Abstract
Background
Patients with systemic lupus erythematosus (SLE) are at risk of developing COVID-19 due to underlying immune abnormalities and regular use of immunosuppressant medications. We aimed to evaluate the presence of SARS-CoV-2 IgG antibodies in patients with SLE with or without previous COVID-19-related symptoms or RT-PCR-confirmed SARS-CoV-2 infection.
Methods
For this analysis, we included patients with SLE from two cohorts based in New York City: the Web-based Assessment of Autoimmune, Immune-Mediated and Rheumatic Patients during the COVID-19 pandemic (WARCOV) study; and the NYU Lupus Cohort (a prospective registry of patients at NYU Langone Health and NYC Health + Hospitals/Bellevue). Patients in both cohorts were tested for SARS-CoV-2 IgG antibodies via commercially available immunoassays, processed through hospital or outpatient laboratories. Patients recruited from the NYU Lupus Cohort, referred from affiliated providers, or admitted to hospital with COVID-19 were tested for SARS-CoV-2 IgG antibodies as part of routine surveillance during follow-up clinical visits.
Findings
329 patients with SLE were included in this analysis, 146 from the WARCOV study and 183 from the NYU Lupus Cohort, and were tested for SARS-CoV-2 antibodies between April 29, 2020, and Feb 9, 2021. 309 (94%) were women and 91 (28%) were of Hispanic ethnicity. 51 (16%) of 329 patients had a positive SARS-CoV-2 IgG antibody test. Seropositive patients were more likely than seronegative patients to be Hispanic (24 [47%] of 51 vsz 67 [24%] of 278). Other demographic variables, SLE-specific factors, and immunosuppressant use were not associated with SARS-CoV-2 positivity. Of the 29 patients with COVID-19 previously confirmed by RT-PCR, 18 (62%) were on immunosuppressants; 24 (83%) of 29 patients tested positive for SARS-CoV-2 IgG antibodies. Of 17 patients who had symptoms of COVID-19 but negative concurrent RT-PCR testing, one (6%) developed an antibody response. Of 26 patients who had COVID-19-related symptoms but did not undergo RT-PCR testing, six (23%) developed an antibody response. Of 83 patients who had no symptoms of COVID-19 and no RT-PCR testing, four (5%) developed an antibody response. Among 36 patients who were initially SARS-CoV-2 IgG positive, the majority maintained reactivity serially (88% up to 10 weeks, 83% up to 20 weeks, and 80% up to 30 weeks). Seven (70%) of ten patients with confirmed COVID-19 had antibody positivity beyond 30 weeks from disease onset.
Interpretation
Most patients with SLE and confirmed COVID-19 were able to produce and maintain a serological response despite the use of a variety of immunosuppressants, providing reassurance about the efficacy and durability of humoral immunity and possible protection against re-infection with SARS-CoV-2.
Funding
National Institute of Arthritis and Musculoskeletal and Skin Diseases, National Institutes of Health, and Bloomberg Philanthropies COVID-19 Response Initiative Grant.
Introduction
New York City was the epicentre of the COVID-19 pandemic from March 1 to June 21, 2020, with more than 200 000 confirmed and probable cases and 17 000 deaths reported by June 21, 2020.1 Despite these high numbers, during the outbreak peak in New York City, only around 12 000 individuals were tested for active infection daily, with positivity rates as high as 41–66%; undoubtedly, large numbers of people with COVID-19 were therefore not identified.1 Serological evaluation to detect previous infections should therefore provide better insight into the prevalence of and risk factors associated with COVID-19, and enable assessment of the competency of individuals in mounting an antiviral immune response. SARS-CoV-2 IgG antibodies are generally detected 2 weeks after infection, with higher titres identified in patients with severe disease.2 ELISA-based antibody tests have greater than 95% specificity for COVID-19, although neutralising antibodies have been undetectable in a small proportion of mild cases.3 The role of serology in determining SARS-CoV-2 prevalence is still unclear, especially among subpopulations with altered immunological responses.
Research in context.
Evidence before this study
Patients with systemic lupus erythematosus (SLE) are at an increased risk of developing viral infections due to immunological abnormalities and immunosuppressant use, which might affect humoral immune responses. To evaluate previous research related to the risk of infections, response to vaccinations and COVID-19 in patients with SLE and rheumatic disease, we searched PubMed for articles published from Jan 1, 1980, to March 14, 2021. Search terms included “systemic lupus erythematosus,” and “rheumatic disease” in combination with “viral infections,” “vaccination immune response,” “COVID-19,” “SARS-CoV-2 IgG” and “COVID-19 antibodies.” We also reviewed the online dashboard for the New York City COVID-19 case and death numbers between March 1 and June 21, 2020. Previous descriptive studies noted frequent hospital admission in patients with SLE andRT-PCR-confirmed SARS-CoV-2 infection, with risk factors for hospital admission including non-White or Hispanic ethnicity, a higher body-mass index, and having at least one other medical comorbidity. Immunosuppressant medication use was not associated with risk of hospital admission for COVID-19.
Added value of this study
To the best of our knowledge, this study represents the largest cohort of patients with SLE evaluated for SARS-CoV-2 IgG antibodies. Most patients with SLE and confirmed COVID-19 were able to produce a serological response despite the use of a variety of immunosuppressants. The majority of patients with SLE had antibody positivity beyond 30 weeks from COVID-19 onset.
Implications of all the available evidence
The ability of patients with SLE to mount an immune response against COVID-19 provides some reassurance about the durability of humoral immunity against SARS-CoV-2 and protection of these patients against re-infection.
Systemic lupus erythematosus (SLE) is a chronic autoimmune inflammatory condition characterised by activation of the innate and adaptive immune systems. Despite heightened production of antiviral type I interferons (IFNs), patients with SLE are at an increased risk of developing viral infections, and this risk is exacerbated by frequent exposure to immunosuppressant medications.4 Use of these medications along with the perturbance of immune mechanisms inherent to SLE might complicate the capability of patients to produce long-term immunity both to SARS-CoV-2 infection and to COVID-19 vaccines. To date, most studies of COVID-19 done in patients with SLE have been limited to relatively small case series.5, 6, 7 We previously reported on a cohort of 226 patients with SLE from the New York University (NYU) hospitals system.8 Similar to other reports, hospital admission was frequent in patients with RT-PCR confirmed SARS-CoV-2 infection (24 [59%] of the convenience sample); risk factors for admission to hospital included non-White or Hispanic ethnicity, a higher body-mass index, and having at least one other medical comorbidity.8 Because of the scarcity of available testing at the time, unambiguous assessment of the number of asymptomatic carriers or RT-PCR-confirmed cases of SARS-CoV-2 infection in all symptomatic patients was not possible.
We aimed to evaluate the presence of SARS-CoV-2 IgG antibodies in patients with SLE with or without COVID-19 symptoms or previously RT-PCR-confirmed SARS-CoV-2 infection, to ascertain whether patients with SLE effectively produce antibodies. We also measured SARS-CoV-2 IgG antibodies in patients with or without COVID-19-related symptoms who were either not tested or were RT-PCR negative.
