Abstract
Little is known about the surgical challenges and outcomes of kidney transplantation (KT) in the face of severe iliac occlusive disease (IOD). We aim to examine our institution's experience and outcomes compared with all KT patients. Retrospective review of our multi-institutional transplant database identified patients with IOD requiring vascular surgery involvement for iliac artery endarterectomy at time of KT from 2000 to 2018. Clinical data, imaging studies, and surgical outcomes of 22 consecutive patients were reviewed. Our primary end-point was allograft survival. Secondary end-points included mortality and perioperative complications. A total of 6,757 KT were performed at our three sites (Florida, Arizona, and Minnesota); there were 22 (0.32%) patients receiving a KT with concomitant IOD requiring iliac artery endarterectomy. Mean patient age was 61.45 ± 7 years. There were 13 (59.1%) male patients. The most common etiology of renal failure was diabetic nephropathy in 10 patients (45.5%) followed by a combination of hypertensive/diabetic nephropathy in five patients (22.7%), and hypertensive nephrosclerosis in three patients (13.6%). The majority ( n = 16, 72.7%) of patients received renal allografts from deceased donors and six (27.3%) were recipients from living donors. Mean time from dialysis to transplantation was 2.9 ± 2.9 years. Mean follow-up was 3.5 ± 2.5 years. Mean length of hospital stay was 6.3 ± 4.3 days (range: 3–18 days). Graft loss within 90 days occurred in two (9.1%) patients, one due to renal vein thrombosis and another due to acute tubular necrosis. Overall allograft survival was 90.1% at 1-year and 86.4% at 3-year follow-up. Overall mortality occurred in 6 (27.3%) patients. Perioperative complications (Clavien-Dindo Grade 2–4) occurred in 13 (59.1%) patients, including 10 (45.5%) with acute blood loss anemia requiring transfusion, 2 (9.1%) reoperations for hematoma evacuation, 1 (4.5%) ischemic colitis requiring total abdominal colectomy, and 1 (4.5%) renal vein thrombosis requiring nephrectomy. IOD patients selected for KT are not common and although challenging, they have similar outcomes to our standard KT patients. The 1- and 3-year allograft survivals were 90.1 and 86.4% versus 96.0 and 90.3% in the general KT patient population. With these excellent outcomes, we recommend expanding the criteria for KT to include patients with IOD with prior vascular surgery consultation to prevent progression of IOD or prevention of wait list removal in select patients who are otherwise good candidates for KT.
Keywords: peripheral arterial disease, renal failure, transplant
Atherosclerosis is frequent in patients with end-stage renal disease awaiting kidney transplantation (KT). 1 The increasing incidence of iliac occlusive disease (IOD) in KT patients is related to the advanced age of recipients and the increased incidence of atherosclerosis in chronic renal failure. 2 Furthermore, there is increased incidence of atherosclerotic comorbidities including diabetes, hypertension, and hypercholesterolemia. 3 Cardiovascular events are a primary cause of mortality in these patients and often preclude KT. 3 4 5 6 Despite preoperative preparations, IOD is commonly encountered at KT that requires concomitant treatment of the vascular lesion to assure proper allograft vascular in- and outflow. 4 Current literature demonstrates a range from 1.3 to 13% incidence of significant IOD at the time of KT. 5 6 We sought to review our institutional experience for this significant risk factor for renal allograft survival.
Methods
We identified all patients from our three transplant centers at our regional sites (Florida, Arizona, and Minnesota) with IOD requiring iliac artery endarterectomy undergoing KT between January 1, 2000 and November 30 , 2018. Data collection included details of each patient's clinical presentation, comorbidities, KT evaluation, surgical management, and follow-up through a retrospective chart review. This study was approved by the Mayo Clinic Institutional Review Board (IRB#17–007736). Informed consent was waived by the IRB as this study was deemed minimal risk to patients.
Our primary end-point was allograft survival. Our secondary end-points included mortality and perioperative complications. Data are reported using means and standard deviations for continuous variables or as frequencies for categorical variables. Differences between categorical variables were tested using χ 2 test and differences between continuous variables were tested using Student's t -test when deemed appropriate. Long-term outcomes were performed descriptively due to the relatively few patients in our cohort. Analyses were performed using JMP 14 (SAS Institute, Cary, NC).
