Abstract
Pain is a common symptom in people with autosomal dominant polycystic kidney disease (ADPKD), but it is assessed and reported inconsistently in research, and the validity of the measures remain uncertain. The aim of this study was to identify the characteristics, content, and psychometric properties of measures for pain used in ADPKD. We conducted a systematic review including all trials and observational studies that reported pain in people with ADPKD. Items from all measures were categorized into content and measurement dimensions of pain. We assessed the general characteristics and psychometric properties of all measures. 118 studies, we identified 26 measures: 12 (46%) measures were developed for a non-ADPKD population, 1 (4%) for chronic kidney disease, 2 (8%) for polycystic liver disease and 11 (42%) specifically for ADPKD. Ten anatomical sites were included, with the lower back the most common (10 measures [39%]), four measurement dimensions (intensity (23 [88%]), frequency (3 [12%]), temporality (2 [8%]), and sensory (21 [81%]), two pain types, nociceptive including visceral (15 [58%]) and somatic (5 [20%]), and neuropathic (2 [8%]), and twelve impact dimensions, where the most frequent was work (5 [31%]). The validation data for the measures were variable and only the ADPKD Impact Scale reported all psychometric domains. The measures for pain in ADPKD varied in terms of content and length, and most had not been validated in ADPKD. A standardized psychometrically robust measure that captures patient-important dimensions of pain is needed to evaluate and manage this debilitating complication of ADPKD.
Introduction
Pain is a debilitating symptom that is experienced by more than 60% of people with autosomal dominant polycystic kidney disease (ADPKD) by the age of 40 years old [1]. The progressive growth of cysts in the kidneys and cyst complications including infection and rupture [2] can cause extreme acute or persistent chronic pain [3] if pain lasts for longer than 4–6 weeks [4]. ADPKD-related pain impacts on sleep quality and physical activity, impairs well-being and overall quality of life due to its recurrent nature and severity [5], necessitating regular analgesics in up to 30% of people with ADPKD [6, 7]. People with ADPKD report pain in a range of sites including the lower back (71%), abdomen (61%), head (49%), and chest (30%) [8], and the onset of pain can be sudden and unpredictable [9]. Cyst-related pain is often persistent and aggravated by standing and walking and the source of pain in ADPKD is often unable to be determined compared with other general pain [10].
The Standardized Outcomes in Nephrology-PKD (SONG-PKD) initiative identified pain as one of the four core outcomes in PKD [11], defined as outcomes of critical importance to all key stakeholders, including patients/caregivers, health professionals, policy makers/funders from recently completed consensus workshop. Pain is the only patient-reported outcome (PROM) in the core outcome set, which was highly prioritised due to its significant and adverse impact on daily and social activities [12]. Despite being identified as a critically important outcome [13], pain is often under recognized and poorly managed [13]. Although there are strategies available to manage pain, including non-pharmacologic treatments (management of diet and lifestyle), analgesics and surgery [14], people with ADPKD still report pain and there is no a systematic approach for the clinical assessment of pain in this setting [3].
Despite its critical importance and being highly prioritized by all stakeholders, pain was reported in only 16 (24%) of randomized trials involving people with ADPKD according to a recent systematic review [15]. Pain has been identified as a core outcome in ADPKD, which means it is to be measured and reported in all trials involving people with ADPKD, using a consistent, validated outcome measurement tool. The aim of this study was to identify the content, general characteristics, and validity of measures used to assess pain in people with ADPKD, to select a robust and feasible outcome measure to use in all clinical trials in this setting. The identification of a suitable measure to capture crucial aspects of pain will ensure an accurate assessment and better understanding of factors associated with pain, and identify interventions targeting to manage pain in people with ADPKD.
Materials and methods
Selection criteria
We searched for all study designs (interventional and non-interventional studies) that involved patients aged at least 18 years with ADPKD and included a patient-reported outcome measure (questionnaire) that assessed any type of pain. Global measures (e.g. a composite measure for health-related quality of life or health status that assessed multiple domains including pain) were included if they reported a pain-specific item, even if they were not designed for ADPKD population. However, only items related to pain were assessed. Studies published in peer-reviewed journals without language restrictions were included. Abstract-only citations were included only if we were able to extract sufficient information about the measure (characteristics and content) used to assess pain.
Study sources and measures
We conducted searches in MEDLINE, Embase, PsycINFO and the CKT register from database inception to February 2020. Google Scholar and reference lists of relevant studies and reviews were also searched. The search strategies are provided in S1 Table. Three authors (PN, EH, AT) independently screened all abstracts and excluded those not meeting the inclusion criteria, then assessed remaining full-text articles for eligibility. Any uncertainties and disagreements about the inclusion of articles were discussed until we reached consensus.
Data extraction and analysis
We extracted the following characteristics from each study included: publication year, country, study design, sample size, type of intervention (if applicable), measure used to assess pain and study duration. To summarize characteristics for the measures identified, PN and EH referred to the source study and key references to extract the following information: response format scale, number of items, recall period, cost of license to use the measure, completion time, language and number of studies that used the measure. PN performed a distinct search for validation studies for each measure and extracted psychometric data in people with ADPKD, using the Consensus-based Standards of health Measurement Measures-Core outcome measures in Effectiveness Trials (COSMIN-COMET) [16]. The data were independently cross-checked by authors EH and AB.
Dimensions of pain
To determine the dimensions assessed in the measures of pain, PN and EH initially extracted all items (questions) on pain, including items from the pain subscale of global measures. The range of items were sorted into dimensions inductively derived. The dimensions identified were the site of pain, measurement (e.g. frequency, intensity), type of pain, and impact of pain. The frequency of each dimension was recorded.
Assessment of psychometric properties
As recommended by COSMIN-COMET [16] guidelines, we examined the available evidence for validity and reliability of the identified measures by examining psychometric properties: content validity, structural validity, criterion validity, reliability including test-retest and internal consistency, measurement error, cross-cultural validity and responsiveness.
Results
Characteristics of studies
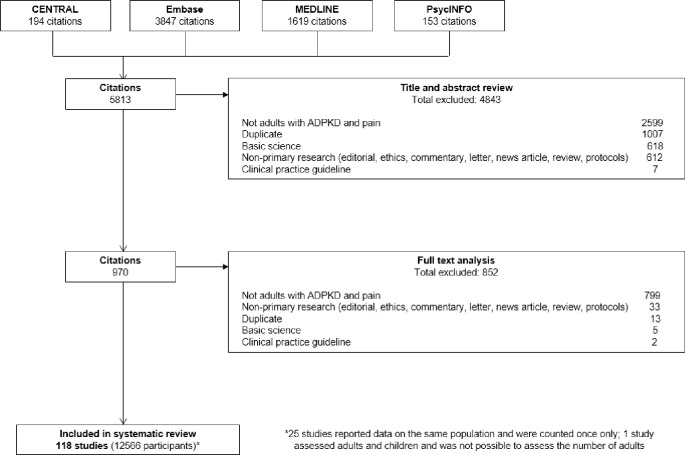
We identified 4806 potential relevant citations after removing the duplicates. We included 118 studies involving a total of 12,566 participants with ADPKD across 35 countries. Of the included studies, 36 (30%) were interventional studies and 82 (70%) were non-interventional studies. The search results are shown in Fig 1. The study characteristics and measures used to assess pain are provided in S2 and S3 Tables. The PRISMA Checklist is provided in S6 Table.
