Abstract
A small percentage of people living with HIV-1 can control viral replication without antiretroviral therapy (ART). These patients are called elite controllers (ECs) if they are able to maintain viral suppression without initiating ART and posttreatment controllers (PTCs) if they control HIV replication after ART has been discontinued. Both types of controllers may serve as a model of a functional cure for HIV-1 but the mechanisms responsible for viral control have not been fully elucidated. In this review, we highlight key lessons that have been learned so far in the study of ECs and PTCs and their implications for HIV cure research.
Since the early days of the HIV epidemic, unusual individuals were reported who were able to suppress plasma viremia to very low or undetectable levels and maintain their CD4+ T cell counts for a prolonged period of time without antiretroviral therapy (ART) (1, 2). Over the years, these individuals have been known by a number of names, including HIV controllers, spontaneous controllers, elite controllers (ECs), elite suppressors, long-term nonprogressors, long-term asymptomatic, and long-term survivors, depending on the clinical and laboratory definition. Not long after combination ART became widely available, an intriguing letter was published regarding an individual in whom ART was initiated during early HIV infection with multiple viral loads greater than 80,000 HIV-1 RNA copies/mL (known as the first “Berlin patient”). The patient elected to stop treatment and, surprisingly, was able to sustain HIV suppression (3), in contrast to the rapid rebound reported in most individuals after ART discontinuation (4). While it is unclear how this individual would have fared without ART (5), this case represented the first report regarding a group of individuals now known as HIV posttreatment controllers (PTCs), individuals who are able to maintain HIV suppression despite stopping ART. There is some variability between studies in the minimum length of time that individuals must remain off ART, with some requiring 24 or more weeks of viral suppression (6) and others requiring 24 or more months (7). Since noncontrollers will experience rapid viral rebound within a median of 3–4 weeks after treatment interruption (4), these definitions are all expected to adequately capture this rare group of PTCs. While HIV ECs are rare, PTCs have been even more difficult to identify and study, largely because it is recommended that patients stay on ART lifelong and these individuals have largely been identified only as part of analytic treatment interruption (ATI) trials. While there may be some overlap between ECs and PTCs, there is increasing evidence that a substantial fraction of PTCs might have been unable to achieve posttreatment control without the initial ART. Together, ECs and PTCs have already provided important insights about the mechanisms behind HIV remission. In this Review, we highlight five key lessons that have been learned so far and how they inform our search for an HIV cure.
ART-free control of replication-competent virus is possible
The existence of HIV ECs has given hope to the field and provided clear evidence that HIV can be controlled without the use of medications and that ART-free HIV remission is possible. In order to recognize controllers as legitimate models of a functional cure for HIV-1, these patients have to be controlling fully pathogenic virus. While there are studies suggesting that patients infected with attenuated HIV variants are more likely to become HIV controllers or long-term nonprogressors (8), there are also several lines of evidence to suggest that some controllers are infected with replication-competent virus (9). Multiple laboratories have cultured virus from a subset of these patients (10–14), and full genome sequence analysis of virus isolated from some controllers has not revealed any large deletions or signature mutations (10). There have also been several transmission pair studies in which transmission of virus between patients with progressive disease and HIV controllers has been documented (15–19). In cases in which replication-competent virus was isolated from both partners, comparable viral fitness was seen in three out of four of these pairs (15–17). There has also been documentation of evolution of plasma virus in cohorts of ECs (20–22). In some cases, this evolution has been in response to selective pressure by CD8+ T cells (20, 21). In another study, replication-competent viral isolates from ECs were shown to replicate vigorously and cause substantial CD4+ T cell depletion in humanized mice (14). Finally, reports of transient (23) or permanent loss of virologic control in ECs (24–27) indicate that the virus they were infected with was in fact replication competent.
There is evidence that PTCs also harbor HIV with preserved viral fitness. This evidence includes data reported from two of the more comprehensive studies to date on HIV PTCs: the French VISCONTI study of 14 early-treated individuals (7) and the Control of HIV after Antiretroviral Medication Pause (CHAMP) study of 67 PTCs from a consortium of North American studies (6). First, in both of these studies and others (28), PTCs were found to have high levels of pre-ART viremia that were similar to levels found in posttreatment noncontrollers (NCs). In the VISCONTI study, levels of pre-ART viral load were markedly higher in the PTCs compared with the viral loads of HIV ECs during acute infection. Second, a substantial subset of CHAMP PTCs were found to have early viral load rebound before regaining control, including 33% of individuals who had early viral rebound peaks above 10,000 HIV-1 RNA copies/mL. Third, robust HIV production could be induced from ex vivo stimulated cells of PTCs from the VISCONTI study (7) and other studies (6, 29–32). Fourth, the analysis of transmission pairs has also shown that the same HIV viral strain infecting a PTC has robust replication kinetics (33) and can lead to end-stage HIV in a partner (30). Fifth, posttreatment control can be maintained despite an onslaught of viral pressure, including evidence of HIV evolution, dual infection, and even superinfection (34). Finally, eventual loss of viral control has been documented in a subset of PTCs, confirming the pathogenicity of the infecting viruses (6, 35). Together, these results demonstrate that an attenuated strain of HIV is not the primary mechanism behind HIV elite and posttreatment control and affirm that these patients represent important models of sustained HIV remission.
