Abstract
Background:
Effective postoperative analgesia leads to early mobilization, fewer pulmonary complications, and shorter hospital stay.
Aims:
We compared the analgesic effects of ultrasound-guided intercostal nerve (ICN) blocks, subcostal transversus abdominis plane (SCTAP) block, and a control group in open cholecystectomy.
Settings and Design:
This was a prospective, randomized controlled, double-blind, multi-arm and parallel study.
Materials and Methods:
The study was conducted on patients of American Society of Anaesthesiology Physical Status Classes I and II, either sex, 18–60 years of age, and body mass index 18–30 kg.m−2. Exclusion criteria were infection at the injection site, coagulopathy, thrombocytopenia, and allergy to the drugs used. Group I (n = 41) received ICN blocks, Group T (n = 41) SCTAP block, and Group C (n = 41) no postoperative block. The duration of analgesia was the primary outcome, and the analgesic consumption, the pain intensity, adverse events, and patient satisfaction were the secondary outcomes.
Statistical Analysis:
For the continuous data, analysis of variance was used for multiple group comparison and intergroup data were analyzed by Student's t-test. Kruskal-Wallis and Mann-Whitney U tests were applied for ordinal data. P = 0.05 or less was considered statistically significant.
Results:
The duration of postoperative analgesia was significantly longer in the ICN (mean = 441.6 min; 95% confidence interval [CI], 407.71, 475.49) and SCTAP block (mean = 417.6 min; 95% CI, 390.94, 444.26) as compared to control (mean = 33.98 min; 95% CI, 26.64, 41.32) (P = 0.00) with no significant intergroup difference between the two intervention groups (P = 0.278). The cumulative analgesic consumption was not significantly different between the intervention groups but was significantly reduced in the study groups when compared with the control group (P < 0.001). No notable adverse events were observed. Patients with both the techniques were very satisfied in comparison with the control group (P = 0.00).
Conclusion:
Both the ICN and SCTAP blocks have similar results in terms of analgesia and patient satisfaction for cholecystectomy.
Keywords: Early ambulation, intercostal nerve, pain management, postoperative pain, prospective studies
INTRODUCTION
Open surgeries still outnumber laparoscopic procedures in developing countries or places of limited resources. Various reasons such as technical difficulties, biliary tract injury, and bleeding also make it necessary to resort to a conversion of technique from laparoscopic to open cholecystectomy (between 0.8 and 12% according to different studies).[1] Postoperative analgesia is a challenging and unmet need in such cases.
Postoperative pain management is vital as several postoperative organ dysfunctions such as pulmonary, cardiac, and gastrointestinal are directly influenced by it. However, optimal postoperative analgesia modalities following cholecystectomy are not known. For postoperative analgesia in an open cholecystectomy, intercostal nerve (ICN) blocks have been used in the past.[2] The use of ultrasound increases the accuracy of these blocks. A relatively newer subcostal transversus abdominis plane (SCTAP) approach of the block has been studied for pain relief in upper abdominal surgeries with the use of ultrasonography (USG).[3]
To our knowledge, the effectiveness of ultrasound-guided ICN and SCTAP blocks has never been compared earlier in cases of open cholecystectomy. In this prospective comparative investigation, the aim was to compare the efficacy of USG-guided ICN and SCTAP blocks for postoperative pain relief in open cholecystectomy. A control group was also used for comparison to avoid confounding factors.
MATERIALS AND METHODS
This was a prospective, randomized, controlled, double-blinded, parallel, multi-arm study conducted in the anesthesia department of a tertiary care center. It was registered with the Clinical Trials Registry of India (CTRI/2018/08/015303). After approval from the Institutional Ethics Committee (L No. 400/2018-19) and obtaining written informed consent, patients posted for open cholecystectomy were enrolled from August 18, 2018, to August 17, 2019. All 140 patients of American Society of Anesthesiologists (ASA) physical status Classes I and II, either sex, ages 18–60 years, and with body mass index (BMI) 18–30 kg.m−2 were enrolled in this study. Exclusion criteria were localized infection at the proposed site of injection, patients with known coagulopathy or thrombocytopenia, inability to comprehend the scoring systems to be employed, inability to accurately describe postoperative pain to investigators (e.g., language barrier or neuropsychiatric disorder), known allergy to the drugs used, opioid tolerance/dependence, preexisting neuralgia, and a history of chronic pain.
