Abstract
Adipose tissue is composed of diverse cell types and plays a major role in energy homeostasis and inflammation at the local and systemic levels. Adipose tissue serves as the main site for vitamin D storage and is among the most important extraskeletal targets of vitamin D which can modulate multiple aspects of adipose tissue biology. Vitamin D may exert inhibitory or stimulatory effects on adipocyte differentiation depending on cell type, stage of differentiation, and the treatment time point. Moreover, vitamin D controls energy metabolism in adipose tissue by affecting fatty acid oxidation, expression of uncoupling proteins, insulin resistance, and adipokine production. Adipose tissue inflammation can have a significant impact on the metabolic disorders often associated with obesity, and vitamin D can modulate the inflammatory response of immune cells and adipocytes within the adipose tissue. This review discusses the role of adipose tissue in vitamin D metabolism, as well as the regulatory role of vitamin D in adipocyte differentiation, adipose tissue energy metabolism, and inflammation, thereby providing insights into the importance of vitamin D in adipose tissue biology.
Keywords: Vitamin D, Adipose tissue, Metabolism, Inflammation
INTRODUCTION
Adipose tissue is composed of various cell types including adipocytes (preadipocytes and mature adipocytes), immune cells (including macrophages, natural killer [NK] cells, and T cells), fibroblasts, smooth muscle cells, and endothelial cells.1 Adipose tissue plays an important role as an energy reservoir, a modulator of energy homeostasis, and an endocrine organ. Therefore, adipose tissue is no longer considered an inert storage organ, and is instead now recognized as a critical player in whole-body energy metabolism and inflammation, with significant implications for chronic diseases.
The role of vitamin D in calcium homeostasis and bone health is widely acknowledged. However, the extraskeletal actions of vitamin D emerged as a topic of interest due to its reported impact on cancer, cardiovascular diseases, diabetes, obesity, and immune and inflammatory responses. Adipose tissue is one of the major extraskeletal targets of vitamin D. The expression of the vitamin D receptor (VDR) gene and vitamin D–metabolizing enzymes in adipocytes and immune cells suggests that vitamin D can have a significant impact on adipose tissue biology. VDR gene expression has been reported in white and brown adipose tissue in mice and 3T3-L1 adipocytes.2
This review focuses on the role of adipose tissue in vitamin D metabolism and the role of vitamin D in adipocyte differentiation, energy metabolism in adipose tissue, and inflammation in adipose tissue, thereby providing insights into the importance of vitamin D in adipose tissue biology.
VITAMIN D STORAGE AND METABOLISM IN ADIPOSE TISSUE
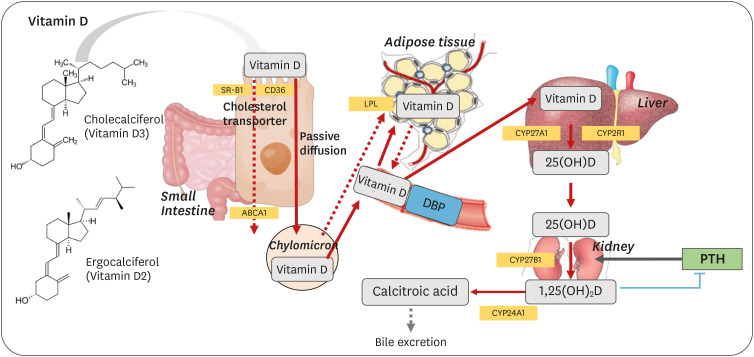
Vitamin D is a fat-soluble vitamin that can be either obtained from the diet or synthesized from 7-dehydrocholesterol in the skin via exposure to ultraviolet (UV)-B light.3 Dietary vitamin D is absorbed in the upper small intestine with other dietary lipids through passive diffusion.4,5,6,7 However, recent studies have demonstrated that cholesterol transporters such as scavenger receptor class B type I (SR-BI), cluster of differentiation 36 (CD36), and Niemann–Pick C1-Like 1 (NPC1-L1) are also involved in vitamin D transport across the enterocyte.8,9,10 The absorbed vitamin D is incorporated into chylomicrons and secreted into the lymph.11,12 When adipose tissue and skeletal muscle uptake chylomicron lipids, a fraction of vitamin D is taken up by these tissues as well; however, the specific mechanism by which vitamin D is stored in adipose tissue has not been elucidated. Moreover, few studies have directly quantified vitamin D levels in adipose tissue.13 Most early studies identified adipose tissue as a major vitamin D storage organ by indirectly measuring the radioactivity of radiolabeled vitamin D3 in adipose tissue.14,15,16 When rats were given 5 µg of vitamin D3 per day for 12 days, the tissue with the highest concentration of vitamin D was the kidney, followed by adipose tissue, blood, and the liver. After deprivation of vitamin D, radioactivity disappeared rapidly in the first 3 weeks in most tissues (blood, liver, and kidney). In contrast, the radioactivity of the adipose tissue did not decrease nearly as substantially after 80 days.17 Heaney et al.18 reported the tissue distribution of vitamin D based on previous studies that measured vitamin D levels in human tissues, as well as high-performance liquid chromatography measurements in pigs fed with approximately 2,000 IU of vitamin D per day. Despite the limitations of quantifying vitamin D levels in tissues from different species, vitamin D has been found to be predominantly stored in adipose tissue as cholecalciferol (vitamin D3), but has also been found in muscle and liver tissues. Using liquid chromatography-mass spectrometry (LC-MS), Piccolo et al.19 demonstrated that total body fat loss (13%) after 12 weeks of caloric restriction did not significantly affect 25-hydroxyvitamin D (25[OH]D) concentrations in human subcutaneous fat and serum; therefore, the authors suggested increasing dietary vitamin D intake or vitamin D synthesis from the skin to improve vitamin D levels instead of weight loss. After long-term supplementation with a high dose of vitamin D3 (20,000 IU/week) for 3–5 years, the median vitamin D3 concentration in the abdominal subcutaneous tissue was more than six times higher in the supplemented group than in the placebo group (209 vs. 32 ng/g tissue).20 Simultaneously, 25(OH)D3 concentrations in serum and fat showed a modest but significant difference between the supplemented and placebo groups. The median concentrations of serum 25(OH)D3 and fat 25(OH)D3 were 99 nmol/L and 3.8 ng/g tissue in the supplemented group and 62 nmol/L and 2.5 ng/g tissue in the placebo group, respectively. Therefore, subcutaneous fat appeared to store a large amount of vitamin D3. In addition to LC-MS quantification, Malmberg et al.21 implemented time of flight secondary ion mass spectrometry (TOF/SIMS) to measure vitamin D3, 25(OH)D3, and 1,25-dihydroxyvitamin D3 (1,25[OH]2D3) levels in human adipose tissue. Using this approach, the authors demonstrated that vitamin D3 and its metabolites were located in adipocyte lipid droplets.