HIV controllers have unusual reservoir characteristics
Newer assays enable investigators to better characterize the reservoir and distinguish between intact provirus and those with large deletions and/or hypermutations (36–38). Using the intact proviral DNA assay (38), Kwaa et al. reported that the frequency of intact HIV DNA was about 20-fold lower than the frequency seen in people living with HIV (PLWH) on ART (39). Jiang and colleagues performed full proviral genome sequencing on large numbers of ECs and chronic progressors (CPs) and also found much lower levels of intact DNA in elites. Interestingly, one EC had a single intact proviral clone detected from 1.02 billion CD4+ T cells, and another EC had no proviral clones detected from 1.5 billion CD4+ T cells (40). This study is the best characterization of a subset of ECs called exceptional elite controllers (EECs) who appear to have smaller reservoirs than the majority of ECs (41–44). Further studies will be needed to determine whether these patients have in fact achieved a sterilizing cure. Several studies have looked at integration sites in HIV controllers and provided evidence of clonal expansion in these participants (31, 40, 45). Studies looking at total and intact proviral DNA found that the frequency of clonally expanded clones in ECs exceeds the frequency seen in PLWH on ART (31, 40) and that the percentage of intact proviral clones that were integrated into non-genic or pseudogenic regions was higher in ECs than in PLWH on ART (40). There was also more enrichment of integration sites in satellite DNA and genes in the zinc finger family in ECs compared with CPs (40). These integration sites would be more likely to result in transcriptional repression, and in fact there was evidence of a lower ratio of viral transcripts to viral DNA. It should be emphasized that these characteristics are more likely a consequence rather than a cause of elite control.
PTCs also have unusual features related to both the size of the reservoir and distribution within T cell subsets. In a study of ten PTCs identified from AIDS Clinical Trials Group (ACTG) ATI trials, PTCs had a restricted reservoir size, featuring an approximately 7-fold smaller total and intact proviral reservoir compared with NCs, although the proportion of intact proviruses was not significantly different between PTCs and NCs (29). As with ART-suppressed individuals, clonally expanded populations of HIV-infected cells appear in PTCs, and likely at a frequency similar to that in NCs (29). The VISCONTI study also reported that PTCs had low levels of HIV DNA that continued to decline off ART (7). Interestingly, the VISCONTI study and others (32) described differences in the reservoir distribution between T cell subsets, with PTCs having smaller reservoirs in the longer-lived naive and central memory populations. Similarly to the EECs, PTCs have also been reported with extremely low levels of HIV reservoirs, which included sampling from tissue compartments (46). However, it is important to note that detectable HIV DNA and cell-associated RNA do not preclude the possibility of posttreatment control (7, 28–30). Finally, nonhuman primate (NHP) studies have also reported limited SIV reservoir size in animals able to maintain viral control after treatment interruption (47). The evidence that HIV reservoirs in HIV controllers are severely restricted in size and demonstrate features of deep latency in ECs is intriguing. These findings highlight the potency of antiviral responses in HIV controllers, but also that transcriptional silencing of HIV proviruses may play a role in long-term viral control. Additional studies in PTCs of HIV reservoir maintenance and transcriptional regulation are needed, including studies of proviral integration sites.
Early ART or immune response is important for control
The few studies of ECs during primary infection suggest that these individuals do not have the high levels of viremia seen in patients with progressive disease (48). Furthermore, these ECs appear to achieve virologic control very early in the course of infection (49, 50). It is almost unheard of for a person who is viremic more than a year after primary infection to subsequently control viral replication without ART.
Since the description of the original acutely treated “Berlin patient,” the majority of reports have profiled pediatric or adult patients who initiated ART during early infection (3, 7, 30, 32, 33, 51–55). This phenomenon does not appear to be restricted to early-treated individuals, as there are cases of PTCs who initiated ART during chronic infection (56, 57), including an individual who had previously progressed to AIDS (46). The largest study to date comparing the frequency of posttreatment control in those initiating ART during early versus chronic infection was the CHAMP study, which confirmed that early treatment was associated with a greater frequency of posttreatment control with 13% of early-treated versus 4% of chronic-treated participants meeting the study’s definition of posttreatment control (6). Several areas of uncertainty remain, including the timing of early ART initiation, as the majority of PTCs were treated during Fiebig stages III to V of infection while studies of individuals who initiated ART during the earliest Fiebig stages had rapid rebound after ATI (58). These reports are also bolstered by NHP studies of early antibody treatment that induced posttreatment control (59, 60). Early suppression of viral replication likely benefits individuals in multiple ways. First, early ART initiation substantially restricts the size of the HIV reservoir (61) with greater than 100-fold difference in the frequency of cells harboring HIV DNA 3 years after infection (62). In addition, early ART initiation is likely to have long-term benefits for preserving functional cellular immune responses to HIV (63, 64). These results provide additional support for early initiation of ART for all PLWH and suggest that early-treated individuals have a lower barrier to HIV remission and may be ideal candidates for HIV curative studies.
Sex and genetics matter … kind of
Recent studies highlight important sex differences in HIV pathogenesis (reviewed in ref. 65) and indicate that females are more likely than males to achieve controller status in both adult (66, 67) and pediatric (68) cohorts. Interestingly, the female partner has been the controller in all four transmission pairs we have studied (15–17). HLA-B*57 and HLA-B*27 were overrepresented in ECs in both cohort studies (69, 70) and genome-wide association studies (71, 72). However, these alleles alone incompletely explain elite control, as they are also present in many CPs. In order to define additional factors that may modulate elite control, Martin et al. performed whole human genome sequencing on large numbers of HLA-B*57 ECs and HLA-B*57 CPs and identified a single variant, present in the NK cell receptor KIR3DL1, as the only notable difference between the two cohorts of patients (73). However, this variant does not fully explain why the majority of patients with protective HLA alleles do not become ECs.