Fig 1. PRISMA flowchart.
Characteristics of the measures
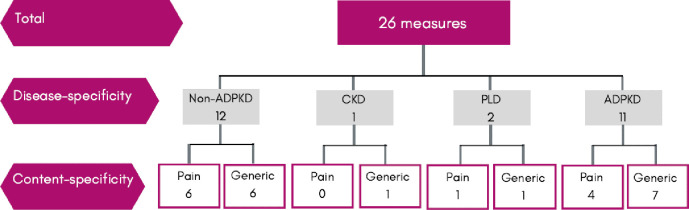
Across the 118 studies, there were 26 different patient-reported outcome measures used to assess pain. Of these, 16 (62%) were established measures and 10 (38%) measures were designed by the authors for use in their study only, without validation. Twelve (46%) measures were developed for a non-ADPKD population, one (4%) for all stages of chronic kidney disease, two (8%) for polycystic liver disease and 11 (42%) were developed for people with ADPKD (Fig 2). Eleven (42%) measures were developed specifically to assess general pain and 15 (58%) assessed broader outcomes such as quality of life and symptoms, in which pain was a subscale. Regarding measures that were not specifically designed to assess pain, number of items ranged from one (n = 4 measures [17–20]), two (n = 3 measures [21–23]), three (n = 5 measures [24–28]), four (n = 1 measure [29]), five (n = 1 measure [30]), and 10 items (n = 1 measure [31]).
Fig 2. Type of measures used to assess pain in patients with ADPKD.
Type of measures used to assess pain in patients with ADPKD. Abbreviations and definitions: non-ADPKD, measures developed for patients without autosomal dominant polycystic kidney disease; CKD, measures developed for patients with chronic kidney disease; PLD, measures developed for patients with polycystic liver disease; ADPKD, measures developed for patients with autosomal dominant polycystic kidney disease.
The 36-Item Short Form Health Survey (SF-36) was the most frequently used measure reported in 16 (33%) studies, followed by Visual Analog Scale (VAS) in 11 (22%) studies, EuroQol-5 Dimension Questionnaire (EQ-5D) in seven (14%) studies and Gastrointestinal questionnaire (GI-Q) in six (12%) studies. The time taken for completion of each measure ranged from two minutes to 30 minutes.
The number of items in the questionnaires varied from nine (Short-form Brief Pain Inventory (BPI-SF)) to 80 (Kidney Disease Quality of life—Short Form (KDQOL-SF)). The recall period ranged from the day of assessment to 10 years. Most of the measures (23, 88%) were free of charge for non-commercial use, some of which required study registration. Characteristics of measures and frequency of use are provided in Table 1.
Table 1. Characteristics of the measures.
| Measure | Response format scale | N of items | Recall period | Cost of license | Completion time (min) | Pain specific | CKD specific | PLD specific | ADPKD specific | Language | Frequency of use (number of studies) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Established measures | |||||||||||
| SF-36 [22] | Y/N, 3-/5-/6- point Likert | 36 | Various | Annual license fee a | 10–15 min | No | No | No | No | English, Italian, German, Japanese | 16 (33%) |
| VAS b | 0–10 numerical scale | - | Before/after treatment (up to 120 months) | Contact author | - | Yes | No | No | No | English | 11 (22%) |
| EQ-5D [20] | VAS | 16 | Various | Licensing fee based on quote | <5 min | No | No | No | No | English, Danish, Dutch, Swedish | 7 (14%) |
| GI-Q [27] | 7-point Likert | 11 | Various | Contact author | ~2–3 min | No | No | No | No | - | 6 (12%) |
| KDQOL-SF [29] | Y/N, 3-/4-/5-/6 point Likert | 80 | Various | Free | ~30 min | No | Yes | No | No | Italian | 2 (4%) |
| ADPKD-IS [24] | 5-point Likert | 18 | Various | Contact author | <5 min | No | No | No | Yes | English | 2 (4%) |
| PLD-Q [31] | Numerical scale | 13 | Before/after treatment | Contact author | <5 min | No | No | Yes | No | Dutch, English | 2 (4%) |
| EORTC QLQ-C30 [23] | 4-point Likert | 30 | Before/after treatment | License fee for non-academic users depends on number of patients | ~6 min | No | No | No | No | Dutch, English | 2 (4%) |
| BPI-SF [32]* | Y/N; 0–10 numerical scale | 9 | Before/after treatment | Contact author | ~2 min | Yes | No | No | No | English | 1 (2%) |
| MPQ-DV [33] | VAS | 17 | Before/after treatment (up to 12 months) | Contact author | 2–5 min | Yes | No | No | No | Dutch, | 1 (2%) |
| SF-MPQ-2 [34]* | VAS | 22 | Before/after treatment | Contact author | 2–5 min | Yes | No | No | No | English | 1 (2%) |
| SF-12 [18] | Y/N; 3-,5-,6-point Likert | 12 | Current | License fee upon request | ~2–3 min | No | No | No | No | English, Danish, Swedish | 1 (2%) |
| GSRS (Revised) [28] | 2-,4-point Likert | 11 | Current | Contact author | ~2–3 min | No | No | No | Yes | Korean | 1 (2%) |
| HAM-D [19] | 3-,5-poin Likert | 21 | Before/after treatment (up to 6 months) | Contact author | 15–20 min | No | No | No | No | - | 1 (2%) |
| Wisconsin BPS (Revised) [6] | 6-point Likert | - | Current | Contact author | - | Yes | No | No | Yes | English | 1 (2%) |
| GI-Q (Revised for PLD) [35] | 7-point Likert | 11 | Before/after treatment (up to 30 months) | Contact author | ~2–3 min | Yes | No | Yes | No | - | 1 (2%) |
| Author-developed measures | |||||||||||
| D’Agnolo 2016 [36]#!c | 1–10 numerical scale | - | Current | Contact author | - | Yes | No | No | Yes | - | 2 (4%) |
| D’Agnolo 2017 [37]!c | |||||||||||
| (ADPKD-related pain) | |||||||||||
| Torres 2012 [7]!d | 0–10 numerical scale | - | Before/after treatment (up to 36 months) | Contact author | - | Yes | No | No | Yes | Multiple languages | 2 (4%) |
| Torres 2011 [38]!d | |||||||||||
| (Kidney pain score) | |||||||||||
| Suwabe 2013 [21] | 4-, 5-point Likert | 12 | Current | Contact author | ~2–3 min | No | No | No | Yes | Japanese | 1 (2%) |
| Suwabe 2017 [25] | 4-, 5-point Likert | 15 | Before/after treatment (up to 12 months) | Contact author | <5 min | No | No | No | Yes | Japanese | 1 (2%) |
| Haseebuddin 2012 [39] | - | - | After treatment | Contact author | - | Yes | No | No | Yes | English | 1 (2%) |
| Iliuta 2019 [26] | 5-point Likert | - | Before/after treatment | Contact author | - | No | No | No | Yes | English | 1 (2%) |
| Sakuhara 2015 [17] | 0–10 numerical scale | - | Before/after treatment (up to 24 months) | Contact author | - | No | No | No | Yes | - | 1 (2%) |
| Taylor 2005 [30] | Y/N | - | Current | Contact author | - | No | No | No | Yes | English | 1 (2%) |
| Abraham 2015 [40] | Numerical scale | - | First 48 hours | Contact author | - | Yes | No | No | No | - | 1 (2%) |
| Walsh 2012 [41] | 0–10 numerical scale | - | - | Contact author | - | Yes | No | No | No | English | 1 (2%) |
(-) Not stated, unclear, or unable to ascertain
* The name of questionnaire was extracted from the protocol, since it was not clearly stated in the primary publication (only “Pain questionnaire” was reported)
# Abstract
! More than one studied referred to the same publication; Y/N: Questions requiring yes/no answers. Note: The language is referred to the language that was used in the studies rather than the available languages for the measures.
