Abstract
Purpose
To report real-world compliance to radiation in gynecologic cancers during the complete lockdown phase of COVID-19 pandemic.
Methods and Materials
From March 23, 2020, until June 30, 2020, complete lockdown was imposed in India. During this period there was restructuring of cancer care and radiation oncology department due to operational policies prevalent in the institution, and the care for gynecological cancer was based on the evolving international recommendations. Institutional review board approval was obtained to audit patterns of care during the complete lockdown phase. Descriptive variables were used to report on patient characteristics, compliance, delays, toxicity, and observed deviations in recommended care.
Results
During the lockdown period spanning 100 days, treatment of 270 and telephonic follow-up of 1103 patients with gynecological cancer was undertaken. Of 270 new patients, due to travel restrictions, 90 patients were referred to the facilities in vicinity of their residence. Of the remaining 180 patients, 138 were planned for complete treatment at our institution and 42 were referred to our center for brachytherapy. Of 138 patients, only 106 (76%) completed the planned external radiation. Twenty-four (26%) patients completed full course of concurrent chemotherapy, 11 (12%) received chemotherapy dose reduction, and 57 (62%) received no concurrent chemotherapy. Treatment delay of up to 3 weeks was noted in 8.6% patients due to COVID-19 infection. No grade 4 to 5 acute sequelae were observed. No excess adverse effects were observed in high-risk population. Low rate of symptom burden was observed among 1103 patients on telephonic follow-up. With 100 (9.6%) patients reporting symptoms, among these, 54% (54 of 100) had complete resolution of symptoms within 4 weeks of teleconsultation, and 10% had disease progression.
Conclusions
Low compliance with planned treatment was observed for radiation and concurrent chemotherapy due to lockdown and fear of contracting COVID-19 and will likely lead to increased risk of cancer-related mortality. Rapid restructuring of care is needed to prevent the same as COVID-19 pandemic further evolves.
Introduction
The year 2020 was affected by the pandemic caused by coronavirus (COVID-19). Due to increasing cases in India in March 2020, a complete nationwide lockdown was imposed beginning March 23, 2020.1 The lockdown was imposed in 4 phases, which was planned to be phased out from June 1, 2020, by selectively releasing restrictions in each phase. The city of Mumbai reported the highest cases in any city in India, exceeding 50,000 cases the first week of June and reporting about 93,000 by July 15,2 constituting about 20% of all cases across the country. Expectedly, overall cancer care and delivery of fractionated radiation treatment was affected owing to compounded effect of fear of attending hospitals, no access to public transport, and limited availability of paid accommodation in the city of Mumbai. As a vast majority of patients with gynecological (cervical cancer) are from lower socioeconomic status, the effect of many of these changes were anticipated to be higher.
During this period, there was also a change in operational workflow of the hospital,3,4 which included reduced staffing and redesignation of inpatient beds for COVID-19 care. This led to reduced staff and bed capacity for administering concurrent chemotherapy or admitting patients for brachytherapy. In keeping with the evolving recommendations for radiation for gynecological cancers during the COVID-19 pandemic,5 the policy for delivery of care was reorganized both for patient treatment and follow-up. However, owing to prolonged lockdown the implementation of COVID-19 guidelines at ground level had distinct challenges.
Methods
The study included consecutive patients who were planned for treatment of gynecologic cancers with radiation and those scheduled for routine follow-up within the gynecologic radiation oncology group at the institution from March 23, 2020, to June 30, 2020, during which the complete lockdown was imposed. All patients >18 years of age were included.
During this period, broad principles that governed the delivery of care within the gynecological radiation oncology unit. The existing staff (staff radiation oncologists, specialists, in training residents, physicists and dosimetrists, nurses, and support staff) services were reorganized such that at a given time only 50% of the staff were functional to handle operations of outpatient department, treatment planning, and brachytherapy applications. This was based on institutional recommendations to ensure patient care at all times in case a staff member tests positive and the need of other contacts to be quarantined, which would introduce challenges in operations and functionality. Institutional review board approval was obtained (TMC-IRB Project No. 3565) to audit patterns of care during the complete lockdown phase. Although the institution was part of developing the guidelines for the prioritization of radiation and brachytherapy for gynecological cancers,5 the physician multidisciplinary group suggested the following recommendations based on evolving and continued review of local and national effect of pandemic: (1) temporary suspension of all concurrent chemotherapy for cervical, endometrial, vulvo-vaginal during radiation because most of the patients on treatment were from geographic locations identified as “COVID-19 hotspots”; (2) preferential use of hypofractionated external beam treatment schedules for very elderly patients (age >75 years) to minimize need for travel on multiple days for treatment; (3) increase in referral for external radiation to nearby facilities when feasible; (4) COVID-19 testing only for symptomatic patients on external radiation, as per institutional and national guidelines6 between March and April 2020; (5) COVID-19 testing for all patients before admission for brachytherapy or inpatient care with effect from May 2020; (6) to delay definitive external radiation or brachytherapy in case a patient tests positive or needs to be isolated; (7) retesting of patients tested positive or quarantined on the 14th day for resumption of treatment; (8) use of extra physical barrier shielding during general anesthesia to minimize aerosol generation during initial days of pandemic followed by planned transition to spinal anesthesia for all brachytherapy procedures to minimize aerosol generation; (9) preferential use of single application multiple fractions of intracavitary interstitial brachytherapy and multiple applications with reduced time interval to prevent multiple inpatient admissions and procedures; (10) due to the anticipated effect of policy revision on institutional and international clinical trials, recruitment on interventional trials was temporarily suspended in a planned way; and (11) no modifications were made on choice of treatment technique or use of imaging for treatment execution.