It has been challenging to translate the favorable host genetics of ECs into broadly applicable therapies. So it was particularly interesting that the VISCONTI, ACTG, and many other studies did not report an increased frequency of protective HLA alleles in PTCs (7, 29, 30, 33, 53, 74). This lack of protective gene candidates raises the possibility that posttreatment control mechanisms may differ between ECs and PTCs and that the lessons learned from PTCs may translate better into treatment strategies. The role that sex and genetics play in posttreatment control has also been highlighted in two studies of African participants. The first study analyzed participants of the PROMISE trial, which enrolled virologically suppressed postpartum women, the majority of whom were from Africa. Interestingly, 25% of women in the PROMISE study were virologically suppressed 12 weeks after ATI compared with only 6% of ACTG participants, who were predominantly male and from the United States (75). A second study found a greater probability of posttreatment control in female participants in the SPARTAC study who were African versus those who were non-African (76). However, neither study was able to fully differentiate between the potential effects of sex, race, and host or viral genetics. Additional studies are needed to determine whether EC and PTC prevalence differs between men and women, and between individuals infected with different HIV subtypes (77).
Immune mediators may differ between ECs and PTCs
The mechanisms responsible for HIV replication control in ECs are still not fully understood, and it has been challenging to distinguish between cause and consequence of control. In fact, most studies are not performed during primary infection while control is being achieved. As described above, the most objective data suggest that class I HLA alleles are associated with protection. Since class I HLA proteins present peptides to CD8+ T cells, it stands to reason that CD8+ T cells play a key role in control. Indirect evidence for the role of CD8+ T cells comes from the monkey model of elite control, in which protective MHC class I alleles have also been described (78). Depletion of CD8+ T cells in EC monkeys results in transient loss of control, suggesting that these cells are playing a role in suppressing viral replication (79, 80). In humans, there is strong evidence that CD8+ T cells from ECs are much better at controlling viral replication in vitro than CD8+ T cells from PLWH on ART (31, 81–85). This enhanced control has been associated with distinct CD8+ T cell metabolic (86–89) and transcriptional (90–92) profiles. Not all ECs have protective HLA alleles, but one study suggested that many of these ECs have HIV-specific CD8+ T cells that kill as effectively as HIV-specific CD8+ T cells in ECs with protective HLA alleles (85). This cytotoxic capacity may be due to the fact that CD8+ T cells from controllers with and without protective HLA alleles target highly constrained epitopes that do not tolerate mutations (93, 94). While peripheral CD8+ T cells can control viral replication by killing infected CD4+ T cells, recent studies suggest that CD8+ T cells from lymph nodes do not have robust cytolytic function (95) and are phenotypically similar to tissue-resident memory CD8+ T cells (96). Interestingly, ECs have higher levels of HIV-specific tissue-resident memory CD8+ T cells than CPs in secondary lymphoid organs, suggesting that these cells control viral replication in the occupied tissue (96, 97). ECs with weak CD8+ T cell responses have been noted (98), but a study suggests that these subjects still possess strong HIV-specific central memory responses (99).
There is evidence that HIV-specific T cells can play a role in determining viral set point after treatment interruption (100). Early ART can preserve polyfunctional T cell responses in PTCs (101), and some harbor CD8+ T cells with the ex vivo capacity to suppress HIV replication (31). In addition, depletion of CD8+ T cells in NHP models of antibody-mediated posttreatment control have led to viral rebound (60). However, the immune mediators of HIV posttreatment control remain unclear, as most of the available data in PTCs have demonstrated relatively modest HIV-specific T cell responses (7, 28, 30, 32, 33, 46, 52, 55). In the patient known as the “Mississippi child,” HIV-specific T cell and antibody responses were largely undetectable during the 21.9 months of remission and returned only at the time of viremic rebound (35). The immune mediators of posttreatment control remain an enduring mystery, and a priority area of research.
The viral control demonstrated in ECs is not without cost, as ECs show increased T cell activation and inflammation (102–104). The impact of sustained immune activation and inflammation in noncontrollers has been associated with poor clinical outcomes (105–108) and extends to ECs, who may have an increased risk of cardiovascular disease (109) and hospitalization (110), although the risk remains undetermined in some studies (111–113). Intriguingly, PTCs can maintain viral control despite generally exhibiting blunted levels of immune activation compared with ECs (7, 28, 32, 33, 55, 99), but additional studies are needed to assess the long-term clinical implications of HIV posttreatment control and whether patients can achieve HIV remission without increased risk of systemic inflammation and clinical events.
