Abstract
Backgrounds and study aims Gel immersion endoscopy is a novel technique to secure the visual field during endoscopy. The aim of this study was to develop a dedicated gel for this technique.
Methods To identify appropriate viscoelasticity and electrical conductivity, various gels were examined. Based on these results, the dedicated gel “OPF-203” was developed. Efficacy and safety of OPF-203 were evaluated in a porcine model.
Results In vitro experiments showed that a viscosity of 230 to 1900 mPa·s, loss tangent (tanδ) ≤ 0.6, and hardness of 240 to 540 N/cm 2 were suitable. Ex vivo experiments showed electrical conductivity ≤ 220 μS/cm is appropriate. In vivo experiments using gastrointestinal bleeding showed that OPF-203 provided clear visualization compared to water. After electrocoagulation of gastric mucosa in OPF-203, severe coagulative necrosis was not observed in the muscularis but limited to the mucosa.
Conclusions OPF-203 is useful for gel immersion endoscopy.
Introduction
During endoscopy for gastrointestinal bleeding, it is often difficult to secure the visual field due to massive bleeding in the lumen. Although the water immersion technique has been used 1 2 3 , it often fails to secure the visual field, because injected/infused water is rapidly mixed with blood and residue. Although endoscopic resection with the water (water or saline) immersion technique has become popular 4 5 6 7 , it is difficult to secure the visual field in case of intraprocedural bleeding 7 . In order to solve these problems, we developed a novel technique called “gel immersion endoscopy” 8 . Clear gel is injected through the accessory channel instead of water. The viscous gel displaces luminal blood, clots and residue. In the space occupied by the gel, we can then easily secure the visual field and perform endotherapy calmly and effectively. Its usefulness has already been reported 9 10 11 .
Although the gel for this technique could be also made by mixing water and a generic gelling agent, it is challenging to uniformly reproduce a gel with suitable clarity and appropriate viscosity. We initially used a jelly-like drink “OS-1 jelly” (Otsuka Pharmaceutical Factory, Japan) for this technique. This is a dedicated rehydration-supplement for patients with dehydration composed of a gelling agent (polysaccharide-thickener), electrolytes, carbohydrates, and water. It has appropriate viscosity for this technique. However, in this gel, cauterization using mono-polar hemostatic forceps is less effective, due to dissipation of electrical current due to its high electrolyte content. Therefore, OS-1 jelly should be replaced with gas or water before using the cautery 9 or bipolar devices should be used in OS-1 jelly 11 .
The requirements of a dedicated gel for gel immersion endoscopy includes safe for use within the digestive tract, safe for use for instruments, transparent, appropriate viscosity, and low electrical conductivity. We developed and evaluated a dedicated gel.
Material and methods (ex vivo)
Gels generally have mechanical properties that combine elasticity and viscosity, with the storage modulus (G') indicating an elastic component and the loss modulus (G") indicating a viscous component, the ratio of which (G"/G') is called the loss tangent (tanδ). The tanδ is the phase difference between stress and strain, a measure of how much external force applied to the gel is preserved (or lost) and indicates a qualitative difference of the gel, specifically the relative properties of the gel in viscous form, such as starch syrup, and in elastic form, such as rubber. To identify appropriate viscoelasticity and electrical conductivity which are required for gel immersion endoscopy, various gelling agents (xanthan gum, locust bean gum, guar gum, tara gum and/or carboxymethyl cellulose) and their ratios were examined in vitro .
To verify ability to secure the visual field and injectability through the catheter, 16 gels with various viscoelastic properties were injected through a 2.5-mm, 1000-mm-long catheter into a Dean-Stark trap filled with 1 % Evans Blue ( Fig. 1 ).
Fig. 1.

a Dean-Stark trap, filled with 1 % Evans Blue. b Injection of pilot gel with optimal viscoelasticity creates a transparent space. c When injecting pilot gel with unsuitable viscoelasticity, it mixes with the pigment immediately.
Results (ex vivo)
Of the 16 gels with various viscoelastic properties, 10 gels had both the ability to secure the visual field and to be injectable through the channel. All the tanδ of the viscoelastic gels that were concluded to be “acceptable” in securing the field of view were about 0.6 or less, while those with a higher tanδ were considered “unacceptable.” Gels considered to have “suitable” injectability had a viscosity of about 1900 mPa·s or less and a hardness of about 550 N/cm or less ( Fig. 2 ). It was found from these results that viscoelastic properties including a viscosity of 230 to 1900 mPa·s, tanδ ≤ 0.6 and hardness of 240 to 540 N/cm 2 were needed to meet the goals of a dedicated gel.
Fig. 2.