The storage of vitamin D in adipose tissue is considered as one of the main factors explaining the association between vitamin D deficiency and obesity. Obese subjects exhibited vitamin D insufficiencies (serum 25[OH]D concentrations between 20–30 ng/mL) and deficiencies (serum 25[OH]D concentrations below 20 ng/mL), and serum 25(OH)D levels showed negative correlations with body mass index and body fat mass.22,23,24 According to the results of a meta-analysis,25 the prevalence of vitamin D deficiency was 35% and 24% higher in obese and overweight persons, respectively, than in normal-weight subjects. When obese subjects were exposed to UV-B light or supplemented with 50,000 IU vitamin D, the increase in serum 25(OH)D levels was lower than that of the normal-weight group. Therefore, it was suggested that vitamin D sequestration in body fat might decrease its bioavailability.26 In contrast, Drincic et al.24 proposed “volumetric dilution” as the reason for the low serum 25(OH)D concentration in obese persons, meaning that the distribution of cholecalciferol in serum and body fat depends on body size. An inverse association between serum 25(OH)D levels and body weight was explained via a hyperbolic model, and not by quantifying tissue vitamin D levels. In fact, only a few studies have directly quantified vitamin D levels in adipose tissue to evaluate differences between obese and non-obese patients.27,28 The total amount of cholecalciferol (ng) in the adipose tissue of obese humans and mice was greater than that of normal-weight controls; however, vitamin D concentrations (ng/g of fat tissue) did not vary significantly. These findings support the volumetric dilution hypothesis as the primary cause of vitamin D deficiency in obese subjects, rather than the hypothesis of sequestration of vitamin D in body fat.27
Adipose tissue plays an important role as a vitamin D storage site. Moreover, 1,25(OH)2D, the bioactive form of vitamin D, is involved in adipocyte differentiation and immune responses. Although the liver and kidney are the primary tissues involved in vitamin D metabolism (e.g., 25-hydroxylation of vitamin D to 25(OH)D and 1-hydroxylation of 25(OH)D into 1,25(OH)2D), both 25-hydroxylase (CYP27A1)29 and 1-hydroxylase (CYP27B1)30 are expressed and active in 3T3 L1 pre-adipocytes, subcutaneous adipose tissue (SAT), and visceral adipose tissue (VAT) in rats and humans.31 Additionally, VDR expression has been observed in 3T3-L1 adipocytes,32 as well as in human pre-adipocytes and differentiated adipocytes.2 Therefore, vitamin D can regulate the transcription of many target genes in adipose tissue via the interactions between 1,25(OH)2D and VDRs, both through endocrine mechanisms and through autocrine and paracrine mechanisms. The absorption and activation process of dietary vitamin D is depicted in Fig. 1.
Fig. 1. Dietary vitamin D absorption activation process.
ABCA1, ATP-binding cassette transporter; CD36, cluster of differentiation 36; CYP27A1, 25-hydroxylase; CYP2R1, 25-hydroxylase; CYP27B1, 1-hydroxylase; CYP24A1, 24-hydroxylase; DBP, vitamin D binding protein; LPL, lipoprotein lipase; PTH, parathyroid hormone; SR-B1, scavenger receptor class B type 1.
MODULATION OF ADIPOCYTE DIFFERENTIATION BY VITAMIN D
Adipogenesis is a highly controlled 2-step process in which the sequential induction of transcription factors regulates the expression of adipocyte-specific markers and the expression of lipogenic genes increases fatty acid synthesis. First, fibroblast-like cells such as mesenchymal stem cells are committed to the adipocyte lineage and become pre-adipocytes. In the second step, pre-adipocytes differentiate into mature adipocytes by undergoing growth arrest, accumulating lipids, and becoming insulin-responsive.33,34 During adipogenesis, several transcriptional factors regulate and activate a sequential series of gene expression.35 This cascade begins with the early and transient expression of CCAAT/enhancer-binding protein (C/EBP) β and C/EBPδ. This leads to the induction of nuclear receptor peroxisome proliferator-activated receptor γ (PPARγ) and C/EBPα, as critical transcriptional regulators of adipogenesis, followed by triglyceride (TG) accumulation resulting from the enhanced expression of various genes associated with adipocyte phenotypes, including fatty acid binding protein (FABP4), acetyl CoA carboxylase (ACC), lipoprotein lipase (LPL), glucose transporter 4 (GLUT4), and fatty acid synthase (FASN or FAS).36 PPARγ and C/EBPα are necessary for adipogenesis, as well as for maintaining the differentiated state of adipocytes.37
Many previous studies have reported that 1,25(OH)2D, the active form of vitamin D, regulates adipocyte differentiation by binding to the nuclear VDR with high affinity. The VDR gene is expressed in 3T3-L1 cells,38 human mammary pre-adipocytes and adipocytes,2,39 and human SAT and VAT.31 VDR expression has been observed in the earliest stages of 3T3-L1 adipocyte differentiation (during the first 4 hours of insulin, dexamethasone, and 3-isobutyl-1-methylxanthine treatment).38,40 In line with these findings, when 1,25(OH)2D-VDR forms a heterodimer with retinoid X receptor (RXR), the resulting complex can regulate the expression of various genes in adipocytes by binding with the vitamin D response element. Lipid accumulation was reduced in VDR knockdown 3T3-L1 cells. Additionally, VDR knockout mice exhibited enhanced energy expenditure, reduced body fat mass, and resistance to high-fat diet (HFD)-induced obesity,41,42 suggesting that the 1,25(OH)2D-VDR signaling pathway plays a role in adipogenesis. However, contradictory results have been reported regarding the role of 1,25(OH)2D and VDR in adipocyte differentiation. Table 1 summarizes the in vitro effects of 1,25(OH)2D on adipocyte differentiation and adipogenesis. Most studies that used 3T3-L1 mouse pre-adipocytes consistently reported that 1,25(OH)2D inhibited adipocyte differentiation by reducing lipid accumulation and decreasing the expression of genes related to lipogenesis, including PPARG and CEBPA.40,43,44,45 Both primary porcine pre-adipocytes46 and human breast adipocytes39 cultured with 1,25(OH)2D3 exhibited reduced lipid accumulation or downregulation of the expression of PPARG, RXRA, and other adipogenesis-related genes. The differentiation of human mammary pre-adipocytes was inhibited both by 1,25(OH)2D3 and 25(OH)D3.39 However, several studies using human pre-adipocytes demonstrated that 1,25(OH)2D promoted the differentiation of pre-adipocytes into mature adipocytes.47,48,49,50 Nimitphong et al.47 demonstrated that 25(OH)D or 1,25(OH)2D treatment enhanced adipogenesis in human and mouse preadipocytes by increasing TG accumulation and expression of PPARG, FABP, LPL, and SREBP. In contrast, 1,25(OH)2D had the opposite effect in mouse 3T3-L1 cells. The authors concluded that the aforementioned differences between human primary preadipocytes and 3T3-L1 cell outcomes might be attributed to the differences in the stage of differentiation of each cell type. For instance, primary adipocytes, which showed increased adipogenesis after 1,25(OH)2D treatment, were at a more advanced stage of differentiation than the 3T3-L1 cells. Moreover, these different outcomes may have also been due to methodological differences related to the time-point of 1,25(OH)2D treatment and variation in VDR expression during adipocyte differentiation.6 VDR was shown to be expressed in the earliest stages of 3T3-L1 adipocyte differentiation and decreased with adipocyte maturation38; thus, exposing 3T3-L1 cells to 1,25(OH)2D3 at an early stage of differentiation is crucial for the expected inhibitory effect on adipogenesis.44,47,51 Although 1,25(OH)2D treatment decreased the expression of C/EBPβ and PPARγ in 3T3-L1 cells, it is possible that vitamin D can promote adipogenesis because CYP27B1 and VDR downregulation by 1,25(OH)2D treatment can decrease the response to vitamin D in adipocytes.52 Nonetheless, the exact mechanisms behind the different effects of vitamin D on adipogenesis must be further clarified.
Table 1. Effects of in vitro 1,25(OH)2D treatment on adipocyte differentiation and adipogenesis.