Concluding remarks
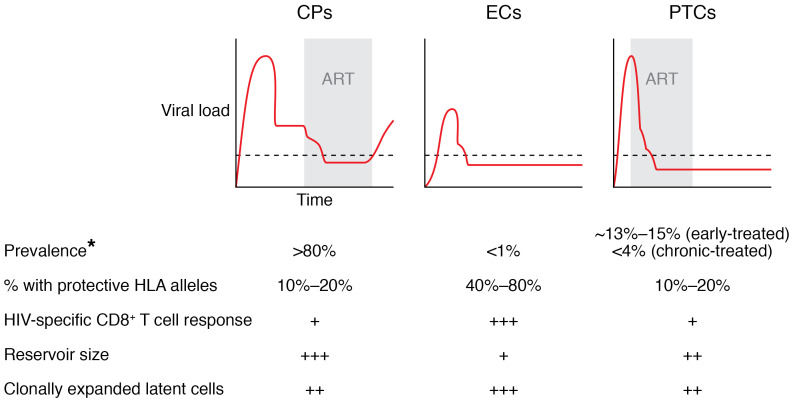
The study of medical outliers with unusual disease phenotypes or responses to treatment has led to important medical advances. In the oncology field, research on exceptional responders to cancer therapeutics has led to a deeper understanding of the molecular basis of treatment response and helped revolutionize the personalized medicine approach to the selection of treatment regimens (114, 115). Similarly, ECs and PTCs represent outliers in HIV natural infection and treatment outcomes (Figure 1). These individuals can maintain HIV suppression of pathogenic, replication-competent virus and serve as models of a functional cure for HIV. Studies of these patients have already led to important insight into the natural control of HIV, and the experience with ECs suggests that combining ART, initiated during primary infection, with a therapeutic vaccination strategy that induces potent CD8+ T cell responses targeting conserved epitopes may be a viable strategy. While protective HLA alleles are overrepresented in ECs, it is reassuring that these alleles are not necessary to achieve long-term remission. An enhanced immune response may lead to HIV remission not only by the clearance of activated HIV-infected cells, but also potentially by selecting for HIV-infected cells that are in a deeper state of HIV latency. Studies of PTCs have highlighted the importance of initiating ART during primary infection, as we may be able to induce posttreatment control in more than 10% of patients (27, 28). The low levels of immune activation and systemic inflammation detected in PTCs represent a hopeful sign that we may be able to induce HIV remission without the clinical risks observed in some HIV ECs. However, PTCs remain understudied as a group, and a concerted international effort is needed to identify these individuals and uncover the mechanisms behind their ability to achieve sustained HIV remission.
Figure 1. Virologic and immunologic profiles of CPs, ECs, and PTCs.
ART is normally started in chronic progressors (CPs) during the chronic phase of infection, and a rebound in viremia is seen when therapy is discontinued. In contrast, elite controllers (ECs) are ART-naive subjects who control viral replication naturally. Posttreatment controllers (PTCs) are more often patients in whom ART is initiated during primary infection. These patients maintain control of viral replication when ART is discontinued. *Estimates depend on definition of EC and PTC. +, ++, and +++ indicate relative magnitude of each parameter.
Acknowledgments
JNB is supported by the Johns Hopkins University Center for AIDS Research (P30AI094189) and National Institute of Allergy and Infectious Diseases (NIAID) award R01AI120024. JZL is supported by NIAID award R01AI150396.
Version 1. 06/01/2021
Electronic publication
Footnotes
Conflict of interest: JZL has consulted for Abbvie and JanBiotech.
Copyright: © 2021, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2021;131(11):e149414.https://doi.org/10.1172/JCI149414.
Contributor Information
Jonathan Z. Li, Email: jli@bwh.harvard.edu.
Joel N. Blankson, Email: jblanks@jhmi.edu.
References
- 1.Cao Y, et al. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N Engl J Med. 1995;332(4):201–208. doi: 10.1056/NEJM199501263320401. [DOI] [PubMed] [Google Scholar]
- 2.Hogervorst E, et al. Predictors for non- and slow progression in human immunodeficiency virus (HIV) type 1 infection: low viral RNA copy numbers in serum and maintenance of high HIV-1 p24-specific but not V3-specific antibody levels. J Infect Dis. 1995;171(4):811–821. doi: 10.1093/infdis/171.4.811. [DOI] [PubMed] [Google Scholar]
- 3.Lisziewicz J, et al. Control of HIV despite the discontinuation of antiretroviral therapy. N Engl J Med. 1999;340(21):1683–1684. doi: 10.1056/NEJM199905273402114. [DOI] [PubMed] [Google Scholar]
- 4.Li JZ, et al. The size of the expressed HIV reservoir predicts timing of viral rebound after treatment interruption. AIDS. 2016;30(3):343–353. doi: 10.1097/QAD.0000000000000953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jessen H, et al. How a single patient influenced HIV research—15-year follow-up. N Engl J Med. 2014;370(7):682–683. doi: 10.1056/NEJMc1308413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Namazi G, et al. The Control of HIV After Antiretroviral Medication Pause (CHAMP) study: posttreatment controllers identified from 14 clinical studies. J Infect Dis. 2018;218(12):1954–1963. doi: 10.1093/infdis/jiy479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saez-Cirion A, et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI Study. PLoS Pathog. 2013;9(3):e1003211. doi: 10.1371/journal.ppat.1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaunders J, et al. The Sydney Blood Bank Cohort: implications for viral fitness as a cause of elite control. Curr Opin HIV AIDS. 2011;6(3):151–156. doi: 10.1097/COH.0b013e3283454d5b. [DOI] [PubMed] [Google Scholar]
- 9.Woldemeskel BA, et al. Viral reservoirs in elite controllers of HIV-1 infection: implications for HIV cure strategies. EBioMedicine. 2020;62:103118. doi: 10.1016/j.ebiom.2020.103118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blankson JN, et al. Isolation and characterization of replication-competent human immunodeficiency virus type 1 from a subset of elite suppressors. J Virol. 2007;81(5):2508–2518. doi: 10.1128/JVI.02165-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamine A, et al. Replication-competent HIV strains infect HIV controllers despite undetectable viremia (ANRS EP36 study) AIDS. 2007;21(8):1043–1045. doi: 10.1097/QAD.0b013e3280d5a7ac. [DOI] [PubMed] [Google Scholar]
