Abstract
Dyslipidemia is a major cause of cardiovascular diseases which represent a leading cause of death in humans. Diverse immune cells are known to be involved in the pathogenesis of cardiovascular diseases such as atherosclerosis. Conversely, dyslipidemia is known to be tightly associated with immune disorders in humans, as evidenced by a higher incidence of atherosclerosis in patients with autoimmune diseases including psoriasis, rheumatoid arthritis, and systemic lupus erythematosus. Given that the dyslipidemia-related autoimmune diseases are caused by autoreactive T cells and B cells, dyslipidemia seems to directly or indirectly regulate the adaptive immunity. Indeed, accumulating evidence has unveiled that proatherogenic factors can impact the differentiation and function of CD4+ T cells, CD8+ T cells, and B cells. This review discusses an updated overview on the regulation of adaptive immunity by dyslipidemia and proposes a potential therapeutic strategy for immune disorders by targeting lipid metabolism.
Keywords: Dyslipidemia, T cell, B cell Dendritic cell, Immune disorder
INTRODUCTION
Lipids such as cholesterols, fatty acids, and phospholipids are essential to higher organisms for energy storage, organ physiology, cellular proliferation and numerous aspects of cellular biology. In the cell, lipid serves as a critical energy source, and the main component of cellular membranes. Moreover, some lipids act as agonists or antagonists for transcription factors to meet diverse demands from various tissues and to maintain cellular homeostasis. On the other hand, chronic dyslipidemia triggers cardiovascular diseases including atherosclerosis-related coronary and cerebral artery diseases which represent the leading cause of death worldwide.1,2 The prevalence of dyslipidemia increases steadily in developed countries, likely due to westernized dietary patterns. In addition, it is evident that aberrant activation of innate and adaptive immune responses contributes to the pathogenesis of atherosclerosis.3,4,5,6 Proatherogenic factors have been shown to exhibit both pro- and anti-inflammatory effects on the innate immune system. For instance, several lipid species are known to stimulate macrophages to produce interleukin (IL)-1β through MyD88 or the inflammasome.6,7,8,9 On the contrary, lipid species can activate the PPARɣ pathway and inhibit inflammatory responses in macrophages.10,11,12 Thus, lipid species can exert both pro-inflammatory and anti-inflammatory functions in a context-dependent manner.
Of note, numerous epidemiologic studies indicate a strong incidental correlation between atherosclerosis and chronic autoimmune disorders, indicating a mutual regulation between immune and cardiovascular systems. For instance, a higher incidence of atherosclerosis is observed in patients with pre-existing autoimmune disorders such as rheumatoid arthritis,13,14,15,16 psoriasis17,18,19,20 systemic lupus erythematosus (SLE),21,22,23 and diabetes mellitus. In addition, dyslipidemia induced by a high-fat diet accelerates the progression of autoimmune lupus, arthritis and encephalomyelitis.24,25,26,27 More importantly, treatment of hyperlipidemia in patients with psoriasis leads to clinical improvement,28,29,30,31 indicating a pathogenic role of dyslipidemia on the development and/or progression of autoimmune diseases in humans as well as in animal models. Given that the dyslipidemia-related immune disorders are mediated by aberrantly activated self-reactive T cells and/or self-reactive B cells, it is feasible to surmise that dyslipidemia directly or indirectly regulates the differentiation and function of adaptive immune cells in vivo.
In this review, we will provide an updated view on the role of dyslipidemia and lipid species in regulating T cells and B cells. In the first half, we discuss the association of dyslipidemia and immune disorders including autoimmune diseases, cancers, and infections. In particular, the suggested potential mechanisms by which lipid metabolism impacts such immune disorders will be discussed. In the second half, we discuss the role of cholesterol metabolism in the context of regulating the differentiation and functions of immune cells including dendritic cells, T cells, and B cells.
DYSLIPIDEMIA REGULATES IMMUNE-MEDIATED DISEASE BY SHAPING THE ADAPTIVE IMMUNE SYSTEM
Lipid homeostasis in adaptive immune cells is crucial, and any disruption to the balance may lead to an engenderment or exacerbation of immune-mediated diseases. This tipping may occur in antigen-presenting dendritic cells or effector cell population such as follicular helper T cells (Tfh) and B cells to mediate autoimmune diseases, tumor microenvironment, and infection.
Autoimmune disease
Autoimmune diseases including multiple sclerosis, SLE, and psoriasis are positively correlated with a risk of cardiovascular disease. Immune-mediated diseases including rheumatoid arthritis and lupus are known to be driven by pathogenic CD4+ T cells. Often, lipid-lowering treatments including low-fat diet and statins are utilized in the treatment of psoriasis and SLE.32,33 The link between autoimmunity and dyslipidemia is well corroborated in various reports.34,35,36,37,38,39
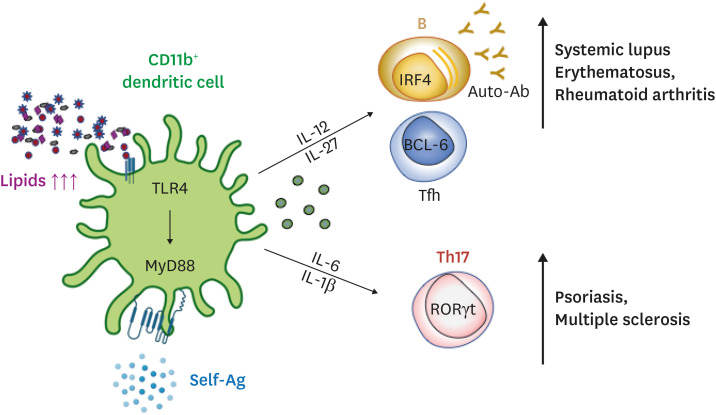
Hyperlipidemic condition promotes autoimmune phenotypes in dendritic cells which in turn, affect Tfh and B cells.34,36,37 For one, dendritic cells with augmented intracellular cholesterols drive lupus-like phenotypes such as glomerulonephritis and a surge in plasma anti-dsDNA antibodies. These splenic dendritic cells are mostly CD11b+ with enhanced ability to induce T-cell activation and disrupt immune tolerance, exacerbating the pathogenesis.36 Cholesterol buildup in CD11c+MHCII+ cells impairs antigen presentation and activates toll-like receptor signaling, driving the production of Baff and April. Eventually, they facilitate the priming of autoreactive T cells, expansion of B cells, and production of autoantibodies.40 Not only that, uptake of oxidized low-density lipoproteins (LDLs) and LDLs by dendritic cells significantly increases Th17 and Tfh differentiation via IL-6 and IL-1β secretion. These pathogenic Tfh and Th17 cells in turn can enhance the susceptibility to other autoimmune diseases such as psoriasis and rheumatoid arthritis (Fig. 1).36,39,41 Furthermore, a double-transgenic mouse expressing human CD1b, CD1c, and CD1b-autoreactive T cell receptor (TCR) in the Apolipoprotein E (ApoE)-deficient background spontaneously develops psoriasiform characterized by an upregulation of IL-6 and IL-17A secretion by CD1b+ dendritic cells and CD1b-autoreactive T cells.41
Fig. 1. Hyperlipidemia impacts autoimmune Tfh cells and Th17 cell responses.
Atherogenic dyslipidemia can be recognized by CD11b+ dendritic cells through pattern recognition receptors, leading to the increased production of pro-inflammatory cytokines including IL-12, IL-27, IL-6, and IL-1. These cytokines in turn promote the differentiation of autoimmune Tfh and Th17 cells in vivo. Thus, dyslipidemia contributes to the onset or progression of Tfh- or Th17-mediated autoimmune diseases.
Tfh, follicular helper T cell; IL, interleukin.
One of our recent studies provides a potential mechanism by which atherogenic conditions mediate Tfh cell differentiation and the following autoimmune response. Bone marrow cells from lupus-prone BXD2 mouse were transferred into bone marrow-ablated ApoE-deficient or wild-type mouse. Compared to the wild-type mouse, the ApoE-deficient mouse displayed a surge of autoreactive CXCR3+ Tfh cells and B cells that led to an escalation of total IgG and IgG2c autoantibodies to dsDNA and rheumatoid factors. Among the elevated cytokines in the sera of dyslipidemic mice, IL-27 produced by Toll-like receptor 4-dependent CD11b+ splenic dendritic cell alone was sufficient to generate germinal center reactions and Tfh cells in ApoE-deficient atherogenic mouse (Fig. 1). This inflation of IL-27 is not only observed in atherogenic mice but also in humans as patients with hypercholesterolemia display a correlation between increased IL-27 and circulating IgG.37 Therefore, we postulate that hyperlipidemic condition facilitates the generation of autoimmune Tfh cell responses via IL-27 and that this proposed axis has relevance in both humans and mice (Fig. 1).
