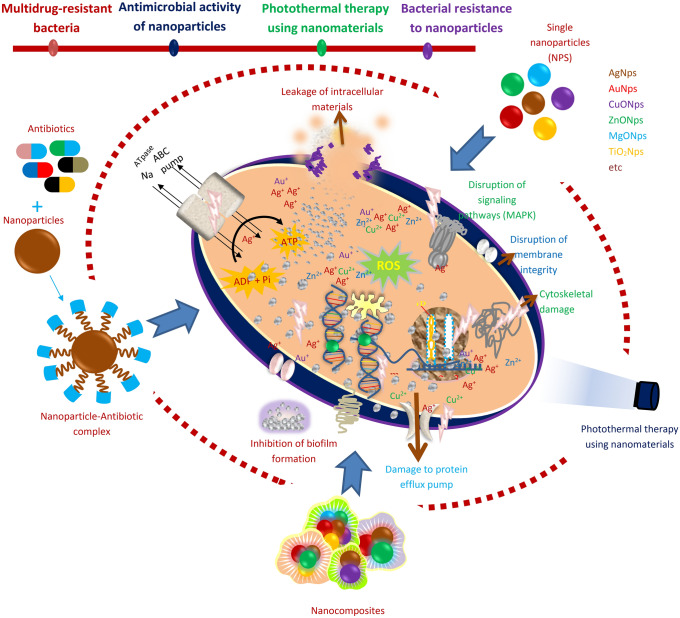
Abstract
Resistance to antimicrobial agents has been alarming in recent years and poses a huge public health threat globally according to the WHO. The increase in morbidity and mortality resulting from microbial infections has been attributed to the emergence of multidrug-resistant microbes. Associated with the increase in multidrug resistance is the lack of new and effective antimicrobials. This has led to global initiatives to identify novel and more effective antimicrobial agents in addition to discovering novel and effective drug delivery and targeting methods. The use of nanoparticles as novel biomaterials to fully achieve this feat is currently gaining global attention. Nanoparticles could become an indispensable viable therapeutic option for treating drug-resistant infections. Of all the nanoparticles, the metals and metal oxide nanoparticles appear to offer the most promise and have attracted tremendous interest from many researchers. Moreover, the use of nanomaterials in photothermal therapy has received considerable attention over the years. This review provides current insight on antimicrobial resistance as well as the mechanisms of nanoparticle antibacterial activity. It offers an in-depth review of all the recent findings in the use of nanomaterials as agents against multi-resistant pathogenic bacteria. Also, nanomaterials that can respond to light stimuli (photothermal therapy) to kill microbes and facilitate enhanced drug delivery and release are discussed. Moreover, the synergistic interactions of nanoparticles with antibiotics and other nanomaterials, microbial adaptation strategies to nanoparticles, current challenges, and future prospects were extensively discussed.
Graphic abstract
Keywords: Nanoparticles, Antimicrobial resistance, Conjugated nanoparticles/nanocomposites, Photothermal therapy, Antibacterial activity, Bacterial resistance to nanoparticles
Introduction
Nanoparticles are biomaterials with dimensions between 1 and 100 nm (nm). Considerable attention has been given to nanomaterials due to their wide application in agriculture, pharmaceuticals, consumer products, transportation, energy, cosmetics, and more importantly as antimicrobial agents. They are currently regarded as viable substitutes and/or supplements to existing antimicrobials (Li et al. 2017). Generally, nanoparticles’ antimicrobial or biomedical properties depend on their methods of synthesis and formulation conditions, such as the nature of the reducing agent, temperature, concentration, and solvent type (Lee et al. 2019; Qing et al. 2018; Kedziora et al. 2018). The actions and activity of these nanoparticles also mostly depend on their chemical composition, shape and size (Shobha et al. 2014). Several conditions and parameters need to be modified and varied to produce a nanoparticle with effective size, distribution, morphologies, and yield. Factors like temperature, pH and metal ion concentration, cell age, and time of reaction also affect the antimicrobial activity of nanoparticles. For an increase in their activity, nanoparticles can be coated with several coating agents (Jaworski et al. 2018). Surface stabilizers, surfactants, polymers and oligonucleotides can be used as coating agents. These capping agents help to stabilize the nanoparticles against agglomeration.
Nanoparticles can either be organic (e.g., liposomes, polymeric, micelles, ferritin) or inorganic (e.g., metal nanoparticles). Both types of nanoparticles have been influential in treating several health conditions (Anselmo and Mitragotri 2016). Organic nanoparticles have been used to increase the bioavailability of drugs, enhance efficient drug delivery and improve antibacterial activity (Yetisgin et al. 2020). They have also been used in treating fungal infection (Mba and Nweze 2020) and have shown promises against viral infections [e.g., severe acute respiratory syndrome 2 (SARS-CoV-2)] infection (Mba et al. 2021). Currently, available nanoparticulate antibacterial systems include liposomes, polymeric NPs, micelles, solid lipid NPs (SLNs), nanostructured lipid carriers (NLCs), nanocapsules, nanotubes, quantum dots, dendrimers, emulsions, nanogels, and vesicles. They are nano-scale drug delivery systems that offer a slow-release and the delivery of drugs to the targeted cells.
The metallic nanoparticles appear to be the most promising. They exhibit diverse activities against several multi-drug resistant pathogens (Liao et al. 2019; Rasheed et al. 2017; Mba and Nweze 2020). The most widely studied metal nanoparticles are silver nanoparticles (AgNps) and gold nanoparticles (AuNps). Metallic oxide nanoparticles with proven antibacterial activities include copper oxide nanoparticles (CuONps), zinc oxide nanoparticles (ZnONps), titanium oxide nanoparticles (TiO2Nps), and magnesium oxide nanoparticles (MgONps). Others include calcium oxide nanoparticles (CaONps), iron oxide nanoparticles (Fe2O3NPS) and manganese oxide (MnO2Nps). AgNps is the most studied and most widely used among all the nanoparticles (Mohler et al. 2018). Nanoparticles can also be combined with antibiotics and other nanomaterials for improved antibacterial activity.
Moreover, the light-responsive technique specifically has been used in the development of drugs. It has also found application in the design of drug carrier systems. The light-responsive structure can easily be regulated, and it has low invasiveness (Thang et al. 2019). Its mechanism of activity is based on the alteration of the light-sensitive molecules when stimulated by light, thus enabling the release of the encapsulated or conjugated drug (Linsley and Wu 2017). Over the years, the use of nanomaterials in photothermal therapy has received considerable attention. Several organic and polymeric nanoparticles have been reported to exhibit inherent photothermal ability (Zhao et al. 2018).
Therefore, in the face of increasing resistance to frontline antimicrobial agents and the rise in infections mostly caused by multidrug-resistant organisms, researchers have made efforts to develop alternative therapeutic approaches. The application of nanotechnology appears to be a viable solution due to the distinctive properties of nanomaterials. This review provides an important update on the issue of antimicrobial resistance. Based on recent literature, the mechanism of antimicrobial activity displayed by nanoparticles are discussed. Recent investigations reporting the activity of nanoparticles against drug-resistant bacteria are also highlighted. Moreover, insight on the synergistic activity of nanoparticles with antibiotics and other nanomaterials are also discussed extensively. Also, nanomaterials that can respond to light stimuli (photothermal therapy) to kill microbes and facilitate enhanced drug delivery and release are discussed. Finally, the recently emerging adaptive response tactics of bacteria to nanoparticles and current challenges in the use of nanoparticles as well as their future prospects are also presented.
The burden of antibiotic resistance and the need for antibacterial nanomaterials
The response of microbes to antimicrobial attack is an important illustration of adaptation and the pinnacle of evolution. Studies have shown that microbial infection is responsible for about 3 million deaths in developing countries annually. It also causes about 10 million deaths annually, mostly in the tropical countries (Dye 2014; Global Health Estimate 2016; Fenollar and Mediannikov 2018) despite the vast advances in diagnoses and therapeutics that have been achieved over the years. Developed countries are not spared either. Antimicrobial drug resistance has become a major global public health issue in medicine. In the US alone, the estimated economic burden associated with multidrug-resistant (MDR) microbes is about $20 billion dollars yearly (Munita and Arias 2016). The issue of resistance often leaves clinicians with no reliable alternative to manage infected patients. Also, the emergence of multidrug resistant bacteria and super bacteria (bacteria resistant to almost all antibiotics) has compounded the problem. This is broadly associated with the excessive use of antibiotics which subsequently facilitates the generation and evolution of strains with genotypic and phenotypic diversities (Wang et al. 2017a, b).
Therefore, emergence of MDR and extensively-drug resistant (XDR) bacteria persist as a critical challenge in public health as it is associated with high mortality, morbidity, and high cost of treatment (Sanchez et al. 2013; Roca et al. 2015). This is further exacerbated by the ability of several bacterial strains to form biofilms, which is associated with about 65–80% of human infections (Lebeaux et al. 2013). Cells in biofilms are usually 100–1000 times less susceptible to antibiotics than planktonic cells (Saginur et al. 2006). The increase in resistance of drugs by biofilms is due to several factors: decrease in drug penetration across the extracellular matrix, reduction in drug concentration, reduction in metabolic rates of bacteria, and transfer of resistant genes (Hall-Stoodley et al. 2004; Hall and Mah 2017). MDR S. aureus and P. aeruginosa are the leading causes of chronic biofilm-associated infections worldwide, often characterized by a slower rate of wound healing, failure of catheters, and prolonged hospital stays (Nathwani et al. 2014; Sanchez et al. 2013).
Furthermore, antimicrobial drug resistance is among the three most important global public health threats identified in the twenty-first century by the World Health Organization (WHO). The ESKAPE group (Enterococcus, Staphylococcus, Klebsiella, Actinobacter, Pseudomonas, Enterobacter) are the most critical and have raised the most concerns. They are all associated with a high mortality rate. Based on reports from WHO, about 80% of the MDR or XDR microbes are due to misuse and overuse of antibiotics, and these infections are associated with severe adverse effects. Currently, there are limited therapeutic and prevention options due to the expansion of MDR bacteria and other resistant pathogens. There is a need for alternative therapeutic options for microbial pathogens. The failure of most antibiotics necessitates the search for better treatment options. Also, for effective infection control, drugs that can treat infection with the smallest possible dose is an appropriate approach (Morgan et al. 2011; WHO 2015).
Nanotechnology is a promising therapeutic strategy due to its high efficacy and therapeutic index against microbes (Hussain et al. 2018). Nanoparticles offer a viable alternative in the management of most bacterial infections, especially those involving multi-drug resistant organisms. Nanoparticles can be used singly or combined with antibiotics providing excellent synergistic effects. Nanomaterials that can respond to diverse endogenous and exogenous stimuli to killed microbes and also facilitate enhanced drug deliver and release are promising strategies (Hsiao et al. 2015; Qiu et al. 2018).
Metallic and metal oxide nanoparticles are promising antibacterial agents
AgNps are currently seen as the next generation antibiotics. This is because of their high effectiveness in inhibiting microorganisms. Currently, AgNps are the leading nanoparticles among all the commercialized nanomaterials (Arya et al. 2019). Research into their use as antimicrobial agents has intensified over the years due to their reduced toxicity when compared to other nanoparticles. Attachment and penetration of the AgNps nanoparticles to microbial membrane surface is usually the first step in its cytotoxic mechanism (Singh et al. 2015). The damage to internal components is caused by the released Ag+ ions which trigger the generation of ROS and subsequent oxidative stress induction which affect the Na+/K+ ATPase pump and signal transduction pathways (Singh et al. 2015; Flores-Lopez et al. 2019). Ag+ ions and AgNps also interact with DNA (phosphorus-containing compounds) leading to protein inactivation and subsequent cell death. In fact, sulphur, chlorine, thiols, and oxygen can interact with a great effect on Ag+ ion release (Maurer and Meyer 2016).
Also, the rate at which this Ag + ion is released is largely affected by the size (Sriram et al. 2012; Abuayyash et al. 2018). Small size AgNps can easily penetrate the cell wall. They also alter the structural integrity and membrane architecture causing an increase in permeability and subsequent cell apoptosis. The type of bacterial species also influences AgNps activity. This is because of the different cell wall composition, thickness, and arrangement (Tamayo et al. 2014). The precise antimicrobial activity of AgNps beside the generation of ROS is associated with the cell wall and plasma membrane damage according to Hamouda et al. (2019). This is because of protein inactivation and membrane lipid peroxidation. These activities modify the structural membrane integrity leading to the disorder of the transport proteins. It also causes potassium leakage.
AuNps are among the most widely researched nanoparticles with good antimicrobial activity (Shamaila et al. 2016; Tao 2018; Bilal et al. 2017). AuNps exhibit several shapes including triangular, spherical, hexagonal, and even rod-like shapes (Abdel-Raouf et al. 2017). The triangular-shaped AuNps as previously reported by Smitha and Gopchandran (2013) exhibit strong antibacterial activity against several bacteria compared to spherically-shaped AuNps. AuNps adhere to the membrane via electrostatic interaction and disrupt membrane integrity (Kundu 2017). They can cause leakage of intracellular components by generating holes in the membrane. AuNps can bind to DNA inhibiting replication and transcription. They can also aggregate with biofilm formed by microbes. Interactions between AuNps also provoked the formation of ROS essential for cell death. They alter membrane potential and decrease ATP synthase activities thereby reducing several metabolic activities. AuNps just like AgNps disrupt cell membrane integrity and structure (Rattanata et al. 2016). AuNps prevent rRNA from binding to its subunits, thus preventing translation (Cui et al. 2012). AuNps also interact with sulphur or phosphorus-containing nucleotides. AuNps supplemented with antibiotics usually shows strong antibacterial activity. According to a study by Brown et al. (2012), ampicillin integrated with AuNp was strongly effective against bacteria (P. aeruginosa, E. coli, Enterobacter aerogenes and MRSA) resistant to ampicillin. The AuNp-AMP complex disrupts and inhibits the transmembrane pump catalyzing drug efflux. The AuNp-AMP also overwhelmingly neutralizes the high β-lactamase expressed by the bacteria.
Furthermore, copper/copper oxide nanoparticles (CuNps/CuONps) exhibit antimicrobial activity (El-Batal et al. 2017; Asemani and Anarjan 2019). Available report suggests that its antimicrobial activity is derived via the electrostatic attraction between the nanoparticle and the cell (Bogdanovic et al. 2014). The Cu2+ ion can also bypass the lipid bilayer and gain access into the cell. Upon penetration into the cell, it triggers the production of ROS. Lipid peroxidation and protein oxidation is also evident (DeAlba-Montero et al. 2017). The ability of Cu to alternate between + 1 and + 2 oxidation state is also responsible for its antimicrobial activity. A recent investigation reported that CuONps can interact with amino acids with a great influence on its bacterial activity (Badetti et al. 2019).
ZnO nanoparticle is also a promising antibacterial agent (Bhuyan et al. 2015). It was previously reported that surface coating of ZnONps could prevent their interactions with biological fluid. However, Pranjali et al. (2019) found that PEGylated ZnONps strongly bind and interact with peritoneal dialysis (PD) fluid, lactic and citric acids leading to agglomeration. In addition, a drastic decrease in the bacterial inhibition effect was observed for both the ZnONps and the PEG-coated ZnONps dispersed in biological fluid. ZnONps release Zn2+ ions when in contact with the microbial cell. The Zn2+ ions disrupt the cell membrane. The interaction between the ions and several intracellular components further causes more harm to the cell (Li et al. 2011). In addition, Zn2+ causes the generation of ROS (Kumar et al. 2011; Singh et al. 2020). Recently, Nejabatdoust et al. (2019) reported that ZnONPs conjugated with thiosemicarbzide and functionalized by glutamic acid modifies the expression of efflux pump genes in multiple drug-resistant S. aureus.
TiO2Np produces ROS which is responsible for cell membrane damage and disruption of oxidative phosphorylation (Singh et al. 2018). TiO2Np also inactivates signaling pathways, reduces the co-enzyme-independent respiratory network as well as the take-up and transport of Fe and P. It also decreases the biosynthesis and degradation of heme group (Foster et al. 2011). Its activity is also photo dependent. The generation of free radicals has also been reported (Wu et al. 2010). Evidence is also available that it can damage the peptidoglycan, lipopolysaccharide, in addition to the phospholipid bilayer (Liu et al. 2010). Similarly, MgONp can produce ROS that is highly detrimental to cells (Krishnamoorthy et al. 2012; He et al. 2016). Nguyen et al. (2018) reported that MgONps reduce biofilm forming ability of S. epidermidis and damages the membrane of E. coli causing cell apoptosis. The authors suggested that the production of ROS, Ca2+ concentrations and quorum sensing are the mechanisms contributing to their antimicrobial activity. In addition, the MgONp cell surface attachment damages membrane integrity and cause leakage of intracellular components. In a recent study, MgONps and MnONps were biosynthesized using Matricaria chamomilla L extract. The results showed that the nanoparticles invade the cells and damage the membrane. This led to the leakage of intracellular cytoplasmic content (Ogungemi et al. 2019). According to Ogungemi et al. (2019) MgONp antimicrobial activity is also achieved by the production of Mg2+ ion, cell membrane interaction and pH changes.