Results of the in vitro experiment for identification of appropriate viscoelasticity. Loss tangent (tanδ) is defined as the ratio between the loss modulus (G’’) and the storage modulus (G’).
Animals
All animal studies and procedures were approved by the Committee on the Care and Use of Laboratory Animals, Otsuka Pharmaceutical Factory, Inc. in full compliance with Japanese regulations. Male SD rats (Charles River Laboratories Japan, Yokohama, Japan) and female LWD pigs (National Federation of Agricultural Cooperative Associations, Okayama, Japan) were housed for a minimum of 1 week before experiments under laboratory conditions (temperature, 23 ± 3°C; humidity, 55 ± 15 %; 12-hour light-dark cycle) and fed a standard laboratory diet and water ad libitum .
Material and methods (ex vivo)
After the rats were euthanized, the liver was removed and a plastic cylinder (1.5-cm diameter and 3 cm long) was put on the surface of the liver, and each test sample including pilot gels was extracted into the cylinder. To verify the efficacy of electrocoagulation, high frequency electrical current using the dry mode 20 W (SurgiStat II, Medtronic Japan, Tokyo, Japan) from a monopolar electrosurgical pencil (E1450X, Medtronic, Dublin, Ireland) was applied for two seconds on the surface of a rat liver.
Results (ex vivo)
In air, distilled water and pilot gel (224 μS/cm or less), electrocoagulation was effective. In the saline or OS-1 jelly, electrocoagulation was not effective ( Table 1 and Fig. 3 ).
Table 1. Identification of appropriate electrical conductivity.
| Test Material | Electric conductivity (µS/cm) | Electrocoagulation |
| Air | 0 | Effective |
| Distilled water | < 10 | Effective |
| Pilot gel | 18 | Effective |
| 53 | Effective | |
| 103 | Effective | |
| 224 | Effective | |
| 608 | Not effective | |
| Saline | 16 000 | Not effective |
| OS-1 jelly | 7130 | Not effective |
Fig. 3.

Results of the ex vivo experiment. In air, distilled water and pilot gel (224 μS/cm or less), the electrocoagulation was effective. In the saline or OS-1 jelly, electrocoagulation was not effective.
Materials and methods (in vivo)
After identifying appropriate viscoelasticity and electrical conductivity, the standardized dedicated gel “OPF-203” was produced. Its ingredients include xanthan gum, locust bean gum, concentrated glycerin, and distilled water. Its electrical conductivity was adjusted to less than 200 μS/cm. Efficacy and safety of OPF-203 were evaluated in a porcine model.
A porcine model of gastrointestinal bleeding was used to evaluate the ability to identify the bleeding point and injectability through the accessory channel. After insertion of the endoscope under anesthesia, a 6 Fr enteral feeding extension tube was fixed to the gastric or colonic mucosa. A thread was tied to the tip of the tube, the endoscope was inserted while grasping the thread with hemostatic forceps, and the thread was fixed to the mucosa with a hemo-clip. Fifty-mL heparinized porcine blood was pre-infused through the catheters, followed by continuous infusion at a rate of 15 mL/min for 1 second with a 1-second pause. An endoscope (GIF-H260Z, Olympus, Tokyo, Japan) with an accessory channel 2.8 mm in diameter was used to inject 200 to 400 mL of OPF-203 into the stomach or colon. To test the gel in a more difficult setting than water, a cylindrical hood was used for the water immersion technique and not used in the gel immersion technique using OPF-203.
Electrocoagulation performance was evaluated using normal stomach. After insertion of the endoscope under anesthesia, the stomach was filled with air, OPF-203 or saline. High frequency electrical current was applied to the gastric mucosa using monopolar hemostatic forceps (FD-410LR, Olympus, Tokyo, Japan) with the soft coagulation mode 80 W (effect 7, VIO300 D, ERBE, Germany) for two seconds.
A porcine colonic mucosal injury model was used to assess the biological safety of OPF-203. After insertion of the endoscope under anesthesia, a biopsy was taken using Radial Jaw 4 (Boston Scientific, Massachusetts, United States). After taking five biopsy specimens in the colon, 200 mL of OPF-203 was injected in four animals as the OPF-203 group, and nothing was injected in the other four animals as the control group. Endoscopic observation and euthanasia were performed one day afterward in two animals in both groups, three days after in the remaining two animals of both groups. Damaged colonic mucosa was evaluated histopathologically.
Results (in vivo)
Video 1 In the porcine model of gastrointestinal bleeding, it was impossible to see the bleeding point by using the water immersion technique. In contrast, it was possible to see the bleeding point by using the gel immersion technique with OPF-203, even without a cylindrical hood.
