Abstract
Cardiovascular diseases play major roles in the health problems worldwide especially in Indonesia. Percutaneous coronary intervention (PCI) is a minimally invasive procedure with relatively low complications. However, high inflammatory response post-PCI has showed adverse events even after administration of standard medication. Previous studies showed that curcumin was able to reduce inflammatory response in adult patients with stable coronary heart disease (CHD). This article determines the efficacy of oral administration of curcumin in reducing inflammatory response post-PCI with stable CHD. A double-blind randomized controlled trial on 50 adult patients comparing curcumin and placebo was performed in Cipto Mangunkusumo General Hospital and Jakarta Heart Center within April and June 2015. Either curcumin (45 mg/day) or placebo was given 7 days prior to PCI until 2 days after PCI. Inflammatory markers (high-sensitivity C-reactive protein [hsCRP] and soluble CD40 ligand [sCD40L]) were measured in three phases (7 days prior PCI, 24 hours post-PCI, and 48 hours post-PCI). There were no significant differences in the reduction of hsCRP and sCD40L between curcumin and placebo groups in three phases of measurement. Curcumin significantly reduce the serum hsCRP ( p = 0.006) and sCD40L ( p = 0.002) 7 days before PCI to 48 hours post-PCI. The decrement of hsCRP (−14.2% vs. –7.4%) and sCD40L (−24.3% vs. –13.2%) from 24 to 48 hours post-PCI was higher in the curcumin group than placebo group. The administration of curcumin 45 mg dose daily for 7 days prior PCI until 48 hours post-PCI is useful in reducing inflammatory response post-PCI with stable CHD.
Keywords: curcumin, inflammatory response, PCI, stable CHD
Cardiovascular diseases play major roles in health problems worldwide. An estimated 17.5 million people died of cardiovascular disease in 2012 contributing to 46% of all noncommunicable disease and 7.4 million of these death were caused by coronary heart disease (CHD), dominated in the countries with low to moderate income. 1 Indonesian Basic Health Research (Riset Kesehatan Dasar) in 2013 showed that CHD is the most common type of cardiovascular disease with prevalence of approximately 1.5% of total population. 2
Percutaneous coronary intervention (PCI) is a minimally invasive procedure that was first described in 1977. The advancement in the techniques, technology, and adjuvant medical therapy resulted in low complications rate which is less than 1 to 2%. 3 4 However, an inflammatory response post-PCI has been shown persistently high in about one-third of patients undergoing PCI. High inflammatory response post-PCI was associated with adverse events, approximately 7.5% cause myocardial infarction (MI) and 2.6% cause mortality at 1 year. 5 Increased inflammatory response post-PCI occurs even after standard medication such as clopidogrel, aspirin, and statin before procedure. 6 7 8 9 10
Previous study reported that curcumin, a yellow pigment isolated from Curcuma longa , is a herbal derivative with well-recognized anti-inflammatory and antioxidant properties. 11 Several in vivo and in vitro studies have shown the potential benefits of curcumin in cardiovascular disease. 12 13
This study aims to determine the efficacy of oral administration of curcumin in reducing inflammatory response following PCI in stable CHD. This is the first clinical study that investigated the effects of curcumin on inflammatory response following PCI in stable CHD.
Methods
Study Design
A double-blind randomized controlled trial was performed in Cipto Mangunkusumo General Hospital and Jakarta Heart Center within April and June 2015. This study has been approved by the Ethics Committee of Faculty of Medicine University of Indonesia, reference number 101/UN2.F1/ETIK/2015.
The patients enrolled in this study must fulfill the following criteria: adult patients with stable CHD undergoing PCI with coronary lesion consisting of stenosis > 70%, measured at 18 to 38 mm, angled 45 to 60 degrees, and without moderate calcification. Exclusion criteria comprises of stable CHD patients with history of myocardial infarct or unstable angina within the last 3 months, total occlusion in angiography, new left bundle branch block, use of steroid or immunosuppressant, and those with condition of increased inflammatory parameters and free radicals such as acute or chronic infection, malignancy, hyperthyroidism, and acute or chronic renal disease ( Fig. 1 ).
Fig. 1.