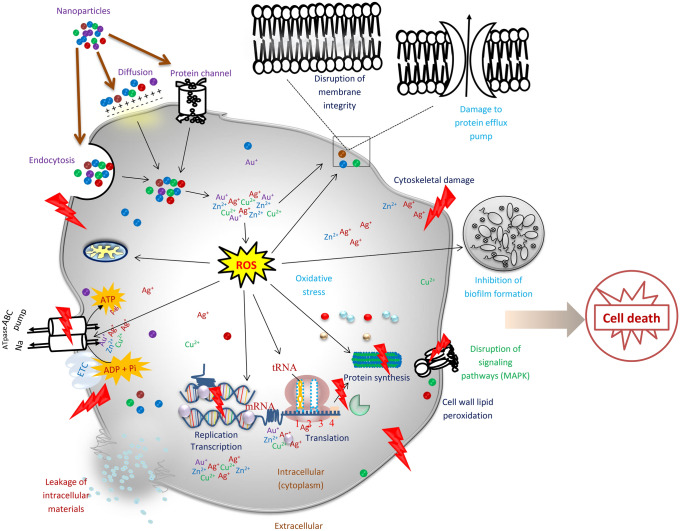
Also, a recent study showed that Fe3O4Nps reduce H+-flux through bacterial membrane. Fe3O4Nps specifically inhibit ATP-associated metabolism. There was also a decrease in membranes associated H2 production (Gabrielyan et al. 2019). Several researchers have investigated the antimicrobial activity of biosynthesized FeNps and Fe2O3Nps (Sathishkumar et al. 2018; Jagathesan and Rajiv 2018; Madivoli et al. 2019). Studies are also available reporting the antimicrobial potentials of calcium oxide nanparticles (Butt et al. 2015; Balaganesh et al. 2018; Pasupathy and Rajamanickam 2019; Gurav et al. 2020). Figure 1 summarizes the mechanisms of antibacterial activity of nanoparticles while Table 1 shows the recent studies reporting the antimicrobial activity of nanoparticles.
Fig. 1.
Mechanism of nanoparticles activity against bacteria
Table 1.
Recent studies on nanoparticles showing the source, shape and size, organisms tested, antimicrobial potential and other key findings (cytotoxicity and reported mechanisms)
| Nps | Source | Shape and size | Bacteria isolates tested | Antibacterial activity (IZD/MIC/MBC) and other key findings | Reference |
|---|---|---|---|---|---|
| AgNps | Acacia rigidula plant extract |
Spherical Size distribution:8–66 nm with mean of 22.46 nm Diameter size: 15–25 nm |
E. coli, P. aeruginosa, Clinically MDR strain of P. aeruginosa and B. subtilis |
In vitro: 62.5, 15.6, 7.8, and 0.5 ppm for E. coli, P. aeruginosa, multi-drug resistant P. aeruginosa and B. subtilis respectively In vivo: antimicrobial effect against the resistant pathogens tested in a murine skin infection model. Effective and safety use of Nps as therapeutic agents in animal models |
Escarcega-Gonzalez et al. (2018) |
| AgNps | Biosynthesized using Pseudoduganella eburnean MAHUQ-39 |
Spherical 8–24 nm |
Multidrug resistant pathogenic microbes S. aureus, P. aeruginosa, E. coli |
MIC of S. aueus and P. aeruginosa were 100 µg/ml respectively. MBC of S. aureus and and P. aeruginosa were 200 and 50 µg/ml respectively Mechanism: structural alterations Disruption of the membrane integrity of strains S. aureus and P. aeruginosa |
Huq (2020) |
| AgNps | Biosynthesized using Sphingobium sp-MAH-11 |
Spherical 7–22 nm |
Drug resistant microbes P. aeruginosa, E. coli, S. aureus |
MIC of E. coli and S. aureus were 6.25 and 50 µg/ml respectively and MBC of E. coli and S. aureus were 25 and 100 µg/ml respectively Mechanism: using E. coli and S. aureus, causes morphological alterations and disrupt membrane integrity of the isolates |
Akter and Huq (2020) |
| AgNps | Green synthetic method and casein hydrolysate as a reducing reagent and NaOH as a catalyst |
Spherical Average sizes: 10 ± 5 nm, 30 ± 5 nm, 60 ± 5 nm, 90 ± 5 nm |
Vibrio natriegens |
MIC and MBC were dose dependent. The smaller the particle the more bacterial damage. MIC ranges from 1 to 11.5 µg/ml and MBC, 1.1–11.7 µg/ml Mechanism: generation of ROS by bacteria and bacteria membrane damage |
Dong et al. (2019) |
| AgNps | Synthesized using aqueous and ethanolic extract of Adrographis paniculata stem |
Spherical Ag-bAgNps- 24.90 nm Et-bAgNps-25.24 nm |
Enteropathogenic E. coli, S. typhi, S. aureus, V. cholerae, E. faecalis, Hafnia alvei, Acninetobacter baumannii, E. coli DH5α, E. coli K12 and B. cereus |
Lowest MIC for both the bAgNps was 0.125 µg. Et-bAgNps had the highest antibacterial activity against S. aureus at 60 µg after 16 h and IZD was 28 mm Cytotoxicity: showed excellent hemocompatibility against human as well as rat RBC. No significant cytoxicity observed when the levels of rat serum ALT, AST, ϒ-GT (liver function biomarkers) and creatinine (kidney function biomarker) were evaluated |
Hossain et al. (2019) |
| AgNps | Synthesized using methanolic extract of Oscillatoria spp. |
Spherical 10 nm |
S. aureus, E. coli 11,775, E. coli 35,218, P. aeruginosa, Citrobacter Spp., Salmonella typhi 14,028, B. cereus |
Effective antibacterial activity against all pathogens with IZD ranging from 1 to 21 mm Antibiofilm: exhibit strong antibiofil activity Cytotoxicity: Using Artemia salina (brine shrimp), it was observed to be insignificant with the highest mortality at 4000 µg/ml and LC50 of 2630.3 µg/ml |
Adebayo-Tayo et al.(2019) |
| AgNps | Synthesized using mycelial extract of endophytic fungus Talaromyces purpureogenus |
Triangular shaped 25 nm |
S. aureus, B. cereus, S. enterica, P. aeruginosa, E. coli |
MIC of 16.12 µg/ml for gram positive and 13.98 µg/ml for gram negative Cytotoxicity: Not toxic to normal NIH3T3 cells. Showed cytotoxicity in A549 cells even at the lowest concentration of 2 µg/ml. cytotoxicity increases with the increase of Nps concentration. IC50 for AgNps and AgNO3 was 376.24 and 250.31 µg/ml respectively. 5.92% of cell apoptosis was induced by the Nps |
Hu et al. (2019) |
| AgNps | Fusarium scirpi (fungi) |
Quasi-spherical 2-20 nm |
Uropathogenic E. coli | MIC of 25 mg/ml. Sub-MIC concentration (7.5 mg/l) was enough to inhibit the pathogen biofilm formation about 97% or produce the disruption of 80% of mature biofilm | Rodriguez-Serrano et al. (2020) |
| AgNps | Aqueous extract of Cyanobacterium oscillatoria |
Spherical 3.30–17.97 nm |
E. coli, B. cereus |
IZD of 22 mm and 20 mm Mechanism: damage to cell membrane, leakage of cytoplasm exterior to cell, Internal diffusing of AgNps to cell, cell disruption, disintegration, Shrinking of protoplasm and, detachment of cellular membrane Cytotoxicity: hemolytic activity showed that it was non-toxic to human RBC in low concentrations |
Hamouda et al., (2019) |
| AgNps | Aqueous extract of black pomegranate peels |
Spherical 32–85 nm |
P. aeruginosa |
Showed strong inhibition against biofilm formation at 0.1–0.5 mg/ml Cytotoxicity: no significant toxicity against L929 cell line at 400 µg/ml |
Habibipour et al. (2019) |
| AgNps | Synthesized by Fusarium solani |
Spherical 13.70 nm |
Multidrug resistant P. aeruginosa and S. aureus |
Showed significant effect against P. aeruginosa (22.4 mm) and MIC of 21.33 µg/ml Mechanism: formation of cracks and pits in the cell wall when nanoparticles were internalized |
El-Sayed and El-Sayed (2020) |
| AgNps | Biosynthesized using Xianghaiensis OF1 strain |
Spherical 64 nm |
P. aeruginosa, M. furfur, B. subtilis, E. coli, S. aureus and K. pneumonia |
MIC of 16, 32, 64, 64, 256, 26 µg/ml respectively Cytotoxicity: in vitro cytotoxicity against mouse fibroblasts and cancer HeLa cell lines showed dose dependent activity. IC50 was found in concentration of 4 and 3.8 µg/ml |
Wypig et al. (2018) |
| AuNps | Aqueous extract of Euprasia officinalis |
Quasi-spherical 49.72 ± 1.2 nm |
P. aeruginosa, E. coli, S. aureus and Vibrio parahaemolyticus |
Antibacterial activity: 15.3 ± 0.5 ppm, 11.7 ± 0.5 ppm, 14.7 ± 0.9 ppm and 13.7 ± 1.1 ppm Cytotoxicity: inhibit human cervical cancer cells (HeLa) at 10 µg/ml but did not inhibit human lung cancer cells (A549) |
Singh et al. (2018) |
| AuNps | Leaf extract of Annona muricata |
Spherical 25.5 nm |
S. aureus, Clostridium sporogenes, E. faecalis and K. pneumonia | Exhibit good antimicrobial activity with increase in concentration | Folorunso et al. (2019) |
| ZnONps | Albizia lebbeck stem bark |
Spherical 66.25 nm |
B. cereus, S. aureus, E. coli, K. pneumonia, and S. typhi |
Exhibit strong antimicrobial activity which was dose dependent Cytotoxicity: MDA-MB231 and MCF-7 cell lines Cytotoxic effect was concentration dependent |
Umar et al. (2019) |
| ZnONps | Bacillus haynesii |
Spherical 50 ± 5 nm |
E. coli ATCC 35,218, S. aureus ATCC 29,213 | MIC and MBC values were > 8 and 16 mg/ml respectively for E. coli and 4 and 8 mg/ml respectively for S. aureus | Rehman et al. (2019) |
| ZnONps | Cinnamomum verum bark extract |
Hexagonal wurtzite 45 nm |
E. coli MTCC 7443 and S. aureus MTCC 7410 | MIC of 125 µg/ml for E. coli and MIC OF 62.5 µg/ml for S. aureus | Ansari et al. (2020) |
| ZnONps | Boswellia ovalifoliolata |
Spherical 20.3 nm |
Sphingobacterium sp., Acinetobacter sp., Ochrobactrum sp. | IZD: 3 mm, 1.7 mm and 4 mm | Supraja et al. (2016) |
| ZnONps | Withania somnifera (ws) leaf extract |
Hexagonal wurtzite 15.6 nm |
E. faecalis, S. aureus, E. coli and P. aeruginosa | A greater antibacterial effect of ws-ZnONps was noticed against E. faecalis and S. aureus at 100µg/ml. The biofilm of E. faecalis and S. aureus were greatly inhibited at 100µg/ml compared to E. coli and P. aeruginosa. Cytotoxicity: laval and pupal development delayed at 25µg/ml. A complete mortality (100%) was observed at 25µg/ml. ws-ZnONps showed least LC50 value (9.65µg/ml) compared to the uncoated ZnONps (38.8µg/ml) and leaf extract (13.06µg/ml) | Malaikozhundan et al. (2020) |
| CuONps | Aqueous extract of Abutilon indicum |
Hexagonal, wurtzite and sponge crystal structure 16.78 |
Klebsiella, E. coli, S. aureus, B. subtilis | Significant bactericidal activity of nanoparticle against Klebsiella and B. subtilis with IZD of 14 ± 005 and 15 ± 0.11 mm respectively. At 5 mg, the CuONps showed effective activity against S. aureus, Klebsiella, B. subtilis with IZD of 10 ± 0.11, 14 ± 0.05 and 15 ± 0.11 mm respectively | Ijaz et al. (2017) |
| CuONps | Aqueous extract of Tamarindus indica fruit |
Cube shaped 40-50 nm |
Proteus mirabilis, S. aureus |
Significant synergistic effect with β-lactam antibiotics Reduction in biofilm formation of the two organisms by 85% and 93% respectively |
Selvaraj et al. (2019) |
| CuONps | Leaf extract of Aloe barbadensis |
Spherical 33.4–64.9 nm |
Pseudomonas, Klebsiella, Staphylococcus, E. coli | IZD of 11, 12, 8 and 9 mm respectively | Saruchi et al. (2019) |
| TiO2Nps | Synthesized using S. aureus |
Spherical 20 nm-30 nm |
E. coli, B. cereus, S. aureus |
Highly effective against B. subtilis (9 mm) and E. coli (14 mm) Showed antibiofilm activity against the pathogens |
Landage et al. (2020) |
| TiO2Nps | Laser ablation |
Round (circular) 36 nm |
S. aureus, E. coli | In distilled water, the MIC is 9.45 mg/ml and 18.91 mg/ml for E. coli and S. aureus respectively. In alcohol, the MIC is 4.72 mg/l for E. coli and 9.45 mg/l for S. aureus | Abdul-Hussan et al. (2018) |
| MgONps | Aqueous extract of Swertia chirayaita |
Spherical < 20 nm |
S. aureus MTCC9442, S. epidermidis MTCC 2639, B. cereus MTCC-9017, E coli MTCC 9721, Proteus vulgaris MTCC7299, K. pneumonia MTCC9751 |
Antimicrobial activity was dose dependent The IZD of the various MgONps concentrations (10, 20, 30 and 40 µl)(0.25 µg/ml) were 18 mm for E. coli and 17 mm for S. aureus, which were also the maximum IZD for all the tested bacteria respectively. Moreover, a 16 mm IZD was obtained for B. cereus, K. pneumonia and P. vulgaris |
Sharma et al. (2017) |
| MgONps | Leaf extracts of Rhododendron arboretum |
Sphere shaped – |
E. coli, Streptococcus mutants, Proteus vulgaris | Inhibition was dose-dépendent and increased with increase in concentration. At 10 mg/ml of MgONps, IZD were 36 mm, 32 mm, 24 mm for E. coli, S. mutans and Proteus vulgaris respectively | Singh et al. (2019) |
| MgONps | Trigonella foenum-graecum leaf extract |
Spherical 13 nm |
E. coli, Bacillus, S. aureus | Good antibacterial activity: 125 µg, 250 µg, 125 µg | Vergheese ans Vishal (2018) |
| MgONps | Commercial |
Polyhedral morphology 20 nm |
E. coli, P. aeruginosa, S. aureus, S. epidermidis, MRSA |
The MIC varied from 0.5 to 1.2 mg/ml. The minimal lethal concentration (MLC) at 90% killing varied from 0.7 to 1.4 mg/ml against the various pathogens. The most potent concentration (MPC) was 1.4 and or 1.6 mg/ml—this depends on the organism tested Mechanism: reduction in adhesion, disruption of biofilm formation, production of ROS, quorum sensing and Ca2+ concentrations |
Nguyen et al. (2018) |
Synergistic antimicrobial activity of nanoparticles
The activities of nanoparticles can be greatly enhanced when conjugated or coated with other materials. In fact, combining nanoparticles with antibiotics can help reduce microbial resistance. In resistant strains, variation in the mode of action of antibiotics and the nanoparticles enhance the susceptibility of the microbe. If a particular strain is resistant to one antimicrobial agent, another antimicrobial agent could trigger the killing by using a different mechanism. The nanoparticles can also act as carriers of antibiotics thereby facilitating access to bacterial cell walls. The antibiotic in turn damages the cell wall enabling easy entry of the nanoparticles and its complex.
In a study that combined amoxicillin with AgNps, a significant reduction in growth of bacteria was evident (Li et al. 2005). The transport of amoxicillin across the microbial membrane was facilitated by the hydrophobic nature of the AgNps which interact with the cell membrane. Duran et al. (2010) showed that AgNps and amoxicillin synergistic activity was due to sulfur bridge formation between the two agents. Fayaz et al. (2010) showed that AgNps-ampicillin complex inhibits the formation of crosslinks in the peptidoglycan layer leading to cell death. This complex also prevented the unwinding of DNA. It was reported by Panacek et al. (2015) that a very low concentration of AgNps is needed for synergistic activity with antibiotics with no cytotoxic effect. Wan et al. (2016) showed the synergistic activity of AgNps combined with polymyxin B and rifampicin against A. baumannii. It was also reported by Banoee et al. (2010) that ZnONps-ciprofloxacin complex interrupts with the endogenous efflux transporter (NorA) and the Omf proteins which facilitate the entrance of ciprofloxacin into the cell. The synergistic activity of TiO2Nps and several antibiotics against MRSA was also studied by Roy et al. (2010). Significant antibacterial activity was observed. However, the exact mechanism of its synergistic activity is yet to be elucidated. Chamundeeswari et al. (2010) reported that AgNps-ampicillin complex exhibit greater antimicrobial activity against S. aureus, E. coli and K. mobilis than single amoxicillin.
In a more recent investigation, Farzana et al. (2017) reported the antimicrobial behavior of ZnONps and β-lactam antibiotics against bacteria. AuNps-antibiotics complex also exhibited excellent antimicrobial activity (Shaikh et al. 2019). Mohamed (2020) reported that AuNps conjugated with ampicillin/amoxicillin showed significant antibacterial activity and was biocompatible with treated cells. Farooq et al. (2019) showed that rifampicin conjugated AgNps produced anti-biofilm activity against MRSA and K. pneumonia. Surwade et al. (2019) reported that AgNps combined with ampicillin showed very strong synergistic effects against MRSA. A similar result was provided by Sajjad et al. (2019) who studied the synergistic activity of AgO2Nps and ceftriaxone against E. coli. Several other researchers have reported the synergistic effects of nanoparticles with antibiotics (Shahbazi et al. 2019; Gounani et al. 2018). Table 2 summarizes other studies that reported the synergistic activity of nanoparticles with antibiotics. Moreover, nanoparticles conjugated with specific antibody exhibit excellent activity against resistant pathogens. Al-Sharqi et al. (2019) reported that AgNps conjugated with specific antibody showed good antimicrobial activity against S. aureus.
Table 2.
