Abstract
Germline mutations in PTEN account for ~10% of cases of autism spectrum disorder (ASD) with coincident macrocephaly. To explore the importance of nuclear PTEN in the development of ASD and macrocephaly, we previously generated a mouse model with predominantly cytoplasmic localization of Pten (Ptenm3m4/m3m4).Cytoplasmic predominant Pten localization results in a phenotype of extreme macrocephaly and autistic-like traits. Transcriptomic analysis of the Ptenm3m4/m3m4 cortex found upregulated gene pathways related to myeloid cell activation, myeloid cell migration, and phagocytosis. These transcriptomic findings were used to direct in vitro assays on Pten wild-type and Ptenm3m4/m3m4 microglia. We found increased Iba1 and C1q expression with enhanced phagocytic capacity in Ptenm3m4/m3m4 microglia, indicating microglial activation. Moreover, through a series of neuron-microglia co-culture experiments, we found Ptenm3m4/m3m4 microglia are more efficient at synaptic pruning compared with wild-type controls. In addition, we found evidence for neuron-microglia cross-talk, where Ptenm3m4/m3m4 neurons elicit enhanced pruning from innately activated microglia. Subsequent in vivo studies validated our in vitro findings. We observed a concurrent decline in the expression of Pten and synaptic markers in the Ptenm3m4/m3m4 cortex. At ~3 weeks of age, with a 50% drop in Pten expression compared with wild-type levels, we observed enhanced activation of microglia in the Ptenm3m4/m3m4 brain. Collectively, our data provide evidence that dysregulated Pten in microglia has an etiological role in microglial activation, phagocytosis, and synaptic pruning, creating avenues for future studies on the importance of PTEN in maintaining microglia homeostasis.
Subject terms: Autism spectrum disorders, Genetics, Neuroscience
Introduction
The gene encoding Phosphatase and TENsin homolog deleted on chromosome TEN (PTEN) is a well-recognized, syndromic risk allele for autism spectrum disorder (ASD), a neurodevelopmental disorder defined by deficits in two core symptom domains: social communication/interaction and restricted/repetitive behavior [1–4]. Carrying a germline PTEN mutation is also the molecular criterion for a diagnosis of PTEN Hamartoma Tumor Syndrome (PHTS), irrespective of clinical phenotype. PHTS (OMIM 601728) is an autosomal dominant, cancer predisposition syndrome, where patients present variably but generally have macrocephaly, benign hamartoma of all three germ layers, elevated risks for specific malignancies, and macrocephalic ASD, the latter occurring in about 20% of PHTS individuals [5–7]. Conversely, ~17% of individuals with ASD and macrocephaly harbor a germline PTEN mutation [2]. Follow-up studies confirmed this genetic association, adjusting the weighted average for PTEN mutation in ASD individuals with coincident macrocephaly to ~10% [4, 8–10]. These genetic data highlight PTEN as one of the most prevalent and penetrant ASD-risk genes.
To explore the importance of PTEN in the development of ASD and macrocephaly, we generated a mouse model with a knock-in mutation disrupting two of the four putative nuclear localization signals of Pten [11–13]. The consequent Ptenm3m4 mouse is characterized by Pten expression predominantly restricted to the cytoplasm with substantial depletion from the nucleus. This cytoplasmic-predominant model presents with macrocephaly due to megencephaly, hypertrophy of neural somas, gliosis, and autistic-like behavior [12]. RNA sequencing of the Ptenm3m4/m3m4 hemi-brain at 2 weeks of age (i.e., P14) and cortex at 6 weeks of age (i.e., P40) demonstrated that the Ptenm3m4/m3m4 transcriptome is reflective of idiopathic autism with many of the same genes differentially expressed [13]. Earlier analyses of the transcriptome of the cortex from Ptenm3m4/m3m4 mice at 6 weeks of age showed increased expression of genes associated with neuroinflammation and significantly decreased expression of genes associated with synaptic transmission [13].
Our earlier studies on the Ptenm3m4 model identified activated microglia in the cortex and hippocampus of the Ptenm3m4/m3m4 mouse [12]. Microglia, the resident immune cells of the brain, participate in synaptic pruning, an important neurodevelopmental process. During synaptic pruning, microglia utilize C1q as the regulator of complement-mediated pruning to target immature synapses for engulfment and subsequent removal [14, 15]. It has been demonstrated that altered complement function and C1q expression in microglia are associated with deficits in synaptic transmission and ASD as a consequence of inappropriate synaptic pruning [16–20]. In one such study, the authors show that C1q protein expression is elevated in the serum of children with ASD compared with age-matched controls, further stressing the necessity of proper complement function for neuronal plasticity [16]. There are no published data on the role of Pten as a regulator of microglia function and synaptic pruning. Based on the transcriptomic and preliminary in vivo findings, we hypothesize that constitutive Pten dysfunction predisposes microglia to aberrant activation, leading to exaggerated participation in synaptic pruning during neurodevelopment. Using both in vitro and in vivo models, we demonstrate that Pten activity in microglia is essential for regulating synapse targeting and engulfment, as well as microglial morphology throughout development.
Materials and methods
Transcriptomic data analysis
The cortical transcriptome of 6-week-old Ptenm3m4 mice (GSE59318) mimics that of idiopathic autism, as described in detail in Tilot et al. [13]. We performed PANTHER GO classification on the list of differentially expressed genes in the cortex of Pten homozygous mutant and wild-type mice. Genes annotated as related to biological process and cellular components of interest were identified by immune system processes (GO: 0002376) and the synapse (GO: 0045202), respectively. Next, we performed a “core analysis” using Ingenuity Pathway Analysis (IPA) software (Qiagen, Redwood City, California). The core analysis was mined for significantly enriched pathways relevant to microglia activity, migration, and function, which were used to inform further in vivo and in vitro experiments.
Animals
Generation and characterization of the Ptenm3m4 mouse on a CD1 background has been described previously [12]. The Ptenm3m4 mutation is located within exon 7 of Pten and consists of five nucleotide substitution mutations, resulting in four nonsynonymous and one synonymous amino acid changes in the third and fourth putative nuclear localization sequences of Pten [11, 12]. Genotyping was performed on genomic DNA from clipped toes per the Jackson Laboratory protocol using modified PCR primers. Wild-type allele primers: mPten-F5, 5′-TGGCAGACTCTTCATTTCTGTGGC-3′, and mPten-R6, 5′-ACTTCTTCACAACCACTTCTTTCAAC-3′. Mutant allele primers are mPten-F3, 5′-TACCCGGTAGAATTTCGACGACCT-3′, and mPten-R6, 5′-ACTTCTTCACAACCACTTCTTTCAAC-3′. Mice were maintained on a 14:10 light:dark cycle with access to food and water ad libitum. The room temperature was maintained between 18 and 26 °C. Animals were euthanized via CO2 asphyxiation followed by cervical dislocation. All experiments were not blinded but randomized and conducted under protocols approved by the Institutional Animal Care and Use Committee (IACUC) at Cleveland Clinic.
We utilized roughly equal numbers of male and female mice for our experiments. We show in a previous study no significant differences between Ptenm3m4/m3m4 male and female gross cellular phenotype so all available samples were utilized accordingly [12]. These data are further supported by western blot experiments which denote the sex of each biological replicate used. (Supplementary Figs. 1a–e and 2a, b).
Western blot analysis
Cortical and hippocampal regions of the brain were isolated, snap-frozen and stored at −80 °C. For making tissue lysates, the tissue was thawed on ice and lysed in RIPA buffer (10 mM Tris-Cl [pH 8], 1 mM EDTA, 0.5 mM EGTA, 1% Triton X-100, 0.1% sodium deoxycholate, 0.1% SDS, 140 mM NaCl, before use add 1 mM PMSF) with phosphatase inhibitor #2 (#P5726-5ML, Sigma, St. Louis, Missouri), phosphatase inhibitor #3 (#P0044-5ML, Sigma) and protease inhibitor (Sigma, #P8345-5ML). All lysates were quantified for protein content using BCA assays, equalized for protein content and 15 μg of protein per sample was loaded on a 4–12% gradient polyacrylamide gel. The separated proteins were transferred to a nitrocellulose membrane, and the membrane was blocked overnight in 2.5% milk diluted in Tris-buffered saline, containing 0.2% Tween-20 (TBST) at 4 °C. Membranes were then washed with TBST and incubated with experiment specific primary antibodies diluted in TBST overnight at 4 °C. The following antibodies were used: Pten (1:5000, #ABM-2025, Cascade Bioscience, Winchester, Massachusetts), Synaptophysin (1:5000, #ab32127, Abcam, Cambridge, Massachusetts), Psd-95 (1:1000, #810401, Biolegend, San Diego, California), and C1q (1:500, #ab71940, Abcam). We removed the primary antibody solution and performed three washes, for 10 min per wash, with TBST. Blots were probed with Goat Anti-Mouse secondary antibody IRDye800CW (1:20,000, #213965, LI-COR, Lincoln, Nebraska) or Goat Anti-Rabbit IRDye680 (LI-COR, #213971) diluted in TBST, for 2 h at room temperature. The membranes were washed three times, 10 min each in TBST and imaged using the Odyssey CLx imaging system (LI-COR). Using ImageJ (National Institute of Health, Bethesda, Maryland, 1995), we performed densitometry analysis on these images to quantify protein expression.