- 12.Julg B, et al. Infrequent recovery of HIV from but robust exogenous infection of activated CD4(+) T cells in HIV elite controllers. Clin Infect Dis. 2010;51(2):233–238. doi: 10.1086/653677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chun TW, et al. Effect of antiretroviral therapy on HIV reservoirs in elite controllers. J Infect Dis. 2013;208(9):1443–1447. doi: 10.1093/infdis/jit306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salgado M, et al. HLA-B*57 elite suppressor and chronic progressor HIV-1 isolates replicate vigorously and cause CD4+ T cell depletion in humanized BLT mice. J Virol. 2014;88(6):3340–3352. doi: 10.1128/JVI.03380-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bailey JR, et al. Transmission of human immunodeficiency virus type 1 from a patient who developed AIDS to an elite suppressor. J Virol. 2008;82(15):7395–7410. doi: 10.1128/JVI.00800-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buckheit RW, 3rd, et al. Host factors dictate control of viral replication in two HIV-1 controller/chronic progressor transmission pairs. Nat Commun. 2012;3:716. doi: 10.1038/ncomms1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walker-Sperling VE, et al. Factors associated with the control of viral replication and virologic breakthrough in a recently infected HIV-1 controller. EBioMedicine. 2017;16:141–149. doi: 10.1016/j.ebiom.2017.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yue L, et al. Transmitted virus fitness and host T cell responses collectively define divergent infection outcomes in two HIV-1 recipients. PLoS Pathog. 2015;11(1):e1004565. doi: 10.1371/journal.ppat.1004565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bendenoun M, et al. What is the most important for elite control: genetic background of patient, genetic background of partner, both or neither? Description of complete natural history within a couple of MSM. EBioMedicine. 2018;27:51–60. doi: 10.1016/j.ebiom.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bailey JR, et al. Maintenance of viral suppression in HIV-1-infected HLA-B*57+ elite suppressors despite CTL escape mutations. J Exp Med. 2006;203(5):1357–1369. doi: 10.1084/jem.20052319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Connell KA, et al. Control of HIV-1 in elite suppressors despite ongoing replication and evolution in plasma virus. J Virol. 2010;84(14):7018–7028. doi: 10.1128/JVI.00548-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mens H, et al. HIV-1 continues to replicate and evolve in patients with natural control of HIV infection. J Virol. 2010;84(24):12971–12981. doi: 10.1128/JVI.00387-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smith NM, et al. Proof-of-principle for immune control of global HIV-1 reactivation in vivo. Clin Infect Dis. 2015;61(1):120–128. doi: 10.1093/cid/civ219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bailey JR, et al. Evolution of HIV-1 in an HLA-B*57-positive patient during virologic escape. J Infect Dis. 2007;196(1):50–55. doi: 10.1086/518515. [DOI] [PubMed] [Google Scholar]
- 25.Rosás-Umbert M, et al. Mechanisms of abrupt loss of virus control in a cohort of previous HIV controllers. J Virol. 2019;93(4):e01436–18. doi: 10.1128/JVI.01436-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leon A, et al. Rate and predictors of progression in elite and viremic HIV-1 controllers. AIDS. 2016;30(8):1209–1220. doi: 10.1097/QAD.0000000000001050. [DOI] [PubMed] [Google Scholar]
- 27.Borrell M, et al. High rates of long-term progression in HIV-1-positive elite controllers. J Int AIDS Soc. 2021;24(2):e25675. doi: 10.1002/jia2.25675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Frange P, et al. HIV-1 virological remission lasting more than 12 years after interruption of early antiretroviral therapy in a perinatally infected teenager enrolled in the French ANRS EPF-CO10 paediatric cohort: a case report. Lancet HIV. 2016;3(1):e49–e54. doi: 10.1016/S2352-3018(15)00232-5. [DOI] [PubMed] [Google Scholar]
- 29.Sharaf R, et al. HIV-1 proviral landscapes distinguish posttreatment controllers from noncontrollers. J Clin Invest. 2018;128(9):4074–4085. doi: 10.1172/JCI120549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salgado M, et al. Prolonged control of replication-competent dual- tropic human immunodeficiency virus-1 following cessation of highly active antiretroviral therapy. Retrovirology. 2011;8:97. doi: 10.1186/1742-4690-8-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veenhuis RT, et al. Long-term remission despite clonal expansion of replication-competent HIV-1 isolates. JCI Insight. 2018;3(18):e122795. doi: 10.1172/jci.insight.122795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chéret A, et al. Combined ART started during acute HIV infection protects central memory CD4+ T cells and can induce remission. J Antimicrob Chemother. 2015;70(7):2108–2120. doi: 10.1093/jac/dkv084. [DOI] [PubMed] [Google Scholar]
- 33.Persaud D, et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med. 2013;369(19):1828–1835. doi: 10.1056/NEJMoa1302976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Etemad B, et al. Final Program. Paper presented at: 9th Edition: HIV Persistence During Therapy: Reservoirs & Eradication Strategies Workshop; December 10–13, 2019; Miami, Florida, USA. https://www.hiv-persistence.com/program-final Accessed April 13, 2021.