Cancer
Depending on a specific signal or a factor provided to the adaptive immune cells, they could either enhance or inhibit tumorigenesis. For instance, cytokines such as interferon gamma (IFN-γ) and tumor necrosis factor alpha (TNF-α) enable cytotoxic CD8+ T cells to lyse tumor cells, but type 2 cytokines or IL-2 could induce pro-tumorigenic Th2 and regulatory T cells. Like the cytokine, cholesterol acts as a signal to the adaptive immune cells to mediate immune responses against the tumor cells.42,43,44,45,46,47
Tumor cells are known to upregulate cholesterol synthesis through sterol regulatory-element binding protein (SREBP) and 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR), inducing tumor-infiltrating CD8+ T cell exhaustion (Fig. 2).43,44,45,46,47 In order to reverse this pro-tumorigenic process, statins are used to inhibit HMGR, which is a rate-limiting enzyme in converting 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) into mevalonate. As a result, the use of statins is negatively correlated with cancer grade, cancer risk, and cancer-specific mortality.48,49,50 One prime study utilizes an adoptive T-cell transfer model in which hgp10025-33 pulsed splenocytes from Pmel-1 mice are injected intravenously into melanoma infected C57BL/6 mice. Intracellular cholesterol levels and exhaustion phenotypes of tumor-infiltrating CD8+ T cells are significantly higher than those of CD8+ T cells in the spleen or draining lymph node, suggesting that the immune checkpoint expression elevation and intracellular cholesterol buildup occur after they enter the tumor bed.47 Also, treatment of avasimibe enhances cytotoxicity of CD8+ T cells in melanoma-bearing mouse. Avasimibe is a pharmacological drug to treat atherosclerosis by inhibiting acetyl-CoA acetyltransferase 1 (ACAT1), a key cholesterol esterification enzyme. Thus, avasimibe administration decreases plasma membrane cholesterol level in CD8+ T cell alone, which causes enhanced T-cell receptor clustering and signaling as well as more efficient formation of the immunological synapse to effectively kill melanoma.43
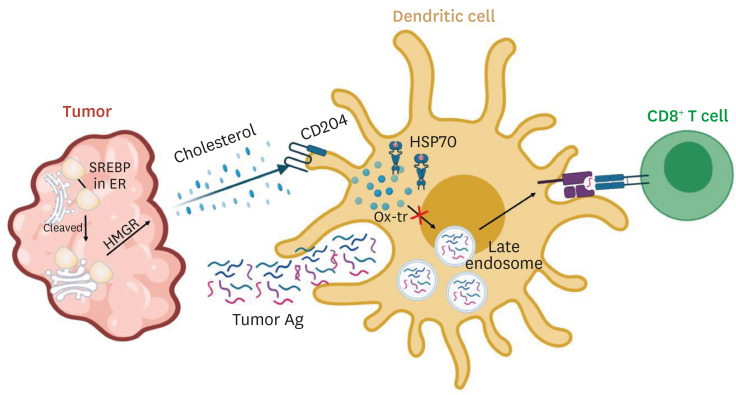
Fig. 2. Tumors incapacitate anti-tumor immunity by regulating lipid metabolism.
The tumor itself creates a hyperlipidemic tumor microenvironment via cleavage of SREBP and the subsequent conversion of HMG-CoA into mevalonate. This tumor intrinsic-pathway elevates the uptake of oxidatively truncated lipids by overexpressing CD204 in dendritic cells and inhibits their cross-presentation by impeding the translocation of lysosomes with amassed pMHC. Prevention of lipid buildup in the dendritic cells may play a crucial role in anti-tumor immunity.
SREBP, sterol regulatory-element binding protein; HMG-CoA, 3-hydroxy-3-methylglutaryl-CoA; pMHC, peptide-MHC class I complexes.
Dendritic cells act as immune surveillance owing to their ability to detect and present tumor-associated antigens to enable various antitumor effector cells. However, tumor-associated dendritic cells in both humans and mice are known to be defective in their capacity to present antigens.51,52,53,54,55 Emerging evidence show that intracellular lipid accumulation is the primary cause for this dysfunctionality.56,57,58 Dendritic cells from tumor-bearing mice and patients with non-small-cell lung carcinoma and head and neck cancer all upregulate their expression of scavenger receptor A, rapidly taking in extracellular triglycerides. And inhibition of acetyl-CoA carboxylase, a critical element in the fatty acid synthesis, revives the anti-tumor activity of the dendritic cells.56 Cross-presentation, crucial for its activation of cytotoxic CD8+ T cells, is also compromised in various tumors. This defect is due to the deposition of lipid bodies containing electrophilic oxidatively truncated (ox-tr) lipids inside the dendritic cells by overexpressing CD204. Ox-tr covalently attaches to chaperone heat shock protein 70, which stabilizes lysosomes trafficking peptide-MHC class I complexes (pMHC). The interaction causes the accumulation of pMHC inside the lysosome and prevents its translocation to the cell surface (Fig. 2).58 Not only that, dendritic cells cultured in the presence of adipocyte-conditioned media from obese subjects or colorectal patients enhance their expression of PD-L1 and PD-L2 and reduce their secretion of IL-12 and IL-10 ratio.57
Infection
Epidemiological studies demonstrate that cholesterol, LDL, and high-density lipoprotein (HDL) levels all increase the likelihood of developing an infection. Also, mortality among patients with sepsis, one of the direst symptoms of an infection, is foreseen by the degree of reduction in the HDL and the total cholesterol levels.59,60,61,62 Cholesterol-reducing drugs such as statins, ezetimibe, and zetia are all implicated in the amelioration of various infections such as typhoid fever and murine cytomegalovirus.63,64 This link between dyslipidemia and infection susceptibility is well-documented in numerous studies. For instance, ApoE- and LDL receptor-deficient mice are susceptible to L. monocytogenes, K. pnemoniae, C. albicans, and lymphocytic choriomeningitis mammarenavirus (LCMV), and those with impaired immunity such as SCID mice, IFN-γ-deficient, and OVA-specific TCR transgenic mice are heavily affected by tuberculosis.65,66,67,68,69,70
T cells play a central role in our fight against infections by killing infected cells using granzyme B and perforin or by releasing pro-inflammatory mediators that recruit other effector cells to the infected site.68,69,71 Hypercholesterolemic ApoE- and LDL receptor-deficient mice do not exhibit any change during the initial spread of LCMV, but the clearance of the virus is significantly delayed in the spleen and nonlymphoid organs including the liver. Activation and recruitment of LCMV-specific CD8+ T cells are suppressed in hypercholesteremic mice with impaired IFN-γ production and cytotoxicity.66 Not only that, ApoE knockout mouse fed with high cholesterol diet displays the direst symptoms associated with tuberculosis (TB) including heavy bacterial burden, severe lung inflammation, and early mortality. Surprisingly, the mortality rate of the hypercholesterolemic mouse matches that of the IFN-γ deficient mouse, the most TB-susceptible strain. Impaired priming of the adaptive immune system is the cause of the susceptibility since, despite an effective Th1 response, ApoE-deficient mouse fed with high cholesterol diet (HC) has a much-delayed immune response. Also, OT-II cells, which are introduced to ApoE-deficient HC mice via intravenous injection and stimulated by OVA-coated iron beads via subcutaneous injection, do not proliferate contrary to those in WT HC mice.72 CD4+ T cells with increased cholesterol efflux via ATP-binding cassette transporter 1 (ABCA1) reduce the susceptibility to HIV-1 infection. The effect was reversible with cholesterol replenishment.73
Dendritic cells are responsible for activating T cells by presenting virus-specific antigens via MHC molecules and differentiating T cells by cytokine stimulation to effectively destroy infected cells.74,75 Once stimulated via toll-like receptors, dendritic cells from the obese individuals compared to the lean individuals', upregulate twofold more of IL-10, inducing a heightened level of IL-4 production from the T cells that dampens anti-viral Th1-type immunity.76 Cholesterol accumulation in dendritic cells of ApoE-deficient mice reduces activation of CD8α- dendritic cells. This inactivation impairs Th1 cell responses while enhancing Th2 cell response, consequently leading to an increased susceptibility to L. major. The dampened immune response is driven by oxidized low-density lipoprotein (oxLDL) via inhibition of nuclear factor kappa light chain enhancer of activated B cells (NF-κB) nuclear translocation and toll-like receptor (TLR)-mediated signaling in CD8α- dendritic cells.77
CHOLESTEROL METABOLISM IN ADAPTIVE IMMUNITY
Cholesterol is one of the major components of the plasma membrane, particularly in lipid rafts, and a critical source of energy. It is well-known that dyslipidemia triggers pro- or anti-inflammatory responses from the innate and adaptive immune system. Particularly, TLR activation in macrophages leads to cholesterol accumulation via inhibition of cholesterol efflux, resulting in enhanced inflammasome activation and subsequent inflammatory responses.78,79,80 Cholesterol efflux (reverse cholesterol transport) in macrophages via ATP-binding cassette (ABC) transporters such as ABCA1 and ATP-binding cassette subfamily G member 1 (ABCG1) induces anti-inflammatory effects by suppressing TLR signaling.81 Genes encoding these transporters are known to be induced by a transcription factor called liver X receptor (LXR), which is activated by intermediates from the cholesterol biosynthetic pathway such as desmosterol and cholesterol-derived oxysterols. Although TLR activation decreases cholesterol efflux and enhances cholesterol accumulation, LXR is also activated by cholesterol accumulation and suppresses inflammatory responses. Desmosterol accumulation by inhibiting desmosterol reductase via cholesterol loading causes LXR activation and reduction in the expression of pro-inflammatory genes.82 Since cholesterol metabolism's impact on the innate immune system is well-established, we will direct our focus on defining the relationship between cholesterol metabolism and the adaptive immune system.