Cell culture
Mixed glia were obtained by trypsinization of P2 cortices followed by plating on poly-D-lysine coated T-75 culture flasks. Mixed glia cultures, were maintained in DMEM with 10% FBS and 1% Penicillin and Streptomycin (Pen/Strep). Once the mixed glia cultures reached DIV 10, they were agitated at 170 RPM for 1 h in order to isolate primary microglia. Isolated microglia were seeded on poly-D-lysine coated glass cover slips and used for immunofluorescent (IF) staining and phagocytic assays at DIV 3 post-shaking.
We isolated primary neurons from E14.5 cortical tissue. Cortical tissue was mechanically dissociated, trypsinized, and isolated primary neurons were seeded on poly-D-lysine coated cover slips. We grew the neurons in neurobasal media (Thermo-Fisher, #21103–049) supplemented with B27 (1×) (Thermo-Fisher, #17504–044), glutamax (1×) (Thermo-Fisher, #35050061), and 1% Pen/Strep. Neuronal cell cultures were maintained until DIV 14, after which we performed IF staining. If designated for neuron/microglia co-culturing experiments, we isolated microglia via shaking and added them to the neuronal cultures at DIV 7 in a 1:1 ratio (25,000 cells/well in 12-well plate). All mixed glia, primary microglia, and primary neurons were cultured in 5% CO2 and 100% humidity at 37 °C. Neurons and microglia remained cultured together in the presence of neurobasal media supplemented with B27 (1×), glutamax (1×), and 1% Pen/Strep to DIV 14, till analysis by IF.
In vitro immunofluorescent staining
We cultured microglia or neurons on poly-D-lysine (PDL)-coated cover slips until DIV 14. Microglia were washed with ice-cold PBS and fixed in ice-cold methanol for 2 min. This was followed by three washes for 5 min each with ice-cold PBS. We then permeabilized the microglia with 0.03% Triton X-100 dissolved in PBS for 4 min. Next the cultures were blocked with 10% normal goat serum for 1 h at room temperature, followed by incubation with primary antibody: Pten (1:100, #ABM-2025, Cascade Bioscience), Synaptophysin (1:500, #ab32127, Abcam), Iba1 (1:500, #019–19741, Wako, Bellwood, Virginia), Iba1 (1:250, #MABN92, EMD Millipore, Burlington, Massachusetts) or C1q (1:250, #ab71089, Abcam), NeuN (1:500, #MAB377, EDM Millipore), cleaved caspase-3 (1:250, #9664S, Cell Signaling, Danvers, Massachusetts) and Psd-95 (1:250, #810401, Biolegend) diluted in 10% normal goat serum in PBS. We incubated cells in primary antibody overnight at 4 °C. Next, we washed the cells with PBS and then added goat anti-mouse Alexa Fluor 568 secondary antibody (1:2000, #A11031, Thermofisher) and goat anti-rabbit Alexa Fluor 488 (1:2000, #A11008, Thermofisher) diluted in 10% normal goat serum in PBS. The cells were incubated in secondary antibody for 2 h at room temperature (in dark). Post-secondary incubation the cells were washed three times with PBS for 5 min. Finally, the coverslips containing the cells were mounting with Vectashield mounting media with DAPI (Vector Laboratories, Burlingame, California).
Quantification of functional synapses
We fixed neuron/microglia co-cultures and stained for the synaptic markers, Synaptophysin at 1:500 dilution of primary antibody, and Psd-95 at 1:250 dilution of primary antibody once the neurons had reached DIV 14. Neuron/microglial cultures were imaged using confocal microscopy (Leica Biosystems, Richmond, Ilinois) and analyzed using the Volocity v6.3.0 (Quorum Technologies Inc., Puslinch, Ontario, Canada) to count functional synapses (co-localized Syn and Psd-95) along individual neurites. Functional synapse number was then normalized to the length of the respective neurite.
Phagocytosis assay
We plated primary microglia at a density of 1 × 105 in a 12-well dish with PDL-coated coverslips for 48 h in a 37 °C cell incubator with 5% CO2 and 100% humidity. Next, we blocked 1 μm fluorescent beads (#L1030, Sigma-Aldrich) in FBS for 1 h at 37 °C at a ratio of 1:5 v/v. Florescent beads were diluted with DMEM to reach a final concentration of 0.01% (v/v). Microglial culture media was replaced with 250 μl DMEM containing beads, and incubated for 1 h at 37 °C in a cell incubator. Cultures were washed thoroughly five times with ice-cold PBS and fixed in ice-cold methanol prior to immunofluorescent staining for Iba1 (1:500, #019–19741, Wako).
Immunofluorescent staining of brain tissue
Mice were euthanized and perfused with ~50 ml of PBS. Brain tissue was extracted and fixed in 4% PFA (pH = 7) for 24 h at 4 °C. PFA was washed three times with PBS, and brain tissue was cryoprotected in 30% sucrose dissolved in PBS for 94 h at 4 °C. 10 μm frozen coronal serial sections were cut on a cryostat and mounted on polarized glass slides (Fisherbrand Superfrost Plus microscope slides, #12–550–15, Fisher Scientific, Waltham, MA). We removed OCT by washing slides in PBS for 10 min. Tissue was permeabilized by incubating the slides with 3% Triton-X dissolved in PBS for 10 min. Slides were washed three times for 5 min each in PBS and probed with experiment specific primary antibodies: Synaptophysin (1:500, #ab32127 Abcam), Iba1 (1:500, #019–19741, Wako), or Iba1 (1:250, #MABN92, EMD Millipore). The slides were incubated overnight at 4 °C in primary antibody, followed by washing PBS and incubation with specific secondary antibodies for 2 h: goat anti-mouse Alexa Fluor 568 (1:2000, #A11031, Thermofisher) and goat anti-rabbit Alexa Fluor 488 (1:2000, #A11008, Thermofisher). Post incubation, slides were washed and mounted in Vectashield medium with DAPI (Vector Laboratories), coverslipped, and sealed with nail polish.
Immunofluorescent quantification
We captured images of brain sections as well as cells grown in vitro as confocal images using a Leica TCS-SP8-AOBS inverted confocal microscope (Leica Microsystems, GmbH, Wetzlar, Germany). Brain section, primary neuron, and/or microglial co-cultures were imaged with a minimum of n = 5 biological replicates. We used ImageJ software to measure area and intensity of the stain and calculated integrated density of brain images. In addition, ImageJ was used to measure area of stain per microglia in vitro and in vivo to assess morphological changes beyond “bushy” and “amoeboid” [21]. Primary neuron/microglial co-cultures were analyzed using the Volocity 3D imaging software in order to count the number of functional synapses and normalize these values to neurite length.
Due to the low abundance of primary microglia resulting from our isolation protocol, we pooled microglia from various cultures where appropriate. These microglia were maintained in culture and stained accordingly. We captured a minimum of five images per cultured microglia genotype. Finally, we quantified these images using ImageJ to measure signal, area and integrated density.
Statistical analysis
Sample size was determined according to our previous studies utilizing the Ptenm3m4 model [12, 13, 22]. These studies were conducted in a non-blinded manner with sample utilization being randomized. Statistical tests are justified according to the distribution of the data, and also if data meets the assumptions of each analysis. We analyzed normally distributed data using a one-way analysis of variance (ANOVA) or Student’s t-test, where appropriate (Graph Pad Prism 8). After performing a one-way or two-way ANOVA when appropriate, we performed a post-hoc Tukey–Kramer analysis (F). When data were not normally distributed, we performed non-parametric analyses including Mann–Whitney U and Kruskall–Wallis tests (H), where appropriate (Graph Pad Prism 8). P values that are less than 0.05 were considered statistically significant. We calculated effect sizes as the mean difference with 95% confidence intervals using the R package DABESTR [23]. In addition, Spearman rho R correlation matrix was used to determine correlations between data plots when appropriate (Graph Pad Prism 8).
