Abstract
The synthesis, crystal structure, and antimicrobial efficacy are reported for a novel material comprising a 1:2 ratio of chlorhexidine (CHX) to N-cyclohexylsulfamate (i.e., artificial sweetener known as cyclamate). The chemical structure is unambiguously identified by incorporating a combination of single-crystal X-ray diffraction (SC-XRD), electrospray ionization mass spectrometry (ESI-MS), 1H nuclear magnetic resonance (NMR) spectroscopy, correlation spectroscopy (COSY), and attenuated total reflection Fourier-transform infrared spectroscopy (ATR-FTIR). The new material: 1) is amongst only several reported structures identified to date incorporating the vital chlorhexidine antimicrobial drug; 2) exhibits broad spectrum antimicrobial activity at concentrations less than 15 μg/mL; and 3) provides a unique delivery method for the essential active pharmaceutical ingredient (API). Furthermore, substitution of inactive gluconate with bioactive cyclamate counterion potentially provides the additional benefit of improving the taste profile of chlorhexidine.
Keywords: Chlorhexidine, N-cyclohexylfulfamate, cyclamate, antimicrobial agent
Graphical Abstract

The facile synthesis of chlorhexidine dicyclamate yields a material with applications as a broad spectrum antimicrobial agent. The minimum inhibitory concentrations (MICs) of the newly discovered material is comparable to chlorhexidine digluconate for Streptococcus mutans while slightly reducing the overall amount of the drug since cyclamate exhibits a smaller molecular weight than gluconate. Additionally, the substitution of the biologically inert gluconate anion with the bioactive artificial sweetener cyclamate (i.e., N-cyclohexylsulfamate,) counterpart yields a material which can potentially enhance the taste profile.
INTRODUCTION
Chlorhexidine is a chemical disinfectant and antiseptic with broad antimicrobial activity against a variety of microorganisms including fungi and bacteria.1 Since its introduction in the 1950’s, it has become increasingly ubiquitous in cosmetic, healthcare, and pharmaceutical industries as a preservative, disinfectant, and antiseptic.2–5 CHX is widely used in mouth rinses for the prevention of plaque formation and development of gingivitis.6 In fact, CHX was included in the “World Health Organization (WHO) Model List of Essential Medicines” for antiseptic (i.e., 5% digluconate solution) and neonatal umbilical cord care (i.e., 7.1% digluconate solution or gel) applications.7 Due to the fact that the neutral chlorhexidine molecule exhibits low water solubility (i.e., less than 0.1 g/L),8 it is typically delivered as an aqueous dication (H2CHX) salt of an appropriate counterion (Figure 1). For example, water soluble salts can be formed by protonating the guanidine groups with gluconic acid (i.e., chlorhexidine digluconate), acetic acid, or hydrochloric acid. Largely attributed to this low solubility and propensity to form micelles in solution, chlorhexidine does not typically crystallize and only five crystal structures of chlorhexidine salts have been reported in the literature over the past 60 years despite its widespread use in global healthcare.9
Figure 1.

Molecular structure of chlorhexidine-cyclamate salt.
In 2008, Dupont et al. reported the crystal structures of complexes between CHX and three anionic calix[4]arene derivatives.10 Nearly a decade later, in 2016, Cattaneo et al. reported crystallographic characterization of the hydrated salts of CHX with SO42− and CO32−.11 However, the effect of the anion on the antimicrobial activity of CHX was not investigated. Herein, we report the crystal structure of a chlorhexidine salt of cyclamate as well as an investigation of the antimicrobial activity of dicyclamate (i.e., CHX-cyclamate or CHC) counterion as compared to digluconate (i.e., CHX-gluconate or CHG) and dihydrochloride (i.e., CHX-HCl) counterparts.