Dendritic cell (DC)
Dendritic cells are antigen-presenting cells that link the innate immune system to the adaptive immune system. One study proposed that the ability of antigen presentation to CD4+ T cells is comparable between DCs isolated from normal and hypercholesterolemic mice.83 As in macrophages, cholesterol accumulation in DCs leads to inflammasome activation and enhanced secretion of IL-1β and IL-18. The deficiency of cholesterol transporters, ABCA1 and ABCG1 that excrete cholesterol from the cells, causes cholesterol accumulation in dendritic cells and skews DCs to CD11b+ inflammatory DCs, resulting in the activation of NLRP3 inflammasome. Furthermore, the lack of cholesterol efflux promotes the secretion of cytokines such as IL-12, IL-8, and IL-23. These pro-inflammatory cytokines drive T cell differentiation towards Th1 and Th17 cells, which mediate autoimmune responses in vivo.36
Cellular sterols are known to be sensed by lipid-activated transcription factor such as LXR, and several lines of evidence suggest that LXR regulates the function of DCs. In particular, LXRα is upregulated during the differentiation of human monocyte-derived DCs, while LXRβ remains at a low level.84 Although LXR activation inhibits LPS-dependent DC maturation and hinders its ability to stimulate T cells, it does not change the expression of antigen-presenting molecules including major histocompatibility complex class I and II. During the DC maturation via LPS, LXR inhibits the expression of actin-bundling protein fascin, an essential component in immune synapse formation, reducing its ability to activate T cells.84 In addition, LXR is known to repress the production of pro-inflammatory cytokines such as TNFα and IL-6 in DCs and inhibit the TLR-induced expression of CCR7, undermining their migration to the chemokine CCL19/CCL21-expressing tissues.85,86 Also, prostaglandin E2 (PGE2), a crucial component in the chemokine induced-migration of DCs, has a role in inhibiting the expression of LXRα in human dendritic cells.86,87 Although these recent findings strongly propose a link between cholesterol metabolism and immune responses in DCs, further studies will be needed to uncover the underlying molecular mechanisms and define its role in the pathogenesis of dyslipidemia-related immune disorders in humans.
T cell
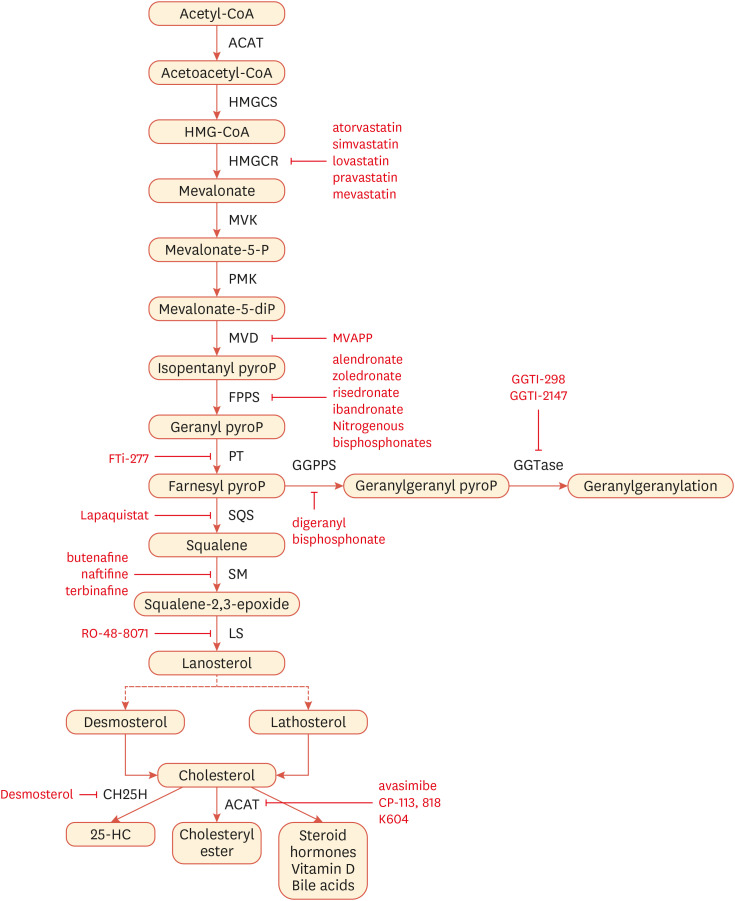
Since newly activated T cells require biosynthetic programs and energy for their clonal expansion, metabolism reprogramming is one of the prerequisites for T cell activation.88,89 Also, cholesterol forms lipid rafts in which TCR signaling complexes are clustered.90,91 Therefore, it is feasible to surmise that cholesterol metabolism is important for controlling T cell proliferation and functions. T cells enhance cholesterol biosynthesis upon activation for their growth and proliferation. For instance, αCD3-induced stimulation of T lymphocytes results in the induction of HMG-CoA reductase, an enzyme involved in the cholesterol biosynthesis required for cell cycle progressionocation to the cell surfac (Fig. 3). Inhibition of the mevalonate pathway via statin, an inhibitor of HMG-CoA reductase, or cholesterol derivatives suppresses αCD3-induced T cell mitogenesis.92 Here, we delve into the role of cholesterol metabolism in T cells in detail.
Fig. 3. Cholesterol synthesis pathways can be targeted by small molecules.
Cholesterol biosynthesis takes place in all mammalian cells including immune cells. The enzymes that mediate the synthesis of indicated sterol intermediates and inhibitors of each enzyme are shown.
CD4+ T cell
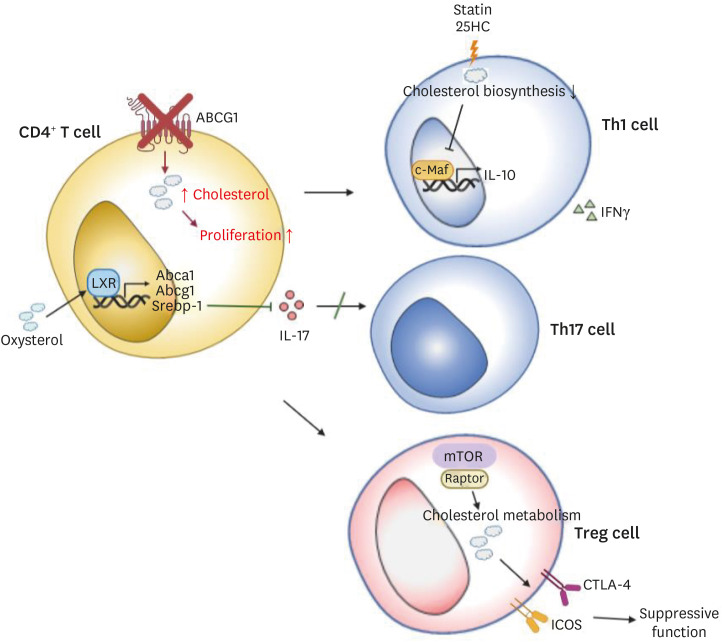
Although activated T cells induce cholesterol biosynthetic pathways for their growth, LXR inhibits T lymphocyte proliferation by promoting cholesterol efflux via cholesterol transporters such as ABCA1 and ABCG1. T cell activation promotes the induction of sulfotransferase family 2B member 1 (SULT2B1), which is an oxysterol-metabolizing enzyme, and thus suppresses the LXR pathway.93 ABCG1, which is induced by LXR, is known as a negative regulator of CD4+ T cell proliferation. Cholesterol accumulation by the removal of Abcg1 in CD4+ T cells enhances proliferation of the T cells (Fig. 4) via enhanced lipid raft formation and phosphorylation of TCR signaling molecules such as zeta-chain-associated protein kinase 70 (ZAP70) and extracellular signal-regulated protein kinases 1 and 2 (ERK1/2).94 By contrast, another study has shown that LXRβ-deficient CD4+ T cells exhibit reduced proliferation compared to LXRβ-sufficient ones.95
Fig. 4. Intracellular cholesterol metabolism controls the proliferation and the differentiation of helper or regulatory T cells.
