Abstract
Background:
Despite extensive evaluation processes to determine candidacy for kidney transplantation, variability in graft failure exists. The role of patient socioeconomic status (SES) in transplantation outcomes is poorly understood due to limitations of conventional SES measures.
Methods:
This population-based retrospective cohort study assessed whether a validated objective and individual-level housing-based SES index (HOUSES), would serve as a predictive tool for graft failure in patients (n=181) who received a kidney transplant in Olmsted County, Minnesota (January 1, 1998 to December 8, 2016). Associations were assessed between HOUSES (quartiles: Q1 [lowest] to Q4 [highest]) and graft failure until last follow up date (December 31, 2016) using Cox proportional hazards. The mean age (SD) was 46.1 (17.2) years, 109 (60.2%) were male, 113 (62.4%) received a living kidney donor transplant, and 40 (22.1%) had a graft failure event.
Results:
Compared to Q1, patients with higher HOUSES (Q2-Q4) had significantly lower graft failure rates (adjusted hazard ratio, 0.47; 95% CI, 0.24–0.92; P < 0.029), controlling for age, sex, race, previous kidney transplantation, and donor type.
Conclusions:
Although criteria for kidney transplant recipients are selective, patients with higher HOUSES had lower graft failure rates. Thus, HOUSES may enable transplantation programs to identify a target group for improving kidney transplantation outcomes.
Introduction
Kidney transplantation is the most frequently performed solid organ transplant procedure with an estimated 16 804 grafts received in 2017.1 Kidney transplantation is an expensive medical procedure such that in 2017 the total cost for combined billed and utilization charges for the time 30 days prior to 6 months posttransplant was reported to be approximately $414 800 per transplant.1 Although kidney transplantation is the preferred treatment option for End Stage Renal Disease (ESRD) patients,2 the 6-month all-cause graft failure rate was 4.3% for recipients of a transplant in 2016, and the 10-year all-cause graft failure rate was 49.7% and 34.1% for recipients of a deceased or living donor transplant in 2007, respectively.3 Given the impact on patients and the healthcare system, identifying high-risk patients for graft failure could be highly beneficial for patients, providers, transplant centers, payers, and society—as it would enable clinical care teams to allocate resources to candidates with greater medical and psychosocial needs.
In this respect, apart from the frequently cited clinical risk factors for poor graft and patient survival (e.g., living versus deceased donor or ESRD etiology),3 one’s socioeconomic status (SES) can be an important yet modifiable predictor for transplantation outcomes. Specifically, SES as a key element of social determinants of health (SDH)4 is defined as one’s abilities to access desired resources (human, materialistic and social)5 which can be altered with proper support. Indeed, SES has been reported to impact kidney transplantation outcomes (e.g., poorer graft & recipient survival among those experiencing neighborhood poverty,6 social deprivation,7,8 & low educational attainment9–11). Yet, the currently available measures of SES have significant limitations due to their difficulty to obtain, significant within-group heterogeneity (i.e., inaccurate or imprecise measurement) causing misclassification,12–14 lack of responsiveness to changes of SES (e.g., educational level),12 and extrapolation from neighborhood SES measures.15–17 Collectively, these measurement errors obscure true clinical and biological heterogeneity and cause inconsistent study results, thus hampering their clinical utility as a tool for predicting transplantation outcomes among kidney transplant recipients.
Our study addresses these challenges by applying an objective, granular, and individual HOUsing-based index of SocioEconomic Status (HOUSES),18 a validated individual-level SES measure which was derived from the County’s publicly available real property data for individual housing unit. Conceptually, it captures wealth and income, access to social and environmental resources and health effects of building features.18 We hypothesized that HOUSES independently identifies high-risk patients for graft failure among kidney transplantation recipients. If our hypothesis is supported, HOUSES may enable care teams to identify high-risk patients among acceptable candidates based on current clinical guidelines and proactively allocate additional resources and support—before and after transplantation—to mitigate potential for poor outcomes. We assessed whether HOUSES would be a significant and independent predictor of cumulative graft failure during the study period.
Materials and Methods
Study Setting and Population
Olmsted County, MN is a mixed rural-urban setting with a high median family income of $60 252 compared to the national average of $53 046 in 2009–2013 and racial composition consisting of 86% non-Hispanic white, 5% Asian, and 4% Hispanic,19 and low racial segregation across census tracks indicated by a White/Black dissimilarity index score of 29.5 in 2010.20 Olmsted County is not classified as a Medically Underserved Area, and many of its residents work in the healthcare industry and have health insurance (27% & 95%, respectively).21,22 The Olmsted County, MN population has shown to be representative of the state of Minnesota and Midwestern populations.23
Olmsted County is an ideal setting for conducting population-based epidemiologic studies. The Rochester Epidemiology Project (REP), funded by the National Institutes of Health (NIH), links medical records for the Olmsted County population from healthcare facilities—where the majority of care is delivered (self-contained healthcare environment)—including Mayo Clinic, Olmsted Medical Center, and other affiliates.23–25 We used REP census to identify residents of Olmsted County, Minnesota, who received a kidney transplant at a single center between January 1st 1998 and December 31st 2016 as kidney transplantation is not conducted in other healthcare facilities in Olmsted County. We chose this study period as electronic files of real property data and corresponding HOUSES data for Olmsted County are available during this timeframe.
Study Design
This was a retrospective population-based cohort study to assess the differential impact of individual-SES as measured by HOUSES on graft failure in kidney transplant recipients. This study was approved by the Institutional Review Boards at Mayo Clinic (17–004336) and Olmsted Medical Center (048-OMC-17).
Kidney Transplant Case Identification
Corresponding International Classification of Diseases, Ninth Revision (ICD-9) and International Statistical Classification of Diseases, Tenth Revision (ICD-10) codes, as well as Current Procedural Terminology (CPT) codes of a renal transplant procedure, were used to initially identify participants during the study period (see Table S1 in Supplemental Materials and Methods). Then, we performed a comprehensive medical record review to confirm transplantation status and obtain data to characterize transplant cases.
Eligibility
Subjects met inclusion criteria if they were a resident of Olmsted County and a recipient of a kidney transplant during the above indicated study period. Subjects who did not give permission to use their medical records via the Minnesota Research Authorization form or who were not a resident of Olmsted County for at least 1 year prior to the transplant procedure were excluded to reduce selection bias due to referral cases (n=22). Subjects were aged 18 years or older and could be a recipient of a previous kidney transplant (n=23).