In some cases of chlorhexidine (digluconate) oral treatments, patients often report an initial unpleasant bitter taste while prolonged use often produces taste disturbances which may last for several hours.12,13 The presence of these side effects may lead to reduced patient compliance and incomplete antimicrobial effect causing a reduction in overall treatment efficacy. In the current work, an artificial sweetener was utilized as a counterion for chlorhexidine in attempts to mitigate chlorhexidine side effects and thus enhance its oral treatment compliance. Hence, the biologically inactive gluconate or acetate counterions are replaced by the bioactive and functional cyclamate anion.
Sodium cyclamate is a relatively stable and inexpensive artificial sweetener produced by the sulfonation of cyclohexylamine.14–15 Besides its potential capability to mask the bitter taste of CHX, it is known and well-studied that the combination of molecules can have an enhancing or synergistic effect on chemical and physical properties (e.g., antimicrobial activity).16 In fact, Cavicchioli et al. reported how the complexation of cyclamate with Ag(I) caused more than a four-fold reduction in minimum inhibitory concentration (MIC) against mycobacterium tuberculosis as compared to AgNO3.17
Due to the critical role that CHX plays in human health, a considerable amount of research has been devoted to understanding its antibacterial mechanism.18 Isotopic labeling studies demonstrated that the uptake of CHX by bacteria occurs rapidly, reaching maximum binding at ca. 20 s, and is concentration-dependent.19 At low concentrations, CHX affects the intracellular cytoplasmic membrane integrity, while at high concentrations it causes congealing of cytoplasm.1 On the other hand, CHX has little effect on the germination of bacterial spores, exhibits low activity against many viruses, and its effect against mycobacteria is bacteriostatic. It is therefore highly desirable to modulate the chemistry of CHX (e.g., conjugation or complexation with other molecules) as to discover synergistic effects.
MATERIALS AND METHODS
Synthesis
Synthesis of CHX-cyclamate was carried out in both methanol and water environments. Chlorhexidine digluconate (20 wt.%), chlorhexidine dihydrochloride, and sodium N-cyclohexylsulfamate (referred to as sodium cyclamate herein) were supplied by Sigma-Aldrich (St. Louis, MO). All materials were used as received by manufacturer without further purification.
The aqueous synthesis entailed dropwise addition of an aqueous 1 wt.% sodium cyclamate solution to a 20 wt.% CHG solution, to achieve a 2:1 molar ratio in accordance with charge balance considerations, yielding a precipitate. The heterogeneous mixture was filtered, washed with copious amounts of water, and dried in a 40 °C vacuum. Synthesis in methanol was conducted by combining dilute solutions of chlorhexidine dihydrochloride (0.2 wt.%) and sodium cyclamate (0.14 wt.%) to achieve a final solution comprising 0.1 wt.% chlorhexidine dihydrochloride and (chlorhexidine)(cyclamate)2 stoichiometry. Slow evaporation of solvent yielded crystal formation. Although the syntheses in water and methanol yielded the same product (Figure 7), the crystals from the aqueous synthesis were used for subsequent analyses.
Figure 7.

FTIR spectra of sodium N-cyclohexylsulfamate, CHX-HCl, CHX-gluconate, CHX-cyclamate crystals prepared from methanol and water. Spectra are offset for clarity.
Characterization
The X-ray diffraction data were collected using Bruker D8 Venture PHOTON 100 CMOS system equipped with a Cu Kα INCOATEC ImuS micro-focus source (λ = 1.54178 Å). The data was collected at 100 K. Indexing was performed using APEX3 (Difference Vectors method).20 Data integration and reduction were performed using SaintPlus 6.01.21 Absorption correction was performed by multi-scan method implemented in SADABS.22 Space group was determined using XPREP implemented in APEX3.20 The structure was solved using SHELXT (direct methods) and was refined using SHELXL-201723–25 (full-matrix least-squares on F2) through OLEX2 interface program.26 All non-hydrogen atoms were refined anisotropically. Hydrogen atoms were placed in geometrically calculated positions and were included in the refinement process using riding model.