Methods
Study design and participants
For this analysis we recruited patients with SLE from two cohorts based in New York City: the Web-based Assessment of Autoimmune, Immune-Mediated and Rheumatic Patients during the COVID-19 pandemic (WARCOV) study,8, 9 and the NYU Lupus Cohort. Patients with SLE from the WARCOV Cohort (outpatients at NYU Langone Health and NYC Health + Hosptials/Bellevue and patients admitted to NYU Langone Health or NYC Health + Hospitals/Bellevue for COVID-19) completed questionnaires approved by the NYU Langone Health institutional review board, which included information about demographic characteristics, comorbidities, SLE medications used, symptoms related to COVID-19, contact exposure, and RT-PCR testing. Full inclusion criteria are summarised in the appendix (p 1). In the WARCOV study, patients with SLE admitted to hospital and those receiving outpatient care were identified by review of medical records and contacted serially for 1–9 weeks between April 13 and June 14, 2020, to prospectively capture symptom onset. Additional patients from the NYU Lupus Cohort (a prospective registry of patients at NYU Langone Health and NYC Health + Hospitals/Bellevue), who did not participate in the WARCOV study, were tested for the presence of SARS-CoV-2 antibodies as standard of care and evaluated by review of electronic medical records for previous SARS-CoV-2 RT-PCR testing and admission to hospital. Full inclusion criteria of the NYU Lupus Cohort are summarised in the appendix (p 1). COVID-19 symptoms were not systematically captured in this group. Previously obtained information in the cohort registry included data about sex, age, race or ethnicity by self report (American Indian or Alaska Native, Asian, Black, White, or other for race, and Hispanic or not Hispanic for ethnicity, regardless of race), criteria for the 1997 revised American College of Rheumatology (ACR) SLE classification, SLE disease activity from a clinical visit up to 26 weeks before antibody testing as measured by the hybrid Systemic Lupus Erythematosus Disease Activity Index (SLEDAI), and medication use, with a focus on glucocorticoids, hydroxychloroquine, and immunosuppressants (azathioprine, mycophenolate mofetil, methotrexate, belimumab, tacrolimus, other miscellaneous medications taken at the time, and cyclophosphamide or rituximab within 1 year of COVID-19 symptoms or RT-PCR when available or antibody testing).10, 11, 12, 13 Data on comorbid conditions and components of the hybrid SLEDAI and Systemic Lupus International Collaborating Clinics (SLICC) Damage Index were obtained by physicians via patients' medical history, physical examinations, and chart review.14 Patients provided written consent to participate in the study. This study was approved by the NYU Langone Health institutional review board (number s20-00389 for the WARCOV study and s14-00487 for the NYU Lupus Cohort registry).
Serological testing
Commercial assays for SARS-CoV-2 IgG antibody testing were done between April 29, 2020, and Feb 9, 2021. The Abbott Architect SARS-CoV-2 IgG chemiluminescent microparticle immunoassay (Abbott Park, IL, USA) targeting the viral nucleocapsid protein was used for the majority of antibody tests. In this assay, the resulting chemiluminescent reaction is measured in relative light units (RLUs). The RLU of patients' samples is then divided by the calibrator signal and the calculated signal to cutoff ratio (index value), which is compared to the RLU in the reaction to the calibrator (ie, a signal to cutoff ratio <1·4 is defined as negative and ≥1·4 defined as positive). This test was granted an FDA emergency use authorisation, with expected performances of 100·0% sensitivity and 99·6% specificity, and showed strong correlation with neutralising antibody assays.15, 16, 17 Information about other antibody assays used is summarised in the appendix (p 1). Samples were processed through hospital or outpatient laboratories and reported in electronic medical records. Most tests were done at the NYU Langone Health and Bellevue Hospital central laboratories, and a small number of samples were processed in commercial laboratories: Quest Diagnostics (Secaucus, NJ, USA) and Labcorp (Burlington, NC, USA).
Statistical analysis
Categorical variables were summarised by computing counts and proportions of patients (%) and continuous variables expressed as means with SDs or medians with IQRs. Baseline characteristics were compared between SARS-CoV-2 IgG positive and negative patients in bivariate analyses with Fisher's exact tests for categorical variables and Mann-Whitney U tests for continuous variables. A p value less than 0·05 was considered as significant. Statistical analyses were done with IBM SPSS Statistics, version 25.
Role of the funding source
The funding sources for this study did not have any role in study design, data collection, data analysis, data interpretation, writing of the report, or the decision to submit the paper for publication.
Results
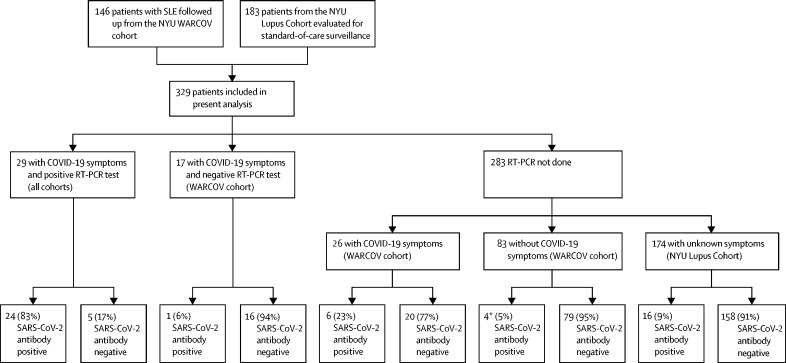
329 patients with SLE were included in this analysis, 146 from the WARCOV study8, 9 and 183 from the NYU Lupus Cohort, and were tested for SARS-CoV-2 antibodies between April 29, 2020, and Feb 9, 2021 (figure 1 ). 309 (94%) were women and 91 (28%) were of Hispanic ethnicity. 346 individual antibody tests were done, of which 298 (86%) were done with the Abbott Architect SARS-CoV-2 IgG chemiluminescent microparticle immunoassay. In the combined cohort, 51 (16%) of 329 patients had a positive SARS-CoV-2 IgG antibody test, and 278 (84%) were negative for SARS-CoV-2 antibodies (figure 1; table 1 ). SARS-CoV-2 IgG antibody test results from eight patients included in this study were previously reported.8 24 (47%) of 51 SARS-CoV-2 antibody-positive patients were of Hispanic ethnicity, compared with 67 (24%) of 278 SARS-CoV-2 antibody-negative patients (p=0·0010). The mean titre for positive quantitative IgG antibody tests was a signal to cutoff ratio of 4·1 (SD 2·1; range 1·7–10·6), obtained at 15·4 (SD 9·8) weeks. Overall, demographic and clinical characteristics were similar across SARS-CoV-2-positive and SARS-CoV-2-negative patients (table 1). Less than 10% of patients in both groups were on prednisone doses higher than 7·5 mg per day (25 [9%] of 278 SARS-CoV-2 antibody-negative patients and three (6%) of 51 SARS-CoV-2 antibody-positive patients). The most prevalent components of the SLICC Damage Index in the seropositive group included avascular necrosis (five [23%] of 22 patients with at least one site), estimated or measured glomerular filtration rate less than 50%, and end-stage renal disease (four [18%] of 22 patients for each variable). In the seronegative group, end-stage renal disease (in 13 [13%] of 99 patients), scarring alopecia (in nine [9%] of 99), and avascular necrosis (in eight [8%] of 99 with at least one site) were most prevalent.