In addition, complete cessation of planned physical follow-up was done and telephonic follow-up facilities were set up by the institution. A structured telephonic interview was performed to assess patients for the following symptoms: (1) any discharge from vagina, (2) abdominal pain or pain in the back radiating to lower limbs, (3) vomiting, and (4) blood in urine/stools. Due to sociocultural reasons and anticipated emotional and psychological effect of ongoing pandemic questions related to sexual functioning were not enquired. The results of the telephonic follow-up were reviewed by radiation oncology staff consultant to identify symptomatic patients for whom additional video calling was organized by staff radiation oncologist or in case of nonresolution of symptoms a physical follow-up to nearest facility was advised.
An expediated institutional review board permission was sought to report on the “real-world pattern of care” during the lockdown period of COVID-19 pandemic. The gynecology radiation oncology database, electronic medical records, and radiation oncology information system was reviewed to summarize the patterns of care during this period of COVID-19 pandemic, including the observed COVID-19 infection rate in patients and gynecology unit health care workers. These patients are planned to be followed up to 36 months and will be assessed for local control, disease-free survival, overall survival, and late radiation associated toxicities.
Results
During the complete lockdown period spanning 100 days, records of 270 patients of gynecological cancer who were either actively on treatment or awaiting treatment initiation were reviewed. Of these only 25.2% patients (n = 68) were from city of Mumbai, and 74.8% (n = 202) patients were from out of Mumbai, the details of which are listed in Table 1. The median age of the cohort was 54 years (30-87). Although 209 patients (71.8%) had no pre-existing comorbidities, 11.5% (n = 31) were hypertensive and 27 (10%) were diabetic. A small proportion of patients had history of ischemic heart disease (n = 6; 2.2%), chronic obstructive pulmonary disease (n = 2; 0.7%), hypothyroidism (n = 9; 3.3%), and human immunodeficiency virus infection (n = 4; 1.5%). The summary of American Society of Anesthesia score of the patients treated within our institution is summarized in Table 1.
Table 1.
Comparative characteristics of patients referred outside and treated within the institution
| No. (%) |
||
|---|---|---|
| Characteristic | Referred outside (N = 90) | Treated within Institution (N = 180) |
| Residence | ||
| Within Mumbai (≤50 km) | 34 (37.8) | 34 (18.9) |
| Outside Mumbai (>50 and ≤500 km) | 28 (31.1) | 44 (24.4) |
| Outside Mumbai (501 to >2000 km) | 28 (31.1) | 102 (56.7) |
| Monthly income (INR) | ||
| Median (range) | 7500 (0-80,000) | 6000 (0-1,50,000) |
| Age (y) | ||
| Median (range) | 53 (30-82) | 54 (31-87) |
| <60 | 70 (77.8) | 135 (75.0) |
| 61-70 | 13 (14.4) | 31 (17.2) |
| 71-80 | 6 (6.7) | 13 (7.2) |
| >80 | 1 (1.1) | 1 (0.6) |
| Comorbidity | ||
| None | 58 (64.4) | 132 (73.3) |
| Hypertension | 11 (12.2) | 16 (8.9) |
| Diabetes | 13 (14.4) | 18 (10) |
| IHD | 1 (1.1) | 5 (2.8) |
| COPD/asthma | 0 (0) | 2 (1.1) |
| Hypothyroidism | 6 (6.7) | 3 (1.7) |
| HIV | 1 (1.1) | 3 (1.7) |
| Primary site of cancer | ||
| Cervix | 72 (80) | 141 (78.3) |
| Endometrium | 15 (16.7) | 30 (16.7) |
| Others | 3 (3.3) | 9 (5) |
| Treatment intent | ||
| Radical | 53 (58.9) | 107 (59.4) |
| Adjuvant | 16 (17.8) | 23 (12.8) |
| Salvage | 12 (13.3) | 13 (7.2) |
| Palliative | 9 (10) | 37 (20.6) |
Abbreviations: COPD = chronic obstructive pulmonary disease; HIV = human immunodeficiency viruses; IHD = ischemic heart disease; INR = Indian Rupee.
Of these 270 patients, 213 (78.9%) had primary diagnosis of cervical cancer and 45 patients (16.7%) had endometrial cancer. Of all the patients, 160 patients (59.3%) needed radical treatment, and 39 (14.4%) needed postoperative radiation. In addition, 25 (9.3%) and 46 (17%) patients needed salvage and palliative radiation respectively. Of these 270 patients who visited us for treatment during the lockdown phase of pandemic, 90 (33.3%) patients were referred to radiation oncology facilities that were in the vicinity of their residence. A comparison between characteristics of patients treated at our institute and referred outside are depicted in Table 2.
Table 2.
