Abstract
We examined the relationship of regulatory and review characteristics to postmarketing safety-related regulatory actions for 61 new therapeutic biologics (NTBs) approved between October 1, 2002 and December 31, 2014. We also compared NTBs with small-molecule new molecular entities (NMEs) on these measures. Postmarketing safety-related regulatory actions were defined as a safety-related withdrawal or a safety-related update to a safety section of the label through June 30, 2018. Four NTBs were withdrawn, two for safety reasons. At least one safety-related update was added to the labels of 54 (88.5%) NTBs. Label updates occurred throughout the follow-up period. Time to the first safety-related regulatory action was shorter for NTBs approved under accelerated approval. The occurrence of safety events was more likely to occur with NTBs than with NMEs. This may be explained in part by the higher proportion of NTBs in the anatomical therapeutic chemical (ATC) classification categories with higher frequency of safety-related updates. NTBs also had shorter time to safety events than NMEs. These findings underscore the importance of continued development of the lifecycle safety surveillance system for both drugs and biologics with consideration for product type and its characteristics, including pharmacologic action.
Keywords: biologics, safety, labeling, regulatory, postmarket, pharmacovigilance
Introduction
Therapeutic biologics are used in the treatment, prevention and cure of diseases. Unlike chemically synthesized, small-molecule drugs that have well-defined structures, biologics are generally derived from living material and are complex molecules or mixtures of molecules1. Prior studies have examined the relationship of drug development characteristics such as expedited pathway designation to the development of postmarketing safety issues2–11. The majority included only small-molecule drugs and excluded biologics 3–5,7,10,11. Several studies that included both small-molecule drugs and biologics did not specifically look at the development of postmarketing safety issues among biologics2,6,9.
Few studies have evaluated the safety-related actions in biologics or contrasted these outcomes with those seen in small-molecule drugs. Giezen found that 23.6% of biologics approved in the U.S. and the EU had a safety-related regulatory action, with 70.7% of actions issued in the first 5 years after approval8. Downing reported that postmarketing safety events occurred more frequently in biologics6. Pinnow examined the relationship of drug development characteristics and expedited pathways to postmarketing safety-related regulatory actions, but did not include NTBs in that study11.
In this study, we sought to describe the timing of safety-related regulatory actions for NTBs and to explore the relationship between review and regulatory pathways and postmarketing safety-related regulatory actions for NTBs approved between October 1, 2002 and December 31, 2014. We also aimed to compare the results to those with new molecular entity (NME) drugs using extended safety-related outcome follow-up through June 30, 2018.
Methods
Postmarketing safety-related regulatory actions for NTBs approved by the FDA Center for Drug Evaluation and Research (CDER) between October 1, 2002, and December 31, 2014 were reviewed through June 30, 2018. Biosimilars and over-the-counter products were excluded from the study. Using publicly available data12–14 we recorded whether the NTB was designated under one or more expedited program: priority review, accelerated approval using a surrogate endpoint, fast-track, or breakthrough therapy15. Indication for use of each NTB was classified as being a serious or life-threatening disorder or not. Orphan therapeutic product designation was obtained from the orphan drug database16. Products approved with multiple indications were classified as non-orphan if any of the indications were not granted orphan designation. We classified NTBs as to whether they were intended for long-term use (chronic or repeated intermittent use for longer than 6 months for the approved indication); whether the application met the Prescription Drug User Fee Act (PDUFA) goal date for the first review cycle, considering any goal-date extension associated with the receipt of a major amendment; and whether it was initially approved with a Boxed Warning. We determined whether the U.S. was the country of first approval and whether the biologic was first marketed in the U.S. We recorded the pharmacologic class of the biologic using the Anatomical Therapeutic Chemical (ATC) Classification System17, whether the biologic was deemed a first-in-class biologic (an indicator of the innovative nature of the biologic), and whether the biologic had a near-deadline approval, defined as being approved within the 59-day period prior to the first-cycle PDUFA goal date. Internal FDA data were used only to clarify ambiguities in less than 1% of the data collected. The detailed methods of determining the regulatory characteristics were previously reported11; definitions of these characteristics are provided in Table S1.
A postmarketing safety-related regulatory action was defined as withdrawal of the drug from the market due to safety concerns, or an update to the drug’s label that included the addition of a new distinct safety-related issue to any of the following safety-related sections of the label from the time of approval through June 30, 2018: Boxed Warning, Contraindications, Warnings and Precautions, Adverse Reactions, or Drug Interactions. We reviewed label updates posted on two public FDA websites: Drug Safety-related Labeling Changes database13,14 and Drugs@FDA12. We reviewed the relevant sepdfuactions of the label as previously described. The following were not considered safety issues and were therefore excluded from the analyses: previously known issues where different terminologies were used in the updated label (i.e., fainting vs. syncope), changes in frequency of a known issue, increases in severity of a known issue not representing a more complex syndrome, updates representing instructions to patients, and additional information related to safety and effective use of the drug related only to treatment of new indications.
For each biologic, a second independent examiner reviewed the abstracted individual safety issues and any discrepancies between the reviewers were resolved. A single label update could include one or more new safety issues, and a single safety issue could result in an update to one or more sections of the label. For each biologic, the number of issues per section and the overall number of issues were determined for each label update. The overall number of updates, as well as the number of updates per section of the label involved, were determined for each biologic. We performed analyses covering all available follow-up, and, to account for the varying length of follow-up, at 3.5 years as this was the minimum follow-up for all products. To compare safety-related regulatory actions between NTBs and NMEs, we updated the previously reported safety data on NMEs through June 30, 2018. We analyzed the 5 previously defined safety-related regulatory actions that included a varying number of safety-related sections of the label11 (Table S1).
The approval status of each NTB on June 30, 2018 (active or withdrawn) was ascertained by checking Purple Book18 and internal FDA records. Withdrawals for safety reasons were considered safety endpoints; withdrawal for other reasons were not. Withdrawal date was the date the sponsor requested withdrawal of the NTB.
Statistical analysis
The overall and yearly (since approval) number of label updates and new safety issues were summarized for each NTB. The length of time from the date of approval through June 30, 2018, or withdrawal from the market, whichever occurred first, defined the length of follow-up for each NTB. The association between postmarketing safety-related regulatory actions and categorical NTB characteristics was assessed using chi-square and Fisher’s exact tests; the Wilcoxon rank-sum test was used for between-group comparisons of continuous variables. We used bivariate logistic regression to assess the relationship between the safety-related regulatory actions and each of the following: ATC classification, regulatory and review characteristics, expedited approval programs, orphan drug designation, and first-in-class status. Multivariable logistic regression was planned but not conducted due to limitations in sample size. Kaplan–Meier estimates with Gehan-Breslow tests were used to analyze the association between regulatory review pathway and time to first safety-related regulatory action.
