Graphical abstract
Keywords: Antiphospholipid antibodies, COVID-19, COVID-19 vaccine, Immunothrombosis
Abstract
Antiphospholipid antibodies (aPLs), present in 1–5 % of healthy individuals, are associated with the risk of antiphospholipid syndrome (APS), which is the most common form of acquired thrombophilia. APLs may appear following infections or vaccinations and have been reported in patients with COronaVIrus Disease-2019 (COVID-19). However, their association with COVID-19 vaccination is unclear. Notably, a few cases of thrombocytopenia and thrombotic events resembling APS have been reported to develop in recipients of either adenoviral vector- or mRNA-based COVID-19 vaccines.
The aim of this review is therefore to speculate on the plausible role of aPLs in the pathogenesis of these rare adverse events.
Adenoviral vector-based vaccines can bind platelets and induce their destruction in the reticuloendothelial organs. Liposomal mRNA-based vaccines may instead favour activation of coagulation factors and confer a pro-thrombotic phenotype to endothelial cells and platelets. Furthermore, both formulations may trigger a type I interferon response associated with the generation of aPLs. In turn, aPLs may lead to aberrant activation of the immune response with participation of innate immune cells, cytokines and the complement cascade. NETosis, monocyte recruitment and cytokine release may further support endothelial dysfunction and promote platelet aggregation. These considerations suggest that aPLs may represent a risk factor for thrombotic events following COVID-19 vaccination, and deserve further investigations.
1. Introduction
The year 2021 started with the hope of definitely defeating the COronaVIrus Disease 2019 (COVID-19) pandemic with introduction of a global vaccination plan. COVID-19 is an infectious disease driven by an enveloped single stranded (ss) RNA virus, called Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). The outbreak initiated in Wuhan in the Fall of 2019 has rapidly spread across the globe [1]. The disease is characterized by high morbidity and mortality rates, but its course is extremely unpredictable and ranges from asymptomatic individuals to cases with life-threatening complications, like disseminated intravascular coagulopathy (DIC) or acute respiratory distress syndrome (ARDS) [2]. The elderly, being male and chronic comorbidities are considered risk factors for worse prognosis [3]. The tendency towards a hyper-coagulable status represents one of the most emblematic aspects of COVID-19, and negatively influences the prognosis of the disease [4]. Although antiphospholipid antibodies (aPLs) have been detected in the serum of COVID-19 patients, their role in driving thrombosis is uncertain [[5], [6], [7], [8]]. APLs are notoriously associated with the antiphospholipid syndrome (APS), an immune-mediated thrombophilia with predisposition to recurrent episodes of arterial and venous thrombosis, foetal loss, as well as other obstetric complications [9]. APS has a multifactorial pathogenesis and infections are recognized to be among the environmental triggers of the disease [10]. Molecular mimicry may induce generation of cross-reacting antibodies directed against microbial and self-epitopes, including protein-phospholipid complexes. Furthermore, in asymptomatic and untreated patients having pre-existent aPLs, infections may trigger pro-inflammatory cascades able to promote development of a full-blown APS. It is possible that the same scenario can take place following parenteral inoculation of vaccines, including those formulated against SARS-CoV-2. Several months after the introduction of mass COVID-19 vaccination campaign, concerns were raised as to the plausible association between unexpected thromboembolic events and COVID-19 vaccines. Currently available COVID-19 vaccination strategies include mRNA-based (BNT162b2 and mRNA-1273) and adenoviral vector-based (ChAdOx1-S and Ad26.COV2) formulations. The safety profile of ChAdOx1-S was recently reviewed by the European Medicines Agency (EMA) Pharmacovigilance Risk Assessment Committee (PRAC), due to several reports of blood clotting events developing days to weeks after the first or second injection of the vaccine [11]. Given the numerical imbalance in clinical trials, venous thromboembolism was also included amongst the safety concerns of the Risk Management Plan (RMP) of the adenoviral vector-based COVID-19 vaccine Ad26.COV2 [12]. On April 14th 2021 the Food and Drug Administration (FDA) paused the use of the Ad26.COV2 vaccine in the United States (U.S.), following the notification of 6 cases of thrombocytopenia and cerebral venous sinus thrombosis occurring in female recipients aged 18 through 48 years [13]. The link connecting COVID-19 vaccines to thrombotic events is however unclear, although an immune-mediated mechanism, akin to what happens in APS, could be a possibility. Furthermore, the majority of vaccinated people who developed severe coagulation disorders were young women, who also represent the target population of APS [14,15].
This review aims to discuss the potential role of aPLs as a pathogenetic contributor to the thrombotic events following COVID-19 vaccination.
2. Antiphospholipid antibodies and antiphospholipid syndrome
APLs consist of a heterogeneous group of autoantibodies, recognizing anionic phospholipids or aggregates of proteins and phospholipids. These antibodies represent the pathognomonic biomarker of APS, the most common cause of acquired thrombophilia in individuals who are less than 50 years old [16]. APS can be associated with systemic lupus erythematosus (SLE) in 10–15 % of cases, although an isolated positivity of aPLs is far more common in these patients [17]. The aforementioned infections sustained by bacteria, viruses and yeasts can elicit production of aPLs based on a mechanism of molecular mimicry [10]. Specifically, the cofactor β2-glycoprotein I, which consists of a highly immunogenic five domain-glycoprotein, displays molecular homology with several microbial peptides [10]. β2-glycoprotein I is able to bind either the endothelium or anti-cardiolipin antibodies (aCLs) and can mediate aPL-induced damage on endothelial cells [10]. Infections can also lead to synthesis of anti-β2-glycoprotein I antibodies, which have been recognized as the most pathogenic in APS [18]. APLs may temporally appear during infections or also following vaccinations [19]. Importantly, detection of these antibodies has also been reported in patients with COVID-19 [7,8], but the connection with thromboembolic events is unclear. The solely presence of aPLs in the bloodstream is in fact not sufficient for the development of APS. It has been estimated that aPLs can be detected in 1–5 % of young healthy individuals and their prevalence increases in the elderly and in people with chronic diseases [20]. A “two hit theory” has therefore been proposed, according to which, in addition to circulating aPLs, a second trigger would be necessary for the development of a full-blown APS [21]. Infections or chronic diseases, like SLE or cancer, might create a favourable pro-inflammatory background, and contribute together with aPLs to the coagulation cascade [19]. Furthermore, such pathological conditions may also represent precipitating factors for catastrophic APS, a life-threatening disorder occurring in less than 1% of APS patients and characterized by the massive activation of the coagulation cascade that can lead to rapid multiorgan failure [19].
The variable spectrum of aPL-related manifestations, going from asymptomatic cases of seropositivity to DIC, has been attributed to the heterogeneity of these antibodies [9,22]. APLs that are routinely measured through laboratory tests include aCL and anti-β2-glycoprotein I IgA, IgM and IgG, while the lupus anticoagulant (LAC) clotting assay can indirectly show the presence of additional aPLs in case of a paradoxical elongation of the activated partial thromboplastin time (aPTT) [9]. It has been shown that the triple positivity in these tests, an anti-β2-glycoprotein I IgG isotype, LAC positivity and high antibody titres are associated with the risk of APS clinical manifestations [23]. Though anti-β2-glycoprotein I antibodies, especially if directed against the D1 domain of β2-glycoprotein I, are considered the most pathogenic, other studies have suggested that non-β2-glycoprotein I-binding aPLs may trigger pro-inflammatory and pro-thrombotic cascades in APS individuals as well [9].