- 35.Luzuriaga K, et al. Viremic relapse after HIV-1 remission in a perinatally infected child. N Engl J Med. 2015;372(8):786–788. doi: 10.1056/NEJMc1413931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ho YC, et al. Replication-competent noninduced proviruses in the latent reservoir increase barrier to HIV-1 cure. Cell. 2013;155(3):540–551. doi: 10.1016/j.cell.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Einkauf KB, et al. Intact HIV-1 proviruses accumulate at distinct chromosomal positions during prolonged antiretroviral therapy. J Clin Invest. 2019;129(3):988–998. doi: 10.1172/JCI124291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bruner KM, et al. A quantitative approach for measuring the reservoir of latent HIV-1 proviruses. Nature. 2019;566(7742):120–125. doi: 10.1038/s41586-019-0898-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kwaa AK, et al. Elite suppressors have low frequencies of intact HIV-1 proviral DNA. AIDS. 2020;34(4):641–643. doi: 10.1097/QAD.0000000000002474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang C, et al. Distinct viral reservoirs in individuals with spontaneous control of HIV-1. Nature. 2020;585(7824):261–267. doi: 10.1038/s41586-020-2651-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mendoza D, et al. Comprehensive analysis of unique cases with extraordinary control over HIV replication. Blood. 2012;119(20):4645–4655. doi: 10.1182/blood-2011-10-381996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Canouï E, et al. A subset of extreme human immunodeficiency virus (HIV) controllers is characterized by a small HIV blood reservoir and a weak T-cell activation level. Open Forum Infect Dis. 2017;4(2):ofx064. doi: 10.1093/ofid/ofx064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zaunders J, et al. Possible clearance of transfusion-acquired nef/LTR-deleted attenuated HIV-1 infection by an elite controller with CCR5 Δ32 heterozygous and HLA-B57 genotype. J Virus Erad. 2019;5(2):73–83. doi: 10.1016/S2055-6640(20)30056-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Casado C, et al. Permanent control of HIV-1 pathogenesis in exceptional elite controllers: a model of spontaneous cure. Sci Rep. 2020;10(1):1902. doi: 10.1038/s41598-020-58696-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boritz EA, et al. Multiple origins of virus persistence during natural control of HIV infection. Cell. 2016;166(4):1004–1015. doi: 10.1016/j.cell.2016.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uruena A, et al. Prolonged posttreatment virologic control and complete seroreversion after advanced human immunodeficiency virus-1 infection. Open Forum Infect Dis. 2021;8(1):ofaa613. doi: 10.1093/ofid/ofaa613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Strongin Z, et al. Virologic and immunologic features of simian immunodeficiency virus control post-ART interruption in rhesus macaques. J Virol. 2020;94(14):e00338-20. doi: 10.1128/JVI.00338-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goujard C, et al. Spontaneous control of viral replication during primary HIV infection: when is “HIV controller” status established? Clin Infect Dis. 2009;49(6):982–986. doi: 10.1086/605504. [DOI] [PubMed] [Google Scholar]
- 49.Walker-Sperling VE, et al. Factors associated with the control of viral replication and virologic breakthrough in a recently infected HIV-1 controller. EBioMedicine. 2017;16:141–149. doi: 10.1016/j.ebiom.2017.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morley D, et al. Rapid development of HIV elite control in a patient with acute infection. BMC Infect Dis. 2019;19(1):815. doi: 10.1186/s12879-019-4374-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hocqueloux L, et al. Long-term immunovirologic control following antiretroviral therapy interruption in patients treated at the time of primary HIV-1 infection. AIDS. 2010;24(10):1598–1601. doi: 10.1097/QAD.0b013e32833b61ba. [DOI] [PubMed] [Google Scholar]
- 52.Goujard C, et al. HIV-1 control after transient antiretroviral treatment initiated in primary infection: role of patient characteristics and effect of therapy. Antivir Ther. 2012;17(6):1001–1009. doi: 10.3851/IMP2273. [DOI] [PubMed] [Google Scholar]
- 53.Maenza J, et al. How often does treatment of primary HIV lead to post-treatment control? Antivir Ther. 2015;20(8):855–863. doi: 10.3851/IMP2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martin GE, et al. Post-treatment control or treated controllers? Viral remission in treated and untreated primary HIV infection. AIDS. 2017;31(4):477–484. doi: 10.1097/QAD.0000000000001382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Violari A, et al. A child with perinatal HIV infection and long-term sustained virological control following antiretroviral treatment cessation. Nat Commun. 2019;10(1):412. doi: 10.1038/s41467-019-08311-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maggiolo F, et al. Post treatment controllers after treatment interruption in chronically HIV infected patients. AIDS. 2018;32(5):623–628. doi: 10.1097/QAD.0000000000001743. [DOI] [PubMed] [Google Scholar]
- 57.Perkins MJ, et al. Brief report: Prevalence of posttreatment controller phenotype is rare in HIV-infected persons after stopping antiretroviral therapy. J Acquir Immune Defic Syndr. 2017;75(3):364–369. doi: 10.1097/QAI.0000000000001393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Colby DJ, et al. Rapid HIV RNA rebound after antiretroviral treatment interruption in persons durably suppressed in Fiebig I acute HIV infection. Nat Med. 2018;24(7):923–926. doi: 10.1038/s41591-018-0026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haigwood NL, et al. Passive immune globulin therapy in the SIV/macaque model: early intervention can alter disease profile. Immunol Lett. 1996;51(1–2):107–114. doi: 10.1016/0165-2478(96)02563-1. [DOI] [PubMed] [Google Scholar]
