Abstract
Gut microbes dictate critical features of host immunometabolism. Certain bacterial components and metabolites (termed postbiotics) mitigate cardiometabolic diseases whereas others potentiate pathological processes. In this review, we discuss key aspects related to the usefulness of bacterial-related molecules strategically positioned as promising treatment strategies for cardiometabolic diseases.
Keywords: Obesity; Diabetes mellitus, type 2; Akkermansia; Lipopolysaccharides; Peptidoglycan
INTRODUCTION
To this date, Akkermansia muciniphila is the only bacterial strain shown to impact markers of human cardiometabolic disease to a high degree of reliability and efficacy. Oral administration of A. muciniphila to human individuals with obesity/overweight improved insulin resistance and reduced circulating cholesterol.1,2 Similar results were reported using pre-clinical models of diet-induced obesity and genetic-induced dyslipidemia, whereby A. muciniphila improved blood glucose control,3,4 facilitated hepatic triglyceride clearance5 and reduced atherogenesis.6 Findings from both pre-clinical models and human trials have pointed to pasteurization (i.e. exposure to mild heat) as an effective way to enhance the cardiometabolic benefits of A. muciniphila.2,3 Pasteurization render bacteria non-replicant while retaining the health benefits of certain probiotic strains.7 Because bioactivity is preserved, it is likely that pasteurization retain the molecular structure of bacterial components needed to elicit immune regulation. These findings are of great importance stressing the utility of bacterial metabolites, cell wall components and bacterial peptides—referred to as postbiotics—as promising strategies to treat metabolic complications linked to overweight and obesity. Postbiotics, however, must not be confused with probiotics or prebiotics. Indeed, probiotics define bacteria that, when administered alive, confer health benefits to the host, whereas the latter term refers to dietary compounds that can favor the expansion of beneficial bacteria in the gut. This article puts into perspective evidence relevant to the usefulness of certain postbiotics against cardiometabolic complications. The impact of short-chain fatty acids (SCFA)—arguably the most studied bacterial postbiotics—on cardiometabolic diseases has been extensively examined elsewhere8,9 and is not addressed in this review.
MAIN TEXT
Host immunity bridges gut microbial components to host metabolic regulation.10 Activation of Toll-like receptor (TLR) 4 by bacterial lipopolysaccharides (LPS) is a classic example of this interaction. In the presence of obesity and poor dietary habits, gut microbial LPS can breach the gut barrier and elicit innate immune activation in key insulin-responsive organs, causing a mild and chronic raise in circulating LPS termed metabolic endotoxemia (ME).11 This moderate, yet persistent, increase in circulating LPS appears to be an important feature of metabolic syndrome as ME is increasingly recognized in the constellation of risk factors involved in cardiovascular diseases (CVD) (Fig. 1).12,13 In agreement, pre-clinical mouse studies have shown that ME is sufficient to initiate and aggravate the low-grade inflammatory state linked to insulin resistance, dysglycemia11 and atherogenesis.6