| Cell type or species | Treatment dose (duration) | Effects | References | |
|---|---|---|---|---|
| 1,25(OH)2D inhibits adipocyte differentiation | ||||
| 3T3-L1 preadipocytes | 1 µM (0–40 hours) | • Repressed up-regulated protein expression of PPARγ2 | Hida et al.43 | |
| 3T3-L1 preadipocytes | 0.01, 0.1, 1, 10, 100 nM (6, 12 hours, 1–10 days) | • Lipid accumulation and the expression of PPARγ, C/EBPα, • FABP4 and SCD-1 were inhibited | Ji et al.40 | |
| • No change in C/EBPβ and C/EBPδ gene expression levels | ||||
| 3T3-L1 preadipocytes | 0.01, 0.1, 1, 10, 100 nM (24, 28, 72 hours) | • Blocks 3T3-L1 cell differentiation into adipocytes (C/EBPα and PPARγ upregulation) in a dose-dependent manner | Kong and Li44 | |
| 3T3-L1 preadipocytes | 10 nM (1, 2, 4, 6, 12 hours, 1, 2, 4, 5 days) | • Down-regulating both C/EBPβ mRNA expression and protein levels | Blumberg et. al.51 | |
| • Up-regulation of ETO (C/EBPβ co-repressor) | ||||
| 3T3-L1 preadipocytes | 10 nM (2, 4, 6 days) | • Inhibited lipid droplet formation and the expressions of adipocyte maker protein, FABP4, PPARγ and C/EBPα | Lee et al.45 | |
| • Inhibited downregulation of WNT/β-catenin pathway | ||||
| Primary porcine preadipocytes | 0.1 nM–1 μM (2, 4, 6, 8 days) | • Suppressed the expression of PPARγ, RXR, LPL, SREBP mRNA | Zhuang et al.46 | |
| Human breast preadipocyte | 100 nM (7, 14 days) | • Reduced intracellular triglyceride on day 7 but not on day 14 | Ching et al.39 | |
| 3T3-L1 preadipocytes | • Dose and time dependent inhibition of adipogenesis | Nimitphong et al.47 | ||
| 1,25(OH)2D promotes adipocyte differentiation | ||||
| Human pre-adipocyte, primary mouse preadipocytes | 0.1, 10 nM (14 days) | • Increased the expression of PPARγ, FABP, LPL, SREBP mRNA and TG accumulation | Nimitphong et al.47 | |
| 10, 100 nM (7 days) | ||||
| Human adipose-derived mesenchymal progenitor cells | 10 nM (7, 14 days) | • Promoted lipid accumulation and enhanced the expression of FABP4, FASN, and PPARγ | Narvaez et al.48 | |
| Human preadipocyte SGBS cells | 10, 100 nM (24 hours) | • Induced VDR, CEBPα, and CEBPβ expression in the preadipocyte stage | Felicidade et al.49 | |
| • Upregulated CEBPα during adipogenesis | ||||
| Mesenchymal stem cells isolated from bone marrow of pig | 10, 100 nM (3, 6, 9, 12 days) | • Increased PPARγ, LPL, AP in dose dependent manner | Mahajan and Stahl50 | |
1,25(OH)2D, 1,25-dihydroxyvitamin D; PPAR, peroxisome proliferator-activated receptor; C/EBP, CCAAT/enhancer-binding protein; FABP, fatty acid binding protein; RXR, retinoid X receptor; LPL, lipoprotein lipase; SREBP, sterol regulatory element-binding protein; TG, triglyceride; VDR, vitamin D receptor; SGBS, Simpson-Golabi-Behmel syndrome.
REGULATION OF ENERGY HOMEOSTASIS BY VITAMIN D
Adipose tissue is among the major contributors to energy homeostasis. White adipose tissue stores excess energy as TG using fatty acids taken up from circulating TG in chylomicrons and very-low-density lipoprotein (VLDL) and/or fatty acids synthesized in the adipose tissue via de novo lipogenesis. When the body requires energy, white adipose tissue releases fatty acids to be used as an energy source by other organs, as well as glycerol for gluconeogenesis.1 Brown adipose tissue (BAT) is involved in thermogenesis. Cold exposure promotes fat uptake and stimulates the expression of uncoupling protein 1 (UCP1) in BAT, which leads to heat generation.53
The regulation of energy metabolism by adipose tissue is orchestrated by several hormones and adipokines. Insulin stimulates glucose uptake into adipose tissue through GLUT4, and GLUT4 expression regulates the expression of carbohydrate response element binding protein (ChREBP), a glucose-responsive transcription factor that regulates fatty acid synthesis and glycolysis.54 Leptin, which is mainly produced in adipose tissue, is another important hormone for energy homeostasis. Leptin signals the brain to decrease food intake, increases energy expenditure through sympathetic nerve activity, and stimulates thermogenesis by increasing UCP1 in BAT. Leptin also affects glucose and lipid metabolism by suppressing gluconeogenesis, increasing peripheral glucose uptake, inhibiting de novo lipogenesis, and stimulating lipolysis and fatty acid oxidation.55 Adiponectin, which is also produced in adipose tissue, increases glucose uptake by skeletal muscle, decreases gluconeogenesis in the liver, and stimulates fatty acid oxidation and glucose utilization in skeletal muscle and the liver.56
The importance of vitamin D in the regulation of energy metabolism is supported by previously published experiments on Vdr knockout and overexpression models.57 Vdr knockout mice exhibited a reduction in adipose tissue mass, lower serum leptin levels, and higher food intake than wild-type mice. Similar effects were observed in mice unable to generate 1,25(OH)2D due to a lack of Cyp27b1 expression.41 These results strongly imply that vitamin D is an important regulator of lipid storage in adipose tissue. Targeted expression of human VDR in mouse adipocytes resulted in higher body weight, fat mass, and leptin levels despite no differences in food intake. This increase in fat mass was mainly due to reduced fatty acid oxidation and lipolysis in the adipose tissue. Moreover, the expression of genes involved in lipolysis (e.g., Atgl and Hsl) and energy utilization (e.g., Cpt, Ucp, Hk, and Pk) was lower in human VDR transgenic mice.58
Vitamin D appears to affect energy metabolism in several different ways. Vitamin D supplementation has been reported to enhance fatty acid oxidation.59 Lower weight gain and adipose tissue mass were observed in mice fed with an HFD supplemented with vitamin D (15,000 IU/kg diet), which was due to increased energy expenditure and the preferential use of lipids as an energy source. The expression of genes involved in energy metabolism, fatty acid oxidation, and mitochondrial biogenesis—including Ppara, Pgc-1a, Pgc-1b, Cpt1b, Mcad, Lcad, and Pdk4—were upregulated in BAT and muscle upon vitamin D supplementation. However, when pregnant rats were subcutaneously injected with vitamin D3 (1 µg/kg/d), the expression of various lipogenic genes, including Fas, Scd1 and Acc1, in adipose tissue was reduced. Additionally, the regulators of lipogenic enzymes were differentially modulated by vitamin D, as Pparg and Insig2 mRNA levels were increased by vitamin D injection, but there was no difference in Srebp1c and Cebpa expression between vitamin D-treated and control rats.60 Vitamin D has been reported to affect insulin secretion and sensitivity. For instance, vitamin D supplementation improved insulin sensitivity in healthy subjects and type 2 diabetes patients.61 Vitamin D is a potential modulator of depolarization-induced insulin secretion via the regulation of intracellular Ca2+ levels in pancreatic β-cells.62 Moreover, vitamin D can modulate adipokine secretion. For example, 1,25(OH)2D stimulates adipose leptin production in a VDR-dependent manner, as it led to markedly increased mRNA expression and secretion of leptin in cultures of adipose tissues obtained from wild-type mice, but not from VDR-null mice.63 However, previous studies reported inconsistent results regarding the effect of vitamin D on adiponectin production. For example, no significant effect of 1,25(OH)2D was found on the adiponectin expression of differentiated human adipocytes.64 In contrast, when overweight/obese and vitamin D-deficient adults received vitamin D supplementation for 16 weeks, there was a greater increase in adiponectin and leptin in the vitamin D group than in the placebo group after adjustment for baseline values, season, sun exposure, and dietary vitamin D intake.65 Nonetheless, adiposity should also be considered when interpreting the effects of vitamin D on leptin and adiponectin levels in human and animal models, as obesity is often associated with vitamin D deficiency and levels of leptin and adiponectin are closely associated with fat mass.66
MODULATION OF INFLAMMATION BY VITAMIN D IN ADIPOSE TISSUE
Adipose tissue functions as an endocrine organ that secretes more than 260 adipokines.67 Obesity causes adipose tissue enlargement through hyperplasia (i.e., an increase in the number of adipocytes) and hypertrophy (i.e., an increase in adipocyte size). 68 In obese individuals, hypertrophic adipocytes exhibit necrotic cell death and local hypoxia, which induces inflammation, followed by increased secretion of monocyte chemoattractant protein (MCP)-1, interleukin (IL)-6, IL-1β, resistin, and tumor necrosis factor alpha (TNF-α).69 Moreover, dietary lipids bind to toll-like receptors (TLR2 and TLR4) on adipocytes and macrophages, thereby activating the nuclear factor (NF)-κB signaling pathway and promoting the expression of inflammatory cytokines.70 Upregulation of pro-inflammatory cytokines induces adipose tissue inflammation by recruiting monocytes/macrophages into the adipose tissue, and activating them toward the pro-inflammatory M1 type.71 Furthermore, infiltration of other immune cells, including CD8+ T cells, neutrophils, and NK cells, precedes macrophage accumulation and contributes to macrophage activation.72 In the adipose tissue of obese subjects, the secretion of pro-inflammatory cytokines, including IL-1β, IL-6, and MCP-1, is increased by both adipocytes and macrophages that reside within the adipose tissue. Macrophages account for more than 50% of immune cells in obese adipose tissue, but constitute fewer than 10% of immune cells in lean adipose tissue.73 Low-grade chronic adipose tissue inflammation is a significant risk factor for metabolic diseases including insulin resistance and type 2 diabetes.