Nanoparticles combined with antibiotics for antimicrobial activity
| Nanoparticles | Synthesis | Antibiotics | Organisms tested/Activity | Reference |
|---|---|---|---|---|
| AuNps | – | Ampicillin |
2 strains of S. aureus MRSA Significant antibacterial activity, cytocompatible against human dermal fibroblasts |
Fan et al. (2019) |
| CuNps | Green synthesis |
Erythromycin Azithromycin Norfloxacin |
Klebsiella, Pseudomonas, E. coli, Shigella Staphylococcus At 50, 100, 200, 400 µg/ml, all bacteria were resistant to antibiotics |
Kaur et al. (2019) |
| AgNps | Synthesized using corn leaf waste of zea mays extract |
Kenamycin Rifampicin |
Bacillus cereus, E. coli, S. aureus, L. monocytogenes, S. typhimurium | Patra and Baek (2017) |
| AgNps | Synthesized using Adiantum philippense extract | Amoxicillin | MRSA | Kalita et al. (2016) |
| AgNps | Leaf extract of Cassia roxburghii |
Ampicillin, Polymyxin, Clotrimazole Amikacin, Chloramphenicol, Penicillin-G, Tetracycline, Amoxiclav, Cefpirome Gentamycin, clotrimazole |
S. aureus, B. subtilis, E. coli, P. aeruginosa | Moteriya et al. (2017) |
| AgNps |
Spherical shaped Nps 8.57 ± 1.17 nm |
Ampicillin, Amikacin |
E. faecium, S. aureus, A. baumannii,, Enterobacter cloacae, E. coli, K. pneumonia Morganella morganii, P. aeruginosa Excellent antimicrobial activity |
Lopez-Carrizales et al. (2018) |
| CuNps | Synthesized using Camellia sinensis (green tea) and β-cyclodextrin |
Penicillin, Streptomycin, Ampicillin Amoxicillin, Gentamicin, Ciprofloxacin |
S. pyrogenes, E. coli, S. typhi, Micrococcus lutus Streptococcus mutans |
Mandava et al. (2017) |
| Bimetallic Ag-Au nanoparticle | Synthesized using cell free supernatant of P. veronii strain AS41G on Annona squamosal L |
Kanamycin, Bacitracin, Gentamycin Streptomycin,Erythromycin, Chloramphenicol |
E. coli, B. subtilis, K. pneumoniae | Sharma et al. (2017) |
| ZnONps | – |
Ciprofloxacin Ampicillin |
E. coli (MTCC 739), Klebsiella pneumonia (MTCC 109), P. aeruginosa (MTCC 741), Salmonella typhi (MTCC 98), S. aureus (MTCC 737), B. subtilis (MTCC 736) Synergistic effects observed No antagonistic effect observed |
Sharma and Jandaik (2016) |
| ZnONps | – |
Ciprofloxacin Ceftazidime |
A. Baumannii Combination caused increased uptake of antibiotic It changes the cells from rod to cocci form |
Ghasemi and Halal (2016) |
| AgNps | – |
B-lactam (ampicillin and penicillin) Quinolone (enoxacin) Aminoglycoside (kenamycin and neomycin) Polypeptide (tetracycline) |
Salmonella typhimurium DT104 Kenamycin, Enoxacin, neomycin and tetracycline exhibited synergistic activity against the pathogen. Ampicillin and penicillin do not show any synergistic activity AgNps form complex with antibiotics (AgNps-tetracycline). The AgNps-tetracycline interact strongly with Salmonella causing Ag+ release. The increase in Ag + concentration in the cell wall facilitate bacterial growth inhibition and subsequent death |
Deng et al. (2016) |
While nanoparticle-antibiotic complex provides a strong synergism against pathogenic microbes, the combination of two different nanoparticles can help improve their activity while reducing their toxicity. Coating nanoparticles with biomaterials can also enhance their activity. Coating AgNps with TiO2 or other metal or metallic oxide materials enhances its antimicrobial activity. Hu et al. (2017) showed that ZnO/Ag bimetallic nanoflowers exhibit improved antibacterial activity. Recent studies by Jaworski et al. (2018) and Cobos et al. (2020) showed that AgNps decorated with graphene oxide nanocomposites exhibit strong antibacterial activity. Nunez et al. (2019) also showed that nanohybrids mediated AgNps was effective against Gram positive and Gram-negative microbes (Nunez et al. 2019). Table 3 summarizes some recent studies on nanocomposites/nanohybrids.
Table 3.
Nanocomposites/combined nanoparticles and their antimicrobial activity and other key finding (cytotoxic activity and mechanisms)
| Nps | Method of synthesis | Shape and size | Bacteria isolates tested | Antibacterial activity (IZD/MIC/MBC) and other key findings | Reference |
|---|---|---|---|---|---|
| Biopolymer-Ni, Zn Nps biocomposite | Biosynthesized using Rhodotorula mucilaginosa UANL-00IL exopolysaccharide | Polymorphic arrangement without definite shape 8–26 nm |
Resistant strains S. aureus P. aeruginosa |
Ni-EPS Showed antimicrobial and antibiofilm activity against the two pathogens at 3 and 2 mg/ml respectively Zn-EPS also showed activity against resistant S. aureus at 1 mg/ml Cytotoxicity: no toxicity, as renal function showed no differences between treatments and control in vivo assays with male rat tests in the study at a concentration of 24 mg/kg of body weight |
Garza-Cervantees et al. (2019) |
| AgNps/ZnONps | Aqueous extract of Ulva fasciata alga |
AgNps: spherical, 15 ± 0.55 nm ZnONps: rod-shape, 187 ± 0.5 nm |
S. aureus Salmonella enterica sub sp. Bukuru E. coli |
Both had increased antibacterial activity with an increase in concentration against E.coli and Salmonella spp. Both demonstrated a good syngergistic effect with antibiotics |
Abo-Shama et al. (2020) |
| Tungsten carbide (Wc), silver (Ag) and copper (Cu) in combination | Commercially obtained |
Wc: hexagonal with average diameter of 250 nm AgNps: rod shape, 80–90 nm CuNps:10–20 nm |
S. aureus P. aeruginosa |
Significant antimicrobial activity | Bankier et al. (2019) |
| Ag/TiO2 | Horizontal vapor phase growth (HVPG) technique |
Nanorods geometrical shape 24.8 µm–0.22 nm |
S. aureus |
Sharp-end nanorods can eradicate bacteria with > 50% efficiency Mechanism: nanorods eliminate bacteria because of their geometrical shape-sharp ends. Sharp-end nanorods with optimal geometrical shape can naturally pierce the cell membrane of bacteria leading to shrinking. This shows that bacteria can be killed not only by the release of ions from Nps but by the ability of utilizing the shape of Nps in killing them |
Muflikhun et al. (2019) |
| Ag-Au/ZnO nanostructure | Justicis adhatoda plant extract |
Nano stick shape 20–25 nm |
E. coli S. aureus |
Good antimicrobial activity | Pandiyan et al. (2019) |
| α-BiO2-ZnO nanostructure | Chemical synthesis | Monoclinic and hexagonal wurtzite | S. aureus | 1.5 cm IZD for 1 mg/l | Chauhan et al. (2019) |
| T-β-D-glu-ZnONps (Trichoderma-β-D-glucan-zinc oxide nanoparticles | Fungal mycellial water extract derived from T. harzianum (SKCGW009) |
Spherical 30.34 nm |
S. aureus |
Inhibit the growth of S. aureus inside of roundworm and enhanced growth of roundworm Cytotoxicity: not toxic to NIH3T3 cells Exhibited the dose-dependent inhibitory effect to human pulmonary Carcinoma A549 cells IC50 of T-ZnoNps and T-β-D-glu-ZnONps against A549 cells was 158 and 56.25 µg/ml respectively |
Saravanakumar et al. (2020) |
| ZnO/Fe3O4/rGO nanocomposites | Hydrothermal method |
Hexagonal wurtzite Inverse spinal structure Rod-shaped morphology Spherical-shaped morphology |
E. coli S. aureus |
Better cidal effect on E. coli when compared to S. aureus after treatment with 1 mg/ml concentration Addition of rGO intensified antibacterial effect to a great extent |
Rajan et al. (2019) |
| Ag/TiO2 | Aqueous extract of Acacia nilotica |
Spherical 20–40 nm |
E. coli, MRSA, P. aeruginosa |
IZD of 24, 20 and 15 mm respectively Maximum IZD was shown at 500 µg/ml concentration. MIC was found to be 64 µg/ml against E. coli and S. aureus while 128 µg/ml against P. aeruginosa Mechanism: Decrease in the level of glutathione, triggered ROS production and lipid peroxidation |
Rao et al. (2019) |
|
ZEO-AgNps, ZEO-CuNps and ZEO-ZnNps (silver, copper and zinc zeolite nanocomposites) |
– |
Spherical 3–15 nm |
Vibrio cholera |
Each nanocomposite type had a distinctive antimicrobial effect altering each V. cholera lifestyle differently. Exhibit antimicrobial and antibiofilm activity Mechanisms: modification of the relative expression of genes that plays a role in biofilm formation Alteration in the level of outer membrane proteins (OmpT, OmpA, OmpU and OmpW) |
Meza-villezcas et al. (2019) |
| Copper-doped chitosan- gelatin (CSG) nanocomposite coatings (Cu-doped CSG nanocomposite coating) | Green synthesis |
E. coli S. aureus |
Antibacterial activity was dependent on Cu concentration Cytocompatibility assessment in vitro showed that the activities of bone marrow stromal cells were not impaired on Cu-doped coatings Improved biological performance of Ti-based materials |
Huang et al. (2020) |
Photothermal therapy using nanomaterials is a promising approach to combat antimicrobial resistance
Over the years, the use of nanomaterials in photo thermal therapy has received considerable attention (Wei et al. 2018; Canaparo et al. 2019; Ramezani et al. 2020). The light-responsive technique specifically has been used in the development of drugs. It has also found application in the design of drug carrier systems and as an antibacterial agent (Qi et al. 2019). The light-responsive structure can be easily regulated and it has low invasiveness (Bao et al. 2016). Its mechanism of activity is based on the alteration of the light-sensitive molecules when stimulated by light, thus enabling the release of the encapsulated or conjugated drug (Chen and Zhao 2018).
For the treatment of cancer, photothermal therapy uses the thermal stress caused by irradiation of light of a specific wavelength (Cheng et al. 2014a). They have also been used for the delivery of anticancer drugs (Yu et al. 2020). Although photothermal therapy has found application in the treatment of cancer, it is effective at killing pathogens irrespective of their drug resistance level or their metabolic state within the biofilm (Galanzha et al. 2012). Halstead et al. (2016) showed that blue light has a broad-spectrum antimicrobial activity against all six ESKAPE members. Further investigations also showed that low penetrating blue light of about 415 ± 10 nm is preferable for the treatment of wound infections as it is associated with low damage to the tissue cells (Wang et al. 2017a, b; Katayama et al. 2018). There was an inhibitory effect on bacterial growth in a study that exposed P. aeruginosa to light-emitting diode (LED) (Sueoka et al. 2018). The increase in antimicrobial activity was possible because the bacteria that withstand the initial PDT were subsequently affected by singlet oxygen produced due to the excitation of the remaining PS. Photothermal therapy leverage the plasmon resonance features of metals, especially AuNps. There is usually the absorption of energy in the visible light spectrum and the omission of energy as heat energy to the immediate medium (Jain et al. 2007; Mocan et al. 2014).
Several organic and polymeric nanoparticles have been reported to exhibit inherent photothermal ability (Zhao et al. 2018). Also, AuNps, iron oxide, black phosphorus, graphene, and many other polymeric nanoparticles with potential for photothermal conversion have been developed for antibiotic drug delivery (Ji et al. 2016; Hu et al. 2013) and chemo-phothermal therapy against pathogens under irradiation by NIR (Hu et al. 2013; Chiang et al. 2015; Meeker et al. 2016). Near-infrared (NIR) light is an excellent exogenous stimulus with promising potential against drug-resistant pathogens. The induction/activation of photothermal therapy (PTT) by NIR enables the PTT to enter deep into the tissue with little cytotoxicity. PTT can disrupt membrane permeability and signaling cascade of pathogenic organisms, disrupt key enzymes and proteins, and cause cell death (Ray et al. 2012; Kim et al. 2015; Korupalli et al. 2017). Kuang et al. (2017) demonstrated the photothermal therapeutic potential of IR-780 iodide (IR780) (a NIR fluorescence dye) encapsulated in cRGD-conjugated solid lipid nanoparticle. The low cytotoxicity, ease of been wrapped by hydrophobic carriers (inherent lipophilicity) and ease of degradation in the cell make IR780 a suitable agent for in vivo photothermal therapy (Ray et al. 2012). Also, under near-infrared (NIR) light irradiation, graphene-based nanomaterials exhibit high photothermal conversion efficiency and outstanding, amphiphilicity. This attribute enables them to attach to the cell membrane of organisms enhancing nanoscale delivery of antimicrobial agents (Yuan et al. 2018; Ran et al. 2017).
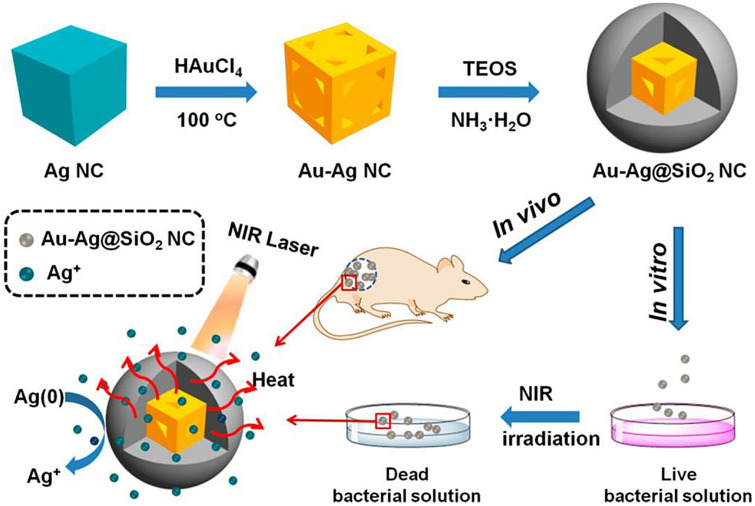
Recently, it was shown that silica-coated gold-silver nanocages (Au–Ag@SiO2 NCs) under NIR laser irradiation showed reliable increases in microbial resistance compared to Au–Ag NCs alone (Wu et al. 2019). Coating the Au–Ag NCs with silicon dioxide improved the surface plasmon resonance of Au–Ag NCs (Fig. 2). Also, upon irradiation of Au–Ag@SiO2 NCs with NIR laser for 10 min, there was a swift temperature rise. Importantly, it was noticed that the increase in the concentration of Au–Ag@SiO2 NCs and the time of laser irradiation correlated with a rise in temperature. This showed that the heat produced by this nanomaterial was rapid and able to eliminate E. faecium, S. aureus, K. pneumoniae, A. baumannii, P. aeruginosa, and Enterobacter spp. (ESKAPE) pathogens.
Fig. 2.
Silica-coated gold-silver nanocages (Au–Ag NCs) showing antibacterial activity by a photothermal effect.
Reproduced from Wu et al. (2019) with permission
Reports on bacterial resistance to nanoparticles are gradually emerging
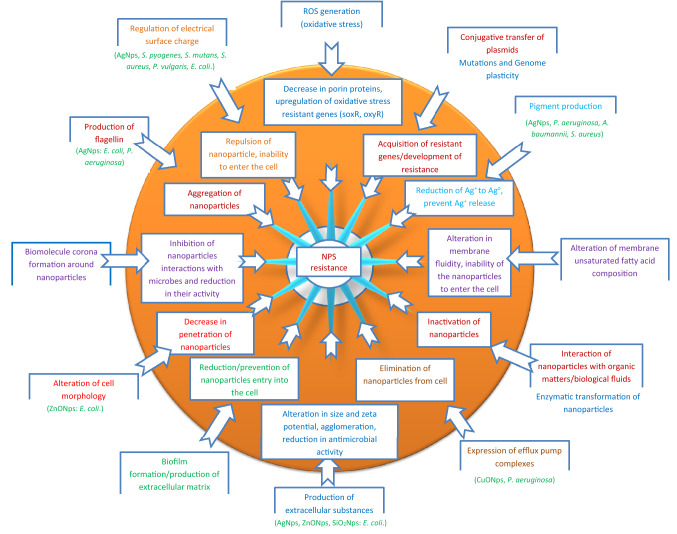
Not minding the effectiveness of an antimicrobial agent, most microbes often find strategies to maneuver these agents. The flexibility of microbial genome and the selective pressure exerted by most biomaterials often facilitate the emergence of resistant. Thus, irrespective of the different mechanisms of nanoparticle activity against microbes, resistance of microbes to nanoparticles have been reported. Efflux pumps, biofilm formation/adaptation, electrostatic repulsion, alterations of morphology and mutations are some of the reported microbial resistance mechanisms to nanoparticles as reported by Nino-Martinez et al. (2019) in a review.
Previously it was shown by Li et al. (1997) that the down regulation of genes coding for the porin proteins causes a decrease in nanoparticle penetration into the cell. Jordan et al. (2008) showed that the presence of envelop stress response (ESR) mechanisms help to maintain bacterial membrane integrity, reducing their interaction with positively charged nanoparticles and alterations of lipopolysaccharide (LPS) and its constituents. In a subsequent investigation by Li et al. (2010) it was also reported that the oxidation and interaction of nanoparticles with organic matters influence its antimicrobial activity. Further investigation by Hachicho et al. (2014) revealed that P. aeruginosa exposed to nanoparticles altered its membrane unsaturated fatty acid composition. This alteration causes a change in membrane fluidity making it difficult for the nanoparticles to enter the cell. Graves et al. (2015) also reported that the exposure of microbes to non-lethal concentration of nanoparticles can facilitate the increase in resistance due to the development of mutations that lead to the upregulation and downregulation of many genes.
The upregulation of RND and CDF transporters, P-type ATpase efflux complexes, czcABC and RND family efflux system was also reported as the resistant mechanism of P. aeruginosa exposed to different concentrations of CuONps (Yang et al. 2012; Guo et al. 2017). Other reported mechanisms of bacterial resistance to nanoparticles include, enzymatic transformation of nanoparticles (Palomo-Siguero et al. 2016), biofilm formation/adaptation (Wang et al. 2016), electrostatic repulsion (Abbaszadegan et al. 2015; Nabavizadeh et al. 2017), pigment production (Ellis et al. 2018), and formation of biomolecule corona around the nanoparticles (Siemer et al. 2019). Panacek et al. (2018) showed that the production of flagellin (adhesive flagellum protein) by E. coli and P. aeruginosa provoked aggregation of nanoparticles leading to resistance. Faghihzadeh et al. (2018) reported that the production of extracellular substances by E. coli altered the size and zeta potential of AgNps leading to agglomeration and subsequent resistant to AgNps. Furthermore, E. coli exposed to sublethal concentration of ZnONps was reported to facilitate conjugative transfer of plasmids housing resistant genes (Wang et al. 2018). Qiu et al. (2015) in a similar investigation that exposed E. coli to TiO2Nps, decrease in growth rate of bacteria with subsequent increase in conjugative transfer of genes coding for antibiotic resistance was also noted.