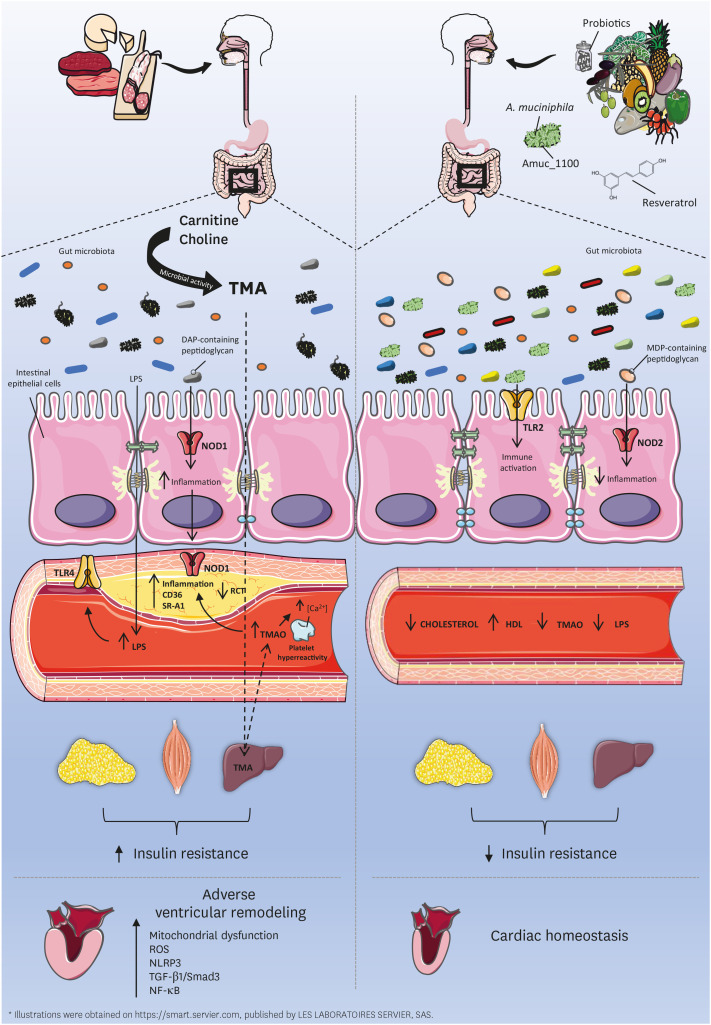
Fig. 1. Bacterial postbiotics influence cardiometabolic disorders. Poor dietary habits (left) contribute to the impairment of gut barrier homeostasis, which favors the leakage of gut microbial LPS into circulation (metabolic endotoxemia). Conversely, healthy dietary habits (right) are linked to preserved gut barrier function. Metabolic endotoxemia is increasingly recognized in the constellation of risk factors involved in cardiometabolic diseases and insulin resistance. Dietary carnitine and choline (left) are metabolized into TMA through microbial activity in the gut. TMA is later converted into proatherogenic TMAO in the liver. Once in circulation, TMAO facilitates atherosclerotic plaque deposition by reducing RCT, increasing inflammation, upregulating scavenger receptors (CD36, SRA1) and promoting platelet hyperreactivity. TMAO further impairs cardiac function by stimulating oxidative stress (mitochondrial dysfunction, ROS), inflammation (NLRP3, NF-kB) and the profibrotic TGF-β1/Smad3 pathway. Consumption of certain probiotics and resveratrol (right) were shown to reduce serum levels of TMAO. Amuc_1100 (right), a cell wall component of A. muciniphila, was shown to recapitulate the beneficial metabolic effects of this bacterium in mice. Likewise, the interaction between NOD receptors and some peptidoglycans, key constituents of the bacterial cell wall, also influence host metabolism. Gut microbiota-derived meso-DAP acid-containing muropeptides act via NOD1 to increase inflammation and insulin resistance, whereas NOD-2 interaction with MDP-containing peptidoglycan resulted in protection against inflammation and insulin resistance.
LPS, lipopolysaccharide; TMA, trimethylamine; TMAO, trimethylamine-N-oxide; RCT, reverse cholesterol transport; CD36, cluster of differentiation 36; SR-A1, scavenger receptor-A1; ROS, reactive oxygen species; NLRP3, nucleotide-binding oligomerization domain-like receptor family pyrin domain containing 3; NF-κB, nuclear factor-κB; TGF-β1, transforming growth factor-β1; NOD, nucleotide-binding oligomerization domain-containing; DAP, diaminopimelic; MDP, muramyl dipeptide; TLR, toll-like receptor; HDL, high-density lipoprotein.
While the cardiometabolic benefits of A. muciniphila are, at least in part, linked to its capacity to strengthen the gut barrier and lower ME,2,3,4,14 an isolated component from the cell wall of this bacterium (Amuc_1100) was shown to recapitulate the metabolic benefits reported after administration of pasteurized A. muciniphila to diet-induced obese mice.3 Amuc_1100, which appears to elicit immune activation via TLR2, increased high-density lipoprotein (HDL) cholesterol, improved insulin sensitivity and alleviated ME in diet-induced obese mice (Fig. 1).3 While more mechanistic pre-clinical studies and clinical trials are warranted, Amuc_1100 holds promise as a postbiotic from A. muciniphila that may curb several features of metabolic syndrome.
