Abstract
BACKGROUND
Recently the α1 adrenergic receptor antagonist terazosin was shown to activate PGK1, a possible target for the mitochondrial deficits in Parkinson disease related to its function as the initial enzyme in ATP synthesis during glycolysis. An epidemiological study of terazosin users showed a lower incidence of Parkinson disease when compared with users of tamsulosin, an α1 adrenergic receptor antagonist of a different class that does not activate PGK1. However, prior research on tamsulosin has suggested that it may in fact potentiate neurodegeneration, raising the question of whether it is an appropriate control group.
METHODS
To address this question, we undertook an epidemiological study on Parkinson disease occurrence rate in 113,450 individuals from the United States with 5 or more years of follow-up. Patients were classified as tamsulosin users (n = 45,380), terazosin/alfuzosin/doxazosin users (n = 22,690), or controls matched for age, sex, and Charlson comorbidity index score (n = 45,380).
RESULTS
Incidence of Parkinson disease in tamsulosin users was 1.53%, which was significantly higher than that in both terazosin/alfuzosin/doxazosin users (1.10%, P < 0.0001) and matched controls (1.01%, P < 0.0001). Terazosin/alfuzosin/doxazosin users did not differ in Parkinson disease risk from matched controls (P = 0.29).
CONCLUSION
These results suggest that zosins may not confer a protective effect against Parkinson disease, but rather that tamsulosin may in some way potentiate Parkinson disease progression.
FUNDING
This work was supported by Cerevel Therapeutics.
Keywords: Genetics, Neuroscience
Keywords: Epidemiology, Parkinson disease
Introduction
Parkinson disease (PD) is the second most common neurodegenerative disorder after Alzheimer disease. Due to the aging of the world population, the incidence of PD is increasing, with 6.1 million affected individuals in 2016 versus 2.5 million affected individuals in 1990 (1). Development of disease-modifying therapies is the greatest unmet need in treatment of neurodegenerative disease; however, the high cost and long timeline of drug development limit the number of therapeutic hypothesis that can be rigorously evaluated in clinical studies (2). Furthermore, when an ineffective approach is pursued, this is often not apparent until completion of large phase II or III trials. Therefore, early identification of treatment targets with increased probability of success is critical to minimize negative resource and capital opportunity cost of failure, unproductive allocation of scarce resources of time and capital, and to increase the speed to finding effective therapeutics for patients with significant unmet need.
Recent evidence from animal models and retrospective epidemiological studies has suggested that phosphoglycerate kinase 1 (PGK1) is a target of interest for disease modification in PD (3). PGK1 is the first enzyme in ATP generation via glycolysis, and therefore PGK1 potentiation may ameliorate cellular pathophysiology in disorders with an established mitochondrial deficit/phenotype such as PD through increasing ATP production in the context of reduced cellular energy production. In 2015, terazosin, an α1 adrenergic receptor antagonist frequently prescribed for benign prostatic hyperplasia (BPH), was found to bind to PGK1 and potentiate its kinase activity (4). This PKG1 pharmacology has also been demonstrated in related drugs harboring quinazoline motifs, such as alfuzosin and doxazosin (4). Furthermore, an analysis of the Truven database and Danish nationwide health registries demonstrated that individuals treated with terazosin, alfuzosin, or doxazosin showed lower rates of both the development of PD and PD-related diagnoses when compared with patients treated with another BPH treatment, tamsulosin, which does not interact with PGK1 (3, 5). To that end, a clinical study evaluating the safety and tolerability of terazosin, 5 mg once daily for 12 weeks, in patients with PD (Hoehn and Yahr stage I–III) has been initiated (NCT03905811).
While the prospect that terazosin may have a protective effect in PD is promising, it is important to closely review the evidence for possible alternative explanations for the observed risk imbalance. Notably, in the analysis by Cai et al. (3), there was no control group of age-matched patients who were not on a BPH treatment. Therefore, although a protective effect associated with terazosin use is possible, an alternative interpretation could be an increased incidence or rate of progression of PD in patients using tamsulosin as the comparison group (3). This idea is not without precedent; Duan and colleagues found a positive correlation between tamsulosin use and dementia risk when compared with terazosin, doxazosin, alfuzosin, or no BPH medication (6). The inclusion of a propensity score–matched no-BPH-drug cohort is critical for interpretation of differences between drug effects (i.e., protection via terazosin vs. increased risk via tamsulosin).