Kidney Transplantation Selection
Evaluation of KT candidates at the Mayo Clinic is based on a multidisciplinary patient care approach standardized across all Mayo Clinic sites (Florida, Arizona, and Minnesota). All patients considered for transplantation undergo a multipart evaluation with the following objectives: (1) to confirm severely limited kidney function, (2) to assess the patient's ability to tolerate operative intervention, (3) to ensure that there is good long-term allograft and patient survival, and (4) to ensure that there is a good social and financial support to successfully sustain the transplanted organ.
A transplant committee comprised of a transplant nurse coordinator, nephrologist, surgeon, pharmacist, psychiatrist, social worker, and nutritionist is involved in the evaluation process for potential KT recipients based on the following criteria:
The transplant recipient should meet the medical indications of the United Network of Organ Sharing
The transplant recipient must be younger than 80 years of age. Should he/she be older than 80 years, the recipient is evaluated on a case-by-case basis.
The transplant recipient must have kidneys with severely diminished function. The glomerular filtration rate should be less than or equal to 20 mL/min/m 2 .
The transplant recipient should have a body mass index (BMI) of less than or equal to 40 kg/m 2 . Those individuals with BMIs between 40 and 45 kg/m 2 will be considered on a case-by-case basis.
The transplant recipient should be able to comply with transplant-related management and medical follow-up.
The transplant recipient should have a good social support system. There should be no psychiatric/psychological barriers for transplantation.
The transplant recipient should have adequate financial resources including health care insurance with medication coverage.
Once the recipient starts the evaluation process, a myriad of tests and consultations is prompted, which is included in Table 1 . Based on these evaluations, the decision to proceed with transplantation is reached. Absolute contraindications for transplantation include active infection, active malignancy, active substance use/abuse, reversible renal failure, uncontrolled psychiatric disorders, documented treatment nonadherence, and/or short life expectancy. A relative contraindication for KT has included severe calcifications of the iliac arteries observed on noncontrast computed tomography.
Table 1. Laboratory, cardiovascular, pulmonary, imaging studies, and consultations required for kidney transplantation (KT) evaluation.
| Laboratory testing | |
|---|---|
| Metabolic panel | Serologies |
| Total and direct bilirubin Aspartate aminotransferase (AST) Alanine aminotransferase (ALT) Alkaline phosphatase Albumin and total protein Total cholesterol High-density lipoprotein (HDL) cholesterol Triglycerides, low-density lipoprotein (LDL) cholesterol Albumin Blood urea nitrogen Calcium Chloride Potassium Sodium Phosphorus Creatinine Glucose Bicarbonate Rheumatologic workup as indicated If patient has good pastures, order anti-glomerular basement membrane (GBM) antibody ABO blood group testing drawn on two separate occasions Human leukocyte antigen (HLA) typing Single antigen bead Troponin T Hemoglobin A1C C peptide (if diabetic) Thyroid-stimulating hormone (TSH) Parathyroid hormone (PTH) Pregnancy testing in females under 60 (unless they have had a hysterectomy) Serum protein electrophoresis (SPEP) with reflex for age > 50, history of Monoclonal gammopathy of undetermined significance (MGUS), myeloma, amyloid considered in patients with history of proteinuric kidney disease of unknown etiology |