Figure 1.
Flow diagram of included participants and proportion of patients with and without SARS-CoV-2 IgG antibodies
NYU=New York University hospitals system. SLE=systemic lupus erythematosus. WARCOV=Web-based Assessment of Autoimmune, Immune-Mediated, and Rheumatic Patients during the COVID-19 Pandemic. *One asymptomatic patient was on intravenous immunoglobulin.
Table 1.
Demographic characteristics of the overall SLE cohort and associations with SARS-CoV-2 IgG positivity
| Overall SLE cohort (n=329) | SARS-CoV-2 antibody-positive patients (n=51) | SARS-CoV-2 antibody-negative patients (n=278) | ||
|---|---|---|---|---|
| Sex | ||||
| Female | 309 (94%) | 48 (94%) | 261 (94%) | |
| Male | 20 (6%) | 3 (6%) | 17 (6%) | |
| Age, years | 43·1 (14·0) | 43·2 (15·7) | 43·1 (13·8) | |
| Ethnicity | ||||
| Hispanic* | 91 (28%) | 24 (47%) | 67 (24%) | |
| Not Hispanic | 238 (72%) | 27 (53%) | 211 (76%) | |
| Race | ||||
| White | 145 (44%) | 25 (49%) | 120 (43%) | |
| Black | 108 (33%) | 18 (35%) | 90 (32%) | |
| Asian | 51 (16%) | 4 (8%) | 47 (17%) | |
| Other | 25 (8%) | 4 (8%) | 21 (8%) | |
| History of lupus nephritis | 161 (49%) | 24 (47%) | 137 (49%) | |
| Medications | ||||
| Immunosuppressants† | 183 (56%) | 30 (59%) | 153 (55%) | |
| Hydroxychloroquine | 271 (82%) | 44 (86%) | 227 (82%) | |
| Glucocorticoid | 101 (31%) | 17 (33%) | 84 (30%) | |
| Dose of prednisone equivalent, mg | 5 (5–10) | 5 (5–9) | 5 (5–10) | |
| Mean hybrid SLEDAI | 3·0 (4·4); n=177 | 2·9 (4·0); n=26 | 3·0 (4·4); n=151 | |
| Mean SLICC Damage Index | 1·2 (1·5); n=121 | 1·9 (2·2); n=22 | 1·0 (1·3); n=99 | |
| History of antiphospholipid antibodies | 74/269 (28%) | 12/37 (32%) | 62/232 (27%) | |
Data are n (%) or n/N (%) for categorical variables and mean (SD) or median (IQR) for continuous variables. Categorical variables compared with Fisher's exact test; continuous variables compared with Mann Whitney U test. SLE=systemic lupus erythematosus. SLEDAI=Systemic Lupus Erythematosus Disease Activity Index. SLICC=Systemic Lupus International Collaborating Clinics.
p=0·0010, Fisher's exact test.
Immunosuppressants include azathioprine, mycophenolate mofetil, methotrexate, belimumab, tacrolimus, tofacitinib, cyclophosphamide, obinutuzumab, and rituximab.
29 patients with confirmed SARS-CoV-2 RT-PCR positivity underwent subsequent SARS-CoV-2 IgG antibody testing. Of these, ten (34%) were admitted to hospital, 26 (90%) were taking hydroxychloroquine, ten (34%) were taking glucocorticoids, and 18 (62%) were on immunosuppressant medications. 24 (83%) tested positive for SARS-CoV-2 IgG antibodies and five (17%) tested negative for SARS-CoV-2 IgG antibodies (figure 1, table 2 ). Initial antibody testing was done 0·0–30·3 weeks after the positive RT-PCR test result (mean 12·7 [SD 8·7] weeks). Of particular clinical relevance, three patients had been treated with rituximab in the year before a positive SARS-CoV-2 RT-PCR test and all produced a positive SARS-CoV-2 antibody response with measurements at 0, 2, and 6 weeks, respectively (appendix p 2). The most recent doses of rituximab these patients received were 17·3 weeks, 21·4 weeks, and 38·9 weeks relative to their first SARS-CoV-2 IgG antibody test.
Table 2.
SARS-CoV-2 serological status in patients with SLE and SARS-CoV-2 RT-PCR positivity
| SARS-CoV-2 antibody-positive patients (n=24) | SARS-CoV-2 antibody-negative patients (n=5) | ||
|---|---|---|---|
| Sex | |||
| Female | 23 (96%) | 4 (80%) | |
| Male | 1 (4%) | 1 (20%) | |
| Age, years | 44·2 (15·7) | 42·6 (14·3) | |
| Ethnicity | |||
| Hispanic | 6 (25%) | 3 (60%) | |
| Not Hispanic | 18 (75%) | 2 (40%) | |
| Race | |||
| White | 10 (42%) | 2 (40%) | |
| Black | 10 (42%) | 2 (40%) | |
| Asian | 2 (8%) | 0 (0%) | |
| Other | 2 (8%) | 1 (20%) | |
| Medications | |||
| Immunosuppressants* | 15 (63%) | 3 (60%) | |
| Hydroxychloroquine | 21 (88%) | 5 (100%) | |
| Glucocorticoid | 7 (29%) | 3 (60%) | |
| Dose of prednisone equivalent, mg | 5 (4–5) | 5 (5–5) | |
| Time from SARS-CoV-2 RT-PCR to first antibody test, weeks | 12·4 (8·9) | 13·8 (8·3) | |
Data are n (%) for categorical variables and mean (SD) or median (IQR) for continuous variables. SLE=systemic lupus erythematosus.
Immunosuppressants include azathioprine, mycophenolate mofetil, methotrexate, belimumab, tacrolimus, cyclophosphamide, obinutuzumab, and rituximab.