- 60.Nishimura Y, et al. Early antibody therapy can induce long-lasting immunity to SHIV. Nature. 2017;543(7646):559–563. doi: 10.1038/nature21435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pinzone MR, et al. Monitoring integration over time supports a role for cytotoxic T lymphocytes and ongoing replication as determinants of reservoir size. J Virol. 2016;90(23):10436–10445. doi: 10.1128/JVI.00242-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ananworanich J, et al. HIV DNA set point is rapidly established in acute HIV infection and dramatically reduced by early ART. EBioMedicine. 2016;11:68–72. doi: 10.1016/j.ebiom.2016.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trautmann L, et al. Profound metabolic, functional, and cytolytic differences characterize HIV-specific CD8 T cells in primary and chronic HIV infection. Blood. 2012;120(17):3466–3477. doi: 10.1182/blood-2012-04-422550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Streeck H, et al. Immunological and virological impact of highly active antiretroviral therapy initiated during acute HIV-1 infection. J Infect Dis. 2006;194(6):734–739. doi: 10.1086/503811. [DOI] [PubMed] [Google Scholar]
- 65.Scully EP. Sex differences in HIV infection. Curr HIV/AIDS Rep. 2018;15(2):136–146. doi: 10.1007/s11904-018-0383-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Madec Y, et al. Spontaneous control of viral load and CD4 cell count progression among HIV-1 seroconverters. AIDS. 2005;19(17):2001–2007. doi: 10.1097/01.aids.0000194134.28135.cd. [DOI] [PubMed] [Google Scholar]
- 67.Madec Y, et al. Natural history of HIV-control since seroconversion. AIDS. 2013;27(15):2451–2460. doi: 10.1097/01.aids.0000431945.72365.01. [DOI] [PubMed] [Google Scholar]
- 68.Vieira VA, et al. Strong sex bias in elite control of paediatric HIV infection. AIDS. 2019;33(1):67–75. doi: 10.1097/QAD.0000000000002043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kaslow RA, et al. Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat Med. 1996;2(4):405–411. doi: 10.1038/nm0496-405. [DOI] [PubMed] [Google Scholar]
- 70.Migueles SA, et al. HLA B*5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc Natl Acad Sci U S A. 2000;97(6):2709–2714. doi: 10.1073/pnas.050567397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fellay J, et al. A whole-genome association study of major determinants for host control of HIV-1. Science. 2007;317(5840):944–947. doi: 10.1126/science.1143767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.International HIV Controllers Study. et al. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science. 2010;330(6010):1551–1557. doi: 10.1126/science.1195271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martin MP, et al. Killer cell immunoglobulin-like receptor 3DL1 variation modifies HLA-B*57 protection against HIV-1. J Clin Invest. 2018;128(5):1903–1912. doi: 10.1172/JCI98463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Etemad B, et al. Abstract 347. Paper presented at: Conference on Retroviruses and Opportunistic Infections (CROI 2016); February 22–25, 2016; Boston, Massachusetts, USA. https://i-base.info/htb/29879 Accessed April 13, 2021.
- 75.Le CN, et al. Time to viral rebound and safety after antiretroviral treatment interruption in postpartum women compared with men. AIDS. 2019;33(14):2149–2156. doi: 10.1097/QAD.0000000000002334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gossez M, et al. Virological remission after antiretroviral therapy interruption in female African HIV seroconverters. AIDS. 2019;33(2):185–197. doi: 10.1097/QAD.0000000000002044. [DOI] [PubMed] [Google Scholar]
- 77.Dos Santos JS, et al. Host factor predictors in long-term nonprogressors HIV-1 infected with distinct viral clades. Curr HIV Res. 2017;15(6):440–447. doi: 10.2174/1570162X16666171206120024. [DOI] [PubMed] [Google Scholar]
- 78.Mudd PA, Watkins DI. Understanding animal models of elite control: windows on effective immune responses against immunodeficiency viruses. Curr Opin HIV AIDS. 2011;6(3):197–201. doi: 10.1097/COH.0b013e3283453e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Friedrich TC, et al. Subdominant CD8+ T-cell responses are involved in durable control of AIDS virus replication. J Virol. 2007;81(7):3465–3476. doi: 10.1128/JVI.02392-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pandrea I, et al. Functional cure of SIVagm infection in rhesus macaques results in complete recovery of CD4+ T cells and is reverted by CD8+ cell depletion. PLoS Pathog. 2011;7(8):e1002170. doi: 10.1371/journal.ppat.1002170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Migueles SA, et al. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat Immunol. 2002;3(11):1061–1068. doi: 10.1038/ni845. [DOI] [PubMed] [Google Scholar]
- 82.Betts MR, et al. Blood. 2006;107(12):4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sáez-Cirión A, et al. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc Natl Acad Sci U S A. 2007;104(16):6776–6781. doi: 10.1073/pnas.0611244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Migueles SA, et al. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity. 2008;29(6):1009–1021. doi: 10.1016/j.immuni.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Migueles SA, et al. CD8(+) T-cell cytotoxic capacity associated with human immunodeficiency virus-1 control can be mediated through various epitopes and human leukocyte antigen types. EBioMedicine. 2014;2(1):46–58. doi: 10.1016/j.ebiom.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chowdhury FZ, et al. Metabolic pathway activation distinguishes transcriptional signatures of CD8+ T cells from HIV-1 elite controllers. AIDS. 2018;32(18):2669–2677. doi: 10.1097/QAD.0000000000002007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tarancon-Diez L, et al. Immunometabolism is a key factor for the persistent spontaneous elite control of HIV-1 infection. EBioMedicine. 2019;42:86–96. doi: 10.1016/j.ebiom.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Angin M, et al. Metabolic plasticity of HIV-specific CD8+ T cells is associated with enhanced antiviral potential and natural control of HIV-1 infection. Nat Metab. 2019;1(7):704–716. doi: 10.1038/s42255-019-0081-4. [DOI] [PubMed] [Google Scholar]