Treatment characteristics of those offered radical treatment
| Characteristic | No. (%) 138 |
|---|---|
| EBRT | |
| Planned | 138 (100) |
| Completed | 106 (76.8) |
| Did not visit facility after lockdown/fear of COVID-19 | 32 (23.2) |
| Concurrent chemotherapy (eligible patients) | 92 (100) |
| Full dose chemotherapy | 24 (26.2) |
| Reduced dose chemotherapy | 11 (11.9) |
| No chemotherapy | 57 (61.9) |
| ASA fitness score* | |
| ASA grade 1 | 53 (29.4) |
| ASA grade 2 | 32 (17.8) |
| ASA grade 3 | 5 (2.8) |
| Brachytherapy applications (total) | 184 (100) |
| ICA | 125 (68) |
| Intracavitary-interstitial application | 9 (5) |
| Interstitial application | 4 (2) |
| Central vaginal source application | 46 (25) |
| Fractionation of ICA/IC-IS | |
| Single application 1 Fr (7 Gy × 4# in 4 implants) | 41 (31%) |
| Single application 2 Fr (7 Gy × 4# in 2 implants) | 86 (64%) |
| Single application ≥3 Fr (8.5 Gy × 3#, 1 implant) | 7 (5%) |
| EQD2 (Gy)† (multiple implants with 1-2 # delivered each implant) | |
| Point A | 78.4 (68.4-87.3) |
| Bladder (2 cc) | 82.9 (61.2-98.7) |
| Rectum (2 cc) | 68.0 (53.64-79.4) |
| Sigmoid (2 cc) | 69.5 (44.0-87.4) |
| EQD2 (Gy; single implant with delivery of all fractions) | |
| Point A | 75.6 (68.0-82.1) |
| Bladder (2 cc) | 85.4 (76.5-92.0) |
| Rectum (2 cc) | 67. (55.0-76.3) |
| Sigmoid (2 cc) | 68.12 (53.0-77.9) |
| OTT, d‡ | 92 (100) |
| ≤50 | 31 (33.7) |
| 51-60 | 12 (13) |
| 61-70 | 16 (17.4) |
| 71-80 | 15 (16.3) |
| 81-90 | 10 (10.9) |
| 91-100 | 4 (4.3) |
| 101-120 | 3 (3.3) |
| >120 | 1 (1.1) |
| OTT >56 d (reasons) | 58 (100) |
| Waitlisted for brachytherapy | 10 (17.2) |
| Treatment induced toxicity | 6 (10.3) |
| Logistics caused by COVID-19 | 37 (63.8) |
| COVID-19 infection (test positive) | 5 (8.6) |
| Acute toxicities (CTCAE, version 5.0) | |
| Grade 2 | 8 (4.4%) |
| Grade 3 | 2 (1.1%) |
| Grade 4 and 5 | 0 (0%) |
Only for patients who underwent brachytherapy under anesthesia.
Excluding palliative and noncompliant and referred out patients EQD2 delivered is lesser than that generated by physical dose addition of the treatment regimen as most of the treatment plans were optimized to ensure the best therapeutic ratio between the target and organ at risk.
Abbreviations: ASA = American Society of Anesthesiology; CTCAE = common terminology criteria for adverse events; EBRT = external beam radiation therapy; EQD2 = equivalent dose in 2Gray fraction; ICA = intracavitary application; IC-IS = intracavitary-interstitial application; OTT = overall treatment time.
Compliance in treatment
Out of 180 patients who were considered for treatment at our institution, radical external radiation or brachytherapy was considered for 107 patients (59.4%), and postoperative adjuvant radiation was planned for 23 (12.8%) patients. Although 13 patients were planned for salvage radiation for recurrence after surgery (either external or in combination with brachytherapy) and 37 were planned for palliative radiation.
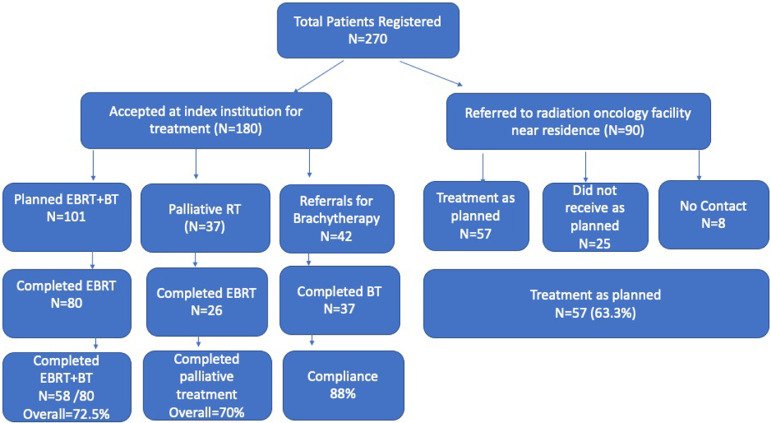
A summary of treatment flow of the patients is depicted in Fig. 1. Of the 180 patients, 138 were planned for complete treatment at our institution and 42 were referred from outside institutions for performing brachytherapy. Of 138 patients who started on external radiation in our institution (101 radical/adjuvant or salvage radiation and 37 palliative intent treatment) only 106 completed the planned external radiation. Of these, 80 patients were in definitive, adjuvant, or salvage setting and 26 in palliative setting. Of the 80 patients who were planned for combination of external radiation and brachytherapy, 22 decided not to go ahead with brachytherapy treatment due to either fear of contracting COVID-19 or travel restrictions. Therefore, overall only 58 of 80 (72.5%) patients who were planned for full course external radiation and brachytherapy managed to complete their entire treatment. Of those undergoing palliative treatment 26 of 37 (70.2%) completed their planned treatment. Of the 42 patients referred for brachytherapy from outside institutions, 5 did not complete brachytherapy (12%; Table 2).