Infrared spectra were collected using a Bruker Vertex 70 FTIR spectrometer (Bruker Optics, Billerica, MA) equipped with a GladiATR diamond ATR accessory (Pike technologies, Madison, WI). The spectral range was 80–4000 cm−1 with a resolution of 4 cm−1. All measurements were carried out at room temperature on as-prepared samples.
1H NMR measurements were performed on 1 wt.% samples in deuterated dimethyl sulfoxide (DMSO) solution. All NMR spectra were acquired on a Bruker Avance spectrometer (Bruker–Biospin, Billerica, MA, USA) with a 5 mm BBI probe operating at 500.0 MHz for 1H at 25 °C. The 1H NMR resonance of the compounds were further assigned using the homonuclear shift correlation 2D NMR (COSY) method.
Antimicrobial Assays
Salmonella enterica serovar Typhimurium LT2,27 Staphylococcus aureus USA300_LAC28 and Streptococcus mutans Clark (ATCC) were used to study the effect of chlorhexidine compounds on survival. S. enterica and S. aureus were cultured in Muller Hinton media (Sigma-Aldrich) and S. mutans was cultured in Reinforced Clostridial Media (Oxoid). Stock solutions for chlorhexidine·2HCl (CHX-HCl; 2.2 mg mL−1) and chlorhexidine cyclamate (CHC; 2 mg mL−1), were prepared by dissolving the compounds in DMSO prior to use. Chlorhexidine gluconate (CHG; 2 mg mL−1) was provided as a 19% w/v solution and further diluted in deionized water.
Growth analyses were conducted as previously described with slight alterations.29 Single bacterial colonies were inoculated into 2 mL of medium in 10 mL capacity culture tubes. Inoculated cultures of S. aureus and S. enterica were grown aerobically at 37 °C with shaking at 200 rpm for 24 hours. S. mutans was cultured statically for 48 hours. End-point Minimum Inhibitory Concentrations (MICs) were determined for CHX-HCl, CHC and CHG using the broth microdilution method from Clinical and Laboratory Standards Institute.30 Overnight cultures were standardized, in triplicates, to 0.5 McFarland standards (OD600= 0.1). MICs were determined in cultures grown in 96-well microtiter plates. 100 μL of the standardized culture was sub-cultured into wells containing 100 μL of medium containing the antimicrobial compound. Control wells containing 200 μL of media only or media with antimicrobial compound were used to standardize the data. The microtiter plates were aerobically incubated statically at 37 °C. The S. aureus and S. enterica cultures were analyzed after 20 hours and S. mutans was analyzed after 48 hours. Culture optical densities (A600) were determined using a Biotek EPOCH 2 microplate reader.
RESULTS AND DISCUSSION
Synthesis of CHX-cyclamate was carried out in aqueous and organic (i.e., methanol) solvent using commercially available precursors. Initial observation of CHX-cyclamate salt formation occurred upon mixing cationic chlorhexidine precursors with sodium cyclamate, which yielded precipitation.
The single crystal X-ray diffraction (SC-XRD) analysis, carried out at 100 K, shows that CHX-cyclamate crystallizes in the monoclinic P21/c space group. The asymmetric unit consists of half a molecule of protonated chlorhexidine (CHX) cation and one molecule of cyclamate anion. Disordered solvent is also present which was modeled as a water molecule (atom O1) with an occupancy of ~0.5. The hydrogen atoms of the water could not be modeled accurately. The unit cell parameters and crystallographic details are listed in Table S1.
The overall structure consists of symmetrically diprotonated CHX molecule surrounded by two cyclamate units (Figure 2) and the structural formula can be described as [C22H32N10Cl2]·[C7H13O3S]2. The CHX molecules adopt a spiral conformation as observed in previous reported structures with the simple CO32− and SO42− anions but instead of the U-shaped coils,11 they arrange into S-shaped coils (Figure 3a). The two Cl ends of the CHX are antiparallel to each other and show weak C-H---Cl hydrogen bonding interactions (3.168 Å)31 with the hydrogens of the cyclamate ring (Figure 3b).
Figure 2.

View of the CHX unit with two cyclamate units.
Figure 3.