Abbreviations: KDQOL-SF: Kidney Disease Quality of life—Short Form; GI-Q: Gastrointestinal questionnaire; VAS: Visual Analog scale; SF-36: Medical Outcomes Study Form, 36 items health survey; ADPKD-IS: ADPKD Impact Scale; PLD-Q: Polycystic Liver Disease questionnaire; EQ-5D: European Quality of Life-5 Dimension Questionnaire that may include a VAS scale for pain; EORTC QLQ-C30: European Organization for Research and Treatment of Cancer quality of life questionnaire core-30; BPI-SF: Short-form Brief Pain Inventory; SF-MPQ: Short Form of the McGill Pain Questionnaire; MPQ-DV: McGill Pain Questionnaire (Dutch version); SF-12: Health Survey Short Form 12; GSRS: Gastrointestinal Symptom Rating Scale; HAM-D: The Hamilton Depression Rating Scale; Wisconsin BPS: Modified version of Wisconsin Brief Pain Survey.
a Upon registration for version 2, users can obtain a quote for the license fee that applies to their project; version 1 can be obtained for free
b VAS scale was clearly reported to assess pain in all studies except Qian 2015 [42]. VAS item with open-ended response questions vary
c This author-developed instrument was reported in two studies as ADPKD-related pain
d This author-developed instrument was reported in two studies as Kidney pain score.
Dimensions of pain
Pain-related items from the measures were classified into four content dimensions [43], which capture the site of pain (e.g. abdomen, lower back, thorax, head and face, generalized body and non-specified), measurement (intensity, frequency, temporality and sensory), type (nociceptive and neuropathic pain) and impact (life participation, sleep and mental).
The measures assessed 10 sites dimensions: abdomen including general abdomen (8 [31%] measures), upper abdomen (4 [15%]), lower abdomen (2 [8%]), flank (7 [27%]); lower back (10 [39%]); thorax including chest (1 [4%]) and rib cage (2 [8%]); head and face (3 [12%]); generalized body (8 [31%]); and non-specified pain (9 [35%]). The measures assessed four measurement dimensions: intensity (23 [88%]), frequency (3 [12%]), temporality (2 [8%]), and sensory (21 [81%]); and two dimensions for type: nociceptive including visceral (15 [58%]) and somatic (5 [20%]); and neuropathic (2 [8%]).
The measures assessed 12 impact dimensions that were reported only in the established measures: life participation including daily activity (4 [25%]), social activity (3 [19%]), work (5 [31%]), walking ability (2 [13%]), physical function (2 [13%]), and strenuous physical activity (1 [6%]), and one dimension for sleep (3 [19%]). The measures assessed mental impact including mood (2 [13%]), bother (2 [13%]), anxiety (1 [6%]), affective (2 [13%]), and enjoyment of life (2 [13%]). None of the author-developed measures assessed impact dimensions. The definitions of each dimension are given in Tables 2–4. Dimensions of pain assessed by measures are provided in S5 Table.
Table 2. Dimensions of pain assessed by measures: Site of pain.
| Measure | Site | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Abdomen | Lower back* | Thorax | Head and Face | Generalized body | Non-specified | |||||
| General | Upper abdomen | Lower abdomen | Flank | Chest | Rib cage (behind/below)^ | |||||
| Established measures | ||||||||||
| SF-36 [22]c | ● | |||||||||
| VAS d,f,g,h,i,j,k,s | ● | ● | ● | ● | ||||||
| EQ-5D [20]d | ● | |||||||||
| GI-Q [27]t | ● | ● | ● | |||||||
| KDQOL-SF [29]b,c | ● | ● | ||||||||
| ADPKD-IS [24]o | ● | |||||||||
|
PLD-Q [31]n |
● | ● | ● | ● | ||||||
| EORTC QLQ-C30 [23]d | ● | |||||||||
| BPI-SF [32]d | ● | ● | ||||||||
| MPQ-DV [33]r | ● | |||||||||
| SF-MPQ-2 [34]o | ● | |||||||||
| SF-12 [18] | ● | |||||||||
|
GSRS (Revised) [28]l,p |
● | ● | ● | ● | ||||||
| HAM-D [19]b | ● | ● | ● | |||||||
| Wisconsin BPS (Revised) [6] | ● | ● | ||||||||
| GI-Q (Revised for PLD) [35] | ● | ● | ||||||||
| Author-developed measures | ||||||||||
| D’Agnolo 2016 [36]#! | ● | ● | ● | ● | ● | ● | ||||
| D’Agnolo 2017 [37]!m,p | ||||||||||
| Torres 2012 [7]! | ● | ● | ● | |||||||
| Torres 2011 [38]!a | ||||||||||
| Suwabe 2013 [21]p | ● | |||||||||
| Suwabe 2017 [25]q | ● | |||||||||
| Haseebuddin 2012 [39] | ● | |||||||||
|
Iliuta 2019 [26] |
● | ● | ● | |||||||
|
Sakuhara 2015 [17] |
● | ● | ||||||||
| Taylor 2005 [30]e | ● | ● | ● | ● | ● | |||||
|
Abraham 2015 [40] |
● | |||||||||
| Walsh 2012 [41] | ● | |||||||||
* Lower back pain was attributed to kidney pain
^ Pain behind or below the rib cage was attributed to liver pain
# Abstract
! More than one studied referred to the same publication
● Data were available for the selected field.