The mechanism through which aPLs induce thrombophilic status in a patient relies on an extremely intricate pathway which merges coagulation with immune response and is referred to as immunothrombosis [24]. The endothelium appears as a central player in this scenario, since it coordinates both immune cell behaviour and coagulation/fibrinolytic pathways. Hence, aPLs may drive thrombophilia as a result of a massive endothelial dysfunction. Under physiological conditions, the endothelium prevents coagulation, masking the pro-coagulant domains of the extracellular matrix (ECM) and hampering platelet aggregation. Key-molecules in this process are nitric oxide (NO) and prostaglandin I2 (PGI2) [25]. Endothelial cells also synthesize anticoagulant mediators, like protein C and anti-thrombin III (ATIII) and the enzyme adenosine diphosphatase, which counteracts the adenosine diphosphate (ADP)-mediated aggregation of platelets [26]. However, following a vessel wall damage or during inflammation, endothelial cells take on a pro-thrombotic phenotype, producing high amounts of platelet activating factor (PAF) and von Willebrand factor (WF), which are instead usually embedded into ECM, with the following activation of platelets [27]. Furthermore, systemic inflammation can stimulate the secretion of tissue factor (TF) from endothelial cells and drive initiation of the coagulation cascade. The final conversion of pro-thrombin into thrombin can further induce endothelial cells to express adhesion molecules for platelets and leukocytes, increasing vascular permeability and cell diapedesis. These effects would be mediated by the proteolytic activity played by thrombin on the protease-activated receptors (PAR) expressed on the plasma membrane of either platelets or endothelial cells [28]. Finally, endothelial cells may provide coagulation factors with the optimal substrate for starting the catalytic process of auto-activation. These steps are illustrated in Fig. 1 .
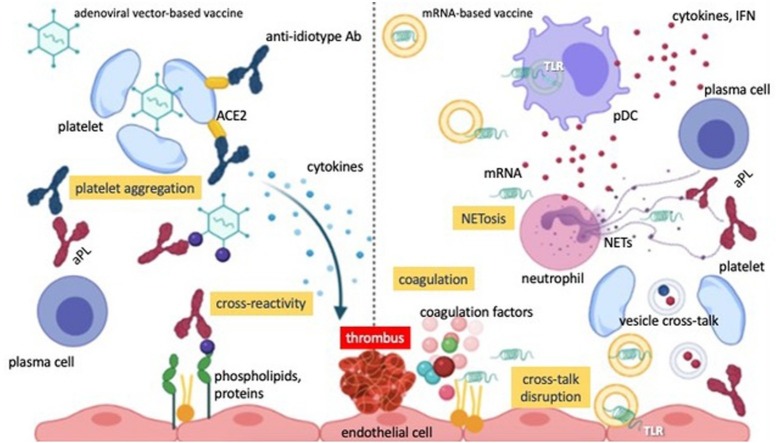
Fig. 1.
The pro-coagulant and anticoagulant properties of endothelium in health and disease.
The endothelium physiologically prevents coagulation by releasing vasodilating and anticoagulant mediators, such as nitric oxide, protein C and anti-thrombin III, and by masking pro-coagulant mediators (tissue factor, platelet activating factor and von Willebrand factor) embedded in the subendothelial extracellular matrix. Following a break in this homeostasis as a result of injury, systemic inflammation or sepsis, the endothelium acquires a pro-coagulant and pro-inflammatory phenotype, unmasking or even secreting pro-coagulant mediators, attracting and activating polymorphs and platelets and inducing the coagulation cascade. Activated neutrophils may release neutrophil extracellular traps, reactive oxygen species, cytokines and enzymes, further contributing to local inflammation and thrombus development.
Abbreviations: NO, nitric oxide; ATIII, anti-thrombin III; TF, tissue factor; ECM, extracellular matrix; PAF, platelet activating factor; WF, von Willebrand factor; PLT, platelet; ROS, reactive oxygen species; CKs, cytokines; NET, neutrophil extracellular trap.
Pro-inflammatory cytokines, like tumor necrosis factor-α (TNF-α) and interferon-γ (IFN-γ), enhance expression of fragment crystallizable (Fc)-receptors on the surface of endothelial cells of the arterial, venous and microvascular tree [29]. These receptors can mediate the interaction with aPLs, resulting in endothelial dysfunction. Specifically, aPLs may favour expression of adhesion molecules for leukocytes and platelets and unbalance the secretion of vasoconstrictor and vasodilator mediators from endothelial cells, like endothelin-1 (ET-1) and NO, respectively. Moreover, aPLs may directly antagonize anticoagulant factors, like protein C, protein S and β2-glycoprotein I [30,31], and activate the innate immune system by triggering neutrophil extracellular trap (NET)osis and the complement cascade [32], Fig. 2 .
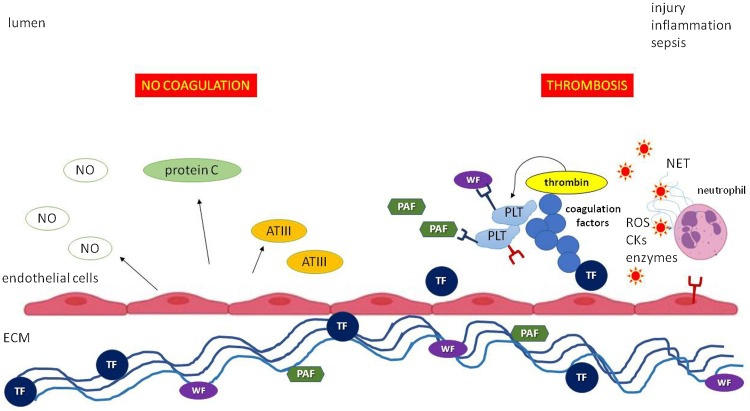
Fig. 2.
The potential role of aPLs in the pathogenesis of immunothrombosis.
Autoantibodies recognize the β2 glycoprotein I fixed to anionic lipids and attract mononuclear cells, platelets and neutrophils to the endothelium by binding the FcR expressed on these cells. Following this interaction, monocytes release cytokines, further recruiting immune cells and polarizing endothelial cells towards a pro-inflammatory and pro-coagulant phenotype. Neutrophils are induced to release neutrophil extracellular traps under the stimulus of IFNα and IFNβ, in turn produced by plasmacytoid dendritic cells, and of other mediators, including the complement factors activated by immunocomplexes. Platelets secrete platelet factor 4, which binds to β2 glycoprotein I and favours the attachment of anti-β2 glycoprotein I antibodies. Platelets may also directly bind anticardiolipin antibodies. Finally, the release of endothelin 1 and tissue factor at the expense of nitric oxide and prostaglandin I2 triggers the coagulation cascade.
Abbreviations: aCL, anticardiolipin antibody; anti-β2GPI, anti-β2 glycoprotein I antibody; β2GPI, β2 glycoprotein I; FcR, fragment crystallizable receptor; pDC, plasmacytoid dendritic cell; IFN, interferon; NETs, neutrophil extracellular traps; ET1, endothelin 1; TF, tissue factor; NO, nitric oxide; PGI2, prostaglandin I2; ROS, reactive oxygen species; PF4, platelet factor 4.
Anti-β2-glycoprotein I antibodies interact with the open conformation of the β2-glycoprotein I. When this glycoprotein is attached to anionic phospholipids with its D5 domain, the concomitant binding of anti-β2-glycoprotein I antibodies on the remaining domains may create a bridge for monocytes that carry Fc receptors, consequently attracting these cells to the endothelium. On the other hand, after binding aPLs, monocytes would receive an activating signal, eventually resulting in secretion of pro-inflammatory cytokines, like interleukin-1β (IL-1β), TNF-α, and TF. In doing so, monocytes may additionally drive endothelial cells to the acquisition of a pro-coagulant phenotype.
