical charts as structured data. The study protocol was approved by the Ethics Committee of Hitachi General Hospital (2017–95). The need for informed consent was waived based on the study’s retrospective design.
Study participants
We included all adult ICU patients (aged ≥20 years). According to the previous literature, we excluded patients with diabetic ketoacidosis or hyperosmolar hyperglycemic syndrome [9]. We also excluded patients with early ICU discharge (<48 hours) because of no need for glycemic control or early death regardless of glycemic control. In addition, when a patient had multiple ICU admissions, we included only the first ICU admission and excluded all subsequent ICU admissions.
Data collection
We extracted patients’ physical information (age, sex, and body mass index [BMI]), disease severity (acute physiology score and acute physiology and chronic health evaluation [APACHE Ⅱ] score), disease category, and comorbidity according to the Charlson Comorbidity Index. APACHE II scores were calculated based on the worst values of vital signs and blood tests (as well as age and comorbidities) occurring during the first 24 hours of ICU admission. Using the measured blood glucose values, we calculated the mean blood glucose, glycemic variability, time above range over 220 mg/dL, time below range under 60 or 40 mg/dL, and TIR 70–180 mg/dL. We also calculated the coefficient of variation as the glycemic variability, which is defined as the standard deviation of blood glucose divided by the mean blood glucose. In addition, we extracted 28-day mortality, the length of ICU and hospital stay, and patient disposition. All of these data were extracted from electronic health records by using information system produced by TXP Medical Co., Ltd., which is linked to an electronic medical record system [14]. In addition, we have also reviewed medical records manually to confirm the extracted information.
Glycemic control in the ICU
In the ICU of Hitachi General Hospital, staff members routinely collect blood gas every eight hours (three times per day) from patients with arterial line insertions. All blood glucose was measured using a blood gas analyzer (ABL90 FLEX, Radiometer, CPH, DK). Insulin was usually administered as a continuous infusion at a 1 U/mL concentration using a syringe pump with a target range of 70–180 mg/dL. The standard protocol of glycemic control in the ICU is shown in S1 Fig. Briefly, 1) we start continuous intravenous (IV) insulin when blood glucose is >180 mg/dL (for patients receiving continuous nutrition); 2) discontinue IV insulin if blood glucose falls <100 mg/dL; and 3) IV dextrose if blood glucose falls ≤70 mg/dL.
Exposure and outcome
The primary exposure was TIR 70–180 mg/dL. The TIR was defined as the amount of time that blood glucose was between 70 and 180 mg/dL divided by the total ICU stay. The primary outcome was 28-day mortality.
Statistical analysis
Summary statistics were used to describe the characteristics of the study participants as appropriate. Missing height and weight data occurred in 23.2% of these patients, but there were no missing data or marked outliers in other variables including blood glucose data.
According to the previous literature, analyses were stratified by HbA1c ≥6.5% (not the diagnosed diabetes) [9]. We examined the association between TIR ≥80% and 28-day mortality using two multivariable models: 1) logistic regression and 2) Cox proportional hazards models. In addition, we also examined the association between TIR and mortality using a logistic regression model in patients with HbA1c <6.5%, stratifying by the presence of diagnosed diabetes. We adjusted for age, sex, APACHE Ⅱ score, Charlson comorbidity index, and the primary diagnosis category (sepsis, cerebrovascular diseases, cardiac diseases, respiratory diseases, gastrointestinal diseases, trauma, postoperative, and others). Kaplan-Meier survival curves were graphed for these populations.
In the sensitivity analyses, first, we repeated the analyses using TIR as a 1) category with 10% incremental of TIR (<60%, 60%-69%, 70%-79%, 80%-89%, and ≥90%) and as a 2) category based on quartiles of patient distribution (<53%, 53%-80%, 81%-93%, and ≥94%; S2 Fig). Second, to assess the linear association of TIR with the outcome, we repeated the analyses using TIR (10% decremental) as a continuous variable. Third, we repeated the primary analysis using TIR during the first three days from the ICU admission, assuming that survived patients were more likely to have stable TIRs. In addition, to test the hypothesis that survived patients were more likely to have stable TIRs, we depicted the association of ICU length-of-stay with the median TIR and examined the correlation using Spearman’s rank test.
Furthermore, to clarify the association between the quality of glycemic control achieved as a result and mortality, we assessed the association between TIR 70–140 mg/dL and mortality using a logistic regression model in patients with HbA1c <6.5% managed with a target glycemic range of 70–180 mg/dL.
A two-sided p-value of <0.05 was considered statistically significant. Statistical analyses were conducted using R version 4.0.2. (The R Foundation for Statistical Computing).
Results
Patient characteristics
From 1 January 2016 through 31 October 2019, 2,288 patients were admitted to the ICU. Of these, we excluded 48 patients under the age of 20 years, 43 patients with diabetic ketoacidosis and hyperosmolar hyperglycemic syndrome, 893 patients those who were discharged from the ICU within 48 hours (808 were good recovery and 85 died), and 74 multiple ICU admissions. The remaining 1,230 patients were eligible for the analysis (S3 Fig). Their median age was 76 years; 65% were male, with median BMI of 22.1 (Table 1). The median HbA1c on admission was 5.9%; 250 patients (20.2%) had HbA1c ≥6.5%. No significant differences were found in age, BMI, comorbidity, or severity (e.g., APACHE Ⅱ score) between patients with HbA1c <6.5% and those with HbA1c ≥6.5%. Patients with HbA1c ≥6.5% had significantly higher mean blood glucose levels and glycemic variability than those with HbA1c <6.5%. Median TIR was 85.7% for patients with HbA1c <6.5% and 41.8% for patients with HbA1c ≥6.5% (p <0.001). Patients with HbA1c ≥6.5% had higher 28-day mortality than those with <6.5% (32.0% vs. 22.9%; p = 0.004).