a Measure reported kidney pain without other specific description. According to the definition reported in other studies, kidney pain was described as flank, back and abdominal pain
b Generalized pain included muscle aches
c Generalized pain included bodily pain
d Qian 2015 [42] non-specified if VAS was a “pain specific instrument”, and no any additional information were reported about pain
e Other pain was assessed as non-specified pain
f Chrispijn 2013 [27] used VAS scale used to assess pain to record abdominal pain
g Kucuk 2016 [44], Dunn 2000 [45], Lee 2003 [46], Lee 2004 [47] reported that patients had abdominal pain and flank pain. Although no further information were reported about VAS scale used to assess pain, we can assume that authors used this instrument to assess abdominal and flank pain
h Dunn 2001 [48] reported that patients had abdominal pain. Although no further information were reported about VAS scale used to assess pain, we can assume that authors used this instrument to assess abdominal pain
i Lipke 2007 [49], Yu 2018 [50] reported that patients had kidney pain. Although no further information were reported about VAS scale used to assess pain, we can assume that authors used this instrument to assess kidney pain
j Rehman 2001 [51] reported that patients had flank pain with or without back pain
k Sulikowski 2006 [52] reported that patients had back and abdominal pain. Although no further information were reported about VAS scale used to assess pain, we can assume that authors used this instrument to assess abdominal and back pain
l Upper abdomen pain included epigastric soreness and right upper abdomen quadrant pain
m Upper abdomen pain was reported as right upper abdomen pain, behind or below the rib cage, and was attributed to liver pain
n Rib cage pain was reported as pain or pressure in the rib cage
o Pain location was not reported
p Generalized pain included heartburn
q Generalized pain included bodily pain and heartburn
r Pain location was not reported
s VAS item with open-ended response questions vary
t Generalized pain included heartburn and epigastric pain.
Table 4. Dimensions of pain assessed by measures: Impact of pain*.
| Measure | Impact | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Life participation | Sleep | Mental | ||||||||||
| Daily activity | Social activity | Work$ | Walking ability | Physical function | Strenuous physical activity | Mood | Bother | Anxiety | Affective | Enjoyment of life | ||
| Established measures | ||||||||||||
| SF-36 [22] | ● | |||||||||||
| VAS £ | ||||||||||||
| EQ-5D [20] | ||||||||||||
| GI-Q [27] | ||||||||||||
| KDQOL-SF [29] | ● | |||||||||||
| ADPKD-IS [24]a | ● | ● | ● | ● | ||||||||
| PLD-Q [31] | ● | |||||||||||
| EORTC QLQ-C30 [23] | ● | |||||||||||
| BPI-SF [32]b | ● | ● | ● | ● | ● | ● | ● | |||||
| MPQ-DV [33]d | ● | |||||||||||
| SF-MPQ-2 [34]c | ● | |||||||||||
| SF-12 [18] | ● | |||||||||||
| GSRS (Revised) [28] | ● | |||||||||||
| HAM-D [19] | ● | |||||||||||
| Wisconsin BPS (Revised) [6]b | ● | ● | ● | ● | ● | ● | ● | ● | ● | |||
| GI-Q (Revised for PLD) [35] | ||||||||||||
* No author-developed measures reported impact as a dimension of pain
$ Work included both work outside the home and housework
£ VAS item with open-ended response questions vary
● Data were
available for the selected field. Note: Physical function included normal or mild exercises.
a Daily activity included the patient’s need to modify lifestyle
b Social activity included relations with others and hobbies
c Affective descriptors of pain included tiring-exhausting, sickening, fearful and punishing-cruel
d Affective descriptors of pain included tension, autonomic, fear, punishment.
Table 3. Dimensions of pain assessed by measures: Measurement and type of pain.
| Measure | Measurement | Type | |||||
|---|---|---|---|---|---|---|---|
| Intensity | Frequency | Temporality* | Sensory§ | Nociceptive | Neuropathic | ||
| Visceral^ | Somatic$ | ||||||
| Established measures | |||||||
|
SF-36 [22]s |
● | ● | |||||
| VAS s,ç, £ | ● | ● | ● | ||||
|
EQ-5D [20]s |
● | ● | |||||
| GI-Q [27]a,q | ● | ● | ● | ||||
|
KDQOL-SF
[29]b,s,t |
● | ● | ● | ● | |||
| ADPKD-IS [24] | ● | ||||||
| PLD-Q [31]c,r | ● | ● | ● | ● | |||
|
EORTC QLQ-C30 [23]s |
● | ● | |||||
| BPI-SF [32]d,u | ● | ● | ● | ||||
| MPQ-DV [33]¥ | ● | ● | |||||
| SF-MPQ-2 [34]e | ● | ● | ● | ||||
| SF-12 [18] | ● | ||||||
|
GSRS (Revised) [28]f,r |
● | ● | ● | ● | |||
|
HAM-D [19]g,r,t |
● | ● | ● | ● | |||
|
Wisconsin BPS (Revised) [6]h,r,z |
● | ● | ● | ● | ● | ||
|
GI-Q (Revised for PLD) [35]i,q |
● | ● | ● | ||||
| Author-developed measures | |||||||
| D’Agnolo 2016 [36]#! | ● | ● | |||||
| D’Agnolo 2017 [37]!j | |||||||
| Torres 2012 [7]! | ● | ● | |||||
| Torres 2011 [38]!k | |||||||
|
Suwabe 2013 [21]l,q,r |
● | ● | ● | ||||
|
Suwabe 2017 [25]m,q,r,s |
● | ● | ● | ||||
| Haseebuddin 2012 [39]s,v | ● | ● | |||||
| Iliuta 2019 [26]n,r | ● | ● | ● | ||||
| Sakuhara 2015 [17]o,r | ● | ● | ● | ||||
| Taylor 2005 [30]p | ● | ● | |||||
| Abraham 2015 [40]s | ● | ● | |||||
| Walsh 2012 [41]s | ● | ● | |||||
* Temporality included continuous, recurring, irregular, intermittent pain
§ Sensory included the sensation of pain as burning, sharp, intense, aching
^Visceral pain included injury/damage to internal organs (liver, kidney), abdomen, chest, epigastric soreness, heartburn
$ Somatic pain included injury/damage to muscle, rib cage, headaches, sprains, cramps
# Abstract
! More than one studied referred to the same publication
£ VAS item with open-ended response questions vary
● Data were available for the selected field.