HOUSES and Psychometric Properties
HOUSES data were used to assess individual-SES at the time of transplant. For generating HOUSES,18 addresses for study subjects at the time of transplantation were retrieved from the REP,23–25 which collects and maintains all historical individual addresses during residence in Olmsted County. HOUSES is a robust individual measure of SES represented by a single factor made up of 4 items (number of bedrooms, number of bathrooms, square footage of the unit, & estimated building value of the unit) ascertained from the county Assessor’s office.18 Upon its inception, HOUSES was derived from a principal component factor analysis such that each item had a factor loading ≥0.40 and eigenvalues >1.0, respectively. Variables were then weighted and aggregated into an overall z-score such that a higher HOUSES score indicated higher SES. The HOUSES was then grouped into quartiles (Q1 : lowest SES). HOUSES has shown strong psychometric properties. HOUSES demonstrated criterion validity such that there were moderate to good correlations with education, income, Hollingshead Index (HS), and Nakao-Treas Index (NT) in Olmsted County, MN (R = 0.29–0.54, P < 0.001) and Jackson County, MO (R = 0.39–0.59, P < .001), respectively.18 For comparison, in Jackson County, MO—an urban setting encompassing Kansas City that is more socioeconomically diverse than Olmsted County, MN (e.g., 75.9% vs 88.7% White alone, 17.5% vs 6.7% without any college education, and 7.8% vs 1.8% with a family income less than $24 999, respectively)—HOUSES demonstrated its applicability to both urban and mixed urban-rural settings. External validity of HOUSES was independently demonstrated by data showing that HOUSES predicted poorly controlled childhood asthma by the Asthma Control Test (ACT) in Sioux Falls, South Dakota.26 Since the original validation, HOUSES has shown to be associated with a broad range of health outcomes known to be inversely associated with SES in adults and children (see Table S2 in Supplemental Materials and Methods).18,27–36
Additional SES Measures
Individual-level SES
We obtained educational levels of study subjects at the time of transplantation from medical record review. Educational levels as a discrete variable were categorized into 1) no high school, 2) high school, 3) some college, 4) college, and 5) graduate or higher education.
Aggregate-level SES
We ascertained Census-block group level education (proportion of associate degree) and median family income in continuous variable from 2010 Census. Then, we categorized the percentage of associate degrees into quartiles and incomes above the median household income into quartiles per census block group where study subjects resided at the time of transplantation.
Covariates
Additional covariates thought to be associated with HOUSES or graft survival were retrieved from patients’ medical records closest to the time of transplant. For this study, early rejection was determined by an episode indicated in a patient’s biopsy report within 1 year of transplant with a grade of 1A or higher, that necessitated treatment documented in the patient’s medical record. Other covariates retrieved from the patient’s medical record included age, sex, race (white/Caucasian, African American, Asian, American Indian/Alaskan Native, more than 1 race, unknown), body mass index (BMI), previous kidney transplant, dialysis duration (preemptive transplant, 0–6 months, 6–12 months, 12–18 months, and greater than 18 months), ESRD etiology (diabetes, hypertension, cystic/hereditary, and other), number of human leukocyte antigen (HLA) mismatches (0–6), diabetes, hypertension, delayed graft function (requirement for dialysis within 7 days of transplant), induction agent (anti-rabbit thymocyte globulin, alemtuzumab, or other), immunosuppression regimen (tacrolimus-based, cyclosporine-based, or other), recipient’s cytomegalovirus virus (CMV) pairing with donor (recipient and donor both negative, recipient positive and donor positive, recipient positive and donor negative, and recipient negative and donor positive), donor type (living or deceased), donor age, and relationship to donor (related or unrelated).
Graft Failure
The primary endpoint of interest was graft failure which consisted of the time from transplantation until a requirement for dialysis or retransplant verified in the patient’s medical record. Study participants were censored on last day of follow-up and at death (if the recipient had a functioning graft at death, a graft failure event was not counted).
Statistical Analysis
Analysis of Variance (ANOVA) and t- tests for continuous variables, and Pearson χ2, and Fisher exact tests for categorical variables, were performed to measure associations between HOUSES in quartiles and characteristics of study subjects at the time of transplantation. Survival analysis was performed using methods for nonparametric data including the Kaplan-Meier life table method with the log rank test of significance, and univariate and multivariate analysis with Cox’s proportional hazards models. Based on the increased rates of graft failure observed among the lowest SES recipients in previous literature (e.g., recipients who live in regions with higher social deprivation),8 in addition to assessing HOUSES as a single ordinal variable in the univariate survival analysis, we also dichotomized HOUSES to compare Q2–4 to Q1. This comparison would help identify a specific socially and medically underserved subgroup of transplant recipients. The HOUSES variable with the largest effect on graft failure was retained for the multivariate models. Variables significantly (P < 0.05) associated with graft failure in univariate analyses or known to vary by SES (e.g., age, sex, & race) or graft failure (e.g., donor type & previous kidney transplant), were entered into the multivariate Cox’s proportional hazards models. The proportionality assumption was assessed and not violated. All analyses were generated using SAS® v9.4 software (SAS Institute, Cary, NC).
Results
Characteristics of Study Subjects
Characteristics of participants at the time of transplant are displayed in Table 1. A total of 216 Olmsted County residents received a kidney transplant and granted authorization for research during the study period. 22 participants were excluded due to not having an Olmsted County address within 1 year prior to transplantation (i.e., referral patients), and of the remaining 194, HOUSES was successfully matched for 93.1% recipients, resulting in 181 eligible subjects. 109 (60%) were male and 75 (40%) were female, and the mean (SD) age at the time of transplantation was 46.1 (17.2) years. In addition, 113 (62.4%) received a living donor kidney transplant (LDKT), and 68 (37.6%) received a deceased donor kidney transplant (DDKT). Overall, 40 (22.1%) recipients had a graft failure event during the study over a median follow up duration of 81 months (range: 0–219). As displayed in Table 1, recipients in lower quartiles tended to have less favorable baseline characteristics such that lower individual-SES was significantly associated with longer dialysis duration (P = 0.005) and deceased donor type (P = 0.003). In addition, HOUSES was significantly associated with age (P = .017) and race (P = 0.035) such that the average age tended to be lower and there were a higher proportion of African American recipients in the first quartile (lowest SES) (Table 1). Additional covariates were not significantly associated with individual SES.
Table 1.