For all patients Cytomegalovirus (immunoglobulin G [IgG] only) Herpes simplex virus ½ (IgG only) Varicella zoster virus (IgG only) Measles and rubella (IgG only) Ebstein–Barr virus (antibody profile—Epstein–Barr virus viral capsid antigen IgG/IgM and Epstein–Barr nuclear antigen) HIV ½ antibody Syphilis IgG HBsAb, HBs antigen, HBcAb Hepatitis C virus (HCV) antibody (Ab) HCV RNA if suspected to have recent HCV infection Hepatitis A virus (HAV)—total antibody For selected candidates (any candidate at risk for infection by virtue of residency or travel): Coccidioides immitis serology (EIA—enzyme immunoassay, ID—immunodiffusion, and CF—complement fixation) Strongyloides serology Schistosoma serology Trypanosoma cruzi serology Hematology Prothrombin time (PT)/international normalized ratio (INR) Complete blood count (CBC) with differential Urine tests Urinalysis Random urine protein and creatinine Gram stain (reflex urine culture) |
| Cardiovascular and pulmonary testing | |
| Electrocardiogram (EKG) Echocardiogram to evaluate valves and pulmonary pressures Stress test for patients with diabetes, age >59, prior coronary artery disease (CAD), or tobacco use Carotid duplex ultrasound for all patients with a history of CAD, stroke, transient ischemic attack (TIA), diabetes, or severe peripheral arterial disease (PAD) Thrombophilia workup: for history of lupus, clotting of access, miscarriages or prior clotting, consider thrombophilia clinic or order the following tests/thrombophilia profile | |
| Antithrombin activity plasma Cardiolipin (phospholipid) antibody Coagulation survey, lupus like anticoagulants Protein C activity Protein A free plasma Prothrombin G20210A nucleotide Beta 2 glycoprotein 1 antibodies (IgG, IgM, and IgA) | |
| Radiology imaging studies | |
| Chest X-ray posterior–anterior and lateral views Duplex scan of kidneys to evaluate for kidney size and presence of cysts Computed tomography (CT) of the abdomen/pelvis without contrast in patients who are older than 65 years of age or with diabetes mellitus (DM), PAD, and if they have been on hemodialysis or peritoneal dialysis for more than 3 years. If complex renal cysts are present, then abdominal CT with contrast should be done annually with urology evaluation Magnetic resonance angiography (MRA) of the brain without contrast should be obtained for all autosomal dominant polycystic kidney disease (ADPKD) patients, who have not had one completed | |
| Cancer screening | |
| Usual screening according to the American Cancer Society guidelines | |
| Consultations | |
| Transplant nephrology Transplant surgery Transplant nurse coordinator Transplant pharmacist Transplant nutrition Transplant financial coordinator Infectious diseases (if indicated) Psychology and psychiatry consultation (if indicated) Cardiology, if patient has history of cardiac problems or abnormal echocardiogram/stress test Other consultations and tests, as indicated | |
Results
There was a total of 6,757 KT at our three sites (Florida, Arizona, and Minnesota). From these, 22 (0.32%) patients had concomitant IOD requiring iliac artery endarterectomy at the time of transplantation. The demographic data, comorbidities, and the etiology of kidney failure are presented in Table 2 . Thirteen (59.1%) patients were male and the mean age at KT was 61.5 ± 7 years. The most common etiology of kidney failure was diabetic nephropathy in 10 patients (45.5%) followed by a combination of hypertensive/diabetic nephropathy in five patients (22.7%) and hypertensive nephrosclerosis in three patients (13.6%). The mean time from dialysis to transplantation was 2.9 ± 2.9 years. Sixteen patients (72.7%) received renal allografts from deceased donors and six (27.3%) were recipients from living donors.
Table 2. Patient characteristics of our kidney transplant (KT) cohort.