a Visceral pain was defined as lower and upper abdominal pain and heartburn
b Visceral pain was defined as chest pain. Somatic pain was defined as cramps
c Visceral pain was defined as back, flank and abdominal pain. Somatic pain was defined as pain or pressure in rib cage
d Somatic pain was defined as headaches, sprains
e Sensory descriptors of pain included:1) continuous pain descriptors (throbbing, cramping, gnawing, aching, heavy and tender pain); 2) intermittent pain descriptors (shooting, stabbing, sharp, splitting, electric-shock and piercing pain); 3) predominantly neuropathic pain descriptors (hot-burning and cold-freezing pain, pain caused by light touch, itching, tingling or pins and needles, numbness
f Visceral pain was defined as right upper quadrant, back, flank pain and epigastric soreness
g Visceral pain was defined as back pain. Somatic pain was defined as muscle pain, headaches and cramps
h Visceral pain was defined as back and abdominal pain
i Visceral pain was defined as lower and upper abdominal pain and heartburn
j Visceral pain was defined as liver (defined as right upper quadrant pain, behind or below the rib cage) and kidney pain (defined as back, flank and abdominal pain)
k Visceral pain was defined as kidney pain
l Visceral pain was defined as heartburn
m Visceral pain was defined as heartburn
n Visceral pain was defined as back, flank and abdominal pain
o Visceral pain was defined as back and abdominal pain
p Visceral pain was defined as back, flank and abdominal pain. Somatic pain was defined headaches
q Heartburn was assessed as sensory pain
r Abdominal distension/fullness/ heavy feelings in abdomen were assessed as sensory pain
s Bodily/generalized pain was assessed as sensory pain
t Cramps and was assessed as sensory pain
u Sprains was assessed as sensory pain
v Pain intensity was reported as degree of subjective pain relief
z Neuropathic pain was reported as radicular pain
ç Rehman 2001 [51] reported intermittent pain
¥ Sensory descriptors of pain included temporal, spatial, punctate pressure, incisive pressure, constrictive pressure, traction pressure, thermal, dullness, stiffness, continuity.
Psychometric properties
The validity and reliability for each measure in people with ADPKD is shown in S4 Table. Of the 16 measures, only two (13%) were validated in the ADPKD population. A summary of the psychometric data for each of these measures is shown in Table 5. The validation data for the measures were variable and only one measure, the ADPKD Impact Scale (ADPKD-IS) [24], provided information across all psychometric domains. The Polycystic Liver Disease questionnaire (PLD-Q) [31] was also evaluated for psychometric properties in people with ADPKD.
Table 5. Development/validation data on psychometric properties.
| Measure/Psychometric proprerty | Validity | Reliability | ||||||
|---|---|---|---|---|---|---|---|---|
| Content | Construct | Criterion | Test-retest | Internal consistency | ||||
| Convergent | Discrimination | Predictive | Concurrent | |||||
| ADPKD-IS [24] | ● | ● | ● | ● | ● | ● | ||
| PLD-Q [31] | ● | ● | ● | ● | ● | ● | ||
Note: Validation studies were excluded if they were not available in full, were for a translation of the original measure or were not written in English
● Data were available for the selected field.
The ADPKD-IS was a 18 items measure created specifically for the ADPKD population, and the development of the measure included quantitative and qualitative studies, with the involvement of clinicians with expertise in ADPKD and people with ADPKD [24]. Confirmatory Factor Analysis supported a three-factor structure rather than the hypothesised two-factor structure, with the addition of four domain-independent items that were retained in the measure. Item discrimination for all items was adequate and each item was adequately correlated to its respective domain, supporting construct validity. Discriminant validity was supported through correlations between changes in the physical domain score and changes in pain intensity measured by pain ratings in the BPI-SF. As predicted, higher scores on all domains were associated with more advanced disease stage. Correlations between ADPKD-IS domains and summary scores on the Health Survey Short Form 12 (SF-12) indicated sufficient concurrent validity. The ADPKD-IS has high test-retest reliability and high internal consistency, both overall and within each domain [24].
The PLD-Q was not created for ADPKD patients without polycystic liver disease, however people with ADPKD were included in the validation study. The PLD-Q was designed based on qualitative and quantitative studies, including two validation studies. Factor analysis supported a unidimensional structure of the measure. As expected, ADPKD patients scored higher for pain than healthy controls but lower than patients with PLD, supporting discriminant validity [31]. Concurrent validity was supported by the high correlation between total PLD-Q score and the European Organization for Research and Treatment of Cancer quality of life questionnaire core-30 (EORTC QOL30) symptom burden score, as well as between the total PLD-Q and the VAS global health scale. Test-retest reliability and internal consistency were both high.
Discussion
The measures of pain differed in content, length, response format scale, number of items, recall period, cost of licence, completion time and available psychometric proprieties. Our analysis showed that only 119 studies in people with ADPKD measured pain, and of these 36 (30%) were randomized trials. Across these studies, 26 different measures were used, which demonstrates inconsistencies in how pain in ADPKD is assessed. Only 11 (42%) were developed for an ADPKD population, of which most (eight measures) were non-validated and developed de novo for the author’s study. More than half of the measures (58%) were not designed specifically for pain. Over one-third of measures (35%) had at least 15 items and four (15%) measures had an estimated completion time of more than five minutes. The sites of pain included lower back and non-specified region/s, and the measurement properties including intensity and types were reported in at least one-third of the measures. The impact of pain on life participation and mental wellbeing were assessed in 12 (75%) measures, and all in established measures only.
Evidence for psychometric validation of the measure for pain in the ADPKD was available for only two measures. Two measures [24, 31], including one that was designed for PLD, assessed psychometric robustness in ADPKD based on the COSMIN-COMET properties. One measure reported data on all psychometric domains in ADPKD. Both measures had more than 10 items and costs of licence were not reported, and they might not be feasible as a core outcome measure or applicable in low income countries. The suitability of other measures to evaluate pain in ADPKD remains unclear as content validity and internal consistency may not be transferable across different patient populations because of range of clinical conditions and treatments. Pain is infrequently evaluated as a separate construct in ADPKD, and often it is incorporated as an item of health-related quality of life. Our findings suggest that pain has been not adequately assessed and a standardized and psychometrically valid measure that addresses key dimensions of pain in ADPKD is needed. However, it is still unclear if a new ADPKD-specific measure may be more appropriate compared to the current measures.