Baseline Characteristics Stratified by HOUSES Quartile
| Baseline Characteristics | Q1 56 (30.9) | Q2 48 (26.5) | Q3 51 (28.2) | Q4 26 (14.4) | P value |
|---|---|---|---|---|---|
| Recipient-level | |||||
| Age at Transplant (Mean, SD) | 40.9 (16.8) | 49.9 (15.4) | 49.6 (18.3) | 43.67 (16.8) | 0.017a |
| Sex (Female) | 22 (39.3) | 22 (45.8) | 18 (35.3) | 10 (38.5) | 0.76b |
| Race | 0.035c | ||||
| White | 41 (73.2) | 39 (81.3) | 44 (86.3) | 23 (88.5) | |
| Black/African American | 9 (16.1) | 0 | 1 (2.0) | 0 | |
| Asian | 1 (1.8) | 6 (12.5) | 3 (5.9) | 2 (7.7) | |
| American Indian/Alaskan Native | 1 (1.8) | 1 (2.1) | 1 (2.0) | 0 | |
| More than One | 3 (5.36) | 2 (4.17) | 2 (4.0) | 1 (3.85) | |
| Unknown | 1 (1.79) | 0 | 0 | 0 | |
| Education | 0.070b | ||||
| No High School | 2 (3.9) | 2 (4.3) | 4 (7.8) | 0 | |
| High School | 19 (36.5) | 13 (27.7) | 9 (17.7) | 5 (19.2) | |
| Some College | 16 (30.8) | 16 (34.0) | 22 (43.1) | 10 (38.5) | |
| College | 5 (9.6) | 6 (12.8) | 12 (23.5) | 9 (34.6) | |
| Graduate or Higher | 10 (19.2) | 10 (21.3) | 4 (7.8) | 2 (7.7) | |
| BMI (Mean, SD) | 29.24 (14.7) | 27.23 (5.3) | 28.98 (16.0) | 27.09 (6.8) | 0.81a |
| Previous Kidney Transplant | 9 (16.1) | 3 (6.3) | 7 (13.7) | 4 (15.4) | 0.46c |
| Early Rejection | 2 (4.1) | 6 (13.0) | 2 (4.1) | 1 (4.0) | 0.27c |
| Dialysis Duration (months) | 0.005b | ||||
| Preemptive | 10 (17.9) | 21 (43.8) | 25 (49.0) | 12 (46.2) | |
| >0–6 | 8 (14.3) | 5 (10.4) | 12 (23.5) | 8 (30.8) | |
| >6–12 | 11 (19.6) | 7 (14.6) | 3 (5.9) | 3 (11.5) | |
| >12–18 | 5 (8.9) | 2 (4.2) | 2 (3.9) | 0 | |
| >18 | 22 (39.3) | 13 (27.1) | 9 (17.7) | 3 (11.5) | |
| ESRD Etiology | 0.75b | ||||
| Diabetes | 14 (25.0) | 13 (27.1) | 12 (23.5) | 3 (11.5) | |
| Hypertension | 3 (5.4) | 4 (8.3) | 4 (7.8) | 0 | |
| Cystic/Hereditary | 3 (5.4) | 7 (14.6) | 7 (13.7) | 3 (11.5) | |
| Other | 36 (64.3) | 24 (50.0) | 28 (54.9) | 20 (76.9) | |
| HLA Mismatch (Mean, SD) | 3.39 (1.9) | 3.24 (1.9) | 3.25 (1.9) | 2.83 (2.0) | 0.75a |
| Diabetes | 36 (64.3) | 36 (75.0) | 29 (56.9) | 13 (50.0) | 0.13c |
| Hypertension | 56 (100.0) | 47 (26.3) | 50 (98.0) | 26 (100.0) | 0.64b |
| Delayed Graft | 7 (12.5) | 6 (12.5) | 2 (3.9) | 0 | 0.10c |
| Induction | 0.39b | ||||
| Thymoglobulin | 37 (74.0) | 24 (54.6) | 30 (65.2) | 14 (56.0) | |
| Campath | 6 (12.0) | 8 (18.2) | 4 (8.7) | 5 (20.0) | |
| Other | 7 (14.0) | 12 (27.3) | 12 (26.1) | 6 (24.0) | |
| Immunosuppression | 0.84b | ||||
| Tacrolimus-based | 51 (91.1) | 41 (85.4) | 47 (92.2) | 23 (88.5) | |
| Cyclosporine-based | 3 (5.4) | 3 (6.3) | 3 (5.9) | 2 (7.7) | |
| Other | 2 (3.6) | 4 (8.3) | 1 (2.0) | 1 (3.9) | |
| Calcineurin Inhibitor | 53 (94.6) | 45 (93.8) | 49 (96.1) | 25 (96.2) | 0.95b |
| CMV | 0.72b | ||||
| R−/D− | 9 (22.0) | 9 (25.0) | 10 (23.8) | 7 (26.9) | |
| R+/D+ | 15 (36.6) | 14 (38.9) | 11 (26.2) | 7 (26.9) | |
| R+/D− | 10 (24.4) | 7 (19.4) | 11 (26.2) | 10 (38.5) | |
| R−/D+ | 7 (17.1) | 6 (16.8) | 10 (23.8) | 2 (7.7) | |
| Donor-level | |||||
| Type (Deceased) | 28 (50.0) | 22 (45.8) | 15 (29.4) | 3 (11.5) | 0.003b |
| Age (Mean, SD) | 39.09 (15.4) | 37.57 (21.3) | 41.13 (13.4) | 39 (12.9) | 0.95a |
| Relationship to Donor (Unrelated) | 6 (21.4) | 8 (30.8) | 14 (38.9) | 8 (22.2) | .51c |
Q=Quartile
ANOVA test
Pearson’s Chi-Square test
Fisher’s Exact test for comparing association between HOUSES quartiles and each characteristic
Association Between HOUSES and Risk of Graft Failure
Table 2 displays the univariate analyses for unadjusted graft failure rates by study subject characteristic. Unadjusted and adjusted proportional hazards from the multivariate analysis are displayed in Table 3. In the initial unadjusted proportional hazards model, individual SES as measured by HOUSES quartiles, as a single ordinal variable, was significant (HR, 0.72, 95% CI, 0.52–0.99; P = 0.047) but the main differences in the risk of graft failures were between Q2–4 vs. Q1 groups (See Figure S1 in Supplemental Materials and Methods for the unadjusted graft failure rates by individual HOUSES quartile using the log-rank method). After collapsing the highest 3 HOUSES quartiles and comparing this group to the high-risk subgroup of transplant recipients in quartile 1, the highest SES quartiles compared to the lowest quartile was significantly associated with lower graft failure rates in Model 1 (Q2–4: HR, 0.48; 95% CI, 0.26–0.90; P = 0.021). These data are further displayed in the Kaplan-Meier plots (Figure 1). After adjustment for age, sex, race, previous kidney transplant, and donor type in Model 3, the highest 3 SES quartiles compared to the lowest quartile (Q2–4: HR, 0.47; 95% CI, 0.24–0.92; P < 0.029) continued to be significantly and independently associated with lower graft failure rates.
Table 2.