(a) The S-shaped CHX coils; and (b) depiction of the C-H---Cl hydrogen bonding interactions.
The SO3− anions from the cyclamate ring form strong to moderate hydrogen bonds (2.059 to 2.706 Å) with the −NH/NH2 groups (2.064/2.153 Å respectively) of three adjacent chlorhexidine cations all arranged in a left-handed conformation. Each CHX unit interacts with four cyclamate units and each of the cyclamate unit shows interaction with two cyclamate units. Interestingly, the cyclamate molecules also show strong hydrogen bonding amongst each other whereby the oxygens of the SO3− of one cyclamate interacts with the hydrogens from the −CH2 of the other cyclamate, forming dimers extending along the c-axis. Adjacent cyclamate molecules alternate with the sulfonate groups pointing at opposing ends giving rise to the dimer network along the b-axis. The water oxygen also shows hydrogen bonding with the hydrogens from the unprotonated −NH2 of the CHX. All these different kinds of hydrogen bonding interactions32 are represented in Figure 4. The resulting structure with alternating CHX coils and cyclamate dimers gives rise to a three dimensional network with extensive hydrogen bonding. The structural arrangement is significantly different from the analogous sulfonate-derivatized calixarenes that are arranged into bilayers and from inclusion complexes with CHX.10 The packing arrangement is depicted in Figures 5 and 6.
Figure 4.

(a) The cyclamate showing interaction with three CHX units; (b) the cyclamate dimer; (c) extension of dimers along the c-axis; and (d) alternating cyclamate molecules along the b-axis.
Figure 5.
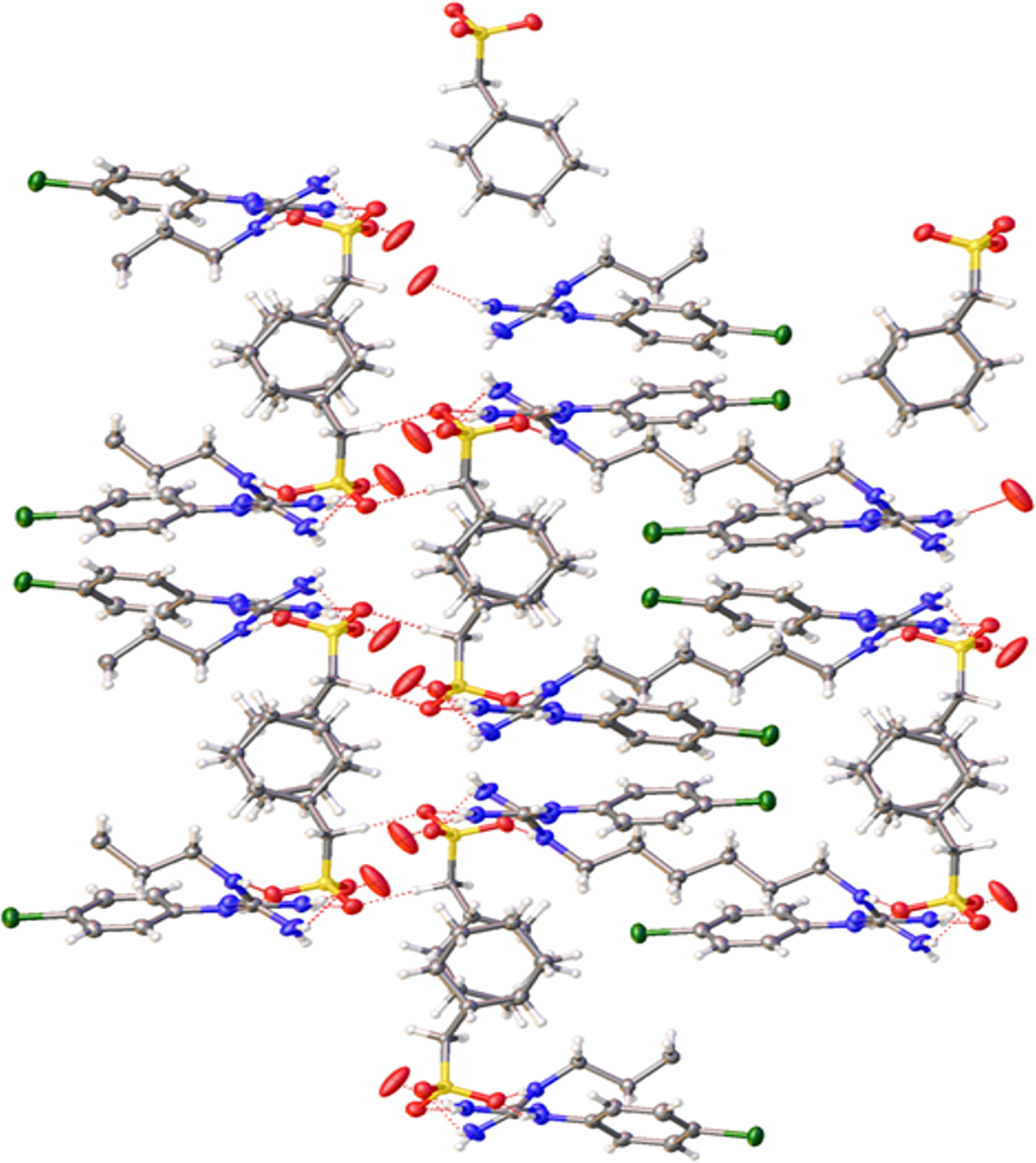
Packing arrangement showing the network formation with CHX coils and cyclamate dimers.
Figure 6.

View of packing along (100) plane showing alternating cyclamate units.
No significant pi-pi interactions were observed in the structure. The C-N bond lengths within the biguanidine units of CHX showed some delocalization of single and double bonds (1.319–1.363 Å) indicating resonance between the protonated forms. The selected bond lengths and angles are summarized in Tables S2 and S3, respectively.