Table 1. Patient characteristics, glycemic control metrics, and outcomes, according to HbA1c levels.
| All patients | HbA1c <6.5% | HbA1c ≥6.5% | P value | |||
|---|---|---|---|---|---|---|
| (n = 1,230) | (n = 980) | (n = 250) | ||||
| Patient characteristics | ||||||
| Age, years | 76 [64, 82] | 75 [64, 82] | 76 [64, 82] | 0.49 | ||
| Male sex | 797 (64.8) | 618 (63.1) | 179 (71.6) | 0.01 | ||
| Body mass index | 22.1 [20, 23] | 22.1 [20, 23] | 22.1 [21, 24] | 0.08 | ||
| Acute physiology score | 13 [9, 18] | 13 [9, 18] | 13 [9, 19] | 0.26 | ||
| APACHE Ⅱ score | 18 [13, 23] | 18 [14, 23] | 18.5 [13, 24] | 0.32 | ||
| Charlson comorbidity index score | 3 [2, 5] | 3.5 [2, 5] | 3 [2, 5] | 0.13 | ||
| HbA1c, % | 5.9 [5.4, 6.4] | 5.7 [5.3, 6.0] | 7.2 [6.8, 8.5] | <0.001 | ||
| Diagnosed Diabetes | 376 (30.6) | 209 (21.3) | 167 (66.8) | <0.001 | ||
| Diagnosis | 0.01 | |||||
| Sepsis | 310 (25.2) | 233 (23.8) | 77 (30.8) | |||
| Cerebrovascular diseases | 122 (9.9) | 102 (10.4) | 20 (8.0) | |||
| Cardiac diseases | 96 (7.8) | 71 (7.2) | 25 (10) | |||
| Cardiac arrest | 126 (10.2) | 91 (9.3) | 35 (14) | |||
| Respiratory diseases | 173 (14.1) | 147 (15) | 26 (10.4) | |||
| Gastrointestinal diseases | 58 (4.7) | 47 (4.8) | 11 (4.4) | |||
| Trauma | 63 (5.1) | 49 (5.0) | 14 (5.6) | |||
| Postoperative | 46 (3.7) | 39 (4.0) | 7 (2.8) | |||
| Others | 236 (19.2) | 201 (20.5) | 35 (14) | |||
| Glycemic control metrics | ||||||
| The number of BG tests per day | 3 [3, 3] | 3 [3, 3] | 3 [3, 3] | 0.89 | ||
| Mean blood glucose, mg/dL | 155 [136, 181] | 149 [134, 170] | 199 [161, 225] | <0.001 | ||
| Coefficient of variation, % | 31.2 [20, 50] | 28.6 [19, 43] | 52.7 [30, 79] | <0.001 | ||
| Time above 220 mg/dL, % | 0 [0, 20] | 0 [0, 11] | 31.3 [4, 48] | <0.001 | ||
| Hypoglycemia <70 mg/dL | 116 (9.4) | 82 (8.4) | 34 (13.6) | 0.02 | ||
| Hypoglycemia <40 mg/dL | 54 (4.4) | 35 (3.6) | 19 (7.6) | 0.009 | ||
| Time in range 70–180 mg/dL, % | 80.7 [53, 93] | 85.7 [65, 95] | 41.8 [27, 76] | <0.001 | ||
| Time in range 70–180 mg/dL ≥80% | 644 (52.4) | 586 (59.8) | 58 (23.2) | <0.001 | ||
| Outcomes | ||||||
| ICU length-of-stay, days | 6 [4, 10] | 6 [4, 10] | 6 [4, 10] | 0.48 | ||
| Hospital length-of-stay, days | 20 [11, 41] | 20.5 [11, 41] | 18.5 [10, 38] | 0.49 | ||
| 28-day mortality | 304 (24.7) | 224 (22.9) | 80 (32.0) | 0.004 | ||
Data are presented as median (interquartile range) or number (percentage). The P values are not adjusted for multiple comparisons.
APACHE, Acute Physiology and Chronic Health Evaluation; BG, Blood glucose; HbA1c, glycosylated hemoglobin; ICU, Intensive care unit.
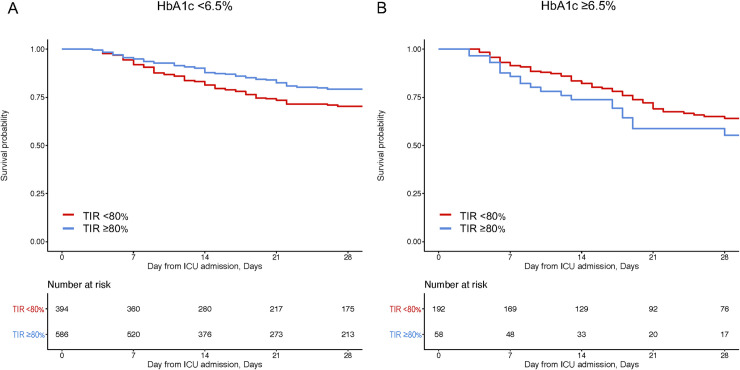
Association of TIR 70–180 mg/dL ≥80% with 28-day mortality
Compared with TIR ≥80%, TIR <80% was associated with the worse 28-day mortality in patients with HbA1c <6.5% (unadjusted odds ratio [OR], 2.23 [95%CI 1.65–3.01]; Table 2 and Fig 1). This association remained significant in the multivariate logistic regression model with a similar effect size (adjusted OR, 2.21 [95%CI 1.60–3.05]). By contrast, in patients with HbA1c ≥6.5%, there was no significant association between TIR ≥80% and 28-day mortality in either unadjusted or adjusted logistic regression models. These associations of TIR with 28-day mortality were consistent with results obtained using Cox proportional hazards models (S1 Table). In patients with HbA1c <6.5%, TIR <80% was significantly associated with worse 28-day mortality than TIR ≥80%, regardless of the presence of diagnosed diabetes (S4 Fig). This association remained significant in the multivariable models (S2 Table).
