Abstract
Information about the effects of angiotensin II receptor blocker (ARB) therapy on the hemodynamic and cardiac structure in patients with chronic aortic regurgitation (CAR) and isolated systolic hypertension (ISH) is limited.
This study planned to test the hypothesis that l -arginine could further enhance the beneficial effect of an ARB, losartan, and provide a favorable effect on the natural history of CAR and ISH.
Sixty patients with CAR and ISH were enrolled in a randomized, double-blind trial comparing hemodynamic and ultrasonic change in two treatment arms: losartan + l -arginine and losartan-only treated groups. Serial echocardiographic and hemodynamic studies were evaluated before and after treatment.
Both groups had a significant reduction in systolic blood pressure (SBP) and diastolic blood pressure (DBP), left ventricular end-diastolic volume index (LVEDVI), LV end-systolic volume index (LVESVI), LV mass index (LVMI), and LV mean wall stress after 6- and 12-month treatment ( p <0.01 in all comparisons). Both groups had a significant increase in LV ejection fraction and exercise duration after 6- and 12-month treatment ( p < 0.01 in all comparisons). Using multivariate linear regression analysis, only losartan + l -arginine therapy achieved a significantly lower LVESVI (38.89 ± 0.23 mL/m 2 ), LVEDVI (102.3 ± 0.3 mL/m 2 ), LVMI (107.6 ± 0.3 g/m 2 ), SBP (123.5 ± 1.0 mm Hg), and greater exercise duration (7.38 ± 0.02 minutes) than those of the losartan-only treated groups ( p <0.01 in all comparisons).
These findings suggest that early co-administrative strategy provides a beneficial approach to favorably influence the natural history of CAR.
Keywords: aortic regurgitation, isolated systolic hypertension, left ventricular mass, echocardiography, l -arginine
Chronic aortic regurgitation (CAR) and isolated systolic hypertension (ISH) can lead to progressive volume and pressure overload of the left ventricle (LV) and may subsequently cause LV dilatation and hypertrophy, congestive heart failure (CHF), and even sudden cardiac death. 1 2 3 Several studies indicate that patients with CAR may benefit from vasodilators. 3 4 5 6 Vasodilators have the potential to reduce afterload and decrease wall stress, thereby limiting LV dilatation and hypertrophy. 1 Vasodilators such as angiotensin-converting enzyme inhibitors (ACEI) and calcium-channel blockers have been suggested by some experts to prevent disease progression and delay the need for aortic valve replacement (AVR). 1 4 6
An angiotensin II receptor blocker (ARB) is a pharmaceutical agent that modulates the renin-angiotensin-aldosterone system (RAAS), which is responsible for blood pressure (BP) regulation and fluid and electrolyte homeostasis. ARBs have been widely used in treating hypertension and CHF. 7 8 The reports of the effect of ARBs in evaluating the LV functional change in aortic regurgitation were limited. 9 10 Only one article has studied the effects of losartan + l -arginine in patients with heart failure secondary to coronary heart disease. 11 However, little is known about the clinical efficacy of ARBs in patients with CAR and ISH.
The discovery of the l -arginine nitric oxide (NO) pathway has had a profound impact on biological science. 12 13 NO reduces stress, minimizes vascular resistance, and optimizes regional blood flow. Moreover, several long-term studies have demonstrated that chronic oral administration of l -arginine can improve clinical symptoms of certain cardiovascular diseases. 14 15 To the best of our knowledge, there is no report describing the effect of co-administration of ARBs and l -arginine in treating patients with CAR and ISH. We hypothesize that long-term co-administration of ARBs and l -arginine may enhance ARB efficacy in patients with CAR and ISH. To test the hypothesis, we conducted a randomized, double-blind trial with two treatment arms that included a losartan plus l -arginine (L + A) group and a losartan (L) group.
Methods
Study Patients
Patients with Doppler echocardiography–documented Aortic Regurgitation (AR) and preserved LV ejection fraction (LVEF > 50%) who had a baseline (resting) systolic BP (SBP) ≥140 mm Hg and diastolic BP (DBP) <90 mm Hg were enrolled. Those with worsening AR within the past 6 months, with other significant valvular lesions, and with histories of significant coronary heart disease, atrial fibrillation, CHF, or diabetes mellitus were excluded. From January, 2016, to December 31, 2018, 68 patients were included. To achieve a homogenous population, six patients with rheumatic heart disease were excluded. Sixty-two patients with degenerative CAR and ISH underwent this age-matched, double-blind treatment trial. Only two patients were withdrawn due to relocation. Sixty asymptomatic patients with CAR and ISH from Yuan's General Hospital, in Kaohsiung City, Taiwan, and An-Fa Institution of Preventive Medicine, Taipei City, Taiwan, were enrolled in the study.
The study protocol was approved by the Review Committee on Ethics of the Yuan's General Hospital and the An-Fa Institution of Preventive Medicine. All patients gave written informed consent before participation in this clinical trial. The cause of CAR was due to degeneration of the valve structure. Patients with chronic degenerative AR were selected by exclusion of other causes of AR from medical history, radiography, electrocardiography, and echocardiographic appearance. The exclusion criteria included history of rheumatic heart disease, dissecting aortic aneurysm, and AR combined with mitral stenosis. Degenerative AR typically presented with echocardiographic features of aortic valve thickening change and/or dilated aortic root.
This study used the AR jet height/LV outflow tract (LVOT) height ratio in the parasternal long-axis view and AR jet area/LVOT area ratio in the parasternal short-axis view as criteria for judging the extent of AR. Ratios of <0.25, 0.26 to 0.64 and >0.65 of AR jet height/LVOT height and ratios of <0.50, 0.50 to 0.59 and >0.60 of AR jet area/LVOT area indicated mild, moderate, and severe AR, respectively. 16 The AR duration was established by chart records obtained from medical history taken directly from study patients. Only asymptomatic AR patients and ISH were included in this clinical trial. The duration of hypertension was also obtained if BP was >140/90 mm Hg from chart records in all patients. None of the patients underwent cardiac catheterization because they were asymptomatic.
Study Protocol
Losartan (COZAAR, Merck and Co, NJ) is an ARB with antihypertensive effect. The l -arginine supplement (Synginine) is a sustained release (SR) product (Synmosa Biopharma Co.). The initial assessments of all patients included a complete history, physical examination, blood chemistries, urinalysis, 12-lead electrocardiogram (ECG), treadmill exercise testing (Bruce protocol), M-mode, 2-dimensional, and Doppler transthoracic echocardiogram. Similar assessments were screened intermittently to select patients without underlying liver or kidney disease initially and to observe whether they developed adverse effects after treatment.
Before we conducted this study, a pair of participants of similar age (grouped in 10 years) and sex were used as an age- and sex-matched group and subsequently randomized based on the stratification with computer assignment. Sixty asymptomatic patients with CAR and ISH were matched by age and sex and stratified into one of two treatment arms: L + A group or L- group. A double-blind design was used, and neither the physicians nor the patients were aware of their treatment regimen. The clinical characteristics of all patients who were enrolled and randomized in the trial are listed in Table 1 . Echocardiographic evaluation, SBP, DBP, heart rate (HR) measurements, and treadmill exercise duration (ED) (Bruce protocol) were studied at baseline and repeated 6- and 12-month, post randomization.