Figure 7 compares the infrared absorption spectra of CHX-cyclamate samples prepared in two different solvents (methanol and water) to the sodium N-cyclohexylsulfamate, chlorhexidine dihydrochloride and lyophilized chlorhexidine digluconate raw materials. Presence of both components (i.e., chlorhexidine and cyclamate) is immediately apparent in the spectra of CHX-cyclamate samples. As an example, chlorhexidine bands corresponding to v(C=C), v(C=N) and δ(NH2) vibrations are clearly evident in the region above 1480 cm− where sodium cyclamate has no infrared absorption.33–35 Similarly, N-H stretching vibrations of −NH, =NH and NH2 functional groups of chlorhexidine can be identified in the 3000–3500 cm−1 high frequency range. The cyclamate component is manifested for instance, by the two prominent sets of bands near the 1030 and 1170 cm−1 region, associated with as/sym (SO2) vibrations.36–37 Evidence of both precursors in the prepared samples along with the fact that the vibrational bands are significantly different in their shape and positions from the initial raw materials suggests the formation of the salt between chlorhexidine and cyclamate ions. Finally, comparison of the spectrum of CHX-cyclamate synthesized in methanol versus water reveals that the two samples display overall similar vibrational profiles with some variations in their relative bands’ intensities likely originating from the small differences in the purity and local structure of the two samples.
1H NMR spectroscopy (Figure 8) and COSY (Figure S6) confirmed that both chlorhexidine and cyclamate exist in the crystal dissolved in DMSO. The 1H NMR chemical shifts corresponding to specific protons of chlorhexidine and cyclamate are indicated in Figure 8. Specifically, the 1H NMR spectrum showed the presence of signals for the benzene rings of CHX, resonating at 7.34 and 7.38 ppm, as well as characteristic signals of methylene protons of CHX at 1.04, 1.46, and 3.06 ppm. In addition, the methylene protons of the cyclohexane ring of cyclamate were identified at 1.16, 1.61, 1.88, 2.87 ppm. Due to the line broadening of the sample, the proton J coupling was not clearly observed. COSY was further performed to confirm the peak assignment of the NMR spectrum. According to peak integrals in Figure 8, the stoichiometric ratio between chlorhexidine and cyclamate is 1:2.