Univariate Proportional Hazards of Graft Failure
| Predictor | HR (95%CI) | P value |
|---|---|---|
| Recipient | ||
| HOUSES (Single Ordinal Variable) | 0.72 (0.52–0.99) | 0.041 |
| HOUSES (Q1 vs Q2–4) | ||
| Q1 | Reference | |
| Q2–Q4 | 0.55 (0.25– 1.22) | 0.021 |
| Age | 0.99 (0.97–1.01) | 0.37 |
| Sex | ||
| Male | Reference | |
| Female | 0.96 (0.51–1.82) | 0.90 |
| Race | ||
| White-Caucasian | Reference | |
| Non-White Caucasian | 1.03 (0.45– 2.34) | 0.95 |
| Educational Attainment | ||
| No High School | Reference | |
| High School | 0.68 (0.19–2.48) | 0.56 |
| Some College | 0.88 (0.26–3.01) | 0.84 |
| College | 0.51 (0.12– 2.16) | 0.36 |
| Graduate or Higher | 0.32 (0.07–1.45) | 0.14 |
| Associate Degree (% Per Census Block) | ||
| Q1 | Reference | |
| Q2–Q4 | 0.80 (0.41–1.57) | 0.52 |
| Income (% Per Census Block) | ||
| Q1 | Reference | |
| Q2–Q4 | 0.55 (0.29–1.03) | 0.063 |
| BMI | 1.00 (0.98–1.03) | 0.70 |
| Previous Kidney Transplant | 4.27 (2.19–8.30) | <.0001 |
| Early Rejection | 1.37 (0.49–3.85) | 0.55 |
| Dialysis Duration (months) | ||
| Preemptive | Reference | |
| >0–6 | 1.22 (0.48– 3.11) | 0.67 |
| >6–12 | 2.10 (0.83 −5.34) | 0.12 |
| >12–18 | 1.30 (0.35– 4.81) | 0.69 |
| >18 | 1.72 (0.73– 4.06) | 0.22 |
| ESRD Etiology | ||
| Diabetes | Reference | |
| Hypertension | 0.92 (0.25 −3.36) | 0.90 |
| Cystic/Hereditary | 0.27 (0.06 −1.24) | 0.092 |
| Other | 0.79 (0.38–1.66) | 0.54 |
| HLA Mismatch | 1.00 (0.76–1.33) | 0.98 |
| Delayed Graft | 1.62 (0.58 −4.56) | 0.36 |
| Induction | ||
| Thymoglobulin | Reference | |
| Campath | 0 | 0.99 |
| Other | 0.79 (0.33–1.93) | 0.61 |
| Immunosuppression | ||
| Tacrolimus-based | Reference | |
| Cyclosporine-based | 2.17 (0.842 −5.593) | 0.11 |
| Other | 1.50 (0.458–4.937) | 0.50 |
| Calcineurin Inhibitor | 0.49 (0.17– 1.38) | 0.17 |
| CMV | ||
| R−/D− | Reference | |
| R+/D+ | 0.60 (0.18–1.98) | 0.40 |
| R+/D− | 1.29 (0.43–3.86) | 0.65 |
| R−/D+ | 0.91 (0.25–3.27) | 0.88 |
| Donor | ||
| Type (Deceased) | 1.46 (0.78 −2.73) | 0.24 |
| Age | 1.05 (0.99– 1.12) | 0.09 |
| Relationship to Donor (Unrelated) | 1.48 (0.62– 3.54) | 0.38 |
Q=Quartile
Table 3.
Multivariate Proportional Hazards of Graft Failure
| Predictor | HR (95% CI) | P value |
|---|---|---|
| Model 1 | ||
| Q1 | Reference | |
| Q2–4 | 0.48 (0.26–0.90) | 0.021 |
| Model 2 | ||
| Q1 | Reference | |
| Q2–4 | 0.50 (0.26–0.96) | 0.038 |
| Age at Transplant | 1.0 (0.98–1.02) | 0.79 |
| Female | Reference | |
| Male | 0.95 (0.49–1.83) | 0.88 |
| White Caucasian | Reference | |
| Non-White Caucasian | 0.83 (0.36–1.95) | 0.67 |
| Model 3 | ||
| Q1 | Reference | |
| Q2–4 | 0.47 (0.24–0.92) | 0.029 |
| Age at Transplant | 1.0 (0.98–1.02) | 0.97 |
| Female | Reference | |
| Male | 1.05 (0.54–2.03) | 0.89 |
| White Caucasian | Reference | |
| Non-White Caucasian | 0.81 (0.35–1.89) | 0.63 |
| No Previous Kidney Transplant | Reference | |
| Previous Kidney Transplant | 4.45 (2.20–8.99) | <0.0001 |
| Living Donor | Reference | |
| Deceased Donor | 1.28 (0.67–2.47) | 0.46 |
Q=Quartile
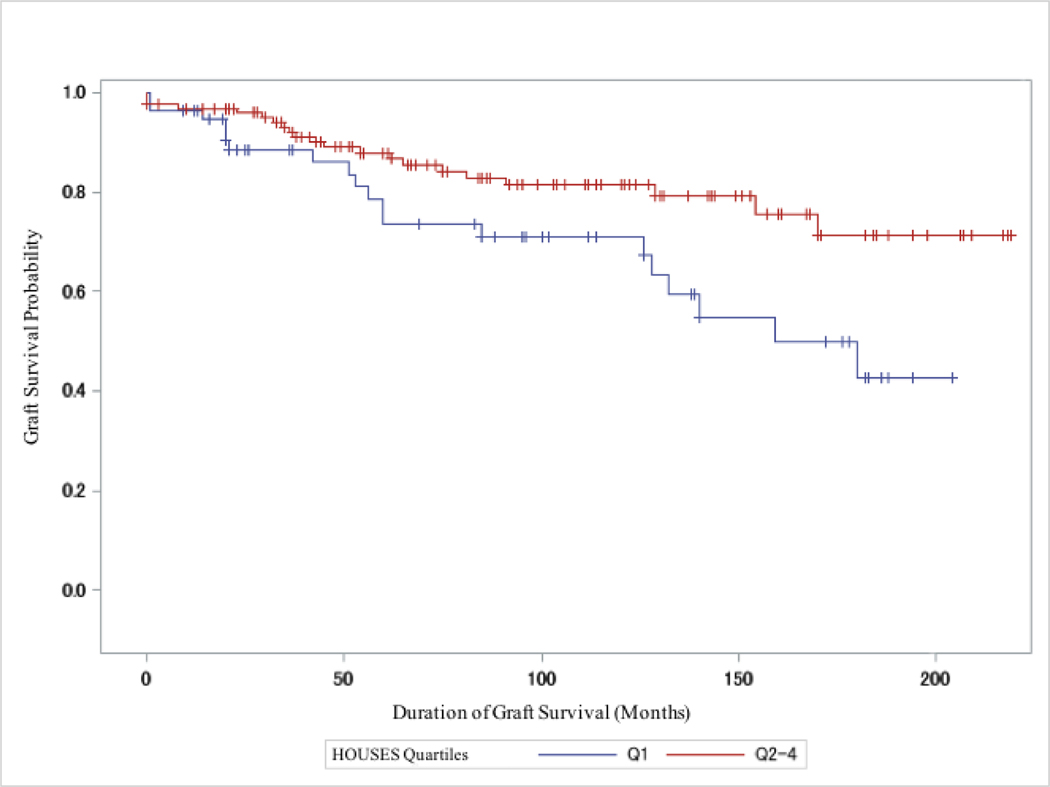
Figure 1.