To further study the implications of tamsulosin and terazosin use for PD risk, we designed a large, retrospective study looking at the incidence of PD in 3 nonoverlapping groups of patients: those treated with terazosin, alfuzosin, or doxazosin (Zosin Cohort); those treated with tamsulosin (Tamsulosin Cohort); and an appropriately matched Control Cohort with no BPH drug treatment.
Results
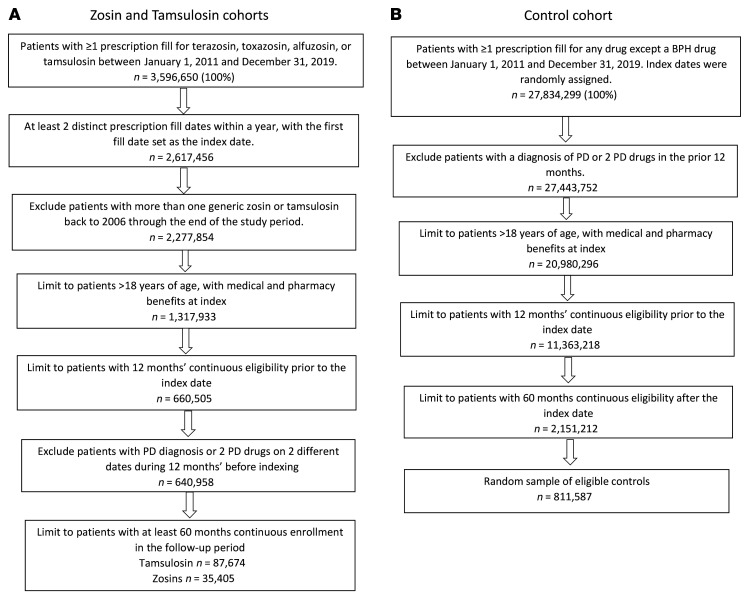
This epidemiological study on risk of PD included 113,450 individuals with 5 or more years of follow-up. Figure 1 shows the sample selection process for the Zosin, Tamsulosin, and Control Cohorts, based on defined inclusion and exclusion criteria. Propensity score matching (PSM) was used to balance the cohorts in demographic and clinical differences (7). Before the PSM procedure was conducted, the initial sample of patients taking tamsulosin was 87,674, and 35,405 were taking a zosin medication (7). From among the 2,151,212 patients identified as eligible controls, the SAS survey select procedure was used to select a random sample of 811,587 patients, stratified by age groups corresponding to the Zosin Cohort after matching to the Tamsulosin Cohort (8).
Figure 1. Initial sample selection and attrition.
PSM results.
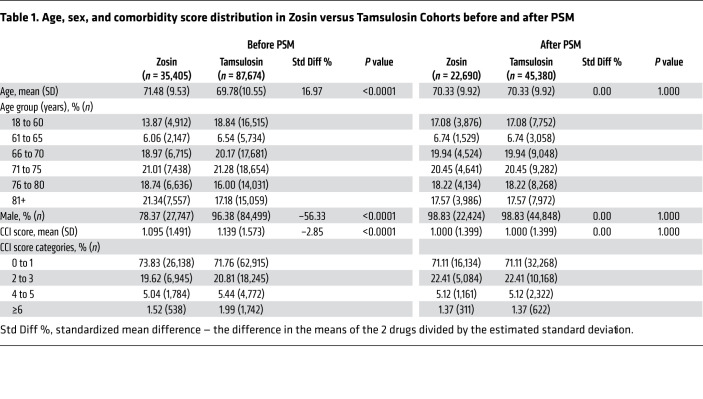
As a result of the PSM procedure, the final Zosin Cohort included 22,690 patients, and the Tamsulosin and Control Cohorts each included 45,380 patients (Tables 1 and 2). Before matching, the Zosin Cohort was older, had fewer males, and lower Charlson comorbidity index (CCI) scores than the Tamsulosin Cohort (9). Similarly, before matching, the Zosin Cohort was older, had more males, and higher CCI scores than the Control Cohort.
Table 1. Age, sex, and comorbidity score distribution in Zosin versus Tamsulosin Cohorts before and after PSM.