Given that both adipocytes and macrophages express VDR and CYP27B1, 1,25(OH)2D or 25(OH)D can control inflammatory responses in adipose tissue. The anti-inflammatory effects of vitamin D on monocytes/macrophages are supported by consistent results from in vitro studies (Table 2). Most of these studies reported that 1,25(OH)2D reduced the expression and secretion of pro-inflammatory cytokines from monocytes/macrophages in a dose-dependent manner through several mechanisms. First, vitamin D modulates the expression of pro-inflammatory cytokines by regulating TLRs, innate immune pattern recognition receptors, which enable the recognition of the pathogen-associated molecular patterns (PAMPs) produced by microorganisms or damage-associated molecular patterns (DAMPs) derived from dead cells or tissue injury.81 1,25(OH)2D3 downregulates the protein and mRNA levels of TLR2 and TLR4 in human monocytes in a time- and dose-dependent manner, followed by a reduction of IL-6 and TNF-α levels.74,75 However, this downregulation of TLR2 and TLR4 by vitamin D was inhibited when a VDR antagonist was added, indicating that the immunomodulatory effect of 1,25(OH)2D on TLR is VDR-dependent.74 Tlr2 expression was also decreased by 1,25(OH)2D treatment in adipose tissue-resident immune cells.79 Second, vitamin D inhibits the NF-κB and MAPK signaling pathways, which are downstream of TLR-mediated pathways. 1,25(OH)2D inhibits NF-κB activation by blocking NF-κB/RelA nuclear translocation and upregulating the expression of IκBα, which is a potent NF-κB inhibitor.74,77,79,82,83 Additionally, 1,25(OH)2D upregulates MAPK phosphatase-1 (MKP-1), which inactivates MAPK signaling via inhibition of p38 activation. A putative VDR binding site has been identified in human and mouse MKP-1 promoters.76
Table 2. Effects of in vitro 1,25(OH)2D treatment on inflammatory responses in monocytes/macrophages.
| Cell type | Treatment dose (duration) | Effects | Reference |
|---|---|---|---|
| Human monocyte (PBMC) | 0.01–100 nM (12, 24, 48, 72 hours) | • Reduced TLR2, TLR4 protein and mRNA | Sadeghi et al.74 |
| • Inhibited NF-κB/RelA translocation | |||
| PBMC | 100 nM (24, 48, 72 hours) | • Down-regulated TLR2, TLR4, and TLR9 expression | Dickie et al.75 |
| • Decreased IL-6 production | |||
| PBMC | 0.1–10 nM (24 hours) | • Decreased IL-6 and TNF-α production | Zhang et al.76 |
| • Increased MKP-1 | |||
| Murine macrophage cells (P388D1) | 100 nM (16 hours preincubation) | • Decreased NF-κB-p65 protein levels in the nucleus | Cohen-Lahav et al.77 |
| • Increased NF-κB-p65 protein levels in the cytosol | |||
| • Increased IκBα protein levels in cytosol | |||
| Human monocytic THP-1 cells | 10 nM (24 hours) | • Decreased IL1β, IL-6, and TNF-α levels | Villaggio et al.78 |
| Mouse stromal vascular cell | 10 nM (24 hours) | • Decreased IL-6 and MCP-1 levels | Park et al.79 |
| • Increased Dusp1 and IkBa expression | |||
| PBMC | 48 hours after 1,25(OH)2D3 | • Decreased proinflammatory cytokines (TNF-α, IL-1α, IL-1β, and IL-6) production | Neve et al.80 |
| Stimulation (10−7–10−11 M) |
1,25(OH)2D, 1,25-dihydroxyvitamin D; PBMC, peripheral blood mononuclear cell; TLR, Toll-like receptor; NF, nuclear factor; IL, interleukin; TNF, tumor necrosis factor; MKP-1, MAPK phosphatase-1; MCP, monocyte chemoattractant protein.
Table 3 summarizes a selection of studies that have investigated the effects of in vitro vitamin D treatment on inflammatory responses. The in vivo effects of vitamin D supplementation on adipose tissue inflammation are listed in Table 4. Whether vitamin D decreases the secretion of pro-inflammatory cytokines by adipocytes is not known conclusively, as previous studies using 3T3-L1 and human adipocytes have reported contradictory results. The mRNA expression of pro-inflammatory cytokines including IL-6, IL-8, MIF, and CD14, and the protein levels of M-CSF, MIP, IL-6, and MCP-1 were increased by treating human adipocytes and differentiated 3T3-L1 cells with 10 nM 1,25(OH)2D3.64,84 In contrast, other studies using human adipocytes and 3T3-L1 cells reported that vitamin D (10–100 nM of 1,25[OH]2D) treatment inhibited IL-6, MCP-1, and IL-1β production and inactivated NF-κB by inducing IκBα.86,87,88 Although the reasons for these contradictory results are not clear, pre-incubation of adipocytes with 1,25(OH)2D for 24 or 48 hours before inflammatory stimuli (e.g., TNF-α or IL-1β) may have mediated the anti-inflammatory activity of vitamin D. Consistent with the anti-inflammatory mechanisms of vitamin D in monocytes/macrophages, vitamin D regulates NF-κB and MAPK signaling in adipocytes. 1,25(OH)2D suppressed the activation of NF-κB signaling by increasing IκBα expression and reducing p65 phosphorylation and inhibited MAPK signaling by upregulating MAPK phosphatase expression (Dusp1 and Dusp10) and downregulating phosphorylated p38 MAPK and phosphorylated Erk1/2 in pre-adipocytes and differentiated adipocytes.79,86,87,88
Table 3. Effects of in vitro (or ex vivo) 1,25(OH)2D treatment on inflammatory responses in preadipocyte/adipocyte.
| Cell type | Treatment (dose, duration) | Effects | Reference |
|---|---|---|---|
| Differentiated 3T3-L1 cells and human adipocytes | 10 nM (48 hours) | • Increased Il6 and Tnfα expression in 3T3-L1 | Sun and Zemel64 |
| • Increased IL6 and IL8 expression in human adipocytes | |||
| Differentiated 3T3-L1 cells (co-cultured with RAW 264.7 cell) and human adipocytes | 10 nM (48 hours) | • Increased CD14, MIF expression in human adipocytes | Sun and Zemel84 |
| • Increased Mcsf, Mcp, Tnfα, Il6, and Mcp1 expression in 3T3-L1 | |||
| Preadipocytes isolated from human subcutaneous WAT | 100 nM (24, 48 hours) | • Reduced CCL2 expression | Lorente-Cebrian et al.85 |
| • Reduced MCP-1 and adiponectin production | |||
| 3T3-L1 cells (co-culture with RAW 264.7 cell) and human preadipocytes | 1, 10, 100 nM (24 hours) | • Decreased IL-6, MCP-1, IL-1b mRNA levels | Marcotorchino et al.86 |
| • Decreased IL-6, MCP-1 production | |||
| Human preadipocytes derived from subcutaneous adipose tissue | 10 nM (24 hours) | • Reduced production of MCP-1, IL-8 and IL-6 | Gao et al.87 |
| • Inactivated NF-κB by upregulation of IκBa | |||
| Human preadipocytes differentiated to mature adipocytes | 0.01, 10 nM (48 hours) | • Reduced MCP1, IL8, RANTES, IL6, and IL1β expression | Ding et al.88 |
| 3T3-L1 cells, human adipocytes | 1, 100 nM (48 hours) | • Decreased chemokine mRNA levels in 3T3-L1 and human adipocyte | Karkeni et al.89 |
| Mouse epididymal adipose tissue | 0.1, 1, 10, 100 nM (24, 48 hours) | • Reduction of leptin secretion and mRNA levels | Kong et al.63 |
| Human visceral adipose tissue | 100 nM (12 hours) | • Alleviated oxidative stress in VAT and vascular preparations and also improved the vascular function | Ionica et al.90 |
1,25(OH)2D, 1,25-dihydroxyvitamin D; MIF, macrophage inhibitory factor; MCSF, macrophage colony-stimulating factor; MCP, monocyte chemoattractant protein; IL, interleukin; NF, nuclear factor; VAT, visceral adipose tissue.