Kaplan Meier Estimates Comparing Graft Failure Rates Between Highest HOUSES Quartiles (Q2–4) Versus Lowest HOUSES quartile (Q1) in all Recipients (N=181). Q2–4 had lower graft failure rates (P = 0.019). + = Censored.
Association Between HOUSES and Risk of Graft Failure by Donor Type
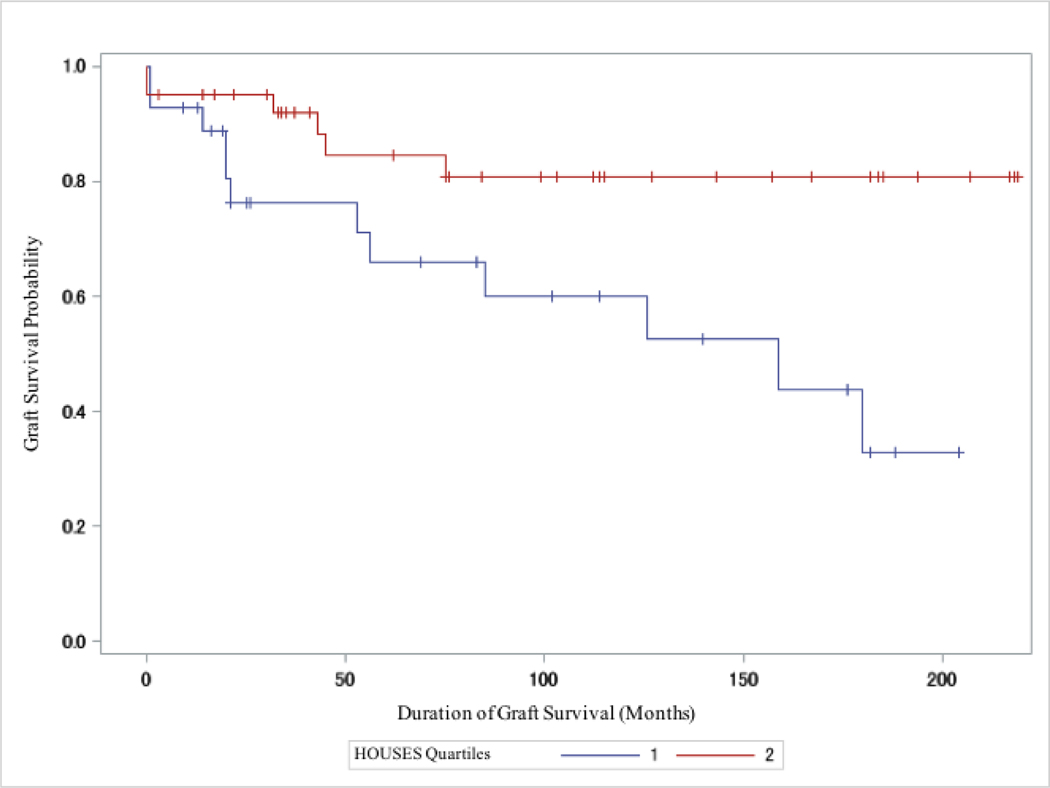
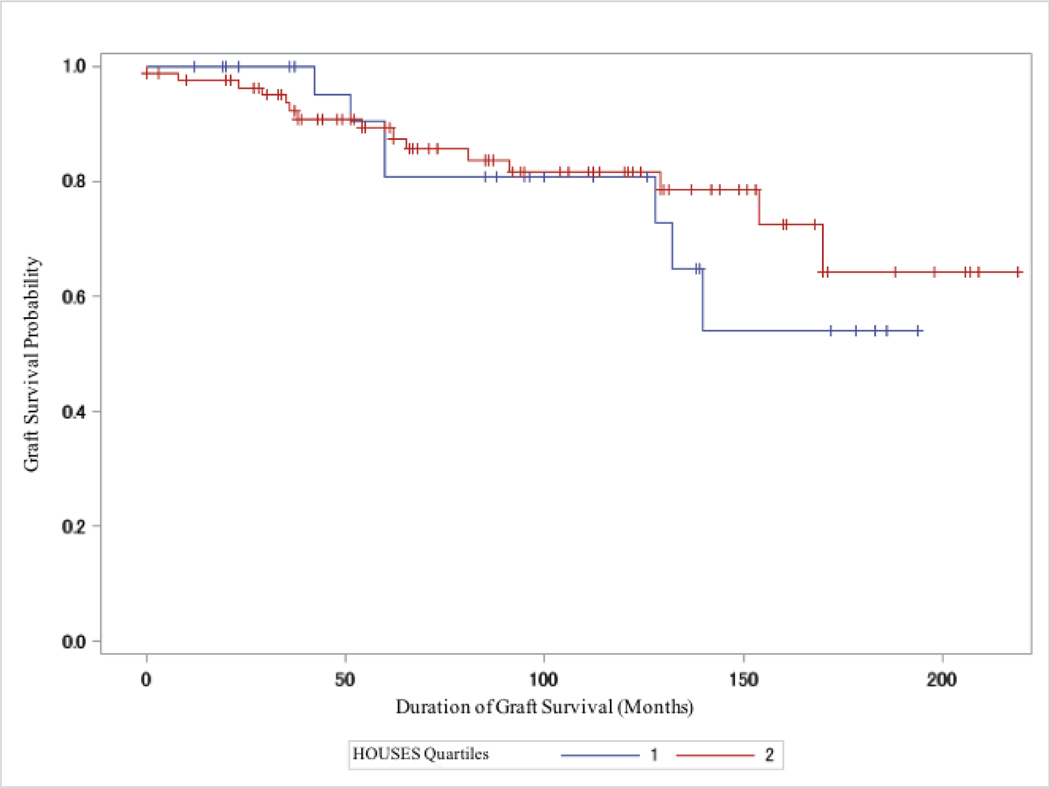
In the subgroup analysis, where we stratified by donor type, deceased donor recipients showed significantly lower graft failure rates in the highest 3 SES quartiles compared to the lowest (Q2–4 vs Q1: P = 0.013; Figure 2); however, living donor recipients did not show differential outcomes based on individual SES as measured by HOUSES (Q2–4 vs Q1: P = 0.56; Figure 3). (See Figure S2 and S3 in Supplemental Materials and Methods for the unadjusted graft failure rates by individual HOUSES quartile restricted to deceased and living donor type, respectively, using the log-rank method).
Figure 2.
Kaplan Meier Estimates Comparing Graft Failure Rates Between Highest HOUSES Quartiles (Q2–4) Versus Lowest HOUSES quartile (Q1) in DDKT Recipients (N=68). Q2–4 had lower graft failure rates (P = 0.013). + = Censored.
Figure 3.
Kaplan Meier Estimates Comparing Graft Failure Rates Between Highest HOUSES Quartiles (Q2–4) Versus Lowest HOUSES quartile (Q1) in LDKT Recipients (N=113; P = 0.56). + = Censored.