A person not involved in the data collection or inpatient care randomly assigned the patients in blocks of 3, into three groups of equal size according to a computer-generated block randomization list provided by www.random.org.[4] The block size (3) was also randomized using a list of random numbers. The medication was prepared by a designated nurse who was not involved in direct patient care. The nurse opened the opaque sealed envelope containing group allocation and drew the study medication into syringes. Two of the anesthesiologists performed all intraoperative assessments, and two other investigators assessed and recorded all the postoperative data. Block size and randomization codes were not revealed to the investigators until all measurements and calculations had been entered into the database for all patients. The patients, investigators, and all medical caregivers were blinded to group allocation. All data were entered into the excel sheet prior to decoding the series of randomization.
Patients were explained about the concerned technique and were instructed to keep fasting for 6 h before surgery and premedicated with alprazolam 0.25 mg per oral the night before surgery. On the morning of surgery, premedication with alprazolam 0.25 mg and tablet ranitidine 150 mg per oral was done. In the operation theater, an intravenous (i.v.) line was established, and i.v. fluids were started. Standard ASA monitors were attached to the patients. Baseline heart rate (HR), mean arterial pressure (MAP), and oxygen saturation (SpO2) were recorded. Continuous electrocardiogram and temperature monitoring were also instituted.
Patients were administered midazolam 1 mg and fentanyl 2 μg.kg−1 i.v. After 3 min of preoxygenation, induction was carried with 2 mg.kg−1 propofol, and endotracheal intubation was facilitated using vecuronium bromide 0.1 mg.kg−1 body weight. Capnography was instituted after intubation. Anesthesia was maintained using nitrous oxide in oxygen (66%: 33%) along with isoflurane (0.5%–1.2%) and incremental doses of vecuronium bromide, as and when required. All the patients were given injections paracetamol 15 mg.kg−1 and ondansetron 0.15 mg.kg−1 i.v. 30 min before the expected completion of surgery.
At the end of the operation, patients were administered one of the blocks under study or no block according to the randomly allocated group. The anesthesiologist who administered the blocks was not involved in the encoding of the data, and the observers who recorded all pain scores were blinded to the used method. Blocks were performed using ultrasound machine (SonoSite M-Turbo, Bothell, Washington, USA) with a linear 6–13 MHz sterile transducer.
Group I (n = 41): Patients received USG-guided right-sided ICN blocks with 0.25% bupivacaine, 3 mL for each segment (T5–T11) using the mid-axillary approach
Group T (n = 41): Patients received USG-guided right-sided oblique SCTAP block with 20 mL of 0.25% bupivacaine
Group C (n = 41): Patients received no postoperative block.
In order to perform the blocks, abdominal skin was prepared with 5% povidone-iodine solution and covered with sterile drapes. ICN blocks were administered in the supine position. A linear ultrasound probe was placed in the sagittal plane, and the needle (Sonoplex STIM, Pajunk, Germany) of 80-mm 22G, was advanced in-plane caudal-cranial 1 cm away from the probe. The external, internal, and innermost intercostal muscle layers between ribs were identified. Power Doppler or B-mode was used to localize the blood vessels, keeping in mind that caudal to the arteries lie the ICN. Seeing the spread through the fascial space between the innermost intercostal muscle and internal intercostal muscle layers a total of 3 mL of the local anesthetic to each segment intended to block was injected, following negative aspiration for blood or air. An indentation in the pleura was often shown in the sonogram after the intercostal block was performed. The needle insertion points were marked at the level of T5–T11, 6–8 cm away from the midline.
Ultrasound-guided SCTAP block was also performed using a ultrasound linear array probe obliquely placed on the upper abdominal wall. The neurofascial layer was identified as lying between the rectus abdominis (posterior rectus sheath) and the transversus abdominis muscle. A 21G needle, (Sonoplex STIM, Pajunk, Germany) of 100 mm was advanced using an in-plane technique till the tip laid within the neurovascular fascial plane. After negative aspiration, a test injection with 1 mL of 0.9% normal saline was performed to confirm the needle location. Then, 20 mL of 0.25% bupivacaine was injected with intermittent aspiration every 3–5 mL while observing the expansion of the intermuscular plane.
The residual muscle relaxation was reversed at the end of surgery with neostigmine 0.05 mg.kg−1 and glycopyrrolate 0.01 mg.kg−1 body weight. After reversal of anesthesia, the sensory change was assessed on the side of the block, between dermatome T4 and dermatome L4. A blunt needle was used to test pinprick and cold with disinfectant swabs at 10, 20, and 30 min after the blocks by one clinical investigator. If the intended sensation did not decrease in surgical dermatomes after 30 min, the patient was regarded to have a failed block and was excluded from the study.