Cholesterol accumulation promotes proliferation in CD4+ T cells. LXR, an important transcription factor regulating cholesterol metabolism, inhibits Th17 differentiation. Moreover, cholesterol biosynthesis regulates the immune resolution by coordinating the switching of IFNγ+ into IL-10 in Th1 cells. mTORC1 also contributes to the immune resolution in Treg cells via regulation of cholesterol metabolism.
LXR, liver X receptor; IFN, interferon; mTOR, mechanistic target of rapamycin; IL, interleukin.
In addition to the T cell activation and proliferation, cholesterol metabolism is also related to T cell differentiation and effector functions. It is reported that LXR suppresses Th17 cell polarization of naïve CD4+ T cells and thus mitigates the onset of experimental autoimmune encephalomyelitis in mice.96 LXR negatively regulates Th17 cell differentiation in mice by reducing the expression of retinoic acid-related orphan receptor gamma t (RORγt) which is a crucial transcription factor for Th17 cell differentiation and Th17-related genes including Il17, Il22, Il23r, and Ahr in Th17 cells. LXR regulates the expression of SREBP-1 isoforms, SREBP-1a and SREBP-1c. SREBP-1 binds to Il17 promoter and suppresses Th17 cell differentiation (Fig. 4), and it also inhibits AhR, a positive regulator of the Th17 differentiation. This effect of LXR is also shown in human Th17 cells.96 These findings suggest the potential role of LXR in the mitigation of autoimmunity by suppressing Th17 cell differentiation via SREBP-1. On the one hand, a recent study reports that immune activation is exacerbated due to the dysfunctional regulatory T cells (Treg), while the number of CD4+ T cells is decreased in LXRβ-deficient mice.95 SREBPs also contribute to the regulation of Treg cell functions, specifically in the context of the tumor microenvironment.97 In tumors, steroid receptor RNA activator protein/SREBP signaling is important in Treg cells to maintain their suppressive ability and programmed cell death protein 1 (PD-1) expression. By contrast, SCAP is not necessary for steady-state Treg cells, indicating a context-specific requirement of this pathway in Treg cell homeostasis. SREBP cleavage-activating protein (SCAP)/SREBP signaling induces PD-1 expression in Treg cells in a TCR signaling-dependent manner. Furthermore, geranylgeranyl pyrophosphate-dependent geranylgeranylation induced by SCAP/SREBP promotes PD-1 expression in Treg cells. SREBP and PD-1 suppress IFNγ expression in Treg cells by controlling phosphatidylinositol-3-kinase (PI3K) signaling in the tumors. Targeting SCAP/SREBP signaling in Treg cells presents antitumor effects without unleashing any autoimmune responses in vivo,97 suggesting that it can be a potent target for cancer immunotherapy. The mechanistic target of rapamycin (mTOR) also links cholesterol metabolism to Treg cell functions. Raptor/mTORC1 in Treg cells plays an important role in the proliferation of Treg cells and their suppressive activity by promoting cholesterol metabolism.98 Moreover, treatment of atorvastatin of 25-hydroxycholesterol in human Th1 cells to inhibit cholesterol biosynthetic pathways impedes immune resolution by suppressing the switch from IFNγ+ to IL-10+ phenotype in Th1 cells via decreased c-Maf, a master transcription regulator for IL-10 in CD4+ T cells (Fig. 4).91 Therefore, disruption in cholesterol metabolism may trigger inflammatory diseases such as rheumatoid arthritis (RA) in humans.99
CD8+ T cell
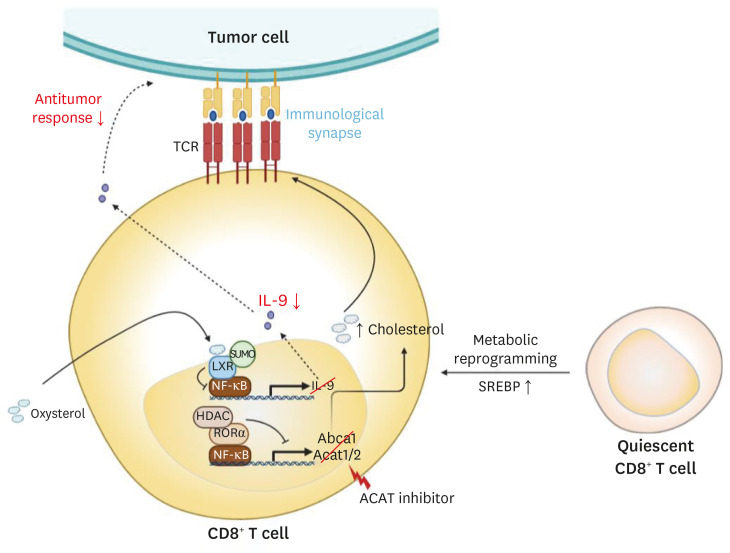
SREBP can activate the expression of genes encoding cholesterol metabolism-related molecules. SREBP has an important role in CD8+ T cell activation by controlling the lipid biosynthesis program in a context-dependent manner as observed in Treg cells.97 SREBP is not necessary for quiescent CD8+ T cells, but for activated CD8+ T cells. Therefore, SREBP contributes to the metabolic reprogramming of CD8+ T cells during activation.100 In addition to the role of LXR in innate immune cells and CD4+ T cells, LXR also contributes to controlling the activation and functions of CD8+ T cells (Fig. 5). As described above, LXR and SREBP are regulated reciprocally during T cell activation, and LXR inhibits lymphocyte proliferation but not its activation (Fig. 4).93 In particular, LXR regulates CD8+ T cell functions by promoting IL-9 secretion. Cholesterol or cholesterol-derived oxysterol inhibits the expression of IL-9 by reducing p65 binding to Il9 promoter via LXR SUMOylation.46 Consistently, Tc9 cells, which exert stronger antitumor responses than Tc1 cells, have lower levels of cholesterol compared to Tc1 cells in mice (Fig. 5).46
Fig. 5. Cholesterol metabolism regulates the activation of CD8+ T cells and the antitumor activity of Tc9 cells. Cholesterol biosynthetic pathways are required for CD8+ T cell in a context-dependent manner, and so they play a crucial part in its anti-tumor efficacy. Increased cholesterol levels promote strong TCR signaling and consequently result in enhanced effector functions of CD8+ T cells. On the other hand, LXR SUMOylation by cholesterol-induced oxysterol reduces the antitumor response in Tc9 cells.
TCR, T cell receptor; LXR, liver X receptor; HDAC, histone deacetylase; RORα, retinoic acid-related orphan receptor α; SREBP, sterol regulatory-element binding protein; IL, interleukin.
Although both cholesterol biosynthesis and efflux are crucial to the regulation of the adaptive immune responses, cholesterol esterification is equally paramount in immune cell activation and function. Cholesterol esterification converts cholesterol into cholesteryl ester, one of the constituents of lipoproteins. ACAT is an enzyme mediating cholesterol esterification, and its two isoforms are ACAT1 and ACAT2. Ablation of ACAT1 in T cells or inhibition of cholesterol esterification by ACAT inhibitors can enhance the proliferation of CD8+ T cells and CD8+ effector function, resulting in the suppression of tumor growth (Fig. 5).43 As described above, cholesterol clusters TCR signaling complexes.90,91 Inhibition of cholesterol esterification in T cells also enhances TCR clustering and promotes efficient immunological synapse formation for CD8+ T cell proliferation and effector function.43 Unlike CD8+ T cells, ACAT1-deficient CD4+ T cells do not display any differences in their function. It might be due to the compensation of ACAT2 for ACAT1 deficiency.43 Lee et al.101 recently showed that retinoic acid-related orphan receptor α (RORα) regulates cholesterol levels in CD8+ T cells by controlling the expression of NF-κB target genes containing Acat1 and Abcg1. They suggest that RORα and histone deacetylase (HDAC) enhance cholesterol levels in CD8+ T cells, resulting in an enhanced TCR signaling, proliferation, and effector functions of CD8+ T cells (Fig. 5). Thus, cholesterol metabolism and sterol-sensing transcription factors have critical regulatory roles in the differentiation and function of T cells.
B cell
Cholesterol biosynthetic pathway is well-known to be involved in the formation of germinal center (GC) B cell. RHO-associated coiled-coil-containing protein kinase 2 (ROCK2) in GC B cells is known to promote GC formation and cholesterol biosynthesis by regulating transcriptional programs.102 ROCK2 phosphorylates IRF8 and supports its interaction with SREBP2, leading to the enhanced expression of cholesterol biosynthesis-related genes.102 Since it is known that IRF8 is linked to inflammation particularly in vascular cells,103 it is possible that ROCK2 links cholesterol biosynthesis to inflammatory responses via IRF8 and SREBP.