Table 2. Characteristics of patients comparing Zosin to Control Cohorts before and after PSM.
PD diagnoses during follow-up period.
As shown in Table 3, diagnoses of PD were observed more frequently in the Tamsulosin Cohort than the Zosin Cohort (1.52% vs. 1.10%, respectively, all patients; P < 0.0001), but they were also observed more frequently in the Tamsulosin Cohort as compared with the Control Cohort (1.53% vs. 1.01%, respectively, all patients; P < 0.001). The rate observed here in the Control Cohort is consistent with that seen in other published studies (10).
Table 3. Proportion of cohorts with PD diagnosis.
The Zosin Cohort did not differ in PD risk from matched controls when examining the entire cohort (P = 0.29) or when the analysis was restricted to those over 60 years of age (P = 0.60). In contrast, PD diagnoses were significantly more frequent among the Tamsulosin Cohort (1.53%) as compared with the matched Control Cohort (1.01%, P < 0.0001) and the Zosin Cohort (1.10%, P < 0.0001), and this result held when analysis was restricted to those over the age of 60 (1.78% for tamsulosin vs. 1.24% for zosins, P < 0.0001; and vs. 1.19% for matched controls, P < 0.0001). There was some evidence of an increased frequency of PD diagnoses in those under the age of 60 for individuals taking either tamsulosin (P = 0.0164) or a zosin (P = 0.0115) when compared with matched controls; however, this result should be taken with caution due to the small number of patient diagnoses with PD in this age group (n = 9 controls, 15 zosin, 23 tamsulosin).
Discussion
Cai et al., using the Truven database, investigated whether patients taking terazosin, alfuzosin, or doxazosin had similar proportions of patients who developed PD as compared with a tamsulosin control group (3). Their finding that patients taking a zosin medication had fewer PD diagnoses than those taking tamsulosin was significant; however, their follow-up time was less than 1 year. By extending follow-up to 5 years, the current study enables examination of PD diagnoses in the longer term, which increases confidence in the diagnosis. Approximately 20% of diagnoses of PD are incorrect within the first year, but this proportion decreases to near zero after a few years. Also, their study did not have a BPH medication–free control group. Because our study included comparisons to matched control subjects, our results suggest that zosins may not confer a protective effect against PD, as proposed by Cai et al., but rather that tamsulosin may in some way potentiate neurodegeneration, in particular among patients over age 61.
This suggestion is supported by a database study by Duan et al. (6), which found that the risk of dementia (not specifically in association with PD) was higher among patients taking tamsulosin, as compared with a control cohort that took no BPH medication as well as individual cohorts taking alternative BPH medications, including doxazosin, terazosin, and alfuzosin, specifically among patients over 65 years of age. The finding was significant when analyses controlled for comorbidity burden and use of chronic medications known to increase the risk of developing dementia. Thus, use of tamsulosin appears to increase the risk of cognitive adverse effects, including the development of dementia, which may not have been observed in a relatively short follow-up in clinical trials of tamsulosin or the study by Cai et al. (3).
Limitations of the current study include matching only for age, sex, and CCI score. Although CCI was considered a proxy for health conditions, it would be useful to match for specific conditions, lifestyle choices (such as smoking), socioeconomic factors, and medications that are likely to affect the risk of developing PD. Furthermore, because the drugs in this study are primarily prescribed for BPH, the cohort was almost entirely male and the findings therefore can only be applied to men. Retrospective observational studies have limitations and claims data do not include all possible outcome variables that would permit more in-depth analyses. The current study determined the risk of developing PD based solely on diagnostic codes; coding may be subject to error and reimbursement bias, as claims are not created for the purposes of research, but for administration and billing. The results obtained are specific to patients with the type of insurance included; they may not be applicable to different populations. Importantly, this study points out that because of these limitations in retrospective studies, careful attention to inclusion of all necessary control groups is essential to correctly understand the implications of any results. Because findings such as these can influence the decisions made by patients and their physicians concerning their care, it is critical that alternative interpretations are fully assessed.
This study challenges the notion that PGK1 may be a valid target for disease modification in PD and raises the question as to the best choice for BPH medications for long-term use by elderly patients. A mechanism explaining the differences we and others have observed for the association of tamsulosin use and neurodegeneration may be further elucidated by longitudinal real-world evidence, controlling for more variables associated with risk of PD and dementia.