Of the five patients who were negative for SARS-CoV-2 antibodies, the first had mild symptoms of fever, sore throat, diarrhoea, anosmia, and ageusia. SARS-CoV-2 IgG antibody testing done 24 weeks following positive RT-PCR was positive (1·2 relative to the signal to cutoff ratio), but lower than the positive threshold on the Abbott assay (≥1·4; appendix p 2). The second presented to the emergency department with musculoskeletal chest pain without upper respiratory symptoms but on routine testing was found to be SARS-CoV-2 RT-PCR positive. The patient's SARS-CoV-2 IgG antibody test was negative after weeks 6 and 20. The third was admitted to hospital with fever, cough, shortness of breath, anosmia, and ageusia. The patient previously underwent renal transplantation. SARS-CoV-2 IgG antibody testing done at weeks 7 and 9 after a positive RT-PCR result resulted in antibody titres rising from a signal to cutoff ratio of 0·4 to 1·0, although not reaching the threshold for positivity. The fourth was a patient with end-stage renal disease on peritoneal dialysis who was admitted to hospital with fever, nausea, vomiting, anosmia, and ageusia. Antibody testing done 21 weeks later was negative at a signal to cutoff ratio of 1·2. The fifth was on hydroxychloroquine, mycophenolate mofetil, and the anti-CD20 agent obinutuzumab for lupus nephritis and was admitted to hospital with fever and lobar pneumonia with a positive SARS-CoV-2 RT-PCR test. This patient was re-admitted to hospital 10 weeks later with anaemia and radiographic findings consistent with COVID-19, although at that time the patient was negative on both RT-PCR testing and serological IgG testing (0·1 signal to cutoff ratio). The most recent obinutuzumab dose had been administered 10 weeks before the patient's first IgG antibody test.
17 patients who reported symptoms indicative of COVID-19 or had known exposure to SARS-CoV-2 during the spring 2020 pandemic peak (as reported in WARCOV questionnaires) but had a negative RT-PCR test result subsequently underwent SARS-CoV-2 IgG testing (0·6–37·7 weeks after symptoms, mean 19·6 [SD 11·6] weeks). Of these, one (6%) tested positive for SARS-CoV-2 antibodies; the patient presented with shortness of breath, sore throat, and chills, and had a positive SARS-CoV-2 IgG antibody test 18 weeks following symptom onset (table 3 ). The patient did not have additional COVID-19-related symptoms between the initial RT-PCR and the serological test.
Table 3.
Patients with SLE positive for SARS-CoV-2 IgG antibodies without a previous positive RT-PCR test
| Age, years | Sex | Ethnicity and race | Time from COVID-19 symptoms to antibody status, weeks | Concurrent medications | Hospital admission for COVID-19 | Highest antibody titre (Abbott Architect; nL <1·4 specimen calibrator) | |
|---|---|---|---|---|---|---|---|
| COVID-19-related symptoms or SARS-CoV-2 exposure with negative RT-PCR test | |||||||
| Patient 1 | 35–40 | Female | Hispanic/White | 18·0 | Hydroxychloroquine | No | 2·2 |
| COVID-19-related symptoms with no RT-PCR Testing | |||||||
| Patient 1 | 40–45 | Female | Not Hispanic/Black | 14·6 | Hydroxychloroquine | No | 2·3 |
| Patient 2 | 30–35 | Female | Hispanic/White | 15·0 | Hydroxychloroquine | No | 2·2 |
| Patient 3 | 60–65 | Female | Hispanic/Black | 23·0 | Hydroxychloroquine, belimumab | No | 3·1 |
| Patient 4 | <20 | Female | Hispanic/White | 7·1 | Hydroxychloroquine, mycophenolate mofetil | No | 4·9 |
| Patient 5 | 45–50 | Female | Not Hispanic/White | 6·4 | None | No | NA |
| Patient 6 | 45–50 | Female | Hispanic/Other | 31·7 | Hydroxychloroquine | No | 3·0 |
| No COVID-19-related symptoms with no RT-PCR testing | |||||||
| Patient 1 | 30–35 | Female | Not Hispanic/Asian | NA | Hydroxychloroquine, mycophenolate mofetil, cyclophosphamide, prednisone, intravenous immunoglobulin | No | NA |
| Patient 2 | 40–45 | Female | Hispanic/Black | NA | None | No | 1·8 |
| Patient 3 | 30–35 | Female | Not Hispanic/Black | NA | Hydroxychloroquine | No | 3·0 |
| Patient 4 | 30–35 | Male | Hispanic/White | NA | Hydroxychloroquine, tofacitinib, prednisone | No | 2·8 |
NA=not available. SLE=systemic lupus erythematosus.
Overall, 283 patients with SLE who were not tested for SARS-CoV-2 infection by RT-PCR were subsequently tested for SARS-CoV-2 IgG antibodies (figure 1). These included patients with COVID-19-related symptoms, no symptoms, and those with unknown symptomatology.
26 patients from the WARCOV cohort who reported symptoms consistent with COVID-19 and whose treating physician suspected COVID-19 but who were unable to undergo RT-PCR testing at the time later underwent SARS-CoV-2 antibody testing. Six (23%) tested positive for SARS-CoV-2 antibodies, 6·4–31·7 weeks after symptom onset (mean 16·3 [SD 9·7] weeks; table 3), with five (83%) of these six patients on hydroxychloroquine, two (33%) on immunosuppressants, and none on glucocorticoids. All six patients reported symptoms of cough, anosmia, and ageusia. Other prevalent symptoms included rhinorrhoea, fever, shortness of breath, and diarrhoea. Of the 20 patients who reported symptoms consistent with COVID-19 who did not test positive for SARS-CoV-2 IgG antibodies 6·1–40·1 weeks (mean 18·8 [SD 10·1] weeks) after symptom onset, 17 (85%) were on hydroxychloroquine, ten (50%) were on immunosuppressants, and four (20%) were on glucocorticoids. Only six (30%) of these antibody-negative patients reported symptoms of anosmia or ageusia. One patient treated with hydroxychloroquine and mycophenolate mofetil reported diarrhoea only and had negative SARS-CoV-2 antibodies at a signal to cutoff ratio of 1·3 (just below the positivity threshold) 16 weeks following symptom onset.
Of the 83 patients from the WARCOV cohort who reported no clinical symptoms consistent with COVID-19, four (5%) had positive antibodies (table 3). None had any documented exposures; one patient was receiving intravenous immunoglobulin 3 weeks before antibody testing.
174 patients with unknown symptomatology during the pandemic, who did not participate in WARCOV questionnaires (ie, from the NYU Lupus Cohort), were evaluated for SARS-CoV-2 antibodies as part of standard-of-care surveillance, with 16 (9%) testing positive. Although COVID-19-related symptoms were not captured in these patients, none required admission to hospital (appendix p 3).
23 (45%) of 51 patients with a positive SARS-CoV-2 IgG antibody test underwent serial antibody testing (mean 15·8 [SD 6·0] weeks between first and last test). Six (26%) of 23 patients with SARS-CoV-2 antibodies subsequently seroconverted to become negative upon repeat testing (measured at 18·0 [SD 5·4] weeks, compared to 14·9 [6·1] weeks in 17 patients who maintained a response). Notably, all six patients who required admission to hospital for COVID-19 sustained SARS-CoV-2 antibody positivity, whereas six (35%) of 17 patients who only required ambulatory care did not maintain an antibody response. Patients whose antibody reactivity became undetectable had lower initial titres than those who sustained a response (2·6 [SD 0·73] vs 4·6 [2·0] signal to cutoff ratio). Of the six patients who did not maintain a response, three (50%) were taking immunosuppressant medications (one on anakinra and cyclosporine, one on azathioprine and rituximab, and one on mycophenolate mofetil and tacrolimus). Among the 17 patients who maintained a response, 11 (65%) were on immunosuppressants.