Anti-β2-glycoprotein I antibodies may also induce NETosis. β2-glycoprotein I has in fact been detected on neutrophil surfaces, and thrombi developing in APS murine models were reported to contain a large amount of NETs [32]. NETosis may give rise to disseminated coagulopathy in a physiological attempt to constrain the dissemination of pathogens. NETs can stimulate platelets, activate the coagulation factors in a contact-phase manner, stabilize thrombi and impede their degradation by fibrinolytic enzymes [32]. Some studies also suggest that NETosis may be associated with aPL-mediated thrombosis through an unbalanced synthesis of cyclic AMP (cAMP) in neutrophils [33]. Other researchers showed that the anti-β2-glycoprotein I/β2-glycoprotein I complex may stimulate the generation of reactive oxygen species (ROS) in neutrophils by means of an alternative pathway to p38, AKT, ERK1/2 or zinc signaling [34]. Neutrophils of APS patients display enhanced adhesion to endothelium and hyper-express several membrane markers, like CD64, Mac-1 and β2-glycoprotein I, which may mediate the interaction with aPL IgG [35]. Cofactor-independent aPLs might activate plasmacytoid dendritic cells through an alternative pathway and favour the secretion of type I IFN and the activation of the complement cascade, both crucial steps for recruitment of neutrophils to endothelium and NETosis. Like anti-β2-glycoprotein I antibodies, cofactor-independent antibodies could induce platelet activation and switch endothelial cells towards a pro-coagulant and vasoconstrictor phenotype [9].
APS is often associated with a depletion in circulating platelets and aPLs may be detected in up to 50 % of subjects affected by immune thrombocytopenic purpura [36]. The mechanism underneath thrombocytopenia in APS is partially understood and likely dependent on the massive destruction, aggregation and sequestration of platelets in the vascular system. Besides endothelium, platelets are essential protagonists of APS pathogenesis. Platelets may in fact directly interact with aPLs by means of their FcγRIIa or via the binding of anionic phospholipids placed on their membrane with aCL antibodies, which may in turn be engaged with anti-β2-glycoprotein I antibodies [37]. Activated platelets can orchestrate a complex network together with immune and endothelial cells. This scenario encompasses the release of chemokines, like platelet factor 4 (PF4) that has a crucial role in β2-glycoprotein I binding, the expression of leukocyte adhesion molecules and the activation of complement and coagulation cascades. Furthermore, activated platelets are able to secrete microparticles rich in negatively charged phospholipids, receptors, and pro-inflammatory and pro-coagulant mediators. In SLE patients, who often suffer from autoimmune thrombocytopenia, aPLs can favour platelet activation by means of a complex network of interactions, which include the binding to FcγRIIa on platelets' surface and complement deposition [38].
3. Antiphospholipid antibodies and COVID-19
Growing evidence is linking COVID-19 to a status of hypercoagulability. The latter seems to depend on the development of an extremely severe systemic immune response, namely a cytokine storm, which may in turn be the forerunner of DIC. Elevated serum levels of D-dimer and fibrinogen and prolonged prothrombin time and aPTT are common findings in COVID-19 patients with more aggressive disease [4]. SARS-CoV-2 infection may activate numerous signaling pathways within immune cells, which may result in cytokine release, NETosis, complement and coagulation cascade activation, as well as TF secretion, platelet recruitment and fibrinolysis inhibition. NETosis has especially been considered as an important pathogenic step that contributes to COVID-19 hypercoagulability [39]. The stimuli derived from either viral infection or the antiviral immune response can force endothelial cells to acquire a pro-thrombotic phenotype [4]. In this scenario, aPLs may play a further role in sealing the interconnection between inflammation and coagulation. Several studies have shown the presence of aPLs in the serum of COVID-19 patients, yet the association with thrombotic events remains controversial. Nevertheless, the use of heparin, intravenous immunoglobulins and certain antibiotics may be effective in controlling the disease, a result that may be partly due to eradication of superimposed microbial infections, which secondarily trigger production of aPLs [[40], [41], [42]]. A systematic review and meta-summary of literature focusing on the occurrence of acute ischemic stroke in SARS-CoV-2-infected individuals found a high prevalence of aPLs in these patients, with LAC being positive in 41.7 % of cases [43]. The authors reviewed 39 studies for a total of 135 patients (mean age 63.4 ± 13.1 years) and estimated a pooled incidence of ischemic stroke being 1.2 %, however, information regarding the aPL status was available only in 16 cases. ACL IgM, IgG and IgA were found in 20 %, 0% and 42.9 % of tested COVID-19 individuals, respectively, whilst anti-β2-glycoprotein I IgM, IgG and IgA were present in 10 %, 38.5 % and 42.9 % of tested patients, respectively [43]. The clinical meaning of these laboratory data is unknown since a control group was not provided, and the pathogenic role of some detected aPLs, like those belonging to the IgA isotype, is doubtful. Another study analyzed the prevalence of serum aPLs and their epitope specificity in a cohort of 122 COVID-19 patients, 16 of whom had major thrombotic events [44]. The authors found that anti-β2-glycoprotein I IgG/IgA/IgM were the most frequently detected aPLs present in 15.6/6.6/9.0 % of patients, respectively, followed by aCL and anti-phosphatidylserine/prothrombin antibodies. Interestingly, no significant associations between the risk of thrombotic events and aPL positivity were observed. It should be noted that anti-β2-glycoprotein I antibodies identified in these patients displayed a different epitope specificity when compared to aPLs that appear during APS.
4. COVID-19 vaccines and antiphospholipid antibodies
Some preclinical and clinical studies have shown that aPLs, with or without APS clinical manifestations, may appear in serum of individuals that received the tetanus toxoid, seasonal influenza and human papillomavirus vaccines [[45], [46], [47], [48]]. The main underlying mechanism would be cross-reactivity between antigenic epitopes present in the vaccine formulations and self-epitopes [49]. The real pathogenic role of these antibodies is still debated, but they may contribute to thrombotic events following COVID-19 vaccination. However, the generation of aPLs may represent the additional straw that breaks the proverbial “camel's back” within a more complex pathogenetic scenario that involves the expression of SARS-CoV-2 structural proteins, including the spike protein which is used in the current mRNA- and adenoviral vector-based vaccines.
4.1. Adenoviral vector-based vaccines
Recently, several concerns arose regarding the post-marketing side effects of the chimpanzee adenovirus encoding the SARS-CoV-2 spike glycoprotein ChAdOx1-S vaccine which has been marketed by AstraZeneca [50]. Several cases of blood clot formation in unusual vascular districts, like the mesenteric vein or cerebral vein/cerebral venous sinus, in otherwise healthy individuals have been reported throughout the European Union [11]. Such adverse events did not emerge during the phase II/III randomized controlled trial (RCT) [51]. On March 18th 2021 the EMA Safety Committee admitted that the administration of the vaccine may be associated with an increased risk of thrombocytopenia and thrombosis in unusual vascular districts. However, the PRAC also stated that this is a rare event and no causal relationship can be proven [11]. Notably, these events were mainly reported occurring in women less than 50 years old, which imposes a tight post-vaccination follow-up in case of suspicious symptoms in this cohort of individuals [14]. Similarly, the vaccination campaign with the Johnson & Johnson adenoviral vector-based COVID-19 vaccine has been recently halted in the U.S. over rare blood clots developing in young female recipients.
The association between thrombocytopenia and adenoviral vector-based vaccines is however not new. In 2007, Stone et al. showed that the intravenous administration of recombinant adenovirus serotype 5 (Ad5) vectors for gene therapy in hCD46Ge transgenic mice unexpectedly triggered the coagulation cascade, with platelet activation and sequestration in the hepatic reticuloendothelial system [52]. Specifically, the authors showed that inoculation of the Ad5-vaccine induced a rapid aggregation of platelets, which may be at the basis of thrombocytopenia, and increased the serum concentration of the coagulation markers CD62p and D-dimer. The hyper-expression of CD62p in platelets was associated with high circulating levels of WF, endothelial activation and leukocyte recruitment. Finally, macrophages and endothelial cells placed in liver sinusoidal vessels were reported to uptake and remove the aggregates composed by the Ad5-vaccine and platelets [52]. Therefore, platelets may work as transporters of viral particles from the site of inoculation to the reticuloendothelial system, and this mechanism may explain the occurrence of a thrombocytopenia or a full-blown thrombotic thrombocytopenic purpura during adenoviral infections or following vaccinations that exploit this type of viral vectors. Moreover, a phase I RCT investigating the safety of a prototype recombinant adenoviral-vector serotype-35 (rAd35) HIV vaccine described an in vitro aPTT elongation in recipients, in turn related to transient appearance of aPLs. This event was observed in 4 cases out of 25 and resolved in 14–150 days without being associated with any clinical manifestations [53]. Similar results were also reported in another trial using first-generation adenoviral vectors for gene therapy in prostate cancer patients [54].