| Age at the time of transplant (years) | 61.5 ± 7 years |
| Male sex | 13 (59.1%) |
| Race/ethnicity | n (%) |
| Caucasian | 11 (50) |
| African American | 9 (40.1) |
| Latino | 2 (9.1) |
| Body mass index (BMI, in kg/m 2 ) | 29.5 ± 4.5 |
| Comorbidities | n (%) |
| Peripheral artery disease | 22 (100) |
| Hypertension | 21 (95.5) |
| Diabetes mellitus | 14 (63.6) |
| Coronary artery disease | 10 (45.5) |
| Hyperlipidemia | 7 (31.8) |
| Chronic pulmonary obstructive Disease | 2 (9.1) |
| Medication | n (%) |
| Acetylsalicylic acid | 19 (86.4) |
| Beta blockers | 15 (68.2) |
| Statins | 10 (45.5) |
| Calcium channel blockers | 10 (45.5) |
| Clopidogrel | 6 (27.3) |
| Anticoagulation medications | 4 (18.2) |
| Etiology of end-stage renal disease | n (%) |
| Diabetes mellitus | 10 (45.5) |
| Diabetes/hypertension | 5 (22.7) |
| Hypertension | 3 (13.6) |
| Lupus nephritis | 1 (4.5) |
| Interstitial nephritis | 1 (4.5) |
| Proteinuric kidney disease | 1 (4.5) |
| Focal segmental Glomerulosclerosis | 1 (4.5) |
| Type of kidney donor | n (%) |
| Deceased donor | 16 (72.7) |
| Living donor | 6 (27.3) |
| Preoperative computed tomography | 13 (59.1) |
| Preoperative serum creatinine (mg/dL) | 7.4 ± 2.6 |
Preoperative cross-sectional imaging in the form of noncontrast CT was performed in 13 (59.1%) patients as part of their routine transplant evaluation, which includes only patients over the age of 65 years, with complex renal cysts, history of peripheral arterial disease, and diabetes mellitus and hemo- or peritoneal dialysis of over 3 years duration ( Fig. 1 ). There was one (4.5%) patient with planned endarterectomy in our cohort. The rest of our patients required intraoperative consultations with vascular surgery.
Fig. 1.
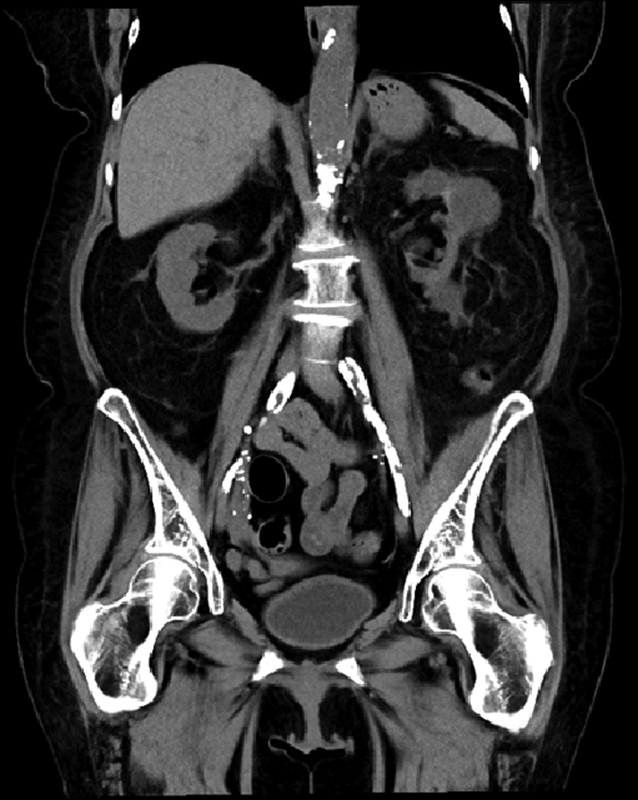
Coronal view of a noncontrast computed tomography scan of the abdomen and pelvis in a patient with significant Iliac occlusive disease (IOD) prior to kidney transplantation.
Immunosuppression is achieved by administration of induction medication followed by maintenance therapy to prevent acute rejection and renal allograft loss. Long-term follow-up after transplantation was managed by our transplant team in 19 (86.4%) patients and in 3 (13.6%) patients by external providers. Follow-up is rigorous and requires good patient adherence: visits every 2 weeks in the first 2 to 4 months; then, monthly visits until the 6th month. Subsequently, visits every 2 months in the first year and every 3 to 4 months until the third year and lastly one visit per semester thereafter. During each follow-up visit, laboratory data are obtained for medication adjustments, if needed. If acute rejection is noted, plasma exchange and/or corticosteroids and/or immunosuppressant medication is initiated depending on the histologic grade of the rejection.