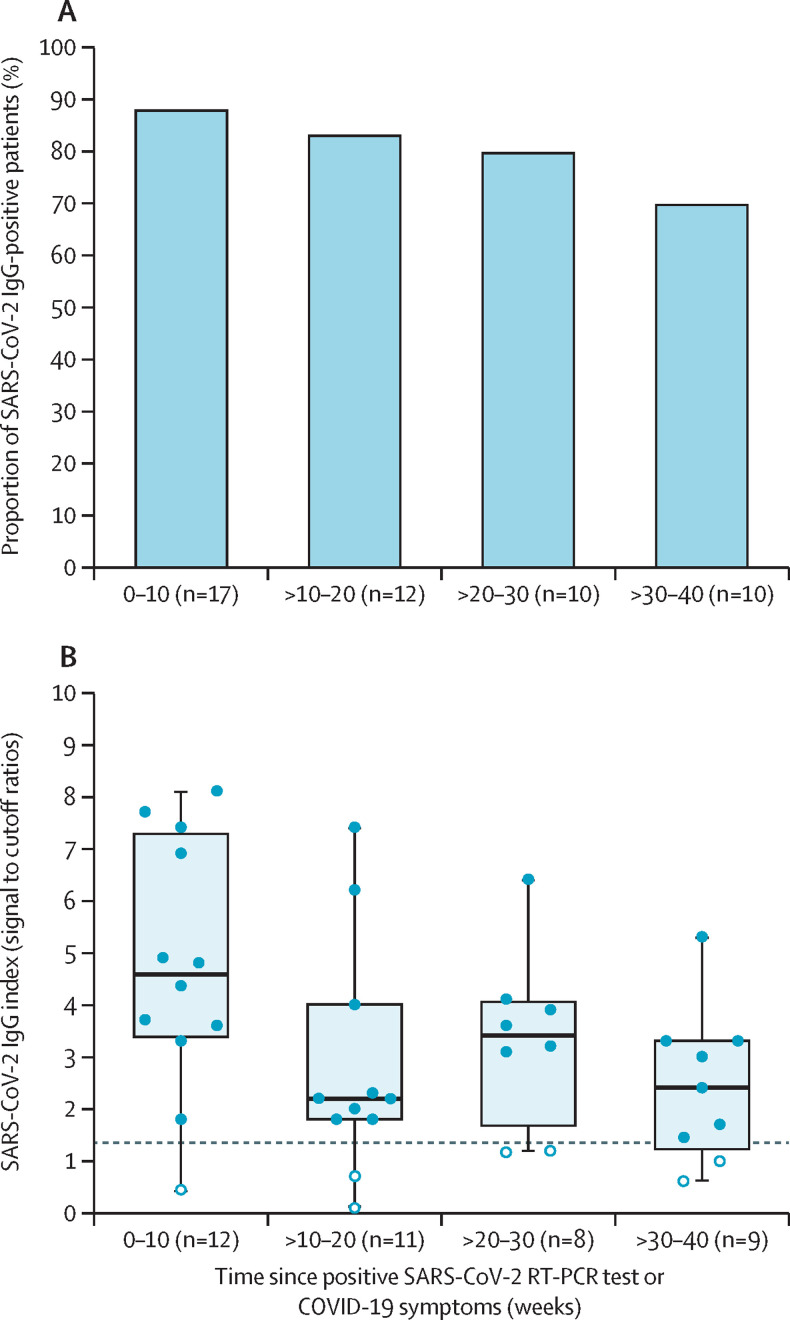
For 36 antibody-positive patients (29 with positive RT-PCR and seven with documented COVID-19 symptoms in the WARCOV study), the timing of symptom onset was known. 49 SARS-CoV-2 IgG antibody tests were done at various timepoints in these patients. 15 (88%) of 17 tests were positive at 0–10 weeks, ten (83%) of 12 were positive at 11–20 weeks, eight (80%) of ten were positive at 21–30 weeks, and seven (70%) of ten were positive at 31–40 weeks after positive RT-PCR or symptoms (figure 2 ). Of the 49 serial antibody tests, 40 were tested with the Abbott Architect assay. Signal to cutoff ratios were highest in patients tested at 0–10 weeks after positive RT-PCR or symptoms, but remained elevated for up to 40 weeks (figure 2).
Figure 2.
Positive SARS-CoV-2 IgG tests after initial presentation in patients with SLE and COVID-19
(A) Proportion of positive SARS-CoV-2 IgG antibodies in 49 patients with confirmed COVID-19, up to 40 weeks after initial presentation. (B) SARS-CoV-2 IgG titres in 40 patients tested with the Abbott Architect platform, up to 40 weeks after initial presentation. The boxplot shows the median (IQR) values, and the error bars show the 95% CI. The dashed line signifies the cutoff for a positive or negative qualitative result (at a signal to cutoff ratio of 1·4). White circles depict negative tests.
Discussion
In this sample of 329 patients with SLE, SARS-CoV-2 IgG prevalence was 16%. Hispanic ethnicity was associated with the presence of SARS-CoV-2 antibodies, possibly representing social determinants of health and increased exposure to the virus due to systemic drivers affecting the living and working conditions of people of Hispanic origin, including larger households, occupations in essential industries precluding remote work, and no paid medical leave, among other factors.18, 19, 20, 21 Sex, age, race, medication use, SLE disease activity, and degree of damage (as measured by the SLEDAI and SLICC Damage Index) were not associated with seropositivity. We observed high rates of SARS-CoV-2 IgG positivity in patients with SLE who had previously RT-PCR-confirmed SARS-CoV-2 infection, despite widespread use of hydroxychloroquine, glucocorticoids, and immunosuppressants. Several patients who tested negative for SARS-CoV-2 antibodies had titres slightly below the positive threshold, suggesting earlier reactivity. Additionally, the majority of serially tested patients sustained their antibody response up to 40 weeks. There was relatively low SARS-CoV-2 antibody development in patients with symptoms suggestive of COVID-19 who were unable to undergo RT-PCR testing. Low positivity was also observed in those with negative RT-PCR tests. These data are indicative of several issues: unreliability of symptom reporting, potential confounding due to an overlap with SLE flares and manifestations of other viral infections, and diminished serological responses in patients with milder COVID-19.