Table 4. Effects of in vivo vitamin D supplementation on adipose tissue inflammation.
| Animal model | Treatment (dose, duration) | Effects | Reference |
|---|---|---|---|
| Swiss mice | HFD supplemented with 1,25(OH)2D3 (0.05 mg/kg of diet) for 4 weeks during 8 weeks feeding | • Decreased IL-6 protein levels in epididymal adipose tissue | Lira et al.91 |
| C57BL/6J mice | Gavage with cholecalciferol (15,000 IU/kg of body weight) for 4 days | • Limited LPS-induced inflammatory responses | Karkeni et al.89 |
| HFD (45% fat) supplemented with cholecalciferol (3,000 IU/kg of body weight) for 10 weeks | • Reduction of chemokine (Ccl2, Ccl5) and M1 macrophage markers | ||
| C57BL/6J mice | HFD (45% fat) supplemented with cholecalciferol (10,000 or 25,000 IU/kg diet) for 13 weeks | • Lower Mcp-1, Rantes expresion | Park et al.79 |
| • No difference in immune cell population in adipose tissue | |||
| Wistar rats | ND or HFD for 4 months, then gavaged with 500 IU/kg/d for 5 weeks | • Lower TNF-α and MCP-1 levels in adipose tissue homogenate | Farhangi et al.92 |
| C57BL/6J mice | High-fat/high-sucrose diet supplemented with 15,000 IU/kg cholecalciferol for 15 weeks | • Lower expression of chemokine (Mcp1, Ccl5) mRNA levels | Marziou et al.93 |
| • No difference in adipocyte size | |||
| Yucatan female microswine | 4,500–5,500 IU of vitamin D3/per day for 12 months | • Higher M2 macrophages in EAT | Gunasekar et al.94 |
| • Lower TNF-α, MCP-1 positive cells in EAT | |||
| Sprague-Dawley rats | 0.3 µg/kg/TIW of 1,25(OH)2D3 by gastric gavage for 10 weeks | • Lower MCP-1, IL-6, and TNF-α production by adipocytes from mesenteric adipose tissue | Su et al.95 |
HFD, high-fat diet; 1,25(OH)2D3, 1,25-dihydroxyvitamin D3; IL, interleukin; LPS, lipopolysaccharide; ND, normal diet; TNF, tumor necrosis factor; MCP, monocyte chemoattractant protein; EAT, epicardial adipose tissue; TIW, three times per week.
Furthermore, dietary vitamin D supplementation can modulate low-grade chronic adipose tissue inflammation induced by obesity. In vivo animal studies have consistently demonstrated the anti-inflammatory effects of vitamin D supplementation (Table 4). Vitamin D supplementation not only decreased the production of pro-inflammatory cytokines (IL-6, IL-1β, and TNF-α) and chemokines (CCL2, CCL5, CXCL10, and CXCL11), but also regulated the recruitment of immune cells into adipose tissue.79,89,91,92,93 Mice fed with an HFD supplemented with cholecalciferol (3,000 IU/kg of body weight) exhibited a decrease in M1 type macrophages and T cells in the adipose tissue when compared with mice fed an HFD with 300 IU of cholecalciferol/kg body weight.89
CONCLUSION
Current studies strongly support the significant impact of vitamin D on multiple aspects of adipose tissue biology. However, the specific roles of vitamin D remain inconclusive and sometimes controversial. For instance, VDR knockout mice exhibited a lower fat mass; nonetheless, vitamin D supplementation or 1,25(OH)2D injection led to similar effects. The effects of vitamin D on adipocyte differentiation and adipogenesis have also not yet been fully elucidated, and inconsistent results have been reported depending on cell types, stage of cell differentiation, and the time point at which vitamin D was administered. The anti-inflammatory effects of vitamin D are more consistent, as most studies (with only a few exceptions) have demonstrated that vitamin D reduces the production of proinflammatory mediators. Therefore, in order to expand the clinical implications of vitamin D for chronic diseases associated with adipose tissue dysfunction, more studies are needed to clarify the aforementioned discrepancies in vitamin D research, establish a causal relationship between vitamin D levels and adipose tissue function, and elucidate the mechanisms by which vitamin D modulates adipose tissue biology.
Footnotes
Funding: This work was funded by a grant from the National Research Foundation (NRF) of Korea (NRF-2018R1D1A1B070491).
Conflict of Interest: The authors have no conflicts of interest to declare.
- Conceptualization: Park CY, Han SN.
- Writing - original draft: Park CY, Han SN.
- Writing - review & editing: Han SN.
References
- 1.Luo L, Liu M. Adipose tissue in control of metabolism. J Endocrinol. 2016;231:R77–R99. doi: 10.1530/JOE-16-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ding C, Gao D, Wilding J, Trayhurn P, Bing C. Vitamin D signalling in adipose tissue. Br J Nutr. 2012;108:1915–1923. doi: 10.1017/S0007114512003285. [DOI] [PubMed] [Google Scholar]
- 3.Institute of Medicine. Dietary reference intakes for calcium and vitamin D. Washington, D.C.: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 4.Haddad JG, Matsuoka LY, Hollis BW, Hu YZ, Wortsman J. Human plasma transport of vitamin D after its endogenous synthesis. J Clin Invest. 1993;91:2552–2555. doi: 10.1172/JCI116492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holick MF, Favus M. Vitamin D: photobiology, metabolism, mechanism of action, and clinical applications. In: Favus MJ, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism. 6th ed. Washington, D.C.: American Society for Bone and Mineral Research; 2003. pp. 129–137. [Google Scholar]
- 6.Abbas MA. Physiological functions of vitamin D in adipose tissue. J Steroid Biochem Mol Biol. 2017;165:369–381. doi: 10.1016/j.jsbmb.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Hollander D, Muralidhara KS, Zimmerman A. Vitamin D-3 intestinal absorption in vivo: influence of fatty acids, bile salts, and perfusate pH on absorption. Gut. 1978;19:267–272. doi: 10.1136/gut.19.4.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reboul E, Goncalves A, Comera C, Bott R, Nowicki M, Landrier JF, et al. Vitamin D intestinal absorption is not a simple passive diffusion: evidences for involvement of cholesterol transporters. Mol Nutr Food Res. 2011;55:691–702. doi: 10.1002/mnfr.201000553. [DOI] [PubMed] [Google Scholar]
- 9.Reboul E, Borel P. Proteins involved in uptake, intracellular transport and basolateral secretion of fat-soluble vitamins and carotenoids by mammalian enterocytes. Prog Lipid Res. 2011;50:388–402. doi: 10.1016/j.plipres.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Kiourtzidis M, Kühn J, Brandsch C, Stangl GI. Vitamin D status of mice deficient in scavenger receptor class B type 1, cluster determinant 36 and ATP-binding cassette proteins G5/G8. Nutrients. 2020;12:E2169. doi: 10.3390/nu12082169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blomstrand R, Forsgren L, Bak TA, Holmberg P, Eriksson G, Blinc R, et al. Intestinal absorption and esterification of vitamin D3-1,2-3H in man. Acta Chem Scand. 1967;21:1662–1663. doi: 10.3891/acta.chem.scand.21-1662. [DOI] [PubMed] [Google Scholar]