To examine the differences between NTBs and NMEs and the five levels of postmarketing safety-related regulatory actions, we conducted bivariate and multivariate logistic regression. We then conducted Kaplan–Meier estimates with Gehan-Breslow tests. Cox proportional hazards modeling were done to adjust for confounders. A P-value of <0.05 was considered significant.
Results
A total of 61 NTBs were approved by the FDA between October 1, 2002 and December 31, 2014. Follow-up ranged from 3.5 to 15.7 years with a mean of 8.6±3.6 years (median, 8.4 years). Forty-nine (80.3%) of the biologics had at least 5 years of follow-up; 20 (32.8%) had at least 10 years of follow-up. Table 1 presents the regulatory and review characteristics of the NTBs and contrasts them with those previously reported for NMEs. Proportions of products approved under accelerated approval using a surrogate endpoint, having breakthrough designation, indicated for long-term use, approved first in the U.S., and marketed first in the U.S., were comparable between NTBs and NMEs. Compared to NMEs, a significantly higher proportion of NTBs were approved with a Boxed Warning (50.8% vs. 26.6%), were indicated for serious conditions (100% vs. 90.3%), had orphan product designation (47.5% vs. 28.1%), were first-in-class (55.7% vs. 34.5%), had fast-track designation (49.2% vs. 30.6%), had priority review designation (68.8% vs. 42.8%), and had been designated in at least one expedited program (70.5% vs. 50.0%). The majority (82.0%) of NTBs were antineoplastic agents or products for serious alimentary tract and metabolic, or musculoskeletal disorders, while the indications for NMEs were spread over a wider range of ATC classes (Table S2).
Table 1:
Regulatory and review characteristics for new therapeutic biologics (NTB) and new molecular entity (NME) drugs approved by the FDA between October 1, 2002 and December 31, 2014
| Variable | New Therapeutic Biologics N = 61 | New Molecular Entities N = 278 | P |
|---|---|---|---|
| Follow-up since approval (years) | |||
| Mean±SD | 8.6±3.6 | 9.1±3.7 | 0.38 |
| Median | 8.4 | 8.9 | |
| IQR | 5.7, 11.3 | 5.7, 12.5 | |
| Range | 3.5, 15.7 | 3.1, 15.7 | |
| Approval time (days)a | |||
| Mean±SD | 422.0±324.5 | 510.6 ± 564.8 | 0.3 |
| Median | 277 | 304 | |
| IQR | 183, 529 | 242, 591 | |
| Range | 75, 1684 | 78, 5590 | |
| <200 | 19 (31.2%) | 58 (20.9%) | |
| 200–399 | 22 (36.1%) | 132 (47.5%) | |
| ≥400 | 20 (32.8%) | 88 (31.6%) | |
| Review time (days)b | |||
| Mean±SD | 327.1±148.2 | 354.5 ± 181.0 | 0.41 |
| Median | 277 | 304 | |
| IQR | 183, 455 | 242, 420 | |
| Range | 75, 652 | 78, 1324 | |
| <200 | 19 (31.1%) | 59 (21.2%) | |
| 200–399 | 24 (39.3%) | 149 (53.6%) | |
| ≥400 | 18 (29.5%) | 70 (25.2%) | |
| Met PDUFA goal first cycle (n (%)) | |||
| Yes | 58 (95.1%) | 256 (92.1%) | 0.59 |
| No | 3 (4.9%) | 22 (7.9%) | |
| Amendment extended PDUFA goal (n (%)) | |||
| Yes | 22 (36.1%) | 59 (21.2%) | 0.01 |
| No | 39 (63.9%) | 219 (78.8%) | |
| Near-regulatory approval [0–59 days] (n (%)) | |||
| Yes | 37 (60.7%) | 159 (57.2%) | 0.62 |
| No | 24 (39.3%) | 119 (42.8%) | |
| Approved with Boxed Warning (n (%)) | |||
| Yes | 31 (50.8%) | 74 (26.6%) | <0.01 |
| No | 30 (49.2%) | 204 (73.4%) | |
| Indicated for serious conditions (n (%)) | |||
| Yes | 61 (100%) | 251 (90.3%) | 0.01 |
| No | 0 | 27 (9.7%) | |
| Indicated for long-term use (n (%)) | |||
| Yes | 47 (77.0%) | 189 (68.0%) | 0.16 |
| No | 14 (23.0%) | 89 (32.0%) | |
| Orphan designation (n (%)) | |||
| Yes | 29 (47.5%) | 78 (28.1%) | <0.01 |
| No | 32 (52.5%) | 200 (71.9%) | |
| First-in-class (n (%)) | |||
| Yes | 34 (55.7%) | 96 (34.5%) | <0.01 |
| No | 27 (44.3%) | 182 (65.5%) | |
| Approved first in the U.S. (n (%)) | |||
| Yes | 42 (69.9%) | 176 (63.3%) | 0.41 |
| No | 19 (31.1%) | 102 (36.7%) | |
| Marketed first in the U.S. (n (%))c | |||
| Yes | 45 (73.8%) | 186 (67.1%) | 0.31 |
| No | 19 (31.2%) | 91 (32.9%) | |
| Fast-track designation (n (%)) | |||
| Yes | 30 (49.2%) | 85 (30.6%) | <0.01 |
| No | 31 (50.8%) | 193 (69.4%) | |
| Priority review designation (n (%)) | |||
| Yes | 42 (68.8%) | 119 (42.8%) | <0.01 |
| No | 19 (31.2%) | 159 (57.2%) | |
| Accelerated approval using a surrogate endpoint (n (%)) | |||
| Yes | 9 (14.8%) | 28 (10.1%) | 0.29 |
| No | 52 (85.2%) | 250 (89.9%) | |
| Breakthrough designation (n (%))d | |||
| Yes | 4 (30.8%) | 8 (18.6%) | 0.44 |
| No | 9 (69.2%) | 35 (81.4%) | |
| One or more expedited program (n (%)) | |||
| Yes | 43 (70.5%) | 139 (50.0%) | <0.01 |
| No | 18 (29.5%) | 139 (50.0%) | |
| Approved first cycle (n (%)) | |||
| Yes | 42 (68.8%) | 189 (68.0%) | 0.9 |
| No | 19 (31.2%) | 89 (32.0%) | |
| ATC classification (n (%))e | |||
| Alimentary tract and metabolism | 10 (16.4%) | 35 (12.6%) | 0.43 |
| Blood and blood forming organs | 3 (4.9%) | 15 (5.4%) | 1 |
| Cardiovascular system | 0 | 20 (7.2%) | 0.03 |
| Dermatological | 0 | 8 (2.9%) | 0.36 |
| Genitourinary system and sex hormones | 0 | 14 (5.0%) | 0.08 |
| Systemic hormonal preparations, excluding sex hormones and insulins | 0 | 7 (2.5%) | 0.36 |
| Antiinfective agents for systemic use | 1 (1.6%) | 36 (12.9%) | 0.01 |
| Antineoplastic and immunomodulation agents | 34 (55.7%) | 57 (20.5%) | <0.01 |
| Musculoskeletal system | 5 (8.2%) | 2 (0.7%) | <0.01 |
| Nervous system | 0 | 39 (14.0%) | <0.01 |
| Antiparasitic products, insecticides and repellents | 0 | 4 (1.4%) | 1 |
| Respiratory system | 1 (1.6%) | 10 (3.6%) | 0.7 |
| Sensory organs | 3 (4.9%) | 9 (3.2%) | 0.46 |
| Various systems | 4 (6.6%) | 22 (7.9%) | 1 |
Approval time is the total time from the receipt of the initially filed application to the date of approval.