Stadlbauer and colleagues22 reported that SARS-CoV-2 seroprevalence in New York City stabilised at about 20% after the initial pandemic peak. Although this figure is likely to be subject to some element of referral bias, it reinforces the profound impact of COVID-19. The rate of SARS-CoV-2 IgG positivity among patients with SLE in New York City captured in our study was lower than this published figure, at 16%. Although the reason for this discrepancy remains unclear, it is plausible that patients with SLE were counselled to exercise increased caution given their increased susceptibility to infections as a result of frequent immunosuppressant use and their underlying immune dysregulation. The evidence for hydroxychloroquine as prophylaxis was largely negative so it is unlikely that this medication accounted for the lower seroprevalence.23
Questions surrounding which patients develop antiviral antibodies, whether these antibodies are protective against future infection, and whether they are sustainable over time are central to discussions about the containment of the COVID-19 pandemic. There have been conflicting reports about the persistence of SARS-CoV-2 antibodies in the general population, with some studies describing a rapid return to baseline levels in those with less severe disease, but others, including a large study based in New York City, showing robust responses for up to 5 months, even in those with mild to moderate disease.24, 25, 26 A study by Seow and colleagues,24 of 65 individuals with RT-PCR-confirmed SARS-CoV-2 infection, showed peak neutralising antibody responses averaging 23·1 days following symptom onset. Nearly all participants had waning neutralising antibody levels afterwards, although patients who had more severe symptoms, and were thus likely to generate a more robust initial neutralising antibody response, generally maintained elevated antibody titres over a follow-up period of up to 94 days.24 Similarly, in our study of patients with SLE, all serially evaluated patients who were admitted to hospital for COVID-19, presumably with more severe disease, maintained antibody responses over time. The ability of these patients to maintain a sustained antibody response after a more intensive initial exposure is potentially reassuring given the high immunogenicity of the currently available COVID-19 vaccines.27
Previous studies have assessed the performance of and validated the SARS-CoV-2 IgG antibody Abbott Architect test in the general population and in RT-PCR-positive patients in both ambulatory and hospital settings. Most of these studies assessed antibody titres within the first few weeks after the onset of symptoms or RT-PCR-positive testing.16, 17, 28, 29 Ng and colleagues16 reported median Abbott IgG values of 3·97 (IQR 1·42–5·23) at week 4 after symptom onset in patients with non-severe COVID-19 and 5·33 (4·9–5·81) in patients admitted to hospital or those who died of COVID-19. Similarly, in another study the mean IgG index values were 4·0 for patients with RT-PCR-confirmed COVID-19 not requiring hospital admission and 5·5 for those admitted to hospital. Since IgG titres measured with the Abbott Architect test decline approximately 6–8 weeks after RT-PCR testing,28 it is reassuring that although SARS-CoV-2 IgG titres in our cohort were obtained at a longer interval after RT-PCR testing, the mean titre of 4·1 was similar to those reported in previous studies, which were not limited to patients with SLE or immunosuppressed individuals.
Even before the onset of the COVID-19 pandemic, there has been interest in the impact of immunosuppressants on the immune response to viral antigens, particularly in the context of serological responses to vaccines. In the case of the influenza vaccine, most patients with rheumatic disease taking disease-modifying antirheumatic drugs (DMARDs) and biologic agents generate an adequate humoral response, with the notable exception of B-cell-depleting agents such as rituximab.30 However, despite a reduction in the antibody response in rituximab-treated patients, the cellular immune response to vaccination appears to be similar among patients treated with B-cell-depleting therapies, DMARDs, and healthy controls.31 In our study, a patient on obinutuzumab with RT-PCR-confirmed SARS-CoV-2 infection did not generate SARS-CoV-2 antibodies 2 months after initial RT-PCR testing, although three patients who received rituximab did produce antibodies (albeit these were measured shortly after infection). Although less is known about the effects of immunosuppressants on the SARS-CoV-2 antibody response, a subset of patients with acute SARS-CoV-2 infection in the study by Seow and colleagues24 received immunomodulating medications for a hyper-inflammatory syndrome, with no apparent impact on the neutralising antibody response, although the effect on antibody longevity was unclear. In the present study, 83% of patients with SLE who had RT-PCR-confirmed SARS-CoV-2 infection had subsequent SARS-CoV-2 IgG antibodies despite 62% being on immunosuppressants. This finding is similar to a recent report in which ten (77%) of 13 RT-PCR-positive patients with a variety of rheumatic diseases developed SARS-CoV-2 antibodies (nine [69%] of 13 were on immunosuppressants).32 Understanding the impact of immunomodulators on antiviral antibody responses will have important implications for determining how best to approach COVID-19 vaccination in patients with SLE, especially since patients on immunosuppressants were excluded from phase 2–3 studies of COVID-19 vaccines.
Although numerous studies have evaluated humoral immunity against SARS-CoV-2, cellular responses remain equally important in combating infection. Previous investigations of the genetically similar viruses SARS-CoV and MERS-CoV have shown that cellular antiviral responses are maintained well beyond a measurable humoral response.33 Ni and colleagues34 reported an increase in the number of IFNγ-secreting nucleocapsid-specific T cells in several patients who had recovered from COVID-19, correlating with neutralising antibody titres and indicating a role for both B and T cells in controlling viral infection. Aberrant T cells are known to play a role in SLE pathogenesis, raising the possibility of overlapping variables that might contribute to severe outcomes in patients with SLE who develop SARS-CoV-2 infection.35 For example, T helper 17 cells, found in greater proportions in patients with active SLE, are also associated with increased disease severity and the potentially fatal hyperinflammation described in a subset of patients with COVID-19.35, 36 A recent study described an increased risk of death from COVID-19 in people with rheumatic diseases with high disease activity and on immunosuppressants.37 Despite this risk, our previous data did not identify SLE-specific factors or immunosuppressants to be associated with an increased risk of admission to hospital for COVID-19.8 Future evaluation of T-cell responses and type I interferon activity in patients with SLE in relation to SARS-CoV-2 infection and vaccination will be essential to elucidate long-term immune responses, particularly if humoral immunity is more likely to decline in these patients.
This study was limited by the small sample size, as well as the variable clinical manifestations, medication regimens, and timing of antibody testing of our cohort, complicating robust evaluation of specific factors affecting antibody formation. Owing to the small sample size, our study was underpowered to detect significant associations and draw fully valid conclusions about the reported findings. We were also only able to do unadjusted analyses and there are several unmeasured variables related to SARS-CoV-2 exposure that are likely to be confounding the association between Hispanic ethnicity and testing positive for SARS-CoV-2 antibodies. These include neighbourhood, occupation, household size, and socioeconomic status, among others. It remains unclear whether negative testing or loss of antibodies is explained by the presence of milder symptoms or is due to immunosuppressive medications. Likewise, the absence of detectable antibodies in patients with clinical features consistent with COVID-19 but no concomitant RT-PCR testing might be due to a rapid decline in antibodies, an inability a priori to mount an immune response, or symptoms from underlying SLE activity or another infectious cause and not COVID-19 per se. Although SARS-CoV-2 IgG testing was generally done as standard of care, there might have been some degree of referral bias with regard to which patients were tested or were willing to travel to the clinic or outpatient laboratories during the pandemic. Moreover, we did not collect information about a non-SLE patient group to serve as a control and compare with the proportion of patients who had a positive antibody test. Although we did not do in-vitro neutralising antibody measurements in our study, the Abbott SARS-CoV-2 IgG antibodies have been shown to have a positive percent agreement between the detection of IgG and neutralising titres greater than 93%.16 Other limitations include inconsistencies about the serological testing methods, requiring comparison of various quantitative and qualitative immunoassays, which might have led to discrepant results.