Postoperative pain was assessed using the verbal rating scale (VRS), consisting of 10-cm scale from 0 (no pain) to 10 (worst imaginable pain). Injection paracetamol 15 mg.kg−1 i.v. TDS was given to all the patients postoperatively. Rescue analgesia was diclofenac sodium 75 mg intramuscular when VRS >3. If that did not relieve pain, additional analgesia in the form of i.v. tramadol 100 mg was slowly administered. Diclofenac up to a maximum of 150 mg and tramadol up to a maximum of 400 mg could be administered in 24 h. If analgesia was still inadequate, injection morphine, 0.1 mg.kg−1 i.v., was administered.
The patient's satisfaction score was assessed using a 7-point Likert VRS ([1] extremely dissatisfied, [2] dissatisfied, [3] somehow dissatisfied, [4] undecided, [5] somehow satisfied, [6] satisfied, [7] extremely satisfied) after 24 h.
Hemodynamic parameters such as HR, MAP, SpO2, and respiratory rate (RR) were taken after the block, and patients were monitored up to 24 h after the block recording was done at 0 min, 15 min, 30 min, 60 min, 6 h, 12 h, and 24 h.
Adverse events such as bradycardia (HR <50 beats/min or 20% decrease from the baseline value), hypotension (fall in blood pressure by 20% from the baseline or an absolute MAP <60 mmHg), bradypnea (RR <8 breaths/min), desaturation (SpO2 <94%), nausea, vomiting, shivering, dryness of the mouth, or any other events during or after the procedure were also noted. Bradycardia was treated with incremental doses of injection Atropine 0.3 mg. Desaturation in any patient was treated with moist O2 inhalation at 5–6 L.min−1. Hypotension was managed with a bolus of i.v. crystalloids or with increments of injection mephentermine 6 mg i.v. The principles for intention-to-treat analysis were followed.
The primary outcome evaluated was the duration of analgesia compared among the ICN block, the SCTAP block, and the control group determined by the time to first rescue analgesia. Secondary outcomes were the total analgesic requirement, pain intensity VRS scores, complications if any, and patient's satisfaction score for 24 h.
The sample size was calculated through an a priori G*Power version 3.0.10 (G*Power, University of Dusseldorf). From the mean of previous studies, the duration of analgesia for the SCTAP block was 248.7 ± 44.0 min, and for the ICN block, the mean was 360 ± 177 min.[5,6] Assuming the alpha error to be 0.05 and the power of the study as 95%, the sample size determined was 72 for the two groups. To address the potential dropouts and withdrawals, we decided to recruit 40 patients per group. The sample size for the control group was also taken to be 40 to eliminate the confounding factors adequately.
Statistical analysis
The statistical analysis was done using Statistical Package for Social Science evaluation version 17.0. (SPSS, Inc., Chicago, IL, USA). The Kolmogorov–Smirnov test was applied to check for the normalcy of distribution and Levene's test for homogeneity of the study population. Parametric data with normal distribution were expressed as mean, standard deviation, and 95% confidence interval. The median and interquartile range was recorded for ordinal data. Frequencies were expressed as number and percentage. For the continuous data, one-way analysis of variance (ANOVA) was used for multiple group comparison, and intergroup data were analyzed by Student's t-test. For ordinal data, Kruskal-Wallis test was used for multiple group comparison and Mann-Whitney U test was applied for comparison between two groups. The goodness of fit was tested by Chi-square test. P = 0.05 or less was considered significant for statistical analysis.
RESULTS
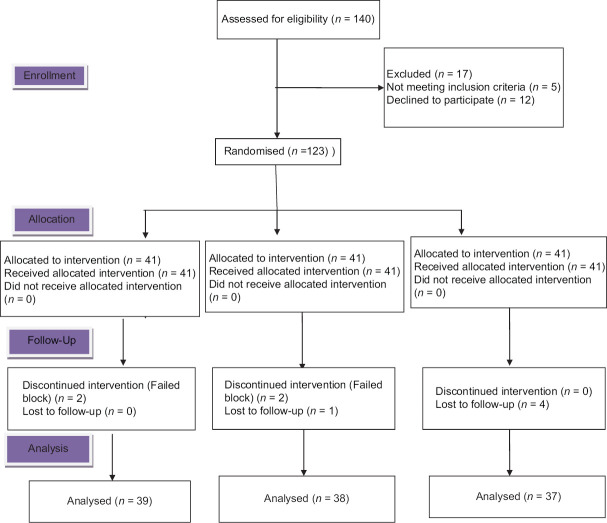
A total of 140 patients were assessed for eligibility; 5 did not meet the inclusion criteria; 12 declined to participate. A total of 9 patients were excluded from the final analysis because 5 of them were not wholly observed during the 30 min observation period, and four had failed blockade. Data were analyzed on 38 patients in Group I, 39 patients in Group T, and 37 patients in Group C [Figure 1].
Figure 1.