Table 2. Associations between time in range 70–180 mg/dL and 28-day mortality using logistic regression models.
| HbA1c <6.5% | HbA1c ≥6.5% | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Time in range | Mortality / n | Mortality rate | Unadjusted OR | Adjusted OR* | Mortality / n | Mortality rate | Unadjusted OR | Adjusted OR* | |
| (95%CI) | (95%CI) | (95%CI) | (95%CI) | ||||||
| Time in range (threshold at 80%) | |||||||||
| <80% | 124 / 394 | 32% | 2.23 (1.65–3.01) | 2.21 (1.60–3.05) | 59 / 192 | 31% | 0.78 (0.42–1.45) | 0.64 (0.30–1.37) | |
| ≥80% | 100 / 586 | 17% | 1 (reference) | 1 (reference) | 21 / 58 | 36% | 1 (reference) | 1 (reference) | |
| Time in range (10% incremental category) | |||||||||
| <60% | 78 / 205 | 38% | 3.76 (2.53–5.58) | 3.62 (2.36–5.53) | 56 / 160 | 35% | 1.77 (0.71–4.38) | 1.19 (0.40–3.53) | |
| 60%-69% | 24 / 86 | 28% | 2.40 (1.39–4.16) | 2.39 (1.33–4.28) | 1 / 13 | 8% | 0.27 (0.03–2.49) | 0.13 (0.01–1.42) | |
| 70%-79% | 22 / 103 | 21% | 1.67 (0.96–2.88) | 1.63 (0.92–2.91) | 2 / 19 | 11% | 0.39 (0.07–2.10) | 0.14 (0.02–0.97) | |
| 80%-89% | 43 / 178 | 24% | 1.96 (1.26–3.06) | 1.83 (1.14–2.93) | 14 / 28 | 50% | 3.29 (1.07–10.1) | 1.81 (0.46–7.06) | |
| ≥90% | 57 / 408 | 14% | 1 (reference) | 1 (reference) | 7 / 30 | 23% | 1 (reference) | 1 (reference) | |
| Time in range (10% decremental) | 1.21 (1.14–1.29) | 1.20 (1.12–1.28) | 1.02 (0.94–1.12) | 1.08 (0.96–1.21) | |||||
| Time in range (quartile category) | |||||||||
| Q1 (<53%) | 60 / 156 | 39% | 4.07 (2.55–6.48) | 3.73 (2.26–6.14) | 53 / 151 | 35% | 1.71 (0.64–4.55) | 1.21 (0.36–4.05) | |
| Q2 (53%-80%) | 70 / 260 | 27% | 2.42 (1.57–3.74) | 2.28 (1.44–3.61) | 6 / 44 | 14% | 0.50 (0.14–1.76) | 0.17 (0.04–0.76) | |
| Q3 (81%-93%) | 55 / 268 | 21% | 1.71 (1.09–2.67) | 1.57 (0.98–2.53) | 15 / 30 | 50% | 3.17 (0.99–10.1) | 2.06 (0.49–8.75) | |
| Q4 (≥94%) | 39 / 296 | 13% | 1 (reference) | 1 (reference) | 6 / 25 | 24% | 1 (reference) | 1 (reference) | |
*Logistic regression adjusted for age, sex, Charlson comorbidity index, APACHE Ⅱ score, and primary diagnosis category (sepsis, cerebrovascular diseases, cardiac diseases, cardiac arrest, respiratory diseases, gastrointestinal diseases, trauma, postoperative, and others).
APACHE, Acute Physiology and Chronic Health Evaluation; HbA1c, glycosylated hemoglobin; OR, odds ratio; CI, confidence interval.
Fig 1. Association of time in range 70–180 mg/dL with 28-day survival according to HbA1c.
Kaplan-Meier curves for patients with and without TIR <80%; TIR <80% (red) and TIR ≥80% (blue), in patients with HbA1c <6.5% (A), patients with HbA1c ≥6.5% (B). TIR, Time in range; HbA1c, glycosylated hemoglobin.
Association of TIR 70–180 mg/dL category with 28-day mortality
10% incremental category
When using 10% incremental TIR as a categorical variable (<60%, 60%-69%, 70%-79%, 80%-89%, and ≥90%), the lower TIR was associated with the worse 28-day mortality compared to TIR ≥90% in patients with HbA1c <6.5% in both unadjusted and adjusted models (e.g., adjusted OR of TIR <60%, 3.62 [95%CI 2.36–5.53]; Table 2). In addition, there was a linear association between 10% TIR decrement and the increased risk of 28-day mortality (adjusted OR per 10% decrement, 1.20 [95%CI 1.12–1.28]). By contrast, no clear association was found between TIR and mortality in patients with HbA1c ≥6.5% in either unadjusted or adjusted logistic regression models. These relations of TIR with 28-day mortality were consistent with results obtained using Cox proportional hazards models (S1 Table and S5 Fig).