Review time is the total number of days FDA took to review the drug, and it is calculated as the time a submission was received to the time a decision was made for each review cycle. Total FDA review time was calculated by summing FDA review time, for all review cycles prior to approval.
NME: n=277; excludes one NME that was approved but was never marketed.
NTB n=13; NME n=43; available only for products filed on or after July 9, 2012.
P-values for each ATC class are based on the comparison of the specific ATC class to all other ATC classes.
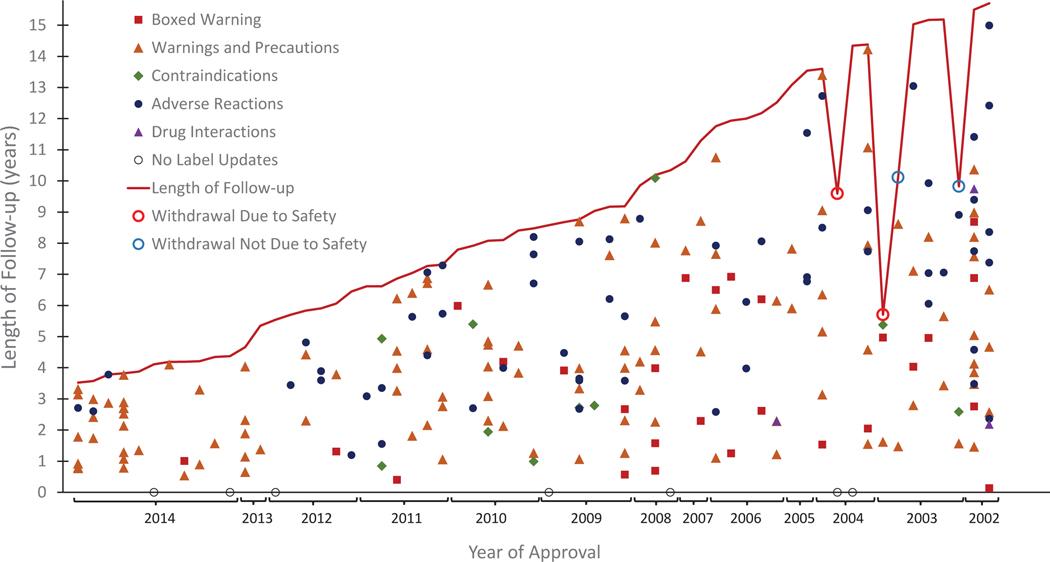
Figure 1 illustrates the timing of all label updates relative to approval. At least one safety-related label update was added to the label of 54 (88.5%) NTBs. Of the seven NTBs without any safety-related labeling updates, follow-up ranged from 4.1 to 14.3 years; two had less than 5 years of follow-up. Label updates occurred as early as 48 days after approval and throughout the follow-up period. The majority of label updates (69.1%) and number of issues added to the label (67.4%) occurred more than three years after approval. As of June 30, 2018, four (6.5%) NTBs were withdrawn from the market, two (3.3%) of which were withdrawn for safety reasons.
Figure 1:
Time to label updates for new therapeutic biologics (NTBs) by section of the label updated as of June 30, 2018. This figure illustrates the time to drug label updates for NTB by section of the label updated as of June 30, 2018. The 61 approved biologics are ordered by length of time since approval in the US (shortest to longest) along the x-axis. The solid markers represent a label update (y-axis indicating the time since approval the update occurred) and the section of the label updated. If more than one section of the label was updated on the same date, the “highest” level is shown according to the following hierarchy “Boxed Warning » Warnings and Precautions » Contraindications » Adverse Reactions » Drug Interactions.” Black circle markers on the x-axis indicates no label updates were made for that NTB during the follow-up period. The continuous line marks the length of time from approval through June 30, 2018 or a withdrawal from the market, whichever came first (i.e., end of follow-up for each NTB). Open red circle markers on this line represent withdrawals from the market due to safety, while the open blue circle markers on this line represent withdrawals from the market not due to safety.
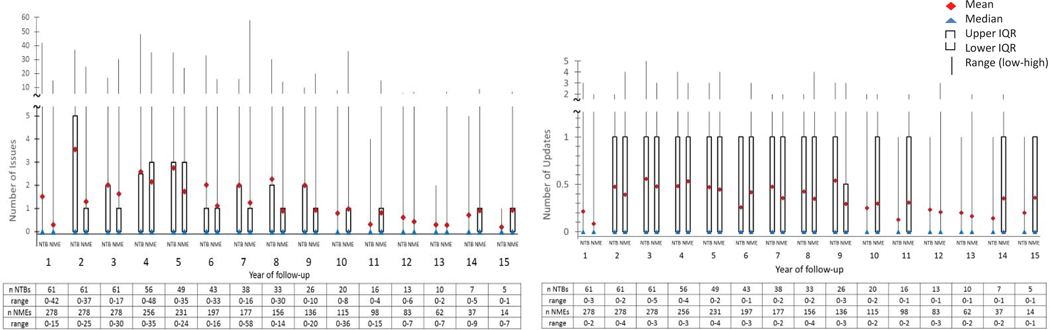
Figure 2 illustrates the number of label updates and issues per year after product approval. The mean number of safety-related label updates per year of follow-up for NTBs ranged from 0.13 to 0.56. The maximum number of label updates made for any one NTB in a year was 5. The mean number of new safety issues per year of follow-up for NTBs ranged from 0.20 to 3.56. The maximum number of issues added to any one NTB in a year was 48, most of which enumerated detailed clinical manifestations of infusion reactions and immunogenicity not in the product’s original label. A median of zero (0) issues and updates per NTB per year reflects the fact that most NTBs did not have safety-related label changes in any given year. The distribution of label updates and issues added to the label over the years of follow-up was similar with that observed with NMEs. While most of the issues were added during the first 9 years since approval, safety-related updates continued to occur 15 years after a product approval.