It must be underlined that the formation of blood clots remains a rare adverse event that developed in a minority of ChAdOx1-S and Ad26.COV2 vaccine recipients. In these subjects, pre-existent aPLs might predispose to this complication, acting as the first trigger in a two hit pathogenetic scenario, although this is not determined a priori. However, it is well-known that adenoviral infections can elicit a de novo synthesis of anti-β2-glycoprotein I antibodies through a molecular mimicry mechanism [10].
A recent paper authored by Greinacher et al. describes a small case series of 9 Austrian and German individuals, who developed thrombotic events and thrombocytopenia 4–16 days after receiving the ChAdOx1-S vaccine [55]. The cohort included 8 females and one male with a median age of 36 years. The coagulation disorder was fatal in 4 cases and deceased subjects had a strong positivity for anti-PF4 antibodies. Notably, one of the individuals included in the case series was also positive for aPLs but the authors did not specify whether this condition was a predisposition for a worse outcome. PF4 is regarded as the main β2-glycoprotein I-interacting factor and, according to in vitro and in silico studies, anti-β2-glycoprotein I antibodies selectively bind β2-glycoprotein I that is complexed to PF4 [56]. The formation of such immunocomplexes can give rise to platelet activation through p38MAPK phosphorylation and release of thromboxane B2 [56]. It may be hypothesized that the binding of anti-β2-glycoprotein I antibodies to their ligand unmasks epitope domains of PF4, inducing the subsequent generation of anti-platelet PF4 antibodies. As a consequence, the dual action of both anti-β2-glycoprotein I and anti-PF4 autoantibodies would greatly increase the risk of disseminated thrombosis.
4.2. mRNA-based vaccines
The effect of COVID-19 mRNA-based vaccines on the coagulation pathway is still raising some level of discussion. Although there were no safety issues that emerged in clinical trials compared to the placebo group [57,58], one case report did describe the development of deep vein thrombosis in a 66-year-old woman with no prior coagulation defects one day after receiving the second dose of the BNT162b2 vaccine [59]. However, several studies have shown that extracellular RNA may take part in the coagulation cascade. Extracellular RNA may be produced in high amounts during sepsis or cancer, two pathological conditions typically associated with a pro-coagulant status [60,61]. Nakazawa et al. observed that RNA obtained from different sources (rRNA, tRNA, viral and artificial RNA) and at least 100 nucleotides in size can trigger the extrinsic coagulation cascade through an alternative pathway [62]. This mechanism encompasses the RNA-mediated activation of the factor VII-activating protease (FSAP), which in turn can activate the coagulation factor VII regardless of the presence of TF and with a coagulation factor VIII-inhibitor bypassing activity [62]. In another study, different types of eukaryotic and prokaryotic extracellular RNAs containing at least 50 nucleotides, but not DNA, were shown to interact with fibrillar collagen and fibrin, coagulation factors XII and XI, prekallikrein and kininogen [63]. RNA molecules were reported to work as a natural foreign substrate promoting the protease activation of the coagulation factors XI and XII and thus initiating the coagulation cascade. These effects were typically inhibited by RNases, which may be constitutionally secreted by endothelial cells [64], reinforcing the central role played by the endothelium in counterbalancing the pro-coagulant activity of foreign or self RNA released during infections or cell damage. It is also plausible that mRNA-based vaccines may not be exempted from the risk of a coagulopathy unleashed through these two mechanisms. Although this phenomenon would mostly follow parenteral inoculation of naked mRNA formulations [65], a leak in the liposomal delivery carriers allowing the outer release of mRNA cannot be excluded for COVID-19 vaccines.
The relationship between mRNA vaccinations and aPLs is unknown. Ribonucleic acids, like mRNAs, may trigger a type I IFN response in target cells following interaction with pattern recognition receptors (PRR) placed in the cytosol and endosomes [66]. Several studies have underscored the connection between a type I IFN signature and primitive or SLE-related APS. In SLE-related APS, a type I IFN signature was associated with moderately high titres of anti-β2-glycoprotein I antibodies, APS disease activity and time to onset, and obstetric complications [67]. Additionally, nucleic acids may foment NETosis, which represents a facilitating mechanism for thrombosis [32].
Due to their nucleic acid structure, mRNA-based vaccines are highly immunogenic and might precipitate the development of APS in asymptomatic aPL-positive individuals following the instauration of a markedly pro-inflammatory background (second hit). A few papers reported the detection on 18F-fluorodeoxyglucose positron emission tomography/computerized tomography (PET/CT) of local (deltoid muscle and lymph nodes) and systemic signs of inflammation in patients being inoculated with both mRNA-1273 and BNT162b2 vaccines [[68], [69], [70], [71]]. Notably, in contrast with the imaging findings and notwithstanding the elevated serum levels of D-dimer and C-reactive protein (CRP) and increased erythrocyte sedimentation rate (ESR) in one case [68], patients complained of no to minimal clinical side effects.
A further argument linking mRNA-based vaccines to immunothrombosis is their molecular resemblance to cellular vesicles, which play a central role in endothelial and platelet communication and homeostasis. The secretion and exchange of vesicles encapsulating molecular mediators has in fact been described as a crucial means of establishing a cross-talk between these cells [38,72]. Based on their diameter, these vesicles can be classified as microparticles (> 100 nanometers) or exosomes (< 100 nanometers) [72]. Both microparticles and exosomes may carry activated cytokines, like IL-1β, and nucleic acids, like RNA. Studies have shown that inflammation and ROS generation may drive platelets to release microparticles rich in oxidized mitochondrial DNA, which may engage Toll-like receptor (TLR)9 and drive a type I IFN response in plasmacytoid dendritic cells [38]. Furthermore, during viral infections, platelets establish a tight contact with neutrophils and express TLR7 that is able to recognize ssRNA. In consideration of the role of aPLs in inducing a type I IFN response and promoting translocation of TLR7 and TLR8 to the endosome in human monocytes and plasmacytoid dendritic cells [73], a role for aPLs in this delicate balance is certainly possible. The levels of circulating endothelial and platelet microparticles, measured by means of flow cytometry, appear to be significantly increased in patients with APS or aPL-positivity compared to healthy individuals [74]. An elegant in vitro study showed that human anti-β2-glycoprotein I antibodies may induce activation of resting endothelial cells by stimulating the inflammasome platform assembly and the secretion of extracellular vesicles containing IL-1β [72]. Additionally, anti-β2-glycoprotein I antibodies activated resting endothelial cells through a TLR7-mediated pathway, which was attributed, at least in part, to the co-secretion of specific microRNAs [72]. TLR7 may however recognize other ssRNA molecules, including mRNA contained in COVID-19 vaccines [75].
5. Concluding remarks
The aberrant activation of the coagulation pathway is a serious adverse event that rarely occurs following COVID-19 vaccination. However, it does represent an important clinical aspect of COVID-19. The missing link between the two conditions may be a consequence of the circulating aPLs that straddle between the coagulation system and the immune response.
Currently available COVID-19 vaccines have received a conditional or emergency marketing authorization from the EMA and the FDA. The rapid developmental process may indeed raise some concerns, especially about potential long-term safety issues. Still, the benefits of the intervention on halting the spread of COVID-19 pandemic appear to outweigh the risks derived from a more long term characterization of the pharmacological profile of these compounds [76]. Furthermore, post-vaccination thrombotic events are deemed as extremely rare complications and there is uncertainty concerning the underneath pathogenetic mechanism.