The alteration in the dissolution and release rate of nanomaterials in the biological system in addition to their interaction with the biological fluid can allow the microbes to modify and enhance their adaptation and fitness strategies. It is important to know that prolonged/widespread use of nanoparticles induces the expression of antibiotic resistant genes. Already the production of ROS is one of the key mechanisms of action of nanoparticles. The presence of ROS can lead to a decrease in porins proteins and upregulation of oxidative stress resistant genes. However, the ability of microbes to generate ROS when exposed to nanoparticles can be influenced by interaction with the host environment. The availability of oxygen and light can greatly influence ROS production (Yu et al. 2018). Under aerobic conditions ions can be released while anaerobic conditions can reduce the chance of ions release. It was also reported by Chen et al. (2017) that exposure of microbes to AgNps under anaerobic condition decreases the antimicrobial activity of nanoparticles (Chen et al. 2017). Moreover, the alteration in environmental conditions can cause a spontaneous rise in mutation and also trigger genome plasticity which can greatly facilitate resistance to antimicrobial agents and evolutions of strains with increased fitness. Figure 3 summarizes the reported resistant mechanisms of bacteria to nanoparticles. Table 4 shows nanomaterials with photothermal activity as antibacterial agents.
Fig. 3.
Resistant mechanisms of bacteria to nanoparticles
Table 4.
Nanomaterials with photothermal activity as antibacterial agents
| Nanomaterials | Microbes | Size (nm) | Light wavelength (nm) | Mechanisms of antibacterial activity | References |
|---|---|---|---|---|---|
| AuNCs-DNase | Gram positive and Gram-negative bacteria | 808 | Disrupt biofilm and kill gram positive and Gram-negative bacteria | Xie et al. (2020) | |
| Aniline nanogel and chitosan-containing AgNPs | E. coli | 78 | 405 | The release of AgNPs causes Cell membrane damage | Ballesteros et al. (2019) |
| AgNPs | S. aureus, Pseudomonas aeruginosa | 15–20 | 460 |
In vitro investigation showed a 10-log reduction in bacterial count in biofilm In vivo study using mice showed a 100% reduction in 2 h. there was also a reduction in wound burden |
Nour El Din et al. (2016) |
| [AL(OH)Pc(SO3Na)4] + Au–AgNPs + aBL | Pseudomonas aeruginosa | 14 | 660 | There was a 3-log reduction | Maliszewska et al (2018) |
| AgNPs + antibiotic + aBL | S. aureus | 15–20 | 460 |
There was a 100% reduction in bacterial growth within 8 h Excellent synergistic activity |
Akram et al. (2016) |
| Calixarene-NO donor conjugate | E. coli, S. aureus | 270 | 400 | The release of No causes membrane damage | Dahl et al. (1988) |
| PLGA NPs | E. faecalis | – | 665 | There was a 96.7% reduction in growth within 10 min | Gonzalez-Delgado et al. (2016) |
| CMP NPS | B. subtilis, E. coli | – | 1.2 W/cm2 | For B. subtilis, there was a 97% reduction under 120 min while E. coli had a 95% reduction under 120 min | Ma et al. (2016) |
| AuNWs | E. coli, S. aureus | 5 ± 1.5 | 808 | Reduction of bacterial growth in vitro | Liao et al. (2020) |
| Ti-GNRs surface | E. coli, P. aeruginosa, S. epidermidis, S. aureus | 49 ± 4 × 11 ± 2 | 808 | Significant reduction in bacterial growth in vitro | Yang et al. (2019) |
| TC-AuNSs | S. aureus, P. aeruginosa | Average diameter of 120 nm | 808 | Reduction in bacterial growth in vitro within 5 min of exposure to NIR laser irradiation | Manivasagan et al. (2019) |
| PU-Au-PEG | S. aureus, P. aeruginosa | 40 × 10 | 808 |
Significant reduction in bacterial adhesion in vitro and in vivo Effective photothermal killing reduction in biofilm formation |
Zhao et al. (2020) |
In summary, nanoparticles have long been seen as potential solutions to the increasing resistance to conventional antibiotics and the evolution of multi-drug resistant bacteria. However, reports on the issue of microbial resistance to nanoparticles are gradually emerging. Its frequent clinical application raises the issue of resistance to these potential biomolecules (Barros et al. 2018; Finley et al. 2015). Future studies should explore the possible resistance mechanisms of bacteria to nanoparticles. The major challenges in using nanoparticles as antimicrobial agents are summarized in Table 5.
Table 5.
Challenges in using nanoparticles as antimicrobial agents
| S. No | Challenges | |
|---|---|---|
| 1 | Size | The size greatly influences antimicrobial potential of nanoparticles. Small size particle has a larger surface area-to-volume ratio. Parameters like synthesis method and reducing/stabilizing agents also affect the morphology, size and stability of the synthesized nanoparticles. Thus, controlling these parameters is a major challenge for efficient and highly effective nanoparticles synthesis. Nanoparticles often used as antimicrobial agents usually ranges from 1 to 100 nm. However, particle size range from 10 µm to 10 nm are often more effective because they can easily penetrate and interact with cells. However, synthesizing nanoparticle with such size is often an issue |
| 2 | Shape | Nanoparticles are often synthesized in different shapes. AgNps for example have different shapes such as spherical, triangular or pyramid, nanorods, nanowires, flower shaped, octahedral, tetrahedral, nano-prism and nano-bars. These shapes can influence the antimicrobial activity of nanoparticles |
| 3 | Aggregate | Nanoparticles can often form aggregates. The formation of these aggregates cause increase in size, thus reducing penetration into the cell and also increases toxicity |
| 4 | Biodistribution | Loss of function due to poor bioavailability is a major challenge in developing effective nanoparticle. Low retention rates of nanoparticle also reduce efficiency. Even the accumulation of nanoparticles may be detrimental to the host |
| 5 | Bioavailability | Poor dispersion affects nanoparticles activity |
| 6 | Cytotoxicity | Toxicity is a crucial issue in the use of nanomaterials. Local and systemic toxic issues in addition to being detrimental to useful bacteria in human is a major concern (Khan et al. 2016). Both nanoparticle and its degradation products can disrupt pathways involved in blood circulation due to its ability to cause hemolysis. Nanoparticle can also lead to organ dysfunction and damage. Large size nanoparticles are more toxic to the biological system than small size particles (Santos et al. 2014). The use of CuONps, ZnONps and TiO2Nps is mostly limited due to their oxidative and DNA damage (Hemeg 2017). CuONps can specifically trigger hepatoxicity and nephrotoxicity via interaction with components of the cell (Baptista et al. 2018). Although some studies have reported no significant in vivo life-threatening toxicity of nanoparticles, there accumulation could be detrimental to body cells (Zazo et al. 2016; Sengupta et al. 2014; Wei et al. 2015; Zaidi et al. 2017). Nanomaterials administered intravenously can accumulate in different organs in the body. Toxicity evaluation at the cellular and systemic levels is very crucial as it carries great clinical relevance |
| 7 | Clearance | Nanoparticles elimination from the biological system is generally low. This can lead to their prolonged accumulation in the system. The charge and size of nanoparticle greatly affect their elimination from the biological system. The kidney can eliminate some nanoparticles while those that were not degraded will be retained in the body for a prolong period of time (Lin et al. 2015) |
| 8 | Interactions | The rapid agglomeration in the use of nanoparticles is a disadvantage in their utilization as an antimicrobial agent. For example, naked ZnONps can strongly interact with organic acids in biological system which can lead to bioconjugates formation. In addition, the antimicrobial effect of nanoparticles is greatly affected by the presence of amino acids as previously stated. This protein are highly abundant in biological system and are often an issue in getting a safer product design in addition to improved product performance |
| 9 | Dosage | Dosage is major issue in nanoparticle application. Currently the dose of nanoparticles leading to disruption of cells in vitro are very high and almost not possible to use in humans. As of date, only few clinical studies are available on nanoparticles dosing. For vital therapeutic targets and reduction in toxicity, dosage optimization and evaluation is very crucial (Grumezescu 2018; Hua et al. 2018) |
| 10 | Instrumentation | High-throughput technology and equipment are also needed to manufacture nanoparticle. This often make continuous/consistent production of highly quality nanoparticle difficult |
| 11 | Scale-up/optimization | Proper guideline formulation for the production, scale-up, physiochemical property characterization, biocompatibility, standardization and protocols to draw a comparison on data origination from in vivo and in vitro experiments are lacking. Inconsistency in size, shape, morphology and other properties may also be evident during large scale production |
| 12 | Prediction | The efficiency or potency of nanomaterials is mostly very difficult to predict |
| 13 | Quality | Producing nanoparticles with uniform size and desired quality and without aggregates is also a major challenge |
| 14 | Variation in microbes and human diseases | Diversities in strains and infections caused by different microbes may influence nanoparticles activity and also complicates treatment |
Discussion
A large portion of the interest in nanomaterials in clinical practice stems from their drug delivery potential. Interestingly, the use of nanoparticles as a drug delivery system dated back to the early 1990s. Several new generation nanoparticles with new therapeutic modalities have been developed since then. Therapeutic and diagnostic nanoparticles fall under two categories: inorganic (AgNps, AuNps, CuONps, ZnONps, TiO2Nps, MgONps, CaONps, Fe2O3Nps, MnO2Nps, etc.) and organic (liposomes, polymeric NPs, micelles, solid lipid Nps (SLNs), nanostructured lipid carriers (NLCs), nanocapsules, nanotubes, quantum dots, dendrimers, emulsions, nanogels, and vesicles). Several inorganic nanoparticles have been successful in clinical studies and have been developed in the clinic for several applications (Anselmo and Mitragotri 2015). Organic nanoparticles have frequently been used in vaccine production and as drug delivery agents. Organic nanoparticles delivered intravenously as treatments for several diseases are also available (Petros and DeSimone 2010). Organic and inorganic nanoparticles have some distinct advantages over several intravenously administered pharmaceutical products. Compared to free drug counterparts, many organic nanoparticles can be fabricated to provide enhanced drug protection, controlled release, prolonged circulation and enhanced target to specific tissues (Wang et al. 2012). Moreover, the stimuli-responsive functions emanating from the surface plasmon resonance of inorganic nanoparticles give them an advantage over individual drugs or molecules (Torchilin 2014).
Nanomaterials have been effective against several microbes. A study by Sarwar et al. (2017) showed that ZnONps form a complex with cholera toxin, compromises its structure, and stops its interaction with receptors present in the erythrocytes. Also, M. tuberculosis showed in vitro susceptibility to AgNps (Tabaran et al. 2020), TiO2 (Ramalingam et al. 2019), and SeNps (Estevez et al. 2020), although their mechanism of action remains unclear. AgNps loaded into Ti nanotubes showed promise against biofilm cells formed by MRSA. Its mechanism of action was via the release of Ag+ (Cheng et al. 2014b). Also, lipid-coated MSNps loaded with colistin and conjugated with LL-37 showed activity against P. aeruginosa-associated pulmonar infections through isoniazid bactericidal effect (Rathnayake et al. 2020).
Moreover, it was reported that Thymus daenensis oil nanoemulsions were effective against bacteria causing pneumococcal infections, and its action mechanism was via oil-bacterial effect (Ghaderi et al. 2017). FA-CP-FA-coated MSNps loaded with ampicillin were shown to be effective against S. aureus and E. coli and could be utilized in treating S. aureus and E. coli-related infections (Chen et al. 2018). Thus, nanoparticles could become an indispensable tool in the treatment of various infections. The use of nanoparticles in the treatment of both chronic and acute respiratory disease was extensively summarized in a recent article by de Menezes et al. (2021).
Also, the ability of nanoparticles to interact with different components of bacteria and exert their antimicrobial mechanisms increases with an increase in the surface/volume ratio of the nanoparticles (Azam et al. 2012). The smaller the size of the nanoparticles, the greater the surface area/volume ratio. Agnihotri et al. (2014) synthesized different AgNps with different sizes and observed that their antibacterial effect depends on their dose and size. Studies have shown that the size of nanoparticles is one of the main factors responsible for their antibacterial activity. However, this depends on the type of synthesis, precursors, and parameters used. Size remains one of the vital factors responsible for the bactericidal effects of nanoparticles (Helmlinger et al. 2016). Nanoparticles with size < 10 nm can penetrate to the interior of the bacterial cell and exert their antibacterial effect (Khalandi et al. 2017), while that > 10 nm cannot penetrate the interior of the bacterial cell (Butler et al. 2015; Wang et al. 2011). A recent investigation by Osonga et al. (2020) reported that the antimicrobial activities of AgNps and AuNps were dependent on size, with no associated toxicity. Furthermore, the surface charge of nanoparticles is also a crucial factor affecting their antimicrobial activity (Abbaszadegan et al. 2015). Moreover, the shape of nanoparticles also determines their antibacterial activity (Raza et al. 2016).
AgNps are highly effective against bacteria. At lower doses, AgNps show low toxicity towards humans (Shahverdi et al. 2007). Chen et al. showed that AuNps with sizes of 8–37 nm were more toxic while AuNps with sizes between 3 and 100 nm were less toxic (Chen et al. 2009). The toxicity was attributed to the synthesis methods and organic reducing and capping agents during the synthesis. Often, there are conflicts in findings regarding the toxic effects of nanoparticles. These differences are broadly due to a lack of standardized experimental procedures (Tao 2018). Sometimes, similar experiments lead to different conclusions. Differences in experimental techniques, doses, and administration routes have not helped the matter. This issue needs proper attention and may require a regional or international regulatory body.
Studies have shown that nanoparticles conjugated with small molecules (e.g., drugs, antibodies, antibiotics, vaccines) are usually more effective than the individual nanoparticles. The combination of nanoparticles with antibiotics greatly reduces the dosage of antibiotics to be administered. This helps to reduce toxicity associated with several antibiotics and helps to reduce resistance acquisition. Combination therapy will pave the way for nanoparticles to be used as adjuncts to existing antimicrobials; thus, helping to reduce resistance associated with most microbes. Impregnation of already available antibiotics with nanoparticles can help improve antimicrobial activity against resistant microbes. More attention should be given to synergistic interactions of nanoparticles with already available antimicrobial agents. However, it should be emphasized that the antimicrobial effect of antibiotic conjugated (impregnated) with nanoparticles depends on the antibiotics used.
Importantly, before nanoparticles can be efficiently incorporated into biological systems, clear insights regarding their stability and interaction with biological fluids (e.g., plasma, serum, proteins, lipids, electrolytes, and metabolites) are needed. The activity of nanoparticles may be influenced by their interactions with protein molecules present in biological systems. The proteins (ligands) can attach to the surface of nanomaterials and influence their dissolution, as well as their antimicrobial and cytotoxic effects. Although it has been reported that surface coating of nanoparticles could prevent their interactions with biological fluids, recent studies have proven that coating the surface of nanoparticles doesn’t prevent their interaction with biological fluid nor improve their antimicrobial potential. Therefore, the interaction of nanoparticles with biological fluids is a crucial area that needs to be exploited. Future studies should look at undesirable off-target interactions of combined nanoparticles and nanoparticles combined with antibiotics.
It is imperative to also look at the dosage of nanoparticles. Drugs that are useful at low doses may exhibit high toxicity at high doses. The doses reported in most studies vary, and the number of cells exposed is not usually reported. Future studies should look at the toxicity. Due to the promising potentials of nanoparticles, one important goal of the nanomaterials research community is to synthesize nanoparticles or nanoparticles that can conjugate very effectively at low doses (concentration). Studies should focus on non-toxic biological materials that can accelerate the potency of nanoparticles without increasing the concentration that might be toxic to biological systems. The combination of different nanoparticles can also help to reduce the dosage. Nanocomposites are also more effective than individual nanoparticles. Thus, more attention should be given to their formulation. Moreover, synthesizing nanoparticles that can bind to proteins, polysaccharides, or small bioactive compounds may be crucial in enhancing their antimicrobial potentials.
The underlying mechanism behind the activity of nanoparticles is yet to be adequately understood. The non-availability of a precise approach for in vitro analysis, in addition to the complexity of the bacterial membrane, makes it difficult to gain proper insight into the exact mechanism for antimicrobial activity of nanoparticles. To efficiently evaluate the accurate therapeutic potentials of nanoparticles and unmask the microbial response to these agents, in vivo studies are indispensable. In vivo studies are essential to elucidate their utility in biological systems fully. Therefore, further studies on the nanomaterial activity at structural, genetic, and proteomic levels are needed.
Furthermore, the frequent clinical application of nanoparticles raises the issue of resistance to these potential agents. Already, microbial resistance to nanoparticles has been summarized above. However, mutation has been one of the reported bacterial mechanisms of resistance to nanoparticles (Graves et al. 2015). Both metal and metal oxide nanoparticles seem to stimulate the co-selection and co-expression of antibiotic resistance genes. In an investigation by Wang et al. (2018), E. coli cultures and aquatic microbiota were exposed to sublethal concentrations of ZnONps. The exposure triggered the conjugative transfer of drug-resistance plasmids. The exposure causes an increase in the cell membrane permeability, increasing horizontal gene transfer (HGT) frequency. A similar finding was previously reported by Qiu et al. (2015), who exposed E. coli to a high concentration of TiO2Nps.
It has also been reported that bacteria exposed to AgNps upregulate genes responsible for protecting against oxidative stress (soxR, oxyR, sodB, sodA) and genes responsible for converting hydrogen peroxide to oxygen (katE and katG) (Gou et al. 2010). In their investigation, Zhang et al. (2018) showed that Al2O3Nps and ZnONps accelerate mutagenesis and the emergence of multiple resistance. According to the investigation, two nanoparticles increased mutation frequency and an increase in multi-antibiotic resistance in the mutation compared to the controls. The nanoparticles also enhanced intracellular ROS, leading to a rise in the frequency of antibiotic resistance mutagenesis.