Quartile category
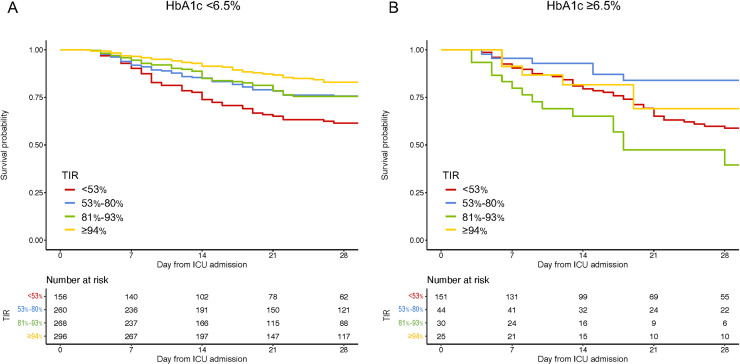
When using TIR as a categorical variable based on a quartile of patients (<53%, 53%-80%, 81%-93%, and ≥94%), the association between the TIR category and 28-day mortality was similar to those using 10% incremental TIR as a categorical variable. For example, TIR <53% was associated with worse 28-day mortality compared to TIR ≥94% (adjusted OR 3.73 [95%CI 2.26–6.14]; Table 2 and Fig 2).
Fig 2. Association of time in range 70–180 mg/dL as a categorical variable (quartile) with 28-day survival according to HbA1c.
Kaplan-Meier curves for patients with each quartile category of TIR; <53% (red), 53%-80% (blue), 81%-93% (green), and ≥94% (yellow), in patients with HbA1c <6.5% (A), patients with HbA1c ≥6.5% (B). TIR, Time in range; HbA1c, glycosylated hemoglobin.
Sensitivity analyses using TIR 70–180 mg/dL of the first three days
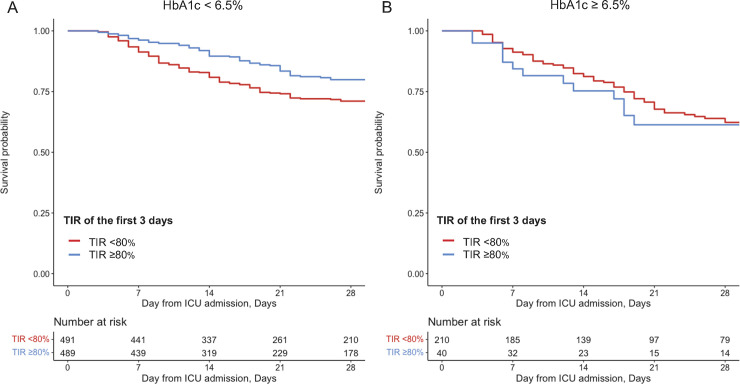
In the analysis using TIR during the first three days from the ICU admission, the overall associations were consistent with those found from the primary analyses (Fig 3). For example, TIR <80% was associated with the worse 28-day mortality in patients with HbA1c <6.5% (e.g., adjusted OR of TIR <80%, 1.88 [95%CI 1.36–2.61]: Table 3). Similarly, the lower TIR was associated with the worse 28-day mortality in patients with HbA1c <6.5% (e.g., adjusted OR of TIR <60%, 2.02 [95%CI 1.33–3.06]; Table 3). In addition, as shown in the S6 Fig, patients with longer ICU length-of-stay were likely to have higher TIR (Spearman’s rank correlation coefficient of 0.55, p = 0.0019).
Fig 3. Association of time in range 70–180 mg/dL of the first three days with 28-day survival according to HbA1c.
Kaplan-Meier curves for patients with and without TIR <80%; TIR <80% (red) and TIR ≥80% (blue), in patients with HbA1c <6.5% (A), patients with HbA1c ≥6.5% (B). TIR, Time in range; HbA1c, glycosylated hemoglobin.
Table 3. Results of sensitivity analyses using time in range during the first three days.
| HbA1c <6.5% | HbA1c ≥6.5% | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Time in range | Mortality / n | Mortality rate | Unadjusted OR | Adjusted OR* | Mortality / n | Mortality rate | Unadjusted OR | Adjusted OR* | |
| Time in range (threshold at 80%) | |||||||||
| <80% | 144 / 491 | 29% | 2.12 (1.56–2.89) | 1.88 (1.36–2.61) | 67 / 210 | 32% | 0.97 (0.47–2.00) | 0.56 (0.23–1.37) | |
| ≥80% | 80 / 489 | 16% | 1 (reference) | 1 (reference) | 13 / 40 | 33% | 1 (reference) | 1 (reference) | |
| Time in range (10% incremental category) | |||||||||
| <60% | 84 / 261 | 32% | 2.32 (1.57–3.42) | 2.02 (1.33–3.06) | 56 / 171 | 33% | 1.32 (0.52–3.33) | 0.65 (0.21–1.97) | |
| 60%-69% | 20 / 92 | 22% | 1.36 (0.77–2.41) | 1.16 (0.63–2.14) | 4 / 15 | 27% | 0.99 (0.23–4.15) | 0.25 (0.05–1.37) | |
| 70%-79% | 40 / 138 | 29% | 2.00 (1.25–3.19) | 1.90 (1.15–3.13) | 7 / 24 | 29% | 1.12 (0.32–3.84) | 0.37 (0.08–1.68) | |
| 80%-89% | 25 / 165 | 15% | 0.87 (0.52–1.46) | 0.88 (0.51–1.51) | 6 / 14 | 43% | 2.04 (0.52–8.00) | 1.09 (0.20–6.07) | |
| ≥90% | 55 / 324 | 17% | 1 (reference) | 1 (reference) | 7 / 26 | 27% | 1 (reference) | 1 (reference) | |
| Time in range (10% decremental) | 1.11 (1.05–1.16) | 1.09 (1.03–1.15) | 1.01 (0.93–1.10) | 1.01 (0.91–1.12) | |||||
| Time in range (quartile category) | |||||||||
| Q1 (<53%) | 61 / 187 | 33% | 2.28 (1.42–3.65) | 2.07 (1.25–3.43) | 50 / 153 | 33% | 1.34 (0.54–3.66) | 0.66 (0.21–2.05) | |
| Q2 (53%-80%) | 83 / 304 | 27% | 1.91 (1.27–2.89) | 1.61 (1.04–2.50) | 17 / 57 | 30% | 1.19 (0.44–3.50) | 0.52 (0.15–1.76) | |
| Q3 (81%-93%) | 25 / 165 | 15% | 1.34 (0.90–1.99) | 1.52 (0.86–2.01) | 6 / 14 | 43% | 1.41 (0.48–4.39) | 0.56 (0.15–2.10) | |
| Q4 (≥94%) | 55 / 324 | 17% | 1 (reference) | 1 (reference) | 7 / 26 | 27% | 1 (reference) | 1 (reference) | |
*Logistic regression adjusted for age, sex, Charlson comorbidity index, APACHE Ⅱ score, and primary diagnosis category (sepsis, cerebrovascular diseases, cardiac diseases, cardiac arrest, respiratory diseases, gastrointestinal diseases, trauma, postoperative, and others).