Results
The Ptenm3m4 neural transcriptome is enriched in neuroinflammation networks, including microglial activation and phagocytic activity
Our earlier studies have revealed a neuroinflammatory signature in the cortical transcriptome of 6-week-old Ptenm3m4/m3m4 mice [4]. Using this platform, we found a significant increase in expression of complement-related proteins, and genes implicated in synaptic pruning in Ptenm3m4/m3m4 mice compared with PtenWT/WT controls (Table 1). In addition, numerous inflammation-related and synapse-related genes also showed differential expression (Supplementary Tables 1 and 2). These results highlight dysregulation of mediators of immune and synaptic function in Ptenm3m4/m3m4 mice.
Table 1.
Expression of complement-related genes in the cortex of Ptenm3m4/m3m4 compared with wildtype.
| Gene ID | Fold change | P value | Q value |
|---|---|---|---|
| C1qa | 2.40 | 5.00E−05 | 0.00191 |
| C1qb | 2.36 | 5.00E−05 | 0.00191 |
| C1qc | 2.40 | 5.00E−05 | 0.00191 |
| C3ar1 | 2.40 | 5.00E−05 | 0.00191 |
| Cx3Cr1 | 1.51 | 5.00E−05 | 0.00191 |
| Itgam | 1.63 | 5.00E−05 | 0.00191 |
| Itgb2 | 1.99 | 5.00E−05 | 0.00191 |
| Trem2 | 1.75 | 5.00E−05 | 0.00191 |
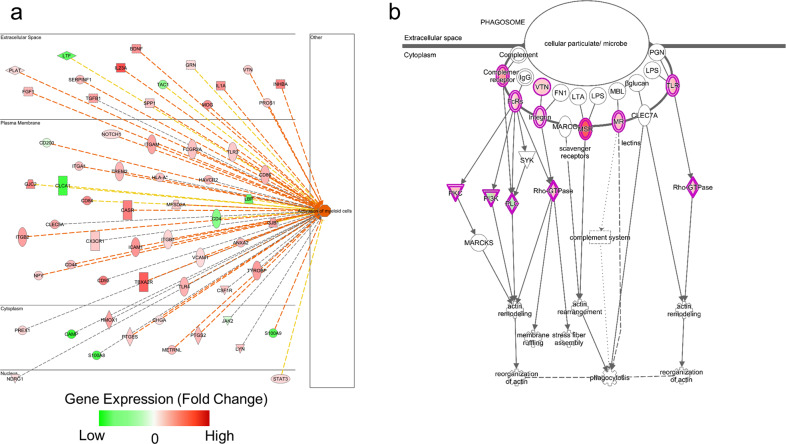
Subsequent gene enrichment and pathway analyses (Ingenuity Pathway Analysis [IPA] software) on the transcriptomic data from the cortices of 6-week-old Ptenm3m4/m3m4 mice, identified significant enrichment of pathways defined as “activation of myeloid cells”, “phagocytosis”, “cell movement of phagocytes” (Fig. 1a, Supplementary Fig. 3a, b) and phagosome formation (Fig. 1b). Collectively, these analyses strongly suggest activation of microglia and increased phagocytosis.
Fig. 1. Ingenuity pathway analysis of Ptenm3m4/m3m4 cortical transcriptome data.
a Increased “Activation of Myeloid Cells” (60 genes; p value = 1.38E−10; z-score = 3.36) pathway enriched in the Ptenm3m4/m3m4 cortex compared with the PtenWT/WT cortex at 6 weeks of age. b Differential expression data overlaid on enriched “Phagosome Formation” (IPA) canonical pathway for Ptenm3m4/m3m4 vs. PtenWT/WT comparison.
Ptenm3m4/m3m4 primary microglia in culture have cytoplasmic predominant Pten localization
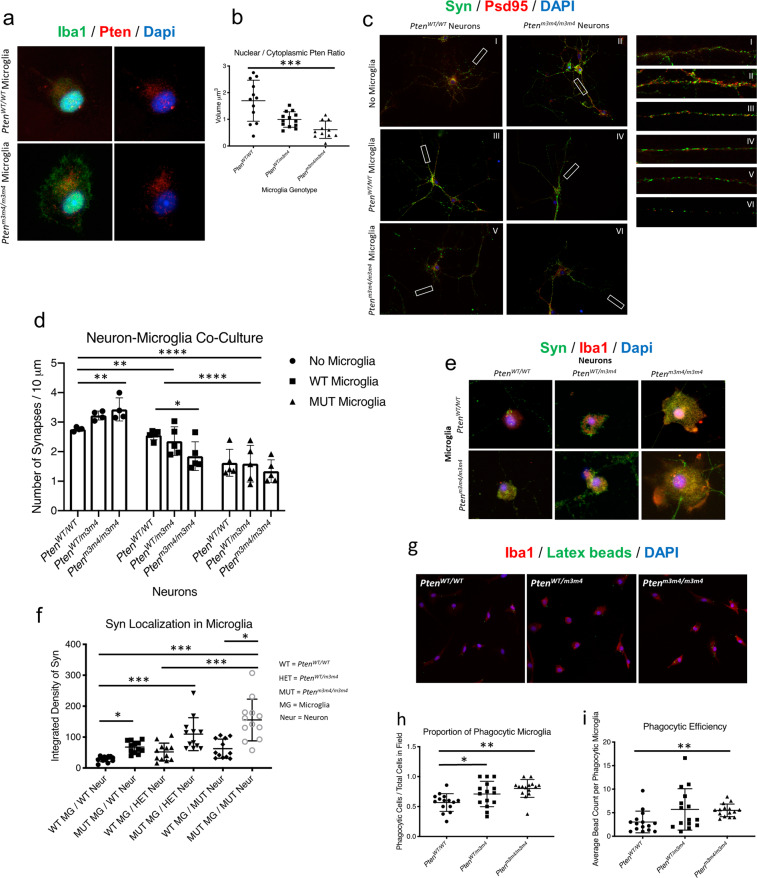
To evaluate Pten localization in mice with Ptenm3m4 mutations, primary microglia from PtenWT/WT, PtenWT/m3m4, and Ptenm3m4/m3m4 mice were co-stained for Pten and Iba1. 3D rendering of the microglia showed Pten localization significantly increased in the cytoplasm compared with the nucleus of Ptenm3m4/m3m4 microglia (mean Pten volume: PtenWT/WT = 1.7 ± 0.77 μm3 vs. Ptenm3m4/m3m4 = 0.61 ± 0.32 μm3; p value = 0.0005) (Fig. 2a, b).
Fig. 2. Cytoplasmic predominant Pten localization results in increased microglia-dependent synaptic pruning and phagocytosis in vitro.