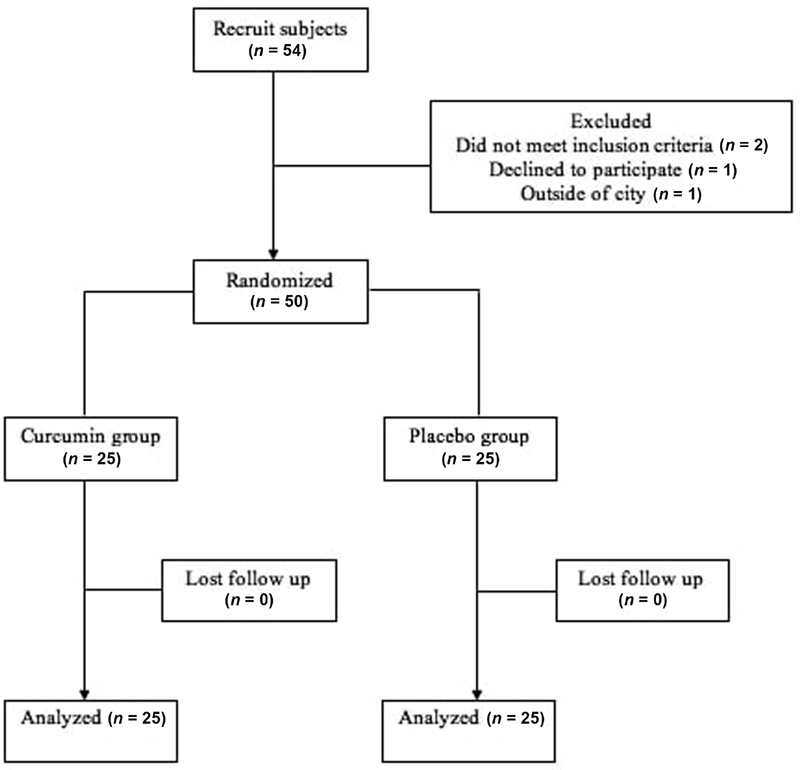
Diagram of trial.
Fifty-four patients were screened, and 4 patients were excluded (2 did not meet inclusion criteria, 1 declined to participate, and 1 dropped out). Fifty patients were randomized to either the curcumin group ( n = 25) or placebo group ( n = 25).
Intervention
Placebo and curcumin (15 mg three times per day) were given from 7 days before PCI until 48 hours post-PCI, as an addition to standard treatment using 100 mg aspirin and 300 mg clopidogrel 12 to 24 hours post-PCI. Before and after stent placement, assessment was done using intravascular ultrasound. PCI was deemed successful when recoil rate was under 30%. Blood samplings for high-sensitivity C-reactive protein (hsCRP) and soluble CD40 ligand (sCD40L) were taken 7 days before curcumin treatment and 24 and 48 hours post-PCI. hsCRP and sCD40L measurement was done using enzyme-linked immunosorbent assay. hsCRP was measured using mg/L as the unit of measurement (normal range < 10 mg/L) and sCD40L was measured using pg/mL as the unit of measurement (normal range < 3,500 pg/mL).
Statistical Analysis
This study was further analyzed by using SPSS statistics for Windows version 21.0. Continuous variables were tested for normal distribution with Kolmogorov–Smirnov test and expressed as mean ± standard deviation if within normal distribution or median (minimum – maximum) if within abnormal distribution. Categorical variables are expressed as frequency (number [%]). The comparison between curcumin and placebo was analyzed using Mann–Whitney test, comparison between three phases of measurement was analyzed using Friedman test, and the variation at different time points were assessed by Wilcoxon test.
Results
Patient Characteristics, Baseline Laboratory Results, and Procedure Characteristics
Fifty patients were enrolled in this study, consisting of 28 male and 22 female patients. Twenty-six patients had history of MI and 20 patients had history of PCI. There was no significant difference in baseline characteristic and PCI procedures ( Table 1 ).
Table 1. Basic and clinical subject characteristics.