Finally, photodynamic light therapy is a promising approach for treating infections, especially those due to ESKAPE pathogens. It is a minimally invasive and inexpensive approach to combat antimicrobial resistance. It is highly effective, especially in topical applications. PDT co-administered or conjugated with antibiotics, nanoparticles, antimicrobial peptides, or efflux pump inhibitors show an excellent effect. However, comparison of the efficacy of the different combinations is always difficult due to lack of standardization.
Conclusion
It is becoming obvious that nanoparticles have the potential to change clinical care by improving current therapies or introducing new therapeutic agents. For translation into clinical practice, studies on the toxicity and biocompatibility of the different combinations are needed. To date, the resistance mechanisms of microbes to nanoparticles have not been properly explored and demands adequate attention. To avoid the issue of resistance associated with conventional antibiotics, an understanding of the adaptive mechanisms of microbial resistance to nanoparticles is warranted and should be exploited in future studies. In the years to come, nanomaterials will innovate the world of technology due to their unique properties. However, one important target area of nanoparticle research should be to reduce their toxic effect on humans and enhance their bioavailability and stability.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abbaszadegan A, Ghabramani Y, Gholami A, Hemmateenejad B, Dorostkar S, Nabavizadeh M, Sharghi H. The effect of change at the surface of silver nanoparticles on antimicrobial activity against gram-positive and gram-negative bacteria: a preliminary study. J Nanomater. 2015;720654:8. [Google Scholar]
- Abdel-Raouf N, Al-Enazi NM, Ibraheem IBM. Green biosynthesis of gold nanoparticles using Galaxaura elongata and characterization of their antibacterial activity. Arabian J Chem. 2017;10:S3029–S3039. [Google Scholar]
- Abdul-Hussan I, Abbas AK, Ibrahim IM, Shallal ZS. Characterization and antimicrobial effects of titanium dioxide nanoparticles produced by laser ablation. Indian J Nat Sci. 2018;8(49):14286–14292. [Google Scholar]
- Abo-Shama UH, El-Gendy H, Mousa WS, Hamouda RA, Yousuf WE, Hetta HF, Abdeen EE. Synergistic and antagonistic effects of metal nanoparticles in combination with antibiotics against some reference strains of pathogenic microorganisms. Infect Drug Resist. 2020;13:351–362. doi: 10.2147/IDR.S234425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abuayyash A, Ziegler N, Gessmann J, Sengstock C, Schildhauer TA, Ludwig A, Köller M. Antibacterial efficacy of sacrifical anode thin films combining silver with platinum group elements within a bacteria-containing human plasma clot. Adv Eng Mater. 2018;20:1700493. [Google Scholar]
- Adebayo-Tayo B, Salaam A, Ajubade A. Green synthesis of silver nanoparticles using Oscillatoria sp. extract, its antibacterial, antibiofilm potential and cytotoxicity activity. Heliyon. 2019;5:e02502. doi: 10.1016/j.heliyon.2019.e02502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnihotri S, Mukherji S, Mukherji S. Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. Rsc Adv. 2014;4:3974–3983. [Google Scholar]
- Akram FE, El-Tayeb T, Abou-Aisha K, El-Azizi M. A combination of silver nanoparticles and visible blue light enhances the antibacterial efficacy of ineffective antibiotics against methicillin-resistant Staphylococcus aureus (MRSA) Ann Clin Microbiol Antimicrob. 2016;15:48. doi: 10.1186/s12941-016-0164-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akter S, Huq MdA. Biologically rapid synthesis of silver nanoparticles by Sphinogobium spp. MAH-11T and their antibacterial activity and mechanisms investigation against drug-resistant pathogenic microbes. Artif Cells Nanomed Biotechnol. 2020;48:1. doi: 10.1080/21691401.2020.1730390. [DOI] [PubMed] [Google Scholar]
- Al-Sharqi A, Apun K, Vincent M, Kanakaraju D, Bilung LM, Sam MSH. Investigation of the antibacterial activity of Ag-Nps conjugated with a specific antibody against Staphylococcus aureus after photoactivation. J Appl Microbiol. 2019;128:102–115. doi: 10.1111/jam.14471. [DOI] [PubMed] [Google Scholar]
- Ansari MA, Murali M, Prasad D, Alzohairy MA, Almatroudi A, Almomary MN, Udayashankar AC, Singh SB, Asiri SMM, Ashwini BS, Gowtham HG, Kalegowda N, Amruthesh KN, Lakshmeesha TR, Niranjana SR. Cinnamomum verum bark extract mediated green synthesis of ZnO nanoparticles and their antibacterial potentiality. Biomolecules. 2020;10:336. doi: 10.3390/biom10020336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anselmo AC, Mitragotri S. A review of clinical translation of inorganic nanoparticles. AAPS J. 2015;17(5):1041–1054. doi: 10.1208/s12248-015-9780-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anselmo AC, Mitragotri S. Nanoparticles in the clinic. Bioeng Transl Med. 2016;1(1):10–29. doi: 10.1002/btm2.10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arya G, Sharma N, Mankamna R, Nimesh S. Antimicrobial silver nanoparticles: future of nanomaterials. In: Prasad R, editor. Microbial nanobionics. Nanotechnology in the life sciences. Cham: Springer; 2019. [Google Scholar]
- Asemani M, Anarjan N. Green synthesis of copper oxide nanoparticles using Juglans regia leaf extract and assessment of their physic-chemical and biological properties. Green Process Synth. 2019;8(1):557–567. [Google Scholar]
- Azam A, Ahmed AS, Oves M, Khan M, Memic A. Size-dependent antimicrobial properties of CuO nanoparticles against gram-positive and-negative bacterial strains. Int J Nanomed. 2012;7:3527. doi: 10.2147/IJN.S29020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badetti E, Calgaro L, Falchi L, Bonetto A, Bettiol C, Leonetti B, Ambrosi E, Zendri E, Marcomini A. Interaction between copper oxide nanoparticles and amino acids: influence on the bacterial activity. Nanomaterials. 2019;9:792. doi: 10.3390/nano9050792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaganesh AS, Sengidaan R, Ranjithkumar R, Chandarshekar B. Synthesis and characterization of porous calcium oxide nanoparticles (CaoNps) Int J Innov Technol Exploring Eng. 2018;8(2S):2278–2757. [Google Scholar]
- Ballesteros CAS, Bernardi JC, Correa DS, Zucolotto V. Controlled release of silver nanoparticles contained in photoresponsive nanogels. ACS Appl Bio Mater. 2019;2(2):644–653. doi: 10.1021/acsabm.8b00366. [DOI] [PubMed] [Google Scholar]
- Bankier C, Matharu RK, Cheong YK, Ren GG, Cloutman-Green E, Ciric L. Synergistic antibacterial effects of metallic nanoparticle combinations. Sci Rep. 2019;9:16074. doi: 10.1038/s41598-019-52473-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banoee M, Seif S, Nazari ZE, Jafari-Fesharaki P, Shahverdi HR, Moballegh A, Moghaddam KM, Shahverdi AR. ZnO nanoparticles enhanced antibacterial activity of ciprofloxacin against Staphylococcus aureus and Escherichia coli. J Biomed Mater Res B. 2010;93:557–561. doi: 10.1002/jbm.b.31615. [DOI] [PubMed] [Google Scholar]
- Bao Z, Liu X, Liu Y, Liu H, Zhao K. Near-infrared light-responsive inorganic nanomaterials for photothermal therapy. Asian J Pharm Sci. 2016;11(3):349–364. [Google Scholar]
- Baptista PV, Mccusker MP, Carvalho A, Ferreira DA, Mohan NM, Martins M, Fernandes AR. Nano-strategies to fight multidrug resistant bacteria—“a battle of the titans”. Front Microbiol. 2018;9:1441. doi: 10.3389/fmicb.2018.01441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros CHN, Fulaz S, Stanisic D, Tasic L. Biogenic nanosilver against multidrug-resistant bacteria (MDRB) Antibiotics (basel) 2018 doi: 10.3390/antibiotics7030069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuyan T, Mishra K, Khanuja M, Prasad R, Varma A. Biosynthesis of zinc oxide nanoparticles from Azadirachta indica for antibacterial and photocatalytic applications. Mater Sci Semicond Process. 2015;32:55–61. [Google Scholar]
- Bilal M, Rasheed T, Iqbal HMN, Hu H, Zhang X. Silver nanoparticles: biosynthesis and antimicrobial potentialities. Int J Pharmacol. 2017;13(7):832–845. [Google Scholar]
- Bogdanović U, Lazić V, Vodnik V, Budimir M, Marković Z, Dimitrijević S. Copper nanoparticles with high antimicrobial activity. Mater Lett. 2014;128:75–78. [Google Scholar]
- Brown AN, Smith K, Samuels TA, Lu J, Obare SO, Scott ME. Nanoparticles functionalized with ampicillin destroy multiple antibiotic-resistant isolates of Pseudomonas aeruginosa and Enterobacter aerogenes and methicillin-resistant Staphylococcus aureus. Appl Environ Microbiol. 2012;78(8):2768–2774. doi: 10.1128/AEM.06513-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt AR, Ejaz S, Baron JC, Ikram M, Ah S. Cao nanoparticles as a potential drug delivery agent for biomedical applications. Dig J Nanomater Biostruct. 2015;10:799–809. [Google Scholar]
- Butler KS, Peeler DJ, Casey BJ, Dair BJ, Elespuru RK. Silver nanoparticles: correlating nanoparticle size and cellular uptake with genotoxicity. Mutagenesis. 2015;30:577–591. doi: 10.1093/mutage/gev020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canaparo R, Foglietta F, Giuntini F, Pepa CD, Dosio F, Serpe L. Recent development in antibacterial therapy: focus on stimuli-responsive drug-delivery systems and therapeutic nanoparitcles. Molecules. 2019;24:1991. doi: 10.3390/molecules24101991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamundeeswari M, Sobhana SS, Jacob JP, Kumar MG, Devi MP, Sastry TP, Mandal AB. Preparation, characterization and evaluation of a biopolymeric gold nanocomposite with antimicrobial activity. Biotechnol Appl Biochem. 2010;55:29–35. doi: 10.1042/BA20090198. [DOI] [PubMed] [Google Scholar]
- Chauhan M, Jasrotia T, Kaur G, Prakash C, Kumar R, Dilbaghi N, Chaudhary GR, Kumar S. Investigating the efficiency of α-Bismuth zinc oxide heterostructure composite/UV-LED in methylene blue dye removal and evaluation of its antimicrobial activity. Environ Res. 2019;180:108857. doi: 10.1016/j.envres.2019.108857. [DOI] [PubMed] [Google Scholar]
- Chen H, Zhao Y. Applications of light-responsive systems for cancer theraostics. ACS Appl Mater Interfaces. 2018;10:21021–21034. doi: 10.1021/acsami.8b01114. [DOI] [PubMed] [Google Scholar]
- Chen Y-S, Hung Y-C, Liau I, Huang GS. Assessment of the in vivo toxicity of gold nanoparticles. Nanoscale Res Lett. 2009;4:858. doi: 10.1007/s11671-009-9334-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Yang P, Yuan Z, Guo J. Aerobic condition enhances bacteriostatic effects of silver nanoparticles in aquatic environment: an antimicrobial study on Pseudomonas aeruginosa. Sci Rep. 2017;7:7398. doi: 10.1038/s41598-017-07989-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Liu Y, Lin A, Huang N, Long L, Gang Y, Liu J. Folic acid-modified mesoporous silica nanoparticles with ph-responsiveness loaded with amp for an enhanced effect against anti-drug-resistant bacteria by overcoming efflux pump systems. Biomater Sci. 2018;6:1923–1935. doi: 10.1039/c8bm00262b. [DOI] [PubMed] [Google Scholar]
- Cheng L, Wang C, Feng L, Yang K, Liu Z. Functional nanomaterials for phototherapies of cancer. Chem Rev. 2014;114:10869–10939. doi: 10.1021/cr400532z. [DOI] [PubMed] [Google Scholar]
- Cheng H, Li Y, Huo K, Gao B, Xiong W. Long-lasting in vivo and in vitro antibacterial ability of nanostructured titania coating incorporated with silver nanoparticles: antibacterial ability of silver nanoparticles. J Biomed Mater Res. 2014;102:3488–3499. doi: 10.1002/jbm.a.35019. [DOI] [PubMed] [Google Scholar]
- Chiang W-L, Lin T-T, Sureshbabu R, Chia W-T, Hsiao H-C, Liu H-Y, Yang C-M, Sung H-W. A rapid drug release system with a NIR light-activated molecular switch for dual-modality photothermal/antibiotic treatments of subcutaneous abscesses. J Controlled Release. 2015;199:53–62. doi: 10.1016/j.jconrel.2014.12.011. [DOI] [PubMed] [Google Scholar]
- Cobos M, De-La-Pinta I, Quindos G, Fernandez MJ, Fernendez MD. Graphene oxide-silver nanoparticle nanohybrids: synthesis, characterization and antimicrobial properties. Nanomaterial. 2020;10:376. doi: 10.3390/nano10020376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Zhao Y, Tian Y, Zhang W, Lü X, Jiang X. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials. 2012;33(7):2327–2333. doi: 10.1016/j.biomaterials.2011.11.057. [DOI] [PubMed] [Google Scholar]
- Dahl TA, Midden WR, Neckers DC. Comparison of photodynamic action by rose Bengal in gram-positive and gram-negative bacteria. Photochem Photobiol. 1988;48:607–612. doi: 10.1111/j.1751-1097.1988.tb02870.x. [DOI] [PubMed] [Google Scholar]
- De Menezes BRC, Rodrigues KF, Schatkosi VM, Pereira RM, Ribas RG, Montanheiro TLA, Thim GP. Current advances in drug delivery of nanoparticles for respiratory disease treatment. J Mater Chem B. 2021;9:1745–1761. doi: 10.1039/d0tb01783c. [DOI] [PubMed] [Google Scholar]
- DeAlba-Montero I, Guajardo-Pacheco J, Morales-Sanchez E, Araujo-Martinez R, Loredo-Becerra GM, Martinez-Castanon GA, Ruiz F, Compean Jasso ME. Antimicrobial properties of copper nanoparticles and amino acid chelated copper nanoparticles produced by using a soya extract. Bioinorg Chem Appl. 2017;2017:1064918. doi: 10.1155/2017/1064918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng H, McShan D, Zhang Y, Sinha SS, Arslan Z, Ray PC, You H. Mechanistic study of the synergistic antibacterial activity of combined silver nanoparticles and common antibiotics. Environ Sci Technol. 2016;50(16):8840–8848. doi: 10.1021/acs.est.6b00998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Zhu H, Shen Y, Zhang W, Zhang L. Antibacterial activity of silver Nanoparticles of different particles against Vibrio natriegens. PLoS ONE. 2019;14(9):e0222322. doi: 10.1371/journal.pone.0222322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos CA, Seckler MM, Ingle AP, Gupta I, Galdiero S, Galdiero M, Gade A, Rai M. Silver nanoparticles: therapeutical uses, toxicity, and safety issues. J Pharm Sci. 2014;103:1931–1944. doi: 10.1002/jps.24001. [DOI] [PubMed] [Google Scholar]
- Durán N, Marcato PD, Conti RD, Alves OL, Costa FTM, Brocchi M. Potential use of silver nanoparticles on pathogenic bacteria, their toxicity and possible mechanisms of action. J Braz Chem Soc. 2010;21:949–959. [Google Scholar]
- Dye C. After 2015: infectious diseases in a new era of health and development. Philos Trans R Soc Lond B. 2014;369:20130426. doi: 10.1098/rstb.2013.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Batal AI, El-Sayyad GS, El-Ghamery A, Gobara M. Response surface methodology optimization of melanin production by Streptomyces cyaneus and synthesis of copper oxide nanoparticles using gamma radiation. J Clust Sci. 2017;28:1083–1112. [Google Scholar]
- Ellis DH, Maurer-Gardner EI, Sulentic CE, Hussain SM. Silver nanoparticle antibacterial efficacy and resistance development in key bacterial species. Biomed Phys Eng Express. 2018;5:015013. [Google Scholar]
- El-Sayed MT, El-Sayed AS. Biocidal activity of metal nanoparticles synthesized by Fusarium against multidrug-resistant bacteria and mycotoxigenic fungi. J Microbiol Biotechnol. 2020;30(2):226–236. doi: 10.4014/jmb.1906.06070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escarcega-Gonzalez CE, Garza-Cervantes JA, Vazquez-Rodriguez A, Montelongo-peralta LZ, Trerino-Gonzalez MT, Barriga-Castro DE, Saucedo-Salazar EM, Chavez-Morales RM, Regalado-Soto DI, Trevino-Gonzalez FM, Carvazco-Rosales JL, Villalobos-Cruz R. In vitro antimicrobial activity of silver nanoparticles produced via a green chemistry synthesis using Acacia rigidula as a reducing and capping agent. Int J Nanomed. 2018;13:2349–2363. doi: 10.2147/IJN.S160605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estevez H, Palacios A, Gil D, Anguita J, Vallet-Regi M, González B, Prados-Rosales R, Luque-Garcia JL. Antimycobacterial effect of selenium nanoparticles on mycobacterium tuberculosis. Front Microbiol. 2020;11:800. doi: 10.3389/fmicb.2020.00800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faghihzadeh F, Anaya N, Astudillo-Castro C, Oyanedel-Craver V. Kinetic, metabolic and macromolecular response of bacteria to chronic nanoparticle exposure in continuous culture. Environ Sci Nano. 2018;5:1386–1396. [Google Scholar]