Figure 8.

1H NMR spectroscopy of crystal dissolved in deuterated DMSO.
The ability of CHX-HCl, CHC and CHG to inhibit the growth of the bacterial pathogens Staphylococcus aureus LAC, Streptococcus mutans, and Salmonella enterica serovar Typhimurium was examined. S. aureus LAC is a gram-positive community-associated methicillin-resistant CA-MRSA strain and a representative strain of the USA300 clone, which is a leading cause of skin and soft tissue infections in North America.38 S. mutans is also gram-positive and the leading causes of dental caries.39 Salmonella enterica serovar Typhimurium is a gram-negative and a primary enteric pathogen affecting humans.40
The MICs of CHX-cyclamate, CHX-gluconate, and CHX-HCl were determined in a liquid culture after static growth. All of the three bacteria displayed typical dose-responses to the compounds utilized (Figure 9a–b and Figure S8). The MICs for CHX-cyclamate, CHX-gluconate, and CHX-HCl are reported in Table 1 and Table S4 for S. mutans, S. aureus, and S. enterica. CHX-HCl demonstrated the lowest MIC values for all tested bacteria, potentially due to the additional cellular toxicity provided by the coordinated strong acid. The gluconate and cyclamate counterparts exhibited parity efficacy versus the oral strain S. mutans. CHX-cyclamate was slightly less effective against S. aureus and more so against S. enterica. Nevertheless, all of the three tested compounds were on the same order of efficacy with efficient gram-positive and gram-negative bacteria inhibition at ppm concentration levels. Based on this data, we posit that the cyclamate counterion does not deactivate chlorhexidine’s antimicrobial mode of action. The MIC values for CHX-gluconate are in good agreement with those reported in literature.8
Figure 9.

MIC assays for CHX-cyclamate, CHX-gluconate, and CHX-HCl with (a) S. mutans and (b) S. aureus.
Table 1.
MIC values for CHX-cyclamate, CHX-gluconate, and CHX-HCl
| Compound | Minimum inhibitory concentration (MIC) | |
|---|---|---|
|
S. mutans (μg mL−1) |
S. aureus (μg mL−1) |
|
| CHX-HCl | 1.5 | 2.0 |
| CHX-gluconate | 2.5 | 3.5 |
| CHX-cyclamate | 2.5 | 5.0 |
CONCLUSION
A novel analog of an essential antimicrobial drug chlorhexidine digluconate was synthesized, characterized, and evaluated for its antibacterial properties. Substitution of the biologically inert gluconate anion with the bioactive N-cyclohexylsulfamate, an artificial sweetener known as cyclamate, counterpart yielded a material which can potentially enhance the taste profile while maintaining parity antimicrobial efficacy against S. mutans which is known to cause dental caries. The novel material is an important progression to the solid state understanding of an indispensable biocide which does not easily crystallize under normal conditions.41 Moreover, the newly developed technology furthers our understanding of CHX and paves the way for further research to improve the current leading antimicrobial treatment in a worldwide campaign to promote oral and overall healthcare.
Supplementary Material
Funding Sources
NIAID award 1R01AI139100-01 from the National Institutes of Health.