The current measures show a very broad range of dimensions in terms of the site of pain, measurement properties, type and impact. Pain complaints are complex and some patients with ADPKD develop a so called chronic pain syndrome, including chronic pelvic pain syndrome or irritable bowel syndrome, that can exacerbate pain. Many of these measures do not reflect the dimensions of pain important to people with ADPKD, are burdensome to administer and complete, and lack evidence to support the psychometric properties. It remains unclear whether some of these measures were able to discriminate between kidney specific and not kidney specific pain, and central sensitization and mental status have not been adequatelly investigated. The pain subscale in the SF-36 questionnaire, which was the most commonly used measure, included only two questions and it is unclear if they assess the dimensions of critical importance to people with ADKPD, and the specific subscale has not been validated. Pain should be collected in “real-time” using tools such as mobile apps, especially because people with ADPKD have both a chronic level of pain and experience acute pain episodes which have to be adequately distinguished.
This review yields a comprehensive assessment of measures that have been used to evaluate pain in people with ADPKD. However, there are some potential limitations. We did not include study protocols and unpublished studies, so some measures of pain used in people with ADPKD may not have been captured and other measures could be potentially adequate to use in ADPKD setting (e.g. the Central Sensitization Inventory [53]). We searched for primary validation studies and did not conduct systematic search for the validation studies for each measure. We recognize that it would not be possible to find all relevant studies that reported validation data for all the measures, and there are only limited data on the psychometric properties because of the limited number of measures validated in ADPKD. We acknowledge that people with ADPKD experience frequently adaptation to recurrent acute and chronic pain and, despite the severity of symptoms, the real impact of pain could be underestimated in this setting.
To improve consistency in reporting patient-important outcomes such as pain, core outcome measures for pain have been established in other non-CKD patient populations. In rheumatology, as part of the Outcome Measures in Rheumatology (OMERACT) [54] initiative, focus groups and interviews were conducted with patients and clinical researchers to establish patient-relevant outcome domains and to identify a measure that captured all critical aspects to assess pain in patients with hip or knee osteoarthritis [55]. However, there were no optimal existing measures in osteoarthritis and a new measure was developed addressing intensity, frequency, impact on quality of life and sleep, and the extent of worry and frustration on pain recurrence, which were of importance to patients [56]. Several PROMIS pain measures have been established and demonstrated to be psychometrically robust in other chronic conditions, such as cancer [57]. Although these measures have not been validated in ADPKD and did not provide further dimensions, the PROMIS item bank for pain can be considered as a reference for the list of possible questions to evaluate in each dimension, considering intensity and life participation as essential dimensions also in our setting. The impact of pain in sexual intercourse and central sensitization have not been explored and may be included in the future research.
As part of the SONG-PKD initiative, pain was established as a core outcome based on a consensus among patients, caregivers and health professionals [12]. This systematic review is the first phase in identifying or developing a standardized, validated measure for pain, and provides detailed and comprehensive information about the domains to potentially address when measuring pain in people with ADPKD. Subsequent work will be based on the COMET-COSMIN framework [16] for establishing core patient-reported outcome measures and will involve an international consensus workshop with patients with ADPKD and health professionals, to discuss potential patient-reported outcome measures for pain to capture all of the relevant domains that are considered important to patients. The proposed measure will be piloted and validated in people with ADPKD, to assess if the measure is appropriate and reliable to evaluate pain in this setting. A standardized and validated measure to assess pain in people with ADPKD will improve consistency in the assessment and reporting of pain in research, and may lead to better pain management and patient outcomes.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOC)
Acknowledgments
We thank Tomasz Jurys, Rachel Beanland, Can Karaca, Ting Ting Wang, Kentaro Iwami Hokkaido, Natalie Sokolskaia, Ellena Fox, Alexandra Bodnaruc and Stijn Van De Velde for their support in the translations of foreign papers.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
AT 1106716 National Health and Medical Research Council (NHMRC) fellowship. YC 1126256 NHMRC Early Career Fellowship.
References
- 1.Bajwa ZH, Gupta S, Warfield CA, Steinman T. Pain management in polycystic kidney disease. Kidney Int. 2001;60(5):1631–44. 10.1046/j.1523-1755.2001.00985.x [DOI] [PubMed] [Google Scholar]
- 2.Kramers BJ, van Gastel MDA, Boertien WE, Meijer E, Gansevoort RT. Determinants of Urine Volume in ADPKD Patients Using the Vasopressin V2 Receptor Antagonist Tolvaptan. Am J Kidney Dis. 2019;73(3):354–62. 10.1053/j.ajkd.2018.09.016 . [DOI] [PubMed] [Google Scholar]
- 3.Tong A, Rangan G, Ruospo M, Saglimbene V, Strippoli GFM, Palmer SC, et al. A painful inheritance—patient perspectives on living with polycystic kidney disease: thematic synthesis of qualitative research. Nephrol Dial Transplant. 2015;30:790–800. 10.1093/ndt/gfv010 [DOI] [PubMed] [Google Scholar]
- 4.Casteleijn NF, Visser F, Drenth JP, Gevers TJ, Groen GJ, Hogan MC, et al. A stepwise approach for effective management of chronic pain in autosomal-dominant polycystic kidney disease. Nephrol Dial Transplant. 2014;29:iv142–iv53. 10.1093/ndt/gfu073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rizk D, Jurkovitz C, Veledar E, Bagby S, Baumgarten DA, Rahbari-Oskoui F, et al. Quality of life in autosomal dominant polycystic kidney disease patients not yet on dialysis. Clin J Am Soc Nephrol. 2009;4(3):560–6. 10.2215/CJN.02410508 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miskulin DC, Abebe KZ, Chapman AB, Perrone RD, Steinman TI, Torres VE, et al. Health-related quality of life in patients with autosomal dominant polycystic kidney disease and CKD stages 1–4: A cross-sectional study. Am J Kidney Dis. 2014;63(2):214–26. 10.1053/j.ajkd.2013.08.017 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, et al. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367(25):2407–18. 10.1056/NEJMoa1205511 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hogan MC, Masyuk TV, Page LJ, Kubly VJ, Bergstralh EJ, Li X, et al. Randomized clinical trial of long-acting somatostatin for autosomal dominant polycystic kidney and liver disease. J Am Soc Nephrol. 2010;21(6):1052–61. 10.1681/ASN.2009121291 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hogan MC, Norby S. Evaluation and Management of Pain in Autosomal Dominant Polycystic Kidney Disease. Adv Chronic Kidney Dis. 2010;17(3):e1–e16. 10.1053/j.ackd.2010.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chapman AB, Devuyst O, Eckardt KU, Gansevoort RT, Harris T, Horie S, et al. Autosomal-dominant polycystic kidney disease (ADPKD): executive summary from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int. 2015;88:17–27. 10.1038/ki.2015.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Standardized Outcomes in Nephrology-PKD (SONG-PKD) initiative. https://songinitiativeorg/projects/song-pkd/ [last access March 2021].