- 12.Compston JE, Merrett AL, Hammett FG, Magill P. Comparison of the appearance of radiolabelled vitamin D3 and 25-hydroxy-vitamin D3 in the chylomicron fraction of plasma after oral administration in man. Clin Sci (Lond) 1981;60:241–243. doi: 10.1042/cs0600241. [DOI] [PubMed] [Google Scholar]
- 13.Blum M, Dolnikowski G, Seyoum E, Harris SS, Booth SL, Peterson J, et al. Vitamin D3 in fat tissue. Endocrine. 2008;33:90–94. doi: 10.1007/s12020-008-9051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blum M, Dallal GE, Dawson-Hughes B. Body size and serum 25 hydroxy vitamin D response to oral supplements in healthy older adults. J Am Coll Nutr. 2008;27:274–279. doi: 10.1080/07315724.2008.10719700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Landrier JF, Marcotorchino J, Tourniaire F. Lipophilic micronutrients and adipose tissue biology. Nutrients. 2012;4:1622–1649. doi: 10.3390/nu4111622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mawer EB, Backhouse J, Holman CA, Lumb GA, Stanbury SW. The distribution and storage of vitamin D and its metabolites in human tissues. Clin Sci. 1972;43:413–431. doi: 10.1042/cs0430413. [DOI] [PubMed] [Google Scholar]
- 17.Rosenstreich SJ, Rich C, Volwiler W. Deposition in and release of vitamin D3 from body fat: evidence for a storage site in the rat. J Clin Invest. 1971;50:679–687. doi: 10.1172/JCI106538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heaney RP, Horst RL, Cullen DM, Armas LA. Vitamin D3 distribution and status in the body. J Am Coll Nutr. 2009;28:252–256. doi: 10.1080/07315724.2009.10719779. [DOI] [PubMed] [Google Scholar]
- 19.Piccolo BD, Dolnikowski G, Seyoum E, Thomas AP, Gertz ER, Souza EC, et al. Association between subcutaneous white adipose tissue and serum 25-hydroxyvitamin D in overweight and obese adults. Nutrients. 2013;5:3352–3366. doi: 10.3390/nu5093352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Didriksen A, Burild A, Jakobsen J, Fuskevåg OM, Jorde R. Vitamin D3 increases in abdominal subcutaneous fat tissue after supplementation with vitamin D3 . Eur J Endocrinol. 2015;172:235–241. doi: 10.1530/EJE-14-0870. [DOI] [PubMed] [Google Scholar]
- 21.Malmberg P, Karlsson T, Svensson H, Lönn M, Carlsson NG, Sandberg AS, et al. A new approach to measuring vitamin D in human adipose tissue using time-of-flight secondary ion mass spectrometry: a pilot study. J Photochem Photobiol B. 2014;138:295–301. doi: 10.1016/j.jphotobiol.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 22.González-Molero I, Rojo-Martínez G, Morcillo S, Gutierrez C, Rubio E, Pérez-Valero V, et al. Hypovitaminosis D and incidence of obesity: a prospective study. Eur J Clin Nutr. 2013;67:680–682. doi: 10.1038/ejcn.2013.48. [DOI] [PubMed] [Google Scholar]
- 23.Mai XM, Chen Y, Camargo CA, Jr, Langhammer A. Cross-sectional and prospective cohort study of serum 25-hydroxyvitamin D level and obesity in adults: the HUNT study. Am J Epidemiol. 2012;175:1029–1036. doi: 10.1093/aje/kwr456. [DOI] [PubMed] [Google Scholar]
- 24.Drincic AT, Armas LA, Van Diest EE, Heaney RP. Volumetric dilution, rather than sequestration best explains the low vitamin D status of obesity. Obesity (Silver Spring) 2012;20:1444–1448. doi: 10.1038/oby.2011.404. [DOI] [PubMed] [Google Scholar]
- 25.Pereira-Santos M, Costa PR, Assis AM, Santos CA, Santos DB. Obesity and vitamin D deficiency: a systematic review and meta-analysis. Obes Rev. 2015;16:341–349. doi: 10.1111/obr.12239. [DOI] [PubMed] [Google Scholar]
- 26.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72:690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 27.Carrelli A, Bucovsky M, Horst R, Cremers S, Zhang C, Bessler M, et al. Vitamin D storage in adipose tissue of obese and normal weight women. J Bone Miner Res. 2017;32:237–242. doi: 10.1002/jbmr.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park CY, Shin Y, Kim JH, Zhu S, Jung YS, Han SN. Effects of high fat diet-induced obesity on vitamin D metabolism and tissue distribution in vitamin D deficient or supplemented mice. Nutr Metab (Lond) 2020;17:44. doi: 10.1186/s12986-020-00463-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Di Nisio A, De Toni L, Sabovic I, Rocca MS, De Filippis V, Opocher G, et al. Impaired release of vitamin D in dysfunctional adipose tissue: new cues on vitamin D supplementation in obesity. J Clin Endocrinol Metab. 2017;102:2564–2574. doi: 10.1210/jc.2016-3591. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Byrne ME, Chang E, Jiang Y, Donkin SS, Buhman KK, et al. 1α,25-dihydroxyvitamin D hydroxylase in adipocytes. J Steroid Biochem Mol Biol. 2008;112:122–126. doi: 10.1016/j.jsbmb.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wamberg L, Christiansen T, Paulsen SK, Fisker S, Rask P, Rejnmark L, et al. Expression of vitamin D-metabolizing enzymes in human adipose tissue -- the effect of obesity and diet-induced weight loss. Int J Obes. 2013;37:651–657. doi: 10.1038/ijo.2012.112. [DOI] [PubMed] [Google Scholar]
- 32.Kamei Y, Kawada T, Kazuki R, Ono T, Kato S, Sugimoto E. Vitamin D receptor gene expression is up-regulated by 1, 25-dihydroxyvitamin D3 in 3T3-L1 preadipocytes. Biochem Biophys Res Commun. 1993;193:948–955. doi: 10.1006/bbrc.1993.1717. [DOI] [PubMed] [Google Scholar]
- 33.Ghaben AL, Scherer PE. Adipogenesis and metabolic health. Nat Rev Mol Cell Biol. 2019;20:242–258. doi: 10.1038/s41580-018-0093-z. [DOI] [PubMed] [Google Scholar]
- 34.Gregoire FM, Smas CM, Sul HS. Understanding adipocyte differentiation. Physiol Rev. 1998;78:783–809. doi: 10.1152/physrev.1998.78.3.783. [DOI] [PubMed] [Google Scholar]
- 35.Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–273. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosen ED, Spiegelman BM. Molecular regulation of adipogenesis. Annu Rev Cell Dev Biol. 2000;16:145–171. doi: 10.1146/annurev.cellbio.16.1.145. [DOI] [PubMed] [Google Scholar]
- 37.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 38.Fu M, Sun T, Bookout AL, Downes M, Yu RT, Evans RM, et al. A nuclear receptor atlas: 3T3-L1 adipogenesis. Mol Endocrinol. 2005;19:2437–2450. doi: 10.1210/me.2004-0539. [DOI] [PubMed] [Google Scholar]
- 39.Ching S, Kashinkunti S, Niehaus MD, Zinser GM. Mammary adipocytes bioactivate 25-hydroxyvitamin D3 and signal via vitamin D3 receptor, modulating mammary epithelial cell growth. J Cell Biochem. 2011;112:3393–3405. doi: 10.1002/jcb.23273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ji S, Doumit ME, Hill RA. Regulation of adipogenesis and key adipogenic gene expression by 1, 25-dihydroxyvitamin D in 3T3-L1 cells. PLoS One. 2015;10:e0126142. doi: 10.1371/journal.pone.0126142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Narvaez CJ, Matthews D, Broun E, Chan M, Welsh J. Lean phenotype and resistance to diet-induced obesity in vitamin D receptor knockout mice correlates with induction of uncoupling protein-1 in white adipose tissue. Endocrinology. 2009;150:651–661. doi: 10.1210/en.2008-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weber K, Erben RG. Differences in triglyceride and cholesterol metabolism and resistance to obesity in male and female vitamin D receptor knockout mice. J Anim Physiol Anim Nutr (Berl) 2013;97:675–683. doi: 10.1111/j.1439-0396.2012.01308.x. [DOI] [PubMed] [Google Scholar]