ABBREVIATIONS
- CHX
chlorhexidine
- CHX-cyclamate
chlorhexidine dicyclamate
- CHX-gluconate
chlorhexidine digluconate
- CHX-HCl
chlorhexidine dihydrochloride
Footnotes
REFERENCES
- 1.McDonnell G; Russell AD, Antiseptics and Disinfectants: Activity, Action, and Resistance. Clin. Microbiol. Rev 1999, 12, 147–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Emilson CG, Potential efficacy of chlorhexidine against mutans streptococci and human dental caries. J. Dent. Res 1994, 73, 682–91. [DOI] [PubMed] [Google Scholar]
- 3.Addy M, Chlorhexidine compared with other locally delivered antimicrobials. A short review. J. Clin. Periodontol 1986, 13, 957–64. [DOI] [PubMed] [Google Scholar]
- 4.Milstone AM; Passaretti CL; Perl TM, Chlorhexidine: expanding the armamentarium for infection control and prevention. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2008, 46, 274–81. [DOI] [PubMed] [Google Scholar]
- 5.Block SS, Disinfection, Sterilization, and Preservation. 5th ed.; Lippincott Williams & Wilkins: Philadelphia, PA. [Google Scholar]
- 6.Addy M, Chlorhexidine compared with other locally delivered antimicrobials. J. Clin. Periodontol 1986, 13, 957–964. [DOI] [PubMed] [Google Scholar]
- 7.WHO Model List of Essential Medicines 2017. World Health Organization. 2017. [cited 2020 Jan 16]. Available from: http://www.who.int/medicines/publications/essentialmedicines/en/ [Google Scholar]
- 8.Paulus W, Directory of Microbicides for the Protection of Materials. Kluwer Academic Publishers: The Netherlands, 2004. [Google Scholar]
- 9.Groom CR; Bruno IJ; Lightfoot MP; Ward SC, The Cambridge Structural Database. Acta Crystallogr. B 2016, 72, 171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dupont N; Lazar AN; Perret F; Danylyuk O; Suwinska K; Navaza A; Coleman AW, Solid state structures of the complexes between the antiseptic chlorhexidine and three anionic derivatives of calix[4]arene. CrystEngComm 2008, 10, 975–977. [Google Scholar]
- 11.Cattaneo D; McOrmick LJ; Cordes DB; Slawin AMZ; Morris RE, Crystal structure resolution of two different chlorhexidine salts. J. Mol. Struct 2016, 1121, 70–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flotra L; Gjermo P; Rolla G; Waerhaug J, Side effects of chlorhexidine mouth washes. Eur. J. Oral Sci 1971, 79, 119–125. [DOI] [PubMed] [Google Scholar]
- 13.Lang N; Brecx MC, Chlorhexidine digluconate–an agent for chemical plaque control and prevention of gingival inflammation. J. Periodontal Res 1986, 21, 74–89. [Google Scholar]
- 14.Chattopadhyay S; Raychaudhuri U; Chakraborty R, Artificial sweeteners - a review. J. Food Sci. Technol 2014, 51, 611–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McKetta JJ, Encyclopedia of Chemical Processing and Design. Marcel Dekker, Inc.: New York, 1996. [Google Scholar]
- 16.Bollenbach T, Antimicrobial interactions: mechanisms and implications for drug discovery and resistance evolution. Curr. Opin. Microbiol 2015, 27, 1–9. [DOI] [PubMed] [Google Scholar]
- 17.Cavicchioli M; Leite CQF; Sato DN; Massabni AC, Synthesis, Characterization and Antimycobacterial Activity of Ag(I)-Aspartame, Ag(I)-Saccharin and Ag(I)-Cyclamate Complexes. Archiv der Pharmazie 2007, 340, 538–542. [DOI] [PubMed] [Google Scholar]
- 18.Ranganthan NS, Handbook of disinfectants and antiseptics. Ascenzi JM, Ed. Marcel Dekker, Inc.: New York, 1996; p 235–264. [Google Scholar]
- 19.Fitzgerald KA; Davies A; Russell AD, Uptake of 14C-chlorhexidine diacetate to Escherichia coli and Pseudomonas aeruginosa and its release by azolectin. FEMS Microbiol. Lett 1989, 60, 327–332. [DOI] [PubMed] [Google Scholar]
- 20.Bruker APEX3 (Version 2015.9), Bruker AXS Inc.: Madison, Wisconsin, USA, 2016. [Google Scholar]