In spite of many anecdotal reports on the development of thrombotic events in recipients of COVID-19 vaccines, scientific evidence relying on published cases on peer-reviewed journals is limited. Nevertheless, lessons from pre−COVID-19 vaccines taught that vaccine-related injuries may be identified several years following the date of the vaccine campaign introduction and after a large number of individuals have been vaccinated [76]. In addition, side effects occurring in a minority of people belonging to specific populations (like aPL-positive subjects) may be underestimated. Regarding the issue of blood clots developing in recipients of COVID-19 vaccines, arterial thrombotic events appeared to be more common amongst older people, while young individuals seem more prone to suffer from venous thrombosis or DIC [77]. This observation is in line with what is reported in APS, in which patients who are older and carry other cardiovascular risk factors tend to develop arterial ischemic events, while younger patients more commonly undergo venous thrombosis [37]. Given the clinical similarities between thrombotic events following COVID-19 vaccination and APS, we provide a pathogenic theory for this rare adverse event that encompasses a pre-existent or a de novo aPL positivity. Specifically, adenoviral vector-based vaccines may contain epitopes able to induce the synthesis of aPLs due to a molecular mimicry mechanism. Furthermore, this vaccine formulation would induce thrombocytopenia and platelet activation. The mRNA-based vaccines may instead trigger a more complex pathway, characterized by activation of TLRs, generation a type I IFN response, NETosis and the direct initiation of the coagulation cascade. Due to their liposomal molecular structure, mRNA vaccines may also disrupt the physiological cross-talk occurring between platelets and endothelial cells, and drive these cells towards a pro-inflammatory and pro-coagulant phenotype. Late coagulation disorders occurring in recipients of COVID-19 vaccines might also be attributed to anti-idiotype antibodies, which usually develop after 1–3 months after immunization. It may be hypothesized that anti-idiotype antibodies reacting against anti-spike antibodies and carrying the internal image of the primitive antigenic determinant might recognize epitopes within the angiotensin-converting enzyme (ACE)2 expressed on platelets. The following binding of anti-idiotype antibodies to platelet ACE2 could favour platelet aggregation and activation in a similar way to SARS-CoV-2 infection, Fig. 3 [78]. Anti-idiotype antibodies may also induce the formation of immunocomplexes [79], which are in turn triggers of the coagulation cascade by means of the engagement with Fc receptors [80]. This event may stack up against aPL-induced thrombophilia, but evidence in support of such an association is lacking.
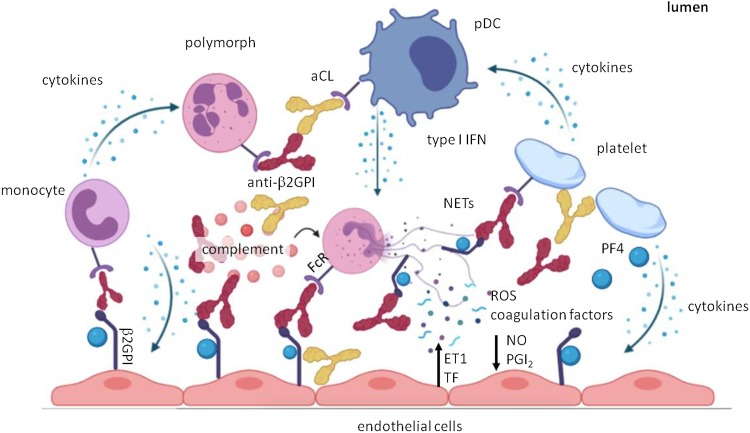
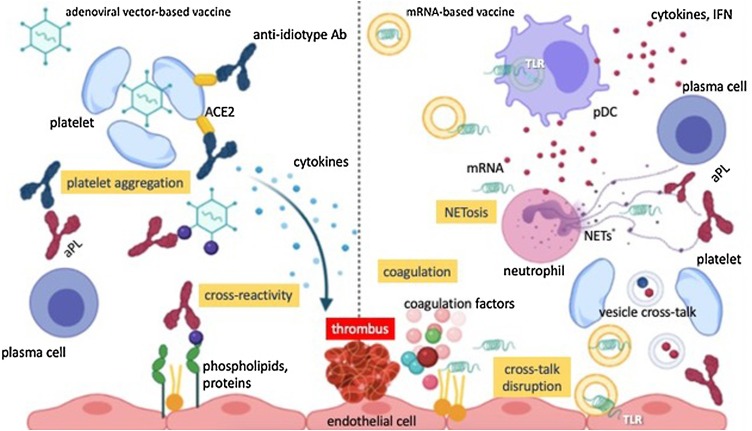
Fig. 3.
Hypothetical immunothrombotic pathways triggered by COVID-19 vaccines.
The left side of the picture depicts the potential scenario occurring after the administration of adenoviral vector-based vaccines. The latter may induce the aggregation of platelets and their sequestration into the reticuloendothelial system, hence contributing to thrombocytopenia and platelet activation. Furthermore, adenoviral epitopes may cross-react with epitopes within self-complexes of proteins and anionic lipids, giving rise to aPLs. Anti-spike antibodies may elicit after several weeks the generation of anti-idiotype antibodies, able to promote platelet activation through the interaction with platelet ACE2. The latter mechanism could potentially take place also after immunization with mRNA-based vaccines.
The right side of the picture instead represents the scenario which may occur with the mRNA-based vaccine formulations. Minimal amounts of mRNA unwantedly released from liposomal capsules may activate Toll-like receptors in plasmacytoid dendritic cells and contribute to the synthesis of type I IFN-related cytokines. These may foment the production of aPLs from plasma cells and NETosis. Furthermore, mRNA may promote the initiation of the coagulation cascade by activating the coagulation factors VII, XI and XII. Finally, liposomal formulations may interfere with the physiological cross-talk occurring between platelets and endothelial cells and favour the activation of the inflammasome platforms into endothelial cells and thus the secretion of pro-inflammatory cytokines.
Abbreviations: Ab, antibody; aPL, antiphospholipid antibody; ACE2, angiotensin-converting enzime 2; TLR, Toll-like receptor; pDC, plasmacytoid dendritic cells; NET, neutrophil extracellular trap; IFN, interferon.
Further research on the interconnectedness between aPLs and COVID-19 vaccines may help identify the subset of predisposed recipients who may be at higher risk of these rare but serious adverse events.
Funding source
None.
Author contributions
RT conceived the idea of the manuscript, performed the bibliographic research, wrote the original draft and drew the figures. ESR edited and critically revised the manuscript. The authors have read and approved the final version of the manuscript.
Declaration of Competing Interest
None.
