To the Editor:
We read with great interest the article by Cornberg et al., recently published in the Journal of Hepatology, reporting the European Association for the Study of the Liver (EASL) position on the use of coronavirus disease 2019 (COVID-19) vaccines in patients affected by chronic liver diseases, and in particular in liver transplant recipients.1 Indeed, despite evidence suggesting that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is not associated with an increased risk of death in patients who received a liver transplant, these patients more frequently required ICU admission and invasive-ventilation compared to matched controls, and therefore do represent a subset of patients that can be considered especially frail, and at high-risk of severe complications of COVID-19.2 Actually, the EASL position paper suggests that the benefit and potential risks of vaccination against COVID-19 should be weighed individually, and that patients with additional risk factors for severe complications, such as advanced age and comorbidities, should be prioritised.1 , 3 Furthermore, the registration studies of the various vaccines against COVID-19 approved by the EMA and FDA did not include patients who had received solid organ transplantation or immune-suppression, and therefore neither the efficacy or safety of the available COVID-19 vaccines have been described in liver transplant recipients, nor can a particular type of vaccine be suggested on a sound basis for these patients.[4], [5], [6] Lastly, there is recent evidence that the immunogenicity of vaccines against COVID-19 is lower in liver transplant recipients compared to healthy controls, and that age, lower glomerular filtration rate, and enhanced immune-suppression are predictors of poor response to vaccination.7
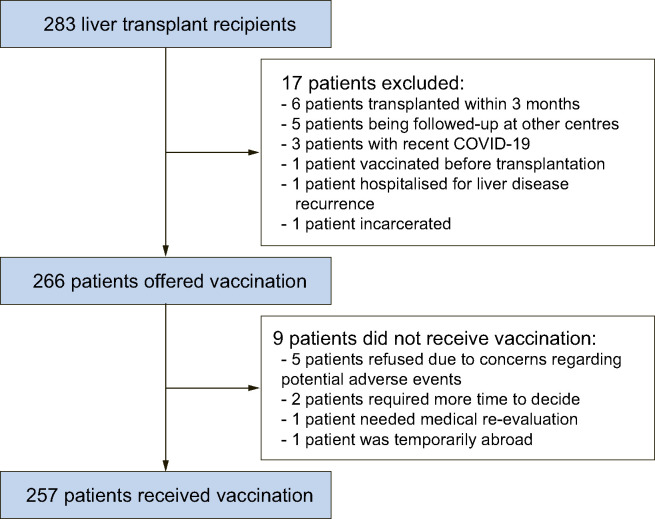
Taking all these considerations into account we wondered how the proposal of vaccination against COVID-19 would be received by liver transplant recipients, given the evidence recently provided by a global survey showing that the overall proportion of individuals willing to receive vaccination against COVID-19 in the general population was 71.2%, with a range between 55% and 90%.8 Thus, we evaluated the candidacy for COVID-19 vaccination in 283 patients who underwent liver transplantation at our liver transplant centre. The COVID-19 vaccination program for liver transplant recipients was carried out using an mRNA-based vaccine (BNT162b2, Pfizer-Bio-NTech), and the vaccination schedule was reserved via a web-based program following a visit with a transplant hepatologist (S.M.) who provided detailed information regarding the vaccination against COVID-19, with the support of material freely available on the webpage of the Italian Association for the Study of the Liver (AISF).9 Vaccinations were carried out at a dedicated facility within our hospital. Among these 283 liver transplant patients, vaccination could not be offered to 17 patients for the following reasons: 6 patients recently received a graft (within 3 months), 5 patients were being followed-up at other liver transplant centres in Italy, 3 patients had recently tested positive for COVID-19, 1 patient had been vaccinated before liver transplantation, 1 patient was hospitalised for complications of liver disease recurrence, and 1 patient was incarcerated. Overall, among the 266 patients who were offered COVID-19 vaccination at our Unit (189 males; median age 62 years; range 27-84 years), 9 patients (3.4%) did not receive the vaccine for the following reasons: 5 patients categorically refused vaccination against COVID-19 due to concerns regarding the potential for severe adverse events, despite having received adequate information and having previously received the vaccinations recommended in liver transplant patients, 2 patients reported concerns over the potential harms of the vaccine – they did not formally refuse vaccination but requested more time to ponder their decision, 1 patient required further work-up for previous allergic reactions to vaccination, and 1 patient was temporarily abroad, while 257 patients (96.6%) accepted and underwent vaccination (Fig. 1 ). All in all, only 5 patients (1.9%) firmly refused COVID-19 vaccination, while in the remaining 4 patients the potential barriers to vaccination could be lifted either by providing further details regarding the vaccination or following resolution of medical and logistical issues.