- 12.Cho Y, Sautenet B, Gutman T, Rangan G, Craig JC, Ong AC et al. Identifying patient-important outcomes in polycystic kidney disease: An international nominal group technique study. Nephrology. 2019:1214–24. 10.1111/nep.13566 [DOI] [PubMed] [Google Scholar]
- 13.Cho Y, Rangan G, Logeman C, et al. Core Outcome Domains for Trials in Autosomal Dominant Polycystic Kidney Disease: An International Delphi Survey. Am J Kidney Dis. 2020. [DOI] [PubMed] [Google Scholar]
- 14.Rangan GK, Alexander S, Campbell KL. KHA-CARI guideline recommendations for the diagnosis and management of autosomal dominant polycystic kidney disease. Nephrology. 2016;21:705–16. 10.1111/nep.12658 [DOI] [PubMed] [Google Scholar]
- 15.Sautenet B, Cho Y, Gutman T, Rangan G, Ong A, Chapman A, et al. Range and variability of outcomes reported in randomized trials conducted in polycystic kidney disease: a systematic review. Am J Kidney Dis. 2020. 10.1053/j.ajkd.2019.12.003 [DOI] [PubMed] [Google Scholar]
- 16.Prinsen CA, Vohra S, Rose MR, et al. How to select outcome measurement instruments for outcomes included in a “Core Outcome Set” ‐ a practical guideline. Trials. 2016;17(1):449. 10.1186/s13063-016-1555-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakuhara Y, Nishio S, Morita K, Abo D, Hasegawa Y, Yuasa N, et al. Transcatheter Arterial Embolization with Ethanol Injection in Symptomatic Patients with Enlarged Polycystic Kidneys. Radiology. 2015;277(1):277–85. 10.1148/radiol.2015141637 . [DOI] [PubMed] [Google Scholar]
- 18.Short Form—12 Health Survey. Available at https://wwwhoagorthopedicinstitutecom/documents/SF12formpdf [last access July 2020].
- 19.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry 1960;23:56–62. 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Euro Quality of Life—5 Dimensions Health Questionnaire. Available at https://euroqolorg/eq-5d-instruments/sample-demo/ [last access July 2020].
- 21.Suwabe T, Ubara Y, Mise K, Kawada M, Hamanoue S, Sumida K, et al. Quality of life of patients with ADPKD-Toranomon PKD QOL study: cross-sectional study. BMC Nephrol. 2013;14:179. 10.1186/1471-2369-14-179 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Short Form—36 Health Survey. Available at https://wwwrandorg/health-care/surveys_tools/mos/36-item-short-form/survey-instrumenthtml [last access July 2020].
- 23.European Organization for the Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30). Available at https://wwweortcorg/app/uploads/sites/2/2018/08/Specimen-QLQ-C30-Englishpdf [last access July 2020].
- 24.Oberdhan D, Cole J, Krasa HB, Cheng R, Czerwiec FS, Hays RD, et al. Development of the Autosomal Dominant Polycystic Kidney Disease Impact Scale: A New Health-Related Quality-of-Life Instrument. Am J Kidney Dis. 2018;71(2):225–35. 10.1053/j.ajkd.2017.08.020 [DOI] [PubMed] [Google Scholar]
- 25.Suwabe T, Ubara Y, Sekine A, Ueno T, Yamanouchi M, Hayami N, et al. Effect of renal transcatheter arterial embolization on quality of life in patients with autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2017;32(7):1176–83. 10.1093/ndt/gfx186 . [DOI] [PubMed] [Google Scholar]
- 26.Iliuta IA, Shi B, Pourafkari M, Akbari P, Bruni G, Hsiao R, et al. Foam Sclerotherapy for Cyst Volume Reduction in Autosomal Dominant Polycystic Kidney Disease: A Prospective Cohort Study. Kidney Medicine. 2019;1(6):366–75. 10.1016/j.xkme.2019.07.015 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chrispijn M, Gevers TJG, Hol JC, Monshouwer R, Dekker HM, Drenth JPH. Everolimus does not further reduce polycystic liver volume when added to long acting octreotide: Results from a randomized controlled trial. J Hepatol. 2013;59(1):153–9. 10.1016/j.jhep.2013.03.004 . [DOI] [PubMed] [Google Scholar]
- 28.Kim H, Park HC, Ryu H, Kim K, Kim HS, Oh KH, et al. Clinical Correlates of Mass Effect in Autosomal Dominant Polycystic Kidney Disease. PLoS ONE. 2015;10(12):e0144526. 10.1371/journal.pone.0144526 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kidney Disease and Quality of Life (KDQOL-SF). Available at https://wwwrandorg/content/dam/rand/www/external/health/surveys_tools/kdqol/kdqol13pdf [last access July 2020].
- 30.Taylor M, Johnson AM, Tison M, Fain P, Schrier RW. Earlier diagnosis of autosomal dominant polycystic kidney disease: importance of family history and implications for cardiovascular and renal complications. Am J Kidney Dis. 2005;46(3):415–23. 10.1053/j.ajkd.2005.05.029 . [DOI] [PubMed] [Google Scholar]
- 31.Neijenhuis MK, Gevers TJ, Hogan MC, Kamath PS, Wijnands TF, van den Ouweland RC, et al. Development and Validation of a Disease-Specific Questionnaire to Assess Patient-Reported Symptoms in Polycystic Liver Disease. Hepatology. 2016;64(1):151–60. 10.1002/hep.28545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brief Pain Inventory (Short Form). Available at http://wwwnpcrcorg/files/news/briefpain_shortpdf [last access July 2020].