Video 2 In the porcine model to assess electrocoagulation by using monopolar hemostatic forceps, sufficient electrocoagulation was observed in both the air and OPF-203 groups. Sufficient electrocoagulation was not achieved in the saline.
In the porcine model of gastrointestinal bleeding, the dedicated gel “OPF-203” easily passed through the accessory channel ≥ 2.8 mm. It was impossible to see the bleeding point in the stomach and the large intestine by using the water immersion technique in three of three attempts. In contrast, it was possible to see the bleeding point by using the gel immersion technique with OPF-203 in three of three attempts in both the stomach and large intestine, even without a cylindrical hood ( Fig. 4 and Video 1 ). Aspiration of OPF-203 through the channel was possible after the procedure.
Fig. 4.
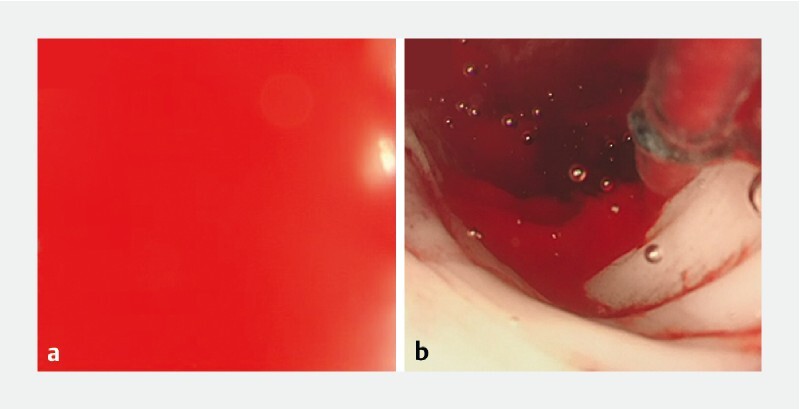
In vivo experiment in the porcine model of gastrointestinal bleeding. a It was impossible to identify the bleeding point by using the water immersion technique. b The OPF-203 enables clear visualization of the bleeding point.
In the porcine model to assess electrocoagulation by using monopolar hemostatic forceps ( Video 2 ), sufficient coagulative necrosis was observed in both the air and OPF-203 groups up to the submucosal tissue. Sufficient coagulation was not achieved in the saline. No coagulative necrosis of the muscularis was observed in all group ( Fig. 5 and Fig. 6 ; Table 2 ). It was easy to grasp the mucosa at the intended site. During the procedure, the visibility through the OPF-203 did not change and the visual field was well maintained.
Fig. 5.

Resected specimen of the stomach in a porcine model to assess electrocoagulation. Effective electrocoagulation by using monopolar hemostatic forceps was observed a in the air and b OPF-203, c not observed in the saline.
Fig. 6.

Histopathological findings of the resected specimen after electrocoagulation. Sufficient coagulative necrosis is observed in both the air and OPF-203 groups up to the submucosal tissue. No coagulative necrosis of the muscularis is observed in all groups (hematoxylin and eosin, × 200).
Table 2. Coagulative necrosis after cauterization in the stomach of the porcine model.
| Tissue layer | Air | OPF-203 | Saline |
| Mucosal epithelium | + + + (3/3) | + + + (3/3) | + (3/3) |
| Lamina propria | + + + (3/3) | + + + (3/3) | + (3/3) |
| Muscularis mucosae to submucosa | + + + (3/3) | + + + (3/3) | − (3/3) |
| Muscularis | − (3/3) | − (3/3) | − (3/3) |
Grade: normal (–), mild (+), moderate (+ +), and severe (+ + +).
In the porcine model for assessing the biological safety of OPF-203, there was no difference in endoscopic findings between OPF-203 and the control group three days after biopsy. There was no difference in histopathological findings comparing the two groups. Findings of tissue healing were observed in both groups three days after biopsy ( Table 3 ). OPF-203 did not interfere with wound healing of damaged colonic mucosa.
Table 3. Histopathological findings after contact in the damaged porcine colonic mucosa.
| Histopathological findings | Group | Control | OPF-203 | ||
| postoperative day | Day 1 | Day 3 | Day 1 | Day 3 | |
| (number of sites) | (10) | (10) | (10) | (10) | |
| Ulcer | Mild | 9 | 10 | 9 | 10 |
| Moderate | 1 | – | 1 | – | |
| Hemorrhage and fibrin exudation with neutrophil infiltration | Normal | – | – | – | 1 |
| Mild | 10 | 10 | 10 | 9 | |
| Fibrosis, submucosa | Normal | 10 | – | 10 | – |
| Mild | – | 10 | – | 10 | |
Grade: normal, mild, moderate and severe.