- 43.Hida Y, Kawada T, Kayahashi S, Ishihara T, Fushiki T. Counteraction of retinoic acid and 1,25-dihydroxyvitamin D3 on up-regulation of adipocyte differentiation with PPARγ ligand, an antidiabetic thiazolidinedione, in 3T3-L1 cells. Life Sci. 1998;62:PL205–PL211. doi: 10.1016/s0024-3205(98)00059-9. [DOI] [PubMed] [Google Scholar]
- 44.Kong J, Li YC. Molecular mechanism of 1,25-dihydroxyvitamin D3 inhibition of adipogenesis in 3T3-L1 cells. Am J Physiol Endocrinol Metab. 2006;290:E916–E924. doi: 10.1152/ajpendo.00410.2005. [DOI] [PubMed] [Google Scholar]
- 45.Lee H, Bae S, Yoon Y. Anti-adipogenic effects of 1,25-dihydroxyvitamin D3 are mediated by the maintenance of the wingless-type MMTV integration site/β-catenin pathway. Int J Mol Med. 2012;30:1219–1224. doi: 10.3892/ijmm.2012.1101. [DOI] [PubMed] [Google Scholar]
- 46.Zhuang H, Lin Y, Yang G. Effects of 1,25-dihydroxyvitamin D3 on proliferation and differentiation of porcine preadipocyte in vitro . Chem Biol Interact. 2007;170:114–123. doi: 10.1016/j.cbi.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 47.Nimitphong H, Holick MF, Fried SK, Lee MJ. 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 promote the differentiation of human subcutaneous preadipocytes. PLoS One. 2012;7:e52171. doi: 10.1371/journal.pone.0052171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Narvaez CJ, Simmons KM, Brunton J, Salinero A, Chittur SV, Welsh JE. Induction of STEAP4 correlates with 1,25-dihydroxyvitamin D3 stimulation of adipogenesis in mesenchymal progenitor cells derived from human adipose tissue. J Cell Physiol. 2013;228:2024–2036. doi: 10.1002/jcp.24371. [DOI] [PubMed] [Google Scholar]
- 49.Felicidade I, Sartori D, Coort SL, Semprebon SC, Niwa AM, D’Epiro GF, et al. Role of 1α,25-dihydroxyvitamin D3 in adipogenesis of SGBS cells: new insights into human preadipocyte proliferation. Cell Physiol Biochem. 2018;48:397–408. doi: 10.1159/000491770. [DOI] [PubMed] [Google Scholar]
- 50.Mahajan A, Stahl CH. Dihydroxy-cholecalciferol stimulates adipocytic differentiation of porcine mesenchymal stem cells. J Nutr Biochem. 2009;20:512–520. doi: 10.1016/j.jnutbio.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 51.Blumberg JM, Tzameli I, Astapova I, Lam FS, Flier JS, Hollenberg AN. Complex role of the vitamin D receptor and its ligand in adipogenesis in 3T3-L1 cells. J Biol Chem. 2006;281:11205–11213. doi: 10.1074/jbc.M510343200. [DOI] [PubMed] [Google Scholar]
- 52.Nobre JL, Lisboa PC, Carvalho JC, Martins MR, Vargas S, Barja-Fidalgo C, et al. Leptin blocks the inhibitory effect of vitamin D on adipogenesis and cell proliferation in 3T3-L1 adipocytes. Gen Comp Endocrinol. 2018;266:1–8. doi: 10.1016/j.ygcen.2018.01.014. [DOI] [PubMed] [Google Scholar]
- 53.Choe SS, Huh JY, Hwang IJ, Kim JI, Kim JB. Adipose tissue remodeling: its role in energy metabolism and metabolic disorders. Front Endocrinol (Lausanne) 2016;7:30. doi: 10.3389/fendo.2016.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Herman MA, Peroni OD, Villoria J, Schön MR, Abumrad NA, Blüher M, et al. A novel ChREBP isoform in adipose tissue regulates systemic glucose metabolism. Nature. 2012;484:333–338. doi: 10.1038/nature10986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park HK, Ahima RS. Physiology of leptin: energy homeostasis, neuroendocrine function and metabolism. Metabolism. 2015;64:24–34. doi: 10.1016/j.metabol.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Adiyaman SC, Ozer M, Saydam BO, Akinci B. The role of adiponectin in maintaining metabolic homeostasis. Curr Diabetes Rev. 2020;16:95–103. doi: 10.2174/1573399815666190702155733. [DOI] [PubMed] [Google Scholar]
- 57.Bouillon R, Carmeliet G, Lieben L, Watanabe M, Perino A, Auwerx J, et al. Vitamin D and energy homeostasis: of mice and men. Nat Rev Endocrinol. 2014;10:79–87. doi: 10.1038/nrendo.2013.226. [DOI] [PubMed] [Google Scholar]
- 58.Wong KE, Kong J, Zhang W, Szeto FL, Ye H, Deb DK, et al. Targeted expression of human vitamin D receptor in adipocytes decreases energy expenditure and induces obesity in mice. J Biol Chem. 2011;286:33804–33810. doi: 10.1074/jbc.M111.257568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marcotorchino J, Tourniaire F, Astier J, Karkeni E, Canault M, Amiot MJ, et al. Vitamin D protects against diet-induced obesity by enhancing fatty acid oxidation. J Nutr Biochem. 2014;25:1077–1083. doi: 10.1016/j.jnutbio.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 60.Kang EJ, Lee JE, An SM, Lee JH, Kwon HS, Kim BC, et al. The effects of vitamin D3 on lipogenesis in the liver and adipose tissue of pregnant rats. Int J Mol Med. 2015;36:1151–1158. doi: 10.3892/ijmm.2015.2300. [DOI] [PubMed] [Google Scholar]
- 61.Yaribeygi H, Maleki M, Sathyapalan T, Iranpanah H, Orafai HM, Jamialahmadi T, et al. The molecular mechanisms by which vitamin D improve glucose homeostasis: a mechanistic review. Life Sci. 2020;244:117305. doi: 10.1016/j.lfs.2020.117305. [DOI] [PubMed] [Google Scholar]
- 62.Szymczak-Pajor I, Drzewoski J, Śliwińska A. The molecular mechanisms by which vitamin D prevents insulin resistance and associated disorders. Int J Mol Sci. 2020;21:6644. doi: 10.3390/ijms21186644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kong J, Chen Y, Zhu G, Zhao Q, Li YC. 1,25-dihydroxyvitamin D3 upregulates leptin expression in mouse adipose tissue. J Endocrinol. 2013;216:265–271. doi: 10.1530/JOE-12-0344. [DOI] [PubMed] [Google Scholar]
- 64.Sun X, Zemel MB. Calcium and 1,25-dihydroxyvitamin D3 regulation of adipokine expression. Obesity (Silver Spring) 2007;15:340–348. doi: 10.1038/oby.2007.540. [DOI] [PubMed] [Google Scholar]
- 65.Mousa A, Naderpoor N, Wilson K, Plebanski M, de Courten MP, Scragg R, et al. Vitamin D supplementation increases adipokine concentrations in overweight or obese adults. Eur J Nutr. 2020;59:195–204. doi: 10.1007/s00394-019-01899-5. [DOI] [PubMed] [Google Scholar]
- 66.Rafiq R, El Haddaoui H, de Mutsert R, Rosendaal FR, Hiemstra PS, Cobbaert CM, et al. Adiposity is a confounding factor which largely explains the association of serum vitamin D concentrations with C-reactive protein, leptin and adiponectin. Cytokine. 2020;131:155104. doi: 10.1016/j.cyto.2020.155104. [DOI] [PubMed] [Google Scholar]