Table 1. Comparison of demographic data, risk factors, and clinical data in two groups a .
| L+ A group (losartan + l -arginine) | L group (losartan) | p -Value | |
|---|---|---|---|
| No. | 30 | 30 | |
| Age (y) | 66.4 ± 9.6 | 66.4 ± 9.4 | 0.500 |
| Sex (M/F) | 23/7 | 24/6 | 0.389 |
| HT duration | 8.4 ± 5.0 | 8.5 ± 4.9 | 0.469 |
| SBP ≧160 mm Hg (No. %) | 11 (36.7%) | 11 (36.7%) | 0.500 |
| Heart rate (bpm) | 77.5 ± 7.2 | 78.6 ± 7.4 | 0.958 |
| AR duration (y) | 6.0 ± 3.2 | 6.1 ± 3.5 | 0.454 |
| Risk factor/laboratory data | |||
| BMI (kg/m 2 ) | 23.8 ± 2.3 | 24.9 ± 2.5 | 0.038 |
| Diabetes mellitus No. (%) | 4 (13.3) | 3 (10.0) | 0.345 |
| Current smoker No. (%) | 14 (46.7) | 13 (43.3) | 0.396 |
| Total cholesterol (mg/dL) | 185 ± 42 | 188 ± 43 | 0.392 |
| LDL-cholesterol (mg/dL) | 115 ± 35 | 114 ± 39 | 0.458 |
| Triglyceride (mg/dL) | 149 ± 40 | 156 ± 48 | 0.270 |
| BUN (mg/dL) | 18.87 ± 6.46 | 17.36 ± 4.75 | 0.151 |
| Creatinine (mg/dL) | 1.35 ± 0.34 | 1.32 ± 0.25 | 0.349 |
| Sodium (mEq/L) | 140.97 ± 3.78 | 141.87 ± 2.94 | 0.152 |
| Potassium (mEq/L) | 4.28 ± 0.35 | 4.32 ± 0.42 | 0.322 |
| GOT (U/L) | 29.32 ± 9.59 | 28.35 ± 8.80 | 0.342 |
| GPT (U/L) | 25.77 ± 9.82 | 28.35 ± 8.80 | 0.400 |
| HbA 1c (%) | 6.54 ± 1.51 | 6.13 ± 1.06 | 0.141 |
| UA (mg/dL) | 6.84 ± 2.10 | 7.10 ± 1.44 | 0.288 |
| Severity of AR | |||
| Mild/moderate/severe, No. | 9/12/9 | 9/12/9 | |
| Dilated aortic root, No. (%) | 10 (33.3) | 9 (30.0) | 0.392 |
| Echo data | |||
| LVEDVI (mL/m 2 ) | 125.5 ± 11.3 | 125.4 ± 11.4 | 0.486 |
| LVESVI (mL/m 2 ) | 51.00 ± 11.02 | 51.23 ± 10.77 | 0.467 |
| LVEF (%) | 59.78 ± 5.41 | 59.60 ± 5.18 | 0.448 |
| LVMWS (K dyne/cm 2 ) | 389.9 ± 45.2 | 390.4 ± 45.5 | 0.483 |
| LV mass index(g/m 2 ) | 131.8 ± 11.9 | 131.7 ± 11.9 | 0.487 |
| Drug regimen | |||
| Aspirin, No. (%) | 10 (33.3) | 11 (36.7) | 0.391 |
| Statin, No. (%) | 14 (46.7) | 15 (50.0) | 0.399 |
| Diuretic drug(added) | 8 (26.7%) | 11 (36.7%) | 0.203 |
| Additive l -arginine | Yes | No | |
| Beta-blocker | 14 (46.7%) | 15 (50.0%) | 0.397 |
Abbreviations: AR, aortic regurgitation; BMI, body mass index; BUN, blood urea nitrogen; F, female; GOT, aspartate aminotransferase; GPT, alanine aminotransferase; HbA 1c , glycated hemoglobin; HT, hypertension; LDL, low density lipoprotein; LVEDVI, left ventricular end-diastolic volume index; LVEF, left ventricular ejection fraction; LVESVI, left ventricular end-systolic volume index; LV, left ventricular; LVMWS, LV mean wall stress; L + A, losartan + l -arginine; L, losartan; M, male; SBP, systolic blood pressure; SD, standard deviation; UA, uric acid.
Data are presented as mean ± SD unless indicated otherwise. There are no significant differences in the demographic information of all data between the 2 groups.
Drug Administration
Study patients were asked to discontinue their current antihypertensive drugs for 2 weeks (the placebo run-in period). Patients were instructed to take chewable nifedipine (Adalat) 5 mg sublingually, every 3 hours, if needed (if systolic BP >160 mm Hg during the placebo run-in period). We instructed patients not to stand for at least 30 to 40 minutes after taking nifedipine sublingually to avoid development of orthostatic hypotension. Additionally, we asked patients to report chest pain or persistent dizziness after use of nifedipine. Of note, we did not enroll patients with hypertensive emergencies or urgencies. None of our patients developed severe adverse effects during the run-in period.
Losartan was started at 25 mg once daily for the first 2 weeks. The dosage was increased to 25 mg twice a day (bid) in week 3 or 4, if BP was >140/90 mm Hg. The l -arginine supplementation with losartan was started at 2.5 g daily and then increased to 5 g bid after 2 weeks if patients could tolerate this dose. After 4 weeks, a diuretic was added and titrated upward if BP remained >140/90 mm Hg. The dosing schedule can be found in Fig. 1 . This higher dose was maintained if no major adverse effect was encountered. The dosage was not increased if there were major adverse effects such as headache, abdominal fullness, diarrhea, or other allergic reaction, or if hemodynamic end points were met (SBP ≤140 mm Hg or BP reduction ≥20% of the initial BP). In other words, the maintenance dosage depended on the physician's clinical assessment, hemodynamic end points, and adverse reactions to the assigned treatment.
Fig. 1.
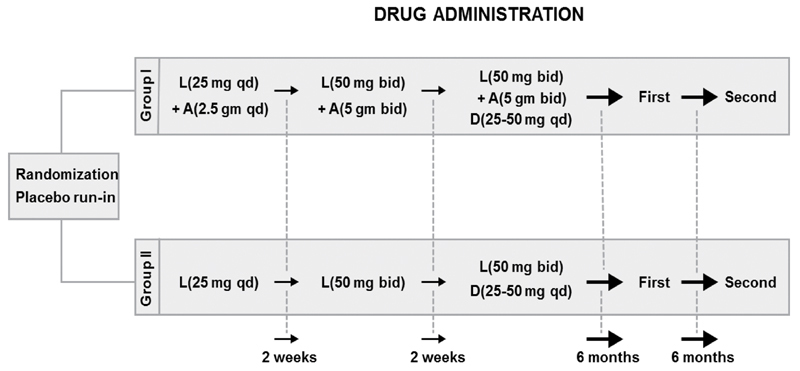
Drug administration protocol shows the administration of the study drugs in these two groups and how dose was initiated and increased at various time intervals. A, l -arginine; bid, twice a day; D, diuretic (hydrochlorothiazide); First, first clinical assessment; L, losartan; qd, once a day; Second, second clinical assessment.