Figure 2:
Boxplots of the number of label updates and issues per year of follow-up for new therapeutic biologics (NTB) and new molecular entities (NME). This figure illustrates the mean and median number of label updates (left) and new safety issues (right) per year of follow-up. The interquartile range (IQR) (difference between the 25th and 75th percentile) and minimum and maximum number of label updates and new safety issues are graphically represented. Yearly rates for label updates and issues incorporated in the label were calculated for each completed year of follow-up after drug approval. The median value of zero reflects that fact that in any given year most drugs were not the subject of a safety-related label update.
Table S3 illustrates the number of label updates and safety issues per section of the label. There were 214 individual label updates addressing 1102 distinct safety-related issues. The Boxed Warning section of the labels was updated 27 times; Warnings and Precautions, 135 times; Contraindications, 25 times; Adverse Reactions, 142 times; and Drug Interactions, 8 times. Of the 1102 safety-related issues added to the label, 94 (8.5%) issues were added to the Boxed Warning section of the label, 424 (38.5%) to Warnings and Precautions, 47 (4.3%) to Contraindications, 652 (59.1%) to Adverse Reactions, and 14 (1.3%) to Drug Interactions.
The number of safety-related label updates per NTB ranged from 0 to 17 (mean±SD, 3.5±3.2; median, 3.0) (Table S4). There were significantly more label updates to the Boxed Warnings (median 0 vs. 0; IQR 0, 1 vs. 0, 0; P<0.001) for NTBs than for NMEs; NMEs had more updates to the Drug Interactions (median 0 vs. 0; IQR 0, 0 vs. 0, 1; P=0.01) than NTBs. Compared to NMEs, NTBs had more new safety issues added to the label overall (median 10 vs. 7; IQR 4, 25 vs. 1, 15; P=0.008), to Boxed Warnings (median 0 vs. 0; IQR 0, 1 vs. 0, 0; P<0.001), to Warnings and Precautions (median 3 vs. 2; IQR 0, 13 vs. 0, 5; P =0.01), and to Adverse Reactions (median 5 vs. 3; IQR 1, 15 vs. 0, 9; P =0.02) and fewer new safety issues added to the Drug Interactions (median 0 vs. 0; IQR 0, 0 vs 0, 1; P =0.01) sections of the label. At 3.5 years of follow-up (available for all products), overall, NTBs had more new safety issues added to the label than NMEs (median 3 vs. 1; IQR 0, 12 vs. 0, 6; P =0.04) though there were no significant differences in the number of label updates (median 1 vs. 1; IQR 0, 2 vs. 0, 2; P =0.27). This included more new safety issues added to the Boxed Warnings (median 0 vs. 0; IQR 0, 0 vs. 0, 0; P =0.003), Warnings and Precautions (median 1 vs. 0; IQR 0, 5 vs. 0, 2; P =0.003), and Adverse Reactions (median 1 vs. 0; IQR 0, 7 vs. 0, 3; P =0.02) sections of the label for NTBs compared to NMEs (Table S4).
For the entire follow-up period, a higher proportion of NTBs, compared to NMEs, had postmarket updates to the Boxed Warnings and Warnings and Precautions sections of the label (32.8% vs. 12.6% and 73.8% vs. 63.1%, respectively) overall and in the ATC categories ‘alimentary tract and metabolism,’ ‘blood and blood forming organs,’ and ‘antineoplastic and immunomodulatory agents’(Table S5).
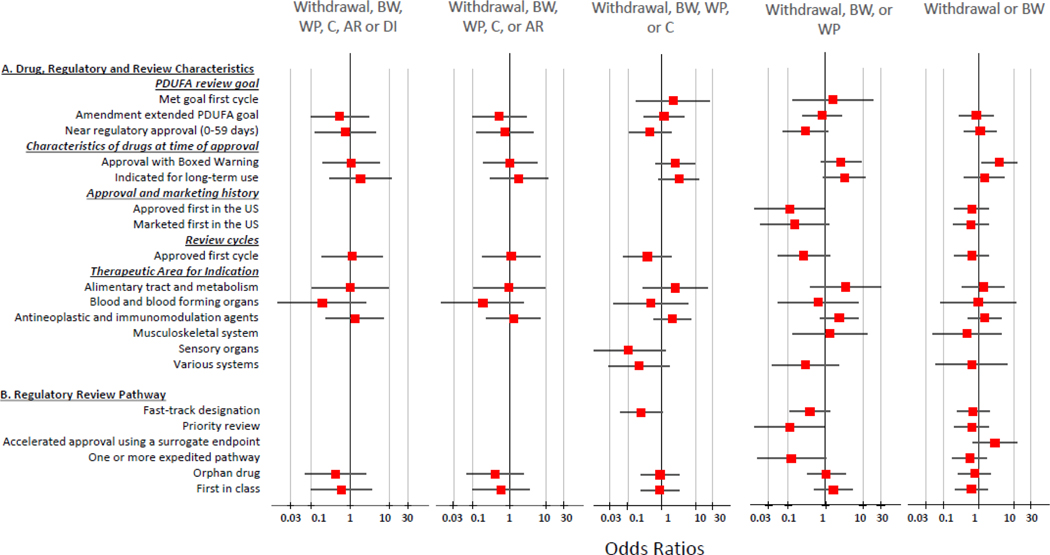
No statistically significant association between a postmarketing safety-related regulatory action and fast-track designation, accelerated approval using a surrogate endpoint, orphan drug designation, designation in at least one expedited pathway, and first-in-class drug status was seen with bivariable logistic regression analyses (Figure 3). There was no relationship between the Prescription Drug User Fee Act (PDUFA) review goal performance (meeting goal date at the first cycle, amendments extending the PDUFA goal date, and approval near the user-fee goal date), nor between marketing first in the U.S., and occurrence of postmarketing safety-related regulatory actions. ATC classification and indication for long-term use were not associated with postmarketing safety-related regulatory actions.
Figure 3:
Relationship of regulatory, review characteristics, and safety-related regulatory actions for new therapeutic biologics (n=61) approved by the FDA between October 1, 2002, and December 31, 2014.
BW, Boxed Warning; WP, Warnings and Precautions; C, Contraindications; AR, Adverse Reactions; DI, Drug Interactions; Forest plot not available for analyses where the independent covariate had a zero value in one of more of the formed 2 X 2 table cells.
Table 2 summarizes the number of label updates and issues added to the NTB labels by specific regulatory pathway. NTBs that were approved under priority review designation had significantly fewer label updates over the total length of follow-up compared with those approved under standard designation (median 2.0 vs. 3.0; P<0.05), though this difference was not seen in the first 3.5 years of follow-up (median 1.0 vs. 1.0; P=0.09). NTBs under accelerated approval using a surrogate endpoint had significantly more label updates (complete follow-up: median 7.0 vs. 2.0; P=0.005; for the first 3.5 years: 2.0 vs. 1.0; P=0.02) and issues added (complete follow-up: median 33.0 vs. 7.0; P=0.002; for the first 3.5 years: median 20.0 vs. 2.0; P=0.001) compared to those that were not.