Peptidoglycan is a key constituent of the bacterial cell wall containing specific muropeptide sequences that are detected by nucleotide-binding oligomerization domain-containing (NOD) proteins. NOD1 recognizes meso-diaminopimelic (meso-DAP) acid-containing muropeptides, whereas NOD2 detects muramyl dipeptide (MDP)-containing peptidoglycan. We have demonstrated in murine models that acute activation of NOD1 causes whole-body and hepatic insulin resistance,15 whereas others have shown that genetic ablation of NOD1 confers protection against diet-induced insulin resistance.16 Concordantly, microbiota-derived muropeptides act on NOD1, but not NOD2, to augment systemic immunity.17,18 In contrast, NOD2 attenuates inflammation induced by other bacterial products and protects against inflammatory colitis19 insulin resistance20 and nonalcoholic fatty liver disease (NAFLD).21 This is, at least in part, linked to NOD2-dependent downstream processing of a wealth of host defense peptides (HDPs) improving gut barrier function.22,23 We have shown that intraperitoneally injected MDP can protect mice from LPS-induced dysglycemia, and that the MDP-based drug mifamurtide can improve glucose tolerance in mice with diet-induced obesity.24 Not surprisingly, the metabolic impact of NOD activation has cardiovascular implications. While NOD1 activation with the synthetic peptidoglycan FK565 caused major site-specific inflammation in the aortic root,25 a recent study showed that depletion of NOD1/2 protected low-density lipoprotein (LDL) receptor knockout mice from developing atherosclerosis.26 Interestingly, both NOD1 and NOD2 signals intracellularly through receptor-interacting serine/threonine-protein kinase 2 (RIPK2). We have shown that nonhematopoietic—but not hematopoietic—RIPK2 mediates the glucose lowering and anti-inflammatory effects of NOD2 activation, whereas LPS and NOD1 synergize in hematopoietic cells to promote insulin resistance.27 Together, these findings show that postbiotics that target NOD receptors are well-positioned as promising strategies to curb CVD (Fig. 1).
The intestinal microbiota metabolizes certain dietary trimethylamines, such as choline and L-carnitine, to produce trimethylamine (TMA). This microbial metabolite crosses the gut barrier and is later oxidized in the liver forming trimethylamine N-oxide (TMAO). Recent epidemiological studies linked TMAO to higher risk of major cardiovascular events.28,29 Several pieces of evidence suggest that gut microbes are necessary to the detrimental effects of certain atherogenic dietary components. Wang et al. found that dietary supplementation with choline and TMAO upregulated both cluster of differentiation 36 (CD-36) and scavenger receptor type-1 (SR-A1) in macrophages while promoting atherosclerosis in a gut microbiota-dependent manner.30 Likewise, mice fed with TMAO, carnitine, or choline exhibited reduced reverse cholesterol transport, which accelerated atherosclerosis only when the gut microbiota was intact.31 TMAO was further reported to directly promote platelet hyperreactivity by enhancing stimulus-dependent release of Ca2+ from intracellular stores, and to upregulate clot formation rate in vivo, contributing to elevated risk of thrombosis.32 The choline-related thrombosis potential was transferred to germ-free mice after fecal microbial transplantation, pointing to a causal role of the gut microbiota.32 The authors further revealed specific gut microbial taxa, such as the SCFA-producing bacteria Lachnospiraceae, Oscilospira and Ruminococcus, to inversely correlate with both TMAO and thrombosis potential.32
Inflammation is a keystone of cardiometabolic disease, and reports from different research groups support the pro-inflammatory potential of TMAO. In rodent models of heart failure, TMAO upregulated profibrotic atrial natriuretic peptide (ANP) and beta-myosin heavy chain (β-MHC),33 as well as nuclear factor-κB (NF-κB) and the transforming growth factor-β1 (TGF-β1)/Smad3 pathway.34 Further, NF-κB and mitogen-activated protein (MAP) kinase were both shown to mediate the impact of TMAO on vascular inflammation.35 Moreover, TMAO activated nucleotide-binding oligomerization domain-like receptor family pyrin domain containing 3 (NLRP3) inflammasome and caspase-1 while increasing mitochondrial-derived reactive oxygen species (ROS) in the aorta.36 In cardiomyocytes, TMAO-derived mitochondrial dysfunction led to impaired pyruvate and fatty acid oxidation.37