Figure 1.
Study patient inclusion workflow.
Also as noted in Table 2, a significant proportion of patients had brachytherapy delivered through 1 or 2 intracavitary applications and with the same application 2 or more fractions were delivered to complete brachytherapy treatment.2, 3, 4 Those who received multiple applications were either part of an ongoing trial or treated through the early phase of lockdown where policies regarding brachytherapy were still being defined.
Despite intention for treatment acceleration, treatment could be completed in <50 days in 33.7% of patients and 13% completed planned treatment within 51 to 60 days. Another 53.3% patients had overall treatment time >60 days. The details of overall treatment time are depicted in Table 2. Overall, in the patients who had delay beyond 70 days, the major contributing reason was the social milieu as the result of pandemic-related fear and lockdown. During this lockdown period only 5 of 138 (4.7%) patients tested positive for novel coronavirus that led to >2 weeks delay in delivery of planned treatment.
It is noteworthy that in this entire period, no grade 4 and 5 acute sequelae of treatment were observed. Grade 2 and 3 toxicities were also reduced to 8 (4.4%) and 2 (1.1%; Table 2), compared with previous studies from the institution.7 This could possibly be attributed to omission of concurrent chemotherapy in a vast majority of patients. The present cohort had 25% patients with age >60 years but no increase in adverse effects was noted in this population.
Within the treated cohort discussed, more than 13 patients were treated for either local relapse (8/13) or locoregional relapse (5/13) after surgery (8/13) or radiation (5/13). All the patients completed salvage treatment. Of the 37 patients considered for palliative treatment, 26 completed planned treatment. The reason for offering palliative radiation are summarized in Table 3.
Table 3.
Details of patients offered palliative radiation during the complete lockdown period
| Characteristic | No. (%) |
|---|---|
| Total patients treated for palliation | 37 (100) |
| Completed planned palliative schedule | 26 (70.27) |
| Could not complete planned dose | 11 (29.73) |
| Reason for noncompliance | |
| Complete relief in symptoms | 2 (18.18) |
| Logistics caused by lockdown | 5 (45.45) |
| Not known | 4 (36.36) |
| Reason for palliation | |
| Adjacent organ infiltration | 17 (45.9) |
| Distant metastasis | 14 (37.8) |
| Poor performance status | 6 (16.3) |
Of the 90 patients referred to other treatment facilities, 57 (63%) patients were able to start the planned treatment within the referred facility within 2 to 3 weeks, and 25 (28%) were unable to reach to the referred cancer care facility due to the practical difficulties caused by lockdown. At the time of submitting this publication, no contact could be made with 8 of 90 (9%) patients.
Teleconsultation
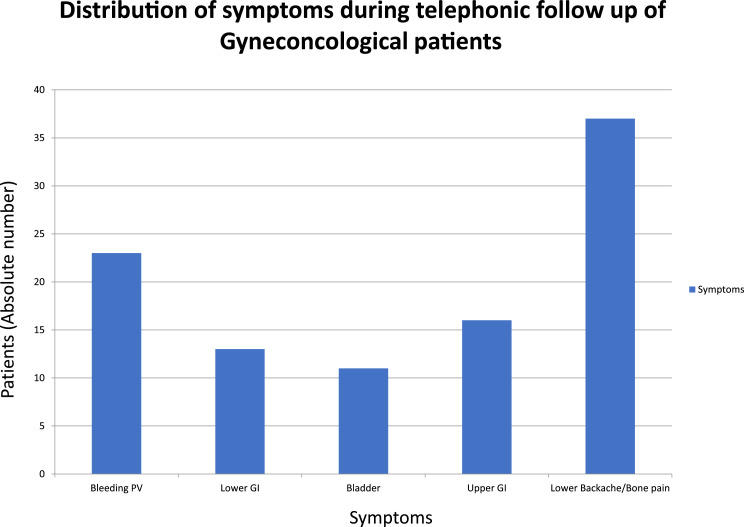
During the lockdown period, as all the scheduled physical planned follow-up was temporarily suspended, 1103 patients who had their planned follow-up were consulted telephonically in a span of 100 days, out of them, 100 were identified to have symptoms (9.6%). The distribution of symptoms during follow up is depicted in Fig. 2. Specifically, the distribution of their predominant symptom is as follows: 37 patients (3.3%) had symptoms of lower backache or bony pain, 23 (2%) complained of vaginal discharge or bleeding, 16 had upper gastrointestinal symptoms, 13 had rectal and lower gastrointestinal symptoms (2.6%), and another 11 (1%) had urinary complaints. Most of these patients were prescribed medications during the telephonic consultation or they were asked to liaise with a local physician or a radiation oncology center if it was feasible. Four weeks after initial telephonic consultation a video call was repeated for symptomatic patients, of which 54 of 100 (54%) had relief of symptoms with the advised line of care. In this cohort, 6 patients had symptom progression and 4 deaths: 3 due to disease progression and 1 due to COVID-19 infection. At the time of submitting this article, 36 patients who had symptoms did not receive the hospital call and as part of the unit policy will be contacted again telephonically within 4 weeks.
