Abstract
Correction for ‘Enantioselective synthesis of isochromans and tetrahydroisoquinolines by C–H insertion of donor/donor carbenes’ by Leslie A. Nickerson et al., Chem. Sci., 2020, 11, 494–498, DOI: 10.1039/C9SC05111B.
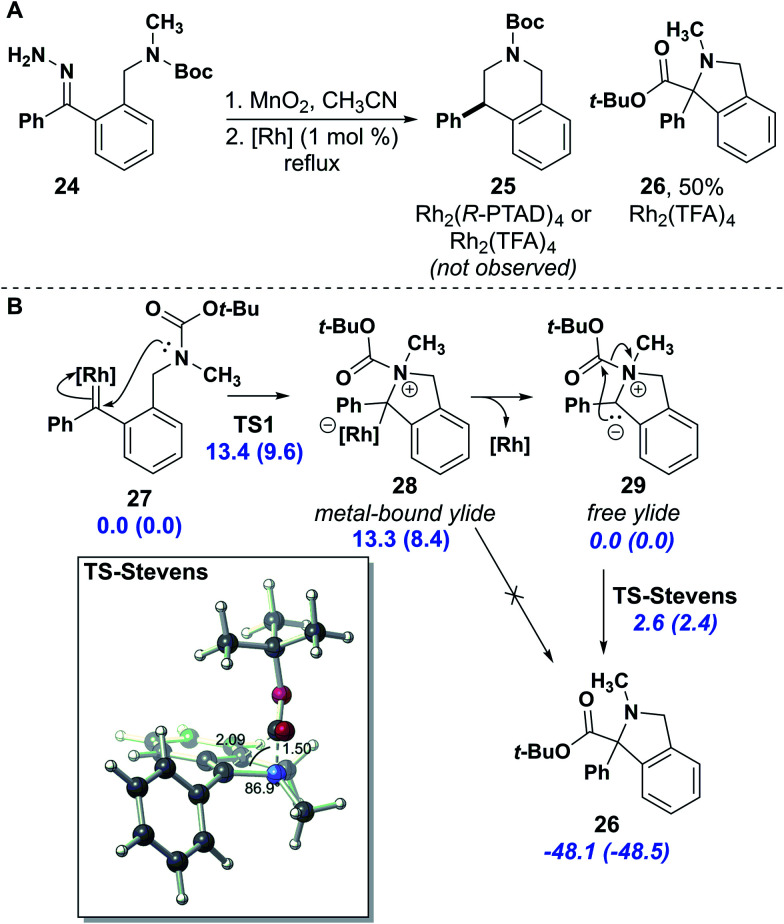
The electron pushing arrows in Fig. 6B were, unbeknownst to the authors, converted by ChemDraw (version 18.0) into double-headed resonance arrows during the final stages of galley proof review and went unnoticed until the article appeared in print. This event was traced to a version-specific bug in the software that has been resolved in a subsequent update. A corrected figure is provided here.
Fig. 1. (A) Stevens rearrangement product synthesis. (B) The DFT (uB3LYP/LANL2DZ[6-31G(d)]) computed mechanism suggests that N-attack to the rhodium carbene and the subsequent Stevens rearrangement is energetically feasible at experimental conditions; relative free energies (electronic energies in parentheses) for metal-bound (normal text) and ylide (italics) reactions are reported in kcal mol−1.
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.