| Subject characteristics | Group | p | |
|---|---|---|---|
| Placebo N = 25 |
Curcumin N = 25 |
||
| Gender | |||
| - Male | 13 (52.0) | 15 (60.0) | 0.6 a |
| - Female | 12 (48.0) | 10 (40.0) | |
| Age (y) | 60.60 ± 7.99 | 62.7 ± 6.46 | 0.3 b |
| History of myocardial infarct | |||
| - Yes | 11 (44.0) | 15 (60.0) | 0.3 a |
| - None | 14 (56.0) | 10 (40.0) | |
| History of ischemic stroke/transient ischemic attack | |||
| - Yes | 0 (0.0) | 1 (4.0) | 1.0 c |
| - None | 25.0 (100) | 24 (96.0) | |
| Smoking | |||
| - Yes | 11 (44.0) | 15 (60.0) | 0.3 a |
| - None | 14 (66.0) | 10 (40.0) | |
| History of percutaneous coronary intervention | |||
| - Yes | 7 (28.0) | 13 (52.0) | 0.09 a |
| - None | 18 (72.0) | 12 (48.0) | |
| History of coronary artery bypass grafting | |||
| - Yes | 0 (0.0) | 0 (0.0) | – |
| - None | 25 (100) | 25 (100) | |
| Body weight (kg) | 66 (38–127) | 67 (54–84) | 0.2 d |
| Body height (cm) | 160.5 ± 7.15 | 161.7 ± 8.72 | 0.6 b |
| Body mass index (kg/m 2 ) | 25.7 (16.9–47.8) | 25.6 (21.2–32.7) | 0.5 d |
| Right ventricle function (%) | 16 (11–22) | 16 (11–22) | 0.3 d |
| Left ventricle function (%) | 55.6 ± 8.17 | 56.3 ± 7.56 | 0.8 b |
| Systolic (mm Hg) | 134.8 ± 14.96 | 134.9 ± 13.31 | 0.9 b |
| Diastolic (mm Hg) | 76.3 ± 8.21 | 77.7 ± 9.89 | 0.6 b |
| Hemoglobin (g/dL) | 14.1 ± 3.39 | 13.6 ± 1.21 | 0.3 b |
| Leukocyte (×103/mL) | 8.2 (4.8–16.5) | 8.2 (3.9–10.5) | 0.6 d |
| Thrombocyte (×103/mL) | 262.4 (135.0–405.0) | 262.0 (116.0–438.0) | 0.6 d |
| Estimated glomerular filtration rate | 68.8 (20.3–111.7) | 68.8 (24.0–98.7) | 0.9 d |
| Triglyceride (mg/dL) | 165.9 (61.0–346.0) | 165.9 (40.0–897.0) | 0.7 d |
| Total cholesterol (mg/dL) | 176.1 (100.0–437.0) | 176.1 (97.0–265.0) | 0.8 d |
| HDL (high-density lipoprotein) (mg/dL) | 48.1 (29.0–82.0) | 48.1 (32.0–75.0) | 0.4 d |
| LDL (low-density lipoprotein) (mg/dL) | 107.6 (37.0–339.0) | 107.6 (39.0–172.0) | 0.9 d |
| HbA1c (hemoglobin A1c) (%) | 7.2 (5.2–11.1) | 7.2 (5.0–10.2) | 0.3 d |
| Blood vessel, n (%) | |||
| Left artery descending (LAD) | 14 (56.0%) | 21 (84.0%) | 1.0 a |
| Left circumflex (LCX) | 3 (12.0%) | 1 (4.0%) | |
| Right coronary artery (RCA) | 8 (32.0%) | 3 (12.0%) | |
| Before dilation | |||
| Balloon inflation (s) | 15 (15–20) | 15 (15–20) | 1.0 d |
| Balloon length (mm) | 15 (15–20) | 15 (15–20) | 1.0 d |
| Balloon pressure (atm) | 12 (8–12) | 12 (8–14) | 0.3 d |
| Stent placement | |||
| Amount of stent | 1 | 1 | 1.0 d |
| Stent length (mm) | 38 (20–38) | 38 (20–38) | 0.6 d |
| Stent length (mm) | 3 (2.5–3.5) | 2.75 (2.5–3.5) | 0.3 d |
| Drug eluting stent | |||
| - Yes | 25 (100%) | 25 (100%) | – |
| - None | 0 (0.0%) | 0 (0.0%) | |
| Deploy stent pressure (atm) | 14 (12–16) | 16 (10–16) | 0.3 d |
| Deploy stent duration (s) | 20 (18–22) | 20 (15–20) | 0.9 d |
Note: Categorical variables are presented with n (%). Numerical variables are presented with mean ± standard deviation if within normal distribution or median (minimum - maximum) if within abnormal distribution.
Chi-square test.
Unpaired t -test.
Fisher's exact test.
Mann–Whitney test.
Main Outcome
hsCRP
There was a significant difference of curcumin's hsCRP between the phase of measurement compared with the baseline ( p = 0.006), but on the contrary, placebo's hsCRP was not significantly different between those phases ( p = 0.05). The increment of hsCRP from baseline to 24 hours post-PCI was lower in the curcumin group than the placebo group (12% vs. 22.7%). On the other hand, the decrement of hsCRP from 24 to 48 hours post-PCI was higher in the curcumin group than placebo group (−14.2% vs. –7.4%) ( Table 2 ). Curcumin group showed significant changes in hsCRP from baseline to 24 hours ( p = 0.01) and 24 to 48 hours post-PCI ( p = 0.02), while hsCRP of the placebo group was significant from 24 to 48 hours post-PCI ( p = 0.002) ( Fig. 2A ).