Figure 2.
Distribution of symptoms during telephonic follow-up of gynecologic oncologist patients. The x axis represents various symptoms, and y axis represents the absolute number of patients
Discussion
Nationwide lockdown due to COVID-19 pandemic posed distinct challenges to delivery of uninterrupted care for patients with solid tumors where fractionated radiation constituted the backbone of curative treatment. Various international societies published their recommendations on prioritizing care of patients with cervical cancer, including brachytherapy whereas adjuvant treatments could hold a relatively lower priority.8,9 Furthermore, special issues of various journals had rapid publications related to organizational and structural changes in patient care, follow-ups, and physician and resident trainings in the institute.4,6 Although there have been publications related to increased risk of adverse effects after surgery and systemic chemotherapy, didactic “real-world reports” of “on-the-ground” implementation of radiation oncology priority procedures is limited.10,11
Mumbai was the worst affected city during the Covid-19 pandemic in India.12 In the first phase of the pandemic, also corresponding to nationwide lockdown, the gynecology radiation oncology unit treated at one-third of its capacity, which is similar to the rate reported in cancer hospitals in the West as a result of reorganization of infrastructural and human resources.13, 14, 15, 16, 17 During this period, as seen in our results, approximately 25% to 30% patients decided not to receive additional treatment due to fear of infection and challenges posed due to nationwide lockdown, reducing patients’ ability to travel for an 8-week treatment. During this phase, interstate and intrastate transportation was stopped. Also, many residential hotels were transformed into either quarantine centers or were asked to remain closed during lockdown, causing inability for travelers to stay in the city and logistical problems. The compliance rate to radiation therapy dropped down to 66% during this period, and was inferior to non-COVID times, which was recorded 85% for the full course of concurrent chemoradiation and brachytherapy.7 Similar responses have been observed in other major cities that became epicenters of COVID-19 within their countries.18 However, unlike in India, in Europe and United States, the functionality of radiation departments continued to exist at an almost similar level in Hong Kong, a country that had previous experience with dealing with SARS virus in 2003.19 The anticipatory fear response throughout the world is likely to have an untoward effect on cancer-specific survival as a result of delayed and interrupted care. Therefore, it is important that as pandemic is evolving we report the “real-world outcomes,” which can help in rapidly shaping treatment policies and response in the next few months also if a second wave or another pandemic in near future were to arise.
During the lockdown period, we continued to treat patients using conventional fractionation (rather than hypofractionation) for external radiation despite recommendations to reduce hospital visits for patients. This decision was made to prevent patients from combined long-term adverse effect of hypofractionated external radiation and brachytherapy, the effect of which on normal tissues was hitherto unknown even from data of other pelvic malignancies (prostate and rectum).20 Furthermore, as cervical cancer has higher alpha/beta ratio10 than rectal or prostate malignancies extreme hypofractionation may not be associated with equivalent tumor response/control. Nevertheless, to reduce the effect of omission of concurrent chemotherapy and to reduce hospital stay we decided to accelerate the treatment to <50 days by altering brachytherapy schedules. This decision was based on the results of a randomized trial from India21, 22, 23 and also observations within the European study on MRI-guided brachytherapy in locally advanced cervical cancer (EMBRACE) study, where reduced overall treatment time was associated with improved local control.24 Based on our institutional experience to treat patients with single application and multiple fractions within and outside clinical trials (CTRI/2017/03/008172), we reduced the number of brachytherapy implants and increased the number of fractions delivered per implant. This reduced the need for repeated hospitalizations and risk of contracting COVID-19 after hospital discharge and before the next procedure. This period also noted a transient reduction in advanced interstitial procedures (from 15%-7%) as a result of reduced staff, reduction in availability of blood products if needed and reduced access to appointments for magnetic resonance imaging. As seen in Table 4, almost 70% of patients received single implant and multiple fractions, including completing all brachytherapy in single application. Nevertheless, despite this attempt, only 33% patients could complete treatment within 50 days and another 13% within 56 days. Inability to reach treatment facility due to lack of transport or fear of contracting COVID-19 led to increase in treatment time. Although the specific causes of prolonged overall treatment time are listed in Table 2, it is noteworthy that COVID-19 infection itself or treatment-induced toxicity led to delay in only 8.2% and 10% of patients, respectively. All other delays happened due to organizational challenges (inability of patients to reach facility, reduced bed capacity, reduced brachytherapy capacity due to staff reorganization). It is expected that this cohort of patients may have reduced local control (estimated 4%, 8%, 12%, 16% in those completing within 61-70 days, 71-80, 81-90, and 91-100 days).25 This detriment may also be further magnified as concurrent chemotherapy was temporarily suspended due to operational reasons during the lockdown, specifically paucity of daycare and in-patient beds and blood products. Concurrent chemotherapy is known to have direct effect on disease-free survival of cervix cancer across stages,26, 27, 28 and this cohort of patients will be at risk of local, nodal, and distant relapse. Careful observation of this cohort may be needed with elective imaging including decisions for salvage hysterectomy or reirradiation in cases of persisting residual disease within 5 to 6 months of follow-up to prevent long-term detriment.
Table 4.