Association Between Other SES Measures and Risk of Graft Failure
As displayed in Table 2, we also assessed the impact of educational attainment and area-level SES measures. In the unadjusted proportional hazards model, patient educational attainment, and the percentage of associate degrees and incomes above the median household income per census block group, were not associated with rates of graft failure.
Discussion
Our study results showed a significant inverse relationship between SES as measured by HOUSES and the risk of graft failure which supports our hypothesis. Specifically, lower rates of graft failure were observed in recipients in the highest 3 HOUSES quartiles compared to those in the lowest HOUSES quartile. Transplantation access disparities by HOUSES were also observed, as indicated by the association between HOUSES with longer dialysis duration and deceased donor type, which may, in part, account for the higher risk of graft failure in recipients with lower SES.
The most notable finding of this study was that individual SES as measured by HOUSES was significantly and independently associated with the risk of graft failure. These findings persisted after adjusting for age, sex, and race, as well as known clinical risk factors (e.g., previous kidney transplantation & donor type) for graft failure. As shown in Figure 1, our data support that differential graft failure rates by HOUSES emerge from an early phase of transplantation and persist up to 15 years posttransplantation. Further, the differences in graft failure rates are increasingly stark with increasing time since transplant as shown by the widening and lack of convergence of the high-SES (Q2–4) and low-SES (Q1) survival curves. These findings have important clinical implications as described below as the significant and independent impact of HOUSES on transplant outcomes persists even with the careful evaluation processes required of candidates for kidney transplantation under current guidelines.
In support of our study findings, previous studies have indicated lower SES to be a potential risk factor for graft failure; however, results have been inconsistent primarily due to significant measurement errors stemming from imprecise or inaccurate measurement of individual SES. For example, some studies found that having higher educational attainment,9–11 private insurance,9 and skilled occupation11 served as protective factors for improved graft and recipient survival. Conversely, other studies found no association between SES (e.g., neighborhood poverty37 & other area-level based measures38–40) and transplant outcomes. As previously mentioned, the use of area-level SES measures may result in misclassification of recipients’ SES,16. Other conventional individual-level SES measures such as educational level or Medicaid eligibility may more proximately capture one’s SES, but still suffer from the aforementioned within-group heterogeneity (variability among an SES stratum) and fail to capture changes in SES over time (e.g., plateau effects observed in educational attainment).12–14 As shown in our study results, educational level and area-level SES measures were not associated with the risk of graft failure. Therefore, the precision of SES measurement in detecting the underlying construct for one’s SES (i.e., abilities to access desired resources including human, materialistic, and social)5 is a key aspect of SES measurement for predicting transplant outcomes in a consistent and reproducible manner. In this respect, we believe HOUSES as an objective, granular, and individual-level SES measure overcomes more of these measurement challenges, better capturing the effect of one’s SES on the risk of graft failure more accurately than other conventional SES measures as demonstrated in our data.18
There are several underlying mechanisms to explain why higher SES recipients as measured by HOUSES had lower graft failure rates. Based on the NIH Centers for Population Health’s conceptual framework, SES as a SDH can serve as an independent risk factor, as well as a fundamental cause (e.g., a social condition influenced by policy) for poor health outcomes.41 HOUSES lends opportunity to intervene—as it captures one’s abilities to access desired resources (i.e., leveraging human, social, & materialistic capital to obtain an asset of value). Specifically, those in Q1 of HOUSES might have limited financial resources (e.g., difficulty continuing immunosuppressive medication beyond the 3-year Medicare cap), health literacy (e.g., nonadherence) and social support (e.g., unbuffered stress or lack of social cohesion) collectively resulting in poor transplantation outcomes. As shown in our results, the limited access to a living donor in those with lower SES (which has lower graft failure rates, compared to deceased donor) is 1 important reason for the inverse association of HOUSES with the higher risk of graft failure. In our study results, the association between lower SES as measured by HOUSES and the risk of graft failure was primarily observed in recipients for deceased donor kidney, while such association was not found in those who received living donor kidneys. It might be difficult to discern whether this divergent observation on the impact of SES on the risk of graft failure by donor type is due to donor type or its associated SES. Nonetheless, we adjusted our study results for donor type suggesting an independent impact of SES on transplantation outcomes. Future studies with a larger sample size are needed to clarify this using stratified analysis.
Our results highlight the need to develop robust strategies to detect and then to reduce disparities in kidney transplant outcomes by SES and have a few important implications. Transplant centers already conduct a rigorous selection in which characteristics (e.g., availability of potential living donors), health status (e.g., medical comorbidities, BMI, & life expectancy) and psychosocial readiness measures (e.g., financial assessment, Stanford Integrated Psychosocial Assessment for Transplant42) in candidates are evaluated. To improve transplantation outcomes in an effective, efficient and equitable manner, HOUSES can supplement these processes by identifying a subset of patients at higher risk for poor outcomes among candidates listed for a transplant, hence, allowing clinical care teams to determine where to allocate (often limited) resources (e.g., increasing medication adherence monitoring & connecting patients to health navigators) to mitigate the unfavorable postprocedural effects of SES in recipients.
Another strategy is to deploy HOUSES to augment risk adjustment models often incorporated in value-based payment systems. For example, National Academy of Medicine and National Quality Forum recommend adjustment for social factors of patient populations when determining the performance of healthcare organizations but acknowledge the lack of suitable individual-level measures for SES as a key challenge.43,44 In this respect, HOUSES could be incorporated in the prospective payment system (pps) bundled payment program set by Centers for Medicare and Medicaid (a dominant payor in transplantation).45 Previous literature has supported the feasibility of incorporating SDHs in payment formulas for government-funded programs such as MassHealth to overcome underpayment limitations (e.g., failure to account for social risk) of a diagnosis-based model.46 Medicare could also consider selectively expanding immunosuppression coverage beyond the 3 year cap posttransplant for beneficiaries with ESRD who are not disabled or over 65, to low-SES recipients as a high-risk group for graft failure.2,47
Lastly, these findings underscore the importance of increasing the number of donors and access to living donor kidney transplants for candidates who experience greater financial barriers or hardships. Consistent with our findings, previous studies have shown that high-SES recipients were more likely to receive a transplant from a living donor and have better survival outcomes.6 Research efforts, policy remedies, and clinical care that would help people from a lower SES background find a living donor should be sought as disparities in kidney transplantation outcomes related to SES could be reduced. Specifically, it is worth elucidating the pathways through which SES as measured by HOUSES operates its impact on transplantation outcomes. In this investigation in the future, HOUSES may reveal unrecognized risk factors.