- Fan Y, Pauer AC, Gonzales AA, Fenniri H. Enhanced antibacterial activity of ampicillin conjugated to gold nanoparticles on PEGylated rosette nanotubes. Int J Nanomed. 2019;14:7281–7289. doi: 10.2147/IJN.S209756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq U, Ahmad T, Khan A, Sarwar R, Shafiq J, Raza Y, Ahmed A, Ullah S, Ur Rehman N, Al-Harrasi A. Rifampicin conjugated silver nanoparticles: a new arena for development of antibiofilm potential against methicillin resistant Staphylococcus aureus and Klebsiella pneumonia. Int J Nanomed. 2019;14:3983–3993. doi: 10.2147/IJN.S198194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzana R, Iqra P, Shafaq F, Sumaira S, Zakia K, Hunaiza T, Husna M. Antimicrobial behavior of zinc oxide nanoparticles and β-lactam antibiotics against pathogenic bacteria. Arch Clin Microbiol. 2017;8(4):57. [Google Scholar]
- Fayaz AM, Balaji K, Girilal M, Yadav R, Kalaichelvan PT, Venketesan R. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: a study against gram-positive and gram-negative bacteria. Nanomedicine. 2010;6:103–109. doi: 10.1016/j.nano.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Fenollar F, Mediannikov O. Emerging infectious diseases in Africa in the 21st century. N Microbe N Infect. 2018;26:S10–S18. doi: 10.1016/j.nmni.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finley PJ, Norton R, Austin C, Mitchell A, Zank S, Durham P. Unprecedented silver resistance in clinically isolated Enterobacteriaceae: major implications for burn and wound management. Antimicrob Agents Chemother. 2015;59:4734–4741. doi: 10.1128/AAC.00026-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Lopez L, Espinoza-Gomez H, Somanathan R. Silver nanoparticles: electron transfer, reactive oxygen species, oxidative stress, beneficial and toxicological effects. Mini Review J Appl Toxicol. 2019;39(1):16–26. doi: 10.1002/jat.3654. [DOI] [PubMed] [Google Scholar]
- Folorunso A, Akintelu S, Oyebamiji AK, Ajaji S, Abiola B, Abdusalam I, Morakinyo A. Biosynthesis, characterization and antimicrobial activity of gold nanoparticles from leaf extracts of Annona muricata. J Nanostructure Chem. 2019;9:111–117. [Google Scholar]
- Foster HA, Ditta IB, Varghese S, Steele A. Photocatalytic disinfection using titanium dioxide: spectrum and mechanism of antimicrobial activity. Appl Microbiol Biotechnol. 2011;90(1):1847–1868. doi: 10.1007/s00253-011-3213-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielyan L, Hakobyan L, Horhannisyan A, Trchounian A. Effects of iron oxide (Fe3O4) nanoparticles on E. coli antibiotic resistant strains. J Appl Microbiol. 2019;26:4. doi: 10.1111/jam.14214. [DOI] [PubMed] [Google Scholar]
- Galanzha EI, Shashkov E, Sarimollaoglu M, Beenken KE, Basnakian AG, Shirtliff ME, Kim J-W, Smeltzer MS, Zharov VP. In vivo magnetic enrichment, photoacoustic diagnosis, and photothermal purging of infected blood using multifunctional gold and magnetic nanoparticles. PLoS ONE. 2012;7(9):e45557. doi: 10.1371/journal.pone.0045557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza-Cervantees JA, Escarcega G, Marichal-Cancino BA, Lopez EDB, Mendiola-Garza G, Marichal-Cancino BA, Lopez-Vazquez MA, Morones-Ramirez JR. Antimicrobial and antibiofilm activity of biopolymer-Ni, Zn nanoparticles biocomposites sythesized using R. mucilaginosa UANL-00IL exopolysaccharide as a capping agent. Int J Nanomed. 2019;14:2557–2571. doi: 10.2147/IJN.S196470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaderi L, Moghimi R, Aliahmadi A, McClements DJ, Rafati H. Development of antimicrobial nanoemulsion-based delivery systems against selected pathogenic bacteria using a thymol-rich Thymus daenensis essential oil. J Appl Microbiol. 2017;123:832–840. doi: 10.1111/jam.13541. [DOI] [PubMed] [Google Scholar]
- Ghasemi F, Halal R. Antimicrobial action of zinc oxide nanoparticles in combination with ciprofloxacin and ceftazidime against multi drug resistant Acinetobacter baumannii. J Glob Antimicrob Resist. 2016;6:118–122. doi: 10.1016/j.jgar.2016.04.007. [DOI] [PubMed] [Google Scholar]
- Global Health Estimates . Deaths by cause, age, sex, by country and by region, 2000–2016. Geneva: World Health Organization; 2016. [Google Scholar]
- González-Delgado JA, Castro PM, Machado A, et al. Hydrogels containing porphyrin-loaded nanoparticles for topical photodynamic applications. Int J Pharm. 2016;510(1):221–231. doi: 10.1016/j.ijpharm.2016.06.037. [DOI] [PubMed] [Google Scholar]
- Gou N, Onnis-Hayden A, Gu AZ. Mechanistic toxicity assessment of nanomaterials by whole-cell-array stress genes expression analysis. Environ Sci Technol. 2010;44:5964–5970. doi: 10.1021/es100679f. [DOI] [PubMed] [Google Scholar]
- Gounani Z, Asadollahi MA, Pedersen JN, Lyngso J, Pedersen JS, Arpanaei A, Meyer RL. Mesoporous silver nanoparticles carrying multiple antibiotics provide enhanced synergistic effect and improved biocompatibility. Colloids Surf B. 2018;175:498–508. doi: 10.1016/j.colsurfb.2018.12.035. [DOI] [PubMed] [Google Scholar]
- Graves JL, Jr, Tajkarimi M, Cunningham Q, Campbell A, Nonga H, Harrison SH, Barrick JE. Rapid evolution of silver nanoparticle resistance in Escherichia coli. Front Genet. 2015;6:42. doi: 10.3389/fgene.2015.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumezescu AM. Nanoscale fabrication, optimization, scale-up and biological aspects of pharmaceutical nanotechnology. London: Elsevier Inc.; 2018. [Google Scholar]
- Guo J, Gao SH, Lu J, Bond PL, Verstraete W, Yuan Z. Copper oxide nanoparticles induce lysogenic bacteriophage and metal-resistance genes in Pseudomonas aeruginosa PAO1. ACS Appl Mater Interfaces. 2017;9:22298–22307. doi: 10.1021/acsami.7b06433. [DOI] [PubMed] [Google Scholar]
- Gurav VL, Samant RA, Manjaer SB, Patil UK, Solkar SR, Moghe SS. Biosynthesis of calcium oxide nanoparticles using Ocimum sanctum (Tulsi) leaf extracts and screening its antimicrobial activity. Asian J Nanosci Mater. 2020;3:115–120. [Google Scholar]
- Habibipour R, Moradi-Haghgou L, Farmany A. Green synthesis of AgNps@PPE and its Pseudomonas aeruginosa biofilm formation activity compared to pomegranate peel extract. Int J Nanomed. 2019;14:6891–6899. doi: 10.2147/IJN.S209912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hachicho N, Hoffmann P, Ahlert K, Heipieper VHJ. Effect of silver nanoparticles and silver ions on growth and adaptive response mechanisms of Pseudomonas putida mt-2. FEMS Microbiol Lett. 2014;355:71–77. doi: 10.1111/1574-6968.12460. [DOI] [PubMed] [Google Scholar]
- Hall CW, Mah TF. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol Rev. 2017;41(3):276–301. doi: 10.1093/femsre/fux010. [DOI] [PubMed] [Google Scholar]
- Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2(2):95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- Halstead FD, Thwaite JE, Burt R, Laws TR, Raguse M, Moeller R, et al. Antibacterial activity of blue light against nosocomial wound pathogens growing planktonically and as mature biofilms. Appl Environ Microbiol. 2016;82:4006–4016. doi: 10.1128/AEM.00756-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamouda RA, Hussein MH, Abo-elmagd RA, Bawazir SS. Synthesis and biological characterization of silver nanoparticles derived from the Cyanobacterium oscillatoria limnetica. Sci Rep. 2019;9:13071. doi: 10.1038/s41598-019-49444-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Ingudam S, Reed S, Gehring A, Strobaugh TP, Jr, Irwin P. Study on the mechanism of antibacterial action of magnesium oxide nanoparticles against foodborne pathogens. J Biotechnol. 2016;14:54. doi: 10.1186/s12951-016-0202-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmlinger J, Sengstock C, Groß-Heitfeld C, Mayer C, Schildhauer T, Köller M, Epple M. Silver nanoparticles with different size and shape: equal cytotoxicity, but different antibacterial effects. RSC Adv. 2016;6:18490–18501. [Google Scholar]
- Hemeg HA. Nanomaterials for alternative antibacterial therapy. Int J Nanomed. 2017;12:8211–8225. doi: 10.2147/IJN.S132163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain MdM, Polash SA, Takikawa M, Shubhra RD, Saha T, Islam Z, Hossain S, Hasan MdA, Takeoka S, Sarker SR. Investigation of the antibacterial activity and in vivo cytotoxicity of biogenic silver nanoparticles as potent therapeutics. Front Bioeng Biotechnol. 2019;9:239. doi: 10.3389/fbioe.2019.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao C-W, Chen H-L, Liao Z-X, Sureshbabu R, Hsiao H-C, Lin S-J, Chang Y, Sung H-W. Effective photothermal killing of pathogenic bacteria by using spatially tunable colloidal gels with nano-localized heating sources. Adv Funct Mater. 2015;25(5):721–728. [Google Scholar]
- Hu B, Zhang L-P, Chen X-W, Wang J-H. Gold nanorod-covered kanamycin-loaded hollow SiO2 (HSKAurod) nanocapsules for drug delivery and photothermal therapy on bacteria. Nanoscale. 2013;5:246–252. doi: 10.1039/c2nr32457a. [DOI] [PubMed] [Google Scholar]
- Hu M, Li C, Li X, Zhou M, Sun J, Sheng F, Shi S, Lu L. Zinc oxide/silver bimetallic nanoencapsulated in Prp/PCL nanofibres for improved antibacterial activity. Artif Cells Nanomed Biotecnol. 2017;46(6):1248–1257. doi: 10.1080/21691401.2017.1366339. [DOI] [PubMed] [Google Scholar]
- Hu X, Saravanakumar K, Jin T, Wang M-H. Mycosynthesis, characterization, anticancer and antibacterial activity of silver nanoparticles from endophytic fungus Talaromyces purpureogenus. Int J Nanomed. 2019;14:3427–3438. doi: 10.2147/IJN.S200817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua S, de Matos MBC, Metselaar JM, Storm G. Current trends and challenges in the clinical translation of nanoparticulate nanomedicines: pathways for translational development and commercialization. Front Pharmacol. 2018;9:790. doi: 10.3389/fphar.2018.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Gao Y, Zhang Y, Cheng T, Ou H, Yang L-J, Liu J, Shi L, Liu J. Silver-decorated polymeric micelles combined with curcumin for enhanced antibacterial agents. J Nanosci Nanotechnol. 2020;15:3574. doi: 10.1021/acsami.7b03347. [DOI] [PubMed] [Google Scholar]
- Huq MdA. Green synthesis of silver nanoparticles using Pseudoduganella eburnean MAHUQ-39 and their antimicrobial mechanisms investigation against drug resistant human pathogens. Int J Mol Sci. 2020;21:1510. doi: 10.3390/ijms21041510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain S, Joo J, Kang J, Kim B, Braun GB, She Z-G, Kim D, et al. Antibiotic-loaded nanoparticles targeted to the site of infection enhance antibacterial efficacy. Nat Biomed Eng. 2018;2:95–103. doi: 10.1038/s41551-017-0187-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijaz F, Shahid S, Khan SA, Ahmad W, Zaman S. Green synthesis of copper oxide nanoparticles using Abutilon indicum leaf extract: antimicrobial antioxidant and photocatalytic dye degradation activities. Trop J Pharm Res. 2017;16(4):743–753. [Google Scholar]
- Jagathesan GP, Rajiv P. Biosynthesis and characterization of iron oxide nanoparticles using Eichhornia crassipes leaf extract and assessing their antibacterial activity. Biocatal Agric Biotechnol. 2018;13:90–94. [Google Scholar]
- Jain PK, Huang X, El-Sayed IH, El-Sayed MA. Review of some interesting surface plasmon resonance enhanced properties of noble metal nanoparticles and their applications to biosystems. Plasmonics. 2007;2(3):107–118. [Google Scholar]
- Jaworski S, Wierzbicki M, Sawosz E, Jung A, Gielerak G, Biernat J, Jaremek H, Lojkowski W, et al. Chwalibog graphene oxide-based nanocomposites decorated with silver nanoparticles as an antibacterial agent. Nanoscale Res Lett. 2018;13:116. doi: 10.1186/s11671-018-2533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji H, Dong K, Yan Z, Ding C, Chen Z, Ren J, Qu X. Bacterial hyaluronidase self-triggered prodrug release for chemophotothermal synergistic treatment of bacterial infection. Small. 2016;12:6200–6206. doi: 10.1002/smll.201601729. [DOI] [PubMed] [Google Scholar]
- Jordan S, Hutchings MI, Mascher T. Cell envelope stress response in Gram-positive bacteria. FEMS Microbiol Rev. 2008;32:107–146. doi: 10.1111/j.1574-6976.2007.00091.x. [DOI] [PubMed] [Google Scholar]
- Kalita S, Kandimalla R, Sharma KK, Kataki AC, Deka M, Kotoky J. Amoxicillin functionalized gold nanoparticles reverts MRSA resistance. Mater Sci Eng. 2016;61:720–727. doi: 10.1016/j.msec.2015.12.078. [DOI] [PubMed] [Google Scholar]
- Katayama B, Ozawa T, Morimoto K, Awazu K, Ito N, Honda N, et al. Enhanced sterilization and healing of cutaneous pseudomonas infection using 5-aminolevulinic acid as a photosensitizer with 410-nm LED light. J Dermatol Sci. 2018;90:323–331. doi: 10.1016/j.jdermsci.2018.03.001. [DOI] [PubMed] [Google Scholar]
- Kaur P, Nene AG, Sharma D, Somani PR, Tuli HS. Synergistic effect of copper nanoparticles and antibiotics to enhance antibacterial potential. Bio Mater Technol. 2019;1(1):33–47. [Google Scholar]
- Kedziora A, Speruda M, Kezyzewska E, Rybka J, Lukowiak A, Bugla-Pl-ploskoriska G. Similarities and differences between silver ions and silver in nanoforms as antibacterial agents. Int J Mol Sci. 2018;19(2):444. doi: 10.3390/ijms19020444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalandi B, Asadi N, Milani M, Davaran S, Abadi AJN, Abasi E, Akbarzadeh A. A review on potential role of silver nanoparticles and possible mechanisms of their actions on bacteria. Drug Res. 2017;11:70–76. doi: 10.1055/s-0042-113383. [DOI] [PubMed] [Google Scholar]
- Khan ST, Musarrat J, Al-Khedhairy AA. Countering drug resistance, infectious diseases, and sepsis using metal and metal oxides nanoparticles: current status. Colloids Surf B. 2016;146:70–83. doi: 10.1016/j.colsurfb.2016.05.046. [DOI] [PubMed] [Google Scholar]
- Kim SH, Kang EB, Jeong CJ, Sharker SMd, Ln I, Park SY. Light controllable surface coating for effective photothermal killing of bacteria. ACS Appl Mater Inter. 2015;7(28):15600–15606. doi: 10.1021/acsami.5b04321. [DOI] [PubMed] [Google Scholar]
- Korupalli C, Huang CC, Lin W-C, Pan W-Y, Lin P-Y, Wan W-L, Li M-J, Chang Y, Sung H-W. Acidity-triggered charge-convertible nanoparticles that can cause bacterium-specific aggregation in situ to enhance photothermal ablation of focal infection. Biomaterials. 2017;116:1–9. doi: 10.1016/j.biomaterials.2016.11.045. [DOI] [PubMed] [Google Scholar]
- Krishnamoorthy K, Manivannan G, Kim SJ, Jeyasubramanian K, Premanathan M. Antimicrobial activity of MgO nanoparticles based on lipid peroxidation by oxygen vacancy. J Nanoparticles Res. 2012;14:1063. [Google Scholar]
- Kuang Y, Zhang K, Cao Y, Chen X, Wang K, Liu M, Pei R. Hydrophobic IR-780 dye encapsulated in cRGD-conjugated solid lipid n for NIR imaging-guided photothermal therapy. ACS Appl Mater Inter. 2017;9:12217–12226. doi: 10.1021/acsami.6b16705. [DOI] [PubMed] [Google Scholar]
- Kumar A, Pandey AK, Singh SS, Shanker R, Dhawan A. Engineered ZnO and TiO(2) nanoparticles induce oxidative stress and DNA damage leading to reduced viability of Escherichia coli. Free Radic Biol Med. 2011;51:1872–1881. doi: 10.1016/j.freeradbiomed.2011.08.025. [DOI] [PubMed] [Google Scholar]
- Kundu S. Gold nanoparticles: their application as antimicrobial agents and vehicles of gene delivery. Adv Biotechnol Microbiol. 2017;4:5. [Google Scholar]
- Landage KS, Arabade GK, Khanna P, Bhongale CT. Biological approach to synthesize TiO2 nanoparticles using Staphylococcus aureus for antibacterial and antibiofilm applications. J Microbiol Exp. 2020;8(1):36–43. [Google Scholar]
- Lebeaux D, Chauhan A, Rendueles O, Beloin C. From in vitro to in vivo models of bacterial biofilm related infections. Pathogens. 2013;2(2):288–356. doi: 10.3390/pathogens2020288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NY, Ko W-C, Hsueh P-R. Nanoparticles in the treatment of infections caused by multidrug-resistant organisms. Front Pharmacol. 2019;10:1153. doi: 10.3389/fphar.2019.01153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XZ, Nikaido H, Williams KE. Silver-resistant mutants of Escherichia coli display active efflux of Ag+ and are deficient in porins. J Bacteriol. 1997;179:6127–6132. doi: 10.1128/jb.179.19.6127-6132.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Li J, Wu C, Wu Q, Li J. Synergistic antibacterial effects of β-lactam antibiotic combined with silver nanoparticles. Nanotechnology. 2005;16:1912. [Google Scholar]