Reasons of non-"?>compliance to radical or adjuvant radiation therapy
| Reason/stage of treatment at dropout | No. (%) N = 62 |
|---|---|
| Reason for noncompliance | 62 (100) |
| Fear on contracting COVID-19 | 10 (16) |
| Logistics caused by lockdown | 40 (65) |
| Referred outside reason for noncompliance Unknown | 3 (5) |
| Unknown | 9 (14) |
| Stage at which treatment was interrupted | 62 (100) |
| Before starting EBRT | 19 (31) |
| During fractionated EBRT | 13 (21) |
| Before starting brachytherapy | 27 (43) |
| During fractionated brachytherapy | 3 (5) |
Abbreviations: EBRT = external beam radiation therapy.
Traditionally, in radiation oncology transition to hypofractionation has been slow. In “normal times” structured prospective trials with rigorous endpoints and long observation period are needed for such transition. However, the pandemic led to fast-paced changes and adaptations that had to be made. Few months before the initiation of COVID-19 pandemic, the gynecological radiation oncology unit had completed accrual toward phase 2 study of single application and multifraction brachytherapy study (CTRI/XXXX). In this study a vast majority of patients received combined intracavitary-interstitial magnetic resonance imaging-based brachytherapy and long-term results would be needed for opening the next phase trials with anticipated full results after a decade. Also, as this transition was envisioned within advanced brachytherapy programs it could be implemented only within settings of advanced brachytherapy programs. However, due to the pandemic we had to transition to this fractionation within days of completing recruitment in the study also within the preview of standard intracavitary and computed tomography-based brachytherapy. Dose volume recommendations from ongoing prospective trials was rapidly adopted for “clinical practice.” Although the present report focusses on lockdown period that had treatment delays, at the time of writing this study more than 150 patients have received brachytherapy treatment with altered fractionation scheme (also more recently completing treatment within 8 weeks) and will form an extremely important cohort that will shape cervix brachytherapy in future as long-term outcomes mature. Similar experiences of rapid adoption of Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST Forward) breast protocol have been observed during COVID-19 pandemic whereas the earlier the UK Standardisation of Breast Radiotherapy (START) Trial B had very slow clinical adoption due to concerns related to toxicity despite availability of structured clinical data.29
During this phase of using radical radiation therapy alone we observed very few acute toxicities and no mortality suggesting that radical radiation therapy and brachytherapy is indeed a treatment that despite its intensity is very well tolerated. Among oncological treatments, radiation is a treatment modality that could be easily deployed and is more resilient in the phase of such pandemics where systemic response to surgery and chemotherapy (including targeted agents and immunotherapy) may be unknown.30, 31, 32 Within our cohort of patients 17.8% and 2.8% patients were categorized American Society of Anesthesia class II and III for brachytherapy. However, no increased adverse effects were observed. Furthermore, 25% of our patients had age >65 years and no increase in adverse effects was noted.
Palliative treatments during this phase could be completed in 70% of patients and overall 9 of 37 (24%) patients who still had symptoms and were on once monthly fractionation could not return to the treatment facility. Another 5 patients had complete relief of symptoms although treatment schedule could not be completed. As the pandemic evolves, there is a clear need of rapid palliative schedules and are presently being tested in ongoing randomized trial in the institution.33
Continuation of radiation treatment including salvage reirradiations among patients who could not be offered surgery or chemotherapy is attributed to the experience of the clinical team. The institutional team has treated patients effectively during and after the human immunodeficiency virus epidemic that taught important lessons about treatment tolerability in patients with viral coinfections.34 All the patients who tested positive for COVID-19 during treatment were reinitiated on external radiation or brachytherapy within 2 to 3 weeks. At the time of writing this article a policy of restarting treatment within 10 days of diagnosing COVID-19 infection has been introduced.
Based on the early observations and lessons on tolerability of radiation during COVID-19 pandemic we also reinitiated use of concurrent chemotherapy from August 2020. Although the results are not part of this article, the treating team has observed no increase in complications with concurrent chemotherapy during the pandemic. Similarly, recruitment to institutional and international trials has been reinitiated. Although the pandemic continues to peak in India with >90,000 new cases per day in September 2020,2 the cancer care is slowly reverting back to normal. It is important to quickly reorganize and deliver appropriate care for cancers, which are the top priority for radiation oncology and brachytherapy, to prevent one public health crisis may from turning into another health crisis.35 It is estimated that in India close to 80,000 women may annually need radiation for cervix cancer.36 Delay of treatment due to fear or lockdown restrictions may lead to an estimated 10% to 12% excess risk of local recurrence. In India, this will put 8000 to 10,000 additional women at risk of relapse and death per year. We believe that early reporting of our observations may help rapid shaping of treatment policies in India and in other countries where COVID-19 is still evolving.