Table 2:
Number of label updates and issues added to labels for original therapeutic biologics, by specific regulatory pathways
| Regulatory Pathway | N | Whole Study Period | 3.5 years of Follow-up | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total number of label updates | Total number of safety issues | Total number of label updates | Total number of safety issues | |||||||||||
| mean±SD | median | Wilcoxon rank-sum P value | mean±SD | median | Wilcoxon rank-sum P value | mean±SD | median | Wilcoxon rank-sum P value | mean±SD | median | Wilcoxon rank-sum P value | |||
| Fast-track designation | Yes | 30 | 2.6±2.3 | 2 | 0.06 | 15.8±20.6 | 7 | 0.16 | 1.1±1.4 | 1 | 0.09 | 5.9±9.9 | 2.5 | 0.19 |
| No | 31 | 4.4±3.7 | 3 | 20.3±18.9 | 16 | 1.8±1.8 | 1 | 11.5±15.1 | 4 | |||||
| Priority review designation | Yes | 42 | 2.9±2.5 | 2 | 0.047 | 16.5±20.1 | 8 | 0.14 | 1.3±1.8 | 1 | 0.09 | 7.6±12.9 | 3 | 0.14 |
| No | 19 | 4.9±4.2 | 3 | 21.5±18.7 | 18 | 1.8±1.4 | 1 | 11.2±13.4 | 7 | |||||
| Accelerated approval using a surrogate endpoint | Yes | 9 | 6.0±2.6 | 7 | 0.005 | 40.4±25.2 | 33 | 0.002 | 3.1±2.7 | 2 | 0.02 | 24.6±19.0 | 20 | 0.003 |
| No | 52 | 3.1±3.1 | 2 | 14.2±15.9 | 7 | 1.2±1.2 | 1 | 6.0±9.5 | 2 | |||||
| One or more expedited program | Yes | 43 | 2.9±2.4 | 2 | 0.052 | 16.3±19.9 | 7 | 0.12 | 1.3±1.7 | 1 | 0.08 | 7.5±12.7 | 3 | 0.10 |
| No | 18 | 5.0±4.3 | 3 | 22.3±18.9 | 19.5 | 1.8±1.4 | 1.5 | 11.7±13.5 | 8 | |||||
| First-in-class | Yes | 34 | 3.4±2.6 | 3 | 0.71 | 19.1±20.2 | 15.5 | 0.57 | 1.4±1.6 | 1 | 0.7 | 9.4±13.8 | 3.5 | 0.79 |
| No | 27 | 3.7±3.8 | 2 | 16.8±19.3 | 8 | 1.6±1.7 | 1 | 7.8±12.1 | 3 | |||||
| Orphan drug | Yes | 29 | 2.7±2.3 | 2 | 0.10 | 17.4±20.9 | 10 | 0.55 | 1.4±2.0 | 1 | 0.16 | 8.3±13.7 | 1 | 0.35 |
| No | 32 | 4.2±3.7 | 3 | 18.7±18.9 | 11.5 | 1.6±1.3 | 1 | 9.1±12.6 | 4 | |||||
NTBs that were approved first in the U.S. and those with priority review designation were less likely to be withdrawn or have any update in the Boxed Warning or Warnings and Precautions than those that were not (OR=0.11; 95% CI 0.01–0.92, for both). Initial approval with a Boxed Warning was associated with a higher likelihood of being withdrawn or having a new safety issue added to the Boxed Warning section (OR=3.75; 95% CI 1.2–11.7). Multivariable logistic regression analysis was not conducted due to limitations in sample size. Because all NTBs were indicated for serious conditions, and because none was classified into many of the ATC codes, analyses for association between safety-related regulatory actions and these characteristics were not possible. Analyses of updated data for NMEs (Figure S1) were generally consistent with previously reported associations.
For NTBs and NMEs combined, we found that antineoplastic or immunomodulatory agents had more label updates (median 3 vs. 2, P=0.001) and new safety issues added to the label (median 11 vs. 6, P<0.001) than other products, and time to first safety event was shorter for antineoplastic or immunomodulatory agents compared to other products.
Unadjusted logistic regression showed that NTBs have a significantly higher odds of a having a postmarketing safety-related regulatory action on three levels of the safety-related regulatory actions (withdrawn or change to 4, 3, and 1 sections of the label) compared to NMEs (Table S6). After adjusting for possible confounders, including length of follow-up, indication for long-term use, approval with a Boxed Warning, and antineoplastic and immunomodulation agent ATC classification, the results remained significant for two levels of safety-related regulatory actions (withdrawn or change to 4, or 1 sections of the label).
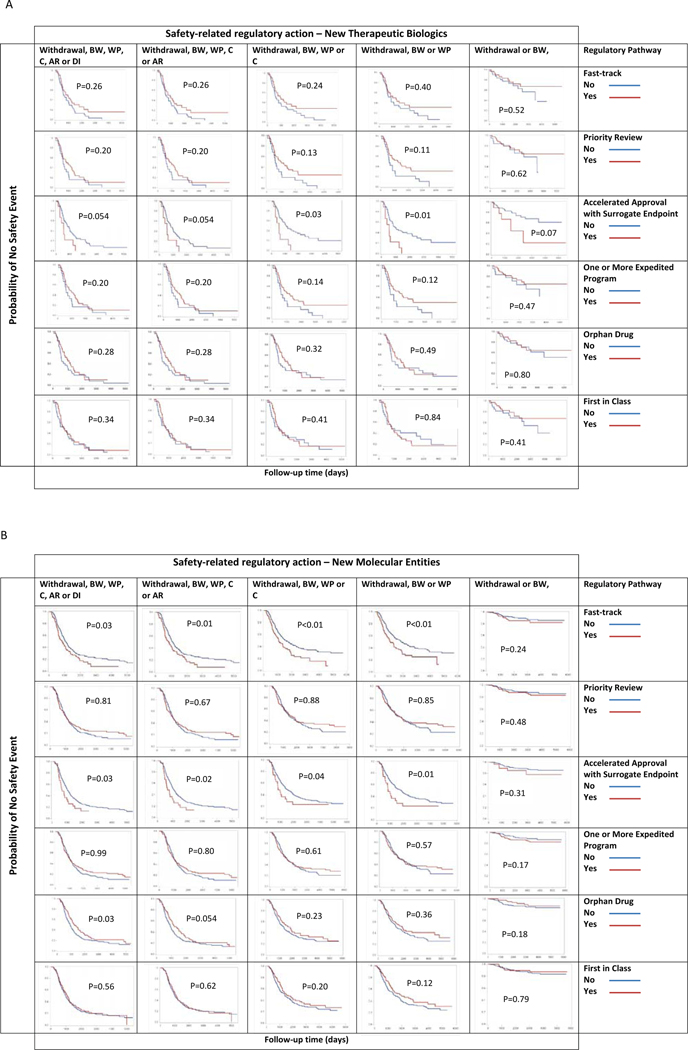
Figure 4a presents the results of the time-to-event analyses for NTBs. Figure 4b presents those for NMEs using additional follow-up data; the results are generally consistent with prior analyses. For both NTBs and NMEs, time to the first safety-related regulatory action was shorter for products approved under accelerated approval using a surrogate endpoint than for those that were not. A notable difference between NTBs and NMEs was that NMEs with fast-track designation had significantly shorter time to a first safety-related regulatory action compared with those approved under the standard regulatory pathway. There were no significant differences between the time to the first safety-related regulatory action between NTBs or NMEs granted priority review designation and those with a standard review status, and between those reviewed or approved under one or more expedited program and those not under any of the expedited programs. There were no significant differences between the time to first safety-related regulatory action between first-in-class and addition-to-class biologics, and those that were not.
