Abstract
Objective:
To use the phenotyping data from the MAPP-II Symptom Patterns Study (SPS) to compare the systemic features between UCPPS with Hunner lesion (HL) versus those without HL.
Methods:
We performed chart review on 385 women and 193 men with UCPPS who enrolled in the MAPP-II SPS. 223 had cystoscopy and documentation of HL status. Among them, 12.5% had HL and 87.5% did not.
Results:
UCPPS participants with HL were older, had increased nocturia, higher Interstitial Cystitis Symptom and Problem Indexes, and were more likely to report “painful urgency” compared to those without HL. On the other hand, UCPPS without HL reported more intense non-urologic pain, greater distribution of pain outside the pelvis, greater numbers of comorbid chronic overlapping pain conditions, higher fibromyalgia-like symptoms, and greater pain centralization, and were more likely to have migraine headache than those with HL. UCPPS without HL also had higher anxiety, perceived stress, and pain catastrophizing than those with HL. There were no differences in sex distribution, UCPPS symptom duration, intensity of urologic pain, distribution of genital pain, pelvic floor tenderness on pelvic examination, quality of life, depression, pain characteristics (nociceptive pain versus neuropathic pain), mechanical hypersensitivity in the suprapubic area during quantitative sensory testing, and 3-year longitudinal pain outcome and urinary outcome between the two groups.
Conclusions:
UCPPS with HL displayed more bladder-centric symptom profiles, while UCPPS without HL displayed symptoms suggesting a more systemic pain syndrome. The MAPP-II SPS phenotyping data showed that Hunner lesion is a distinct phenotype from non-Hunner lesion.
Keywords: Ulcerative interstitial cystitis, interstitial cystitis, chronic prostatitis, Hunner lesion, clinical phenotyping, personalized medicine
Introduction:
Patients with urologic chronic pelvic pain syndrome (UCPPS, which includes interstitial cystitis/bladder pain syndrome [IC/BPS] and chronic prostatitis/ chronic pelvic pain syndrome [CP/CPPS]) are heterogeneous. About 10-20% of UCPPS patients who underwent cystoscopy have one or more Hunner lesion (HL) or “classic interstitial cystitis” inside the bladder. Hunner lesion (also called “classic IC”, “ulcerative IC”, or ESSIC type 3C) is described as a “circumscript, reddened mucosal area having small vessels radiating towards a central scar, and/or fibrin deposit of coagulum attached to this area.”1 HL is typically red stellate in appearance, but may be linear, or bleed like a red waterfall with bladder distention. HL represents a localized, distinct area that is visible on cystoscopy, and histopathology revealed inflammation.1–5
Over three decades ago, Fall et al have described IC/BPS as a heterogeneous syndrome, and illustrated some of the differences between patients with and without HL.6 It has been hypothesized that UCPPS patients with HL might represent a distinct phenotype from those without HL.7–9 Although studies showed that HL patients are typically older and have more severe bladder-centric symptoms (e.g., increased nocturia), it is not clear whether non-HL patients have more systemic manifestation than HL since the data in the literature are conflicting. For example, Lai et al showed that irritable bowel syndrome was less common among HL, but Doiron et al and Peters et al did not show a difference.7–9 Two recent literature reviews did not provide definitive answer to the question of whether non-HL patients have more systemic manifestation than HL, or not.10, 11 To test the hypothesis that non-HL represents a different clinical phenotype from HL, with HL being a more bladder-centric disease while non-HL a systemic pain syndrome, we need more data on their systemic presentation.
The MAPP (Multidisciplinary Approach to the Study of Chronic Pelvic Pain) Research Network Phase II Symptom Patterns Study (SPS) is the most comprehensive phenotyping effort of UCPPS to date. The MAPP-II SPS has collected detailed information on over 600 UCPPS participants, including their non-urologic symptoms, chronic overlapping pain conditions (COPC), intensity and distribution of their systemic pain, pain characteristics (e.g., neuropathic pain versus nociceptive pain), psychosocial symptoms (e.g., stress and catastrophizing), and their quantitative sensory testing (QST) and neuroimaging profiles, plus their 3-year longitudinal pain and urinary outcomes, in addition to their HL status.12 Many of these non-urologic domains have not been examined in details in previous comparative studies. Here we have used the deep phenotyping data from the MAPP-II SPS to address the question whether UCPPS without HL have more systemic manifestation than those with HL.
Methods:
Participants.
The MAPP-II Symptom Pattern Study (SPS) enrolled 385 women and 193 men with UCPPS at six clinical sites across the United States from 2014 to 2019. Participants have a clinical diagnosis of interstitial cystitis/bladder pain syndrome (IC/BPS) in men or women, or chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) in men. The inclusion and exclusion criteria, study design, questionnaires, and detailed study procedures have been described by Clemens et al (2020).12 All participants provided written informed consent following IRB-approved protocols.
Hunner lesion(s) assessment.
We performed medical chart review on UCPPS participants who enrolled in the MAPP-II Study to determine if cystoscopy was performed as part of their clinical care; and if so, whether one or more Hunner lesion (HL) was observed during cystoscopy with or without hydrodistention. Participants who did not have cystoscopy or in whom their HL status was not known based on medical records were excluded from this analysis. MAPP-II participants with HL and without HL were compared.
Measures.