Gut microbial modulation might represent an important strategy to alleviate TMAO production and thereby attenuate CVD (Fig. 1). Individuals classified within an enterotype dominated by Prevotella displayed higher circulating levels of TMAO than subjects with an enterotype enriched in Bacteroides.31 In the same study, omnivorous subjects with enhanced plasma TMAO showed higher abundance of Peptostreptococcaceae and Clostridium and lower Lachnospira as compared to vegan/vegetarian individuals with low circulating TMAO concentration.31 Heianza and colleagues38 recently reported that continuous increase in circulating TMAO over a period of 10 years was associated with higher risk of coronary heart disease, which was shown to be alleviated by healthy dietary patterns. Accordingly, consumption of the polyphenol resveratrol remodeled the gut microbiota in mice, including augmented Lactobacillus and Bifidobacterium abundances, thereby decreasing TMAO levels by mitigating commensal microbial TMA.39 Similarly, four weeks of daily consumption of certain probiotic strains reduced serum TMAO in healthy adult males.40 Further, limiting TMA production with choline analogs was shown as a potential therapeutic strategy against atherosclerosis41 and heart failure.42
CONCLUSION
Host-microbe interaction takes place through engagement of immune responses. Postbiotics, such as MDP and Amuc_1100, are promising candidates to promote cardiometabolic benefits via innate immune activation. Other bacteria derived molecules, such as certain LPS and TMAO, are pathognomonic to immunometabolic mechanisms worsening cardiovascular disease risk. TMAO is well-positioned as a biomarker and a possible treatment target to mitigate cardiometabolic complications.
Footnotes
Funding: None.
Conflict of Interest: The authors have no conflict of interest to declare.
- Writing - original draft: Anhê FF, Jensen BAH, Perazza L.
- Writing - review & editing: Anhê FF, Marette A, Tchernof A, Schertzer J.
References
- 1.Anhê FF, Schertzer JD, Marette A. Bacteria to alleviate metabolic syndrome. Nat Med. 2019;25:1031–1033. doi: 10.1038/s41591-019-0516-1. [DOI] [PubMed] [Google Scholar]
- 2.Depommier C, Everard A, Druart C, Plovier H, Van Hul M, Vieira-Silva S, et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med. 2019;25:1096–1103. doi: 10.1038/s41591-019-0495-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plovier H, Everard A, Druart C, Depommier C, Van Hul M, Geurts L, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2017;23:107–113. doi: 10.1038/nm.4236. [Internet] [DOI] [PubMed] [Google Scholar]
- 4.Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110:9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen J, Tong X, Sud N, Khound R, Song Y, Maldonado-Gomez MX, et al. Low-density lipoprotein receptor signaling mediates the triglyceride-lowering action of Akkermansia muciniphila in genetic-induced hyperlipidemia. Arterioscler Thromb Vasc Biol. 2016;36:1448–1456. doi: 10.1161/ATVBAHA.116.307597. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Lin S, Vanhoutte PM, Woo CW, Xu A. Akkermansia muciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in Apoe−/− mice. Circulation. 2016;133:2434–2446. doi: 10.1161/CIRCULATIONAHA.115.019645. [DOI] [PubMed] [Google Scholar]
- 7.Sakai T, Taki T, Nakamoto A, Shuto E, Tsutsumi R, Toshimitsu T, et al. Lactobacillus plantarum OLL2712 regulates glucose metabolism in C57BL/6 mice fed a high-fat diet. J Nutr Sci Vitaminol (Tokyo) 2013;59:144–147. doi: 10.3177/jnsv.59.144. [DOI] [PubMed] [Google Scholar]