Figure 4:
Time to first safety-related regulatory action, new therapeutic biologics (A) and new molecular entities (B), by regulatory pathway using Gehan-Breslow tests.
BW, Boxed Warning; WP, Warnings and Precautions; C, Contraindications; AR, Adverse Reactions; DI, Drug Interactions.
The time to the first safety-related regulatory action for each of five levels of safety-related regulatory actions was shorter for NTBs compared with NME (Figure S2). Multivariable Cox proportional hazards models showed that NTBs continued to have a higher occurrence of safety-related withdrawal or update to the Boxed Warning section of the label compared to NMEs (Figure S2).
The most common adverse events added to the Boxed Warning or Warnings and Precautions sections of the NTB labels were infections, which were added to the label of 53 (86.9%) NTB labels. These included progressive multifocal encephalopathy; various fungal, viral, and bacterial infections; and reactivation of latent infections. Immediate hypersensitivity reactions were added to the label of 44 (72.1%) NTBs. The most common amongst these included anaphylactic reaction (21.3%), angioedema (13.1%), and hypersensitivity (11.5%).
Discussion
In a set of 61 NTBs approved between 2002 and 2014, postmarketing safety-related regulatory actions occurred throughout the product’s postmarket life. Using a range of definitions of safety-related regulatory actions that consider updates to different safety-related sections of the NTBs’ labels, we found that the second through ninth years after approval were the most active, with a diminution, but not cessation, of activity thereafter. This variability in timing is consistent with previous findings that described the issues and timing of postmarketing addition of Boxed Warnings for biologics approved in the U.S.8 The most common adverse events added to NTB labels are similar to those reported by Patel and colleagues19.
Many of our findings were consistent with our previously reported results concerning NMEs, though we did find some differences between NTBs and NMEs. Over the entire follow-up, NTBs had significantly more label updates than NMEs. NTBs also had more new safety issues added to the label overall, and to the Boxed Warning, Warnings and Precautions and Adverse Reactions sections of the label compared to NMEs. Our finding that proportions of NTBs that have an update to the Boxed Warning section (32.8%) or Warnings and Precaution section (73.8%) are higher than the corresponding proportions for NMEs (12.6% and 63.3%, respectively) is similar to that of Downing, who found that 28 of 183 (15.3%) pharmaceuticals and 15 of 39 (38.5%) biologics were issued at least one incremental Boxed Warning6, and to those of Giezen, who found that 17 of 136 (12.5%) biologics were issued postapproval Boxed Warning8. NTBs consistently had more postmarketing safety-related regulatory actions than NMEs, regardless of the number of sections of the label included, a finding consistent with Downing who reported that postmarketing safety events were more frequent among biologics compared to drugs6.
NTBs had fewer label updates and new safety issues added to the Drug Interactions section of the label compared to NMEs, likely because biologic proteins are catabolized by proteases, while direct cytochrome P450-based mechanisms explain many drug-drug interactions20. The number of safety issues added to the label of first-in-class NTBs was higher than that added to NTBs that are not first-in-class, a finding that was also true for NMEs and that suggests that prior in-class experience informs initial safety-related labeling.
Our data suggest that, on average, new safety issues related to NTBs are identified sooner than those for NMEs. Compared to NMEs, the time to first label update was shorter for NTBs, and the number of safety updates and safety issues during the first 3.5 postmarket years was higher. Our unadjusted time-to-events results are different from those reported by Downing6 who found that time to a postmarketing safety event was similar for biologics and small-molecule drugs. However, Downing used a limited definition of postmarketing safety event, which included withdrawal, an update to the Boxed Warning section or an issuance of a safety communication by the FDA6. In our study these differences did not remain statistically significant after adjustment for several factors, except for Boxed Warnings and withdrawals.
Our data suggest that differences in occurrence and timing of safety-related regulatory actions between NTBs and NMEs is due in part to a higher proportion of NTBs, relative to NMEs, being in ATC classifications that have high frequencies for postmarket updates to the Boxed Warnings and Warnings and Precautions sections of the label. However, even within these ATC categories, the proportion of NTBs with a postmarket label update to these sections was higher for NTBs than for NMEs.
When evaluating regulatory pathways, there were some similarities in the results of our time-to-event analyses between NTBs and NMEs. Like NMEs approved via the accelerated approval pathway using a surrogate endpoint, NTBs approved via this pathway had a shorter time to first safety event than those approved not under this pathway, a finding that was most pronounced for the Boxed Warnings and Warnings and Precautions sections and that persisted throughout follow-up. Products approved under the accelerated approval pathway using a surrogate marker are generally required to conduct post-approval clinical trials to verify the NTB’s clinical benefit21. It is possible that the routine, systematic collection of safety data in clinical trials allows for greater post-approval observation and identification of adverse events. In addition, because surrogate endpoints are often used in clinical trials when clinical outcomes might take a very long time to study15, it is possible that the duration of observation time may not fully identify adverse reactions that are duration-dependent or that depend on cumulative exposure.
NTBs approved via the accelerated approval pathway had more safety-related updates and safety-related issues than those approved not under this pathway, both in the first 3.5 years after approval and throughout follow-up. A similar trend was previously observed for NMEs11. The observation that most (eight of nine) NTBs initially approved under the accelerated approval pathway were in the ATC class ‘antineoplastics and immunomodulators’, a class that has a higher number of safety-related label updates and safety-related issues than NTBs in other ATC classes, may explain this finding.
The earlier identification of safety-related regulatory actions for products under the accelerated approval pathway coupled with the higher proportion of NTBs, relative to NMEs, using that pathway may explain the earlier identification of adverse reactions for NTBs compared to NMEs. Similarly, the higher proportion of NTBs in the ‘antineoplastic and immunomodulation agent’ ATC classification, relative to NMEs, may explain the higher number of safety updates and safety issues amongst NTBs compared to NMEs.