Fig. 1.
Flow of liver transplant patients who were offered vaccination against COVID-19.
Therefore, we have demonstrated that the acceptance rate for COVID-19 vaccination among liver transplant recipients is extremely high, at least 96.6%, and that patients reporting a firm opposition to vaccination represent only a minority of potential vaccine recipients (1.9%). This acceptance rate was higher than expected in the general population, despite potential concerns regarding the lack of safety results available in large series of transplanted patients, and initial evidence of reduced efficacy of vaccination in this population.7 Currently, vaccination of household members and caregivers of these patients is underway, and among this group immediate prioritisation is being given to those who take care of transplanted patients who could not receive COVID-19 vaccination because they had recently received a graft.
We feel that these positive results strongly emphasise how adequate counselling may enhance vaccination uptake and adherence to the recommendations provided by EASL and by national scientific societies.1 , [9], [10], [11] A structured program may support rapid and complete COVID-19 vaccination for liver transplant recipients, protecting this frail population.
Financial support
The authors received no financial support to produce this manuscript.
Authors’ contributions
All authors contributed equally to the concept and design, to the preparation of the manuscript and read and approved the final manuscript.
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2021.05.009.
Supplementary data
The following is the supplementary data to this article:
References
- 1.Cornberg M., Buti M., Eberhardt C.S., Grossi P.A., Shouval D. EASL position paper on the use of COVID-19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipients. J Hepatol. 2021 Apr;74(4):944–951. doi: 10.1016/j.jhep.2021.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Webb G.J., Moon A.M., Barnes E., Barritt A.S., Marjot T. Determining risk factors for mortality in liver transplant patients with COVID-19. Lancet Gastroenterol Hepatol. 2020 Jul;5(7):643–644. doi: 10.1016/S2468-1253(20)30125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Webb G.J., Moon A.M., Barnes E., Barritt A.S., 4th, Marjot T. Age and comorbidity are central to the risk of death from COVID-19 in liver transplant recipients. J Hepatol. 2021 Feb 5 doi: 10.1016/j.jhep.2021.01.036. S0168-8278(21)00085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020 Dec 31;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021 Feb 4;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet (London, England) 2021 Jan 9;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rabinowich L., Grupper A., Baruch R., Ben-Yehoyada M., Halperin T., Turner D., et al. Low immunogenicity to SARS-CoV-2 vaccination among liver transplant recipients. J Hepatol. 2021 Apr 20 doi: 10.1016/j.jhep.2021.04.020. S0168-8278(21)00255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lazarus J.V., Ratzan S.C., Palayew A., Gostin L.O., Larson H.J., Rabin K., et al. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med. 2021 Feb;27(2):225–228. doi: 10.1038/s41591-020-1124-9. Epub 2020 Oct 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Available at: https://www.webaisf.org/wp-content/uploads/2021/02/Vaccino_e_Fegato_AISF_01.02.2021.pdf, accessed on May 12, 2021.
- 10.Russo F.P., Piano S., Bruno R., Burra P., Puoti M., Masarone M., et al. Italian association for the study of the liver position statement on SARS-CoV2 vaccination. Dig Liver Dis. 2021 Apr 5 doi: 10.1016/j.dld.2021.03.013. S1590-8658(21)00128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tacke F., Cornberg M., Sterneck M., Trebicka J., Settmacher U., Bechstein W.O., et al. S1-Leitlinie zur Versorgung von Lebertransplantierten während der COVID-19-Pandemie – AWMF-register Nr. 021-031 – stand: 07.01.21. Z Gastroenterol. 2021 Apr;59(4):345–359. doi: 10.1055/a-1372-5595. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.