As mentioned above, LXR activation also contributes to immune tolerance through its ability to promote apoptotic cell clearance via macrophages.104 LXRα and LXRβ double-deficient mice are shown to spontaneously develop autoantibodies and lupus-like autoimmune inflammation. Engulfment of apoptotic cells by macrophages leads to the accumulation of cellular components including cholesterol and consequently activates LXR signaling. LXR promotes the expression of Mer receptor tyrosine kinase (Mertk) in macrophages leading to the clearance of apoptotic cells, forming a positive feedback loop to further ingest apoptotic cells and suppress inflammatory responses. Accordingly, LXR activation can counteract the development of autoimmune disease by inhibiting autoantibody production by B cells and maintaining immune tolerance.104
On the contrary, lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) is expressed on dendritic cells and promotes humoral responses, leading to B cell differentiation and secretion of immunoglobulin.105 OxLDL, which acts as a ligand of LOX-1, is a well-established risk factor in the development of atherosclerosis. LOX-1 on DCs promotes B cell differentiation into plasmablasts by reducing paired box 5 (Pax5) and enhancing B lymphocyte-induced maturation protein 1 (Blimp-1) expression. In addition, LOX-1 on DCs induces cytokines, proliferation-inducing ligands (APRIL), and B cell-activating factors (BAFF) which all contribute to B cell proliferation, differentiation, class-switching, and plasma cell survival, promoting antibody production. Furthermore, LOX-1 on B cells plays an important part in humoral immune responses by guiding B cells into lymphoid tissues via lymphoid organ homing receptor CCR7.105 Together, LOX-1 stimulated by oxLDL is expressed in both DCs and B cells and contributes to enhanced humoral immune responses in vivo.
Geranylgeranyl pyrophosphate (GGPP) is one of the derivatives of mevalonate. It is required for protein isoprenylation and links cholesterol metabolism and B cell immunity. Geranylgeranylation of proteins such as small GTPase, Ras, and Rho, regulates its functions. In B cells, geranylgeranylation controls CD40-dependent B cell activation. Blocking geranylgeranylation via geranylgeranyl transferase inhibitor (GGTI) suppresses CD40-dependent B cell activation while exerting minute effects on the production of cytokines and chemokines by activated B cells.106 Moreover, GGPP regulates the function of regulatory B cells. The metabolic intermediate of mevalonate, GGPP, enhances IL-10 production in regulatory B cells and promotes their ability to suppress Th1 responses.107 IL-10 is controlled via the RAS-PI3Kδ-protein kinase B-glycogen synthase kinase 3 (RAS-PI3Kδ-AKT-GSK3) pathway by GGPP and in a BLIMP-dependent manner.107 Although not common, Epstein-Barr virus (EBV) leads to the expression of the mevalonate biosynthetic pathway-related genes by targeting transcription factors such as MYC and SREBP by EBV-encoded EBV nuclear antigen 2 (EBNA2) and subsequently promotes B cell proliferation and survival.108 Mevalonate-derived GGPP contributes to the geranylgeranylation of Ras-associated binding (Rab) protein. EBV nuclear antigen 3C (EBNA3C) from EBV enhances the expression of Rab13. Together, EBV leads to the proliferation and survival of the infected B cells by promoting the expression of cholesterol biosynthetic pathway, synthesizing GGPP, and activating Rab.108 Thus, lipid metabolism critically regulates the differentiation of and antibody production from B cells. Additional studies will be needed to address whether targeting lipid metabolism can improve the antibody production from B cells in vaccination settings and ameliorate the pathogenesis of antibody-mediated immune disorders in humans.
CONCLUSION AND PERSPECTIVES
Dyslipidemia is a well-established risk factor of cardiovascular diseases in humans; however, it is becoming clearer that it also significantly impacts the pathogenesis of immune disorders, as discussed above (Fig. 6). In particular, accumulating evidences have demonstrated that a number of lipid species can be sensed by innate immune cells including macrophages and dendritic cells, which in turn can regulate adaptive immune cells. In addition, lipid species within immune cells also play a critical role, not only in the proliferation and signal transduction as shown in other cell types but also in the functions of innate and adaptive immune cells.
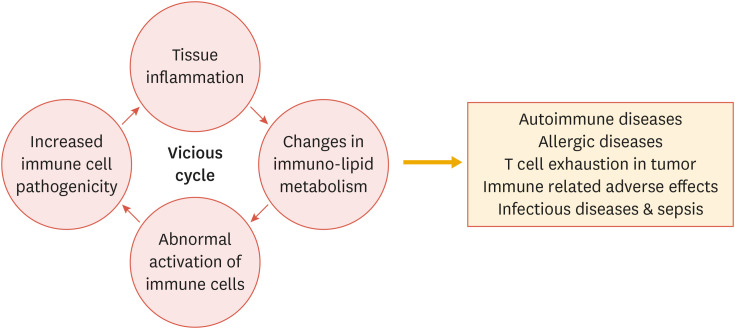
Fig. 6. Lipid metabolism is involved in the pathogenesis of immune disorders.
Environmental dyslipidemia as well as disturbed cellular lipid metabolism critically impacts the aberrant activation and differentiation of immune cells, leading to enhanced pathogenicity of immune cells that triggers tissue inflammation. As a result, unbalanced lipid metabolism contributes to the development of a wide range of immune disorders including autoimmune diseases, allergies, cancers, and infectious diseases. Targeting lipid metabolic pathways may be effective in ameliorating immune disorders in humans.
As a consequence, levels of extracellular, as well as intracellular lipid species, are critical determinants of the outcome of adaptive immune responses. Moreover, drugs targeting lipid metabolism also critically impact our immune system and defensive mechanisms. As discussed above, several FDA-approved drugs demonstrate the potentials to be used as immunological modulators that can improve antitumor immunity, vaccine efficacy, or ameliorate autoimmunity in animal models. Such immunological aspects of lipid metabolism are of great interest since they will uncover a novel immunopathogenesis regulated by lipid metabolism, and will also pave the development of new therapeutic approaches for immune disorders in humans by targeting lipid metabolism. Given that a number of enzymes and transcription factors in lipid metabolism are proven to be effective and safe targets in humans, further interdisciplinary researches involving immunology and lipid metabolism would be an important field of translational and clinical researches.
ACKNOWLEDGEMENTS
We thank the entire Chung lab for suggestion and discussion.
Footnotes
Funding: This study was supported by the National Research Foundation of Korea 2020R1A3B207889011 (Y. C).
Conflict of Interest: The authors declare no competing financial interest.
- Conceptualization: Kim D, Chung H, Lee JE, Kim J, Chung Y.
- Funding acquisition: Chung Y.
- Supervision: Chung Y.
- Visualization: Kim D, Chung H, Chung Y
- Writing - original draft: Kim D, Chung H, Chung Y
- Writing - review & editing: Lee JE, Kim J, Hwang J, Chung Y.