The MAPP-II measures have been described in details by Clemens et al (2020).12 Briefly, UCPPS severity was characterized on the two distinct dimensions of pain and urinary symptoms using the MAPP Pain Severity Score & MAPP Urinary Severity Score respectively.13 Chronic overlapping pain syndromes (COPC), which include irritable bowel syndrome, fibromyalgia, chronic fatigue syndrome, migraine headache, temporomandibular joint disorder, and vulvodynia (in females) were assessed using the Complex Multiple Symptoms Inventory (CMSI) modules.14 The presence and distribution of systemic pain, genital pain, and pelvic floor tenderness was assessed using a CHOIR whole body map,15 gender-specific genital map, and standardized pelvic floor examination, respectively. Widespread pain was operationalized as having pain in 2 or more non-pelvic regions on the CHOIR whole body map. Anxiety and depression were assessed using the Hospital Anxiety and Depression Scale (HADS). Stress and pain catastrophizing were assessed using the Perceived Stress Scale (PSS) and Coping Strategies Questionnaires (CSQ) respectively. Quality of life measures included the SF-12 and GenitoUrinary Pain Index (GUPI).
The Pain Detect Questionnaire (0-38) was used to describe pain characteristics as nociceptive pain (≤12) versus neuropathic pain (≥19).16 Fibromyalgia Symptom scale (FS, 0-31)17, along with its two subscales (Symptom Severity scale SS, and Widespread Pain Index WPI), were used to assess the construct of “fibromyalgia-ness” as a surrogate measure of pain centralization.18 The median of 7 on the Fibromyalgia Symptom scale was used to classify patients as low (≤7) versus high (>7) pain centralization, indicating the degree of central augmentation on the pain experience. Quantitative sensory testing (QST) was used to assess for segmental mechanical hypersensitivity of the suprapubic area. A hand-held algometer with a flat rubber probe was used to deliver a series of pressure stimuli (2 kg, 4 kg) in pseudo-random order to the suprapubic area and to a control site on the forearm. Participants rated the magnitude of pain evoked by each pressure stimulus on a numerical rating scale from 0 to 100 (0 = no pain; 100 = pain as bad as you can imagine).19 An increase in pressure pain ratings indicated segmental mechanical hypersensitivity to the suprapubic area.
Statistical Analyses.
Means and standard deviations were reported for continuous variables and relative frequencies (percentages) for categorical variables. Student’s t-tests and Chi-square tests (or Fisher’s exact tests, if applicable) were used. For the longitudinal symptom pattern comparisons, a dynamic functional clustering algorithm (GitHub https://github.com/jialinyi94/FTSC) was used to classify n=578 UCPPS participants from the MAPP II SPS into 3 unique subgroups (improve, stable, worse), separately for urological pain severity and urinary symptoms severity, using the 3-year longitudinal symptom data. Analyses used SAS software 9.4 (SAS Institute, Cary, NC).
Results:
Among the 385 women and 193 men with UCPPS who enrolled in the MAPP-II SPS, 223 (38.5%) had cystoscopy and documentation of their HL status. Among them, 12.5% (20 women, 8 men) had HL. Among those with and without HL, 28.6% and 25.6% were men respectively, with no sex difference (p=0.74). Their age distribution was shown in Figure 1. The prevalence of HL increased with age. For those with age <50, less than 1 in 20 had HL (4.0%). For those with age 50-59 and 60-69, about 1 in 5 had HL (19.0% and 20.5%). For those with age ≥70, more than half (54.5%) had HL.
Figure 1:
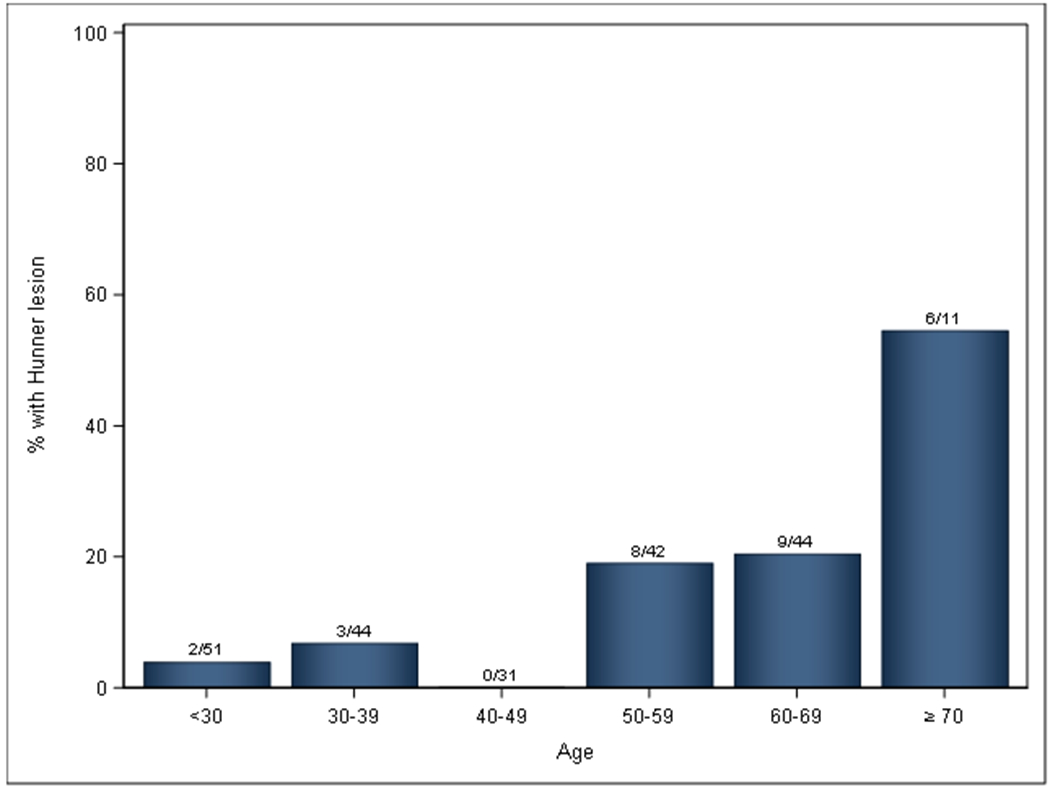
Age distribution of Hunner lesion by decade (No. with HL/No. with known HL status)
Supplemental Table 1 compared the demographics and urologic symptoms between UCPPS participants with and without HL. UCPPS with HL were older (58.0 vs. 43.6), had more nocturia (ICSI question: 3.5 vs. 2.0), higher Interstitial Cystitis Symptom Index (12.7 vs. 9.8) and Problem Index (10.4 vs. 8.4), and were more likely to have “painful urgency” (85.7% vs. 66.2%) compared to those without HL. “Painful urgency” refers to increased urinary urgency due to pain, pressure, or discomfort instead of fear of incontinence.20 There were no differences in UCPPS symptom duration, intensity of urologic pain, distribution of genital pain, pelvic floor tenderness on pelvic examination, and quality of life between UCPPS with and without HL.