Despite the intention to slowly recover back to normalcy, our unit continues to see only 50% of the original number of patients at the time of submission of this manuscript due to restrictions on interstate travel and fear in patients and caregivers of acquiring COVID-19 infection. During the lockdown period hospital and our unit offered free of cost teleconsultation services to almost 1100 patients who were scheduled for clinical follow-up for cervix or endometrial cancer. In this cohort, a low rate of symptom burden was observed in those scheduled for follow-up (9.6%). Although these observations will require validation against clinical follow-up in the near future, the results may open an opportunity to preferentially teleconsult in a vast majority of patients in future. The strategy of teleconsultations for follow-up has been previously validated in lung and oral cancer patients in our institution.37 Although a recently initiated ongoing trial in telephonic follow-up in gynecological cancers will provide structured information in future, this cohort of 1100 patients who were attended through teleconsultations during Covid-19 will allow shaping of follow-up clinics in near future even as pandemic evolves. Similar insights into integrating telemedicine into radiation oncology have recently been reported.38
Conclusions
The results of this audit highlight how strict lockdowns during pandemics and fear based response can adversely affect cancer care. Based on our early observations and results mortality due to gynecological cancer is going to exceed COVID-19 mortality in the near future. During lockdown, use of radiation and brachytherapy was found to be safe and with no increase in complications. Following the norms of social distancing, personal protection, and hygiene, the planned care for gynecological cancers with radiation should be restored back to normal. Effect of delays, change in therapeutic decisions and delivery of clinical care, including teleconsultations needs to be further followed up to understand long-term effect on clinical practice.
Footnotes
Sources of support: This work had no specific funding.
Disclosures: None.
Data Sharing Statement: Research data are stored in an institutional repository and will be shared uponrequest to the corresponding author.
References
- 1.COVID-19 pandemic in India . 2020. Wikipedia, The Free Encyclopedia. Available at: https://en.wikipedia.org/wiki/COVID-19_pandemic_in_India. Accessed March 23, 2020. [Google Scholar]
- 2.Worldometers.Info. 2020. Available at: https://www.worldometers.info/coronavirus/. Accessed July 15, 2020.
- 3.Tata Memorial Centre COVID-19 Working Group The covid-19 pandemic and the tata memorial centre response. Indian J Cancer. 2020;57:123–128. doi: 10.4103/ijc.IJC_250_20. [DOI] [PubMed] [Google Scholar]
- 4.Pramesh CS, Badwe RA. Cancer management in india during covid-19. N Engl J Med. 2020;382:e61. doi: 10.1056/NEJMc2011595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elledge CR, Beriwal S, Chargari C, et al. Radiation therapy for gynecologic malignancies during the COVID-19 pandemic: International expert consensus recommendations. Gynecol Oncol. 2020;158:244–253. doi: 10.1016/j.ygyno.2020.06.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Indian Council of Medical Research NDI . 2020. Indian council of medical research, New Delhi. Available at: https://www.icmr.gov.in/pdf/covid/strategy/Strategey_for_COVID19_Test_v4_09042020.pdf. Accessed April 9, 2020. [Google Scholar]
- 7.Mittal P, Chopra S, Pant S, et al. Standard chemoradiation and conventional brachytherapy for locally advanced cervical cancer: Is it still applicable in the era of magnetic resonance–based brachytherapy? J Glob Oncol. 2018;4:1–9. doi: 10.1200/JGO.18.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uwins C, Bhandoria GP, Shylasree TS, et al. COVID-19 and gynecological cancer: A review of the published guidelines. Int J Gynecol Cancer. 2020;30:1424–1433. doi: 10.1136/ijgc-2020-001634. [DOI] [PubMed] [Google Scholar]
- 9.Chargari C, Chopra S, Viswanathan AN, et al. Brachytherapy issues and priorities in the context of the Coronavirus disease 2019 (COVID-19) outbreak. Adv Radiat Oncol. 2020;5:640–643. doi: 10.1016/j.adro.2020.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Quteimat OM, Amer AM. The impact of the COVID-19 pandemic on cancer patients. Am J Clin Oncol. 2020;43:452–455. doi: 10.1097/COC.0000000000000712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang H, Zhang L. Risk of COVID-19 for patients with cancer. Lancet Oncol. 2020;21:e181. doi: 10.1016/S1470-2045(20)30149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buchholz K. Maharashtra counts one third of COVID-19 deaths in India. Available at: https://www.statista.com/chart/21868/share-of-COVID-19-cases-deaths-in-indian-states-cities/. Accessed December 22, 2020.