Methods
Data source
The Optum Research Database (ORD) is Optum’s proprietary administrative claims research database composed of pharmacy and medical claims. The ORD includes deidentified and HIPAA-compliant medical and pharmacy claims data from 1993 to the present on more than 73 million lives. In 2018, approximately 19% of the US commercially enrolled population, 21% of the Medicare Advantage population, and 22% of the Medicare Part D (with pharmacy claims only) populations were represented in the ORD.
Study design
This was a retrospective observational study including claims data for the period January 1, 2010, to December 31, 2019 (study period). An index event was defined by the first prescription fill for an alpha blocker medication of interest for BPH (terazosin, doxazosin, afluzosin, tamsulosin) during the identification period (see Supplemental Figure 1) January 1, 2011, to December 31, 2019. A baseline period of at least 12 months with continuous medical and pharmacy benefits prior to the index event was set to identify any previous PD diagnosis or PD medication (DOPA decarboxylase/DA precursor, dopamine agonists, MAO B inhibitors, COMT inhibitors, anticholinergics, adamantanes, adenosine A2a agonists; see Supplemental Table 1). A follow-up period of at least 5 years of continuous medical and pharmacy benefits after indexing was used to assess PD diagnosis occurring after the initiation of treatment with the index medication.
Study sample
The study included commercially and Medicare Advantage Part D insured patients (≥18 years of age) taking medications of interest for BPH (terazosin, doxazosin, afluzosin, tamsulosin) who had no evidence of PD during the baseline period. Three mutually exclusive cohorts were created based on presence or absence of BPH medication of interest, along with other criteria: the Zosin Cohort taking terazosin, doxazosin, or alfuzosin; the Tamsulosin Cohort, which was only taking tamsulosin; and the Control Cohort, which was taking none of the 4 drugs of interest. For all cohorts, continuous enrollment with medical and pharmacy benefits for the entire study period, including 5 years of follow-up, were required.
Patients were further included in the Zosin or Tamsulosin Cohorts by at least 2 prescription fills in the same year for a zosin (terazosin, doxazosin, afluzosin) or tamsulosin. They were excluded by the presence of claims containing PD diagnoses codes (ICD code 332 or G20) or 2 or more PD medication fills in the baseline period.
Patients were included in the Control Cohort with at least 1 prescription fill for any prescription, other than the 4 BPH medications of interest during the study period. A random index date was chosen as the date of a prescription. They were excluded by PD diagnosis codes (ICD code 332 or G20) or 2 or more PD medication fills in the baseline period, or fills for terazosin, doxazosin, alfuzosin, or tamsulosin.
Measures/outcomes
Patient characteristics were noted, including age as of the index date, sex, and CCI score at index (9). Mean (± standard deviation [SD]) continuous age; n (%) male; and mean (SD) continuous CCI score were compared. During the follow-up period, the number (%) of patients with PD diagnosis was identified by ICD-9/10 codes 332 or G20 and compared between Zosin and Tamsulosin Cohorts, and between Zosin and Control Cohorts, stratified by age group (all, 18–60 years, and 61+ years).
Analytical methods
Randomization of controls.
From among the 2,151,212 patients identified as eligible controls, the SAS survey select procedure (8) was used for a simple random sample. We stratified by the age group at index (18 to 50, 51 to 55, 56 to 60, 61 to 65, 66 to 70, 71 to 75, 76 to 80, 81+). For the sampling rate, we used the age group distribution of the Zosin Cohorts after matching to the Tamsulosin Cohort. The final random sample of controls included 811,587 patients.
PSM procedure.
PSM was used to balance the cohorts in demographic and clinical differences including age on index date, sex, and mean CCI score (7). A greedy match procedure provided 2 control matches per zosin case. First, patients taking zosins were matched to patients taking tamsulosin. From that matching output, the zosin-matched patients were matched to randomized controls using the same criteria. For any cases without 2 controls, cases and all corresponding controls were dropped.
Statistics
Statistical analyses were conducted comparing matched Zosin Cohort versus Control Cohort, Zosin versus Tamsulosin Cohort, and Tamsulosin versus Control Cohort, with the SAS procedure, proc genmod, comparing patient characteristics used for the PSM procedure (7, 8). Cohorts were compared using χ2 statistics for outcomes of PD diagnoses over the 5-year minimum follow-up period; P less than 0.05 was considered significant.