- 67.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 68.Jo J, Gavrilova O, Pack S, Jou W, Mullen S, Sumner AE, et al. Hypertrophy and/or hyperplasia: dynamics of adipose tissue growth. PLOS Comput Biol. 2009;5:e1000324. doi: 10.1371/journal.pcbi.1000324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jernås M, Palming J, Sjöholm K, Jennische E, Svensson PA, Gabrielsson BG, et al. Separation of human adipocytes by size: hypertrophic fat cells display distinct gene expression. FASEB J. 2006;20:1540–1542. doi: 10.1096/fj.05-5678fje. [DOI] [PubMed] [Google Scholar]
- 70.Baker RG, Hayden MS, Ghosh S. NF-κB, inflammation, and metabolic disease. Cell Metab. 2011;13:11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Watanabe Y, Nagai Y, Takatsu K. Activation and regulation of the pattern recognition receptors in obesity-induced adipose tissue inflammation and insulin resistance. Nutrients. 2013;5:3757–3778. doi: 10.3390/nu5093757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sadeghi K, Wessner B, Laggner U, Ploder M, Tamandl D, Friedl J, et al. Vitamin D3 down-regulates monocyte TLR expression and triggers hyporesponsiveness to pathogen-associated molecular patterns. Eur J Immunol. 2006;36:361–370. doi: 10.1002/eji.200425995. [DOI] [PubMed] [Google Scholar]
- 75.Dickie LJ, Church LD, Coulthard LR, Mathews RJ, Emery P, McDermott MF. Vitamin D3 down-regulates intracellular Toll-like receptor 9 expression and Toll-like receptor 9-induced IL-6 production in human monocytes. Rheumatology (Oxford) 2010;49:1466–1471. doi: 10.1093/rheumatology/keq124. [DOI] [PubMed] [Google Scholar]
- 76.Zhang Y, Leung DY, Richers BN, Liu Y, Remigio LK, Riches DW, et al. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol. 2012;188:2127–2135. doi: 10.4049/jimmunol.1102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cohen-Lahav M, Shany S, Tobvin D, Chaimovitz C, Douvdevani A. Vitamin D decreases NFκB activity by increasing IκBα levels. Nephrol Dial Transplant. 2006;21:889–897. doi: 10.1093/ndt/gfi254. [DOI] [PubMed] [Google Scholar]
- 78.Villaggio B, Soldano S, Cutolo M. 1,25-dihydroxyvitamin D3 downregulates aromatase expression and inflammatory cytokines in human macrophages. Clin Exp Rheumatol. 2012;30:934–938. [PubMed] [Google Scholar]
- 79.Park CY, Kim TY, Yoo JS, Seo Y, Pae M, Han SN. Effects of 1,25-dihydroxyvitamin D3 on the inflammatory responses of stromal vascular cells and adipocytes from lean and obese mice. Nutrients. 2020;12:364. doi: 10.3390/nu12020364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Neve A, Corrado A, Cantatore FP. Immunomodulatory effects of vitamin D in peripheral blood monocyte-derived macrophages from patients with rheumatoid arthritis. Clin Exp Med. 2014;14:275–283. doi: 10.1007/s10238-013-0249-2. [DOI] [PubMed] [Google Scholar]
- 81.Chen GY, Nuñez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen Y, Kong J, Sun T, Li G, Szeto FL, Liu W, et al. 1,25-Dihydroxyvitamin D3 suppresses inflammation-induced expression of plasminogen activator inhibitor-1 by blocking nuclear factor-κB activation. Arch Biochem Biophys. 2011;507:241–247. doi: 10.1016/j.abb.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stio M, Martinesi M, Bruni S, Treves C, Mathieu C, Verstuyf A, et al. The The vitamin D analogue TX 527 blocks NF-κB activation in peripheral blood mononuclear cells of patients with Crohn's disease. J Steroid Biochem Mol Biol. 2007;103:51–60. doi: 10.1016/j.jsbmb.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 84.Sun X, Zemel MB. Calcitriol and calcium regulate cytokine production and adipocyte-macrophage cross-talk. J Nutr Biochem. 2008;19:392–399. doi: 10.1016/j.jnutbio.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 85.Lorente-Cebrián S, Eriksson A, Dunlop T, Mejhert N, Dahlman I, Aström G, et al. Differential effects of 1α,25-dihydroxycholecalciferol on MCP-1 and adiponectin production in human white adipocytes. Eur J Nutr. 2012;51:335–342. doi: 10.1007/s00394-011-0218-z. [DOI] [PubMed] [Google Scholar]
- 86.Marcotorchino J, Gouranton E, Romier B, Tourniaire F, Astier J, Malezet C, et al. Vitamin D reduces the inflammatory response and restores glucose uptake in adipocytes. Mol Nutr Food Res. 2012;56:1771–1782. doi: 10.1002/mnfr.201200383. [DOI] [PubMed] [Google Scholar]
- 87.Gao D, Trayhurn P, Bing C. 1,25-dihydroxyvitamin D3 inhibits the cytokine-induced secretion of MCP-1 and reduces monocyte recruitment by human preadipocytes. Int J Obes. 2013;37:357–365. doi: 10.1038/ijo.2012.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ding C, Wilding JP, Bing C. 1,25-dihydroxyvitamin D3 protects against macrophage-induced activation of NFκB and MAPK signalling and chemokine release in human adipocytes. PLoS One. 2013;8:e61707. doi: 10.1371/journal.pone.0061707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Karkeni E, Marcotorchino J, Tourniaire F, Astier J, Peiretti F, Darmon P, et al. Vitamin D limits chemokine expression in adipocytes and macrophage migration in vitro and in male mice. Endocrinology. 2015;156:1782–1793. doi: 10.1210/en.2014-1647. [DOI] [PubMed] [Google Scholar]
- 90.Ionica M, Aburel OM, Vaduva A, Petrus A, Rațiu S, Olariu S, et al. Vitamin D alleviates oxidative stress in adipose tissue and mesenteric vessels from obese patients with subclinical inflammation. Can J Physiol Pharmacol. 2020;98:85–92. doi: 10.1139/cjpp-2019-0340. [DOI] [PubMed] [Google Scholar]
- 91.Lira FS, Rosa JC, Cunha CA, Ribeiro EB, do Nascimento CO, Oyama LM, et al. Supplementing alpha-tocopherol (vitamin E) and vitamin D3 in high fat diet decrease IL-6 production in murine epididymal adipose tissue and 3T3-L1 adipocytes following LPS stimulation. Lipids Health Dis. 2011;10:37. doi: 10.1186/1476-511X-10-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Farhangi MA, Mesgari-Abbasi M, Hajiluian G, Nameni G, Shahabi P. Adipose tissue inflammation and oxidative stress: the ameliorative effects of vitamin D. Inflammation. 2017;40:1688–1697. doi: 10.1007/s10753-017-0610-9. [DOI] [PubMed] [Google Scholar]
- 93.Marziou A, Philouze C, Couturier C, Astier J, Obert P, Landrier JF, et al. Vitamin D supplementation improves adipose tissue inflammation and reduces hepatic steatosis in obese C57BL/6J Mice. Nutrients. 2020;12:342. doi: 10.3390/nu12020342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gunasekar P, Swier VJ, Fleegel JP, Boosani CS, Radwan MM, Agrawal DK. Vitamin D and macrophage polarization in epicardial adipose tissue of atherosclerotic swine. PLoS One. 2018;13:e0199411. doi: 10.1371/journal.pone.0199411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Su YB, Li TH, Huang CC, Tsai HC, Huang SF, Hsieh YC, et al. Chronic calcitriol supplementation improves the inflammatory profiles of circulating monocytes and the associated intestinal/adipose tissue alteration in a diet-induced steatohepatitis rat model. PLoS One. 2018;13:e0194867. doi: 10.1371/journal.pone.0194867. [DOI] [PMC free article] [PubMed] [Google Scholar]