References
- 1.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 2.Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17:1410–1422. doi: 10.1038/nm.2538. [DOI] [PubMed] [Google Scholar]
- 3.Hansson GK, Libby P. The immune response in atherosclerosis: a double-edged sword. Nat Rev Immunol. 2006;6:508–519. doi: 10.1038/nri1882. [DOI] [PubMed] [Google Scholar]
- 4.Weber C, Zernecke A, Libby P. The multifaceted contributions of leukocyte subsets to atherosclerosis: lessons from mouse models. Nat Rev Immunol. 2008;8:802–815. doi: 10.1038/nri2415. [DOI] [PubMed] [Google Scholar]
- 5.Grundtman C, Wick G. The autoimmune concept of atherosclerosis. Curr Opin Lipidol. 2011;22:327–334. doi: 10.1097/MOL.0b013e32834aa0c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 7.Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stewart CR, Stuart LM, Wilkinson K, van Gils JM, Deng J, Halle A, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagy L, Tontonoz P, Alvarez JG, Chen H, Evans RM. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell. 1998;93:229–240. doi: 10.1016/s0092-8674(00)81574-3. [DOI] [PubMed] [Google Scholar]
- 11.Chawla A, Barak Y, Nagy L, Liao D, Tontonoz P, Evans RM. PPAR-gamma dependent and independent effects on macrophage-gene expression in lipid metabolism and inflammation. Nat Med. 2001;7:48–52. doi: 10.1038/83336. [DOI] [PubMed] [Google Scholar]
- 12.Moore KJ, Rosen ED, Fitzgerald ML, Randow F, Andersson LP, Altshuler D, et al. The role of PPAR-gamma in macrophage differentiation and cholesterol uptake. Nat Med. 2001;7:41–47. doi: 10.1038/83328. [DOI] [PubMed] [Google Scholar]
- 13.Goodson N, Marks J, Lunt M, Symmons D. Cardiovascular admissions and mortality in an inception cohort of patients with rheumatoid arthritis with onset in the 1980s and 1990s. Ann Rheum Dis. 2005;64:1595–1601. doi: 10.1136/ard.2004.034777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.John H, Kitas G, Toms T, Goodson N. Cardiovascular co-morbidity in early rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2009;23:71–82. doi: 10.1016/j.berh.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Nurmohamed MT. Cardiovascular risk in rheumatoid arthritis. Autoimmun Rev. 2009;8:663–667. doi: 10.1016/j.autrev.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 16.Stamatelopoulos KS, Kitas GD, Papamichael CM, Chryssohoou E, Kyrkou K, Georgiopoulos G, et al. Atherosclerosis in rheumatoid arthritis versus diabetes: a comparative study. Arterioscler Thromb Vasc Biol. 2009;29:1702–1708. doi: 10.1161/ATVBAHA.109.190108. [DOI] [PubMed] [Google Scholar]
- 17.Henseler T, Christophers E. Disease concomitance in psoriasis. J Am Acad Dermatol. 1995;32:982–986. doi: 10.1016/0190-9622(95)91336-x. [DOI] [PubMed] [Google Scholar]
- 18.Mallbris L, Akre O, Granath F, Yin L, Lindelöf B, Ekbom A, et al. Increased risk for cardiovascular mortality in psoriasis inpatients but not in outpatients. Eur J Epidemiol. 2004;19:225–230. doi: 10.1023/b:ejep.0000020447.59150.f9. [DOI] [PubMed] [Google Scholar]
- 19.Kremers HM, McEvoy MT, Dann FJ, Gabriel SE. Heart disease in psoriasis. J Am Acad Dermatol. 2007;57:347–354. doi: 10.1016/j.jaad.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 20.Kimball AB, Robinson D, Jr, Wu Y, Guzzo C, Yeilding N, Paramore C, et al. Cardiovascular disease and risk factors among psoriasis patients in two US healthcare databases, 2001-2002. Dermatology. 2008;217:27–37. doi: 10.1159/000121333. [DOI] [PubMed] [Google Scholar]
- 21.Manzi S, Meilahn EN, Rairie JE, Conte CG, Medsger TA, Jr, Jansen-McWilliams L, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol. 1997;145:408–415. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- 22.Esdaile JM, Abrahamowicz M, Grodzicky T, Li Y, Panaritis C, du Berger R, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001;44:2331–2337. doi: 10.1002/1529-0131(200110)44:10<2331::aid-art395>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 23.Roman MJ, Shanker BA, Davis A, Lockshin MD, Sammaritano L, Simantov R, et al. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N Engl J Med. 2003;349:2399–2406. doi: 10.1056/NEJMoa035471. [DOI] [PubMed] [Google Scholar]
- 24.Morrow WJ, Ohashi Y, Hall J, Pribnow J, Hirose S, Shirai T, et al. Dietary fat and immune function. I. Antibody responses, lymphocyte and accessory cell function in (NZB x NZW)F1 mice. J Immunol. 1985;135:3857–3863. [PubMed] [Google Scholar]
- 25.Yumura W, Hattori S, Morrow WJ, Mayes DC, Levy JA, Shirai T. Dietary fat and immune function. II. Effects on immune complex nephritis in (NZB x NZW)F1 mice. J Immunol. 1985;135:3864–3868. [PubMed] [Google Scholar]
- 26.Winer S, Paltser G, Chan Y, Tsui H, Engleman E, Winer D, et al. Obesity predisposes to Th17 bias. Eur J Immunol. 2009;39:2629–2635. doi: 10.1002/eji.200838893. [DOI] [PubMed] [Google Scholar]
- 27.Jhun JY, Yoon BY, Park MK, Oh HJ, Byun JK, Lee SY, et al. Obesity aggravates the joint inflammation in a collagen-induced arthritis model through deviation to Th17 differentiation. Exp Mol Med. 2012;44:424–431. doi: 10.3858/emm.2012.44.7.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naldi L, Parazzini F, Peli L, Chatenoud L, Cainelli T. Dietary factors and the risk of psoriasis. Results of an Italian case-control study. Br J Dermatol. 1996;134:101–106. [PubMed] [Google Scholar]
- 29.Rucević I, Perl A, Barisić-Drusko V, Adam-Perl M. The role of the low energy diet in psoriasis vulgaris treatment. Coll Antropol. 2003;27(Suppl 1):41–48. [PubMed] [Google Scholar]
- 30.Reich K, Nestle FO, Papp K, Ortonne JP, Evans R, Guzzo C, et al. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet. 2005;366:1367–1374. doi: 10.1016/S0140-6736(05)67566-6. [DOI] [PubMed] [Google Scholar]
- 31.Chang SH, Park H, Dong C. Act1 adaptor protein is an immediate and essential signaling component of interleukin-17 receptor. J Biol Chem. 2006;281:35603–35607. doi: 10.1074/jbc.C600256200. [DOI] [PubMed] [Google Scholar]
- 32.Ghazizadeh R, Tosa M, Ghazizadeh M. Clinical improvement in psoriasis with treatment of associated hyperlipidemia. Am J Med Sci. 2011;341:394–398. doi: 10.1097/MAJ.0b013e3181ff8eeb. [DOI] [PubMed] [Google Scholar]
- 33.Yu HH, Chen PC, Yang YH, Wang LC, Lee JH, Lin YT, et al. Statin reduces mortality and morbidity in systemic lupus erythematosus patients with hyperlipidemia: a nationwide population-based cohort study. Atherosclerosis. 2015;243:11–18. doi: 10.1016/j.atherosclerosis.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 34.Tilley BJ, Cook JL, Docking SI, Gaida JE. Is higher serum cholesterol associated with altered tendon structure or tendon pain? A systematic review. Br J Sports Med. 2015;49:1504–1509. doi: 10.1136/bjsports-2015-095100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alvarez P, Genre F, Iglesias M, Augustin JJ, Tamayo E, Escolà-Gil JC, et al. Modulation of autoimmune arthritis severity in mice by apolipoprotein E (ApoE) and cholesterol. Clin Exp Immunol. 2016;186:292–303. doi: 10.1111/cei.12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Westerterp M, Gautier EL, Ganda A, Molusky MM, Wang W, Fotakis P, et al. Cholesterol accumulation in dendritic cells links the inflammasome to acquired immunity. Cell Metab. 2017;25:1294–1304.e6. doi: 10.1016/j.cmet.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaddis DE, Padgett LE, Wu R, McSkimming C, Romines V, Taylor AM, et al. Apolipoprotein AI prevents regulatory to follicular helper T cell switching during atherosclerosis. Nat Commun. 2018;9:1095. doi: 10.1038/s41467-018-03493-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ryu H, Lim H, Choi G, Park YJ, Cho M, Na H, et al. Atherogenic dyslipidemia promotes autoimmune follicular helper T cell responses via IL-27. Nat Immunol. 2018;19:583–593. doi: 10.1038/s41590-018-0102-6. [DOI] [PubMed] [Google Scholar]