Echocardiographic Analysis
Two-dimensional echocardiograms were performed with a commercially available HP (Hewlett-Packard) 7500 ultrasound system with a 2.5-MHz transducer. Measurements were performed at the end of the trial. Echocardiographic examinations were coded and read by two independent observers (M. L. and S.-L.L.), both of whom were blinded to the patient's identity and order of studies. A discrepancy ≥10 mL for LV volume and ≥10 g for LV mass index required repeated analysis of the echocardiographic data by a third observer; H-W.K. Agreement was achieved by consensus. All echocardiographic measurements were made according to the recommendations of the American Society of Echocardiography. 17 LV mean wall stress (MWS) was measured by combining peak systolic arterial pressure (SAP) measurements (Cuff method) of LV radius and posterior wall thickness as follows: MWS (K dyne/cm 2 ) = CF × SAP × Rm/Thm, where CF = conversion factor (CF = 1 K dyne/cm 2 mm Hg) and Rm and Thm = averages of end-diastolic and end-systolic values of the LV radius (R) and wall thickness (Th) (cm). 18 These measurements were done on M-mode tracings derived from two-dimensional visualization of the LV in the parasternal short-axis view, similar to previous methods. 3 18 LV endocardial and epicardial echocardiograms in apical 4-chamber and parasternal short-axis views for 5 cardiac cycles were digitized at end-diastole (R wave peak on ECG) and at end-systole (time of the smallest cavity area) by two independent observers. LV volumes were calculated by an ellipsoid biplane area-length model, and ejection fraction was derived as (EDV-ESV)/EDV × 100%, where EDV and ESV represented end-diastolic and end-systolic volume, respectively. 17 19 LV myocardial mass (LVM) was calculated using a two-dimensional method based on the area-length formula by multiplying the myocardial volume by the specific weight of cardiac muscle (1.05 g/mL). 17 19 20 All values were expressed as an index (m 2 ) by dividing by body surface area.
Statistical Analysis
All values are presented as mean ± standard deviation. One-way analysis of variance was used to compare data between the two groups. The Scheffe test was used to compare the data difference in each group at baseline and following 6- and 12-month treatments. Before using multivariate analysis, we checked the assumptions of our data, including normal distribution, a post-hoc sample size calculation, and residual analysis of the echocardiographic and hemodynamic data. The intensity change of echocardiographic and hemodynamic data at baseline versus after 6-month, baseline versus after 12-month, and 6-month versus after 12-month treatment was calculated and presented as percentage changes. Multivariate regression analysis was used to adjust for age and sex to compare the difference on intensity changes of echocardiographic and hemodynamic data using losartan (L) treatment as the reference group in three stages. Pearson correlation analysis was employed in 30 patients to assess whether the measurements of echocardiographic variables were reproducible. Any difference was considered significant at an α level of <0.05.
Results
Baseline Comparisons and Follow-Up
For the l -arginine supplement, the dose of l -arginine was increased progressively. The l -arginine dose was increased to 5 g bid after 2 weeks and remained unchanged thereafter. Most patients tolerated l -arginine very well. However, some patients developed mild gastrointestinal symptoms, which were relieved with antacids. Thus, 18 patients (60%) used 10 g (5 g bid) daily and 12 patients (40%) received 7.5 g (5 g AM, 2.5 g PM) daily. Clinical characteristics and measurements of LV dimensions and the baseline echocardiographic and hemodynamic examinations in these two groups were similar ( Table 1 ). All participants were asymptomatic, and none required surgical intervention during the 12-month follow-up period. There was no substantial deterioration of liver or renal function (as assessed by the serum aspartate or alanine aminotransferase, blood urea nitrogen, and creatinine levels), sodium or potassium levels, or substantial change in uric acid or glycated hemoglobin levels during the 12-month treatment in either groups. The grading of AR severity of the study patients by the AR jet height/LVOT height ratio from the parasternal long-axis view and by the AR jet area/LVOT area ratio from the parasternal short axis-view was similar for the two groups. The reproducibility of the measurements of echocardiographic variables (in 30 cases) was good. The results of Pearson correlation analysis are shown in Table 2 ; all r values were ≥0.905 and all p values were <0.001.
Table 2. Results of the Pearson correlation analysis testing the reproducibility of the measurements of echocardiographic variables.
| Variables | Interobserver | Intraobserver | ||
|---|---|---|---|---|
| r | p -Value | r | p -Value | |
| LVEDV | 0.961 | <0.001 | 0.945 | <0.001 |
| LVESV | 0.971 | <0.001 | 0.961 | <0.001 |
| LVEF | 0.964 | <0.001 | 0.941 | <0.001 |
| LVM | 0.952 | <0.001 | 0.934 | <0.001 |
| LVMWS | 0.937 | <0.001 | 0.905 | <0.001 |
Abbreviations: LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; LVM, LV mass, LVMWS, LV mean wall stress; r , correlation coefficient.
The echocardiographic and hemodynamic analyses for the two groups are summarized in Tables 3 and 4 . Left ventricular mean wall stress (LVMWS) and left ventricular mass index (LVMI) were significantly reduced in both losartan + l -arginine-treated group and losartan-only–treated group compared with the baseline ( p < 0.01). A statistically significant reduction in LV end-diastolic volume index (LVEDVI) and LV end-systolic volume index (LVESVI) were also observed in these two groups after 6-month and 12-month follow-up evaluations ( p < 0.01). Additionally, a mild increase in ejection fraction and ED was detected in these two groups when compared with the baseline value ( p <0.01). Significant decrease in HRs, SBP, and DBP was also found in both groups compared with those of baseline data ( p < 0.01 in all comparisons, Tables 3 and 4 ).
Table 3. Intensity change of echocardiographic and hemodynamic data in patients treated with losartan + l -arginine during 12-month follow-up period a .