Table 1 compared the systemic presentation of UCPPS participants with and without HL. UCPPS without HL reported more intense non-urologic pain (3.6 vs. 1.8, on a 0-10 pain scale), greater distribution of pain outside the pelvis on the whole body map (7.5 vs. 3.5 pain sites, 2.5 vs. 1.0 pain regions), greater percentages with widespread pain (57.1% vs. 18.5%), greater numbers of COPC (1.2 vs. 0.6), higher Fibromyalgia Symptom scale (FS, 8.7 vs. 5.5) and its two subscales (Fibromyalgia Symptom Severity scale, SS, 5.7 vs. 4.4; Widespread Pain Index, WPI, 2.8 vs. 1.0), greater pain centralization (FS >7, 50.3% vs. 25.9%), and were more likely to have migraine headache (38.9% vs. 11.5%) than those with HL. There was no difference in the percentages with Pain Detect ≤12 (nociceptive pain) or ≥19 (neuropathic pain) between the two groups. With respect to psychosocial symptoms, UCPPS without HL also had higher anxiety (HADS 7.2 vs. 4.1), perceived stress (PSS: 15.9 vs. 12.5), and pain catastrophizing (CSQ: 11.9 vs. 8.3) than those with HL, but there was no difference in depression. For QST, there were no differences in mechanical sensitivity in the suprapubic area or the forearm control site between the HL and non-HL groups (no differences in pressure pain ratings to 2 kg and 4 kg pressure stimuli).
Table 1:
Comparison of systemic presentation and longitudinal trends of UCPPS with and without HL.
| UCPPS without HL (n=195) |
UCPPS with HL (n=28) |
p-value | |
|---|---|---|---|
| Non-Urologic Pain Intensity: | |||
| Overall non-pelvic pain (numeric ratings, 0-10) (SD) | 3.6 (2.7) | 1.8 (2.1) | 0.0006* |
| Non-Urologic Pain Outside the Pelvis: | |||
| No. of non-pelvic pain sites (0-76) | 7.5 (8.7) | 3.5 (5.0) | 0.019* |
| No. of non-pelvic pain regions (0-12) | 2.5 (2.6) | 1.0 (1.6) | 0.0029* |
| % with widespread pain (≥2 non-pelvic regions) | 108 (57.1%) | 5 (18.5%) | 0.0002* |
| COPC (Chronic Overlapping Pain Conditions): | |||
| Irritable bowel syndrome | 75 (40.3%) | 9 (34.6%) | 0.58 |
| Fibromyalgia | 9 (4.7%) | 1 (3.6%) | 0.80 |
| Chronic fatigue syndrome | 29 (15.3%) | 2 (8.0%) | 0.33 |
| Migraine headache | 74 (38.9%) | 3 (11.5%) | 0.0062* |
| Temporomandibular joint disorder | 54 (28.1%) | 3 (11.1%) | 0.059 |
| Vulvodynia (in females) | 21 (16.2%) | 4 (20.0%) | 0.67 |
| Presence of at least one COPC? | 123 (63.1%) | 11 (39.3%) | 0.016* |
| No. of COPC | 1.3 (1.4) | 0.8 (1.1) | 0.039* |
| Pain Characteristics: | |||
| Nociceptive pain (Pain Detect ≤12) | 135 (69.2%) | 21 (75.0%) | 0.19 |
| Neuropathic pain (Pain Detect ≥19) | 25 (12.8%) | 2 (7.1%) | 0.39 |
| Pain Detect scores | 10.0 (7.0) | 8.2 (5.9) | 0.19 |
| Fibromyalgia Symptom scale (FS, 0-31) | 8.7 (5.3) | 5.5 (4.1) | 0.0036* |
| Pain centralization (% with FS >7) | 95 (50.3%) | 7 (25.9%) | 0.02* |
| Fibromyalgia Symptom Severity scale (SS, 0-12) | 5.7 (3.2) | 4.4 (3.1) | 0.043* |
| Widespread Pain Index (WPI, 0-19) | 2.8 (3.4) | 1.0 (1.7) | 0.0073* |
| Psychosocial symptoms: | |||
| Depression, HADS (0-21) | 5.7 (4.5) | 5.1 (4.4) | 0.49 |
| Anxiety, HADS (0-21) | 7.2 (4.7) | 4.1 (3.1) | 0.0013* |
| Perceived Stress, PSS (0-40) | 15.9 (7.9) | 12.5 (7.4) | 0.040* |
| Pain Catastrophizing, CSQ (0-36) | 11.9 (8.4) | 8.3 (6.8) | 0.033* |
| Quantitative Sensory Testing (QST) pressure pain ratings: | |||
| 2 kg to suprapubic area (0-100) | 19.9 (17.0) | 18.8 (14.3) | 0.74 |
| 4 kg to suprapubic area (0-100) | 49.7 (25.9) | 57.5 (19.6) | 0.14 |
| 2 kg to forearm control (0-100) | 13.6 (14.7) | 13.7 (14.1) | 0.98 |
| 4 kg to forearm control (0-100) | 37.3 (25.4) | 40.3 (24.0) | 0.58 |
| Longitudinal Trends: | Regression | ||
| MAPP Pain Severity Score Functional Cluster over 3 years a | p-value | ||
| Improving | 61 (31.3%) | 15 (53.6%) | 0.79 b |
| Stable | 83 (42.6%) | 4 (14.3%) | |
| Worsening | 51 (26.2%) | 9 (32.1%) | |
| MAPP Urinary Severity Score Functional Cluster over 3 years a | |||
| Improving | 55 (28.2%) | 12 (42.9%) | 0.26 c |
| Stable | 99 (50.8%) | 7 (25.0%) | |
| Worsening | 41 (21.0%) | 9 (32.1%) |
p<0.05
Naliboff et al (2017).