- 39.Masson W, Lobo M, Molinero G. Psoriasis and cardiovascular risk: a comprehensive review. Adv Ther. 2020;37:2017–2033. doi: 10.1007/s12325-020-01346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ito A, Hong C, Oka K, Salazar JV, Diehl C, Witztum JL, et al. Cholesterol accumulation in CD11c+ immune cells is a causal and targetable factor in autoimmune disease. Immunity. 2016;45:1311–1326. doi: 10.1016/j.immuni.2016.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bagchi S, He Y, Zhang H, Cao L, Van Rhijn I, Moody DB, et al. CD1b-autoreactive T cells contribute to hyperlipidemia-induced skin inflammation in mice. J Clin Invest. 2017;127:2339–2352. doi: 10.1172/JCI92217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang J, Li L, Lian J, Schauer S, Vesely PW, Kratky D, et al. Tumor-induced hyperlipidemia contributes to tumor growth. Cell Reports. 2016;15:336–348. doi: 10.1016/j.celrep.2016.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang W, Bai Y, Xiong Y, Zhang J, Chen S, Zheng X, et al. Potentiating the antitumour response of CD8(+) T cells by modulating cholesterol metabolism. Nature. 2016;531:651–655. doi: 10.1038/nature17412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baek AE, Yu YA, He S, Wardell SE, Chang CY, Kwon S, et al. The cholesterol metabolite 27 hydroxycholesterol facilitates breast cancer metastasis through its actions on immune cells. Nat Commun. 2017;8:864. doi: 10.1038/s41467-017-00910-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Long J, Zhang CJ, Zhu N, Du K, Yin YF, Tan X, et al. Lipid metabolism and carcinogenesis, cancer development. Am J Cancer Res. 2018;8:778–791. [PMC free article] [PubMed] [Google Scholar]
- 46.Ma X, Bi E, Huang C, Lu Y, Xue G, Guo X, et al. Cholesterol negatively regulates IL-9-producing CD8+ T cell differentiation and antitumor activity. J Exp Med. 2018;215:1555–1569. doi: 10.1084/jem.20171576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma X, Bi E, Lu Y, Su P, Huang C, Liu L, et al. Cholesterol induces CD8(+) T cell exhaustion in the tumor microenvironment. Cell Metab. 2019;30:143–156.e5. doi: 10.1016/j.cmet.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jacobs EJ, Rodriguez C, Bain EB, Wang Y, Thun MJ, Calle EE. Cholesterol-lowering drugs and advanced prostate cancer incidence in a large U.S. cohort. Cancer Epidemiol Biomarkers Prev. 2007;16:2213–2217. doi: 10.1158/1055-9965.EPI-07-0448. [DOI] [PubMed] [Google Scholar]
- 49.Kumar AS, Benz CC, Shim V, Minami CA, Moore DH, Esserman LJ. Estrogen receptor-negative breast cancer is less likely to arise among lipophilic statin users. Cancer Epidemiol Biomarkers Prev. 2008;17:1028–1033. doi: 10.1158/1055-9965.EPI-07-0726. [DOI] [PubMed] [Google Scholar]
- 50.Longo J, van Leeuwen JE, Elbaz M, Branchard E, Penn LZ. Statins as anticancer agents in the era of precision medicine. Clin Cancer Res. 2020;26:5791–5800. doi: 10.1158/1078-0432.CCR-20-1967. [DOI] [PubMed] [Google Scholar]
- 51.Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 52.Knight SC. Specialized perinodal fat fuels and fashions immunity. Immunity. 2008;28:135–138. doi: 10.1016/j.immuni.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 53.Harimoto H, Shimizu M, Nakagawa Y, Nakatsuka K, Wakabayashi A, Sakamoto C, et al. Inactivation of tumor-specific CD8+ CTLs by tumor-infiltrating tolerogenic dendritic cells. Immunol Cell Biol. 2013;91:545–555. doi: 10.1038/icb.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shi Y, Yu P, Zeng D, Qian F, Lei X, Zhao Y, et al. Suppression of vascular endothelial growth factor abrogates the immunosuppressive capability of murine gastric cancer cells and elicits antitumor immunity. FEBS J. 2014;281:3882–3893. doi: 10.1111/febs.12923. [DOI] [PubMed] [Google Scholar]
- 55.McDonnell AM, Lesterhuis WJ, Khong A, Nowak AK, Lake RA, Currie AJ, et al. Tumor-infiltrating dendritic cells exhibit defective cross-presentation of tumor antigens, but is reversed by chemotherapy. Eur J Immunol. 2015;45:49–59. doi: 10.1002/eji.201444722. [DOI] [PubMed] [Google Scholar]
- 56.Herber DL, Cao W, Nefedova Y, Novitskiy SV, Nagaraj S, Tyurin VA, et al. Lipid accumulation and dendritic cell dysfunction in cancer. Nat Med. 2010;16:880–886. doi: 10.1038/nm.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Del Cornò M, D'Archivio M, Conti L, Scazzocchio B, Varì R, Donninelli G, et al. Visceral fat adipocytes from obese and colorectal cancer subjects exhibit distinct secretory and ω6 polyunsaturated fatty acid profiles and deliver immunosuppressive signals to innate immunity cells. Oncotarget. 2016;7:63093–63105. doi: 10.18632/oncotarget.10998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Veglia F, Tyurin VA, Mohammadyani D, Blasi M, Duperret EK, Donthireddy L, et al. Lipid bodies containing oxidatively truncated lipids block antigen cross-presentation by dendritic cells in cancer. Nat Commun. 2017;8:2122. doi: 10.1038/s41467-017-02186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iribarren C, Jacobs DR, Jr, Sidney S, Claxton AJ, Feingold KR. Cohort study of serum total cholesterol and in-hospital incidence of infectious diseases. Epidemiol Infect. 1998;121:335–347. doi: 10.1017/s0950268898001435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grion CM, Cardoso LT, Perazolo TF, Garcia AS, Barbosa DS, Morimoto HK, et al. Lipoproteins and CETP levels as risk factors for severe sepsis in hospitalized patients. Eur J Clin Invest. 2010;40:330–338. doi: 10.1111/j.1365-2362.2010.02269.x. [DOI] [PubMed] [Google Scholar]
- 61.Guirgis FW, Donnelly JP, Dodani S, Howard G, Safford MM, Levitan EB, et al. Cholesterol levels and long-term rates of community-acquired sepsis. Crit Care. 2016;20:408. doi: 10.1186/s13054-016-1579-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaysen GA, Ye X, Raimann JG, Wang Y, Topping A, Usvyat LA, et al. Lipid levels are inversely associated with infectious and all-cause mortality: international MONDO study results. J Lipid Res. 2018;59:1519–1528. doi: 10.1194/jlr.P084277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alvarez MI, Glover LC, Luo P, Wang L, Theusch E, Oehlers SH, et al. Human genetic variation in VAC14 regulates Salmonella invasion and typhoid fever through modulation of cholesterol. Proc Natl Acad Sci U S A. 2017;114:E7746–E7755. doi: 10.1073/pnas.1706070114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blanc M, Hsieh WY, Robertson KA, Watterson S, Shui G, Lacaze P, et al. Host defense against viral infection involves interferon mediated down-regulation of sterol biosynthesis. PLoS Biol. 2011;9:e1000598. doi: 10.1371/journal.pbio.1000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Roselaar SE, Daugherty A. Apolipoprotein E-deficient mice have impaired innate immune responses to Listeria monocytogenes in vivo. J Lipid Res. 1998;39:1740–1743. [PubMed] [Google Scholar]
- 66.Ludewig B, Jäggi M, Dumrese T, Brduscha-Riem K, Odermatt B, Hengartner H, et al. Hypercholesterolemia exacerbates virus-induced immunopathologic liver disease via suppression of antiviral cytotoxic T cell responses. J Immunol. 2001;166:3369–3376. doi: 10.4049/jimmunol.166.5.3369. [DOI] [PubMed] [Google Scholar]
- 67.Mogues T, Goodrich ME, Ryan L, LaCourse R, North RJ. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J Exp Med. 2001;193:271–280. doi: 10.1084/jem.193.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Orme IM. Immunology and vaccinology of tuberculosis: can lessons from the mouse be applied to the cow? Tuberculosis (Edinb) 2001;81:109–113. doi: 10.1054/tube.2000.0257. [DOI] [PubMed] [Google Scholar]
- 69.Pearl JE, Saunders B, Ehlers S, Orme IM, Cooper AM. Inflammation and lymphocyte activation during mycobacterial infection in the interferon-gamma-deficient mouse. Cell Immunol. 2001;211:43–50. doi: 10.1006/cimm.2001.1819. [DOI] [PubMed] [Google Scholar]
- 70.Vonk AG, De Bont N, Netea MG, Demacker PN, van der Meer JW, Stalenhoef AF, et al. Apolipoprotein-E-deficient mice exhibit an increased susceptibility to disseminated candidiasis. Med Mycol. 2004;42:341–348. doi: 10.1080/13693780410001657135. [DOI] [PubMed] [Google Scholar]
- 71.Swain SL, McKinstry KK, Strutt TM. Expanding roles for CD4+ T cells in immunity to viruses. Nat Rev Immunol. 2012;12:136–148. doi: 10.1038/nri3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martens GW, Arikan MC, Lee J, Ren F, Vallerskog T, Kornfeld H. Hypercholesterolemia impairs immunity to tuberculosis. Infect Immun. 2008;76:3464–3472. doi: 10.1128/IAI.00037-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jiang H, Badralmaa Y, Yang J, Lempicki R, Hazen A, Natarajan V. Retinoic acid and liver X receptor agonist synergistically inhibit HIV infection in CD4+ T cells by up-regulating ABCA1-mediated cholesterol efflux. Lipids Health Dis. 2012;11:69. doi: 10.1186/1476-511X-11-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sloan JM, Kershaw MH, Touloukian CE, Lapointe R, Robbins PF, Restifo NP, et al. MHC class I and class II presentation of tumor antigen in retrovirally and adenovirally transduced dendritic cells. Cancer Gene Ther. 2002;9:946–950. doi: 10.1038/sj.cgt.7700509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol. 2020;20:7–24. doi: 10.1038/s41577-019-0210-z. [DOI] [PubMed] [Google Scholar]