- 13.Slotman BJ, Lievens Y, Poortmans P, et al. Effect of COVID-19 pandemic on practice in european radiation oncology centers. Radiother Oncol. 2020;150:40–42. doi: 10.1016/j.radonc.2020.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wakefield DV, Sanders T, Wilson E, et al. Initial impact and operational responses to the COVID-19 pandemic by american radiation oncology practices. Int J Radiat Oncol Biol Phys. 2020;108:356–361. doi: 10.1016/j.ijrobp.2020.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srinivasa GY, Dey T, Suri V, et al. Rationalizing treatment for gynecological cancers during the COVID-19 pandemic: An Indian experience. Indian J Gynecol Oncol. 2020;18:101. doi: 10.1007/s40944-020-00448-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El Majjaoui S, Ismaili N, Benjaafar N. COVID-19, brachytherapy, and gynecologic cancers: A Moroccan experience. SN Compr Clin Med. 2020:1–4. doi: 10.1007/s42399-020-00402-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Malicki J, Martenka P, Dyzmann-Sroka A, et al. Impact of COVID-19 on the performance of a radiation oncology department at a major comprehensive cancer centre in Poland during the first ten weeks of the epidemic. Rep Pract Oncol Radiother. 2020;25:820–827. doi: 10.1016/j.rpor.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sindhu K, Gupta V. 2020. Fear in the age of COVID-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee AWM, Xu Z-Y, Lin L, et al. Advocacy to provide good quality oncology services during the COVID-19 pandemic: Actions at 3-levels. Radiother Oncol. 2020;149:25–29. doi: 10.1016/j.radonc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ling D, Vargo J, Grimm R, et al. Dose-response model for severe late laryngeal toxicity after stereotactic body radiation therapy for previously irradiated head and neck cancer. Int J Radiat Oncol Biol Phys. 2020;108:E6. [PMC free article] [PubMed] [Google Scholar]
- 21.Song S, Rudra S, Hasselle MD, et al. The effect of treatment time in locally advanced cervical cancer in the era of concurrent chemoradiotherapy. Cancer. 2013;119:325–331. doi: 10.1002/cncr.27652. Available at: https://acsjournals.onlinelibrary.wiley.com/doi/full/10.1002/cncr.27652. Accessed July 17, 2012. [DOI] [PubMed] [Google Scholar]
- 22.Lin S-M, Ku H-Y, Chang T-C, et al. The prognostic impact of overall treatment time on disease outcome in uterine cervical cancer patients treated primarily with concomitant chemoradiotherapy: A nationwide Taiwanese cohort study. Oncotarget. 2017;8:85203–85213. doi: 10.18632/oncotarget.19617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez CA, Grigsby PW, Castro-Vita H, Lockett MA. Carcinoma of the uterine cervix. I. Impact of prolongation of overall treatment time and timing of brachytherapy on outcome of radiation therapy. 1995;32:1275–1288. doi: 10.1016/0360-3016(95)00220-S. Available at: https://www.redjournal.org/article/0360-3016(95)00220-S/pdf. Accessed July 30, 1995. [DOI] [PubMed] [Google Scholar]
- 24.Pötter R, Tanderup K, Kirisits C, et al. The EMBRACE II study: The outcome and prospect of two decades of evolution within the GEC-ESTRO GYN working group and the embrace studies. Clin Transl Radiat Oncol. 2018;9:48–60. doi: 10.1016/j.ctro.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.González Ferreira JA, Jaén Olasolo J, Azinovic I, et al. Effect of radiotherapy delay in overall treatment time on local control and survival in head and neck cancer: Review of the literature. Rep Pract Oncol Radiother. 2015;20:328–339. doi: 10.1016/j.rpor.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta S, Maheshwari A, Parab P, et al. Neoadjuvant chemotherapy followed by radical surgery versus concomitant chemotherapy and radiotherapy in patients with stage IB2, IIA, or IIB squamous cervical cancer: A randomized controlled trial. J Clin Oncol. 2018;36:1548–1555. doi: 10.1200/JCO.2017.75.9985. [DOI] [PubMed] [Google Scholar]
- 27.Shrivastava S, Mahantshetty U, Engineer R, et al. Cisplatin chemoradiotherapy vs radiotherapy in FIGO stage IIIB squamous cell carcinoma of the uterine cervix: A randomized clinical trial. JAMA Oncol. 2018;4:506–513. doi: 10.1001/jamaoncol.2017.5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: A systematic review and meta-analysis of individual patient data from 18 randomized trials. [DOI] [PMC free article] [PubMed]
- 29.Coles CE, Choudhury A, Hoskin PJ, et al. COVID-19: A catalyst for change for UK clinical oncology. Int J Radiat Oncol Biol Phys. 2020;108:462–465. doi: 10.1016/j.ijrobp.2020.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dai M, Liu D, Liu M, et al. Patients with cancer appear more vulnerable to Sars-Cov-2: A multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10:783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mehta V, Goel S, Kabarriti R, et al. Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discovery. 2020;10:935. doi: 10.1158/2159-8290.CD-20-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mei H, Dong X, Wang Y, et al. Managing patients with cancer during the COVID-19 pandemic: Frontline experience from Wuhan. Lancet Oncol. 2020;21:634–636. doi: 10.1016/S1470-2045(20)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palliative radiation for advanced cervical cancer. Available at: https://ClinicalTrials.gov/show/NCT03997110. Accessed June 25, 2019.
- 34.Shrivastava SK, Engineer R, Rajadhyaksha S, et al. HIV infection and invasive cervical cancers, treatment with radiation therapy: Toxicity and outcome. Radiother Oncol. 2005;74:31–35. doi: 10.1016/j.radonc.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 35.Sharpless NE. COVID-19 and cancer. Science. 2020;368:1290. doi: 10.1126/science.abd3377. [DOI] [PubMed] [Google Scholar]
- 36.Chopra SJ, Mathew A, Maheshwari A, et al. National cancer grid of india consensus guidelines on the management of cervical cancer. J Glob Oncol. 2018;4:1–15. doi: 10.1200/JGO.17.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malik A, Nair S, Sonawane K, et al. Outcomes of a telephone-based questionnaire for follow-up of patients who have completed curative-intent treatment for oral cancers. JAMA Otolaryngol Head Neck Surg. 2020;146:1102–1108. doi: 10.1001/jamaoto.2020.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orazem M, Oblak I, Spanic T, et al. Telemedicine in radiation oncology post-COVID-19 pandemic: There is no turning back. Int J Radiat Oncol Biol Phys. 2020;108:411–415. doi: 10.1016/j.ijrobp.2020.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]