Consort flow diagram
No significant differences were observed in demographic data regarding age, sex, BMI, and ASA grading among the different groups. The majority of the study participants were female, owing to the higher incidence of cholelithiasis in female [Table 1]. There were no significant differences in incision length and the operative time between groups. The effect of ICN or SCTAP blockade was checked one by one in all the patients. None of the patients had the dermatome sensory distribution blockade as assessed by the pinprick approach in Group C. Two patients had failed blocks in Group T and two in Group I and were excluded from the final analysis.
Table 1.
Demographic profile
| Variables | Group I (n=38) | Group T (n=39) | Group C (n=37) | P |
|---|---|---|---|---|
| Age (years) | 44.43±8.25 | 45.08±6.99 | 42.52±7.75 | 0.326 (ANOVA) |
| Sex (female/male) | 33/5 | 31/8 | 31/6 | 0.685 (χ2) |
| ASA (1/2) | 32/6 | 34/5 | 29/8 | 0.58 (χ2) |
| BMI (kg.m−2) | 25.51±8.02 | 23.41±10.47 | 22.15±8.28 | 0.268 (ANOVA) |
Data are presented as mean±SD and frequency. ASA=American Society of Anesthesiologists, BMI=Body mass index, SD=Standard deviation
There was a significant difference among the groups in the duration of analgesia, the primary outcome (P = 0.00; df = 111). Patients receiving the ICN block (Group I) had a longer duration of analgesia (mean = 441.6; 95% CI, 407.71, 475.49) as compared with the SCTAP block (Group T) (mean = 417.6; 95% CI, 390.94, 444.26), but the difference between the two was insignificant (P = 0.278; df = 75). Both Groups I and T had a significantly longer duration of analgesia when compared with that of the control group (Group C) (mean = 33.98; 95% CI, 26.64, 41.32) (P = 0.00; df = 74 in both). Figure 2 shows the duration of analgesia in the study groups.
Figure 2.
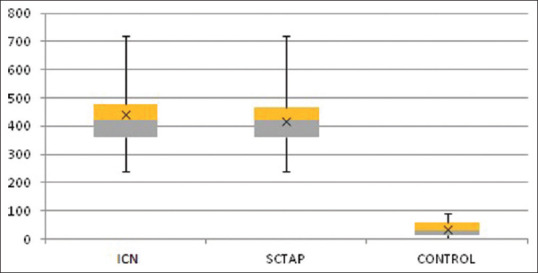
Duration of analgesia (min)
Kaplan–Meier curve for the proportion of patients not receiving the first analgesic request after 1 h and up to 24 h recorded and censored at the 6th and 12th h is presented in Figure 3. No significant difference was demarcated between the curves (log e-rank test, P = 0.78; df = 75) in Group I and Group T. The difference was highly significant between Group I and Group C (P = 0.01; df = 74) and Group T and Group C (P = 0.01; df = 73).
Figure 3.
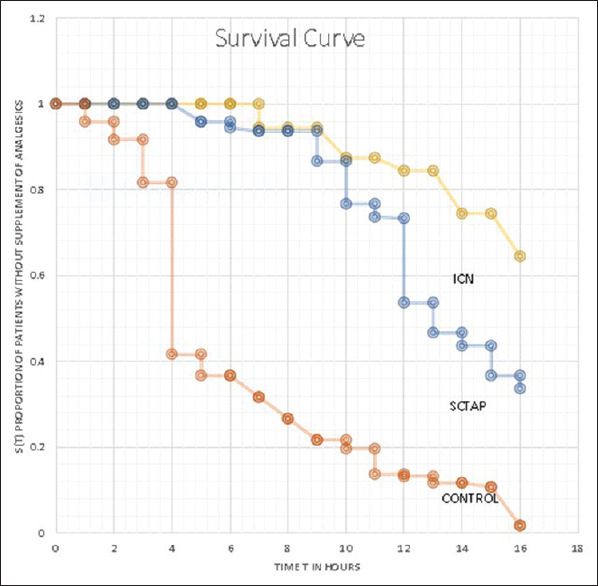
First analgesia requirement at different time intervals
For the determination of cumulative postoperative analgesic consumption, equianalgesic doses were used. A dose of 100 mg of tramadol was taken to be equivalent to 150 mg of diclofenac, each of which was considered to be equal in analgesic efficacy to 10 mg of morphine, and a score of 1 was allotted.[7,8] In this manner, the analgesic consumption in 24 h was formulated. The total tramadol and diclofenac consumption were significantly different among the groups, as shown in Figure 4 (P = 0.000; df = 111). The postoperative analgesic consumption in Group I with the median value of the score obtained was 1 (1.25) was less than that in Group T, 1.5 (1), but the difference was not significant (P = 0.185). There was a highly significant difference in analgesic consumption between Group I and Group C with median value 2.5 (1) (P < 0.001; df = 74) and also between Group T and Group C (P = 0.001; df = 73) [Figure 4]. Group C required significantly more rescue analgesia compared to Groups I and T.