- 89.Loucif H, et al. Lipophagy confers a key metabolic advantage that ensures protective CD8A T-cell responses against HIV-1. Autophagy. 2021;18:1–16. doi: 10.1080/15548627.2021.1874134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Simonetta F, et al. High eomesodermin expression among CD57+ CD8+ T cells identifies a CD8+ T cell subset associated with viral control during chronic human immunodeficiency virus infection. J Virol. 2014;88(20):11861–11871. doi: 10.1128/JVI.02013-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nguyen S, et al. Elite control of HIV is associated with distinct functional and transcriptional signatures in lymphoid tissue CD8+ T cells. Sci Transl Med. 2019;11(523):eaax4077. doi: 10.1126/scitranslmed.aax4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rutishauser RL, et al. TCF-1 regulates HIV-specific CD8+ T cell expansion capacity. JCI Insight. 2021;6(3):136648. doi: 10.1172/jci.insight.136648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gorin AM, et al. HIV-1 epitopes presented by MHC class I types associated with superior immune containment of viremia have highly constrained fitness landscapes. PLoS Pathog. 2017;13(8):e1006541. doi: 10.1371/journal.ppat.1006541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gaiha GD, et al. Structural topology defines protective CD8+ T cell epitopes in the HIV proteome. Science. 2019;364(6439):480–484. doi: 10.1126/science.aav5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Reuter MA, et al. HIV-specific CD8+ T cells exhibit reduced and differentially regulated cytolytic activity in lymphoid tissue. Cell Rep. 2017;21(12):3458–3470. doi: 10.1016/j.celrep.2017.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Buggert M, et al. Identification and characterization of HIV-specific resident memory CD8+ T cells in human lymphoid tissue. Sci Immunol. 2018;3(24):eaar4526. doi: 10.1126/sciimmunol.aar4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kiniry BE, et al. Detection of HIV-1-specific gastrointestinal tissue resident CD8+ T-cells in chronic infection. Mucosal Immunol. 2018;11(3):909–920. doi: 10.1038/mi.2017.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sáez-Cirión A, et al. Heterogeneity in HIV suppression by CD8 T cells from HIV controllers: association with Gag-specific CD8 T cell responses. J Immunol. 2009;182(12):7828–7837. doi: 10.4049/jimmunol.0803928. [DOI] [PubMed] [Google Scholar]
- 99.Ndhlovu ZM, et al. Elite controllers with low to absent effector CD8+ T cell responses maintain highly functional, broadly directed central memory responses. J Virol. 2012;86(12):6959–6969. doi: 10.1128/JVI.00531-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Schooley RT, et al. AIDS Clinical Trials Group 5197: a placebo-controlled trial of immunization of HIV-1-infected persons with a replication-deficient adenovirus type 5 vaccine expressing the HIV-1 core protein. J Infect Dis. 2010;202(5):705–716. doi: 10.1086/655468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Samri A, et al. Polyfunctional HIV-specific T cells in post-treatment controllers. AIDS. 2016;30(15):2299–2302. doi: 10.1097/QAD.0000000000001195. [DOI] [PubMed] [Google Scholar]
- 102.Ostrowski SR, et al. Residual viraemia in HIV-1-infected patients with plasma viral load. Scand J Immunol. 2008;68(6):652–660. doi: 10.1111/j.1365-3083.2008.02184.x. [DOI] [PubMed] [Google Scholar]
- 103.Hunt PW, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008;197(1):126–133. doi: 10.1086/524143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pereyra F, et al. Persistent low-level viremia in HIV-1 elite controllers and relationship to immunologic parameters. J Infect Dis. 2009;200(6):984–990. doi: 10.1086/605446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu Z, et al. CD8+ T-lymphocyte activation in HIV-1 disease reflects an aspect of pathogenesis distinct from viral burden and immunodeficiency. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18(4):332–340. doi: 10.1097/00042560-199808010-00004. [DOI] [PubMed] [Google Scholar]
- 106.Giorgi JV, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179(4):859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 107.Kuller LH, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hazenberg MD, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS. 2003;17(13):1881–1888. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- 109.Pereyra F, et al. Increased coronary atherosclerosis and immune activation in HIV-1 elite controllers. AIDS. 2012;26(18):2409–2412. doi: 10.1097/QAD.0b013e32835a9950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Crowell TA, et al. Hospitalization rates and reasons among HIV elite controllers and persons with medically controlled HIV infection. J Infect Dis. 2015;211(11):1692–1702. doi: 10.1093/infdis/jiu809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Brusca RM, et al. Subclinical cardiovascular disease in HIV controller and long-term nonprogressor populations. HIV Med. 2020;21(4):217–227. doi: 10.1111/hiv.12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dominguez-Molina B, et al. Analysis of non-AIDS-defining events in HIV controllers. Clin Infect Dis. 2016;62(10):1304–1309. doi: 10.1093/cid/ciw120. [DOI] [PubMed] [Google Scholar]
- 113.Crowell TA, et al. Hospitalizations among HIV controllers and persons with medically controlled HIV in the U.S. Military HIV Natural History Study. J Int AIDS Soc. 2016;19(1):20524. doi: 10.7448/IAS.19.1.20524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tsimberidou AM, et al. Defining, identifying, and understanding “exceptional responders” in oncology using the tools of precision medicine. Cancer J. 2019;25(4):296–299. doi: 10.1097/PPO.0000000000000392. [DOI] [PubMed] [Google Scholar]
- 115.Iyer G, et al. Genome sequencing identifies a basis for everolimus sensitivity. Science. 2012;338(6104):221. doi: 10.1126/science.1226344. [DOI] [PMC free article] [PubMed] [Google Scholar]