Long-term use was associated with more safety-related regulatory actions for NMEs. While the same trend seen with the NTBs, the relatively small sample size of the NTB group may have limited our ability to detect a difference. An indication for long-term use was associated with shorter time to first label change for both NMEs and NTBs, which coupled with a higher proportion of NTBs indicated for long-term use may partially explain earlier label updates for NTBs.
Our findings suggest that pharmacological action, accelerated approval pathway, and long-term use, rather than intrinsic differences between NTBs and NMEs, are important factors in timing and proportion with postmarketing safety label changes. It is possible that patients treated in specialized care settings, such as clinics that administer or prescribe anti-neoplastic or immunosuppressive agents, may be more closely monitored, thus providing greater opportunity for identification of previously unknown serious adverse reactions, which then result in more label updates and more new safety issues added to the label.
Our study has several strengths. We examined the association of review and regulatory characteristics with postmarketing safety-related regulatory actions of NTBs, while previous studies of approvals in the United States only examined small-molecule drugs4,5,7,10,11. We also compared regulatory characteristics of NTBs and NMEs, while the few studies that included both small-molecule drugs and biologics in their analysis did not specifically look at the associations with safety among biologics2,6,9. We examined postmarketing safety issues affecting five safety-related sections of the drug product label. The majority of the studies examining postmarketing safety issues focused on the Boxed Warning section even though the majority of the safety issues identified in our study affect other sections of the label. Our relatively long follow-up (up to 15.7 years), with each biologic having at least 3.5 years of follow-up and 80% having at least five years of follow-up, allowed us to examine label updates that may occur further along in the product’s life cycle.
Our study has a number of limitations. First, our analyses are based on counts of safety updates and issues and do not take into account the nature or severity of individual safety issues. Analyses according to specific sections of the label may broadly, though incompletely, account for the range of severity across individual safety issues. Second, our outcome measures, the number and timing of safety-related label changes, are not measures of a drug or biologic’s complete safety profile, nor are they a measure of the product’s risk-benefit balance. For this reason, our comparison between NMEs and NTBs cannot be interpreted as comparisons of safety profiles or benefit-risk profiles between NTBs and NMEs. For these reasons, our descriptive analyses and comparisons between NTBs and NMEs, even when adjusted, do not account for all the differences between NTBs and NMEs. This analysis did not include the size of the premarket safety database nor did it measure postmarket exposure, which may be important determinants in the identification of postmarket adverse events10. Finally, the relatively small number of NTBs that were approved during the study period might have rendered the study underpowered to detect certain differences in outcomes.
This study has described similarities and differences between NTBs and NMEs in both review and regulatory characteristics, as well as in the relationship of these characteristics to safety-related regulatory actions. These findings underscore the importance of continued development of the lifecycle safety surveillance system for both drugs and biologics with consideration for product type and its characteristics, including pharmacologic action.
Supplementary Material
Figure S1: Relationship of regulatory, review characteristics, and safety-related regulatory actions for new molecular entities (n=278) approved by the FDA between October 1, 2002, and December 31, 2014.
Figure S2: Time to first safety-related regulatory action comparing new therapeutic biologics (NTB) and new molecular entities (NME) using Gehan-Breslow tests and Cox proportional hazards model hazard ratios comparing NTB vs. NME. Variables considered potential confounders if they were associated with the time to safety-related regulatory action at P<0.10 or if they had been considered to be important confounders of safety-related regulatory actions in the published literature. Evaluation of the proportional hazards assumptions testing was done by evaluating the graphic presentation of survival curves and through the ASSESS statement in SAS which examines the cumulative sums of martingale residuals over covariate values. Potential confounders that had a P-value of <0.05 were included in the multivariable models.
Table S1: Definitions of regulatory and review characteristics, regulatory review pathways, and safety-related regulatory actions
Table S2: Anatomical Therapeutic Chemical (ATC) Classification and originally approved indication for new therapeutic biologics
Table S3: Number of label updates and safety issues added to each section of the label for new therapeutic biologics (NTB)
Table S4: Number of label updates and number of issues for new therapeutic biologics (NTB) and new molecular entity (NME) by section of the label
Table S5: Number and percent of new therapeutic biologics (NTB) and new molecular entity (NME) drugs with a postmarketing safety update by section of the label update and Anatomical Therapeutic Chemical (ATC) Classification System
Table S6: Logistic regression analysis for association between safety-related regulatory action and therapeutic product type (new therapeutic biologics (NTB) vs. new molecular entity (NME) drugs). Variables were considered potential confounders if they were associated with the safety-related regulatory action at P<0.10, or if they had been considered to be important confounders of the occurrence of any safety-related regulatory actions or serious safety-related regulatory actions in the published literature. A backwards, stepwise logistic regression analysis was performed to determine the independent predictors of the dichotomous outcome. Potential confounders that had a P-value of <0.05 were included in the multivariable models.
Study Highlights:
• What is the current knowledge on the topic?
Prior research examining postmarketing safety-related regulatory actions have focused on new molecular entity (NME) drugs and have not addressed therapeutic biologics.
• What question did this study address?
This study examined the association between regulatory and review characteristics and postmarketing safety-related regulatory actions (e.g. withdrawals and updates to safety-related sections of the labels) in new therapeutic biologics (NTB) and compared the results to these associations in NMEs.
• What does this study add to our knowledge?
At least one safety-related label update was added to the label of the majority of NTBs. Label updates occurred throughout the follow-up period. Time to the first safety-related regulatory action was shorter for NTBs approved under accelerated approval using a surrogate endpoint than for those that were not. The occurrence of safety events was higher among NTBs compared with NMEs. NTBs also had shorter time to safety events than NMEs.
• How might this change clinical pharmacology or translational science?
Our analysis demonstrated the need to have a robust safety surveillance system throughout a product’s lifecycle with consideration for product type and characteristics.
Acknowledgements:
We thank Mike Lanthier at the Food and Drug Administration for his assistance with data collection.
Funding: IB and SC are supported by appointments to the Research Participation Program at the U.S. Food and Drug Administration Center for Drug Evaluation and Research, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the U.S. Food and Drug Administration.
Footnotes
Conflict of interest: All authors declared no competing interests for this work.
Disclaimer: The views in this manuscript are those of the authors and not necessarily those of the U.S. Food and Drug Administration.
This manuscript includes analyses of publicly available data on CDER new molecular entity and new biologic approvals (October 2002-December 2014). The analyzed dataset is a high-level compilation of existing, publicly available data from FDA’s internal databases and document records, and to the best of our knowledge, reflects the state of each application at the time of initial regulatory approval. For additional or more detailed information about an application (e.g., FDA-approved conditions of use, approval letters), consider reviewing information available on Drugs@FDA or in the Orange and Purple Books.