Table 2. Median of serum level in patients with CHD before PCI, 24 and 48 hours post-PCI, and delta of serum level between baseline versus 24 hours post-PCI and 24 versus 48 hours post-PCI.
| Inflammatory markers, median of serum levels a | Delta of serum levels | |||||||
|---|---|---|---|---|---|---|---|---|
| hsCRP (mg/L) | Baseline | 24 h | 48 h | p -Value 2 | Δ1 | Δ2 | ||
| Curcumin | 2.5 (0.4––119) | 2.8 (0.2––155) | 2.4 (0.4––68.2) | 0.006 | +0.3 | 12% | –0.4 | 14.2% |
| Placebo | 2.2 (0––68.4) | 2.7 (0.4––18.2) | 2.5 (0.5––13.9) | 0.05 | +0.5 | 22.7% | –0.2 | 7.4% |
| p -Value 1 | 0.6 | 0.8 | 0.8 | |||||
| sCD40L (pg/dL) | Baseline | 24 h | 48 h | p -Value 2 | Δ1 | Δ2 | ||
| Curcumin | 5604.2 (2056.2–11471.6) | 7584.3 (2898.5–13065.0) | 5738.9 (3830.3–10386.6) | 0.002 | +1980.17 | 35.3% | –1845.49 | 24.3% |
| Placebo | 6398.8 (4085.1–12769.8) | 7525.9 (1721.9–14793.7) | 6534.5 (1810.3–11516.6) | 0.3 | +1127.11 | 17.6% | –991.39 | 13.2% |
| p -Value 1 | 0.2 | 0.6 | 0.3 | |||||
Abbreviations: CHD, coronary heart disease; hsCRP, high-sensitivity C-reactive protein; PCI, percutaneous coronary intervention; sCD40L, soluble CD40 ligand.
Note: Δ1 indicates the intragroup difference between baseline and 24 hours post-PCI. Δ2 indicates the intragroup difference between 24 and 48 hours post-PCI.
Data are presented in median (minimum-maximum).
Fig. 2.
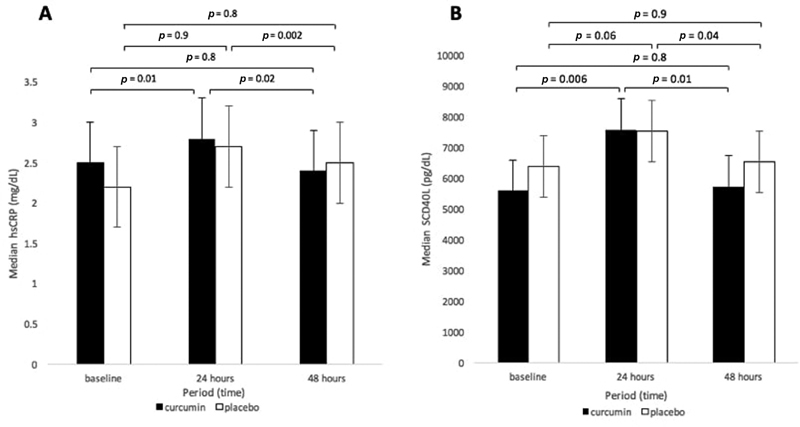
( A ) High-sensitivity C-reactive protein (hsCRP) serum level in curcumin and placebo groups at three times of observation. ( B ) Soluble CD40 ligand (sCD40L) serum level in curcumin and placebo groups at three times observation. *Comparison within group was analyzed using Wilcoxon test.
sCD40L
Both groups showed an increment of sCD40L from baseline to 24 hours post-PCI and decrement of sCD40L at 48 hours post-PCI. There was no significant difference of the sCD40L reduction between the curcumin and placebo groups in the three phases of measurement, the baseline ( p = 0.2), 24 hours post-PCI ( p = 0.6), and 48 hours post-PCI ( p = 0.3). There was a significant difference in sCD40L of the curcumin group from baseline to 24 to 48 hours post-PCI ( p = 0.002) but sCD40L of the placebo group did not show significance difference ( p = 0.3). The increment of sCD40L from baseline to 24 hours post-PCI was higher in the curcumin group than the placebo group (35.3% vs. 17.6%). On the other hand, the decrement of sCD40L serum level from 24 to 48 hours post-PCI was higher in the curcumin group than the placebo group (−24.3% vs. –13.2%) ( Table 2 ). Curcumin group showed significant changes in sCD40L from baseline to 24 hours ( p = 0.006) and 24 to 48 hours post-PCI ( p = 0.01), while sCD40L of the placebo group was significant in hsCRP serum levels from 24 to 48 hours post-PCI ( p = 0.04) ( Fig. 2B ).