In logistic regression model, after adjusting for baseline pain severity, the presence of HL was not associated with significant change in the longitudinal pain trajectory.
In logistic regression model, after adjusting for baseline urinary severity, the presence of HL was not associated with significant change in the longitudinal urinary trajectory.
Table 2 compared the longitudinal trends of MAPP Pain Severity Score and MAPP Urinary Severity Score. There were no differences in Pain or Urinary Severity at baseline, and during follow-ups at 6 months, 18 months and 3 years (p>0.05). The cumulative logistic regression modeling for longitudinal profiles showed lack of proportional odds, both for pain and urinary symptom trajectories (p=0.30 and 0.81 respectively). The linear trends (improving vs. stable vs. worsening) were not significant due to the observation that HL were less likely to be classified in the middle category (stable) compared to either improving or worsening. HL participants were less likely to remain stable but were more likely to have variable symptoms over time compared to non-HL (p=0.012 for pain outcome, p=0.039 for urinary outcome).
Table 2:
Comparison of 3 year longitudinal trends of UCPPS with and without HL.
| Longitudinal Trends: | UCPPS without HL (n=195) |
UCPPS with HL (n=28) |
Linear Effect p-value, b |
Quadratic Effect p-value c |
|---|---|---|---|---|
| MAPP Pain Severity Score Functional Cluster over 3 years a | ||||
| Improving | 61 (31.3%) | 15 (53.6%) | 0.30 | 0.012 |
| Stable | 83 (42.6%) | 4 (14.3%) | ||
| Worsening | 51 (26.2%) | 9 (32.1%) | ||
| MAPP Urinary Severity Score Functional Cluster over 3 years a | ||||
| Improving | 55 (28.2%) | 12 (42.9%) | 0.81 | 0.039 |
| Stable | 99 (50.8%) | 7 (25.0%) | ||
| Worsening | 41 (21.0%) | 9 (32.1%) | ||
| Longitudinal Outcome: | Mean (SD) | p-value | ||
| MAPP Pain Severity Score | ||||
| Baseline | 15.2 (5.9) | 15.1 (6.1) | 0.92 | |
| At 6 month | 13.9 (6.5) | 12.1 (5.6) | 0.20 | |
| At 18 month | 13.1 (6.9) | 12.9 (6.7) | 0.93 | |
| At 3 year | 12.5 (7.2) | 9.7 (6.7) | 0.12 | |
| MAPP Urinary Severity Score | ||||
| Baseline | 12.2 (6.4) | 14.4 (6.9) | 0.10 | |
| At 6 month | 11.7 (6.1) | 13.2 (6.5) | 0.25 | |
| At 18 month | 11.0 (6.6) | 13.3 (6.6) | 0.11 | |
| At 3 year | 11.0 (6.9) | 11.7 (7.4) | 0.69 |
Naliboff et al (2017).
Linear effect p-value: Was HL more or less likely to improve compared to non-HL?
Quadratic effect p-value: Was the middle group (stable) different from the two extremes (improving or worsening)?
Supplemental Table 2 compared participants in whom their HL status was known (e.g., with cystoscopy), versus those in whom their HL status were not known (e.g., did not undergo cystoscopy, no documentation of cystoscopy findings). The two groups presented in the table were age-and sex-matched. There were no differences in symptom duration, prevalence of fibromyalgia or other COPCs, or the number of pain sites between the two groups. Those with unknown HL status had more anxiety and higher Pain Severity than those with known HL status, but the differences were small.
Discussion:
It is hypothesized that UCPPS patients with HL might represent a different clinical phenotype from those without HL.7–9 To support the hypothesis, we think that the following questions need to be addressed: (1) Do HL patients have more bladder centric symptoms to support a bladder-centric disease? (2) Do non-HL patients have more systemic presentation to support a systemic pain syndrome? (3) Do the two phenotypes have different pathophysiology or pathology? (4) Can the identification of different phenotypes potentially guide individualized treatments to optimize outcome?