References:
- 1.FDA. Frequently Asked Questions About Therapeutic Biological Products, <https://www.fda.gov/drugs/therapeutic-biologics-applications-bla/frequently-asked-questions-about-therapeutic-biological-products> (2015). Accessed 1 March 2020.
- 2.Arnardottir AH et al. Additional safety risk to exceptionally approved drugs in Europe? Br. J. Clin. Pharmacol. 72, 490–499, doi: 10.1111/j.1365-2125.2011.03995.x (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berndt ER, Gottschalk AHB, Philipson TJ & Strobeck MW Industry funding of the FDA: effects of PDUFA on approval times and withdrawal rates. Nat. Rev. Drug. Discov. 4, 545–554, doi: 10.1038/nrd1774 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Carpenter D, Chattopadhyay J, Moffitt S & Nall C The Complications of Controlling Agency Time Discretion: FDA Review Deadlines and Postmarket Drug Safety. Am. J. Pol. Sci. 56, 98–114, doi: 10.1111/j.1540-5907.2011.00544.x (2012). [DOI] [PubMed] [Google Scholar]
- 5.Carpenter D, Zucker EJ & Avorn J Drug-Review Deadlines and Safety Problems. N. Engl. J. Med. 358, 1354–1361, doi: 10.1056/NEJMsa0706341 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Downing NS et al. Postmarket Safety Events Among Novel Therapeutics Approved by the US Food and Drug Administration Between 2001 and 2010. JAMA 317, 1854–1863, doi: 10.1001/jama.2017.5150 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frank C et al. Era Of Faster FDA Drug Approval Has Also Seen Increased Black-Box Warnings And Market Withdrawals. Health Aff. (Millwood) 33, 1453–1459, doi: 10.1377/hlthaff.2014.0122 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Giezen TJ et al. Safety-Related Regulatory Actions for Biologicals Approved in the United States and the European Union. JAMA 300, 1887–1896, doi: 10.1001/jama.300.16.1887 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Mol PGM et al. Post-Approval Safety Issues with Innovative Drugs: A European Cohort Study. Drug Saf. 36, 1105–1115, doi: 10.1007/s40264-013-0094-y (2013). [DOI] [PubMed] [Google Scholar]
- 10.Mostaghim SR, Gagne JJ & Kesselheim AS Safety related label changes for new drugs after approval in the US through expedited regulatory pathways: retrospective cohort study. BMJ 358, j3837, doi: 10.1136/bmj.j3837 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinnow E et al. Postmarket Safety Outcomes for New Molecular Entity (NME) Drugs Approved by the Food and Drug Administration Between 2002 and 2014. Clin. Pharmacol. Ther. 104, 390–400, doi: 10.1002/cpt.944 (2018). [DOI] [PubMed] [Google Scholar]
- 12.FDA. Drugs@FDA: FDA Approved Drug Products, <https://www.accessdata.fda.gov/scripts/cder/daf/>. Accessed 1 March 2020.
- 13.FDA. Medical Product Safety Information, <http://wayback.archiveit.org/7993/20170110235327/http://www.fda.gov/Safety/MedWatch/SafetyInformation/default.htm> (2020). Accesed 1 March 2020.
- 14.FDA. Drug Safety-related Labeling Changes (SrLC), <https://www.accessdata.fda.gov/scripts/cder/safetylabelingchanges/> (2016). Accessed 1 March 2020.
- 15.FDA. Guidance for Industry Expedited Programs for Serious Conditions — Drugs and Biologics, <http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm358301.pdf> (2014). Accessed 1 March 2020.
- 16.FDA. Search Orphan Drug Designations and Approvals, <https://www.accessdata.fda.gov/scripts/opdlisting/oopd/> (2020). Accessed 1 March 2020.
- 17.WHO. ATC/DDD Index 2019, <https://www.whocc.no/atc_ddd_index/> (2020). Accessed 1 March 2020.
- 18.FDA. Purple Book: Lists of Licensed Biological Products with Reference Product Exclusivity and Biosimilarity or Interchangeability Evaluations, <https://www.fda.gov/drugs/therapeutic-biologics-applications-bla/purple-book-lists-licensed-biological-products-reference-product-exclusivity-and-biosimilarity-or> (2020). Accessed 1 March 2020.
- 19.Patel SV & Khan DA Adverse Reactions to Biologic Therapy. Immunol. Allergy Clin. Am. 37, 397–412, doi: 10.1016/j.iac.2017.01.012 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Schrieber SJ et al. Considerations for Biologic Product Drug–Drug Interactions: A Regulatory Perspective. Clin. Pharmacol. Ther. 105, 1332–1334, doi: 10.1002/cpt.1366 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Beaver JA et al. A 25-Year Experience of US Food and Drug Administration Accelerated Approval of Malignant Hematology and Oncology Drugs and Biologics: A Review. JAMA Oncol. 4, 849–856, . doi: 10.1001/jamaoncol.2017.5618. (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Relationship of regulatory, review characteristics, and safety-related regulatory actions for new molecular entities (n=278) approved by the FDA between October 1, 2002, and December 31, 2014.
Figure S2: Time to first safety-related regulatory action comparing new therapeutic biologics (NTB) and new molecular entities (NME) using Gehan-Breslow tests and Cox proportional hazards model hazard ratios comparing NTB vs. NME. Variables considered potential confounders if they were associated with the time to safety-related regulatory action at P<0.10 or if they had been considered to be important confounders of safety-related regulatory actions in the published literature. Evaluation of the proportional hazards assumptions testing was done by evaluating the graphic presentation of survival curves and through the ASSESS statement in SAS which examines the cumulative sums of martingale residuals over covariate values. Potential confounders that had a P-value of <0.05 were included in the multivariable models.
Table S1: Definitions of regulatory and review characteristics, regulatory review pathways, and safety-related regulatory actions
Table S2: Anatomical Therapeutic Chemical (ATC) Classification and originally approved indication for new therapeutic biologics
Table S3: Number of label updates and safety issues added to each section of the label for new therapeutic biologics (NTB)
Table S4: Number of label updates and number of issues for new therapeutic biologics (NTB) and new molecular entity (NME) by section of the label
Table S5: Number and percent of new therapeutic biologics (NTB) and new molecular entity (NME) drugs with a postmarketing safety update by section of the label update and Anatomical Therapeutic Chemical (ATC) Classification System
Table S6: Logistic regression analysis for association between safety-related regulatory action and therapeutic product type (new therapeutic biologics (NTB) vs. new molecular entity (NME) drugs). Variables were considered potential confounders if they were associated with the safety-related regulatory action at P<0.10, or if they had been considered to be important confounders of the occurrence of any safety-related regulatory actions or serious safety-related regulatory actions in the published literature. A backwards, stepwise logistic regression analysis was performed to determine the independent predictors of the dichotomous outcome. Potential confounders that had a P-value of <0.05 were included in the multivariable models.