- 76.O'Shea D, Corrigan M, Dunne MR, Jackson R, Woods C, Gaoatswe G, et al. Changes in human dendritic cell number and function in severe obesity may contribute to increased susceptibility to viral infection. Int J Obes. 2013;37:1510–1513. doi: 10.1038/ijo.2013.16. [DOI] [PubMed] [Google Scholar]
- 77.Shamshiev AT, Ampenberger F, Ernst B, Rohrer L, Marsland BJ, Kopf M. Dyslipidemia inhibits Toll-like receptor-induced activation of CD8alpha-negative dendritic cells and protective Th1 type immunity. J Exp Med. 2007;204:441–452. doi: 10.1084/jem.20061737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Castrillo A, Joseph SB, Vaidya SA, Haberland M, Fogelman AM, Cheng G, et al. Crosstalk between LXR and toll-like receptor signaling mediates bacterial and viral antagonism of cholesterol metabolism. Mol Cell. 2003;12:805–816. doi: 10.1016/s1097-2765(03)00384-8. [DOI] [PubMed] [Google Scholar]
- 79.Zhao GJ, Tang SL, Lv YC, Ouyang XP, He PP, Yao F, et al. Antagonism of betulinic acid on LPS-mediated inhibition of ABCA1 and cholesterol efflux through inhibiting nuclear factor-kappaB signaling pathway and miR-33 expression. PLoS One. 2013;8:e74782. doi: 10.1371/journal.pone.0074782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nat Rev Immunol. 2015;15:104–116. doi: 10.1038/nri3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yvan-Charvet L, Welch C, Pagler TA, Ranalletta M, Lamkanfi M, Han S, et al. Increased inflammatory gene expression in ABC transporter-deficient macrophages: free cholesterol accumulation, increased signaling via toll-like receptors, and neutrophil infiltration of atherosclerotic lesions. Circulation. 2008;118:1837–1847. doi: 10.1161/CIRCULATIONAHA.108.793869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Spann NJ, Garmire LX, McDonald JG, Myers DS, Milne SB, Shibata N, et al. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell. 2012;151:138–152. doi: 10.1016/j.cell.2012.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Packard RR, Maganto-García E, Gotsman I, Tabas I, Libby P, Lichtman AH. CD11c(+) dendritic cells maintain antigen processing, presentation capabilities, and CD4(+) T-cell priming efficacy under hypercholesterolemic conditions associated with atherosclerosis. Circ Res. 2008;103:965–973. doi: 10.1161/CIRCRESAHA.108.185793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Geyeregger R, Zeyda M, Bauer W, Kriehuber E, Säemann MD, Zlabinger GJ, et al. Liver X receptors regulate dendritic cell phenotype and function through blocked induction of the actin-bundling protein fascin. Blood. 2007;109:4288–4295. doi: 10.1182/blood-2006-08-043422. [DOI] [PubMed] [Google Scholar]
- 85.Hanley TM, Blay Puryear W, Gummuluru S, Viglianti GA. PPARgamma and LXR signaling inhibit dendritic cell-mediated HIV-1 capture and trans-infection. PLoS Pathog. 2010;6:e1000981. doi: 10.1371/journal.ppat.1000981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bruckner M, Dickel D, Singer E, Legler DF. Converse regulation of CCR7-driven human dendritic cell migration by prostaglandin E2 and liver X receptor activation. Eur J Immunol. 2012;42:2949–2958. doi: 10.1002/eji.201242523. [DOI] [PubMed] [Google Scholar]
- 87.Scandella E, Men Y, Gillessen S, Förster R, Groettrup M. Prostaglandin E2 is a key factor for CCR7 surface expression and migration of monocyte-derived dendritic cells. Blood. 2002;100:1354–1361. doi: 10.1182/blood-2001-11-0017. [DOI] [PubMed] [Google Scholar]
- 88.MacIver NJ, Michalek RD, Rathmell JC. Metabolic regulation of T lymphocytes. Annu Rev Immunol. 2013;31:259–283. doi: 10.1146/annurev-immunol-032712-095956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Geltink RI, Kyle RL, Pearce EL. Unraveling the complex interplay between T cell metabolism and function. Annu Rev Immunol. 2018;36:461–488. doi: 10.1146/annurev-immunol-042617-053019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bietz A, Zhu H, Xue M, Xu C. Cholesterol metabolism in T cells. Front Immunol. 2017;8:1664. doi: 10.3389/fimmu.2017.01664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reboldi A, Dang E. Cholesterol metabolism in innate and adaptive response. F1000 Res. 2018;7:1647. doi: 10.12688/f1000research.15500.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chakrabarti R, Engleman EG. Interrelationships between mevalonate metabolism and the mitogenic signaling pathway in T lymphocyte proliferation. J Biol Chem. 1991;266:12216–12222. [PubMed] [Google Scholar]
- 93.Bensinger SJ, Bradley MN, Joseph SB, Zelcer N, Janssen EM, Hausner MA, et al. LXR signaling couples sterol metabolism to proliferation in the acquired immune response. Cell. 2008;134:97–111. doi: 10.1016/j.cell.2008.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Armstrong AJ, Gebre AK, Parks JS, Hedrick CC. ATP-binding cassette transporter G1 negatively regulates thymocyte and peripheral lymphocyte proliferation. J Immunol. 2010;184:173–183. doi: 10.4049/jimmunol.0902372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Michaels AJ, Campbell C, Bou-Puerto R, Rudensky AY. Nuclear receptor LXRβ controls fitness and functionality of activated T cells. J Exp Med. 2021;218:e20201311. doi: 10.1084/jem.20201311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cui G, Qin X, Wu L, Zhang Y, Sheng X, Yu Q, et al. Liver X receptor (LXR) mediates negative regulation of mouse and human Th17 differentiation. J Clin Invest. 2011;121:658–670. doi: 10.1172/JCI42974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lim SA, Wei J, Nguyen TM, Shi H, Su W, Palacios G, et al. Lipid signalling enforces functional specialization of Treg cells in tumours. Nature. 2021;591:306–311. doi: 10.1038/s41586-021-03235-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zeng H, Yang K, Cloer C, Neale G, Vogel P, Chi H. mTORC1 couples immune signals and metabolic programming to establish T(reg)-cell function. Nature. 2013;499:485–490. doi: 10.1038/nature12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Perucha E, Melchiotti R, Bibby JA, Wu W, Frederiksen KS, Roberts CA, et al. The cholesterol biosynthesis pathway regulates IL-10 expression in human Th1 cells. Nat Commun. 2019;10:498. doi: 10.1038/s41467-019-08332-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kidani Y, Elsaesser H, Hock MB, Vergnes L, Williams KJ, Argus JP, et al. Sterol regulatory element-binding proteins are essential for the metabolic programming of effector T cells and adaptive immunity. Nat Immunol. 2013;14:489–499. doi: 10.1038/ni.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee IK, Song H, Kim H, Kim IS, Tran NL, Kim SH, et al. RORα regulates cholesterol metabolism of CD8+ T cells for anticancer immunity. Cancers (Basel) 2020;12:E1733. doi: 10.3390/cancers12071733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ricker E, Chinenov Y, Pannellini T, Flores-Castro D, Ye C, Gupta S, et al. Serine-threonine kinase ROCK2 regulates germinal center B cell positioning and cholesterol biosynthesis. J Clin Invest. 2020;130:3654–3670. doi: 10.1172/JCI132414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chmielewski S, Piaszyk-Borychowska A, Wesoly J, Bluyssen HA. STAT1 and IRF8 in vascular inflammation and cardiovascular disease: diagnostic and therapeutic potential. Int Rev Immunol. 2016;35:434–454. doi: 10.3109/08830185.2015.1087519. [DOI] [PubMed] [Google Scholar]
- 104.A-Gonzalez N, Bensinger SJ, Hong C, Beceiro S, Bradley MN, Zelcer N, et al. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity. 2009;31:245–258. doi: 10.1016/j.immuni.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Joo H, Li D, Dullaers M, Kim TW, Duluc D, Upchurch K, et al. C-type lectin-like receptor LOX-1 promotes dendritic cell-mediated class-switched B cell responses. Immunity. 2014;41:592–604. doi: 10.1016/j.immuni.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Shimabukuro-Vornhagen A, Zoghi S, Liebig TM, Wennhold K, Chemitz J, Draube A, et al. Inhibition of protein geranylgeranylation specifically interferes with CD40-dependent B cell activation, resulting in a reduced capacity to induce T cell immunity. J Immunol. 2014;193:5294–5305. doi: 10.4049/jimmunol.1203436. [DOI] [PubMed] [Google Scholar]
- 107.Bibby JA, Purvis HA, Hayday T, Chandra A, Okkenhaug K, Rosenzweig S, et al. Cholesterol metabolism drives regulatory B cell IL-10 through provision of geranylgeranyl pyrophosphate. Nat Commun. 2020;11:3412. doi: 10.1038/s41467-020-17179-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang LW, Wang Z, Ersing I, Nobre L, Guo R, Jiang S, et al. Epstein-Barr virus subverts mevalonate and fatty acid pathways to promote infected B-cell proliferation and survival. PLoS Pathog. 2019;15:e1008030. doi: 10.1371/journal.ppat.1008030. [DOI] [PMC free article] [PubMed] [Google Scholar]