a Immunofluorescent co-staining of Iba1 (green) and Pten (red) in PtenWT/WT and Ptenm3m4/m3m4 primary microglia. b Quantification of nuclear and cytoplasmic distribution of Pten within PtenWT/WT (mean volume = 1.7 ± 0.77 μm3; H = 14.1) and Ptenm3m4/m3m4 (mean volume = 0.61 ± 0.32 μm3; H = 14.1, p value = 0.0005). c Immunofluorescent co-staining of Syn (green) and Psd-96 (Red) in neuron-microglia co-cultures. d Quantification of functional synapse number along 10 µm of neurite length of each neuron-microglia co-culture shows microglia account for 68.57% of the observable interaction across all neuronal conditions irrespective of neuronal genotype (p value ≤ 0.0001, F = 52.57): Ptenm3m4/m3m4 microglia compared with no microglia (mean difference in synapses per 10 µm = 1.62 ± 1.23 to 2.01; F = 52.57, p value < 0.0001) and Ptenm3m4/m3m4 microglia compared with PtenWT/WT microglia (mean difference in synapses per 10 µm = 0.74 ± 0.37 to 1.10; F = 52.57, p value < 0.0001) and PtenWT/WT microglia compared with no microglia (mean difference in synapses per 10 µm = 0.88 ± 0.49 to 1.27; F = 52.57, p value < 0.0001). In addition, Ptenm3m4/m3m4 neurons (mean synapses per 10 µm = 1.3 ± 0.4 vs. 3.2 ± 0.2; F = 28, p value < 0.0001) compared with PtenWT/m3m4 neurons (mean synapses per 10 µm = 1.6 ± 0.6 vs. 3.2 ± 0.2; F = 13, p value = 0.001) and PtenWT/WT neurons (mean synapses per 10 µm = 1.6 ± 0.5 vs. 2.8 ± 0.07; F = 20, p value = 0.0004). e Immunofluorescent co-staining in neuron-microglia co-cultures shows Syn (green) and Iba1 (red). f Quantification of Syn localization within microglia co-cultured with primary neurons (mean Syn integrated density: Ptenm3m4/m3m4 microglia with PtenWT/WT neurons = 67.4 ± 19.1; H = 42.0, p value = 0.04; PtenWT/WT microglia with PtenWT/WT neurons = 29.1 ± 9.0; H = 42.0; Ptenm3m4/m3m4 microglia with Ptenm3m4/m3m4 neurons = 155.4 ± 67.4; H = 42.0, p value < 0.0001; PtenWT/WT microglia with Ptenm3m4/m3m4 neurons = 62.5 ± 30.1; H = 42.0). g Phagocytosis assay with primary microglia cultures co-stained for Iba1 (red) and fluorescent latex beads (green). h, i Phagocytosis assay quantifications of phagocytic microglia (mean phagocytic microglia in observed field: PtenWT/WT = 0.6 ± 0.15; H = 12.69; PtenWT/m3m4 microglia = 0.78 ± 0.3; H = 12.69, p value = 0.03; Ptenm3m4/m3m4 microglia = 0.8 ± 0.15; H = 12.69, p value = 0.001) and phagocytic ability of microglia to uptake fluorescent beads (mean phagocytized beads: PtenWT/W = 3.0 ± 2.3; H = 10.5; Ptenm3m4/m3m4 = 5.5 ± 1.3; H = 10.5, p value = 0.005).
Ptenm3m4/m3m4 microglia show evidence of enhanced synaptic pruning activity
To evaluate synaptic pruning capacity of Pten mutant microglia we co-cultured primary neurons and microglia from PtenWT/WT, PtenWT/m3m4, or Ptenm3m4/m3m4 mice in differing combinations for 72 h. Co-localization of pre- and post-synaptic markers, Syn and Psd-95, respectively, was used to determine presence of functional synapse and normalized to neurite length (Fig. 2c, d). Upon analysis of our microglia-neuron co-cultures we found the most dramatic decrease in synapse number with Ptenm3m4/m3m4 microglia co-cultures compared with PtenWT/WT microglia co-cultures (mean difference of synapses per 10 µm = 0.74; F = 57.52, p value ≤ 0.0001) and also compared with neuronal cultures without microglia (mean difference of synapses per 10 µm = 1.62; F = 57.52, p value ≤ 0.0001) (Fig. 2c, d). Subsequent analysis shows the highest numbers of functional synapses per 10 µm of neurite length were observed when no microglia were present in the neuronal cultures of all genotypes (mean synapses per 10 µm: PtenWT/WT = 2.8 ± 0.07; PtenWT/m3m4 = 3.2 ± 0.2; Ptenm3m4/m3m4 = 3.4 ± 0.4). Ptenm3m4/m3m4 neurons show significantly more synapses compared with PtenWT/WT neurons (mean difference of synapses per 10 µm = 0.6; F = 7.865, p value = 0.0095) (Fig. 2c, d and Supplementary Fig. 4a, b, Supplementary Table 3). In microglia co-cultures the number of synapses were substantially reduced compared with neuronal mono-cultures. The largest decrease in synapse number was observed when Ptenm3m4/m3m4 neurons were cultured with Ptenm3m4/m3m4 microglia (mean synapses per 10 µm = 1.3 ± 0.4) compared with PtenWT/m3m4 neurons (1.6 ± 0.6) and PtenWT/WT neurons (1.6 ± 0.5) (Fig. 2c, d and Supplementary Fig. 4c–e, Supplementary Table 3). Interestingly, even in PtenWT/WT microglia co-cultures, the largest decrease was seen with Ptenm3m4/m3m4 neurons (Fig. 2c, d and Supplementary Fig. 4c, d). We confirmed that the decrease in synapse number in the co-culture was due to microglial- pruning, and not due to neuronal death by co-staining for NeuN and Cleaved Caspase-3. No increase in neuronal death was observed (Supplementary Fig. 5a). These data show that neurons from Ptenm3m4/m3m4 mutants form more synapses compared with PtenWT/WT neurons as shown in earlier studies. However, in a systemic germline Ptenm3m4/m3m4 mutant system, our data suggest cross-talk between neurons and microglia, resulting in significantly increased synaptic pruning in Ptenm3m4/m3m4 mutants compared with PtenWT/WT mice.
Ptenm3m4/m3m4 microglia have enhanced ability for synaptic engulfment and phagocytosis
To further confirm that Ptenm3m4/m3m4 microglia have enhanced pruning efficiency, we used the microglia and neuronal co-culture system. We observed increased synaptic engulfment in Ptenm3m4/m3m4 microglia compared with PtenWT/WT microglia when co-cultured with PtenWT/WT neurons (Fig. 2e, f). Interestingly, when Ptenm3m4/m3m4 neurons were present in the co-culture system, there was an observed increase in synaptic pruning by both Ptenm3m4/m3m4 and PtenWT/WT microglia. The highest numbers of engulfed synapses were observed in Ptenm3m4/m3m4 microglia and Ptenm3m4/m3m4 neuron co-cultures (Fig. 2e, f, and Supplementary Table 4). Bead-based phagocytosis assays revealed significantly increased numbers of beads per microglia (PtenWT/WT = 3.0 ± 2.3 vs. Ptenm3m4/m3m4 = 5.5 ± 1.3; p value = 0.005), as well as increased numbers of phagocytic microglia in cultures of Ptenm3m4/m3m4 microglia vs. PtenWT/WT microglia (Ptenm3m4/m3m4 microglia = 0.8 ± 0.15 vs. PtenWT/WT = 0.6 ± 0.15; p value = 0.001) (Fig. 2g–i). Collectively, these data imply that Ptenm3m4/m3m4 microglia have a higher capacity to engulf synapses, and that cross-talk via Ptenm3m4/m3m4 neurons may enhance pruning when co-cultured together regardless of microglia genotype.
Enhanced synaptic pruning by Ptenm3m4/m3m4 microglia is associated with increased C1q production and deposition
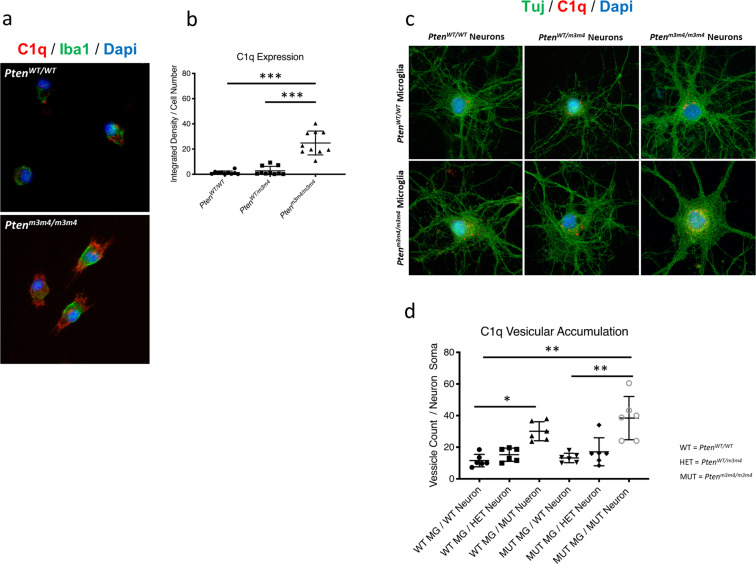
Since complement expression in primary microglia is the key regulator of microglial-dependent synaptic pruning [17–19], we examined C1q protein levels. RNA-seq and ELISA analyses on cortical lysates as well as IF staining showed increases in C1q expression in PtenWT/m3m4 and Ptenm3m4/m3m4 microglia (C1q integrated density: PtenWT/WT = 1.3 ± 1.4; vs. Ptenm3m4/m3m4 = 24.8 ± 9.5, p value = 0.0002) (Fig. 3a, b; Supplementary Fig. 5b). Next, we co-labeled neurons with class III β-tubulin (Tuj1) and C1q. In comparison to PtenWT/WT neurons, Ptenm3m4/m3m4 neurons showed significantly increased C1q foci present on their soma when co-cultured with either of the PtenWT/WT or Ptenm3m4/m3m4 microglia (C1q foci Ptenm3m4/m3m4 neurons: PtenWT/WT microglia = 30.1 ± 6.0, p value = 0.01 vs. Ptenm3m4/m3m4 microglia = 38.4 ± 13.6, p value = 0.02) (Fig. 3c, d). These data suggest that Ptenm3m4/m3m4 microglia are independently predisposed to enhanced synaptic pruning via increased expression of C1q. In addition, the microglial-neuron cross-talk, specifically that involving Ptenm3m4/m3m4 neurons, is able to enhance microglial pruning efficiency irrespective of microglial genotype likely resulting in a “double whammy” for over-pruning of synapses in Ptenm3m4/m3m4 mice.