Comparative studies have shown that HL patients were older and had more severe bladder-centric symptoms (e.g., more nocturia), so point 1 is supported.7–9 Consistent with these findings, MAPP-II data also showed that HL were older, had more nocturia, higher ICSI and ICPI, and were more likely to have “painful urgency” compared to non-HL. Although the underlying pathophysiology of UCPPS is poorly understood, other studies have demonstrated histopathological evidence of bladder inflammation in HL.1–5 In addition, cytokine/chemokine profiles have shown pro-inflammatory changes (e.g., HL patients had elevated urinary CXCL-10, CXCL-1, NGF, IL-6, IL-8, MIF, HB-EGF, and EGF levels),21–29 so point 3 is supported. Non-randomized, uncontrolled clinical studies have shown remarkable pain improvement after triamcinolone and/or fulguration of HL (point 4).30 Data on whether non-HL patients have more systemic presentation (point 2) were conflicting in the literature since studies did not focus on non-urologic manifestation of UCPPS.7–9 Our MAPP-II Study provided deep phenotyping data to further help to answer this question.
The MAPP-II data support the notion that UCPPS patients (males and females) without HL were more likely to have systemic pain outside the pelvis compared to those with HL. This is reflected in their higher non-urologic pain scores, more widespread distribution of pain, more COPC, and higher fibromyalgia-ness scores (using the FS, SS, WPI instruments). Anxiety, perceived stress, and pain catastrophizing were also more severe in non-HL. This observation suggested a systemic pain syndrome in a subset of UCPPS patients where the bladder, prostate and pelvic floor are “bystanders” of a systemic process, and that UCPPS patients without HL are more likely to have this systemic pain syndrome. The MAPP-II data, along with data from other studies, support the hypothesis that UCPPS patients with HL represent a different clinical phenotype from those without HL. Table 3 summarized the key findings of the current and previous comparative studies. One might notice that the findings among the studies are mostly but not completely agreed. It is possible that differences in clinical routines in the present series comparing to studies performed elsewhere may partly explain the heterogeneity of results among studies (e.g., differences to the Peters et al paper).8
Table 3:
Summary of the key findings of the current study and previous comparative studies. “HL” = higher in HL. “Non-HL” = higher in non-HL. ns = no statistical significant difference between HL and non-HL. Empty cell = not reported.
| The current study a |
Moh et al (2018) a | Doiron et al (2016) | Peters et al (2011) | |
|---|---|---|---|---|
| N | 223 | 150 | 359 | 214 |
| % with HL | 12.5% | 27.3% | 12.2% | 16.8% |
| Demographics: | ||||
| Age | HL (older) | HL (older) | HL (older) | HL (older) |
| Sex | ns | ns | ||
| Race | ns | ns | ||
| Pelvic symptoms: | ||||
| Urologic pain intensity | ns | non-HL | HL | |
| Urinary frequency | ns | HL | HL | |
| Urgency | ns | ns | ||
| Nocturia | HL | HL | HL | HL |
| Symptom duration | ns | ns | Non-HL | |
| ICSI | HL | ns | HL | ns |
| ICPI | HL | ns | ns | ns |
| Painful urgency | HL | |||
| Genital pain | ns | |||
| Pelvic floor tenderness | ns | |||
| Systemic presentation: | ||||
| Non-urologic pain intensity | Non-HL | ns (trending non-HL) |
||
| Irritable bowel syndrome | ns | Non-HL | ns | ns |
| Fibromyalgia | ns | ns | Non-HL | |
| Chronic fatigue syndrome | ns | ns | ns | |
| Migraine headache | Non-HL | ns | Non-HL | |
| Temporomandibular joint disorder | ns | Non-HL | ||
| Vulvodynia | ns | ns | ns | |
| No. of COPC | Non-HL | |||
| Anxiety | Non-HL | Non-HL | ns | |
| Depression | ns | ns | ns | |
| Perceived tress | Non-HL | |||
| Catastrophizing | Non-HL | |||
| Widespread pain | Non-HL | ns | ||
| Fibromyalgia-ness | Non-HL | |||
| Quality of life: | ns |
The participants in these two studies did not overlap.
We have reported the results in men with UCPPS. A total of 8 out of 58 men had HL (13.7%). HL can occur in men, and the rate of HL was not different from those in women (p=0.74). In fact, 2 of these HL men had a clinical diagnosis of CP/CPPS. The take home lesson is that men can have IC/BPS, and a portion of men will have HL, so it is important to look for HL in men with cystoscopy in the proper age group. Our data from Figure 1 suggested that cystoscopy has a higher yield of finding HL in UCPPS patients aged 50 or above (about 20% for age 50-69, and over 50% for age ≥70).
It is important to recognize that clinical symptoms alone do not reliably separate HL vs. non-HL patients. For instance, their intensity of urologic pain or the duration of UCPPS symptoms were similar. The differences in Interstitial Cystitis Symptom and Problem Indexes were only 2 to 3 points, which may not be clinically significant. Most patients in both groups had “painful bladder filling”,20 as well as pelvic floor tenderness. Many patients with HL also had pain outside the pelvis. Cystoscopy remains the only reliable way to identify HL. Flexible cystoscopy can be performed in the office to identify HL with minimal discomfort. Cystoscopy allows identification of a subset of men and women with HL for targeted triamcinolone injection and/or fulguration (third-line treatments in the AUA Guideline 30), thus bypassing second-line treatments which has common side effects. Pentosan polysulfate is also less effective in patients with HL compared to non-HL.31 Patients with HL also responded better to cyclosporine A than those without HL.32 Oral cyclosporine A can be considered for HL patients who failed triamcinolone injection and/or fulguration.