APACHE, Acute Physiology and Chronic Health Evaluation; HbA1c, glycosylated hemoglobin; OR, odds ratio; CI, confidence interval.
Association of TIR 70–140 mg/dL with 28-day mortality in patients with HbA1c <6.5%
Compared with TIR 70–140 mg/dL ≥80%, TIR 70–140 mg/dL <80% was associated with the worse 28-day mortality in patients with HbA1c <6.5% (unadjusted OR, 3.54 [95%CI 2.10–6.39]; S3 Table). This association remained significant in the multivariate logistic regression model with a similar effect size (adjusted OR, 3.02 [95%CI 1.75–5.56]; S3 Table). When using 10% incremental TIR 70–140 mg/dL as a categorical variable (<60%, 60%-69%, 70%-79%, 80%-89%, and ≥90%), the lower TIR was associated with the worse 28-day mortality compared to TIR ≥90% in both unadjusted and adjusted models (e.g., adjusted OR of TIR <60%, 2.72 [95%CI 1.18–7.40]; S3 Table). Also, when using TIR as a categorical variable based on a quartile of patients (<20%, 20%-43%, 44%-64%, and ≥65%), the association between the TIR category and 28-day mortality was similar to those using 10% incremental TIR as a categorical variable.
Discussion
This study of 1,230 ICU patients found that TIR 70–180 mg/dL was associated with 28-day mortality in critically ill patients with HbA1c <6.5%, although no apparent association was found in those with HbA1c ≥6.5%. While the causal relationship cannot be determined, based on the analyses conducted using several cut-off values, the optimal range of TIR may be >90%, or >80% is also reasonable for patients with HbA1c <6.5%. Furthermore, similar findings obtained using TIR during the first three days support the use of TIR as a prognostic marker for critically ill patients.
The observed relation of higher TIR with a lower mortality in critically ill patients was consistent with those of earlier studies [7, 9–11]. Krinsley et al. examined mortality between the high-TIR and low-TIR groups and found significantly lower mortality in the high-TIR group than in the low-TIR group among non-diabetic patients (8.5% vs. 15.8%, p<0.001), although no difference was found in mortality between groups among diabetic patients (16.1% vs. 14.4%, p = 0.60) [7]. Similarly, Lanspa et al. found that the relation between TIR >80% and mortality was likely to depend on HbA1c rather than on whether the patient had diabetes mellitus [9]. In fact, in this study, we also analyzed patients with HbA1c<6.5% stratified by the presence of diagnosed diabetes, and found that TIR>80% was significantly associated with mortality, regardless of the diagnosis of diabetes (S2 Table and S4 Fig).
Earlier studies of critically ill patients have described an association between TIR and mortality with a target range of 70–140 mg/dL for blood glucose [7, 9]. Whereas these earlier studies provided concrete evidence on the glucose management for ICU patients, the current national guidelines recommend the more liberal upper target glucose level of 180 mg/dL [13]. We found that TIR 70–140 mg/dL <80% was significantly associated with mortality, and in patients with HbA1c <6.5%, 28-day mortality in patients with TIR 70–140 mg/dL ≥80% was lower than those in patients with TIR 70–180 mg/dL ≥80% (9% vs. 17%, Table 2 and S3 Table). The direct comparison may be difficult because the target range of glycemic control was 70–180 mg/dL in our hospital (not 70–140 mg/dL). However, when assessing the association between glycemic control and mortality, from a physiological standpoint, the resulting level of glycemia achieved may be more important than how we set the glycemic target range [10].
Moreover, few studies have addressed survival bias, by which surviving patients were more likely to have stable TIR [15]. Although we hypothesized that survived patients were more likely to have stable TIR, this survivorship bias might be affected by the glucose management protocol and the definition of TIR. Indeed, a previous study reported that diabetic patients (who are more likely to be out-of-range) were more likely to have longer time on insulin therapy compared to non-diabetic patients [16]. Nevertheless, the observed correlation between the ICU length-of-stay and the median TIR (S6 Fig) and the results using TIR during the first three days should support our inference.