Fig. 3. Increased expression of C1q in Ptenm3m4/m3m4 microglia and enhanced C1q accumulation on synapses of Ptenm3m4/m3m4 neurons.
a Immunofluorescent staining for C1q (red) and Iba1 (green) in primary microglia. b Quantification of C1q expression in primary microglia (mean integrated density of C1q: PtenWT/WT = 1.3 ± 1.4; H = 19.45; PtenWT/m3m4 = 2.8 ± 0.56; H = 19.45, p value = 0.0008; Ptenm3m4/m3m4 = 24.8 ± 9.5; H = 19.45, p value = 0.0002). c Immunofluorescent co-staining for C1q (red) and Tuj1 (green) in neuron-microglia co-cultures. d Quantification of C1q foci on neuronal somas (mean C1q foci number: Ptenm3m4/m3m4 neurons with PtenWT/WT microglia = 30.1 ± 6.0; H = 23.64, p value = 0.01; Ptenm3m4 neurons with Ptenm3m4/m3m4 microglia = 38.4 ± 13.6; H = 23.64, p value = 0.02; PtenWT/WT neuron with PtenWT/WT microglia = 11.6 ± 3.9; PtenWT/WT neuron with Ptenm3m4/m3m4 microglia = 13.2 ± 3.0).
Ptenm3m4/m3m4 mice have reduced expression of synaptic markers and increased microglia activation concurrent with decreased Pten expression in the forebrain
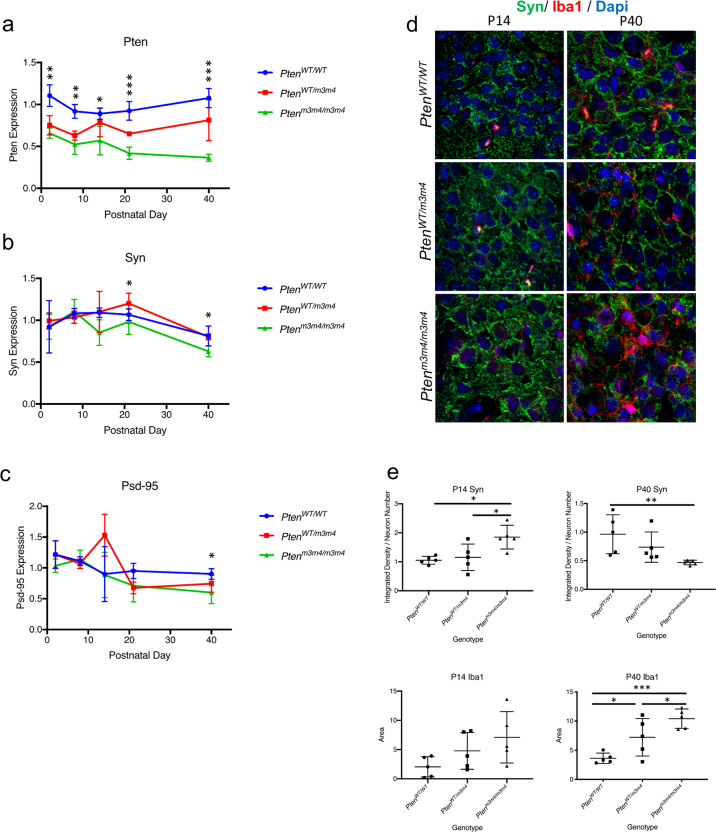
The in vitro data presented above show that Ptenm3m4/m3m4 microglia have enhanced phagocytosis and synaptic pruning capacity with increased C1q expression. These findings were further validated by our Ptenm3m4/m3m4 murine model characterized by a systemic cytoplasmic predominant Pten. Western blot analyses showed that Pten expression is significantly reduced in the hemibrains of Ptenm3m4/m3m4 mice compared with those of PtenWT/WT controls at P2 (relative expression = 0.63, p value = 0.002) and P8 (relative expression = 0.52, p value = 0.002) (Fig. 4a–c) and later ages (Supplementary Fig. 1a–e). As the Ptenm3m4/m3m4 mouse continues to age, Pten expression progressively declines in the cortex until reaching its nadir at P40 (relative expression = 0.36, p value = 0.001) (Fig. 4a). Interestingly, it is also at this time point, the terminal age for this mouse model, we observe a significant decrease in expression of Syn (relative expression = 0.62, p value = 0.02) and Psd-95 (relative expression = 0.60, p value = 0.01) (Fig. 4a–c). This is concordant with increased expression of C1q (relative expression = 4.6, p value = 0.001) (Fig. 5g) in the Ptenm3m4/m3m4 cortex. In order to determine if a correlation existed between the trends in Pten, Syn, Psd95, and C1q expression over time, we performed a Spearman rho R correlation matrix. We find there to be a correlative expression between Pten and Psd95 (r = 0.90), as well as inverse correlative expression between Pten and C1q (r = 0.70), C1q and Syn (r = 0.60), and C1q and Psd95 (r = 0.90) (Supplementary Fig. 2a, b). In addition, we find that only at P40 that a strong correlation exists between decreased expression of Pten and decreased expression of the synaptic marker Syn (r = 0.94, p value = 0.01).
Fig. 4. Dysregulated Pten expression in forebrains of Ptenm3m4/m3m4 mice correlates with decreases in synaptic marker expression and increased microglial activation in vivo.
a Expression of Pten in the forebrain of PtenWT/WT, PtenWT/m3m4, and Ptenm3m4/m3m4 mice quantified by western blot analyses at P2, P8, P14, P21, and P40; P2 (relative expression = 0.63; H = 11.92, p value = 0.002), P8 (relative expression = 0.52; H = 11.70, p value = 0.002), P14 (relative expression = 0.69; H = 7.98, p value = 0.01), P21 (relative expression = 0.41; H = 14.24, p value = 0.0005), P40 (relative expression = 0.36; H = 12.44, p value = 0.001). b Expression of Syn in the forebrain of PtenWT/WT, PtenWT/m3m4, and Ptenm3m4/m3m4 mice quantified by western blot analyses at P2, P8, P14, P21, and P40; P40 (relative expression = 0.62; H = 10.43, p value = 0.02). c Expression of Psd-95 in the forebrain of PtenWT/WT, PtenWT/m3m4, and Ptenm3m4/m3m4 mice quantified by western blot analyses at P2, P8, P14, P21, and P40; P40 (relative expression = 0.60; H = 8.6, p value = 0.01). d Immunofluorescent staining for Syn (green) and Iba1 (red) in PtenWT/WT, PtenWT/m3m4, and Ptenm3m4/m3m4 CA3 hippocampal regions at P14 and P40. e Quantification of Syn in CA3 region of the hippocampus at P14 (mean integrated density of Syn: PtenWT/WT = 1.06 ± 0.13; PtenWT/m3m4 = 1.15 ± 0.46; F = 7.17, p value = 0.02; Ptenm3m4/m3m4 = 1.85 ± 0.41; F = 7.17, p value = 0.01) and P40 (mean integrated density Syn: PtenWT/WT = 0.96 ± 0.33; Ptenm3m4/m3m = 0.47 ± 0.04; F = 4.9, p value = 0.03), as well as microglia cell area P40 (mean Iba1 area: PtenWT/WT = 3.62 ± 0.89; Ptenm3m4/m3m4 = 10.41 ± 1.70; F = 12.5, p value = 0.0009).