Figure 4.
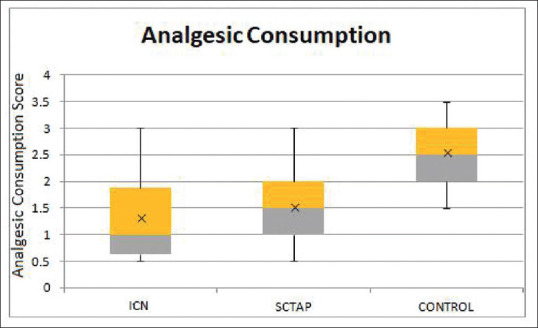
Cumulative analgesic consumption by the groups during 24 hours postoperatively
A Kruskal–Wallis test revealed a significant difference in VRS score at rest in the ICN, SCTAP, and nonblock groups (all P < 0.01; df = 111) [Table 2]. Both Groups I and T delivered excellent analgesia at rest compared with Group C. The SCTAP block and ICN block had significantly reduced postoperative VRS pain scores at all time points. However, when intergroup analysis of Groups I and T was done, the difference was significant in the first 6 h but not after that time.
Table 2.
Comparison median (interquartile range) of verbal rating scale score at different time intervals
| Time (min) | Group I (n=38) | Group T (n=39) | Group C (n=37) | P | |||
|---|---|---|---|---|---|---|---|
| I and T* | T and C* | I and C* | I, T and C† | ||||
| 0 min | 1 (2) | 1 (1) | 3 (2) | 0.08 | 0.000 | 0.000 | 0.000 |
| 15 min | 0 (1) | 1 (1) | 3 (0) | 0.000 | 0.000 | 0.000 | 0.000 |
| 30 min | 0 (0) | 0 (1) | 4 (1) | 0.000 | 0.000 | 0.000 | 0.000 |
| 60 min | 0 (1) | 1 (1) | 3 (1) | 0.005 | 0.000 | 0.000 | 0.000 |
| 6 h | 3 (1) | 3 (2) | 4 (2) | 0.002 | 0.038 | 0.015 | 0.007 |
| 12 h | 4 (2) | 4 (2) | 5 (2) | 0.46 | 0.000 | 0.000 | 0.000 |
| 24 h | 4 (1) | 4 (1) | 5 (1) | 0.4 | 0.000 | 0.000 | 0.000 |
*Mann–Whitney U-test; †Kruskal–Wallis test
Table 3 shows the analysis of satisfaction scores between the groups, which was highly significant, and patients receiving both SCTAP and ICN block were extremely satisfied when compared with no block group (P = 0.00; df = 111).
Table 3.
Patient satisfaction
| Groups | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| ICN (%) (n=38) | - | - | - | 1 (2.7) | 6 (15.7) | 17 (44.8) | 14 (36.8) |
| TAP (%) (n=39) | - | - | - | 2 (5.3) | 12 (30.7) | 13 (33.3) | 12 (30.7) |
| Control (%) (n=37) | - | - | 8 (21.6) | 18 (48.6) | 9 (24.4) | 3 (8.4) | - |
| χ2 (df) | 79.2 (2) | ||||||
| P | 0.000 | ||||||
Patient satisfaction score: 1=Extremely dissatisfied, 2=Dissatisfied, 3=Somehow dissatisfied, 4=Undecided, 5=Somehow Satisfied, 6=Satisfied, 7=Extremely satisfied, df=Degree of freedom, ICN=Intercostal nerve, TAP=Transversus abdominis plane
DISCUSSION
Cholecystectomy is one of the most commonly performed surgeries worldwide, especially in India. To our knowledge, this was the first double-blinded randomized controlled study to compare the use of the ICN and SCTAP blockade for patients undergoing open cholecystectomy.
The duration of postoperative analgesia was significantly longer in the ICN and SCTAP block as compared to that of control (P = 0.00), with no significant intergroup difference between the two intervention groups. The cumulative analgesic consumption was significantly reduced in the study groups when compared with that of the control group (P < 0.001) but was not significantly different between the intervention groups. There were no notable adverse events. Patients with both the chosen techniques were very satisfied when compared with that of the control group (P = 0.00).