Discussion
This study did not show any death or complication due to PCI procedure. The mean age in this study was lower than average of the Indonesian population. No side effects of curcumin administration such as nausea or vomiting were observed. The curcumin intoxication was mild and rare even in dose of 12,000 mg. 14 15 16
Curcumin's Effect against hsCRP Level
Curcumin was shown to blunt the increment in hsCRP from baseline to 24 hours and give greater decrement from 24 to 48 hour post-PCI. PCI could contribute to reactive oxygen species (ROS) production by endothelial stimulation and ischemic-reperfusion process. ROS is known to trigger proinflammatory compounds production including hsCRP with the help of mononuclear cells and T lymphocytes. Tsimikas et al reported that PCI increased oxidized low-density lipoprotein (LDL) which is an immunogenic molecule capable to directly and indirectly triggers proinflammatory molecules and subsequently increase hsCRP. 17 Jiang and colleagues demonstrated that rosuvastatin could reduce hsCRP level at 3 days post-PCI. 18 Mirzabeigi et al reported that curcumin inhibited LDL oxidation and reduced ROS production, therefore reducing hsCRP level. 12 Curcumin effects to metabolic and inflammatory response in acute coronary syndrome had been studied by Alwi and reported that low-dose curcumin (3 × 15 mg) could decreased hsCRP after 1 week of use. 13 Our study show the beneficial effect of low-dose 45 mg curcumin given a week before PCI until 48 hours post-PCI in blunting the inflammatory effect by increasing the anti-inflammation properties of statin.
Curcumin's Effect against sCD40L Level
Our study showed that there is a larger increment of sCD40L concentration in the curcumin group from baseline to 24 hours post-PCI; however, this observation was followed by larger decrement in the curcumin group from 24 to 48 hours post-PCI. sCD40L is a protein within the tumor necrosis factor α ( TNF-α) superfamily, and expressed by activated T-lymphocyte, which later activates endothelial cells and triggers ROS along with proinflammatory chemokines and cytokines production. This process was playing a role in atherogenesis process. A study done by Li et al showed that curcumin could improve atherosclerotic coronary artery permeability that could inhibit lipid deposition and infiltration of inflammatory cell on lesion site, thereby delaying formation of atheroma plaque. The same study also revealed curcumin's role in the prevention of endothelial damage caused by ischemia-reperfusion process by inhibition of matrix metallopeptidase 9 (MMP-9). MMP-9 is synthesized by various matrix cells and macrophages and has roles in extracellular matrix degradation, reduced TNF-α, and CRP level. 19 Study about curcumin's role as an anti-inflammatory agent in reperfusion injury post-PCI on humans and animals has never been done before. 20 Our study show that sCD40L increase within 24 hours post-PCI, in concordance with the inflammation process post-PCI, and then decrease in a larger level when administered low-dose 45 mg curcumin a week before PCI until 48 hours post-PCI.
Conclusion
The administration of curcumin with 45 mg dose daily for 7 days before PCI until 48 hours post-PCI was safe, tolerable, and useful in reducing inflammatory response for patient with stable CHD by decreasing biomarker of inflammation (hsCRP and sCD40L) through the increase of internal anti-inflammation utilization.
Footnotes
Conflict of Interest None declared.