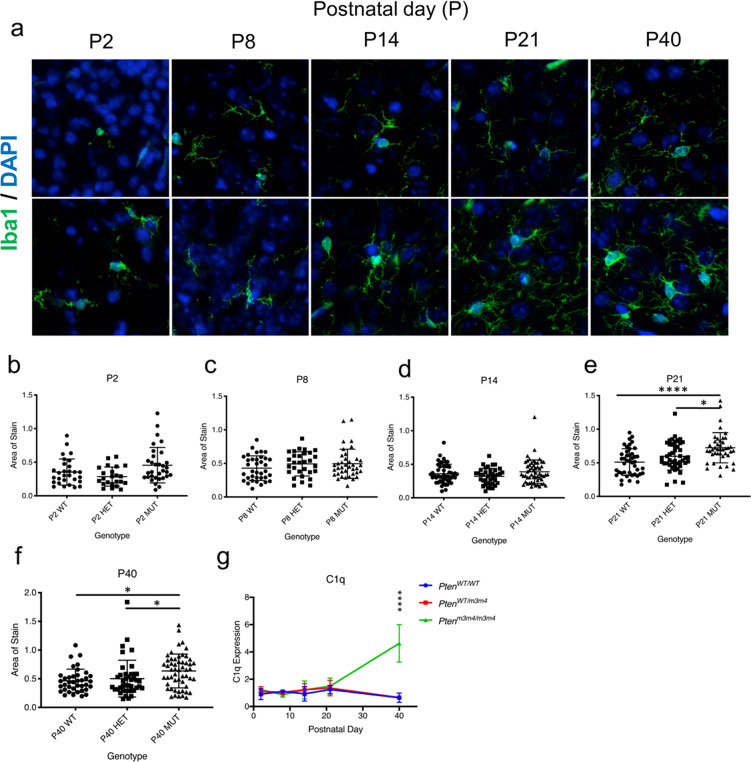
Fig. 5. Increased microglial activation and C1q expression in cortex of Ptenm3m4/m3m4 mice.
a Immunofluorescence staining of Iba1 (green) to examine cell morphology in microglial cultures derived from PtenWT/WT, PtenWT/m3m4, and Ptenm3m/m3m4 cortex tissue developmental stages P2, P8, P14, P21, and P40. b–f Quantification of Iba1 stain area in PtenWT/WT, PtenWT/m3m4, and Ptenm3m/m3m4 cortical microglia at P2, P8, P14, P21, and P40. g Expression of C1q in the forebrain of PtenWT/WT, PtenWT/m3m4, and Ptenm3m4/m3m4 mice quantified by western blot analyses at P2, P8, P14, P21, and P40, (P40 Ptenm3m4/m3m4 relative expression = 4.6 ± 1.4; H = 11.01, p value = 0.009).
In addition, in Ptenm3m4/m3m4 hippocampus, we observe decreases in Syn and Pten expression at the P40 time point relative to PtenWT/WT controls by Western analysis (Supplementary Fig. 6a, b, c, e). Syn expression on the surface of hippocampal CA3 neurons of PtenWT/m3m4 and Ptenm3m4/m3m4 mice actually showed a significant increase in expression by IF analyses during early development at age P14 (Mean Syn: PtenWT/WT = 1.05 ± 0.13 vs. Ptenm3m4/m3m4 = 1.85 ± 0.41, p = 0.01 (Fig. 4d, e). However, by P40 Syn expression in the CA3 neurons is significantly decreases in Ptenm3m4/m3m4 mice compared with their wild type littermates (Mean Syn: PtenWT/WT = 0.96 ± 0.33; Ptenm3m4/m3m = 0.47 ± 0.04, p value = 0.03) (Fig. 4d, e). To further investigate the increased synapse number in hippocampus at P14 followed by significantly decreased expression at P40, we looked at the presence and activation status of the microglial pruning machinery in the hippocampus. We found that at P14, there is no significant increase in activated microglia (Fig. 4d, e). In contrast at P40, Ptenm3m4/m3m4 mice showed a significant increase in microglia activation compared with PtenWT/WT mice as estimated by activated morphological changes and increased cell area at P40 (Iba1 area: PtenWT/WT = 3.62 ± 0.89 vs. Ptenm3m4/m3m4 = 10.41 ± 1.70, p value = 0.001) (Fig. 4d, e), as well as by Iba1 expression levels (relative expression: 1.90 ± 0.44; F = 0.7, p value = 0.002) (Supplementary Fig. 6d). In addition, C1q expression was also found to be significantly increased in Ptenm3m4/m3m4 mice compared with PtenWT/WT at P40 both in the hippocampus (relative expression: 1.78, p value = 0.004) (Supplementary Fig. 6d). Western blot analysis showed no statistically significant increase in C1q expression during earlier developmental time points (Fig. 5g, Supplementary Fig. 1a–e).
These data suggest a correlation in vivo between activation of microglia, increased expression of C1q and decrease in synaptic proteins in the brain, but it is only when the Ptenm3m4/m3m4 mouse reaches P40, near its terminal age and coincidentally the nadir of Pten expression do we observe the largest effect.
Ptenm3m4/m3m4 microglia become activation at P21 in mouse cortex
To understand the role of microglial activation and its temporal effect on synapse numbers in the brain, we analyzed microglial activation across development using IF. No significant differences in microglial cell area were observed at P2, P8, or P14 in PtenWT/m3m4 or Ptenm3m4/m3m4 mouse cortex compared with wild-type cortex (Fig. 5a–f). Although not statistically significant, several “bushy” microglia were observed at P14. Furthermore, by P21, PtenWT/m3m4 and Ptenm3m4/m3m4 mice showed significantly increased microglial activation compared with that of PtenWT/WT (mean cell area: PtenWT/WT = 0.51 ± 0.20; PtenWT/m3m4 = 0.59 ± 0.21; H = 18.15, p value = 0.04; Ptenm3m4/m3m4 = 0.72 ± 0.22, p value < 0.0001) (Fig. 5e, f). This difference between mutant and wildtype mice was also evident by P40 (mean area: PtenWT/WT = 0.46 ± 0.20, H = 10.5; Ptenm3m4/m3m4 = 0.64 ± 0.29; H = 10.5, p value = 0.01) (Fig. 5e, f).
Collectively, our in vivo data suggest that dysregulated Pten expression is correlated with changes in microglial activation status and a subsequent decrease in number of synapses at later ages. Although the Ptenm3m4 mouse is predisposed to increased synapses consistent with observations in neuron-specific Pten mutant models, the decrease in synapses begins to take place by P21, a correlated time point when Pten levels plunge below 50% of wild-type mice (Fig. 4a). Incidentally, synaptic pruning has been shown to peak in mice also at P21 [24]. We show that dysregulated feedback cues between microglia and neurons involving C1q expression may play some role in the exaggerated decrease in synapses in Ptenm3m4 mutants.
Discussion
This study demonstrates that germline Pten mutation that disrupts Pten nuclear localization results in changes in microglial morphology, activation, and function. Using IPA, we re-analyzed the published Ptenm3m4/m3m4 neural transcriptome [13], finding enriched networks related to neuroinflammation and synaptic transmission (Fig. 1a, b). We were able to bring these pathway-based predictions to in vitro and in vivo validation. Initially, we utilized a series of co-culture experiments with microglia and neurons from PtenWT/WT and Ptenm3m4/m3m4 genotypes. Our results demonstrate that cytoplasmic-predominant Pten in Ptenm3m4/m3m microglia leads to a greater propensity to prune synapses (Fig. 2), enhanced phagocytic capacity, and increased expression of C1q (Fig. 3). We next confirmed these in vitro findings using the Ptenm3m4/m3m mouse, showing decreased expression of synaptic proteins and increased microglia activity in the cortex and hippocampus (Fig. 4 and Supplementary Fig. 6d). This study is the first to demonstrate a function for Pten in regulating not only microglial activation but also synaptic pruning.
In this study, we demonstrate the first microglial pathology subsequent to a germline Pten mutation arising from cell-autonomous mechanisms. Other Pten models have observed similar activated microglial morphologies but fail to pursue any inquiry into Pten function in microglia, making them the last neural cell type requiring Pten function to be characterized [25, 26]. Here, utilizing in vitro culture techniques we were careful to separate the Ptenm3m4 microglia from neuronal and glial influences and still demonstrate a microglial pathology (Figs. 2 and 3). Although there is a vast body of literature demonstrating that microglial-dependent synaptopathies are associated with ASD-like behaviors in mice [24], this study does not demonstrate a direct cause–and-effect relationship between our reported microglia pathology and the ASD-like characteristics of the Ptenm3m4 model [4, 12, 13]. These observations are purely associative, and it is unknown if the mutant microglia have an impact on neuronal function and the ASD-like phenotypes reported in our model.
Although this study of the effect of Pten disruption in microglia is unique, there have been other studies on the effect of Pten disruption in myeloid-derived cells, the cellular precursors of microglia. These studies partially corroborate our findings. For instance, when Pten is knocked out in myeloid-derived cells (MDCs), they show increased PI3K signaling, elevated G-CSF secretion, and enhanced proliferation [27]. In addition, Pten knockout in MDCs increases phagocytosis of apoptotic cells and differences in cytokine production [28].