- Li Z, Greden K, Alvarez PJ, Gregory KB, Lowry GV. Adsorbed polymer and NOM limits adhesion and toxicity of nano scale zerovalent iron to E. coli. Environ Sci Technol. 2010;44:3462–3467. doi: 10.1021/es9031198. [DOI] [PubMed] [Google Scholar]
- Li M, Zhu L, Lin D. Toxicity of ZnO nanoparticles to Escherichia coli: mechanism and the influence of medium components. Environ Sci Technol. 2011;45:1977–1983. doi: 10.1021/es102624t. [DOI] [PubMed] [Google Scholar]
- Li S, Zhu T, Huang J, Guo Q, Chen G, Lai Y. Durable antibacterial and UV-protective Ag/TiO2@ fabrics for sustainable biomedical application. Int J Nanomed. 2017;12:2593–2606. doi: 10.2147/IJN.S132035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao S, Zhang Y, Pan X, Zhu F, Jiang G, Liu Q, Cheng Z, Dai G, Wu G, Wang L, Chen L. Antibacterial activity and mechanism of silver nanoparticles against multidrug-resistant Pseudomonas aeruginosa. Int J Nanomed. 2019;14:1469–1487. doi: 10.2147/IJN.S191340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Z, Zhang W, Qiao Z, Luo J, Ai Niwaer AE, Meng X, Wang H, Li X, Zuo F, Zhao Z. Dopamine-assisted one-pot synthesis of gold nanoworms and their application as photothermal agents. J Colloid Interface Sci. 2020;562:81–90. doi: 10.1016/j.jcis.2019.11.055. [DOI] [PubMed] [Google Scholar]
- Lin Z, Monteiro-Riviere NA, Riviere JE. Pharmacokinetics of metallic nanoparticles. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2015;7:189–217. doi: 10.1002/wnan.1304. [DOI] [PubMed] [Google Scholar]
- Linsley CS, Wu BM. Recent advances in light-responsive on–demand drug delivery systems. Ther Deliv. 2017;8(2):89–107. doi: 10.4155/tde-2016-0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Duan W, Wang Q, Li X. The damage of outer membrane of Escherichia coli in the presence of TiO2 combined with UV light. Colloids Surf B. 2010;78:171–176. doi: 10.1016/j.colsurfb.2010.02.024. [DOI] [PubMed] [Google Scholar]
- Lopez-Carrizales M, Velasco KKI, Castillo C, Floves A, Magana M, Martinez-Castanon GA, Martinez-Gutierrez F. In vitro synergism of silver nanoparticles with antibiotics as an alternative treatment in multiresistant uropathogens. Antibiotics. 2018;7:50. doi: 10.3390/antibiotics7020050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma BC, Ghasimi S, Landfester K, et al. Enhanced visible light promoted antibacterial efficiency of conjugated microporous polymer nanoparticles via molecular doping. J Mater Chem B. 2016;4:5112–5118. doi: 10.1039/c6tb00943c. [DOI] [PubMed] [Google Scholar]
- Madivoli ES, Kareru PG, Maina EG, Nyabola AO, Wanakai SI, Nyang’au JO. Biosynthesis of iron nanoparticles using Ageratum conyzoides extracts, their antimicrobial and photocatalytic activity. SN Appl Sci. 2019;1(5):500. [Google Scholar]
- Malaikozhundan B, Vinodhini J, Kalanjiam MAR, Vinotha V, Palanisamy S, Vijayakumar S, Vaseeharan B, Mariyappan A. High synergistic antibacterial, antibiofilm, antidiabetic and antimetabolic activity of withania Somnifera leaf extract-assisted zinc oxide nanoparticle bioprocess. Biosyst Eng. 2020 doi: 10.1007/500449-020-02346-0. [DOI] [PubMed] [Google Scholar]
- Maliszewska I, Kałas W, Wysokiéska E, Tylus W, Pietrzyk N, Popko K, et al. Enhancement of photo-bactericidal effect of tetrasulfonated hydroxyaluminum phthalocyanine on Pseudomonas aeruginosa. Lasers Med Sci. 2018;33:79–88. doi: 10.1007/s10103-017-2337-0. [DOI] [PubMed] [Google Scholar]
- Mandava K, Kadimcharla K, Keesara NR, Sumayya NF, Prathyusha B, Batchu UR. Green synthesis of stable copper nanoparticles and synergistic activity with antibiotics. Indian J Pharm Sci. 2017;79(5):695–700. [Google Scholar]
- Manivasagan P, Khan F, Hoang G, Mondal S, Kim H, Doan VHM, Kim Y-M, Oh J. Thiol chitosanwrapped gold nanoshells for near-infrared laser-induced photothermal destruction of antibiotic-resistant bacteria. Carbohydr Polym. 2019;225:115228. doi: 10.1016/j.carbpol.2019.115228. [DOI] [PubMed] [Google Scholar]
- Maurer LL, Meyer JN. A systematic review of evidence for silver nanoparticle-induced mitochondrial toxicity. Environ Sci Nano. 2016;3:311–322. [Google Scholar]
- Mba IE, Nweze EI. The use of nanoparticles as alternative therapeutic agents against Candida infections: an up-to-date overview and future perspectives. World J Microbiol Biotech. 2020;36:163. doi: 10.1007/s11274-020-02940-0. [DOI] [PubMed] [Google Scholar]
- Mba IE, Sharndama HC, Osondu-Chuka GO, Okeke OP. Immunobiology and nanotherapeutics of severe acute respiratory syndrome 2 (SARS-CoV-2): a current update. Infect Dis. 2021 doi: 10.1080/23744235.2021.1916071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker DG, Jenkins SV, Miller EK, Beenken KE, Loughran AJ, Powless A, Muldoon TJ, et al. Synergistic photothermal and antibiotic killing of biofilm associated Staphylococcus aureus using targeted antibiotic-loaded gold nanoconstructs. ACS Infect Dis. 2016;2:241–250. doi: 10.1021/acsinfecdis.5b00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meza-villezcas A, Gallego-Hernandez AL, Yildiz FH, Jaime-Acuna DE, Raymond-Herrera O, Huerta-Saquero A. Effect of antimicrobial nanocomposites on Vibrio cholera lifestyles: pellicle biofilm, planktonic and surface-attached biofilm. PLoS ONE. 2019;14(6):e0217869. doi: 10.1371/journal.pone.0217869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocan L, Ilie I, Matea C, Tabaran F, Kalman E, Iancu C, Mocan T. Surface plasmon resonance-induced photoactivation of gold nanoparticles as bactericidal agents against methicillin-resistant Staphylococcus aureus. Int J Nanomed. 2014;9:1453–1461. doi: 10.2147/IJN.S54950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed MA. Myco-engineered gold nanoparticles from Jahnula aquatica coated with ampicillin/amoxicillin and their antibacterial and anticancer activity against cancer cells. Biotechnol Lett. 2020;42:151–170. doi: 10.1007/s10529-019-02764-5. [DOI] [PubMed] [Google Scholar]
- Möhler JS, Sim W, Blaskovich MA, Cooper MA, Ziora ZM. Silver bullets: a new lustre on an old antimicrobial agent. Biotechnol Adv. 2018;36(5):1391–1411. doi: 10.1016/j.biotechadv.2018.05.004. [DOI] [PubMed] [Google Scholar]
- Morgan DJ, Okeke IN, Laxminarayan R, Perencevich EN, Weisenberg S. Non-prescription antimicrobial use worldwide: a systematic review. Lancet Infect Dis. 2011;11:692–701. doi: 10.1016/S1473-3099(11)70054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moteriya P, Padalia H, Chanda S. Characterization, synergistic antibacterial and free radical scavenging efficacy of silver nanoparticles synthesized using Cassia roxburghii leaf extract. J Genet Eng Biotechnol. 2017;15(2):505–513. doi: 10.1016/j.jgeb.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muflikhun MA, Frommelt MC, Farman M, Chua AY, Santos GNC. Structures mechanical properties and antibacterial activity of Ag/TiO2 nanocomposite materials synthesized via HVPG technique for coating application. Heliyon. 2019;5:e01475. doi: 10.1016/j.heliyon.2019.e01475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munita JM, Arias CA. Mechanisms of antibiotics resistance. Microbiol Spectr. 2016 doi: 10.1128/microbiolspec.VMBF-0016-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabavizadeh M, Abbaszadegan A, Gholami A, Kadkhoda Z, Mirhadi H, Ghasemi Y, Safari A, Hemmateenejad B, Dorostkar S, Sharghi H. Antibiofilm efficacy of positively charged imidazolium-based silver nanoparticles in Enterococcus faecalis using quantitative real-time PCR. Jundishapur J Microbiol. 2017 doi: 10.5812/jjm.55616. [DOI] [Google Scholar]
- Nathwani D, Raman G, Sulham K, Gavaghan M, Menon V. Clinical and economic consequences of hospital acquired resistant and multidrug-resistant Pseudomonas aeruginosa infections: a systematic review and meta-analysis. Antimicrob Resist Infect Control. 2014;3(32):1–16. doi: 10.1186/2047-2994-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nejabatdoust A, Zamani H, Salehzadeh A. Functionalization of ZnO nanoparticles by glutamic acid and conjugation with thiosemicarbazide alters expression of efflux pump genes in multiple drug-resistant Staphylococcus aureus strains. Microb Drug Resist. 2019;25(7):966–974. doi: 10.1089/mdr.2018.0304. [DOI] [PubMed] [Google Scholar]
- Nguyen N-YT, Gruelling N, Wetteland CL, Rosario R, Liu H. Antimicrobial activities and mechanisms of magnesium oxide nanoparticles (nMgO) against pathogenic bacteria, yeasts, and biofilms. Sci Rep. 2018;8:16260. doi: 10.1038/s41598-018-34567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nino-Martinez N, Orozco MFS, Martinez-Castanon G-A, Mendez FT, Ruiz F. Molecular mechanisms of bacterial resistance to metal and metal oxide nanoparticles. Int J Mol Sci. 2019;20:2808. doi: 10.3390/ijms20112808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nour El Din S, El-Tayeb TA, Abou-Aisha K, El-Azizi M. In vitro and in vivo antimicrobial activity of combined therapy of silver nanoparticles and visible blue light against Pseudomonas aeruginosa. Int J Nanomed. 2016;11:1749–1758. doi: 10.2147/IJN.S102398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunez YAR, Castro RI, Arenas FA, Lopez-Cabana ZE, Carreno G, Carasco-Sanchez V, Marican A, Villasenor J, Vargas E, Santos LS, Duran-Lara EF. Preparation of hydrogel/silver nanohybrids mediated by tunable-size nanoparticles for potential antibacterial applications. Polymers. 2019;11:716. doi: 10.3390/polym11040716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogungemi SO, Zhang F, Abdallah Y, Zhang M, Wang Y, Sun G, Qiu W, Li B. Biosynthesis and characterization of magnesium oxide and manganese dioxide nanoparticles using Matricaria Chamomilla leaf extract and its inhibitoru effect on Acidovorax oryzae strain RS-2. Artif Cells Nanomed Biotechnol. 2019;47(1):2230–2239. doi: 10.1080/21691401.2019.1622552. [DOI] [PubMed] [Google Scholar]
- Osonga FJ, Akgul A, Yazgan I, Akgul A, Eshun GB, Sakhaee L, Sadik OA. Size and shape-dependent antimicrobial activities of silver and gold nanoparticles: a model study as potential fungicides. Molecules. 2020;25:2682. doi: 10.3390/molecules25112682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomo-Siguero M, Gutiérrez AMA, Pérez-Conde C, Madrid Y. Effect of selenite and selenium nanoparticles on lactic bacteria: a multi-analytical study. Microchem J. 2016;126:488–495. [Google Scholar]
- Panacek A, Smekalova M, Kilianova M, Prucek R, Bogdanova K, Vecerova R, Kolar M, Havrdova M, Plaza GA, Chojniak J, Zboril R, Kvitek L. Strong and nonspecific synergistic antibacterial efficiency of antibiotics combined with silver nanoparticles at very low concentrations showing no cytotoxic effect. Molecules. 2015;21:E26. doi: 10.3390/molecules21010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panacek A, Kvitek L, Smekalova M, Vecerova R, Kolar M, Roderova M, Dycka F, Sebela M, Prucek R, Tomanec O, Zboril R. Bacterial resistance to silver nanoparticles and how to overcome it. Nature Nanotechnol. 2018;13:65–71. doi: 10.1038/s41565-017-0013-y. [DOI] [PubMed] [Google Scholar]
- Pandiyan N, Murugesan B, Arumugam M, Sonamuthu J, Samayanan S, Mahalingam S. Ionic liquid. A greener templating agent with Justicia adhatida plant extract assisted green synthesis of morphologically improved Ag-Au/ZnO nanostructures and its antibacterial and anticancer activities. J Photochem Photobiol B Biol. 2019;198:111559. doi: 10.1016/j.jphotobiol.2019.111559. [DOI] [PubMed] [Google Scholar]
- Pasupathy S, Rajamanickam M. Synthesis of pure and bio modified calcium oxide (CaO) nanoparticles using waste chicken egg shells and evaluation of its antibacterial activity. Int J Pharma Sci Res. 2019;1:230. [Google Scholar]
- Patra JK, Baek KH. Antibacterial activity and synergistic antibacterial potential of biosynthesized silver nanoparticles against food borne pathogenic bacteria along with its anticandidal and antioxidant effects. Front Microbiol. 2017;8:167. doi: 10.3389/fmicb.2017.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petros RA, DeSimone JM. Strategies in the design of nanoparticles for therapeutic applications. Nat Rev Drug Discov. 2010;9(8):615–627. doi: 10.1038/nrd2591. [DOI] [PubMed] [Google Scholar]
- Pranjali L, Meher MK, Raj R, Prasad N, Poluri KM, Kumar D, Guleria A. Physiochemical and antibacterial properties of PEGylated zinc oxide nanoparticles dispersed in peritoneal dialysis fluid. ACS Omega. 2019;4:19255–19264. doi: 10.1021/acsomega.9b02615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi M, Chi M, Sun X, Xie X, Weir MD, Oates TW, Zhou Y, Wang L, Bai Y, Xu HHK. Nanomaterial-based antibacterial photodynamic therapies to combat oral biofilms and infectious diseases. Int J Nanomed. 2019;14:6937–6956. doi: 10.2147/IJN.S212807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing Y, Cheng L, Li R, Liu G, Zhang Y, Tang X, Wang J, Liu H, Qin Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int J Nanomed. 2018;13:3311–3327. doi: 10.2147/IJN.S165125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Z, Shen Z, Qian D, Jin M, Yang D, Wang J, Zhang B, Yang Z, Chen Z, Wang X. Effects of nano-TiO2 on antibiotic resistance transfer mediated by RP4 plasmid. Nanotoxicology. 2015;9:895–904. doi: 10.3109/17435390.2014.991429. [DOI] [PubMed] [Google Scholar]
- Qiu M, Wang D, Liang W, Liu L, Zhang Y, Chen X, Sang DK, et al. Novel concept of the smart NIR-light-controlled drug release of black phosphorus nanostructure for cancer therapy. Proc Natl Acad Sci USA. 2018;115:501–506. doi: 10.1073/pnas.1714421115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan AS, Khan A, Asrar S, Raza H, Das RK, Sahu NK. Synthesis of ZnO/Fe3O4/rGo nanocomposites and evaluation of antibacterial activities towards E. coli and S. aureus. IET Nanobiotchnol. 2019;13(7):682–687. doi: 10.1049/iet-nbt.2018.5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalingam V, Sundaramahalingam S, Rajaram R. Size-dependent antimycobacterial activity of titanium oxide nanoparticles against mycobacterium tuberculosis. J Mater Chem B. 2019;7:4338–4346. [Google Scholar]
- Ramezani P, Abnous K, Taghdisi SM, Zahiri M, Ramezani M, Alibolandi M. Targeted MMP-2 responsive chimeric polymersomes for therapy against colorectal cancer. Colloids Surf B. 2020;193:111135. doi: 10.1016/j.colsurfb.2020.111135. [DOI] [PubMed] [Google Scholar]
- Ran X, Du Y, Wang Z, Wang H, Pu F, Ren J, Qu X. Hyaluronic acid-templated Ag nanoparticles/graphene oxide composites for synergistic therapy of bacteria infection. ACS Appl Mater Interfaces. 2017;9:19717–19724. doi: 10.1021/acsami.7b05584. [DOI] [PubMed] [Google Scholar]
- Rao TN, Babji RP, Ahmad N, Khan RA, Hassan I, Shahzad SA, Husain FM. Green synthesis and structural classification of Acacia nilotica mediated-silver doped titanium oxide (Ag/TiO2) spherical nanoparticles: assessment of its antimicrobial and anticancer activity. Saudi J Biol Sci. 2019;26:1385–1391. doi: 10.1016/j.sjbs.2019.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed T, Bilal M, Li C, Iqbal HMN. Biomedical potentialities of Taraxacum officinale-based nanoparticles biosynthesized using methanolic leaf extract. Curr Pharma Biotechnol. 2017;18:14. doi: 10.2174/1389201019666180214145421. [DOI] [PubMed] [Google Scholar]
- Rathnayake K, Patel U, Pham C, McAlpin A, Budisalich T, Jayawardena SN. Targeted delivery of antibiotic therapy to inhibit pseudomonas aeruginosa using lipid-coated mesoporous silica core-shell nanoassembly. ACS Appl Bio Mater. 2020;3:6708–6721. doi: 10.1021/acsabm.0c00622. [DOI] [PubMed] [Google Scholar]
- Rattanata N, Klaynongsruang S, Leelayuwat C, Limpaiboon T, Lulitanond A, Boonsiri P, Chio-Srichan S, Soontaranon S, Rugmai S, Daduang J. Gallic acid conjugated with gold nanoparticles: antibacterial activity and mechanism of action on foodborne pathogens. Int J Nanomed. 2016;27(11):3347–3356. doi: 10.2147/IJN.S109795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray PC, Khan SA, Singh AK, Senapati D, Fan Z. Nanomaterials for targeted detection and photothermal killing of bacteria. Chem Soc Rev. 2012;41:3193–3209. doi: 10.1039/c2cs15340h. [DOI] [PubMed] [Google Scholar]