References
- 1.Estimates G H. Geneva: World Health Organization; 2012. Deaths, Disability-Adjusted Life Year (DALYs), Years of Life Lost (YLL) and Years Lost due to Disability (YLD) by Cause, Age and Sex, 2000–2012. [Google Scholar]
- 2.Ministry of Health Republic of Indonesia . Jakarta: 2013. Basic Health Research. [Google Scholar]
- 3.Gruntzig A.Transluminal dilatation of coronary-artery stenosis Lancet 19781(8058):263. [DOI] [PubMed] [Google Scholar]
- 4.Means G, End C, Kaul P. Management of percutaneous coronary intervention complications. Curr Treat Options Cardiovasc Med. 2017;19(04):25. doi: 10.1007/s11936-017-0526-6. [DOI] [PubMed] [Google Scholar]
- 5.Kalkman D N, Aquino M, Claessen B E. Residual inflammatory risk and the impact on clinical outcomes in patients after percutaneous coronary interventions. Eur Heart J. 2018;39(46):4101–4108. doi: 10.1093/eurheartj/ehy633. [DOI] [PubMed] [Google Scholar]
- 6.Ferns G A, Raines E W, Sprugel K H, Motani A S, Reidy M A, Ross R.Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF Science 1991253(5024):1129–1132. [DOI] [PubMed] [Google Scholar]
- 7.Holmes D R, Jr, Vlietstra R E, Smith H C. Restenosis after percutaneous transluminal coronary angioplasty (PTCA): a report from the PTCA Registry of the National Heart, Lung, and Blood Institute. Am J Cardiol. 1984;53(12):77C–81C. doi: 10.1016/0002-9149(84)90752-5. [DOI] [PubMed] [Google Scholar]
- 8.Levine G N, Bates E R, Blankenship J C. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation. 2011;124(23):e574–e651. doi: 10.1161/CIR.0b013e31823ba622. [DOI] [PubMed] [Google Scholar]
- 9.Nobuyoshi M, Kimura T, Nosaka H. Restenosis after successful percutaneous transluminal coronary angioplasty: serial angiographic follow-up of 229 patients. J Am Coll Cardiol. 1988;12(03):616–623. doi: 10.1016/s0735-1097(88)80046-9. [DOI] [PubMed] [Google Scholar]
- 10.Serruys P W, Luijten H E, Beatt K J. Incidence of restenosis after successful coronary angioplasty: a time-related phenomenon. A quantitative angiographic study in 342 consecutive patients at 1, 2, 3, and 4 months. Circulation. 1988;77(02):361–371. doi: 10.1161/01.cir.77.2.361. [DOI] [PubMed] [Google Scholar]
- 11.Kapakos G, Youreva V, Srivastava A K. Cardiovascular protection by curcumin: molecular aspects. Indian J Biochem Biophys. 2012;49(05):306–315. [PubMed] [Google Scholar]
- 12.Mirzabeigi P, Mohammadpour A H, Salarifar M, Gholami K, Mojtahedzadeh M, Javadi M R. The effect of curcumin on some of traditional and non-traditional cardiovascular risk factors: a pilot randomized, double-blind, placebo-controlled trial. Iran J Pharm Res. 2015;14(02):479–486. [PMC free article] [PubMed] [Google Scholar]
- 13.Alwi I. Universitas Indonesia; 2006. Hubungan faktor metabolik dengan respons infllamasi pada sindrom koroner akut pasien diabetes mellitus tipe 2. [Google Scholar]
- 14.Cheng A LHC, Hsu C H, Lin J K.Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions Anticancer Res 200121(4B):2895–2900. [PubMed] [Google Scholar]
- 15.Lao C D, Ruffin M T, IV, Normolle D. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med. 2006;6:10. doi: 10.1186/1472-6882-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma R A, McLelland H R, Hill K A. Pharmacodynamic and pharmacokinetic study of oral curcuma extract in patients with colorectal cancer. Clin Cancer Res. 2001;7(07):1894–1900. [PubMed] [Google Scholar]
- 17.Tsimikas S, Lau H K, Han K R. Percutaneous coronary intervention results in acute increases in oxidized phospholipids and lipoprotein(a): short-term and long-term immunologic responses to oxidized low-density lipoprotein. Circulation. 2004;109(25):3164–3170. doi: 10.1161/01.CIR.0000130844.01174.55. [DOI] [PubMed] [Google Scholar]
- 18.Kim T H, Jiang H H, Youn Y S.Preparation and characterization of water-soluble albumin-bound curcumin nanoparticles with improved antitumor activity Int J Pharm 2011403(1-2):285–291. [DOI] [PubMed] [Google Scholar]
- 19.Li T T, Xie Y, Guo Y. Effect of probucol on vascular remodeling due to atherosclerosis in rabbits: an intravascular ultrasound study. Chin Med J (Engl) 2011;124(12):1840–1847. [PubMed] [Google Scholar]
- 20.DiSilvestro R A, Joseph E, Zhao S, Bomser J. Diverse effects of a low dose supplement of lipidated curcumin in healthy middle aged people. Nutr J. 2012;11:79. doi: 10.1186/1475-2891-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]