Biography

Rossella Talotta received her Ph.D. in Rheumatology from the University of Padua, Italy. She is doing her postdoctoral training at the University of Messina, Italy. She is currently working at collaborative research projects with the University of Aarhus, Denmark, and the University of Pennsylvania, Philadelphia, USA, aiming to study the immunogenetic and microbial pathogenesis of autoimmune diseases. She wrote more than fifty papers and abstracts published on international peer-reviewed journals. She also received a number of awards from the Italian Society of Rheumatology and Lundbeckfonden for leading research projects in the field of Rheumatology and Immunology.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salzberger B., Buder F., Lampl B., Ehrenstein B., Hitzenbichler F., Hanses F. Epidemiologie von SARS-CoV-2-Infektion und COVID-19. Internist (Berl). 2020;61 doi: 10.1007/s00108-020-00834-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ye Q., Wang B., Mao J., Fu J., Shang S., Shu Q., Zhang T. Epidemiological analysis of COVID‐19 and practical experience from China. J. Med. Virol. 2020;92 doi: 10.1002/jmv.25813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jayarangaiah A., Kariyanna P.T., Chen X., Jayarangaiah A., Kumar A. COVID-19-Associated coagulopathy: an exacerbated immunothrombosis response. Clin. Appl. Thromb. 2020;26 doi: 10.1177/1076029620943293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Y., Xiao M., Zhang S., Xia P., Cao W., Jiang W., Chen H., Ding X., Zhao H., Zhang H., Wang C., Zhao J., Sun X., Tian R., Wu W., Wu D., Ma J., Chen Y., Zhang D., Xie J., Yan X., Zhou X., Liu Z., Wang J., Du B., Qin Y., Gao P., Qin X., Xu Y., Zhang W., Li T., Zhang F., Zhao Y., Li Y., Zhang S. Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N. Engl. J. Med. 2020;382:e38. doi: 10.1056/nejmc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harzallah I., Debliquis A., Drénou B. Lupus anticoagulant is frequent in patients with Covid‐19. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14867. 10.1111/jth.14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van der Linden J., Almskog L., Liliequist A., Grip J., Fux T., Rysz S., Ågren A., Oldner A., Ståhlberg M. Thromboembolism, hypercoagulopathy, and antiphospholipid antibodies in critically Ill coronavirus disease 2019 Patients: a before and after study of enhanced anticoagulation. Crit. Care Explor. 2020;2 doi: 10.1097/CCE.0000000000000308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamadé A., Woehl B., Harzallah I., Talbot M., Tousch J., Jambert L. Antiphospholipid antibodies in patients with coronavirus disease 2019 infection hospitalized in conventional unit. Blood Coagul. Fibrinolysis. 2021;32 doi: 10.1097/MBC.0000000000000963. [DOI] [PubMed] [Google Scholar]
- 9.Lackner K.J., Müller-Calleja N. Pathogenesis of antiphospholipid syndrome: recent insights and emerging concepts. Expert Rev. Clin. Immunol. 2019;15:199–209. doi: 10.1080/1744666X.2019.1546578. [DOI] [PubMed] [Google Scholar]
- 10.Harel M., Aron-Maor A., Sherer Y., Blank M., Shoenfeld Y. The infectious etiology of the antiphospholipid syndrome: links between infection and autoimmunity. Immunobiology. 2005;210 doi: 10.1016/j.imbio.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 11.European Medicines Agency . 2021. EMA/PRAC/157045/2021 Pharmacovigilance Risk Assessment Committee (PRAC). Signal Assessment Report on Embolic and Thrombotic Events (SMQ) with COVID-19 Vaccine (ChAdOx1-S [recombinant]) – COVID-19 Vaccine AstraZeneca (Other Viral Vaccines)https://www.ema.europa.eu/en/documents/prac-recommendation/signal-assessment-report-embolic-thrombotic-events-smq-covid-19-vaccine-chadox1-s-recombinant-covid_en.pdf [Google Scholar]
- 12.2021. COVID-19 Vaccine (Ad26.COV2-S [recombinant]) RISK MANAGEMENT PLAN (RMP)https://www.ema.europa.eu/en/documents/rmp-summary/covid-19-vaccine-janssen-epar-risk-management-plan_en.pdf (Accessed 3 April 2021) [Google Scholar]
- 13.FDA . 2021. Janssen COVID-19 Vaccine Frequently Asked Questions.https://www.fda.gov/emergency-preparedness-and-response/mcm-legal-regulatory-and-policy-framework/janssen-covid-19-vaccine-frequently-asked-questions (Accessed 15 April 2021) [Google Scholar]
- 14.European Medicines Agency . 2021. COVID-19 Vaccine AstraZeneca: Benefits Still Outweigh the Risks Despite Possible Link to Rare Blood Clots With Low Blood Platelets.https://www.ema.europa.eu/en/news/covid-19-vaccine-astrazeneca-benefits-still-outweigh-risks-despite-possible-link-rare-blood-clots [Google Scholar]
- 15.Papadakis E., Banti A., Kioumi A. Women’s issues in antiphospholipid syndrome. Isr. Med. Assoc. J. 2016;18:524–529. [PubMed] [Google Scholar]
- 16.Petri M. Antiphospholipid syndrome. Transl. Res. 2020;225:70–81. doi: 10.1016/j.trsl.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pons-Estel G.J., Andreoli L., Scanzi F., Cervera R., Tincani A. The antiphospholipid syndrome in patients with systemic lupus erythematosus. J. Autoimmun. 2017;76 doi: 10.1016/j.jaut.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 18.Meroni P.L., Borghi M.O., Raschi E., Tedesco F. Pathogenesis of antiphospholipid syndrome: understanding the antibodies. Nat. Rev. Rheumatol. 2011;7 doi: 10.1038/nrrheum.2011.52. [DOI] [PubMed] [Google Scholar]
- 19.Mendoza-Pinto C., García-Carrasco M., Cervera R. Role of infectious diseases in the antiphospholipid syndrome (Including its catastrophic variant) Curr. Rheumatol. Rep. 2018;20:62. doi: 10.1007/s11926-018-0773-x. [DOI] [PubMed] [Google Scholar]
- 20.Gezer S. Antiphospholipid syndrome. Dis. Mon. 2003;49:696–741. doi: 10.1016/j.disamonth.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 21.Meroni P.L., Riboldi P. Pathogenic mechanisms of antiphospholipid syndrome: a new autoimmune disease. Drug Discov. Today Dis. Mech. 2004;1:309–314. doi: 10.1016/j.ddmec.2004.11.006. [DOI] [Google Scholar]
- 22.Lackner K.J., Müller-Calleja N. Pathogenesis of the antiphospholipid syndrome revisited: time to challenge the dogma. J. Thromb. Haemost. 2016;14:1117–1120. doi: 10.1111/jth.13320. [DOI] [PubMed] [Google Scholar]
- 23.Pons-Estel G.J., Andreoli L., Scanzi F., Cervera R., Tincani A. The antiphospholipid syndrome in patients with systemic lupus erythematosus. J. Autoimmun. 2017;76:10–20. doi: 10.1016/j.jaut.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Preissner K.T., Herwald H. Extracellular nucleic acids in immunity and cardiovascular responses: between alert and disease. Thromb. Haemost. 2017;117:1272–1282. doi: 10.1160/TH-16-11-0858. [DOI] [PubMed] [Google Scholar]
- 25.Cines D.B., Pollak E.S., Buck C.A., Loscalzo J., Zimmerman G.A., McEver R.P., Pober J.S., Wick T.M., Konkle B.A., Schwartz B.S., Barnathan E.S., McCrae K.R., Hug B.A., Schmidt A.M., Stern D.M. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. doi: 10.1182/blood.V91.10.3527. [DOI] [PubMed] [Google Scholar]
- 26.Esmon C.T. Regulation of blood coagulation. Biochim. Biophys. Acta - Protein Struct. Mol. Enzymol. 2000;1477:349–360. doi: 10.1016/S0167-4838(99)00266-6. [DOI] [PubMed] [Google Scholar]
- 27.Wu K.K., Thiagarajan P. Role of endothelium in thrombosis and hemostasis. Annu. Rev. Med. 1996;47:315–331. doi: 10.1146/annurev.med.47.1.315. [DOI] [PubMed] [Google Scholar]