- Raza M, Kanwal Z, Rauf A, Sabri A, Riaz S, Naseem S. Size-and shape-dependent antibacterial studies of silver nanoparticles synthesized by wet chemical routes. Nanomaterials. 2016;6:74. doi: 10.3390/nano6040074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehman S, Jermy BR, Akhtar S, Borgio JF, Azeez SA, Ravinayagam V, Jindan RA, Alsalem ZH, Buhameid A, Gani A. Isolaton and characterization of novel thermophile, Bacillus haynesii, applied for the green synthesis of Zno nanoparticles. Artif Cell Nanomed Biotechnol. 2019;47(1):2072–2082. doi: 10.1080/21691401.2019.1620254. [DOI] [PubMed] [Google Scholar]
- Roca I, Akova M, Baquero F, Carlet J, Cavaleri M, Coenen S, Cohen J, et al. The global threat of antimicrobial resistance: science for intervention. N Microbes N Infect. 2015;6:22–29. doi: 10.1016/j.nmni.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Serrano C, Guzman-Moreno J, Angeles-chavez C, Rodriguez-Gonzalez V, Ortega-Sigala JJ, Ramirez-Santoyo RM, Vidales-Rodriguez LE. Biosynthesis of silver nanoparticles by Fusarium scirpi and its poential as antimicrobial agent against uropathogenic Escherichia coli biofilm. PLoS ONE. 2020;15(3):e0230275. doi: 10.1371/journal.pone.0230275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AS, Parveen A, Koppalkar AR, Prasad MA. Effect of nano-titanium dioxide with different antibiotics against methicillin-resistant Staphylococcus aureus. J Biomater Nanobiotechnol. 2010;1:37. [Google Scholar]
- Saginur R, Stdenis M, Ferris W, Aaron SD, Chan F, Lee C, Ramotar K. Multiple combination bactericidal testing of staphylococcal biofilms from implant-associated infections. Antimicrob Agents Chemother. 2006;50(1):55–61. doi: 10.1128/AAC.50.1.55-61.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjad S, Uzair B, Shaukat A, Jamshed M, Leghari SAK, Ismail M, Mansoor Q. Synergistic evaluation of AgO2 nanoparticles with ceftriaxone against CTXM and blaSHV genes positive ESBL producing clinical strains of uropathogenic E. Coli. IET Nanobiotechnol. 2019;13(4):435–440. doi: 10.1049/iet-nbt.2018.5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez CJ, Mende K, Beckius ML, Akers KS, Romano DR, Wenke JC, Murray CK. Biofilm formation by clinical isolates and the implications in chronic infections. BMC Infect Dis. 2013;13:47. doi: 10.1186/1471-2334-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saravanakumar K, Jeevithan E, Hu X, Chelliah R, Oh D-H, Wang M-H. Enhanced anti-lung carcinoma and anti-biofilm activity of fungal molecules conjugated with β-D-glucan from barley. J Photochem Photobiol B. 2020;203:11728. doi: 10.1016/j.jphotobiol.2019.111728. [DOI] [PubMed] [Google Scholar]
- Saruchi, Thakur P, Kumar V. Kinetics and thermodynamics studies for removal of methylene blue dye by biosynthesized copper oxide nanoparticles and its antibacterial activity. J Environ Health Sci Eng. 2019;17:367–376. doi: 10.1007/s40201-019-00354-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarwar S, Ali A, Pal M, Chakrabarti P. Zinc oxide nanoparticles provide anti-cholera activity by disrupting the interaction of cholera toxin with the human GM1 receptor. J Biol Chem. 2017;292:18303–18311. doi: 10.1074/jbc.M117.793240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathishkumar G, Logeshwaran V, Sarathbabu S, Jha PK, Jeyaraj M, Rajkuberan C, Senthilkumar N, Sivaramakrishnan S. Green synthesis of magnetic Fe3O4 nanoparticles using Couroupita guianensis Aubl. Fruit extract for their antibacterial and cytotoxicity activities. Artif Cells Nanomed Biotechnol. 2018;46(3):589–598. doi: 10.1080/21691401.2017.1332635. [DOI] [PubMed] [Google Scholar]
- Selvaraj RCA, Rajendra M, Nagaiah HP. Re-potentiation of β-lactam antibiotic by synergistic combination with biogenic copper oxide nanocubes against biofilm forming multidrug-resistant bacteria. Molecules. 2019;24:3055. doi: 10.3390/molecules24173055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta J, Ghosh S, Datta P, Gomes A, Gomes A. Physiologically important metal nanoparticles and their toxicity. J Nanosci Nanotechnol. 2014;14:990–1006. doi: 10.1166/jnn.2014.9078. [DOI] [PubMed] [Google Scholar]
- Shahbazi E, Morshedzadeh F, Zaeifi D. Bacteriostatic potency of Fe2O3 against Enterococcus faecalis in synergy with antibiotics by DDST method. Avicenna J Med Biotech. 2019;11(2):176–179. [PMC free article] [PubMed] [Google Scholar]
- Shahverdi AR, Fakhimi A, Shahverdi HR, Minaian S. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomed Nanotechnol Biol Med. 2007;3:168–171. doi: 10.1016/j.nano.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Shaikh S, Nazam N, Rizvi SMD, Ahmad K, Bait MH, Lee EJ. Choice. Mechanistic insights into the antimicrobial actions of metallic nanoparticles and their implication for multi drug resistance. Int J Mol Sci. 2019;20:2468. doi: 10.3390/ijms20102468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamaila S, Zafar N, Riaz S, Sharif R, Nazir J, Naseem S. Gold nanoparticles: an efficient antimicrobial agent against enteric bacterial human pathogen. Nanomaterials. 2016;6:71. doi: 10.3390/nano6040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Jandaik S, Kumar S. Synergistic activity of doped zinc oxide nanoparticles with antibiotics: ciprofloxacin, amoxicillin, fluconazole and amphotericin B against pathogenic microorganisms. An Acad Bras Cienc. 2016;88(3):1689–1698. doi: 10.1590/0001-3765201620150713. [DOI] [PubMed] [Google Scholar]
- Sharma G, Soni R, Jasuja ND. Photo assisted synthesis of magnesium oxide nanoparticles with swertia chirayaita. J Taibah Univ Sci. 2017;11:471–477. [Google Scholar]
- Shobha G, Vinutha M, Ananda S. Biological synthesis of copper nanoparticles and its impact—a review. Int J Pharma Sci Invent. 2014;3(8):28–38. [Google Scholar]
- Siemer S, Westmeier D, Barz M, Eckrich J, Wünsch D, Seckert C, Thyssen C, Schilling O, Hasenberg M, Pang C. Biomolecule-corona formation confers resistance of bacteria to nanoparticle-induced killing: implications for the design of improved nanoantibiotics. Biomaterials. 2019;192:551–559. doi: 10.1016/j.biomaterials.2018.11.028. [DOI] [PubMed] [Google Scholar]
- Singh S, Park I, Shin Y, Lee Y. Comparative study on antimicrobial efficiency of AgSiO2, ZnAg, and Ag–Zeolite for the application of fishery plastic container. J Mater Sci Eng. 2015;4:2169. doi: 10.4172/2169-0022.1000180. [DOI] [Google Scholar]
- Singh H, Du J, Singh P, Yi TH. Ecofriendly synthesis of silver and gold nanoparticles by Euphrasia officinalis leaf extract and its biomedical applications. Artif Cells Nanomed Biotechnol. 2018;46(6):1163–1170. doi: 10.1080/21691401.2017.1362417. [DOI] [PubMed] [Google Scholar]
- Singh A, Joshi NC, Ramola M. Magnesium oxide nanoparticles (MgONps): green synthesis, characterizations and antimicrobial activity. Res J Pharm Tech. 2019;12(10):4644–4646. [Google Scholar]
- Singh R, Cheng S, Singh S. Oxidative stress-mediated genotoxic effect of zinc oxide nanoparticles on Deinococcus radiodurans. 3 Biotech. 2020;10:66. doi: 10.1007/s13205-020-2054-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smitha SL, Gopchandran KG. Surface enhance raman scattering, antibacterial and antifungal active triangular gold nanoparticles spectromchim. Spectrochim Acta A. 2013;102:114–119. doi: 10.1016/j.saa.2012.09.055. [DOI] [PubMed] [Google Scholar]
- Sriram MI, Kalishwaralal K, Barathmanikanth S, Gurunathani S. Size-based cytotoxicity of silver nanoparticles in bovine retinal endothelial cells. Nanosci Methods. 2012;1:56–77. [Google Scholar]
- Sueoka K, Chikama T, Latief MA, Ko JA, Kiuchi Y, Sakaguchi T, et al. Time-dependent antimicrobial effect of photodynamic therapy with TONS 504 on Pseudomonas aeruginosa. Lasers Med Sci. 2018;33:1455–1460. doi: 10.1007/s10103-018-2490-0. [DOI] [PubMed] [Google Scholar]
- Supraja N, Prasad TNVKV, Krishna TG, David E. Synthesis, characterization and evaluation of the antimicrobial efficacy of Boswellia ovalifoliolata stem barks extract a existed zinc oxide nanoparticles. Appl Nanosci. 2016;6:581–590. [Google Scholar]
- Surwade P, Ghildyal C, Weikel C, Luxton T, Peloquin D, Fan X, Shah V. Augumented antibacterial activity of ampicillin with silver nanoparticles against methicillin-resistant Staphylococcus aureus (MRSA) J Antibiot (Tokyo) 2019;72(1):50–53. doi: 10.1038/s41429-018-0111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tăbăran A-F, Matea CT, Mocan T, Tăbăran A, Mihaiu M, Iancu C, Mocan L. Silver nanoparticles for the therapy of tuberculosis. Int J Nanomed. 2020;15:2231–2258. doi: 10.2147/IJN.S241183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamayo LA, Zapata PA, Vejar ND, Azócar MI, Gulppi MA, Zhou X, Thompson GE, Rabaghati FM, Paez MA. Release of silver and copper nanoparticles from polyethylene nanocomposites and their penetration into Listeria monocytogenes. Mater Sci Eng C. 2014;40:24–31. doi: 10.1016/j.msec.2014.03.037. [DOI] [PubMed] [Google Scholar]
- Tao C. Antimicrobial activity and toxicity of gold nanoparticles: research, challenges and prospects. Lett Appl Microbiol. 2018;67:537–543. doi: 10.1111/lam.13082. [DOI] [PubMed] [Google Scholar]
- Thang DC, Wang Z, Lu X, Xing B. Precise cell behaviors manipulation through light-responsive nano-regulators: recent advance and perspective. Theranostics. 2019;9(11):3308–3340. doi: 10.7150/thno.33888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torchilin VP. Multifunctional, stimuli-sensitive nanoparticulate systems for drug delivery. Nat Rev Drug Discov. 2014;13(11):813–827. doi: 10.1038/nrd4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umar H, Kavaz D, Rizaner N. Biosynthesis of zinc oxide nanoparticles using Albizia lebbeck stem bark and evaluation of its antimicrobial, antioxidant, and cytotoxic activities on human breast cancer cell lines. Int J Nanomed. 2019;14:87–100. doi: 10.2147/IJN.S186888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergheese M, Vishal SK. Green synthesis of magnesium oxide nanoparticles using Trigonella foenum-graecum leaf extract and its antibacterial activity. J Pharmacogn Phytochem. 2018;7(3):1193–1200. [Google Scholar]
- Wan G, Ryan L, Yin Y, Yang T, Ge M, Cheng X. Effects of silver nanoparticles in combination with antibiotics on the resistant bacteria Acinetobacter baumannii. Int J Nanomed. 2016;11:3789. doi: 10.2147/IJN.S104166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Lawson R, Ray PC, Yu H. Toxic effects of gold nanoparticles on Salmonella typhimurium bacteria. Toxicol Ind Health. 2011;227:547–554. doi: 10.1177/0748233710393395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AZ, Langer R, Farokhzad OC. Nanoparticle delivery of cancer drugs. Annu Rev Med. 2012;63:185–198. doi: 10.1146/annurev-med-040210-162544. [DOI] [PubMed] [Google Scholar]
- Wang Q, Kang F, Gao Y, Mao X, Hu X. Sequestration of nanoparticles by an EPS matrix reduces the particle-specific bactericidal activity. Sci Rep. 2016;6:21379. doi: 10.1038/srep21379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Hu C, Shao L. The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int J Nanomed. 2017;12:1227–1249. doi: 10.2147/IJN.S121956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Harrington OD, Wang Y, Murray CK, Hamblin MR, Dai T. In vivo investigation of antimicrobial blue light therapy for multidrugresistant Acinetobacter baumannii burn infections using bioluminescence imaging. J Vis Exp. 2017;122:e54997. doi: 10.3791/54997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Yang F, Zhao J, Xu Y, Mao D, Zhu X, Luo Y, Alvarez P. Bacterial exposure to ZnO nanoparticles facilitates horizontal transfer of antibiotic resistance genes. NanoImpact. 2018;10:61–67. [Google Scholar]
- Wei L, Lu J, Xu H, Patel A, Chen ZS, Chen G. Silver nanoparticles: synthesis, properties, and therapeutic applications. Drug Discov Today. 2015;20:595–601. doi: 10.1016/j.drudis.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Liu L, Guo X, Wang Y, Zhao J, Zhou S. Light-activated ros-responsive nanoplatform co-delivering apatinib and doxorubicin for enhanced chemo-photodynamic therapy of multidrug-resistant tumors. ACS Appl Mater Interfaces. 2018;10:17672–17684. doi: 10.1021/acsami.8b04163. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) (2015) Antibiotic resistance: multi-country public awareness survey. https://www.who.int/antimicrobial-resistance/publications/baselinessurveynov2015/en/. Accessed 8 Dec 2020
- Wu P, Xie R, Imlay K, Shang JK. Visible-light-induced bactericidal activity of titanium dioxide codoped with nitrogen and silver. Environ Sci Technol. 2010;44:6992–6997. doi: 10.1021/es101343c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Li A, Zhao X, Zhang C, Yu B, Zhao N, Xu F-J. Silica coated gold-silver nanocages as photothermal antibacterial agents for combined anti-infective therapy. ACS Appl Mater Interfaces. 2019;11(19):17177–17183. doi: 10.1021/acsami.9b01149. [DOI] [PubMed] [Google Scholar]
- Wypig M, Czarnecka J, Swiecimska M, Dahm H, Rai M, Golinska P. Synthesis, characterization and evaluation of antimicrobial and cytotoxic activities of biogenic silver nanoparticles synthesized from Streptomyces xinghaiensis OF1 strain. World J Biotechnol. 2018;34:23. doi: 10.1007/s11274-017-2406-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Zheng W, Jiang X. Near-infrared light-activated phototherapy by gold nanoclusters for dispersing biofilms. ACS Appl Mater Interfaces. 2020;12:9041–9049. doi: 10.1021/acsami.9b21777. [DOI] [PubMed] [Google Scholar]
- Yang Y, Mathieu JM, Chattopadhyay S, Miller JT, Wu T, Shibata T, Guo W, Alvarez PJ. Defense mechanisms of Pseudomonas aeruginosa PAO1 against quantum dots and their released heavy metals. ACS Nano. 2012;6:6091–6098. doi: 10.1021/nn3011619. [DOI] [PubMed] [Google Scholar]
- Yang T, Wang D, Liu X. Assembled gold nanorods for the photothermal killing of bacteria. Colloids Surf B. 2019;173:833–841. doi: 10.1016/j.colsurfb.2018.10.060. [DOI] [PubMed] [Google Scholar]
- Yetisgin AA, Cetinel S, Zuvin M, Kosar A, Kutlu O. Therapeutic nanoparticles and their targeted delivery application. Molecules. 2020;25:2193. doi: 10.3390/molecules25092193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Liu J, Yin Y, Shen M. Interactions between engineered nanoparticles and dissolved organic matter: a review on mechanisms and environmental effects. J Environ Sci. 2018;63:198–217. doi: 10.1016/j.jes.2017.06.021. [DOI] [PubMed] [Google Scholar]
- Yu Z, Li Q, Wang J, Yu Y, Wang Y, Zhou Q, Li P. Reactive oxygen species-related nanoparticle toxicity in the biomedical field. Res Lett. 2020;15:115. doi: 10.1186/s11671-020-03344-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Li, Lei T, Zhenduo C, Xianjin Y, Yufeng Z, Kwok YKW, Chu Paul K, Shuilin Wu. Rapid sterilization and accelerated wound healing using Zn2+ and graphene oxide modified g-C3N4 under dual light irradiation. Adv Funct Mater. 2018;28:1800299. [Google Scholar]
- Zaidi S, Misba L, Khan AU. Nano-therapeutics: a revolution in infection control in post antibiotic era. Nanomedicine. 2017;13:2281–2301. doi: 10.1016/j.nano.2017.06.015. [DOI] [PubMed] [Google Scholar]
- Zazo H, Colino CI, Lanao JM. Current applications of nanoparticles in infectious diseases. J Control Release. 2016;224:86–102. doi: 10.1016/j.jconrel.2016.01.008. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Gu AZ, Xie S, Li X, Cen T, Li D, Cgen J. Nano-metal oxides induce antimicrobial resistance via radical-mediated mutagenesis. Environ Int. 2018;121:1162–1171. doi: 10.1016/j.envint.2018.10.030. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Lu Z, Dai X, Wei X, Yu Y, Chen X, et al. Glycomimeticconjugated photosensitizer for specific Pseudomonas aeruginosa recognition and targeted photodynamic therapy. Bioconj Chem. 2018;29:3222–3230. doi: 10.1021/acs.bioconjchem.8b00600. [DOI] [PubMed] [Google Scholar]
- Zhao Y-Q, Sun Y, Zhang Y, Ding X, Zhao N, Yu B, Zhao H, Duan S, Xu F-J. Well-defined gold nanorod/polymer hybrid coating with inherent antifouling and photothermal bactericidal properties for treating an infected hernia. ACS Nano. 2020;14:2265–2275. doi: 10.1021/acsnano.9b09282. [DOI] [PubMed] [Google Scholar]