| (A) Baseline stage | (B) Mid-term stage (6-month) | (C) Final stage(12-month) | Percent change | |||
|---|---|---|---|---|---|---|
| (A) | A vs. B ( p -Value) | A vs. C ( p -Value) | B vs. C ( p -Value) | |||
| Echocardiographic data | ||||||
| LVEDVI (mL/m 2 ) | 125.5 ± 11.3 | 107.6 ± 11.8 | 102.5 ± 11.4 | –14.3 (<0.001) | –18.3 (<0.001) | –4.7 (<0.001) |
| LVESVI (mL/m 2 ) | 51.00 ± 11.02 | 42.00 ± 11.30 | 38.89 ± 11.15 | –17.6 (<0.001) | –23.7 (<0.001) | –7.4 (<0.001) |
| EF (%) | 59.78 ± 5.41 | 61.46 ± 6.66 | 62.80 ± 6.96 | 2.81 (<0.001) | 5.05 (<0.001) | 2.18 (<0.001) |
| LVMI (g/m 2 ) | 131.8 ± 11.9 | 113.1 ± 12.5 | 107.6 ± 11.9 | –14.2 (<0.001) | –18.4 (<0.001) | –4.9 (<0.001) |
| LVMWS (K dyne/cm 2 ) | 389.9 ± 45.2 | 281.3 ± 36.7 | 279.3 ± 35.7 | –27.9 (<0.001) | –28.4 (<0.001) | –0.7 (<0.010) |
| Hemodynamic data | ||||||
| HR (beats/min) | 78.4 ± 7.5 | 76.0 ± 7.2 | 73.9 ± 7.1 | –3.06 (<0.001) | –5.74 (<0.001) | –2.76 (<0.001) |
| SBP (mm Hg) | 175.6 ± 19.2 | 126.4 ± 15.2 | 123.0 ± 14.4 | –28.0 (<0.001) | –30.0 (<0.001) | –2.68 (<0.001) |
| DBP (mm Hg) | 86.5 ± 9.2 | 75.8 ± 9.6 | 73.4 ± 9.5 | –13.5 (<0.001) | –15.1 (<0.001) | –3.16 (<0.001) |
| ED (min) | 6.29 ± 1.42 | 7.11 ± 1.45 | 7.42 ± 1.41 | 13.4 (<0.001) | 18.0 (<0.001) | 4.36 (<0.001) |
Abbreviations: DBP, diastolic blood pressure; ED, exercise duration; EF, ejection fraction; HR, heart rate; LVEDVI, left ventricular end-diastolic volume index; LVESVI, left ventricular end-systolic volume index; LVMI, LV mass index; LVWMS, LV mean wall stress; SBP, systolic blood pressure; SD, standard deviation. Other abbreviation definitions can be found on Table 1 .
Echocardiographic and hemodynamic data are presented as mean ± SD. Intensity changes of echocardiographic and hemodynamic data are presented as percent changes.
Table 4. Intensity change of echocardiographic and hemodynamic data in patients treated with losartan during 12-month follow-up period a .
| (A) Baseline stage | (B) Mid-term stage(6-month) | (C) Final stage(12-month) | Percent change | |||
|---|---|---|---|---|---|---|
| A vs. B ( p -value) | A vs. C ( P value) | B vs. C ( p -value) | ||||
| Echocardiographic data | ||||||
| LVEDVI (mL/m 2 ) | 125.4 ± 11.4 | 108.0 ± 11.5 | 106.0 ± 11.5 | –13.9 (<0.001) | –15.5 (<0.001) | –1.85 (<0.001) |
| LVESVI (mL/m 2 ) | 51.23 ± 10.77 | 42.80 ± 10.90 | 41.13 ± 10.65 | –16.5 (<0.001) | –19.7 (<0.001) | –3.90 (<0.001) |
| EF (%) | 59.60 ± 5.18 | 60.96 ± 6.17 | 61.80 ± 6.14 | 2.28 (<0.001) | 3.69 (<0.001) | 1.37 (<0.001) |
| LVMI (g/m 2 ) | 131.7 ± 11.9 | 113.4 ± 12.1 | 111.3 ± 12.1 | –13.9 (<0.001) | –15.5 (<0.001) | –1.85 (<0.001) |
| LVMWS (K dyne/cm 2 ) | 390.4 ± 45.5 | 286.7 ± 40.5 | 284.5 ± 40.4 | –26.6 (<0.001) | –27.1 (<0.001) | –0.76 (0.011) |
| Hemodynamic data | ||||||
| HR (beats/min) | 78.5 ± 7.5 | 76.1 ± 7.2 | 75.8 ± 7.0 | –2.67 (<0.001) | –3.43 (<0.001) | –0.39 (0.03) |
| SBP (mm Hg) | 175.9 ± 19.0 | 129.5 ± 17.1 | 127.4 ± 16.9 | –26.4 (<0.001) | –27.6 (<0.001) | –1.62 (<0.001) |
| DBP (mm Hg) | 86.9 ± 9.4 | 76.5 ± 9.4 | 74.6 ± 9.3 | –12.0 (<0.001) | –14.2(<0.001) | –2.48 (<0.001) |
| ED (min) | 6.25 ± 1.43 | 6.95 ± 1.44 | 7.06 ± 1.46 | 11.2 (<0.001) | 13.0 (<0.001) | 1.58 (<0.001) |
Abbreviations: DBP, diastolic blood pressure; ED, exercise duration; EF, ejection fraction; HR, heart rate; LVEDVI, left ventricular end-diastolic volume index; LVESVI, left ventricular end-systolic volume index; LVMI, LV mass index; LVWMS, LV mean wall stress; SBP, systolic blood pressure; SD, standard deviation.
Intensity Change of Echocardiographic and Hemodynamic Data
The intensity changes over time (baseline, 6-month, and 12-month follow-up) in echocardiographic and hemodynamic variables presented as “percent change” after 6-month and 12-month treatment are shown in Tables 3 and 4 . For the losartan + l -arginine–treated group, there was a 14.3 and 18.3% decrease in the LVEDVI data after 6-month and 12-month treatment, respectively ( p < 0.01 in both comparisons). A 4.7% decrease in the LVEDVI when comparing 12-month data with 6-month data ( p < 0.01) was also noted. Similar findings for LVESVI data were found in the comparison of 6-month and 12-month data to that of baseline, and 12-month to that of 6-month data ( p < 0.01 in all comparisons) ( Table 3 ). A statistically significant reduction in LVEDVI and LVESVI was observed in the losartan-only–treated group after 6-month and 12-month follow-up evaluations ( p < 0.01 in all comparisons) ( Table 4 ). LVMI decreased significantly after 6-month and 12-month treatment in both groups ( p < 0.001 in all comparisons). Additionally, a further decrease in LVMI was noted when comparing 12-month versus 6-month data in both groups ( p < 0.001 in all comparisons) ( Tables 3 and 4 ). Interestingly, the losartan + l -arginine–treated group had a significant reduction in LVMWS in 12-month versus 6-month data, with a p -value less than 0.01 ( Table 3 ); the p -value was less than 0.05 in the losartan group ( Table 4 ).
For hemodynamic variables, significant decreases in HRs were found in both the losartan + l -arginine–treated group and the losartan-only–treated group compared with baseline data ( P < 0.01 in all comparisons) ( Tables 3 and 4 ). A significant reduction in SBP and DBP were observed in both groups after 6-month and 12-month follow-up ( p < 0.01). Furthermore, both treatment strategies revealed a significant increase in ED after 6-month and 12-month treatment ( p < 0.01 in all comparisons) ( Tables 3 and 4 ). Generally speaking, percent change of echocardiographic and hemodynamic data had time-dependent trends with serial changes over time in patients treated with different regimens. Notably, the intensity of percent change in echocardiographic and hemodynamic data in the group treated with losartan + l -arginine was slightly higher than in the group treated with losartan alone.
Comparison of Echocardiographic and Hemodynamic Data Using the Losartan Treatment Arm as the Reference Group
Table 5 shows the comparison of echocardiographic and hemodynamic data using the losartan treatment arm as the reference group, which were compared with those of the losartan + l -arginine treatment arm following 6-month and 12-month therapy after multivariate linear regression analysis adjusted for baseline indicators, age, and sex. After 6-month treatment, our results showed that there were no significant differences in echocardiographic variables between the L group and the L + A group. For the hemodynamic data, the SBP and ED were significantly better in the L + A–treated group compared with those in the losartan-only–treated group ( p < 0.05 for both comparisons). For the 12-month data, the statistically significant differences in all echocardiographic variables were observed in the L + A group and not in the L group. Additionally, there was a significantly lower SBP and improved ED in the L + A group compared with the L group ( p < 0.01 in both comparisons).