In the study conducted by Jemal et al., the median time for the first analgesic request by Kaplan–Meier analysis after ICN block for cholecystectomy was 6 h, which was closer to what we observed.[6] Contrary to this, Angral et al. found the time to first analgesic request to be 9.45 ± 4.44 h.[9] This might be due to the difference in local anesthesia drug concentration and the surgical procedure (laparoscopic cholecystectomy vs. open cholecystectomy). Another study reported that time to the first analgesic request after the ICN block to be 225 min. One reason for this difference in the duration of time compared to our study may be due to the technique used for ICN (single interspace intercostal block vs. multiple injection intercostal block).[9]
Classic transversus abdominis plane (TAP) block has been broadly accepted in patients who have undergone lower abdominal surgery, as part of the multimodal approach for perioperative analgesia, with a significant decrease in the requirement of opioids within the first 24 h postoperatively.[10,11] An evident decrease of opioid-related side effects, such as postoperative nausea and vomiting, is seen. There are three conventional approaches to perform a TAP blockade: subcostal, classic midaxillary, and ilioinguinal-iliohypogastric. There is variation in the distribution of local anesthetic, and the extent of sensory blockade among these three approaches.[12,13] For the upper abdomen operation, Hebbard originally described the oblique subcostal transverses abdominis plane (OSTAP) approach in 2008, which was thought to exhibit adequate analgesia involving dermatomes T6–T10 (for upper abdominal surgery).[14] Of late, the OSTAP block has been reported to produce analgesia better than the traditional TAP block or i.v. opioid analgesia during the first postoperative 24 h period in patients undergoing laparoscopic cholecystectomy.[15] We chose the subcostal approach to perform a TAP block for open cholecystectomy based on this investigation.[12,13]
Our results were similar to those of the previous findings where TAP blocks have been described to last from 6 h to 24 h using ropivacaine.[12,16,17] Lee et al. demonstrated that maximal dermatome spread was observed at 30 min, which usually regressed by 24 h after SCTAP block.[16] Although few studies have shown the analgesic effect lasting more than 24 h, Stoving et al. demonstrated that the blockade duration of ultrasound-guided single TAP block with 20 mL, 7.5 mg.mL−1 ropivacaine was approximately 10-h with a large variation in healthy volunteers.[18,19] The duration of the peripheral nerve block depends on factors such as the choice of local anesthetics, the site of injection, and the presence of adjuncts, which might be responsible for the differences observed in the duration of analgesia.
Although the two techniques, ICN block and SCTAP block, are different, both target the same lower intercostals nerves in this study. Because in ICN blockade the local anesthetics are more in the vicinity of the nerves, we had expected denser block with prolonged effect in ICN block as compared to SCTAP block. However, the duration was more in ICN block than that in SCTAP block, but it did not differ significantly.
The study done by Jemal et al. demonstrated that ICN decreases pain intensity for the first 6-h and analgesic consumption for up to 24 h during the postoperative period in patients undergoing open cholecystectomy. The median tramadol and diclofenac consumption and pain scores were less in the intercostals block group in the study done by Jemal et al.[6] We had similar findings. Few other previous studies that have examined the the analgesic effects of conventional ICN in patients undergoing open cholecystectomy reported comparable results.[9]
A meta-analysis about the analgesic efficacy of TAP in 31 controlled trials, including 1611 adult participants undergoing abdominal laparotomy, abdominal laparoscopy, or cesarean delivery, was done. It showed that at rest and on movement, pain scores reduced at 6 h postoperatively.[20] Suseela et al. compared the analgesic efficacy of SCTAP block with port-site infiltration of local anesthetic and found it to provide a longer duration of analgesia with lesser requirement of tramadol and lower pain score.[21] Compared with Group C, cumulative analgesic consumption and VRS pain scores till 24 h postoperatively decreased significantly in Group T in our study.
Significantly lower pain scores in the case of ICN blockade as compared to SCTAP block in the first 6 h could also be because of the vicinity of the local anesthetic to the ICN in case of the former as compared to the latter.
The cumulative analgesic consumption, pain scores after 6 h, as well as the patient satisfaction did not vary significantly between the two techniques, though the drugs were more in the vicinity of the nerves in ICN blocks, as the lesser total volume of local anesthetic was used in ICN block than that in SCTAP block.
In the present study, the sensory block was assessed 10 min, 20 min, and 30 min after the block was administered, and two patients in Group I and two in Group T were excluded from the final analysis because of a failed blockade. We opted to assess the effect of the blocks till 30 min of performance of blocks as Lee et al. found that in the SCTAP block, a maximum dermatomal spread was observed at 30 min.[16] We expected the same in the case of ICN block too.