Interestingly, there are also some parallels between the microglial phenotypes in the Ptenm3m4 model and the microglial phenotypes associated with neurodegenerative disorders, such as Alzheimer’s disease [29, 30]. The activation of microglia and increased synaptic pruning are a feature of many models of neurodegeneration. Collectively, these data suggest that Pten functions as a negative regulator of microglial activity in a cell-autonomous fashion. This is manifested most clearly in our in vitro studies of microglia morphology, Iba1 expression, and phagocytosis activity. Additional studies will be required to elucidate the undoubtedly numerous pathways by which Pten disruption contributes to microglial pathology.
In addition, we observed increases in the expression of molecules related to complement and innate immune function: C1qa-c, C3ar1, Cx3cr1, Itgam, Itgb2, and Trem2. This finding is often reported both in ASD and neurodegenerative models with activated microglia [24, 30, 31] (Table 1). The overexpression of C1q is the most telling of these molecules given its status as a critical mediator of synaptic pruning (Fig. 3a, b). This study is the first reported correlation between Pten and C1q. Thus, our work suggests Pten participates in the regulation of synaptic pruning via complement signaling. Another study associated Pten disruption in an MKPOSE model to changes in complement expression, but the genetic complexity of that model previously obfuscated what is now a clear molecular connection [32]. Future studies will be important to dissect out how Pten function regulates C1q expression and determining if there are subsequent physiological consequences relevant to synaptic transmission, neuronal plasticity, and autism behavior.
Here, we show the synaptic pruning by Ptenm3m4/m3m4 microglia is due both to cell-autonomous and non-cell-autonomous mechanisms as outlined by our current working model (Supplementary Fig. 7a–c). It is evident that there is an additive effect on pruning from the combination of Ptenm3m4/m3m4 neurons with Ptenm3m4/m3m4 microglia, illustrated by both the increase in phagocytosis and the increase in C1q foci in these co-cultures (Figs. 2g, and 3c). It is important to note that although we observe decreased nuclear Pten in the brain of the Ptenm3m4 mouse, we also observe decreased cytoplasmic Pten [12]. We believe this to be, in part, due to decreased Pten stability, as well as increased proteasomal degradation as a result of the inability of Pten to evade cytoplasmic ubiquitination. In light of this, it is important to note that both decreased stability and nuclear localization have been observed in a subset of human mutations involving Pten from ASD patients [33, 34]. Therefore, the Ptenm3m4 mouse is currently the best in vivo mouse model for this type of mutation.
In addition, further work is required to dissect whether the enhanced pruning is because of the increase in dendritic branching (i.e., more “weak” synaptic connections) in the Ptenm3m4/m3m4 neurons or because of an unknown “eat me” signal [14, 15]. As discussed earlier, we observed that Ptenm3m4/m3m4 neurons cultured in vitro have more synapses compared with PtenWT/WT and PtenWT/m3m4 (Supplementary Fig. 4a, b). These data are supported by the work of others who have shown Pten deletion in neurons results in increased synaptic spine density and dendritic arborization [35–37].
Interestingly, in vivo, we observe a significant decrease in Syn expression at P21, and by P40, this effect is even greater with significantly decreased Psd-95 expression (Fig. 4b, c). While in vitro, we see significant decreases in synapses at DIV 14 when co-cultured with microglia for 7 days. Perhaps these subtle differences in the timing of decreased synapses may be due to the microenvironment of our neuron-microglia co-culture system, which may not be as complex as the in vivo context [38, 39]. These difference in the timing of synaptic loss may also be partially due to a lack of the milieu of exogenous cellular factors, which also must undergo temporal changes, that would only be present in vivo. The literature has demonstrated that synaptic pruning peaks in mice at P21 [15], when we first see significantly decreased Syn expression and increased microglia activation in the Ptenm3m4/m3m4 model (Figs. 4b and 5a, e). We would like to note, that although there is not a significant change in microglia morphology in the Ptenm3m4/m3m4 until P21, we did observe some microglia activated in the cortex at P14, which is concordant with synaptic pruning in our DIV 14 neuron-microglia co-cultures (Fig. 5a). Despite the primary neuron cultures only having 7 days to incubate with microglia, they show signs of significant synaptic pruning already, suggesting that pruning occurs earlier in vitro than in vivo. This could be explained by one of two non-mutually exclusive contexts: (1) priming of primary microglia due to culture conditions prior to isolation and incubation with primary neurons; or (2) exogenous regulators (other cells and their signals) that are lacking in the in vitro system. The direct mechanism of how synaptic pruning in microglia is initiated by P21 and subsequently repressed remains unclear, however our data suggest that Pten signaling may be a key regulator of this process.
Another avenue to consider in this study and moving forward is that the Ptenm3m4 model is not a conventional “knockout”, rather it is a “knock-in” germline mutant. This is an innovative aspect to our study since the germline model more accurately recapitulates the systemic effects of Pten dysfunction observed in our PTEN germline mutation-positive patients with ASD. It would be interesting to investigate other human-PTEN-ASD mimicking mutant mouse models with respect to Pten structure, function, subcellular localization, and overall phenotype, including the effects on neurons, astrocytes, oligodendrocytes, and microglia. Perhaps some mutations would present in a “spectrum” of minor to severe phenotypes as described in clinical cases of ASD with respect to behavior, and perhaps even to cellular severity of the phenotype.
This study identifies a new role for Pten in influencing microglia activity, especially in the context of synaptic pruning, a normal neurodevelopmental process that supports a healthy synaptic architecture. Although this study is limited in that it cannot cleanly distinguish between the effects of cell-autonomous and exogenous factors on synaptic pruning, it identifies an important role for Pten in microglia, where Pten acts to guard against neuroinflammation, preventing aberrant activation and enhanced phagocytosis. Moreover, this observed microglia pathology may be relevant to neurodevelopmental and synaptopathic phenotypes such as autism.
Supplementary information
Acknowledgements
We thank Drs Anthony Wynshaw-Boris and Hua Lou for helpful discussions. The authors would like to also thank Qi Yu for technical assistance with animal husbandry and breeding. We are grateful to Crystal Cruz for illustrating Supplementary Fig. 7. This study was supported, in part, by the Ambrose Monell Foundation. CE is the Sondra J. and Stephen R. Hardis Endowed Chair of Cancer Genomic Medicine at the Cleveland Clinic, and is an ACS Clinical Research Professor.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version of this article (10.1038/s41380-020-0681-0) contains supplementary material, which is available to authorized users.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. Washington, D.C.: American Psychiatric Association; 2013. xliv, 947 p.