Table 5. Comparison of echocardiographic and hemodynamic data using losartan (l) treatment as reference group after 6-and 12-month treatment, using multivariate linear regression analysis adjusted for baseline data, age, and sex a .
| (A) L + A group( n = 30) | (B) L group( n = 30) | p– Value | |
|---|---|---|---|
| Echocardiography data (6-month) | |||
| LVEDVI (mL/m 2 ) | 107.4 ± 0.3 | 107.9 0.3 | 0.296 |
| LVESVI (mL/m 2 ) | 42.01 ± 0.24 | 42.58 ± 0.24 | 0.074 |
| EF (%) | 61.41 ± 0.21 | 61.10 ± 0.21 | 0.276 |
| LVMI (g/m 2 ) | 113.1 ± 0.4 | 113.4 ± 0.4 | 0.527 |
| LVMWS (K dyne/cm 2 ) | 280.6 ± 2.6 | 284.7 ± 2.5 | 0.238 |
| Hemodynamic data (6-month) | |||
| HR (beats/min) | 76.1 ± 0.2 | 76.0 ± 0.2 | 0.889 |
| SBP (mm Hg) | 126.6 ± 0.9 | 129.5 ± 1.0 | 0.027 |
| DBP (mm Hg) | 75.9 ± 0.6 | 76.3 ± 0.6 | 0.634 |
| ED (min) | 7.06 ± 0.02 | 6.98 ± 0.02 | <0.001 |
| Echocardiographic data (12-month) | |||
| LVEDVI (mL/m 2 ) | 102.3 ± 0.3 | 105.9 ± 0.3 | <0.001 |
| LVESVI (mL/m 2 ) | 38.9 ± 0.23 | 40.95 ± 0.23 | <0.001 |
| EF (%) | 62.75 ± 0.23 | 61.91 ± 0.23 | <0.001 |
| LVMI (g/m 2 ) | 107.6 ± 0.3 | 111.3 ± 0.3 | <0.001 |
| LVMWS (K dyne/cm 2 ) | 278.8 ± 1.6 | 283.0 ± 1.5 | 0.007 |
| Hemodynamic data (12-month) | |||
| HR (beats/min) | 74.0 ± 0.2 | 75.7 ± 0.2 | 0.889 |
| SBP (mm Hg) | 123.5 ± 1.0 | 127.4 ± 1.0 | 0.002 |
| DBP (mm Hg) | 73.5 ± 0.6 | 74.4 ± 0.6 | 0.276 |
| ED (min) | 7.38 ± 0.02 | 7.08 ± 0.02 | <0.001 |
Abbreviations: DBP, diastolic blood pressure; ED, exercise duration; EF, ejection fraction; HR, heart rate; LVEDVI, left ventricular end-diastolic volume index; LVESVI, left ventricular end-systolic volume index; LVMI, LV mass index; LVWMS, LV mean wall stress; SBP, systolic blood pressure; SD, standard deviation.
Discussion
Our results showed that patients treated with losartan + l -arginine demonstrated consistent improvements in echocardiographic and hemodynamic data, which were greater than that seen in the losartan-only group after 6-month and 12-month interventions. After adjusting for baseline echocardiographic and hemodynamic data, age, and sex, our study also found that patients treated with losartan + l -arginine showed greater improvements in echocardiographic and hemodynamic data than the losartan-treated group after 12 months.
Traditionally, ISH is defined as SBP above 140 mm Hg and DBP less than 90 mm Hg. 21 22 23 In October 2018, the American College of Cardiology/American Heart Association Task Force reported new BP guidelines, according to which an SBP of 130 mm Hg was considered hypertensive at all ages. 24 Following this, ISH was defined as SBP above 130 mm Hg and DBP less than 90 mm Hg. However, since our study was initiated in 2016, we used the previous definition of ISH (i.e., baseline [resting] SBP >140 mm Hg and DBP < 90 mm Hg) in our inclusion criteria.
From 2016 through 2018, according to the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, 8 the definition of successful treatment (control) was to treat SBP and DBP to targets of less than 140 and less than 90 mm Hg, respectively. It was for this reason that we chose SBP less than 140 mm Hg, and not 130 mm Hg, as our treatment target.
Our study used the AR jet height/LVOT height ratio and the AR jet area/LVOT area ratio for judging the severity of AR. Since we used the parasternal long-axis plane and short-axis planes to assess the extent of AR, the orthogonal imaging planes likely provided additional information, which provides confidence in quantifying the AR severity. 25 This method has been applied accurately in the past to predict the severity of AR as determined by angiographic grading. 26 27
CAR and ISH induce long-term hemodynamic burden on the LV. 1 2 3 Long-term hemodynamic overload may eventually lead to muscle dysfunction, CHF, and even death. 1 3 4 Therefore, valvular replacement is recommended when more than mild symptoms are present or before the LV is compromised due to severe aortic regurgitation. 1 3 4 Some studies have supported the idea that asymptomatic patients with severe aortic regurgitation and normal LVEF may undergo surgery prior to the development of symptoms or reduction of LVEF. 26 27 This recommendation is based on the fact that reduced LVEF most likely has a marked adverse effect on survival after AVR. 26 27 28 29 However, some studies have indicated that there is no good evidence that early valve replacement provides benefit in symptomatic patients with normal LVEF, 30 31 or in asymptomatic patients with reduced LVEF. 32 33 34 It is also known that hypertension can be associated with complex and diverse cardiovascular structure and function change. 35 36
l -Arginine is the precursor for the endogenous synthesis of NO. NO generated from l -arginine is an important messenger molecule that is involved in functions as diverse as neurotransmission, vasodilation, inflammation, and regulation of gene expression. 12 37 A previous study found that after oral intake, l -arginine is subject to extensive presystemic and systemic elimination by bacteria in the gut and arginase in the gut and liver, respectively. 38 This may limit the bioavailability of l -arginine. Since circulating disappearance of l -arginine is rapid, a previous article found that l -arginine doses of ∼2 to 4 g tid (3 times a day) are required to provide a hypotensive effect. 39 Another article reported that an l -arginine daily dose of 6 to 12 g would be required to produce a favorable hypotensive effect. 40 In our study, the maintenance dose of l -arginine in 25 of 30 cases (83%) was 10 g daily. If patients developed adverse effects like headache, abdominal fullness, diarrhea, or other allergic reactions, the dose was tapered to 7.5 g daily. This way all patients were able to tolerate daily doses ranging from 7.5 to 10 g. Administration of l -arginine bid instead of tid was done to achieve better compliance.