- 28.Coughlin S.R. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 29.Pan L., Kreisle R.A., Shi Y. Expression of endothelial cell IgG Fc receptors and markers on various cultures. Chin. Med. J. (Engl). 1999;112:157–161. [PubMed] [Google Scholar]
- 30.Hochberg M., Gravallese E., Silman A., Smolen J., Weinblatt M., Weisman M. 7th ed. 2019. Rheumatology. [Google Scholar]
- 31.Balasubramanian K., Chandra J., Schroit A.J. Immune clearance of phosphatidylserine-expressing cells by phagocytes: the role of β2-glycoprotein I in macrophage recognition. J. Biol. Chem. 1997;272:31113–31117. doi: 10.1074/jbc.272.49.31113. [DOI] [PubMed] [Google Scholar]
- 32.Bravo-Barrera J., Kourilovitch M., Galarza-Maldonado C. Neutrophil extracellular traps, antiphospholipid antibodies and treatment. Antibodies. 2017;6:4. doi: 10.3390/antib6010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ali R.A., Gandhi A.A., Meng H., Yalavarthi S., Vreede A.P., Estes S.K., Palmer O.R., Bockenstedt P.L., Pinsky D.J., Greve J.M., Diaz J.A., Kanthi Y., Knight J.S. Adenosine receptor agonism protects against NETosis and thrombosis in antiphospholipid syndrome. Nat. Commun. 2019;10 doi: 10.1038/s41467-019-09801-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.You Y., Liu Y., Li F., Mu F., Zha C. Anti‐β2GPI/β2GPI induces human neutrophils to generate NETs by relying on ROS. Cell Biochem. Funct. 2019;37 doi: 10.1002/cbf.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sule G., Kelley W.J., Gockman K., Yalavarthi S., Vreede A.P., Banka A.L., Bockenstedt P.L., Eniola‐Adefeso O., Knight J.S. Increased adhesive potential of antiphospholipid syndrome neutrophils mediated by β2 integrin Mac‐1. Arthritis Rheumatol. 2020;72 doi: 10.1002/art.41057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liebman H.A., Stasi R. Secondary immune thrombocytopenic purpura. Curr. Opin. Hematol. 2007;14 doi: 10.1097/MOH.0b013e3282ab9904. [DOI] [PubMed] [Google Scholar]
- 37.Baroni G., Banzato A., Bison E., Denas G., Zoppellaro G., Pengo V. The role of platelets in antiphospholipid syndrome. Platelets. 2017;28 doi: 10.1080/09537104.2017.1280150. [DOI] [PubMed] [Google Scholar]
- 38.Scherlinger M., Sisirak V., Richez C., Lazaro E., Duffau P., Blanco P. New insights on platelets and platelet-derived microparticles in systemic lupus erythematosus. Curr. Rheumatol. Rep. 2017;19:1–10. doi: 10.1007/s11926-017-0678-0. [DOI] [PubMed] [Google Scholar]
- 39.Gremese E., Ferraccioli G. The pathogenesis of microthrombi in COVID‐19 cannot be controlled by DOAC: NETosis should be the target. J. Intern. Med. 2021;289 doi: 10.1111/joim.13228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Indari O., Jakhmola S., Manivannan E., Jha H.C. An update on antiviral therapy against SARS-CoV-2: how far have we come? Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.632677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carallo C., Pugliese F., Vettorato E., Tripolino C., Delle Donne L., Guarrera G., Spagnolli W., Cozzio S. Higher heparin dosages reduce thromboembolic complications in patients with COVID-19 pneumonia. J. Investig. Med. 2021 doi: 10.1136/jim-2020-001628. [DOI] [PubMed] [Google Scholar]
- 42.Shao Z., Feng Y., Zhong L., Xie Q., Lei M., Liu Z., Wang C., Ji J., Li W., Liu H., Gu Z., Hu Z., Su L., Wu M., Liu Z. Clinical efficacy of intravenous immunoglobulin therapy in critical patients with COVID-19: a multicenter retrospective cohort study. SSRN Electron. J. 2020 doi: 10.2139/ssrn.3576827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tan Y.-K., Goh C., Leow A.S.T., Tambyah P.A., Ang A., Yap E.-S., Tu T.-M., Sharma V.K., Yeo L.L.L., Chan B.P.L., Tan B.Y.Q. COVID-19 and ischemic stroke: a systematic review and meta-summary of the literature. J. Thromb. Thrombolysis. 2020;50 doi: 10.1007/s11239-020-02228-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borghi M.O., Beltagy A., Garrafa E., Curreli D., Cecchini G., Bodio C., Grossi C., Blengino S., Tincani A., Franceschini F., Andreoli L., Lazzaroni M.G., Piantoni S., Masneri S., Crisafulli F., Brugnoni D., Muiesan M.L., Salvetti M., Parati G., Torresani E., Mahler M., Heilbron F., Pregnolato F., Pengo M., Tedesco F., Pozzi N., Meroni P.L. Anti-phospholipid antibodies in COVID-19 are different from those detectable in the anti-phospholipid syndrome. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.584241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inic-Kanada A., Stojanovic M., Zivkovic I., Kosec D., Micic M., Petrusic V., Zivancevic-Simonovic S., Dimitrijevic L. Original article: murine monoclonal antibody 26 raised against tetanus toxoid cross-reacts with β2-Glycoprotein I: its characteristics and role in molecular mimicry. Am. J. Reprod. Immunol. 2008;61 doi: 10.1111/j.1600-0897.2008.00660.x. [DOI] [PubMed] [Google Scholar]
- 46.Zivkovic I., Stojanovic M., Petrusic V., Inic-Kanada A., Dimitrijevic L. Induction of APS after TTd hyper-immunization has a different outcome in BALB/c and C57BL/6 mice. Am. J. Reprod. Immunol. 2011;65 doi: 10.1111/j.1600-0897.2010.00922.x. [DOI] [PubMed] [Google Scholar]
- 47.Bizjak M., Bruck O., Kanduc D., Praprotnik S., Shoenfeld Y. Vaccinations and secondary immune thrombocytopenia with antiphospholipid antibodies by human papillomavirus vaccine. Semin. Hematol. 2016;53 doi: 10.1053/j.seminhematol.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 48.Vista E., Crowe S., Thompson L., Air G., Robertson J., Guthridge J., James J. Influenza vaccination can induce new-onset anticardiolipins but not β2-glycoprotein-I antibodies among patients with systemic lupus erythematosus. Lupus. 2012;21 doi: 10.1177/0961203311429554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cruz-Tapias P., Blank M., Anaya J.-M., Shoenfeld Y. Infections and vaccines in the etiology of antiphospholipid syndrome. Curr. Opin. Rheumatol. 2012;24 doi: 10.1097/BOR.0b013e32835448b8. [DOI] [PubMed] [Google Scholar]
- 50.Wise J. Covid-19: European countries suspend use of Oxford-AstraZeneca vaccine after reports of blood clots. BMJ. 2021 doi: 10.1136/bmj.n699. [DOI] [PubMed] [Google Scholar]
- 51.Ramasamy M.N., Minassian A.M., Ewer K.J., Flaxman A.L., Folegatti P.M., Owens D.R., et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2020;396 doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stone D., Liu Y., Shayakhmetov D., Li Z.-Y., Ni S., Lieber A. Adenovirus-platelet interaction in blood causes virus sequestration to the reticuloendothelial system of the liver. J. Virol. 2007;81:4866–4871. doi: 10.1128/jvi.02819-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crank M.C., Wilson E.M.P., Novik L., Enama M.E., Hendel C.S., Gu W., Nason M.C., Bailer R.T., Nabel G.J., McDermott A.B., Mascola J.R., Koup R.A., Ledgerwood J.E., Graham B.S. Safety and immunogenicity of a rAd35-EnvA prototype HIV-1 vaccine in combination with rAd5-EnvA in healthy adults (VRC 012) PLoS One. 2016;11 doi: 10.1371/journal.pone.0166393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Malaeb B.S., Gardner T.A., Margulis V., Yang L., Gillenwater J.Y., Chung L.W.K., Macik G., Koeneman K.S. Elevated activated partial thromboplastin time during administration of first-generation adenoviral vectors for gene therapy for prostate cancer: identification of lupus anticoagulants. Urology. 2005;66:830–834. doi: 10.1016/j.urology.2005.04.041. [DOI] [PubMed] [Google Scholar]