Operative Procedure
In 19 (86.4%) of 22 patients, a right lower quadrant Gibson incision was made to obtain access to the iliac vessels in the retroperitoneal space in the standard fashion. 7 The other three (13.6%) kidney allografts were placed in the left iliac fossa, two of those secondary to advanced peripheral arterial disease in the right iliac vessels ( Fig. 2 ). There were five patients who required iliac artery patching (two with bovine pericardium and three with renal vein from the donor kidney). A single patient required a distal external iliac artery stent. In all 22 patients, an end-to-side anastomosis was performed from the renal artery and vein directly to the external iliac artery and vein, respectively. The ureter was anastomosed directly into the bladder using a Lich-gregoir technique. Mean operative time was variable at 225.1 ± 104.9 minutes (range: 137–560 minutes).
Fig. 2.
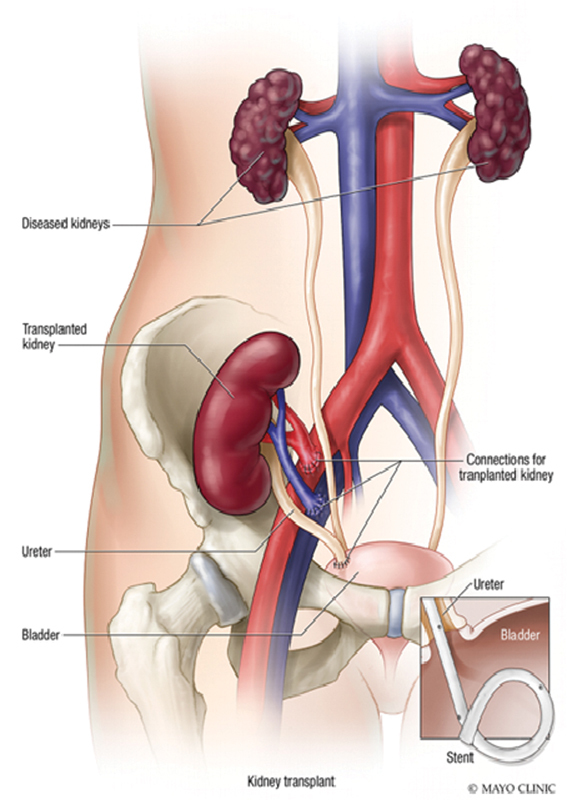
Primary kidney transplantation are performed in the iliac fossa (Used with permission of Mayo Foundation for Medical Education and Research. All rights reserved).
Postoperative and Long-Term Management
Mean length of hospital stay was 6.3 ± 4.3 days (range: 3–18 days). All patients were placed on oral aspirin 81 mg. The significant discrepancy in hospitalization was predominantly due to those patients who suffered from postoperative complications. Complications were graded using the Clavien-Dindo system 8 9 (Clavien-Dindo Grade 2–4) and occurred in 13 (59.1%) patients, including 10 (45.5%) with acute blood loss anemia requiring transfusion, 2 (9.1%) reoperations for hematoma evacuation, 1 (4.5%) ischemic colitis requiring total abdominal colectomy, and 1 (4.5%) renal vein thrombosis requiring nephrectomy ( Table 3 ). Nine (40.9%) patients required hemodialysis in the first week following transplantation.
Table 3. Postoperative complications.
| Postoperative complications | n (%) |
|---|---|
| Acute blood loss anemia, requiring transfusion | 10 (45.5) |
| Perinephric hematoma requiring reoperation | 2 (9.1) |
| Ischemic colitis requiring colectomy | 1 (4.5) |
| Graft thrombosis requiring nephrectomy | 1 (4.5) |
The perinephric hematoma evacuations were on patients with significantly large hematomas. They measured 13 × 3.7 × 7.8 cm and 14 × 11 × 4 cm in size. The patient who developed ischemic colitis was slow to recover following KT. She later developed a leukocytosis of up to 40 × 10 9 /L on postoperative day 9 along with significant abdominal pain. On postoperative day 11, she underwent a flexible sigmoidoscopy that revealed colonic ischemia. She then underwent total abdominal colectomy with end ileostomy.