In summary, the majority of patients with SLE and confirmed COVID-19 in our study were able to produce a serological response despite being on a variety of immunosuppressant treatments. Although a small subset of patients had negative testing on follow-up or lost antibody reactivity on serial examinations, no clear associated patterns were apparent. Additional studies will be required to fully evaluate the immune response of patients with SLE to COVID-19 and vaccination, but these findings provide reassurance about patients' abilities to generate an appropriate antibody response after infection and their possible protection against re-infection with similar strains of SARS-CoV-2.
Data sharing
De-identified participant data will be made available upon request following publication. All data requests should be made via e-mail to the corresponding author.
Acknowledgments
Acknowledgments
PMI and JPB received funding from the National Institutes of Health and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant number P50 AR07059), and JUS and PMI received a Bloomberg Philanthropies COVID-19 Response Initiative Grant. No authors are employed by NIH. We thank all patients who participated in this study, despite the challenging circumstances. We are grateful to the clinicians from NYU Langone Health and NYC Health + Hospitals/Bellevue who referred patients to us. We thank Leora Horwitz for her assistance with the ICD-10 query at NYU. We also acknowledge Tania Moin and Ranit Shriky for assistance in navigating regulatory matters.
THE NYU WARCOV Investigators
Samrachana Adhikari, Jordan Axelrad, Natalie Azar, Rebecca Blank, Lenore Brancato, Konstantin Brodetskiy, Lily Cao, Philip M Carlucci, Steven Carsons, Miao Chang, Shannon Chang, Alan Chen, Michael Colin, Lauren Fried, Bruce Garner, Avram Goldberg, Brian Golden, Michael Golpanian, Mayce Haj-Ali, Jessica Hoey, Yamen Homsi, Simon Hong, David Hudesman, Nazia Hussain, Brian Jaros, Susan Katz, Avani Kolla, Euna Lee, Sicy Lee, Robert Lesser, Robin Lipschitz, Eileen Lydon, Fardina Malik, Keshav Mangalick, Kavini Mehta, Anang Modi, Andrea Neimann, Joshua Novack, Julie Nusbaum, Connor Peterson, Andres Piatti, Benjamin Plotz, Andrew Porges, Lindsey Quintana, Paula Rackoff, Deborah Ramirez, Lauren Rangel, Soumya Reddy, Kimberly Robins, Pamela Rosenthal, Jonathan Samuels, Sabina Sandigursky, Vaish Sekar, Shruti Shankar, Harry Shen, Stephen Smiles, Craig Smuda, Bruce Solitar, Gary Solomon, Jennifer Stein, Alexa Steuer, Janine Sullivan, Katerina Svigos, Andrea Troxel, Stelios Viennas, Lauren Wong, Di Yan, Kaitlyn (Lu) Yin, Trevor Young, Gary Zagon.
Contributors
AS, AG, RF-R, and PMI conceived the study, searched the literature, collected data, created the figures and wrote the original draft. RF-R, AG, MM, KKD, and AJE collected data. MYK assisted in data analysis. All authors contributed to interpretation and analysis of data, reviewed and edited the manuscript, approved the final version to be published and agree to be accountable for all aspects of the work. AS, AG, RF, and PMI have verified the underlying data. AS, AG, RF-R, and PMI had final responsibility for the decision to submit for publication.
Declaration of interests
AS reports consulting fees from Bristol-Myers Squibb, Eli Lilly and Company, GlaxoSmithKline, Kezar Life Sciences, AstraZeneca, and Janssen. RHH reports consulting fees from Janssen. JUS reports consulting fees from Janssen, Novartis, Pfizer, Sanofi, Amgen, UCB, and AbbVie; and has received funding for investigator-initiated studies from Novartis, Sanofi, and Janssen. ADB reports consulting fees from GlaxoSmithKline and Novartis. JPB reports consulting fees from AbbVie, Amgen, Bristol-Myers Squibb, Exagen, GlaxoSmithKline, and Janssen. PMI reports consulting fees from GlaxoSmithKline. All other authors declare no competing interests.
Contributor Information
NYU WARCOV Investigators:
Samrachana Adhikari, Jordan Axelrad, Natalie Azar, Rebecca Blank, Lenore Brancato, Konstantin Brodetskiy, Lily Cao, Philip M. Carlucci, Steven Carsons, Miao Chang, Shannon Chang, Alan Chen, Michael Colin, Lauren Fried, Bruce Garner, Avram Goldberg, Brian Golden, Michael Golpanian, Mayce Haj-Ali, Jessica Hoey, Yamen Homsi, Simon Hong, David Hudesman, Nazia Hussain, Brian Jaros, Susan Katz, Avani Kolla, Euna Lee, Sicy Lee, Robert Lesser, Robin Lipschitz, Eileen Lydon, Fardina Malik, Keshav Mangalick, Kavini Mehta, Anang Modi, Andrea Neimann, Joshua Novack, Julie Nusbaum, Connor Peterson, Andres Piatti, Benjamin Plotz, Andrew Porges, Lindsey Quintana, Paula Rackoff, Deborah Ramirez, Lauren Rangel, Soumya Reddy, Kimberly Robins, Pamela Rosenthal, Jonathan Samuels, Sabina Sandigursky, Vaish Sekar, Shruti Shankar, Harry Shen, Stephen Smiles, Craig Smuda, Bruce Solitar, Gary Solomon, Jennifer Stein, Alexa Steuer, Janine Sullivan, Katerina Svigos, Andrea Troxel, Stelios Viennas, Lauren Wong, Di Yan, Kaitlyn (Lu) Yin, Trevor Young, and Gary Zagon
Supplementary Material
References
- 1.City of New York COVID-19. Data Summary - NYC Health. https://www1.nyc.gov/site/doh/covid/covid-19-data.page
- 2.Qu J, Wu C, Li X, et al. Profile of IgG and IgM antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020;71:2255–2258. doi: 10.1093/cid/ciaa489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu F, Liu M, Wang A, et al. Evaluating the association of clinical characteristics with neutralizing antibody levels in patients who have recovered from mild COVID-19 in Shanghai, China. JAMA Intern Med. 2020;180:1356–1362. doi: 10.1001/jamainternmed.2020.4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danza A, Ruiz-Irastorza G. Infection risk in systemic lupus erythematosus patients: susceptibility factors and preventive strategies. Lupus. 2013;22:1286–1294. doi: 10.1177/0961203313493032. [DOI] [PubMed] [Google Scholar]
- 5.Gartshteyn Y, Askanase AD, Schmidt NM, et al. COVID-19 and systemic lupus erythematosus: a case series. Lancet Rheumatol. 2020;2:e452–e454. doi: 10.1016/S2665-9913(20)30161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gianfrancesco M, Hyrich KL, Al-Adely S, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79:859–866. doi: 10.1136/annrheumdis-2020-217871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathian A, Mahevas M, Rohmer J, et al. Clinical course of coronavirus disease 2019 (COVID-19) in a series of 17 patients with systemic lupus erythematosus under long-term treatment with hydroxychloroquine. Ann Rheum Dis. 2020;79:837–839. doi: 10.1136/annrheumdis-2020-217566. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez-Ruiz R, Masson M, Kim MY, et al. Leveraging the United States epicenter to provide insights on COVID-19 in patients with systemic lupus erythematosus. Arthritis Rheumatol. 2020;72:1971–1980. doi: 10.1002/art.41450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haberman RH, Castillo R, Chen A, et al. COVID-19 in patients with inflammatory arthritis: a prospective study on the effects of comorbidities and disease-modifying antirheumatic drugs on clinical outcomes. Arthritis Rheumatol. 2020;72:1981–1989. doi: 10.1002/art.41456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40 doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 11.Buyon JP, Petri MA, Kim MY, et al. The effect of combined estrogen and progesterone hormone replacement therapy on disease activity in systemic lupus erythematosus: a randomized trial. Ann Intern Med. 2005;142:953–962. doi: 10.7326/0003-4819-142-12_part_1-200506210-00004. [DOI] [PubMed] [Google Scholar]