- 21.Bruker SAINT-V8.35A. Data Reduction Software, Madison, Wisconsin, USA, 2016. [Google Scholar]
- 22.Sheldrick GM SADABS. Program for Empirical Absorption Correction, University of Gottingen: Germany, 1996. [Google Scholar]
- 23.Sheldrick G, Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheldrick G, Phase annealing in SHELX-90: direct methods for larger structures. Acta Cryst. A 1990, 46, 467–473. [Google Scholar]
- 25.Sheldrick G, A short history of SHELX. Acta Cryst. A 2008, 64, 112–122. [DOI] [PubMed] [Google Scholar]
- 26.Dolomanov OV; Bourhis LJ; Gildea RJ; Howard JAK; Puschmann H, OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr 2009, 42, 339–341. [Google Scholar]
- 27.Boyd JM; Teoh WP; Downs DM, Decreased Transport Restores Growth of a Salmonella enterica apbC Mutant on Tricarballylate. J. Bacteriol 2012, 194, 576–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts CA; Al-Tameemi HM; Mashruwala AA; Rosario-Cruz Z; Chauhan U; Sause WE; Torres VJ; Belden WJ; Boyd JM, The Suf Iron-Sulfur Cluster Biosynthetic System Is Essential in Staphylococcus aureus, and Decreased Suf Function Results in Global Metabolic Defects and Reduced Survival in Human Neutrophils. Infect. Immun 2017, 85, e00100–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dubovoy V; Ganti A; Zhang T; Al-Tameemi H; Cerezo JD; Boyd JM; Asefa T, One-Pot Hydrothermal Synthesis of Benzalkonium-Templated Mesostructured Silica Antibacterial Agents. J. Am. Chem. Soc 2018, 140, 13534–13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard - Ninth Edition. Clinical and Laboratory Standards Institute: Wayne, PA, 2012. [Google Scholar]
- 31.Aakeröy CB; Evans TA; Seddon KR; Pálinkó I, The C–H···Cl hydrogen bond: does it exist? New J. Chem 1999, 23, 145–152. [Google Scholar]
- 32.Jeffrey GAJ; Jeffrey GA, An Introduction to Hydrogen Bonding. Oxford University Press: 1997. [Google Scholar]
- 33.Călinescu M; Negreanu-Pîrjol T; Georgescu R; Călinescu O, Synthesis and characterization of new copper(II) complex compounds with chlorhexidine. Part I. Cent. Eur. J. Chem 2010, 8, 543–549. [Google Scholar]
- 34.Luo D; Shahid S; Wilson RM; Cattell MJ; Sukhorukov GB, Novel Formulation of Chlorhexidine Spheres and Sustained Release with Multilayered Encapsulation. ACS Appl. Mater. Interfaces 2016, 8, 12652–60. [DOI] [PubMed] [Google Scholar]
- 35.Rema T; Lawrence JR; Dynes JJ; Hitchcock AP; Korber DR, Microscopic and spectroscopic analyses of chlorhexidine tolerance in Delftia acidovorans biofilms. Antimicrob. Agents Chemother 2014, 58, 5673–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katiyar RS, Raman and infra-red spectra of crystalline potassium sulphamate. Proc. Indian Acad. Sci 1965, 62, 169–175. [Google Scholar]
- 37.Ilczyszyn MM; Ilczyszyn M, Raman, infrared and 13C NMR studies on betaine–sulfamic acid (2:1) crystal and its hydrogen bonds. J. Raman Spectrosc 2003, 34, 693–704. [Google Scholar]
- 38.Planet PJ, Life After USA300: The Rise and Fall of a Superbug. J. Infect. Dis 2017, 215, S71–S77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loesche WJ, Role of Streptococcus mutans in human dental decay. Microbiol. Rev 1986, 50, 353–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fàbrega A; Vila J, Salmonella enterica serovar Typhimurium skills to succeed in the host: virulence and regulation. Clin. Microbiol. Rev 2013, 26, 308–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hao Z; Cheng C; Pan L; Subramanyam R Chlorhexidine-cyclamate complexes and oral care compositions comprising the same. Patent Application WO 2019/125413, June 27, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