There are limitations that should be considered when interpreting these findings. The multivariate analysis did not include all confounding variables due to the small sample size and not all covariates having been collected or available. While other covariates (e.g., ESRD etiology) from the Scientific Registry of Transplant Recipients risk adjustment models48 were considered for the multivariate analysis, a conservative approach was taken such that only variables most statistically significant and clinically relevant were selected. It was also presumed that some variation would be naturally adjusted for in routine transplantation evaluation processes. In addition, given the high percentage of whites/Caucasians (81.67%), race was dichotomized into white/Caucasian and nonwhite/Caucasian, which may not represent true survival differences among specific races. Also, the homogeneity of this sample (e.g., primarily white/Caucasian race from a relatively affluent community) and mixed rural-urban setting in this study, in part, restricts the generalizability of these findings to other populations and areas. For instance, the distribution of transplant candidates across HOUSES quartiles may be different in a metropolitan region which could widen or shrink graft failure disparities. Future research should replicate HOUSES in other settings to assess whether similar transplantation disparities are observed. Along these lines, this is a single-center study and thus, our study findings on transplantation need to be replicated in different study settings.
Notwithstanding, this study overcomes the misclassification barriers commonly associated with traditional area-level SES measures, and affords a more accurate depiction of SES via HOUSES, which has shown strong psychometric properties as discussed in the Method section.18. Further, HOUSES has a few additional innovative features. For example, its use of public property data addresses the limitations frequently associated with conventional individual-level SES measures, which enables implementation of HOUSES on a large scale through geospatial analysis and geocoding as HOUSES uses patients’ address information. Also, HOUSES can capture change of individual SES over time as property data collected by a county Assessor’s office is updated annually and relocation or change of address often reflect change of one’s SES. In addition to studies in Olmsted County, MN, Jackson County, MO and Sioux Falls, SD, HOUSES could be applied to other areas in the US to more precisely quantify SES. The significant results from this research also highlight SES-related access and outcomes disparities in kidney transplantation, in turn, introducing important considerations and new opportunities for achieving high-value care and high-quality research.
In conclusion, the HOUSES helped identify high-risk recipients for graft failure among candidates listed for a transplant. The HOUSES may enable care teams to proactively allocate resources and support to such high-risk patients to mitigate the risk of graft failure. These findings necessitate action to further evaluate and remove access barriers pre- and post transplant, to explore alternative models of financing and delivering healthcare to socioeconomically underserved recipients, and to consider focused investing in health policies that improve graft survival. In this endeavor, HOUSES is a useful tool for research and clinical care in transplantation.
Supplementary Material
Acknowledgments
We thank the Precision Population Science Lab, as well as Lila Rutten, PhD, who helped make this study possible. We also thank Kelly Okeson for her administrative support. In addition, we thank the Rochester Epidemiology Project, supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676, who provided support and resources to make this study possible.
Declaration of all sources of funding: The authors of this manuscript have no conflicts of interest to disclose as described by Transplantation.
Abbreviations:
- ANOVA
Analysis of Variance
- BMI
Body Mass Index
- CI
Confidence Interval
- CKD
Chronic Kidney Disease
- CMV
Cytomegalovirus Virus
- CPT
Current Procedural Terminology
- DF
Degrees of Freedom
- ESRD
End-Stage Renal Disease
- HLA
Human Leukocyte Antigen
- HOUSES
A HOUsing-based index of SocioEconomic Status
- HR
Hazard Ratio
- ICD-9
International Classification of Diseases, Ninth Revision
- ICD-10
International Statistical Classification of Diseases, Tenth Revision
- NIH
National Institutes of Health
- REP
Rochester Epidemiology Project
- SES
Socioeconomic Status
- SDH
Social Determinants of Health
Footnotes
Disclosure Statement: The authors declare no conflicts of interest.
References
- 1.Bentley TS, Phillips SJ, Milliman. 2017 U.S. organ and tissue transplant cost estimates and discussion. http://www.milliman.com/uploadedFiles/insight/2017/2017-Transplant-Report.pdf. Published August 2019. [Google Scholar]
- 2.Gill JS, Tonelli M. Penny wise, pound foolish? Coverage limits on immunosuppression after kidney transplantation. N Engl J Med. 2012;366(7):586–589. [DOI] [PubMed] [Google Scholar]
- 3.Hart A, Smith JM, Skeans MA, et al. OPTN/SRTR 2017 annual data report: kidney. Am J Transplant. 2019;19(Suppl 2):19–123. [DOI] [PubMed] [Google Scholar]
- 4.Warnecke RB, Oh A, Breen N, et al. Approaching Health Disparities From a Population Perspective: The National Institutes of Health Centers for Population Health and Health Disparities. Am J Public Health. 2008;98(9):1608–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oakes JM Rossi PH. The measurement of SES in health research: current practice and steps toward a new approach. Soc Sci Med. 2003;56(4):769–784. [DOI] [PubMed] [Google Scholar]
- 6.Axelrod DA, Dzebisashvili N, Schnitzler MA, et al. The interplay of socioeconomic status, distance to center, and interdonor service area travel on kidney transplant access and outcomes. Clin J Am Soc Nephrol. 2010;5(12):2276–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Begaj I, Khosla S, Ray D, et al. Socioeconomic deprivation is independently associated with mortality post kidney transplantation. Kidney Int. 2013;84(4):803–809. [DOI] [PubMed] [Google Scholar]
- 8.Stephens MR, Evans M, Ilham MA, et al. The influence of socioeconomic deprivation on outcomes following renal transplantation in the United kingdom. Am J Transplant. 2010;10(7):1605–1612. [DOI] [PubMed] [Google Scholar]
- 9.Goldfarb-Rumyantzev AS, Koford JK, Baird BC, et al. Role of socioeconomic status in kidney transplant outcome. Clin J Am Soc Nephrol. 2006;1(2):313–322. [DOI] [PubMed] [Google Scholar]
- 10.Goldfarb-Rumyantzev AS, Sandhu GS, Barenbaum A, et al. Education is associated with reduction in racial disparities in kidney transplant outcome. Clin Transplant. 2012;26(6):891–899. [DOI] [PubMed] [Google Scholar]
- 11.Mistretta A, Veroux M, Grosso G, et al. Role of socioeconomic conditions on outcome in kidney transplant recipients. Transplant Proc. 2009;41(4):1162–1167. [DOI] [PubMed] [Google Scholar]
- 12.US Department of Labor. Median weekly earnings by educational attainment in 2014. US Bureau of Labor Statistics website. https://www.bls.gov/opub/ted/2015/median-weekly-earnings-by-education-gender-race-and-ethnicity-in-2014.htm. Accessed April 20, 2019. [Google Scholar]
- 13.Liberatos P, Link BG, Kelsey JL. The measurement of social class in epidemiology. Epidemiol Rev. 1988;10:87–121. [DOI] [PubMed] [Google Scholar]
- 14.Berkman LF, Macintyre S. The measurement of social class in health studies: old measures and new formulations. IARC Sci Pub. 1997(138):51–64. [PubMed] [Google Scholar]
- 15.Pardo-Crespo MR, Narla NP, Williams AR, et al. Comparison of individual-level versus area-level socioeconomic measures in assessing health outcomes of children in Olmsted County, Minnesota. J Epidemiol Community Health. 2013;67(4):305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geronimus AT. Invited commentary: Using area-based socioeconomic measures--think conceptually, act cautiously. Am J Epidemiol. 2006;164(9):835–840. [DOI] [PubMed] [Google Scholar]
- 17.Narla NP, Pardo-Crespo MR, Beebe TJ, et al. Concordance between Individual vs. Area-Level Socioeconomic Measures in an Urban Setting. J Health Care Poor Underserved. 2015;26(4):1157–1172. [DOI] [PubMed] [Google Scholar]
- 18.Juhn YJ, Beebe TJ, Finnie DM, et al. Development and initial testing of a new socioeconomic status measure based on housing data. J Urban Health. 2011;88(5):933–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.United States Census Bureau. American Fact Finder. https://factfinder.census.gov/faces/nav/jsf/pages/download_center.xhtml#none. Accessed April 20, 2019.