Critically ill patients can easily become hyperglycemic because of various factors [17, 18]. Glucose is a pro-inflammatory mediator with mechanisms such as increasing plasma interleukin-8 levels [19], increasing nuclear factor kappa-light-chain-enhancer of activated B cells [20], and increasing plasma matrix metalloproteinase (MMP) -2 and MMP-9 levels [21]. Although hyperglycemia rarely causes death directly, prolonged hyperglycemia can contribute to morbid conditions such as sepsis and post-intensive care syndrome by causing a toxic cellular environment and impaired immune function [22]. By contrast, hypoglycemia is an iatrogenic complication that should be avoided. The NICE-SUGAR Study investigators showed that moderate hypoglycemia (40–70 mg/dL) and severe hypoglycemia (<40 mg/dL) were both independent risk factors of death (moderate: HR 1.41 [95%CI 1.21–1.62], severe: HR 2.10 [95%CI 1.59–2.77]) [23]. Hypoglycemia presents an independent risk of death, as well as risks of convulsions and arrhythmias [24, 25]. The management of glycemic control to avoid hyperglycemia and hypoglycemia, as clinicians have attempted for many decades, is nothing less than maintaining high TIR management. Therefore, the finding that high TIR was associated significantly with low mortality was clinically plausible.
Importantly, the association between TIR 70–180 mg/dL and mortality was found only in patients with HbA1c <6.5% on admission: not in those with ≥6.5%. Several studies have revealed similar associations [7, 9]. Indeed, unlike patients with diabetes mellitus, TIR 70–140 mg/dL >80% was not associated with mortality in critically ill patients without diabetes mellitus [7]. Possible explanations include that patients with HbA1c ≥6.5% on admission were more likely to have been chronically hyperglycemic. Chronic hyperglycemia may attenuate the extent of cellular toxicity in acute hyperglycemia by downregulating the glucose transporter (GLUT) 1 and GLUT 4 transporters [26]. In addition, patients with a high HbA1c on admission may be less likely to be harmed by a low TIR because they are relatively less harmed by exposure to hyperglycemia [27].
Potential limitations
There are several potential limitations in this study. First, unmeasured factors related to assessment of the association of TIR with outcomes may exist because of the nature of retrospective design. Second, this study calculated TIR using glucose measured using a blood gas analyzer every eight hours. Compared with previous studies, our insulin protocol requires fewer blood glucose measurements per day (three times per day). Because glycemic variability and control measures depend on the frequency of measurements, the lower frequency might result in wider tolerances, looser control, and possibly more unmeasured glycemic variation [7, 9]. Third, there might be a time-varying confounding and exposure-covariate feedback. That is, the lower TIR does not solely depend on only glucose management but also patient condition at each time. Fourth, of the total 18 ICU beds in our hospital, 10 contain beds with a 4:1 patient-nurse ratio, which may affect the ICU’s ability to provide safe and effective blood glucose control. Fifth, because this study was conducted at a single tertiary care institution in Japan, and the patients included may not reflect the Japanese population. Thus, the generalizability of the findings may be limited. Yet, these findings were consistent with previous studies [7, 13] and the observed association may not be substantially varied across hospitals.
Conclusions
We found that lower TIR 70–180 mg/dL was associated with a higher 28-day mortality in critically ill patients with HbA1c <6.5 mg/dl, whereas there was no consistent association in patients with HbA1c ≥6.5%. Further validation studies are needed, but for glycemic control at a range of 70–180 mg/dL, TIR ≥90%, at least ≥80%, may be the target for critically ill patients with HbA1c <6.5%.
Supporting information
BG, blood glucose; ICU, intensive care unit.
(TIFF)
The quartiles of TIR 70–180 mg/dL were as follow, lower quartile, 53.2%; median, 80.7%; upper quartile, 93.3%. TIR, Time in range.
(TIFF)
ICU, intensive care unit; DKA, diabetic ketoacidosis; HHS, hyperosmolar hyperglycemic syndrome.
(TIFF)
Kaplan-Meier curves for patients with and without TIR <80%; TIR <80% (red) and TIR ≥80% (blue), in patients with HbA1c <6.5% and non-DM (A), patients with HbA1c <6.5% and DM (B). HbA1c, glycosylated hemoglobin; DM, diabetes mellitus; TIR, time in range.
(TIFF)
Kaplan-Meier curves for patients with each 10% incremental category of TIR; <60% (red), 60%-69% (blue), 70%-79% (green), 80%-89% (yellow), and ≥90% (gray), in patients with HbA1c <6.5% (A), patients with HbA1c ≥6.5% (B). TIR, Time in range; HbA1c, glycosylated hemoglobin.
(TIFF)
There was a weak correlation between the ICU length-of-stay and median TIR 70–180 mg/dL (Spearman’s rank correlation coefficient of 0.55, p = 0.0019). TIR, time in range; ICU, intensive care unit.
(TIFF)
*Cox proportional hazards model adjusted for age, sex, Charlson comorbidity index, APACHE Ⅱ score, and primary diagnosis category (sepsis, cerebrovascular diseases, cardiac diseases, cardiac arrest, respiratory diseases, gastrointestinal diseases, trauma, postoperative, and others). APACHE, Acute Physiology and Chronic Health Evaluation; HbA1c, glycosylated hemoglobin; HR, hazard ratio.
(DOCX)
*Logistic regression adjusted for age, sex, Charlson comorbidity index, APACHE Ⅱ score, and primary diagnosis category (sepsis, cerebrovascular diseases, cardiac diseases, cardiac arrest, respiratory diseases, gastrointestinal diseases, trauma, postoperative, and others). APACHE, Acute Physiology and Chronic Health Evaluation; HbA1c, glycosylated hemoglobin; OR, odds ratio; CI, confidence interval.
(DOCX)
*Logistic regression adjusted for age, sex, Charlson comorbidity index, APACHE Ⅱ score, and primary diagnosis category (sepsis, cerebrovascular diseases, cardiac diseases, cardiac arrest, respiratory diseases, gastrointestinal diseases, trauma, postoperative, and others). APACHE, Acute Physiology and Chronic Health Evaluation; HbA1c, glycosylated hemoglobin; CI, confidence interval.