- 12.Gladman DD, Ibañez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29:288–291. [PubMed] [Google Scholar]
- 13.Thanou A, James JA, Arriens C, et al. Scoring systemic lupus erythematosus (SLE) disease activity with simple, rapid outcome measures. Lupus Sci Med. 2019;6 doi: 10.1136/lupus-2019-000365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. 1996;39:363–369. doi: 10.1002/art.1780390303. [DOI] [PubMed] [Google Scholar]
- 15.FDA EUA authorized serology test performance. April 28, 2021. https://www.fda.gov/medical-devices/emergency-situations-medical-devices/eua-authorized-serology-test-performance
- 16.Ng DL, Goldgof GM, Shy BR, et al. SARS-CoV-2 seroprevalence and neutralizing activity in donor and patient blood. Nat Commun. 2020;11 doi: 10.1038/s41467-020-18468-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bryan A, Pepper G, Wener MH, et al. Performance characteristics of the Abbott architect SARS-CoV-2 IgG assay and seroprevalence in Boise, Idaho. J Clin Microbiol. 2020;58:e00941–e00950. doi: 10.1128/JCM.00941-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Podewils LJ, Burket TL, Mettenbrink C, et al. Disproportionate incidence of COVID-19 infection, hospitalizations, and deaths among persons identifying as Hispanic or Latino - Denver, Colorado March-October 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1812–1816. doi: 10.15585/mmwr.mm6948a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogedegbe G, Ravenell J, Adhikari S, et al. Assessment of racial/ethnic disparities in hospitalization and mortality in patients with COVID-19 in New York City. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.26881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Selden TM, Berdahl TA. COVID-19 and racial/ethnic disparities in health risk, employment, and household composition. Health Aff. 2020;39:1624–1632. doi: 10.1377/hlthaff.2020.00897. [DOI] [PubMed] [Google Scholar]
- 21.Arasteh K. Prevalence of comorbidities and risks associated with COVID-19 among Black and Hispanic populations in New York City: an examination of the 2018 New York City Community Health Survey. J Racial Ethn Heal Disparities. 2020 doi: 10.1007/s40615-020-00844-1. published online Aug 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stadlbauer D, Tan J, Jiang K, et al. Repeated cross-sectional sero-monitoring of SARS-CoV-2 in New York City. Nature. 2021;590:146–150. doi: 10.1038/s41586-020-2912-6. [DOI] [PubMed] [Google Scholar]
- 23.Mitjà O, Corbacho-Monné M, Ubals M, et al. A cluster-randomized trial of hydroxychloroquine for prevention of Covid-19. N Engl J Med. 2021;384:417–427. doi: 10.1056/NEJMoa2021801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seow J, Graham C, Merrick B, et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol. 2020;5:1598–1607. doi: 10.1038/s41564-020-00813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ibarrondo FJ, Fulcher JA, Goodman-Meza D, et al. Rapid decay of anti-SARS-CoV-2 antibodies in persons with mild Covid-19. N Engl J Med. 2020;383:1085–1087. doi: 10.1056/NEJMc2025179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wajnberg A, Amanat F, Firpo A, et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370:1227–1230. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Walsh EE, Frenck RW, Jr, Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muecksch F, Wise H, Batchelor B, et al. Longitudinal serological analysis and neutralizing antibody levels in coronavirus disease 2019 convalescent patients. J Infect Dis. 2021;223:389–398. doi: 10.1093/infdis/jiaa659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Theel ES, Harring J, Hilgart H, Granger D. Performance characteristics of four high-throughput immunoassays for detection of IgG antibodies against SARS-CoV-2. J Clin Microbiol. 2020;58:e01243–e01320. doi: 10.1128/JCM.01243-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hua C, Barnetche T, Combe B, Morel J. Effect of methotrexate, anti-tumor necrosis factor α, and rituximab on the immune response to influenza and pneumococcal vaccines in patients with rheumatoid arthritis: a systematic review and meta-analysis. Arthritis Care Res. 2014;66:1016–1026. doi: 10.1002/acr.22246. [DOI] [PubMed] [Google Scholar]
- 31.Meroni PL, Zavaglia D, Girmenia C. Vaccinations in adults with rheumatoid arthritis in an era of new disease-modifying anti-rheumatic drugs. Clin Exp Rheumatol. 2018;36:317–328. [PubMed] [Google Scholar]
- 32.D'Silva KM, Serling-Boyd N, Hsu TY-T, Sparks JA, Wallace ZS. SARS-CoV-2 antibody response after COVID-19 in patients with rheumatic disease. Ann Rheum Dis. 2021;80:817–819. doi: 10.1136/annrheumdis-2020-219808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zuo J, Dowell A, Pearce H, et al. Robust SARS-CoV-2-specific T-cell immunity is maintained at 6 months following primary infection. Nature Immunol. 2021;22:620–626. doi: 10.1038/s41590-021-00902-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ni L, Ye F, Cheng ML, et al. Detection of SARS-CoV-2-Specific humoral and cellular immunity in COVID-19 convalescent individuals. Immunity. 2020;52:971. doi: 10.1016/j.immuni.2020.04.023. 77.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fernandez-Ruiz R, Paredes JL, Niewold TB. COVID-19 in patients with systemic lupus erythematosus: lessons learned from the inflammatory disease. Transl Res 2020. 2021;232:13–36. doi: 10.1016/j.trsl.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: an emerging target of JAK2 inhibitor fedratinib. J Microbiol Immunol Infect. 2020;53:368–370. doi: 10.1016/j.jmii.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strangfeld A, Schäfer M, Gianfrancesco MA, et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2020-219498. published online Jan 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified participant data will be made available upon request following publication. All data requests should be made via e-mail to the corresponding author.