- 20.Residental Segregation. Diversity and Disparities Project. http://www.s4.brown.edu/us2010/segregation2010/Default.aspx?msa=10180. Accessed April 20, 2019.
- 21.MUA find. HRSA Data Warehouse website, http://datawarehouse.hrsa.gov/tools/analyzers/MuaSearchResults.aspx. Accessed April 20, 2019.
- 22.Olmsted County, Minnesota Community Health Needs Assessment 2013. https://www.co.olmsted.mn.us/OCPHS/reports/Documents/Community%20Health%20Needs%20Assessment%202013.pdf. Accessed April 21, 2019.
- 23.St Sauver JL, Grossardt BR, Leibson CL, et al. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clinic Proc. 2012;87(2):151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rocca WA, Yawn BP, St Sauver JL, et al. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clinic Proc. 2012;87(12):1202–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.St Sauver JL, Grossardt BR, Yawn BP, et al. Use of a Medical Records Linkage System to Enumerate a Dynamic Population Over Time: The Rochester Epidemiology Project. Am J Epidemiol. 2012;173(9):1059–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bang DW, Manemann SM, Gerber Y, et al. A novel socioeconomic measure using individual housing data in cardiovascular outcome research. Int J Environ Res Public Health. 2014;11(11):11597–11615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghawi H, Crowson CS, Rand-Weaver J, et al. A novel measure of socioeconomic status using individual housing data to assess the association of SES with rheumatoid arthritis and its mortality: a population-based case-control study. BMJ Open. 2015;5(4):e006469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takahashi PY, Ryu E, Hathcock MA, et al. A novel housing-based socioeconomic measure predicts hospitalisation and multiple chronic conditions in a community population. J Epidemiol Community Health. 2016;70(3):286–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ryu E, Juhn YJ, Wheeler PH, et al. Individual housing-based socioeconomic status predicts risk of accidental falls among adults. Ann Epidemiol. 2017;27(7):415–420.e412. [DOI] [PubMed] [Google Scholar]
- 30.Wi CI, Gauger J, Bachman M, et al. Role of individual-housing-based socioeconomic status measure in relation to smoking status among late adolescents with asthma. Ann Epidemiol. 2016;26(7):455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wi CI, St Sauver JL, Jacobson DJ, et al. Ethnicity, Socioeconomic Status, and Health Disparities in a Mixed Rural-Urban US Community-Olmsted County, Minnesota. Mayo Clinic Proc. 2016;91(5):612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Butterfield MC, Williams AR, Beebe T, et al. A two-county comparison of the HOUSES index on predicting self-rated health. J Epidemiol Community Health. 2011;65(3):254–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harris MN, Lundien MC, Finnie DM, et al. Application of a novel socioeconomic measure using individual housing data in asthma research: an exploratory study. NPJ Prim Care Respir Med. 2014;24:14018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson MD, Urm SH, Jung JA, et al. Housing data-based socioeconomic index and risk of invasive pneumococcal disease: an exploratory study. Epidemiol Infect. 2013;141(4):880–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hammer R, Capili C, Wi CI, et al. A new socioeconomic status measure for vaccine research in children using individual housing data: a population-based case-control study. BMC Public Health. 2016;16(1):1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris MN, Lundien MC, Finnie DM, et al. Application of a novel socioeconomic measure using individual housing data in asthma research: an exploratory study. NPJ Prim Care Respir Med. 2014;24:14018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Press R, Carrasquillo O, Nickolas T, et al. Race/ethnicity, poverty status, and renal transplant outcomes. Transplantation. 2005;80(7):917–924. [DOI] [PubMed] [Google Scholar]
- 38.Aitken E, Dempster N, Ceresa C, et al. The impact of socioeconomic deprivation on outcomes following renal transplantation in the West of Scotland. Transplant Proc. 2013;45(6):2176–2183. [DOI] [PubMed] [Google Scholar]
- 39.Laging M, Kal-van Gestel JA, van de Wetering J, et al. Understanding the influence of ethnicity and socioeconomic factors on graft and patient survival after kidney transplantation. Transplantation. 2014;98(9):974–978. [DOI] [PubMed] [Google Scholar]
- 40.Francis A, Didsbury M, Lim WH, et al. The impact of socioeconomic status and geographic remoteness on access to pre-emptive kidney transplantation and transplant outcomes among children. Pediatr Nephrol. 2016;31(6):1011–1019. [DOI] [PubMed] [Google Scholar]
- 41.Warnecke RB, Oh A, Breen N, et al. Approaching health disparities from a population perspective: the National Institutes of Health Centers for Population Health and Health Disparities. Am J Public Health. 2008;98(9):1608–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maldonado JR, Dubois HC, David EE, et al. The Stanford Integrated Psychosocial Assessment for Transplantation (SIPAT): a new tool for the psychosocial evaluation of pre-transplant candidates. Psychosomatics. 2012;53(2):123–132. [DOI] [PubMed] [Google Scholar]
- 43.National Academy of Medicine: Health and Medicine Division. Accounting for Social Risk Factors in Medicare Payment. 2017. [PubMed] [Google Scholar]
- 44.National Quality Fourm. Risk Adjustment for Socioeconomic Status or Other Sociodemographic Factors. Published 2014. [Google Scholar]
- 45.Kirchhoff SM, Congressional Research Service. Medicare Coverage of End-Stage Renal Disease (ESRD). https://fas.org/sgp/crs/misc/R45290.pdf. Published August 16, 2018. Accessed April 15, 2019. [Google Scholar]
- 46.Ash AS, Mick EO, Ellis RP, et al. Social Determinants of Health in Managed Care Payment Formulas. JAMA Intern Med. 2017;177(10):1424–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woodward RS, Page TF, Soares R, et al. Income-related disparities in kidney transplant graft failures are eliminated by Medicare’s immunosuppression coverage. Am J Transplant. 2008;8(12):2636–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scientific Registry of Transplant Recipients. SRTR Risk Adjustment Model Documentation: Posttransplant Outcomes. https://www.srtr.org/reports-tools/risk-adjustment-models-posttransplant-outcomes/. Accessed August 25, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.