- 8.Hernández MA, Canfora EE, Jocken JW, Blaak EE. The short-chain fatty acid acetate in body weight control and insulin sensitivity. Nutrients. 2019;11:1943. doi: 10.3390/nu11081943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canfora EE, Jocken JW, Blaak EE. Short-chain fatty acids in control of body weight and insulin sensitivity. Nat Rev Endocrinol. 2015;11:577–591. doi: 10.1038/nrendo.2015.128. [DOI] [PubMed] [Google Scholar]
- 10.Zheng D, Liwinski T, Elinav E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020;30:492–506. doi: 10.1038/s41422-020-0332-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 12.Ahmadi SF, Streja E, Zahmatkesh G, Streja D, Kashyap M, Moradi H, et al. Reverse epidemiology of traditional cardiovascular risk factors in the geriatric population. J Am Med Dir Assoc. 2015;16:933–939. doi: 10.1016/j.jamda.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McClelland RL, Jorgensen NW, Budoff M, Blaha MJ, Post WS, Kronmal RA, et al. 10-year coronary heart disease risk prediction using coronary artery calcium and traditional risk factors: derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) with validation in the HNR (Heinz Nixdorf Recall) study and the DHS (Dallas Heart Study) J Am Coll Cardiol. 2015;66:1643–1653. doi: 10.1016/j.jacc.2015.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greer RL, Dong X, Moraes AC, Zielke RA, Fernandes GR, Peremyslova E, et al. Akkermansia muciniphila mediates negative effects of IFNγ on glucose metabolism. Nat Commun. 2016;7:13329. doi: 10.1038/ncomms13329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schertzer JD, Tamrakar AK, Magalhães JG, Pereira S, Bilan PJ, Fullerton MD, et al. NOD1 activators link innate immunity to insulin resistance. Diabetes. 2011;60:2206–2215. doi: 10.2337/db11-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amar J, Chabo C, Waget A, Klopp P, Vachoux C, Bermúdez-Humarán LG, et al. Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecular mechanisms and probiotic treatment. EMBO Mol Med. 2011;3:559–572. doi: 10.1002/emmm.201100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hergott CB, Roche AM, Tamashiro E, Clarke TB, Bailey AG, Laughlin A, et al. Peptidoglycan from the gut microbiota governs the lifespan of circulating phagocytes at homeostasis. Blood. 2016;127:2460–2471. doi: 10.1182/blood-2015-10-675173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clarke TB, Davis KM, Lysenko ES, Zhou AY, Yu Y, Weiser JN. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat Med. 2010;16:228–231. doi: 10.1038/nm.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hedl M, Li J, Cho JH, Abraham C. Chronic stimulation of Nod2 mediates tolerance to bacterial products. Proc Natl Acad Sci U S A. 2007;104:19440–19445. doi: 10.1073/pnas.0706097104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denou E, Lolmède K, Garidou L, Pomie C, Chabo C, Lau TC, et al. Defective NOD2 peptidoglycan sensing promotes diet-induced inflammation, dysbiosis, and insulin resistance. EMBO Mol Med. 2015;7:259–274. doi: 10.15252/emmm.201404169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cavallari JF, Pokrajac NT, Zlitni S, Foley KP, Henriksbo BD, Schertzer JD. NOD2 in hepatocytes engages a liver-gut axis to protect against steatosis, fibrosis, and gut dysbiosis during fatty liver disease in mice. Am J Physiol Endocrinol Metab. 2020;319:E305–E314. doi: 10.1152/ajpendo.00181.2020. [DOI] [PubMed] [Google Scholar]
- 22.Grimm MC, Pavli P. NOD2 mutations and Crohn's disease: are Paneth cells and their antimicrobial peptides the link? Gut. 2004;53:1558–1560. doi: 10.1136/gut.2004.043307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen BA, Marette A. Microbial translocation in type 2 diabetes: when bacterial invaders overcome host defence in human obesity. Gut. 2020;69:1724–1726. doi: 10.1136/gutjnl-2020-321288. [DOI] [PubMed] [Google Scholar]
- 24.Cavallari JF, Fullerton MD, Duggan BM, Foley KP, Denou E, Smith BK, et al. Muramyl dipeptide-based postbiotics mitigate obesity-induced insulin resistance via IRF4. Cell Metab. 2017;25:1063–1074.e3. doi: 10.1016/j.cmet.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 25.Nishio H, Kanno S, Onoyama S, Ikeda K, Tanaka T, Kusuhara K, et al. Nod1 ligands induce site-specific vascular inflammation. Arterioscler Thromb Vasc Biol. 2011;31:1093–1099. doi: 10.1161/ATVBAHA.110.216325. [DOI] [PubMed] [Google Scholar]