(DOCX)
Acknowledgments
We were very grateful to Mr. Sato Shuntaro (biostatistician, The University of Nagasaki) for his helpful discussions and comments on the manuscript regarding statistical methods and presentation of our study.
Data Availability
The datasets are not publicly available due to no Institutional Review Board’s approval of data sharing. Data are available from the Institutional Review Board of Hitachi General Hospital (contact via swebkanrisha.nichibyo.gd@hitachi.com) for researchers who meet the criteria for access to confidential data.
Funding Statement
The funder, TXP Medical, was not involved in the study design, data analysis, publication decisions, or manuscript preparation and provided support only in the form of authors' salaries and research materials. This study was not conducted in partnership with a commercial organization.
References
- 1.van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al.: Intensive insulin therapy in critically ill patients. N Engl J Med 2001, 345(19):1359–1367. 10.1056/NEJMoa011300 [DOI] [PubMed] [Google Scholar]
- 2.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, et al.: Intensive insulin therapy in the medical ICU. N Engl J Med 2006, 354(5):449–461. 10.1056/NEJMoa052521 [DOI] [PubMed] [Google Scholar]
- 3.Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, et al. : Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009, 360(13):1283–1297. 10.1056/NEJMoa0810625 [DOI] [PubMed] [Google Scholar]
- 4.Standards of medical care in diabetes—2014. Diabetes Care 2014, 37 Suppl 1:S14–80. [DOI] [PubMed] [Google Scholar]
- 5.Qaseem A, Chou R, Humphrey LL, Shekelle P: Inpatient glycemic control: best practice advice from the Clinical Guidelines Committee of the American College of Physicians. Am J Med Qual 2014, 29(2):95–98. 10.1177/1062860613489339 [DOI] [PubMed] [Google Scholar]
- 6.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. : Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 2017, 43(3):304–377. 10.1007/s00134-017-4683-6 [DOI] [PubMed] [Google Scholar]
- 7.Krinsley JS, Preiser JC: Time in blood glucose range 70 to 140 mg/dl >80% is strongly associated with increased survival in non-diabetic critically ill adults. Crit Care 2015, 19:179. 10.1186/s13054-015-0908-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Omar AS, Salama A, Allam M, Elgohary Y, Mohammed S, Tuli AK, et al.: Association of time in blood glucose range with outcomes following cardiac surgery. BMC Anesthesiol 2015, 15:14. 10.1186/1471-2253-15-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lanspa MJ, Krinsley JS, Hersh AM, Wilson EL, Holmen JR, Orme JF, et al.: Percentage of Time in Range 70 to 139 mg/dL Is Associated With Reduced Mortality Among Critically Ill Patients Receiving IV Insulin Infusion. Chest 2019, 156(5):878–886. 10.1016/j.chest.2019.05.016 [DOI] [PubMed] [Google Scholar]
- 10.Signal M, Le Compte A, Shaw GM, Chase JG: Glycemic levels in critically ill patients: are normoglycemia and low variability associated with improved outocomes? J Diabetes Sci Technol 2012, 6(5):1030–7. 10.1177/193229681200600506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Penning S, Chase JG, Preiser JG, Pretty CG, Signal M, Melot C, et al.: Dose the achievement of an intermediate glycemic target reduce organ failure and mortality? A post hoc analysis of the Glucontrol trial. J Crit Care 2014, 29(3):374–9 10.1016/j.jcrc.2014.01.013 [DOI] [PubMed] [Google Scholar]
- 12.Jacobi J, Bircher N, Krinsley J, Agus M, Braithwaite SS, Deutschman C, et al. : Guidelines for the use of an insulin infusion for the management of hyperglycemia in critically ill patients. Crit Care Med 2012, 40(12):3251–3276. 10.1097/CCM.0b013e3182653269 [DOI] [PubMed] [Google Scholar]
- 13.Association AD: 13. Diabetes Care in the Hospital. Diabetes Care 2016, 39 Suppl 1:S99–104. [DOI] [PubMed] [Google Scholar]
- 14.Goto T, Hara K, Hashimoto K, Soeno S, Shirakawa T, Sonoo T, et al.: Validation of chief complaints, medical history, medications, and physician diagnoses structured with an integrated emergency department information system in Japan: the Next Stage ER system. Acute Med Surg 2020, 7(1):e554. 10.1002/ams2.554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sechterberger MK, Luijf YM, Devries JH: Poor agreement of computerized calculators for mean amplitude of glycemic excursions. Diabetes Technol Ther 2014, 16(2):72–75. 10.1089/dia.2013.0138 [DOI] [PubMed] [Google Scholar]
- 16.Lanspa MJ, Hirshberg EL, Phillips GD, Holmen J, Stoddard G, Orme J: Moderate glucose control is associated with increased mortality compared with tight glucose control in critically ill patients without diabetes. Chest 2013, 143(5):1226–1234. 10.1378/chest.12-2072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCowen KC, Malhotra A, Bistrian BR: Stress-induced hyperglycemia. Crit Care Clin 2001, 17(1):107–124. 10.1016/s0749-0704(05)70154-8 [DOI] [PubMed] [Google Scholar]