- 33.Vanderiet K AH, Carton H, et al. The McGill Pain Questionnaire constructed for the Dutch language (MPQ-DV). Preliminary data concerning reliability and validity. Pain. 1987;30:395–408. 10.1016/0304-3959(87)90027-3 [DOI] [PubMed] [Google Scholar]
- 34.Dworkin RH, Turk D, Trudeau JJ, Benson C, Biondi DM, Katz NP, et al. Validation of the Short-Form McGill Pain Questionnaire-2 (SF-MPQ-2) in Acute Low Back Pain. J Pain. 2015;16(4):357–66 10.1016/j.jpain.2015.01.012 [DOI] [PubMed] [Google Scholar]
- 35.van Aerts RMM, Kievit W, D’Agnolo HMA, Blijdorp CJ, Casteleijn NF, Dekker SEI, et al. Lanreotide Reduces Liver Growth In Patients With Autosomal Dominant Polycystic Liver and Kidney Disease. Gastroenterology. 2019;157(2):481–91.e7. 10.1053/j.gastro.2019.04.018 . [DOI] [PubMed] [Google Scholar]
- 36.D’Agnolo HM, Kievit W, Takkenberg RB, Riano I, Bujanda L, Neijenhuis MK, et al. Ursodeoxycholic acid in advanced polycystic liver disease: A phase 2 multicenter randomized controlled trial. J Hepatol. 2016;65(3):601–7. 10.1016/j.jhep.2016.05.009 . [DOI] [PubMed] [Google Scholar]
- 37.D’Agnolo HMA, Casteleijn NF, Gevers TJG, de Fijter H, van Gastel MDA, Messchendorp AL, et al. The Association of Combined Total Kidney and Liver Volume with Pain and Gastrointestinal Symptoms in Patients with Later Stage Autosomal Dominant Polycystic Kidney Disease. Am J Nephrol. 2017;46(3):239–48. 10.1159/000479436 . [DOI] [PubMed] [Google Scholar]
- 38.Torres VE, Meijer E, Bae KT, Chapman AB, Devuyst O, Gansevoort RT, et al. Rationale and design of the TEMPO (Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and its Outcomes) 3–4 Study. Am J Kidney Dis. 2011;57(5):692–9. 10.1053/j.ajkd.2010.11.029 . [DOI] [PubMed] [Google Scholar]
- 39.Haseebuddin M, Tanagho YS, Millar M, Roytman T, Chen C, Clayman RV, et al. Long-term impact of laparoscopic cyst decortication on renal function, hypertension and pain control in patients with autosomal dominant polycystic kidney disease. J Urol. 2012;188(4):1239–44. 10.1016/j.juro.2012.06.026 . [DOI] [PubMed] [Google Scholar]
- 40.Abraham GP, Siddaiah AT, Das K, Ramaswami K, George DP, Thampan OS. Laparoscopic nephrectomy for autosomal dominant polycystic kidneys in patients with end-stage renal disease on maintenance hemodialysis: 10-year single surgeon experience from an Indian center. J Minim Access Surg. 2015;11(3):187–92. 10.4103/0972-9941.140217 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walsh N, Sarria JE. Management of chronic pain in a patient with autosomal dominant polycystic kidney disease by sequential celiac plexus blockade, radiofrequency ablation, and spinal cord stimulation. Am J Kidney Dis. 2012;59(6):858–61. 10.1053/j.ajkd.2011.12.018 . [DOI] [PubMed] [Google Scholar]
- 42.Qian X, Sheng X, Li R, Liu H, Kong X, Duan L, et al. Which Stage of ADPKD Is More Appropriate for Decortication? A Retrospective Study of 137 Patients from a Single Clinic. PLoS ONE. 2015;10(5):e0120696. 10.1371/journal.pone.0120696 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Merskey H BM, Bonica JJ, Boyd BD, Carmon A, Deathe AB, et al. Classification of chronic pain: descriptions of chronic pain syndromes and definitions of pain terms. Task Force on Taxonomy of the International Association for the Study of Pain (IASP). 1994. [Google Scholar]
- 44.Kucuk EV, Tahra A, Bindayi A, Suceken FY, Onol FF, Boylu U. Long-term functional results of aspiration and sclerotherapy with ethanol in patients with autosomal dominant polycystic kidney disease: a non-randomized pilot clinical study. Int Urol Nephrol. 2016;48(4):457–63. 10.1007/s11255-015-1211-x . [DOI] [PubMed] [Google Scholar]
- 45.Dunn MD, Portis AJ, Elbahnasy AM, Shalhav AL, Rothstein M, McDougall EM, et al. Laparoscopic nephrectomy in patients with end-stage renal disease and autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2000;35(4):720–5. 10.1016/s0272-6386(00)70021-7 . [DOI] [PubMed] [Google Scholar]
- 46.Lee DI, Andreoni CR, Rehman J, Landman J, Ragab M, Yan Y, et al. Laparoscopic cyst decortication in autosomal dominant polycystic kidney disease: impact on pain, hypertension, and renal function. J Endourol. 2003;17(6):345–54. 10.1089/089277903767923100 . [DOI] [PubMed] [Google Scholar]
- 47.Lee DI, Clayman RV. Hand-assisted laparoscopic nephrectomy in autosomal dominant polycystic kidney disease. J Endourol. 2004;18(4):379–82. 10.1089/089277904323056942 . [DOI] [PubMed] [Google Scholar]
- 48.Dunn MD, Portis AJ, Naughton C, Shalhav A, McDougall EM, Clayman RV. Laparoscopic cyst marsupialization in patients with autosomal dominant polycystic kidney disease. J Urol. 2001;165(6 Pt 1):1888–92. 10.1097/00005392-200106000-00011 . [DOI] [PubMed] [Google Scholar]
- 49.Lipke MC, Bargman V, Milgrom M, Sundaram CP. Limitations of laparoscopy for bilateral nephrectomy for autosomal dominant polycystic kidney disease. J Urol. 2007;177(2):627–31. 10.1016/j.juro.2006.09.026 . [DOI] [PubMed] [Google Scholar]
- 50.Yu J, Li B, Xiang YZ, Qi TG, Jin XB, Xiong H. Should kidney volume be used as an indicator of surgical occasion for patients with autosomal dominant polycystic kidney disease? Medicine. 2018;97(27):e11445. 10.1097/MD.0000000000011445 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rehman J, Landman J, Andreoni C, McDougall EM, Clayman RV. Laparoscopic bilateral hand assisted nephrectomy for autosomal dominant polycystic kidney disease: initial experience. J Urol. 2001;166(1):42–7. . [PubMed] [Google Scholar]
- 52.Sulikowski T, Kaminski M, Rozanski J, Zietek Z, Domanski L, Majewski W, et al. Laparoscopic removal of renal cysts in patients with ADPKD as an alternative method of treatment and patient preparation for kidney transplantation: preliminary results. Transplant Proc. 2006;38(1):23–7. 10.1016/j.transproceed.2005.11.082 . [DOI] [PubMed] [Google Scholar]
- 53.Central Sensitisation Inventory. Available at https://wwwphysio-pediacom/Central_Sensitisation_Inventory [last access September 2020].
- 54.OMERACT. Working Group name: Pain. https://omeractorg/working-groups/pain/.
- 55.Dougados M, Hawker G, Lohmander S, Davis AM, Dieppe P, Maillefert JF. OARSI/OMERACT Criteria of Being Considered a Candidate for Total Joint Replacement in Knee/Hip Osteoarthritis as an Endpoint in Clinical Trials Evaluating Potential Disease Modifying Osteoarthritic Drugs. J Rheumatol. 2009:2097–9. 10.3899/jrheum.090365 [DOI] [PubMed] [Google Scholar]
- 56.Hawker GA, Davis A, French MR, Cibere J, Jordan JM, March L. Development and preliminary psychometric testing of a new OA pain measure e an OARSI/OMERACT initiative. Osteoarthritis and Cartilage. 2008;16:409e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.PROMIS pain measures. Available at https://wwwhealthmeasuresnet/search-view-measures [last access September 2020].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOC)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.