Study approval
Only completely deidentified data were accessed for this study, and its conduct aligned with US Health Insurance Portability and Accountability Act regulations. Thus, Institutional Review Board approval was neither obtained nor sought.
Author contributions
DJS and RS initially conceived of the study, analyzed data, and prepared the manuscript. DLG, SD, SSP, and JJR contributed to the initial conceptualization of the study and to manuscript preparation (review/revision). AB, MF, and MIS contributed to study design, data collection and analysis, and manuscript preparation.
Supplementary Material
Acknowledgments
Medical writing support was provided by Caroline Jennermann of Optum HEOR, as funded by Cerevel. Armen Zakharyan was responsible for development of the models used in this work. We thank Monica Frazer for contributions to the development of the manuscript; Phil Iredale, Yong Mi Choi, Georgette Suidan, David Zhang, Kimberly Kerr, and Hanh Nguyen for helpful discussion and for contributions to the design of the study; and Timothy Bancroft for analytical guidance.
Version 1. 04/06/2021
In-Press Preview
Version 2. 06/01/2021
Electronic publication
Funding Statement
All work was internal target validation research at Cerevel. Work done in collaboration with Optum Life Sciences was funded by Cerevel.
Footnotes
Conflict of interest: RS, SD, DLG, SSP, JJR, and DJS were employees of and equity shareholders in Cerevel Therapeutics, LLC, at the time this work was conducted. At the time of the study, AB, MF, and MIS were employed by Optum, which was contracted by Cerevel to perform the analyses. Employment of none of the authors was contingent upon the publication of the manuscript.
Copyright: © 2021, American Society for Clinical Investigation.
Reference information: J Clin Invest. 2021;131(11):e145112. https://doi.org/10.1172/JCI145112.
See the related Commentary at Parkinson disease risks: correctly identifying environmental factors for a chronic disease.
Contributor Information
Rahul Sasane, Email: rahul.sasane@cerevel.com.
Amy Bartels, Email: amy.bartels@optum.com.
Michelle Field, Email: shelli.field@optum.com.
Maria I. Sierra, Email: maria.sierra@optum.com.
Sridhar Duvvuri, Email: Sridhar.Duvvuri@cerevel.com.
David L. Gray, Email: david.gray@cerevel.com.
Sokhom S. Pin, Email: Sokhom.pin@cerevel.com.
John J. Renger, Email: john.renger@cerevel.com.
David J. Stone, Email: david.stone@cerevel.com.
References
- 1.GBD 2016 Causes of Death Collaborators Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1151–1210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Herper M. How much does pharmaceutical innovation cost? A look at 100 companies. Forbes. August 11, 2013. Accessed April 2, 2021. https://www.forbes.com/sites/matthewherper/2013/08/11/the-cost-of-inventing-a-new-drug-98-companies-ranked/?sh=2bc4ddd82f08.
- 3.Cai R, et al. Enhancing glycolysis attenuates Parkinson’s disease progression in models and clinical databases. J Clin Invest. 2019;129(10):4539–4549. doi: 10.1172/JCI129987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen X, et al. Terazosin activates Pgk1 and Hsp90 to promote stress resistance. Nat Chem Biol. 2015;11(1):19–25. doi: 10.1038/nchembio.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simmering JE, et al. Association of glycoloysis-enhancing α-1 blockers with risk of developing Parkinson disease. JAMA Neurol. doi: 10.1001/jamaneurol.2020.5157. [published online February 1, 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duan Y, et al. Tamsulosin and the risk of dementia in older men with benign prostatic hyperplasia. Pharmacoepidemiol Drug Saf. 2018;27(3):340–348. doi: 10.1002/pds.4361. [DOI] [PubMed] [Google Scholar]
- 7.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70(1):41–55. doi: 10.1093/biomet/70.1.41. [DOI] [Google Scholar]
- 8. SAS Enterprise Guide 8.3. SAS Institute Inc; 2021. [Google Scholar]
- 9.Quan H, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
- 10.Hirsch L, et al. The incidence of Parkinson’s disease: a systematic review and meta-analysis. Neuroepidemiology. 2016;46(4):292–300. doi: 10.1159/000445751. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.