As evident from this study, cystoscopy was not commonly performed in the US even among specialized academic centers that treat UCPPS (e.g., <40% of our cohort had cystoscopy and HL status documented). This limitation needs to be addressed in future clinical guidelines because without cystoscopy one cannot really distinguish HL from non-HL. A recent paper has provided a visual atlas to facilitate the proper diagnosis of Hunner lesion on office cystoscopy.33
The identification of HL serves as an example where the use of clinical phenotyping may help to inform individualized treatments to patients with UCPPS. Using a “one size fits all” approach to manage IC/BPS or CP/CPPS without regards for differentiating clinical characteristics may lead to treatment failures. This may explain why many previous randomized controlled trials have failed to demonstrate benefits since many phenotypes were likely mixed in the cohorts recruited using broad inclusion criteria. It is important in future clinical trials and research studies to document the HL status at baseline, and to stratify the enrollment and outcome analysis based on HL status, so researchers may study the differential response to specific treatments in UCPPS patients with and without HL. Designing and enrolling future clinical trials with baseline phenotyping factors in mind will greatly facilitate the development of personalized treatment of urologic syndromes.
Strengths of the study included the size of the multi-institutional MAPP-II cohort, and the detailed phenotyping data and longitudinal outcomes that were available. Potential weakness included reliance on medical chart review. Only 38.5% of the cohort (223 out of 578) had cystoscopy and documentation of their HL status.
Conclusions:
UCPPS with HL displayed more bladder-centric symptom profiles, while UCPPS without HL displayed symptoms suggesting a more systemic pain syndrome. The MAPP-II SPS phenotyping data showed that Hunner lesion UCPPS is a distinct phenotype from non-Hunner lesion UCPPS.
Supplementary Material
Acknowledgement:
Funding for the MAPP Research Network was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health (NIH) [DK082315 (Andriole, G; Lai, H), DK082316 (Landis, J), DK082325 (Buchwald, D), DK082333 (Lucia, M), DK082342 (Klumpp, D; Schaeffer A), DK082344 (Kreder, K), DK082345 (Clauw, D; Clemens, JQ), DK082370 (Mayer, E; Rodriguez L), DK103227 (Moses, M), DK103260 (Anger, J; Freeman, M), DK103271 (Nickel, J)].
Abbreviations:
- COPC
chronic overlapping pain condition(s)
- HL
Hunner lesion
- MAPP
Multidisciplinary Approach to the Study of Chronic Pelvic Pain
- SPS
Symptom Pattern Study
- QST
quantitative sensory testing
- UCPPS
urologic chronic pelvic pain syndrome
Appendix: MAPP-II Research Network Study Group
MAPP Network Executive Committee
J. Quentin Clemens, MD, FACS, MSci, Network Chair, 2013-
Philip Hanno, MD
Ziya Kirkali, MD
John W. Kusek, PhD
J. Richard Landis, PhD
M. Scott Lucia, MD
Robert M. Moldwin, MD
Chris Mullins, PhD
Michel A. Pontari, MD
University of Colorado Denver Tissue Analysis & Technology Core
M. Scott Lucia, MD, Core Dir.
Adrie van Bokhoven, PhD, Co-Dir.
Andrea A. Osypuk, BS
Robert Dayton, Jr
Chelsea S. Triolo, BS
Karen R. Jonscher, PhD
Holly T. Sullivan, BS
R. Storey Wilson, MS
Zachary D. Grasmick, BS
National Institutes of Diabetes & Digestive and Kidney Diseases
Chris Mullins, PhD
John W. Kusek, PhD
Ziya Kirkali, MD
Tamara G. Bavendam, MD
University of Pennsylvania Data Coordinating Core
J. Richard Landis, PhD, Core Dir.
Dina Appleby, MS
Ted Barrell, BA
Ro-Pauline Doe, BA
John T. Farrar, MD: MSCE, PhD
Melissa Fernando, MPH
Lura Gallagher, MPH, CCRP
Philip Hanno, MD
Xiaoling Hou, MS
Tamara Howard, MPH
Thomas Jemielita, MS
Natalie Kuzla, MA
Robert M. Moldwin, MD
Craig Newcomb, MS
Michel A. Pontari, MD
Nancy Robinson-Garvin PhD
Sandra Smith, AS
Alisa Stephens-Shields, PhD
Yanli Wang, MS
Xingmei Wang, MS
DISCOVERY SITES
Northwestern University
David J. Klumpp, PhD, Co-Dir.
Anthony J. Schaeffer, MD, Co-Dir.
Apkar (Vania) Apkarian, PhD
Christina Arroyo
Michael Bass, PhD
David Cella, PhD
Melissa A. Farmer, PhD
Colleen Fitzgerald, MD
Richard Gershon, PhD
James W. Griffith, PhD
Charles J. Heckman II, PhD
Mingchen Jiang, PhD
Laurie Keefer, PhD
Robert Lloyd, PhD
Darlene S. Marko, RN, BSN, CCRC
Jean Michniewicz
Richard Miller, PhD
Todd Parrish, PhD
Frank Tu, MD, MPH
Ryan Yaggie
University of California, LA PAIN Neuroimaging Core
Emeran A. Mayer, MD, Co-Dir.
Larissa V. Rodríguez, MD, Co-Dir.
Jeffry Alger, PhD
Cody P. Ashe-McNalley
Ben Ellingson, PhD
Nuwanthi Heendeniya
Lisa Kilpatrick, PhD
Cara, Kulbacki
Jason Kutch, PhD
Jennifer S. Labus, PhD
Bruce D. Naliboff, PhD
Fornessa Randal
Suzanne R. Smith, RN, NP
University of Iowa
Karl J. Kreder, MD, MBA, Dir.