- 18.Dungan KM, Braithwaite SS, Preiser JC: Stress hyperglycaemia. Lancet 2009, 373(9677):1798–1807. 10.1016/S0140-6736(09)60553-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chettab K, Zibara K, Belaiba SR, McGregor JL: Acute hyperglycaemia induces changes in the transcription levels of 4 major genes in human endothelial cells: macroarrays-based expression analysis. Thromb Haemost 2002, 87(1):141–148. [PubMed] [Google Scholar]
- 20.Yorek MA, Dunlap JA: Effect of increased concentration of D-glucose or L-fucose on monocyte adhesion to endothelial cell monolayers and activation of nuclear factor-kappaB. Metabolism 2002, 51(2):225–234. 10.1053/meta.2002.29958 [DOI] [PubMed] [Google Scholar]
- 21.Aljada A, Ghanim H, Mohanty P, Syed T, Bandyopadhyay A, Dandona P: Glucose intake induces an increase in activator protein 1 and early growth response 1 binding activities, in the expression of tissue factor and matrix metalloproteinase in mononuclear cells, and in plasma tissue factor and matrix metalloproteinase concentrations. Am J Clin Nutr 2004, 80(1):51–57. 10.1093/ajcn/80.1.51 [DOI] [PubMed] [Google Scholar]
- 22.Stevens RD, Dowdy DW, Michaels RK, Mendez-Tellez PA, Pronovost PJ, Needham DM: Neuromuscular dysfunction acquired in critical illness: a systematic review. Intensive Care Med 2007, 33(11):1876–1891. 10.1007/s00134-007-0772-2 [DOI] [PubMed] [Google Scholar]
- 23.Finfer S, Liu B, Chittock DR, Norton R, Myburgh JA, McArthur C, et al. : Hypoglycemia and risk of death in critically ill patients. N Engl J Med 2012, 367(12):1108–1118. 10.1056/NEJMoa1204942 [DOI] [PubMed] [Google Scholar]
- 24.Preiser JC, Devos P, Ruiz-Santana S, Melot C, Annane D, Groeneveld J, et al. : A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med 2009, 35(10):1738–1748. 10.1007/s00134-009-1585-2 [DOI] [PubMed] [Google Scholar]
- 25.Hermanides J, Bosman RJ, Vriesendorp TM, Dotsch R, Rosendaal FR, Zandstra DF, et al.: Hypoglycemia is associated with intensive care unit mortality. Crit Care Med 2010, 38(6):1430–1434. 10.1097/CCM.0b013e3181de562c [DOI] [PubMed] [Google Scholar]
- 26.Klip A, Tsakiridis T, Marette A, Ortiz PA: Regulation of expression of glucose transporters by glucose: a review of studies in vivo and in cell cultures. Faseb j 1994, 8(1):43–53. 10.1096/fasebj.8.1.8299889 [DOI] [PubMed] [Google Scholar]
- 27.Krinsley JS, Fisher M: The diabetes paradox: diabetes is not independently associated with mortality in critically ill patients. Hosp Pract (1995) 2012, 40(2):31–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
BG, blood glucose; ICU, intensive care unit.
(TIFF)
The quartiles of TIR 70–180 mg/dL were as follow, lower quartile, 53.2%; median, 80.7%; upper quartile, 93.3%. TIR, Time in range.
(TIFF)
ICU, intensive care unit; DKA, diabetic ketoacidosis; HHS, hyperosmolar hyperglycemic syndrome.
(TIFF)
Kaplan-Meier curves for patients with and without TIR <80%; TIR <80% (red) and TIR ≥80% (blue), in patients with HbA1c <6.5% and non-DM (A), patients with HbA1c <6.5% and DM (B). HbA1c, glycosylated hemoglobin; DM, diabetes mellitus; TIR, time in range.
(TIFF)
Kaplan-Meier curves for patients with each 10% incremental category of TIR; <60% (red), 60%-69% (blue), 70%-79% (green), 80%-89% (yellow), and ≥90% (gray), in patients with HbA1c <6.5% (A), patients with HbA1c ≥6.5% (B). TIR, Time in range; HbA1c, glycosylated hemoglobin.
(TIFF)
There was a weak correlation between the ICU length-of-stay and median TIR 70–180 mg/dL (Spearman’s rank correlation coefficient of 0.55, p = 0.0019). TIR, time in range; ICU, intensive care unit.
(TIFF)
*Cox proportional hazards model adjusted for age, sex, Charlson comorbidity index, APACHE Ⅱ score, and primary diagnosis category (sepsis, cerebrovascular diseases, cardiac diseases, cardiac arrest, respiratory diseases, gastrointestinal diseases, trauma, postoperative, and others). APACHE, Acute Physiology and Chronic Health Evaluation; HbA1c, glycosylated hemoglobin; HR, hazard ratio.
(DOCX)
*Logistic regression adjusted for age, sex, Charlson comorbidity index, APACHE Ⅱ score, and primary diagnosis category (sepsis, cerebrovascular diseases, cardiac diseases, cardiac arrest, respiratory diseases, gastrointestinal diseases, trauma, postoperative, and others). APACHE, Acute Physiology and Chronic Health Evaluation; HbA1c, glycosylated hemoglobin; OR, odds ratio; CI, confidence interval.
(DOCX)
*Logistic regression adjusted for age, sex, Charlson comorbidity index, APACHE Ⅱ score, and primary diagnosis category (sepsis, cerebrovascular diseases, cardiac diseases, cardiac arrest, respiratory diseases, gastrointestinal diseases, trauma, postoperative, and others). APACHE, Acute Physiology and Chronic Health Evaluation; HbA1c, glycosylated hemoglobin; CI, confidence interval.
(DOCX)
Data Availability Statement
The datasets are not publicly available due to no Institutional Review Board’s approval of data sharing. Data are available from the Institutional Review Board of Hitachi General Hospital (contact via swebkanrisha.nichibyo.gd@hitachi.com) for researchers who meet the criteria for access to confidential data.