Acute and chronic administration of l -arginine has been shown to improve endothelial function in both animal and human models in the field of certain cardiovascular diseases. 12 13 Treatment with l -arginine has improved endothelial function in patients with hypercholesterolemia and CHF. 41 42 However, the efficacy of l -arginine has not been tested in patients with CAR and ISH. Also, little is known about ARB (losartan) efficacy regarding the issues of CAR and ISH. Since the RAAS is usually stimulated in CAR, 43 it appears that angiotensin II inhibitor (losartan) therapy may be beneficial for those with enhanced RAAS activity. 7 In this study, statistically significant beneficial changes in echocardiographic parameters of LV volume, LVMI, and EF, and clinical parameters of SBP and ED were evident in the L + A group versus the L group ( p <0.01 in all comparisons) ( Table 5 ). In other words, our results showed a beneficial effect in both echocardiographic and clinical data in the L + A treatment group compared with the L-treated group. These changes may be due to the improved vascular endothelial function achieved with l -arginine and by losartan's unloading effect to decrease LV mass and limit volume increase. We hypothesize that the co-administrative strategy (ARB + l -arginine) can provide a beneficial approach for afterload reduction in the natural history of CAR and ISH. However, due to the limitation that our study population was relatively small, future studies with larger volumes of patients are needed to make definitive conclusions.
In this study, Tables 3 and 4 show differences in echocardiographic and hemodynamic data in three stages for the L + A group and the L-only group; however, they were not adjusted for other potential confounders. However, Table 5 shows the differences in data of echocardiographic and hemodynamic variables between the L group and the L + A group after 6-month and 12-month treatment, using multivariate analysis. After adjusting for baseline, age, and sex using multivariate analysis, this study demonstrated that differences existed in the data between the two groups after 12-month treatment, but not after 6-month treatment ( Table 5 ).
Previous and Recent Studies on Vasodilator Therapy
LV unloading agents may increase the myocardial efficiency and limit the stimulus to hypertrophy by decreasing LV wall stress. 1 3 In 1990, Scognamiglio et al 4 presented the results of a 12-month, randomized, double-blind, placebo-controlled trial of nifedipine in 72 asymptomatic patients with severe CAR. They demonstrated a significant reduction in LVEDVI and LVMI and an increase in LVEF. Greenberg et al 5 showed that 24 months of treatment with hydralazine reduced the LV volume overload in CAR and suggested that such therapy may have a beneficial effect in the natural history of CAR. These two trials suggest the potential value of long-term treatment with an arteriolar dilator (nifedipine and hydralazine) in asymptomatic patients with CAR. Reske et al 43 first demonstrated that an ACEI-captopril mediated a decrease in regurgitant volume and suppressed the RAAS in patients with CAR. Subsequently, our own study demonstrated that patients with asymptomatic aortic regurgitation who were treated with enalapril for 12 months had a significant reduction in LVEDVI, LVESVI, and LVMI compared with those on hydralazine therapy. 3
The RAAS may play an important role in the regulation of cardiac myocyte growth. 44 It is conceivable that the modulation of RAAS by ACEI and ARB has beneficial effects in cardioprotection, vasodilation, decreased LV afterload, and natriuresis. 3 7 44 Both losartan and l -arginine have pharmacologic effects that can influence reduction of LV afterload; the early co-administrative therapy with l -arginine and losartan was, therefore, considered a reasonable strategy in asymptomatic patients with CAR and ISH.
Comparison of Ultrasonic and Hemodynamic Changes
MWS is a factor that strongly influences LV function in patients with CAR and ISH, and a sustained unloading action may be important in delaying structural damage of the myocardium. 3 7 45 46 LV dilatation, LV mass, and LVEF are important predictors of the need for AVR 1 4 and, when severely affected, can limit the reversibility of LV dysfunction after surgery. 1 2 3 Thus, it seems rational that early treatment resulting in sustained reductions in LVEDI, LVESVI, and mass, as well as improvements of LVEF and ED, may limit the development of irreversible structural myocardial lesions 3 4 6 and delay the need of AVR. 3 29 30 This study demonstrates that treatment with L + A achieved a better decrease in LVESVI, LVEDVI, LVMI, and SBP in addition to producing a better effect on ED and LVEF compared with losartan alone ( p < 0.01 in all comparisons) when using multivariate linear regression analysis. The results of this trial in asymptomatic patients with CAR and ISH showed that long-term losartan + l -arginine unloading therapy favorably affects LV dimensions, hypertrophy, volume, and function in addition to improved ED compared with losartan-only therapy. In other words, the co-administrative strategy achieved better results in terms of echocardiographic and hemodynamic variables.
Rationale of Impact after Adding L-Arginine to Losartan
An experiment using murine models found that following surgical induction of AR, the AR mice developed a greater degree of atherosclerotic plague along the descending thoracic and abdominal aorta compared with the control mice (without AR). This experiment demonstrated that AR did have a positive correlation with atherosclerosis. 47 In addition, emerging data has reported that oxidative stress and vascular inflammation may contribute to the pathogenesis of hypertension. 48 Current evidence suggests that hypertension and endothelial dysfunction are integrally related. 48 This study enrolled AR and ISH patients, as both AR and hypertension have an affirmative relationship with atherosclerosis.
A previous study reported that losartan can improve the endothelial dysfunction of resistance arteries in patients with essential hypertension. 49 Additionally, losartan has been shown to prevent stroke and improve cerebral artery smooth muscle and endothelial function. 50 l -Arginine supplementation was also shown to attenuate the endothelial dysfunction associated with coronary heart disease 51 and some forms of animal hypertension. 52 53 Another study found that a moderate increase in dietary l -arginine significantly lowered BP and had positive effects on renal function and carbohydrate metabolism in healthy volunteers. 13 From the above evidence, we believe that treatment with losartan together with l -arginine is likely beneficial to patients with hypertension-related endothelial dysfunction. Our study demonstrates that this co-administration strategy had considerable improvement in SBP, LV function, LV volume and mass, and ED in contrast to ARB drug therapy alone ( Table 5 ). Our results support the hypothesis that the l -arginine-NO pathway can be reactivated by long-term oral supplementation of l -arginine and losartan treatment in patients with CAR and ISH.
Conclusion
This study demonstrated that both losartan and l -arginine treatments are capable of decreasing LV MWS and improving LV performance. However, it was only the combination strategy(L + A) that achieved a better clinical efficacy demonstrating considerable and sustained LV volume and mass regression over a 12-month period in patients with CAR and ISH. Our study also demonstrates that l -arginine as an adjunctive therapy can enhance losartan efficacy. It indicates that the early unloading co-administrative strategy has greater potential to favorably influence the natural history of asymptomatic patients with CAR and ISH.
Funding Statement
Funding An-Fa Institution of Preventive Medicine
AFSR 2010-2
Yuan's General Hospital, Kaohsiung City, Taiwan
YGH17 - 010
Disclosures
All authors have no conflicts of interest to disclose.
Contributed equally to this work.