- 55.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P., Eichinger S. A prothrombotic thrombocytopenic disorder resembling heparin-induced thrombocytopenia following coronavirus-19 vaccination. Res. Sq. 2021:1–8. doi: 10.21203/rs.3.rs-362354/v1. (Accessed 31 March 2021) [DOI] [Google Scholar]
- 56.Sikara M.P., Routsias J.G., Samiotaki M., Panayotou G., Moutsopoulos H.M., Vlachoyiannopoulos P.G. β2 glycoprotein I (β2GPI) binds platelet factor 4 (PF4): implications for the pathogenesis of antiphospholipid syndrome. Blood. 2010;115:713–723. doi: 10.1182/blood-2009-03-206367. [DOI] [PubMed] [Google Scholar]
- 57.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., McGettigan J., Khetan S., Segall N., Solis J., Brosz A., Fierro C., Schwartz H., Neuzil K., Corey L., Gilbert P., Janes H., Follmann D., Marovich M., Mascola J., Polakowski L., Ledgerwood J., Graham B.S., Bennett H., Pajon R., Knightly C., Leav B., Deng W., Zhou H., Han S., Ivarsson M., Miller J., Zaks T. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck R.W., Hammitt L.L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D.B., Mather S., Dormitzer P.R., Şahin U., Jansen K.U., Gruber W.C. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carli G., Nichele I., Ruggeri M., Barra S., Tosetto A. Deep vein thrombosis (DVT) occurring shortly after the second dose of mRNA SARS-CoV-2 vaccine. Intern. Emerg. Med. 2021:1–2. doi: 10.1007/s11739-021-02685-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Essandoh K., Fan G.C. Role of extracellular and intracellular microRNAs in sepsis. Biochim. Biophys. Acta - Mol. Basis Dis. 2014;1842:2155–2162. doi: 10.1016/j.bbadis.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ng E.K.O., Tsui N.B.Y., Lam N.Y.L., Chiu R.W.K., Yu S.C.H., Wong S.C.C., Lo E.S.F., Rainer T.H., Johnson P.J., Lo Y.M.D. Presence of filterable and nonfilterable mRNA in the plasma of cancer patients and healthy individuals. Clin. Chem. 2002;48:1212–1217. doi: 10.1093/clinchem/48.8.1212. [DOI] [PubMed] [Google Scholar]
- 62.Nakazawa F., Kannemeier C., Shibamiya A., Song Y., Tzima E., Schubert U., Koyama T., Niepmann M., Trusheim H., Engelmann B., Preissner K.T. Extracellular RNA is a natural cofactor for the (auto-)activation of factor VII-activating protease (FSAP) Biochem. J. 2005;385:831–838. doi: 10.1042/BJ20041021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kannemeier C., Shibamiya A., Nakazawa F., Trusheim H., Ruppert C., Markart P., Song Y., Tzima E., Kennerknecht E., Niepmann M., von Bruehl M.-L., Sedding D., Massberg S., Gunther A., Engelmann B., Preissner K.T. Extracellular RNA constitutes a natural procoagulant cofactor in blood coagulation. Proc. Natl. Acad. Sci. 2007;104 doi: 10.1073/pnas.0608647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fischer S., Nishio M., Dadkhahi S., Gansler J., Saffarzadeh M., Shibamiyama A., Kral N., Baal N., Koyama T., Deindl E., Preissner K.T. Expression and localisation of vascular ribonucleases in endothelial cells. Thromb. Haemost. 2011;105:345–355. doi: 10.1160/TH10-06-0345. [DOI] [PubMed] [Google Scholar]
- 65.Zeng C., Zhang C., Walker P.G., Dong Y. In: Current Topics in Microbiology and Immunology. Springer, editor. 2020. Formulation and delivery technologies for mRNA vaccines. Berlin, Heidelberg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Talotta R. Do COVID-19 RNA-based vaccines put at risk of immune-mediated diseases? In reply to “potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases,”. Clin. Immunol. 2021;224 doi: 10.1016/j.clim.2021.108665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xourgia E., Tektonidou M.G. Type I interferon gene expression in antiphospholipid syndrome: pathogenetic, clinical and therapeutic implications. J. Autoimmun. 2019;104 doi: 10.1016/j.jaut.2019.102311. [DOI] [PubMed] [Google Scholar]
- 68.Steinberg J., Thomas A., Iravani A. 18F-fluorodeoxyglucose PET/CT findings in a systemic inflammatory response syndrome after COVID-19 vaccine. Lancet. 2021;397 doi: 10.1016/S0140-6736(21)00464-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Johnson B.J., Van Abel K., Ma D., Johnson D.R. FDG avid axillary lymph nodes after COVID-19 vaccination. J. Nucl. Med. 2021 doi: 10.2967/jnumed.121.262108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Avner M., Orevi M., Caplan N., Popovtzer A., Lotem M., Cohen J.E. COVID-19 vaccine as a cause for unilateral lymphadenopathy detected by 18F-FDG PET/CT in a patient affected by melanoma. Eur. J. Nucl. Med. Mol. Imaging. 2021 doi: 10.1007/s00259-021-05278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ahmed N., Muzaffar S., Binns C., Ilyas M.W., Usmani S. COVID-19 vaccination manifesting as incidental lymph nodal uptake on 18F-FDG PET/CT. Clin. Nucl. Med. 2021 doi: 10.1097/RLU.0000000000003635. [DOI] [PubMed] [Google Scholar]
- 72.Wu M., Barnard J., Kundu S., Mccrae K.R. A novel pathway of cellular activation mediated by antiphospholipid antibody-induced extracellular vesicles. J. Thromb. Haemost. 2015;13:1928–1940. doi: 10.1111/jth.13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Prinz N., Clemens N., Strand D., Pütz I., Lorenz M., Daiber A., Stein P., Degreif A., Radsak M., Schild H., Bauer S., Von Landenberg P., Lackner K.J. Antiphospholipid antibodies induce translocation of TLR7 and TLR8 to the endosome in human monocytes and plasmacytoid dendritic cells. Blood. 2011;118:2322–2332. doi: 10.1182/blood-2011-01-330639. [DOI] [PubMed] [Google Scholar]
- 74.Breen K.A., Sanchez K., Kirkman N., Seed P.T., Parmar K., Moore G.W., Hunt B.J. Endothelial and platelet microparticles in patients with antiphospholipid antibodies. Thromb. Res. 2015;135:368–374. doi: 10.1016/j.thromres.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 75.Fotin-Mleczek M., Duchardt K.M., Lorenz C., Pfeiffer R., Ojkić-Zrna S., Probst J., Kallen K.J. Messenger RNA-based vaccines with dual activity induce balanced TLR-7 dependent adaptive immune responses and provide antitumor activity. J. Immunother. 2011;34:1–15. doi: 10.1097/CJI.0b013e3181f7dbe8. [DOI] [PubMed] [Google Scholar]
- 76.Hampton L.M., Aggarwal R., Evans S.J.W., Law B. General determination of causation between Covid-19 vaccines and possible adverse events. Vaccine. 2021;39 doi: 10.1016/j.vaccine.2021.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.FDA . 2021. Vaccines and Related Biological Products Advisory Committee Meeting February 26. (2021). https://www.fda.gov/media/146217/download (Accessed 5 April 2021) [Google Scholar]
- 78.Zhang S., Liu Y., Wang X., Yang L., Li H., Wang Y., Liu M., Zhao X., Xie Y., Yang Y., Zhang S., Fan Z., Dong J., Yuan Z., Ding Z., Zhang Y., Hu L. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J. Hematol. Oncol. 2020;13 doi: 10.1186/s13045-020-00954-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kamath P.S., Badakere S.S., Mehta B.C. Anti-idiotype antibodies and circulating immune complexes after immunisation with tetanus-toxoid. Ceylon Med. J. 1994;39:97–100. [PubMed] [Google Scholar]
- 80.Cines D.B., Zaitsev S., Rauova L., Rux A.H., Stepanova V., Krishnaswamy S., Sarkar A., Anna Kowalska M., Zhao G., Mast A.E., Blumberg L.J., McCrae K.R., Poncz M., Hubbard J.J., Pyzik M., Blumberg R.S. FcRn augments induction of tissue factor activity by IgG-containing immune complexes. Blood. 2020;135:2085–2093. doi: 10.1182/blood.2019001133. [DOI] [PMC free article] [PubMed] [Google Scholar]