One disadvantage of the ICN technique was the multiple-site injection. SCTAP block was simpler in this respect being a single-injection technique. ICN block can be administered by single-injection technique as done in the previous study where ICN block with a single puncture was done by placing the local anesthetic into the space between the serratus muscle and external intercostal muscles, but the efficacy of this as compared to multiple injection technique is expected to be less and needs to be evaluated.[22]
One of the main concerns about the ICN or SCTAP blockade is the systemic toxicity of the local anesthetics. The study by Griffiths et al. reported potentially toxic ropivacaine concentrations following the use of TAP blocks in gynecologic surgery when a total dose of ropivacaine (3 mg.kg−1) was used.[23] Toju et al. found that the administration of ropivacaine at 3 mg.kg−1 during OSTAP led to rapid increases in its plasma concentration during the first 2 h after the block, and the Cmax almost reached the threshold for systemic toxicity.[24,25] Therefore, the first limitation of our study was that we did not measure plasma bupivacaine concentrations in the groups postoperatively. However, none of the patients had symptoms of local anesthetic systemic toxicity (e.g., tinnitus, seizures, and cardiovascular collapse) either immediately following the ICN or SCTAP block or in the postanesthesia care unit postoperatively. In addition, these data signify the experience at a single academic institution with ASA I and II or patients with normal BMI, and may not be generalized to the broader population. The modest duration of a single-shot ICN and SCTAP blocks is a noteworthy limitation. ICN and SCTAP blocks with a continuous catheter or with long-acting local anesthetics need to be further investigated. We have not determined the analgesic scores during movement, but these scores are expected to be similarly varying in the groups as in resting condition because none of these blocks offer visceral analgesia and just provide sensory blockade of the abdominal wall.[24] The lack of visceral pain analgesia may require the use of additional methods of postoperative pain control.
CONCLUSION
Multiple injection techniques of the thoracic ultrasound-guided ICN block are an effective analgesic technique for open cholecystectomy surgery as SCTAP block, if not significantly better. Both SCTAP and ICN blocks are equally effective techniques with longer postoperative analgesia request time, total analgesic dose requirements, and reduced postoperative pain than those not receiving any block. Both are analgesic and opioid sparing without apparent side effects. The authors, therefore, support the two techniques as valuable alternatives to each other for open cholecystectomy postoperative pain management as part of multimodal analgesia. One can prefer SCTAP block due to its single-injection technique.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Fernández Martín MT, López Álvarez S, Mozo Herrera G, Platero Burgos JJ. Ultrasound-guided cutaneous intercostal branches nerves block: A good analgesic alternative for gallbladder open surgery. Rev Esp Anestesiol Reanim. 2015;62:580–4. doi: 10.1016/j.redar.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 2.Chakraborty A, Khemka R, Datta T. Ultrasound-guided truncal blocks: A new frontier in regional anaesthesia. Indian J Anaesth. 2016;60:703–11. doi: 10.4103/0019-5049.191665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krige A, Daugherty M. Truncal Blocks: Rectus Sheath Catheters. Analgesia in Major Abdominal Surgery. Cham: Springer International Publishing; 2018. pp. 193–215. [Google Scholar]
- 4.Haahr M. Random.org. [Last accessed on 2020 Jun 09]. Available from: https://www.random.org/mads/
- 5.Kamhawy G, El-Taher E, Abdelrahman M. A comparison of oblique subcostal transversus abdominis plane block versus thoracic paravertebral block for postoperative analgesia after open cholecystectomy. Egypt J Anaesth. 2017;33:323–9. [Google Scholar]
- 6.Jemal B, Woldeyohanes M, Shitemaw T, Ayalew N, Awoke Z, Abiy S. Effectiveness of thoracic paravertebral and intercostal nerve blocks as a part of postoperative analgesia in patients undergoing open cholecystectomy under general anesthesia in Addis Ababa, Ethiopia: A prospective cohort study, 2018. Int J Surg Open. 2019;18:1–8. [Google Scholar]
- 7.Equianalgesic. In: Wikipedia. 2020. [Last accessed on 2020 Jun 19]. Available from: https://en.wikipedia.org/w/index.php?title=Equianalgesic&oldid=962241131 .