There were two graft losses (9.1%) that occurred within 90 days of transplant. The first one was described as a “difficult” vascular anastomosis during the transplantation. This patient became oligoanuric within 24 hours and a duplex ultrasound on postoperative day 1 revealed no blood flow to the new transplanted kidney. He underwent allograft nephrectomy on this same day. The pathology report revealed extensive venous thrombosis with cortical and medullary necrosis. The second patient with allograft loss had initially delayed graft function of 7 days. His postoperative course was then unremarkable. However, 30 days later, he was readmitted with acute renal injury. He was treated for acute rejection with steroids, and underwent ultrasound and carbon dioxide angiogram to no avail. The allograft function did not recover and his renal biopsy revealed dense acute tubular necrosis of unknown etiology.
Mean follow-up was 3.5 ± 2.5 years. Overall allograft survival was 90.1% at 1-year and 86.4% at 3-year follow-up. Overall mortality occurred in six (27.3%) patients. Information regarding cause of death could only be found for one patient, who expired from cardiac arrest; all others have unknown causes of death.
Discussion
Little is known about the surgical challenges and outcomes of KT in the face of IOD. As the population continues to age and as more patients present with diabetes, hypertension, and longstanding smoking history, the incidence of kidney transplant recipients presenting with significant IOD should be expected to increase. 10 11 In this study, we have identified 22 patients (0.32%) with extensive IOD at the time of KT. While the safety of simultaneous reconstruction has been questioned previously, 8 9 Clavien et al 9 have demonstrated that concurrent interventions are both safe and feasible.
The primary end-point of our study was graft survival, which we demonstrated to be 90.1% at 1-year and 86.4% at 3-year follow-up, which is consistent with the national means for any high-volume transplant center. 10 Comparatively, our three-site allograft survival is 96.0 and 90.3% at 1- and 3 years post-KT, respectively. Two renal allografts were lost within 90 days secondary to renal vein thrombosis on postoperative day 1 and acute tubular necrosis on postoperative day 30. Despite this being a substantial loss, our early allograft loss is lower than what has been previously reported. 4 This further reaffirms that endarterectomy at the time of KT is both safe, effective, and may lead to long-term allograft survival. Of those patients requiring endarterectomy, only one case was identified and planned in the preoperative setting. This may be due to the infrequent use of cross-sectional imaging (59.1% of patients had preoperative CT) to screen for IOD in our KT population. Since the conduction of the current study, we have included a noncontrast CT as required preoperative assessment and a vascular surgery consultation in those patients with significant IOD in our KT patient population. We believe this highlights the importance of standardizing preoperative screening protocols and the vital role of multidisciplinary approach to patient care in this complex patient population.
Patients undergoing endarterectomy at the time of renal transplant are at increased risk of complications. We recommend baseline surveillance of renal allografts on postoperative day 0 utilizing Doppler ultrasound. Doppler ultrasound should be repeated for any change in renal function, significant ultrasound abnormalities, or delayed allograft function beyond expectations of the specific donor allograft. Doppler ultrasound represents a noninvasive, low-cost screening tool in these complex patients. A low index of suspicion should be maintained for the need to pursue CO 2 contrast or traditional angiography versus early return to the operating room in cases of atypical allograft function. It is worthwhile mentioning that in the current endovascular era of managing many vascular diseases, this option has been mostly used in the post-transplantation setting. Glebova et al 11 illustrate both a renal artery and an iliac artery stenosis managed with balloon angioplasty. In a second review of 10-year data from the University of Michigan, 25 of their 34-patient cohort required endovascular balloon angioplasty due to renal artery and iliac artery stenosis. 12
Mortality on dialysis far exceeds our reported transplant mortality. The United States Renal Data System reports 3-year survival on hemodialysis is only 57%; 3-year survival on peritoneal dialysis is 70%. 13 In contrast, our patients requiring iliac intervention at the time of renal transplant demonstrated 86.4% 3-year survival. This demonstrates significant survival benefit in those patients eligible for combined vascular surgery and transplant over starting/remaining on dialysis. Carefully selected patients have significant potential improvement in both quality of life and overall survival by considering combined endarterectomy and KT.