References
- 1.Mahajerin A, Gurm H S, Tsai T T, Chan P S, Nallamothu B K. Vasodilator therapy in patients with aortic insufficiency: a systematic review. Am Heart J. 2007;153(04):454–461. doi: 10.1016/j.ahj.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Bonow R O, Rosing D R, McIntosh C L. The natural history of asymptomatic patients with aortic regurgitation and normal left ventricular function. Circulation. 1983;68(03):509–517. doi: 10.1161/01.cir.68.3.509. [DOI] [PubMed] [Google Scholar]
- 3.Lin M, Chiang H T, Lin S L. Vasodilator therapy in chronic asymptomatic aortic regurgitation: enalapril versus hydralazine therapy. J Am Coll Cardiol. 1994;24(04):1046–1053. doi: 10.1016/0735-1097(94)90868-0. [DOI] [PubMed] [Google Scholar]
- 4.Scognamiglio R, Fasoli G, Ponchia A, Dalla-Volta S. Long-term nifedipine unloading therapy in asymptomatic patients with chronic severe aortic regurgitation. J Am Coll Cardiol. 1990;16(02):424–429. doi: 10.1016/0735-1097(90)90596-h. [DOI] [PubMed] [Google Scholar]
- 5.Greenberg B, Massie B, Bristow J D. Long-term vasodilator therapy of chronic aortic insufficiency. A randomized double-blinded, placebo-controlled clinical trial. Circulation. 1988;78(01):92–103. doi: 10.1161/01.cir.78.1.92. [DOI] [PubMed] [Google Scholar]
- 6.Scognamiglio R, Rahimtoola S H, Fasoli G, Nistri S, Dalla Volta S. Nifedipine in asymptomatic patients with severe aortic regurgitation and normal left ventricular function. N Engl J Med. 1994;331(11):689–694. doi: 10.1056/NEJM199409153311101. [DOI] [PubMed] [Google Scholar]
- 7.Willenheimer R, Dahlöf B, Rydberg E, Erhardt L. AT1-receptor blockers in hypertension and heart failure: clinical experience and future directions. Eur Heart J. 1999;20(14):997–1008. doi: 10.1053/euhj.1999.1547. [DOI] [PubMed] [Google Scholar]
- 8.Chobanian A V, Bakris G L, Black H R. The Seventh Report of the Joint National Committee Blood Pressure: the JNC 7 report. JAMA. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 9.Plante E, Lachance D, Beaudoin J. Comparative study of vasodilators in an animal model of chronic volume overload caused by severe aortic regurgitation. Circ Heart Fail. 2009;2(01):25–32. doi: 10.1161/CIRCHEARTFAILURE.108.801548. [DOI] [PubMed] [Google Scholar]
- 10.Roberts P A, Lin A CW, Cowan B R, Young A A, Stewart R. Comparison of effects of losartan and metoprolol on left ventricular and aortic function at rest and during exercise in chronic aortic regurgitation. Int J Cardiovasc Imaging. 2018;34(04):615–624. doi: 10.1007/s10554-017-1268-y. [DOI] [PubMed] [Google Scholar]
- 11.Koifman B, Topilski I, Megidish R. Effects of losartan + L-arginine on nitric oxide production, endothelial cell function, and hemodynamic variables in patients with heart failure secondary to coronary heart disease. Am J Cardiol. 2006;98(02):172–177. doi: 10.1016/j.amjcard.2006.01.085. [DOI] [PubMed] [Google Scholar]
- 12.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329(27):2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 13.Siani A, Pagano E, Iacone R, Iacoviello L, Scopacasa F, Strazzullo P.Blood pressure and metabolic changes during dietary L-arginine supplementation in humans Am J Hypertens 200013(5 Pt 1):547–551. [DOI] [PubMed] [Google Scholar]
- 14.Böger R H, Bode-Böger S M. The clinical pharmacology of L-arginine. Annu Rev Pharmacol Toxicol. 2001;41:79–99. doi: 10.1146/annurev.pharmtox.41.1.79. [DOI] [PubMed] [Google Scholar]
- 15.Böger R H, Bode-Böger S M, Frölich J C. The L-arginine-nitric oxide pathway: role in atherosclerosis and therapeutic implications. Atherosclerosis. 1996;127(01):1–11. doi: 10.1016/s0021-9150(96)05953-9. [DOI] [PubMed] [Google Scholar]
- 16.American Society of Echocardiography . Zoghbi W A, Enriquez-Sarano M, Foster E. Recommendations for evaluation of the severity of native valvular regurgitation with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16(07):777–802. doi: 10.1016/S0894-7317(03)00335-3. [DOI] [PubMed] [Google Scholar]
- 17.Lang R M, Badano L P, Mor-Avi V. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(01):1–3.9E15. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Quinones M A, Mokotoff D M, Nouri S, Winters W L, Jr, Miller R R. Noninvasive quantification of left ventricular wall stress. Validation of method and application to assessment of chronic pressure overload. Am J Cardiol. 1980;45(04):782–790. doi: 10.1016/0002-9149(80)90122-8. [DOI] [PubMed] [Google Scholar]
- 19.Chamber Quantification Writing Group ; American Society of Echocardiography's Guidelines and Standards Committee ; European Association of Echocardiography . Lang R M, Bierig M, Devereux R B. Recommendations for chamber quantification: a report from the American Society of Echocardiography's guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Chirinos J A, Segers P, De Buyzere M L. Left ventricular mass: allometric scaling, normative values, effect of obesity, and prognostic performance. Hypertension. 2010;56(01):91–98. doi: 10.1161/HYPERTENSIONAHA.110.150250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yano Y, Stamler J, Garside D B. Isolated systolic hypertension in young and middle-aged adults and 31-year risk for cardiovascular mortality: the Chicago Heart Association Detection Project in Industry study. J Am Coll Cardiol. 2015;65(04):327–335. doi: 10.1016/j.jacc.2014.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bavishi C, Goel S, Messerli F H. Isolated systolic hypertension: an update after SPRINT. Am J Med. 2016;129(12):1251–1258. doi: 10.1016/j.amjmed.2016.08.032. [DOI] [PubMed] [Google Scholar]
- 23.Mancia G, Fagard R, Narkiewicz K. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2013;34(28):2159–2219. doi: 10.1093/eurheartj/eht151. [DOI] [PubMed] [Google Scholar]
- 24.Whelton P K, Carey R M, Aronow W S. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;138(17):e484–e594. doi: 10.1161/CIR.0000000000000596. [DOI] [PubMed] [Google Scholar]
- 25.Perry G J, Helmcke F, Nanda N C, Byard C, Soto B. Evaluation of aortic insufficiency by Doppler color flow mapping. J Am Coll Cardiol. 1987;9(04):952–959. doi: 10.1016/s0735-1097(87)80254-1. [DOI] [PubMed] [Google Scholar]
- 26.Scognamiglio R, Negut C, Palisi M, Fasoli G, Dalla-Volta S. Long-term survival and functional results after aortic valve replacement in asymptomatic patients with chronic severe aortic regurgitation and left ventricular dysfunction. J Am Coll Cardiol. 2005;45(07):1025–1030. doi: 10.1016/j.jacc.2004.06.081. [DOI] [PubMed] [Google Scholar]