- 8.Tripathi KD. Essentials of Medical Pharmacology. 7th ed. New Delhi: Jaypee Brothers Medical Publishers (P) Ltd; 2013. [Google Scholar]
- 9.Angral R, Lachala S, Gupta S. Postoperative analgesic technique in laparoscopic cholecystectomy: Comparison of local instillation with bupivacaine vs intravenous butorphanol vs intercostal nerve block with bupivacaine. Sri Lankan J Anaesthesiol. 2013;19:29. [Google Scholar]
- 10.Chou R, Gordon DB, de Leon-Casasola OA, Rosenberg JM, Bickler S, Brennan T, et al. Management of Postoperative Pain: A Clinical Practice Guideline From the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council. J Pain. 2016;17:131–57. doi: 10.1016/j.jpain.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Baeriswyl M, Kirkham KR, Kern C, Albrecht E. the analgesic efficacy of ultrasound-guided transversus abdominis plane block in adult patients: A meta-analysis. Anesth Analg. 2015;121:1640–54. doi: 10.1213/ANE.0000000000000967. [DOI] [PubMed] [Google Scholar]
- 12.Abdallah FW, Chan VW, Brull R. Transversus abdominis plane block: A systematic review. Reg Anesth Pain Med. 2012;37:193–209. doi: 10.1097/AAP.0b013e3182429531. [DOI] [PubMed] [Google Scholar]
- 13.Urigel S, Molter J. Transversus abdominis plane (TAP) blocks. AANA J. 2014;82:73–9. [PubMed] [Google Scholar]
- 14.Hebbard P. Subcostal transversus abdominis plane block under ultrasound guidance. Anesth Analg. 2008;106:674–5. doi: 10.1213/ane.0b013e318161a88f. [DOI] [PubMed] [Google Scholar]
- 15.Shin HJ, Oh AY, Baik JS, Kim JH, Han SH, Hwang JW. Ultrasound-guided oblique subcostal transversus abdominis plane block for analgesia after laparoscopic cholecystectomy: A randomized, controlled, observer-blinded study. Minerva Anestesiol. 2014;80:185–93. [PubMed] [Google Scholar]
- 16.Lee TH, Barrington MJ, Tran TM, Wong D, Hebbard PD. Comparison of extent of sensory block following posterior and subcostal approaches to ultrasound-guided transversus abdominis plane block. Anaesth Intensive Care. 2010;38:452–60. doi: 10.1177/0310057X1003800307. [DOI] [PubMed] [Google Scholar]
- 17.Li M, Wan L, Mei W, Tian Y. Update on the clinical utility and practical use of ropivacaine in Chinese patients. Drug Des Devel Ther. 2014;8:1269–76. doi: 10.2147/DDDT.S57258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sinha S, Palta S, Saroa R, Prasad A. Comparison of ultrasound-guided transversus abdominis plane block with bupivacaine and ropivacaine as adjuncts for postoperative analgesia in laparoscopic cholecystectomies. Indian J Anaesth. 2016;60:264–9. doi: 10.4103/0019-5049.179464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Støving K, Rothe C, Rosenstock CV, Aasvang EK, Lundstrøm LH, Lange KH. Cutaneous sensory block area, muscle-relaxing effect, and block duration of the transversus abdominis plane block: A randomized, blinded, and placebo-controlled study in healthy volunteers. Reg Anesth Pain Med. 2015;40:355–62. doi: 10.1097/AAP.0000000000000252. [DOI] [PubMed] [Google Scholar]
- 20.Guo JG, Li HL, Pei QQ, Feng ZY. The analgesic efficacy of subcostal transversus abdominis plane block with Mercedes incision. BMC Anesthesiol. 2018;18:36. doi: 10.1186/s12871-018-0499-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suseela I, Anandan K, Aravind A, Kaniyil S. Comparison of ultrasound-guided bilateral subcostal transversus abdominis plane block and port-site infiltration with bupivacaine in laparoscopic cholecystectomy. Indian J Anaesth. 2018;62:497–501. doi: 10.4103/ija.IJA_55_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johansson A, Renck H, Aspelin P, Jacobsen H. Multiple intercostal blocks by a single injection? A clinical and radiological investigation. Acta Anaesthesiol Scand. 1985;29:524–8. doi: 10.1111/j.1399-6576.1985.tb02247.x. [DOI] [PubMed] [Google Scholar]
- 23.Griffiths JD, Barron FA, Grant S, Bjorksten AR, Hebbard P, Royse CF. Plasma ropivacaine concentrations after ultrasound-guided transversus abdominis plane block. Br J Anaesth. 2010;105:853–6. doi: 10.1093/bja/aeq255. [DOI] [PubMed] [Google Scholar]
- 24.Toju K, Shiraishi K, Hakozaki T, Isosu T, Murakawa M. Plasma ropivacaine concentration following ultrasound-guided subcostal transversus abdominis plane block in adults. J Anesth. 2015;29:146–8. doi: 10.1007/s00540-014-1864-0. [DOI] [PubMed] [Google Scholar]
- 25.Finnerty O, Carney J, McDonnell JG. Trunk blocks for abdominal surgery. Anaesthesia. 2010;65(Suppl 1):76–83. doi: 10.1111/j.1365-2044.2009.06203.x. [DOI] [PubMed] [Google Scholar]