Catherine S. Bradley, MD, MSCE
Mary Eno, RN, RA
Kris Greiner, BA
Yi Luo, PhD, MD
Susan K. Lutgendorf, PhD
Michael A. O’Donnell, MD
Barbara Ziegler, BA
Andrew Schrepf, PhD
Isabelle Hardy, MBA
Vince Magnotta, PhD
Brad Erickson, MD
University of Michigan
Daniel J. Clauw, MD, Co-Dir.; Network Chair, 2008-2013
J. Quentin Clemens, MD, FACS, MSci, Co-Dir.; Network Chair, 2013-
Suzie As-Sanie, MD
Sandra Berry, MA
Clara Grayhack,
Megan E. Halvorson, BS, CCRP
Richard Harris, PhD
Steve Harte, PhD
Eric Ichesco, BS
Ann Oldendorf, MD
Katherine A. Scott, RN, BSN
David A. Williams, PhD
University of Washington, Seattle
Dedra Buchwald, MD, Dir.
Niloofar Afari, PhD, UCSD
Tamara Bacus, BS
Todd Edwards, PhD
John Krieger, MD
Kenneth Maravilla, MD
Jane Miller, MD
Donald Patrick, PhD
Xiaoyan Qin, PhD
Stephanie Richey, BS
Rosana Risques, PhD
Kelly Robertson, BS
Susan O. Ross, RN, MN
Roberta Spiro, MS
Eric Strachan, PhD
TJ Sundsvold, MPH
Suzette Sutherland, MD
Claire C. Yang, MD
Washington University, St. Louis
Gerald L. Andriole, MD, Co-Dir., PI
H. Henry Lai, MD, Co-Dir., PI
Rebecca L. Bristol, BA, BS
Robert W. Gereau IV, PhD,
Barry A. Hong, PhD, FAACP
Aleksandra P. Klim, RN, MHS, CCRC
Siobhan Sutcliffe, PhD, ScM, MHS
Joel Vetter
David G. Song
Melissa Milbrandt
Simon Haroutounian, PhD
Pooja Vijairania
Kaveri Parker (Chaturvedi)
Tran Hung
Graham Colditz, MD, PH
Vivien C. Gardner, RN, BSN
Jeffrey P Henderson, MD, PhD
Theresa M. Spitznagle, PT, DPT, WCS
Ratna Pakpahan, MHA
Aimee James PhD, MPH
Yan Yan
Marvin Epolian Langston
Barry Hong, PhD
Susan Mueller
Jan Crowley
Sherri Vogt
Scott Hultgren, PhD
Nang Nguyen, PhD
Gabriel Blasche
Chang Shen Qiu, PhD
Lori Cupps
Song Bok
NON-RECRUITING DISCOVERY SITES
Cedars-Sinai Medical Center
Jennifer Anger, MD, MPH
James Ackerman, MA
A. Lenore Ackerman, MD, PhD
Jeena Cha, BS, CCRP
Karyn Eilber, MD
Michael Freeman, PhD
Vincent Funari, PhD
Jayoung Kim, PhD
Jennifer Van Eyk, PhD
Wei Yang, PhD
Queens University
J. Curtis Nickel, MD, FRCSC, Dir.
Garth D. Ehrlich, PhD, [Drexel COM]
Harvard Medical School/Boston Children’s Hospital
Marsha A. Moses, PhD, Dir.
Andrew C. Briscoe
David Briscoe, MD
Adam Curatolo, BA
John Froehlich, PhD
Richard S. Lee, MD
Monisha Sachdev, BS
Keith R. Solomon, PhD
Hanno Steen, PhD
Stanford University
Sean Mackey, MD, PhD, Dir.
Epifanio Bagarinao, PhD
Lauren C. Foster, BA
Emily Hubbard, BA
Kevin A. Johnson, PhD, RN
Katherine T. Martucci, PhD
Rebecca L. McCue, BA
Rachel R. Moericke, MA
Aneesha Nilakantan, BA
Noorulain Noor, BS
Footnotes
Conflict of Interest: No conflict of interest to the content of the paper
Reference:
- 1.van de Merwe JP, Nordling J, Bouchelouche P et al. : Diagnostic criteria, classification, and nomenclature for painful bladder syndrome/interstitial cystitis: an ESSIC proposal. Eur Urol, 53: 60, 2008 [DOI] [PubMed] [Google Scholar]
- 2.Leiby BE, Landis JR, Propert KJ et al. : Discovery of morphological subgroups that correlate with severity of symptoms in interstitial cystitis: a proposed biopsy classification system. J Urol, 177: 142, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Tomaszewski JE, Landis JR, Russack V et al. : Biopsy features are associated with primary symptoms in interstitial cystitis: results from the interstitial cystitis database study. Urology, 57: 67, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Belknap S, Blalock E, Erickson D: The Challenges of Interstitial Cystitis: Current Status and Future Prospects. Drugs, 75: 2057, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Regauer S, Gamper M, Fehr MK et al. : Sensory Hyperinnervation Distinguishes Bladder Pain Syndrome/Interstitial Cystitis from Overactive Bladder Syndrome. J Urol, 197: 159, 2017 [DOI] [PubMed] [Google Scholar]
- 6.Fall M, Johansson SL, Aldenborg F: Chronic interstitial cystitis: a heterogeneous syndrome. J Urol, 137: 35, 1987 [DOI] [PubMed] [Google Scholar]
- 7.Doiron RC, Tolls V, Irvine-Bird K et al. : Clinical Phenotyping Does Not Differentiate Hunner Lesion Subtype of Interstitial Cystitis/Bladder Pain Syndrome: A Relook at the Role of Cystoscopy. J Urol, 196: 1136, 2016 [DOI] [PubMed] [Google Scholar]
- 8.Peters KM, Killinger KA, Mounayer MH et al. : Are ulcerative and nonulcerative interstitial cystitis/painful bladder syndrome 2 distinct diseases? A study of coexisting conditions. Urology, 78: 301, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Van Moh F, Vetter J, Lai HH: Comparison of urologic and non-urologic presentation in interstitial cystitis/bladder pain syndrome patients with and without Hunner lesions. Neurourol Urodyn, 37: 2911, 2018 [DOI] [PubMed] [Google Scholar]
- 10.Fall M, Nordling J, Cervigni M et al. : Hunner lesion disease differs in diagnosis, treatment and outcome from bladder pain syndrome: An ESSIC working group report. Scand J Urol, 54: 91, 2020 [DOI] [PubMed] [Google Scholar]