- 27.Acar J, Michel P L, Luxereau P. How to manage patients with severe left ventricular dysfunction and valvular regurgitation. J Heart Valve Dis. 1996;5(04):421–429. [PubMed] [Google Scholar]
- 28.Klodas E, Enriquez-Sarano M, Tajik A J, Mullany C J, Bailey K R, Seward J B. Optimizing timing of surgical correction in patients with severe aortic regurgitation: role of symptoms. J Am Coll Cardiol. 1997;30(03):746–752. doi: 10.1016/s0735-1097(97)00205-2. [DOI] [PubMed] [Google Scholar]
- 29.Dujardin K S, Enriquez-Sarano M, Schaff H V, Bailey K R, Seward J B, Tajik A J. Mortality and morbidity of aortic regurgitation in clinical practice. A long-term follow-up study. Circulation. 1999;99(14):1851–1857. doi: 10.1161/01.cir.99.14.1851. [DOI] [PubMed] [Google Scholar]
- 30.Henry W L, Bonow R O, Rosing D R, Epstein S E. Observations on the optimum time for operative intervention for aortic regurgitation. II. Serial echocardiographic evaluation of asymptomatic patients. Circulation. 1980;61(03):484–492. doi: 10.1161/01.cir.61.3.484. [DOI] [PubMed] [Google Scholar]
- 31.Scognamiglio R, Fasoli G, Dalla Volta S. Progression of myocardial dysfunction in asymptomatic patients with severe aortic insufficiency. Clin Cardiol. 1986;9(04):151–156. doi: 10.1002/clc.4960090404. [DOI] [PubMed] [Google Scholar]
- 32.Henry W L, Bonow R O, Borer J S. Observations on the optimum time for operative intervention for aortic regurgitation. I. Evaluation of the results of aortic valve replacement in symptomatic patients. Circulation. 1980;61(03):471–483. doi: 10.1161/01.cir.61.3.471. [DOI] [PubMed] [Google Scholar]
- 33.American College of Cardiology/American Heart Association Task Force on Practice Guidelines . Nishimura R A, Otto C M, Bonow R O. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63(22):2438–2488. doi: 10.1016/j.jacc.2014.02.537. [DOI] [PubMed] [Google Scholar]
- 34.Working Group on Valvular Heart Disease . Iung B, Gohlke-Bärwolf C, Tornos P. Recommendations on the management of the asymptomatic patient with valvular heart disease. Eur Heart J. 2002;23(16):1253–1266. doi: 10.1053/euhj.2002.3320. [DOI] [PubMed] [Google Scholar]
- 35.Tsioufis C, Stougiannos P, Kakkavas A. Relation of left ventricular concentric remodeling to levels of C-reactive protein and serum amyloid A in patients with essential hypertension. Am J Cardiol. 2005;96(02):252–256. doi: 10.1016/j.amjcard.2005.03.054. [DOI] [PubMed] [Google Scholar]
- 36.Schillaci G, Verdecchia P, Porcellati C, Cuccurullo O, Cosco C, Perticone F. Continuous relation between left ventricular mass and cardiovascular risk in essential hypertension. Hypertension. 2000;35(02):580–586. doi: 10.1161/01.hyp.35.2.580. [DOI] [PubMed] [Google Scholar]
- 37.Lin M. Reappraisal of L-arginine in the field of cardiovascular medicine for clinical applications and pharmacology: an update. J Intern Med Taiwan. 2007;18:225–235. [Google Scholar]
- 38.Morris S M., JrEnzymes of arginine metabolism J Nutr 2004134(10, Suppl)2743S–2747S, discussion 2765S–2767S. [DOI] [PubMed] [Google Scholar]
- 39.Jabecka A, Ast J, Bogdaski P. Oral L-arginine supplementation in patients with mild arterial hypertension and its effect on plasma level of asymmetric dimethylarginine, L-citruline, L-arginine and antioxidant status. Eur Rev Med Pharmacol Sci. 2012;16(12):1665–1674. [PubMed] [Google Scholar]
- 40.Ast J, Jabłecka A, Bogdański P, Smolarek I, Krauss H, Chmara E. Evaluation of the antihypertensive effect of L-arginine supplementation in patients with mild hypertension assessed with ambulatory blood pressure monitoring. Med Sci Monit. 2010;16(05):CR266–CR271. [PubMed] [Google Scholar]
- 41.Drexler H, Zeiher A M, Meinzer K, Just H.Correction of endothelial dysfunction in coronary microcirculation of hypercholesterolaemic patients by L-arginine Lancet 1991338(8782-8783):1546–1550. [DOI] [PubMed] [Google Scholar]
- 42.Hambrecht R, Hilbrich L, Erbs S. Correction of endothelial dysfunction in CHF: additional effect of exercise training and oral L-arginine supplementation. J Am Coll Cardiol. 2000;35(03):706–713. doi: 10.1016/s0735-1097(99)00602-6. [DOI] [PubMed] [Google Scholar]
- 43.Reske S N, Heck I, Kropp J. Captopril mediated decrease of aortic regurgitation. Br Heart J. 1985;54(04):415–419. doi: 10.1136/hrt.54.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carey R M, Siragy H M. Newly recognized components of the renin-angiotensin system: potential roles in cardiovascular and renal regulation. Endocr Rev. 2003;24(03):261–271. doi: 10.1210/er.2003-0001. [DOI] [PubMed] [Google Scholar]
- 45.Grinstead W C, Young J B.The myocardial renin-angiotensin system: existence, importance, and clinical implications Am Heart J 1992123(4 Pt 1):1039–1045. [DOI] [PubMed] [Google Scholar]
- 46.Katz A M. Angiotensin II: hemodynamic regulator or growth factor? J Mol Cell Cardiol. 1990;22(07):739–747. doi: 10.1016/0022-2828(90)90086-h. [DOI] [PubMed] [Google Scholar]
- 47.Hoi Y, Zhou Y Q, Zhang X, Henkelman R M, Steinman D A. Correlation between local hemodynamics and lesion distribution in a novel aortic regurgitation murine model of atherosclerosis. Ann Biomed Eng. 2011;39(05):1414–1422. doi: 10.1007/s10439-011-0255-z. [DOI] [PubMed] [Google Scholar]
- 48.Dharmashankar K, Widlansky M E. Vascular endothelial function and hypertension: insights and directions. Curr Hypertens Rep. 2010;12(06):448–455. doi: 10.1007/s11906-010-0150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schiffrin E L, Park J B, Intengan H D, Touyz R M. Correction of arterial structure and endothelial dysfunction in human essential hypertension by the angiotensin receptor antagonist losartan. Circulation. 2000;101(14):1653–1659. doi: 10.1161/01.cir.101.14.1653. [DOI] [PubMed] [Google Scholar]
- 50.Vacher E, Richer C, Giudicelli J-F. Effects of losartan on cerebral arteries in stroke-prone spontaneously hypertensive rats. J Hypertens. 1996;14(11):1341–1348. doi: 10.1097/00004872-199611000-00013. [DOI] [PubMed] [Google Scholar]
- 51.Lerman A, Burnett J C, Jr, Higano S T, McKinley L J, Holmes D R., Jr Long-term L-arginine supplementation improves small-vessel coronary endothelial function in humans. Circulation. 1998;97(21):2123–2128. doi: 10.1161/01.cir.97.21.2123. [DOI] [PubMed] [Google Scholar]
- 52.Chen P Y, Sanders P W. L-arginine abrogates salt-sensitive hypertension in Dahl/Rapp rats. J Clin Invest. 1991;88(05):1559–1567. doi: 10.1172/JCI115467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patel A, Layne S, Watts D, Kirchner K A. L-arginine administration normalizes pressure natriuresis in hypertensive Dahl rats. Hypertension. 1993;22(06):863–869. doi: 10.1161/01.hyp.22.6.863. [DOI] [PubMed] [Google Scholar]