Discussion
In vitro evaluation revealed that gel with a tanδ < 0.6 makes it possible to create a working space with good visibility that prevents mixing with blood. In endoscopic hemostasis, blood cannot penetrate or diffuse into the gel, facilitating identification of a bleeding point in otherwise difficult circumstances.
Ex vivo studies suggest that effective electrocoagulation using mono-polar devices including hemostatic forceps is possible in gel with 220 μS/cm or less conductivity. The electrical conductivity does not necessarily have to be zero. Electrocoagulation worked adequately in the OPF-203 (actual measurement: less than 30 μS/cm).
In vivo studies showed that the gel immersion technique using the dedicated gel OPF-203 is useful to secure the visual field in a porcine model of gastrointestinal bleeding. Electrocoagulation using mono-polar hemostatic forceps is useful in OPF-203. Biological safety was also confirmed in the porcine model. Based on these results, gel immersion technique with OPF-203 enables electrocoagulation using mono-polar devices in the gel. It facilitates control of the bleeding in various settings including intraprocedural bleeding in endoscopic submucosal dissection and endoscopic mucosal resection using the water immersion technique. Since the viscoelasticity of OPF-203 was selected specifically using criteria to facilitate endoscopic use, OPF-203 is easier to inject through the accessory channel than OS-1 jelly.
Conclusions
OPF-203 has been available in the Japanese market as “VISCOCLEAR” since October 2020. We expect that the gel immersion technique using “VISCOCLEAR” facilitates endoscopic hemostasis in difficult circumstances in clinical practice.
Footnotes
Competing interests Drs. Yano and Ohata hold patents for and are the inventors of the dedicated gel for this method.
References
- 1.Frossard J L, Gervaz P, Huber O. Water-immersion sigmoidoscopy to treat acute gastrointestinal bleeding in the perioperative period after surgical colorectal anastomosis. Gastrointest Endosc. 2010;71:167–170. doi: 10.1016/j.gie.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 2.Gor N, Patil A. Endoscopic management of postoperative ileocolonic anastomotic bleeding by using water submersion. Gastrointest Endosc. 2011;74:721–722. doi: 10.1016/j.gie.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 3.Yamamoto H, Koiwai H, Sekine Y et al. Colonoscopy in flowing water for lower gastrointestinal bleeding: a reliable method for confirmation of bleeding points for endoscopic treatment. Gastrointest Endosc. 2000;52:678–681. doi: 10.1067/mge.2000.109873. [DOI] [PubMed] [Google Scholar]
- 4.Binmoeller K F, Weilert F, Shah J et al. “Underwater” EMR without submucosal injection for large sessile colorectal polyps (with video) Gastrointest Endosc. 2012;75:1086–1091. doi: 10.1016/j.gie.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 5.Akasaka T, Takeuchi Y, Uedo N et al. “Underwater” endoscopic submucosal dissection for superficial esophageal neoplasms. Gastrointest Endosc. 2017;85:251–252. doi: 10.1016/j.gie.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 6.Despott E J, Murino A. Saline-immersion therapeutic endoscopy (SITE): An evolution of underwater endoscopic lesion resection. Dig Liver Dis. 2017;49:1376. doi: 10.1016/j.dld.2017.08.035. [DOI] [PubMed] [Google Scholar]
- 7.Nagata M. Usefulness of underwater endoscopic submucosal dissection in saline solution with a monopolar knife for colorectal tumors (with videos) Gastrointest Endosc. 2018;87:1345–1353. doi: 10.1016/j.gie.2017.11.032. [DOI] [PubMed] [Google Scholar]
- 8.Yano T, Nemoto D, Ono K et al. Gel immersion endoscopy: a novel method to secure the visual field during endoscopy in bleeding patients (with videos) Gastrointest Endosc. 2016;83:809–811. doi: 10.1016/j.gie.2015.09.048. [DOI] [PubMed] [Google Scholar]
- 9.Miura Y, Yano T, Takezawa T et al. Gel immersion endoscopy simplifies hemostasis during endoscopic submucosal dissection using the pocket-creation method. Endoscopy. 2018;50:E294–e295. doi: 10.1055/a-0651-0365. [DOI] [PubMed] [Google Scholar]
- 10.Akasaka T, Takeuchi Y, Ishida H et al. A novel gel immersion technique using a bipolar needle-knife in endoscopic submucosal dissection for superficial gastrointestinal neoplasms. Ann Gastroenterol. 2018;31:247. doi: 10.20524/aog.2018.0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto K, Shiratori Y, Ikeya T. Utility of the gel immersion method for treating massive colonic diverticular bleeding. Clin Endosc. 2020 doi: 10.5946/ce.2020.081. [DOI] [PMC free article] [PubMed] [Google Scholar]