- 11.Lai HH, Pickersgill NA, Vetter J: Hunner Lesion Phenotype in Interstitial Cystitis/Bladder Pain Syndrome: A Systematic Review and Meta-Analysis. J Urol, 204: 518, 2020 [DOI] [PubMed] [Google Scholar]
- 12.Clemens JQ, Kutch JJ, Mayer EA et al. : The Multidiscipinary Approach to the Study of Chronic Pelvic Pain (MAPP) Research Network: Design and implementation of the Symptom Patterns Study (SPS). Neurourol Urodyn, In press., 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griffith JW, Stephens-Shields AJ, Hou X et al. : Pain and Urinary Symptoms Should Not be Combined into a Single Score: Psychometric Findings from the MAPP Research Network. J Urol, 195: 949, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams DA, Schilling S: Advances in the assessment of fibromyalgia. Rheum Dis Clin North Am, 35: 339, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collaborative Health Outcomes Information Registry (CHOIR)
- 16.Freynhagen R, Baron R, Gockel U et al. : painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin, 22: 1911, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Wolfe F, Clauw DJ, Fitzcharles MA et al. : Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol, 38: 1113, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Brummett CM, Urquhart AG, Hassett AL et al. : Characteristics of fibromyalgia independently predict poorer long-term analgesic outcomes following total knee and hip arthroplasty. Arthritis Rheumatol, 67: 1386, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lai HH, Gardner V, Ness TJ et al. : Segmental hyperalgesia to mechanical stimulus in interstitial cystitis/bladder pain syndrome: evidence of central sensitization. J Urol, 191: 1294, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai HH, Krieger JN, Pontari MA et al. : Painful Bladder Filling and Painful Urgency are Distinct Characteristics in Men and Women with Urological Chronic Pelvic Pain Syndromes: A MAPP Research Network Study. J Urol, 194: 1634, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niimi A, Igawa Y, Aizawa N et al. : Diagnostic value of urinary CXCL10 as a biomarker for predicting Hunner type interstitial cystitis. Neurourol Urodyn, 37: 1113, 2018 [DOI] [PubMed] [Google Scholar]
- 22.Tyagi P, Killinger K, Tyagi V et al. : Urinary chemokines as noninvasive predictors of ulcerative interstitial cystitis. J Urol, 187: 2243, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vera PL, Preston DM, Moldwin RM et al. : Elevated Urine Levels of Macrophage Migration Inhibitory Factor in Inflammatory Bladder Conditions: A Potential Biomarker for a Subgroup of Interstitial Cystitis/Bladder Pain Syndrome Patients. Urology, 116: 55, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang CO, Li ZL, Kong CZ: APF, HB-EGF, and EGF biomarkers in patients with ulcerative vs. non-ulcerative interstitial cystitis. BMC Urol, 5: 7, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erickson DR, Tomaszewski JE, Kunselman AR et al. : Urine markers do not predict biopsy findings or presence of bladder ulcers in interstitial cystitis/painful bladder syndrome. J Urol, 179: 1850, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lamb LE, Janicki JJ, Bartolone SN et al. : Development of an interstitial cystitis risk score for bladder permeability. PLoS One, 12: e0185686, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pinto R, Lopes T, Costa D et al. : Ulcerative and nonulcerative forms of bladder pain syndrome/interstitial cystitis do not differ in symptom intensity or response to onabotulinum toxin A. Urology, 83: 1030, 2014 [DOI] [PubMed] [Google Scholar]
- 28.Logadottir YR, Ehren I, Fall M et al. : Intravesical nitric oxide production discriminates between classic and nonulcer interstitial cystitis. J Urol, 171: 1148, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Logadottir Y, Delbro D, Lindholm C et al. : Inflammation characteristics in bladder pain syndrome ESSIC type 3C/classic interstitial cystitis. Int J Urol, 21 Suppl 1: 75, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Hanno PM, Erickson D, Moldwin R et al. : Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J Urol, 193: 1545, 2015 [DOI] [PubMed] [Google Scholar]
- 31.Fritjofsson A, Fall M, Juhlin R et al. : Treatment of ulcer and nonulcer interstitial cystitis with sodium pentosanpolysulfate: a multicenter trial. J Urol, 138: 508, 1987 [DOI] [PubMed] [Google Scholar]
- 32.Forrest JB, Payne CK, Erickson DR: Cyclosporine A for refractory interstitial cystitis/bladder pain syndrome: experience of 3 tertiary centers. J Urol, 188: 1186, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Ronstrom C, Lai HH: Presenting an atlas of Hunner lesions in interstitial cystitis which can be identified with office cystoscopy. Neurourol Urodyn, 39: 2394, 2020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