- 2.Butler MG, Dasouki MJ, Zhou XP, Talebizadeh Z, Brown M, Takahashi TN, et al. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. J Med Genet. 2005;42:318–21. doi: 10.1136/jmg.2004.024646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gabrielli AP, Manzardo AM, Butler MG. GeneAnalytics pathways and profiling of shared autism and cancer genes. Int J Mol Sci. 2019;20:E1166. doi: 10.3390/ijms20051166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tilot AK, Frazier TW, 2nd, Eng C. Balancing proliferation and connectivity in PTEN-associated autism spectrum disorder. Neurotherapeutics. 2015;12:609–19. doi: 10.1007/s13311-015-0356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tan MH, Mester J, Peterson C, Yang Y, Chen JL, Rybicki LA, et al. A clinical scoring system for selection of patients for PTEN mutation testing is proposed on the basis of a prospective study of 3042 probands. Am J Hum Genet. 2011;88:42–56. doi: 10.1016/j.ajhg.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tan MH, Mester JL, Ngeow J, Rybicki LA, Orloff MS, Eng C. Lifetime cancer risks in individuals with germline PTEN mutations. Clin Cancer Res. 2012;18:400–7. doi: 10.1158/1078-0432.CCR-11-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yehia L, Eng C. 65 YEARS OF THE DOUBLE HELIX: one gene, many endocrine and metabolic syndromes: PTEN-opathies and precision medicine. Endocr Relat Cancer. 2018;25:T121–40. doi: 10.1530/ERC-18-0162. [DOI] [PubMed] [Google Scholar]
- 8.Orrico A, Galli L, Buoni S, Orsi A, Vonella G, Sorrentino V. Novel PTEN mutations in neurodevelopmental disorders and macrocephaly. Clin Genet. 2009;75:195–8. doi: 10.1111/j.1399-0004.2008.01074.x. [DOI] [PubMed] [Google Scholar]
- 9.Varga EA, Pastore M, Prior T, Herman GE, McBride KL. The prevalence of PTEN mutations in a clinical pediatric cohort with autism spectrum disorders, developmental delay, and macrocephaly. Genet Med. 2009;11:111–7. doi: 10.1097/GIM.0b013e31818fd762. [DOI] [PubMed] [Google Scholar]
- 10.Klein S, Sharifi-Hannauer P, Martinez-Agosto JA. Macrocephaly as a clinical indicator of genetic subtypes in autism. Autism Res. 2013;6:51–6. doi: 10.1002/aur.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chung JH, Eng C. Nuclear-cytoplasmic partitioning of phosphatase and tensin homologue deleted on chromosome 10 (PTEN) differentially regulates the cell cycle and apoptosis. Cancer Res. 2005;65:8096–100. doi: 10.1158/0008-5472.CAN-05-1888. [DOI] [PubMed] [Google Scholar]
- 12.Tilot AK, Gaugler MK, Yu Q, Romigh T, Yu W, Miller RH, et al. Germline disruption of Pten localization causes enhanced sex-dependent social motivation and increased glial production. Hum Mol Genet. 2014;23:3212–27. doi: 10.1093/hmg/ddu031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tilot AK, Bebek G, Niazi F, Altemus JB, Romigh T, Frazier TW, et al. Neural transcriptome of constitutional Pten dysfunction in mice and its relevance to human idiopathic autism spectrum disorder. Mol Psychiatry. 2016;21:118–25. doi: 10.1038/mp.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salter MW, Stevens B. Microglia emerge as central players in brain disease. Nat Med. 2017;23:1018–27. doi: 10.1038/nm.4397. [DOI] [PubMed] [Google Scholar]
- 15.Tay TL, Savage JC, Hui CW, Bisht K, Tremblay ME. Microglia across the lifespan: from origin to function in brain development, plasticity and cognition. J Physiol. 2017;595:1929–45. doi: 10.1113/JP272134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corbett BA, Kantor AB, Schulman H, Walker WL, Lit L, Ashwood P, et al. A proteomic study of serum from children with autism showing differential expression of apolipoproteins and complement proteins. Mol Psychiatry. 2007;12:292–306. doi: 10.1038/sj.mp.4001943. [DOI] [PubMed] [Google Scholar]
- 17.Chu Y, Jin X, Parada I, Pesic A, Stevens B, Barres B, et al. Enhanced synaptic connectivity and epilepsy in C1q knockout mice. Proc Natl Acad Sci USA. 2010;107:7975. doi: 10.1073/pnas.0913449107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bailey A, Le Couteur A, Gottesman I, Bolton P, Simonoff E, Yuzda E, et al. Autism as a strongly genetic disorder: evidence from a British twin study. Psychol Med. 1995;25:63–77. doi: 10.1017/S0033291700028099. [DOI] [PubMed] [Google Scholar]
- 19.Fonseca MI, Chu SH, Hernandez MX, Fang MJ, Modarresi L, Selvan P, et al. Cell-specific deletion of C1qa identifies microglia as the dominant source of C1q in mouse brain. J Neuroinflammation. 2017;14:48. [DOI] [PMC free article] [PubMed]
- 20.Gandal MJ, Haney JR, Parikshak NN, Leppa V, Ramaswami G, Hartl C, et al. Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap. Science. 2018;359:693–7. doi: 10.1126/science.aad6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harry GJ, Kraft AD. Microglia in the developing brain: a potential target with lifetime effects. Neurotoxicology. 2012;33:191–206. doi: 10.1016/j.neuro.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee H, Thacker S, Sarn N, Dutta R, Eng C. Constitutional mislocalization of Pten drives precocious maturation in oligodendrocytes and aberrant myelination in model of autism spectrum disorder. Transl Psychiatry. 2019;9:13. doi: 10.1038/s41398-018-0364-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ho J, Tumkaya T, Aryal S, Choi H, Claridge-Chang A. Moving beyond P values: data analysis with estimation graphics. Nat Methods. 2019;16:565–6. doi: 10.1038/s41592-019-0470-3. [DOI] [PubMed] [Google Scholar]
- 24.Reemst K, Noctor SC, Lucassen PJ, Hol EM. The Indispensable Roles of Microglia and Astrocytes during Brain Development. Front Hum Neurosci. 2016;10:566. doi: 10.3389/fnhum.2016.00566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y, Huang WC, Sejourne J, Clipperton-Allen AE, Page DT. Pten mutations alter brain growth trajectory and allocation of cell types through elevated beta-catenin signaling. J Neurosci. 2015;35:10252–67. doi: 10.1523/JNEUROSCI.5272-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goebbels S, Wieser GL, Pieper A, Spitzer S, Weege B, Yan K, et al. A neuronal PI(3,4,5)P3-dependent program of oligodendrocyte precursor recruitment and myelination. Nat Neurosci. 2017;20:10–5. doi: 10.1038/nn.4425. [DOI] [PubMed] [Google Scholar]
- 27.Tesio M, Oser GM, Baccelli I, Blanco-Bose W, Wu H, Gothert JR, et al. Pten loss in the bone marrow leads to G-CSF-mediated HSC mobilization. J Exp Med. 2013;210:2337–49. doi: 10.1084/jem.20122768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mondal S, Ghosh-Roy S, Loison F, Li Y, Jia Y, Harris C, et al. PTEN negatively regulates engulfment of apoptotic cells by modulating activation of Rac GTPase. J Immunol. 2011;187:5783–94. doi: 10.4049/jimmunol.1100484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perry VH, O’Connor V. The role of microglia in synaptic stripping and synaptic degeneration: a revised perspective. ASN Neuro. 2010;2:e00047. doi: 10.1042/AN20100024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schafer DP, Lehrman EK, Kautzman AG, Koyama R, Mardinly AR, Yamasaki R, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Filipello F, Morini R, Corradini I, Zerbi V, Canzi A, Michalski B, et al. The microglial innate immune receptor TREM2 is required for synapse elimination and normal brain connectivity. Immunity. 2018;48:979–91 e8. doi: 10.1016/j.immuni.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 32.Suryawanshi S, Huang X, Elishaev E, Budiu RA, Zhang L, Kim S, et al. Complement pathway is frequently altered in endometriosis and endometriosis-associated ovarian cancer. Clin Cancer Res. 2014;20:6163–74. doi: 10.1158/1078-0432.CCR-14-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fricano-Kugler CJ, Getz SA, Williams MR, Zurawel AA, DeSpenza T, Jr, Frazel PW, et al. Nuclear excluded autism-associated phosphatase and tensin homolog mutations dysregulate neuronal growth. Biol Psychiatry. 2018;84:265–77. doi: 10.1016/j.biopsych.2017.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mighell TL, Evans-Dutson S, O’Roak BJ. A saturation mutagenesis approach to understanding PTEN lipid phosphatase activity and genotype-phenotype relationships. Am J Hum Genet. 2018;102:943–55. doi: 10.1016/j.ajhg.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kazdoba TM, Sunnen CN, Crowell B, Lee GH, Anderson AE, D’Arcangelo G. Development and characterization of NEX- Pten, a novel forebrain excitatory neuron-specific knockout mouse. Dev Neurosci. 2012;34:198–209. doi: 10.1159/000337229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwon CH, Luikart BW, Powell CM, Zhou J, Matheny SA, Zhang W, et al. Pten regulates neuronal arborization and social interaction in mice. Neuron. 2006;50:377–88. doi: 10.1016/j.neuron.2006.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cupolillo D, Hoxha E, Faralli A, De Luca A, Rossi F, Tempia F, et al. Autistic-like traits and cerebellar dysfunction in Purkinje cell PTEN knock-out mice. Neuropsychopharmacology. 2016;41:1457–66. doi: 10.1038/npp.2015.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fraser MM, Zhu X, Kwon CH, Uhlmann EJ, Gutmann DH, Baker SJ. Pten loss causes hypertrophy and increased proliferation of astrocytes in vivo. Cancer Res. 2004;64:7773–9. doi: 10.1158/0008-5472.CAN-04-2487. [DOI] [PubMed] [Google Scholar]
- 39.Harrington EP, Zhao C, Fancy SP, Kaing S, Franklin RJ, Rowitch DH. Oligodendrocyte PTEN is required for myelin and axonal integrity, not remyelination. Ann Neurol. 2010;68:703–16. doi: 10.1002/ana.22090. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.