Limitations
Our review is a single-center retrospective review and as such, several limitations need to be acknowledged. First, our limited number of KT patients with concomitant IOD that required endarterectomy at the time of transplantation. Second, we believe that although this cohort is limited, most likely many of the KT candidates were not considered in the selection process due to the extensive IOD. In light of our current findings, several practical changes have been introduced that include a noncontrast CT as required preoperative assessment and a vascular surgery consultation in those patients with significant IOD on imaging. Furthermore, we are unable to generalize our outcomes to other transplant practices. Therefore, larger studies with multicenter data are necessary.
Conclusion
IOD patients selected for KT are not common and although challenging, they do have the same outcomes as our standard KT patients. The 1- and 3-year allograft survivals were 90.1 and 86.4% versus 96.0 and 90.3% in the general KT patient population. With these excellent outcomes, we recommend expanding the criteria for KT to include patients with IOD with prior vascular surgery consultation to prevent progression of IOD or prevention of wait list removal in select patients who are otherwise good candidates for KT.
Conflict of Interest None.
Note
Poster Presentation at the 2019 Annual Vascular Meeting on June 12–15 2019 in Washington DC.
References
- 1.Olechnowicz-Tietz S, Gluba A, Paradowska A, Banach M, Rysz J. The risk of atherosclerosis in patients with chronic kidney disease. Int Urol Nephrol. 2013;45(06):1605–1612. doi: 10.1007/s11255-013-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Committee of Renal and Peripheral Arteries, Japan Atherosclerosis Society . Shoji T, Abe T, Matsuo H. Chronic kidney disease, dyslipidemia, and atherosclerosis. J Atheroscler Thromb. 2012;19(04):299–315. doi: 10.5551/jat.10454. [DOI] [PubMed] [Google Scholar]
- 3.Droupy S, Eschwège P, Hammoudi Y, Durrbach A, Charpentier B, Benoit G.Consequences of iliac arterial atheroma on renal transplantation J Urol 2006175(3 Pt 1):1036–1039. [DOI] [PubMed] [Google Scholar]
- 4.Hausberg M, Kisters K, Kosch M, Barenbrock M. [Alterations of the arterial vessel wall in renal failure] Med Klin (Munich) 2000;95(05):279–285. doi: 10.1007/pl00002122. [DOI] [PubMed] [Google Scholar]
- 5.Kidney Transplant Working Group of the Canadian Society of Transplantation . Knoll G, Cockfield S, Blydt-Hansen T. Canadian Society of Transplantation consensus guidelines on eligibility for kidney transplantation. CMAJ. 2005;173(10):1181–1184. doi: 10.1503/cmaj.051291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.ERA-EDTA DESCARTES Working Group . Maggiore U, Abramowicz D, Budde K. Standard work-up of the low-risk kidney transplant candidate: a European expert survey of the ERA-EDTA Developing Education Science and Care for Renal Transplantation in European States Working Group. Nephrol Dial Transplant. 2019;34(09):1605–1611. doi: 10.1093/ndt/gfy391. [DOI] [PubMed] [Google Scholar]
- 7.Wind G GVR. New York: Wolters Kluwer; 2013. Anatomic Exposures in Vascular Surgery. Third edition. [Google Scholar]
- 8.Clavien P A, Barkun J, de Oliveira M L. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(02):187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 9.Clavien P A, Sanabria J R, Strasberg S M. Proposed classification of complications of surgery with examples of utility in cholecystectomy. Surgery. 1992;111(05):518–526. [PubMed] [Google Scholar]
- 10.https://optn.transplant.hrsa.gov/news/two-year-analysis-shows-effects-of-kidney-allocation-system/. Accessed June 23, 2020
- 11.Glebova N O, Brooke B S, Desai N M, Lum Y W. Endovascular interventions for managing vascular complication of renal transplantation. Semin Vasc Surg. 2013;26(04):205–212. doi: 10.1053/j.semvascsurg.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Arya S, Coleman D M, Osborne N H. Outcomes of endovascular interventions for salvage of renal transplant allografts. J Vasc Surg. 2013;57(06):1621–1627. doi: 10.1016/j.jvs.2012.11.117. [DOI] [PubMed] [Google Scholar]
- 13.US Renal Data System. USRDS 2016 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Bethesda, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2016