- 26.Vlacil AK, Schuett J, Ruppert V, Soufi M, Oberoi R, Shahin K, et al. Deficiency of Nucleotide-binding oligomerization domain-containing proteins (NOD) 1 and 2 reduces atherosclerosis. Basic Res Cardiol. 2020;115:47. doi: 10.1007/s00395-020-0806-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cavallari JF, Barra NG, Foley KP, Lee A, Duggan BM, Henriksbo BD, et al. Postbiotics for NOD2 require nonhematopoietic RIPK2 to improve blood glucose and metabolic inflammation in mice. Am J Physiol Endocrinol Metab. 2020;318:E579–E585. doi: 10.1152/ajpendo.00033.2020. [DOI] [PubMed] [Google Scholar]
- 28.Croyal M, Saulnier PJ, Aguesse A, Gand E, Ragot S, Roussel R, et al. Plasma trimethylamine N-oxide and risk of cardiovascular events in patients with type 2 diabetes. J Clin Endocrinol Metab. 2020;105:dgaa188. doi: 10.1210/clinem/dgaa188. [DOI] [PubMed] [Google Scholar]
- 29.Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1584. doi: 10.1056/NEJMoa1109400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576–585. doi: 10.1038/nm.3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165:111–124. doi: 10.1016/j.cell.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Z, Wu Z, Yan J, Liu H, Liu Q, Deng Y, et al. Gut microbe-derived metabolite trimethylamine N-oxide induces cardiac hypertrophy and fibrosis. Lab Invest. 2019;99:346–357. doi: 10.1038/s41374-018-0091-y. [DOI] [PubMed] [Google Scholar]
- 34.Wang G, Kong B, Shuai W, Fu H, Jiang X, Huang H. 3,3-Dimethyl-1-butanol attenuates cardiac remodeling in pressure-overload-induced heart failure mice. J Nutr Biochem. 2020;78:108341. doi: 10.1016/j.jnutbio.2020.108341. [DOI] [PubMed] [Google Scholar]
- 35.Seldin MM, Meng Y, Qi H, Zhu W, Wang Z, Hazen SL, et al. Trimethylamine N-oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-κB. J Am Heart Assoc. 2016;5:e002767. doi: 10.1161/JAHA.115.002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen ML, Zhu XH, Ran L, Lang HD, Yi L, Mi MT. Trimethylamine-N-oxide induces vascular inflammation by activating the NLRP3 inflammasome through the SIRT3-SOD2-mtROS signaling pathway. J Am Heart Assoc. 2017;6:e006347. doi: 10.1161/JAHA.117.006347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Makrecka-Kuka M, Volska K, Antone U, Vilskersts R, Grinberga S, Bandere D, et al. Trimethylamine N-oxide impairs pyruvate and fatty acid oxidation in cardiac mitochondria. Toxicol Lett. 2017;267:32–38. doi: 10.1016/j.toxlet.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 38.Heianza Y, Ma W, DiDonato JA, Sun Q, Rimm EB, Hu FB, et al. Long-term changes in gut microbial metabolite trimethylamine N-oxide and coronary heart disease risk. J Am Coll Cardiol. 2020;75:763–772. doi: 10.1016/j.jacc.2019.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen ML, Yi L, Zhang Y, Zhou X, Ran L, Yang J, et al. Resveratrol attenuates trimethylamine-N-oxide (TMAO)-induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. MBio. 2016;7:e02210–e02215. doi: 10.1128/mBio.02210-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen S, Jiang PP, Yu D, Liao GC, Wu SL, Fang AP, et al. Effects of probiotic supplementation on serum trimethylamine-N-oxide level and gut microbiota composition in young males: a double-blinded randomized controlled trial. Eur J Nutr. Forthcoming2020 doi: 10.1007/s00394-020-02278-1. [DOI] [PubMed] [Google Scholar]
- 41.Wang Z, Roberts AB, Buffa JA, Levison BS, Zhu W, Org E, et al. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell. 2015;163:1585–1595. doi: 10.1016/j.cell.2015.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Organ CL, Li Z, Sharp TE, 3rd, Polhemus DJ, Gupta N, Goodchild TT, et al. Nonlethal inhibition of gut microbial trimethylamine N-oxide production improves cardiac function and remodeling in a murine model of heart failure. J Am Heart Assoc. 2020;9:e016223. doi: 10.1161/JAHA.119.016223. [DOI] [PMC free article] [PubMed] [Google Scholar]