Abstract
Maternal hypertensive disorders during pregnancy (HDP) have been associated with neuropsychiatric problems in offspring. We aim to investigate the associations between specific types of maternal HDP and offspring neurodevelopmental disorders and further examine whether the timing of onset and severity of HDP would affect these associations. The study population consisted of 4,489,044 live-born singletons in Denmark during 1978–2012 and Sweden during 1987–2010. Maternal HDP was categorized into chronic hypertension, gestational hypertension, and pre-eclampsia; pre-eclampsia was further stratified according to timing (early-onset, late-onset), or severity (moderate, severe) of the disease. Neurodevelopmental disorders, including attention-deficit/hyperactivity disorder (ADHD), autism spectrum disorder (ASD), and intellectual disability (ID), were defined by ICD-coded register diagnosis. Cox regression was used to calculate hazard ratios (HR) while adjusting for potential confounders, and sibling analyses assessed the influence of unmeasured shared familial factors. Maternal HDP was associated with increased risks of ADHD (HR, 1.24; 95% confidence interval [CI], 1.20–1.28), ASD (1.29 [1.24–1.34]), and ID (1.58 [1.50–1.66]) in offspring, respectively, which was mostly driven by pre-eclampsia. The strongest associations were observed for early-onset and severe pre-eclampsia, and the corresponding HRs for ADHD, ASD and ID were 1.93 [1.73–2.16], 1.86 [1.61–2.15], and 3.99 [3.42–4.65], respectively. The results were similar in the sibling analyses. The associations between maternal HDP and offspring neurodevelopmental disorders were consistent across the subgroups of sex, preterm status, parity, maternal age and psychiatric disorders. Maternal HDP, especially early-onset pre-eclampsia, are associated with increased risks of ADHD, ASD, and ID in particular, independent of shared familial factors.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10654-021-00756-2.
Keywords: Hypertensive disorders during pregnancy, Pre-eclampsia, Attention-deficit/hyperactivity disorder, Autism spectrum disorder, Intellectual disability, Register-based research
Introduction
Neurodevelopmental disorders, such as attention-deficit/hyperactivity disorder (ADHD) and autism spectrum disorder (ASD), affect about 10% of children worldwide [1]. Neurodevelopmental disorders can lead to a range of adverse outcomes [2–5], including difficulties in academic and career development [3], suicide [4], and premature death [5]. Given the increasing rate of neurodevelopmental disorders [6], gaining knowledge about their etiology is a public health priority. In addition to the genetic susceptibility [7], emerging evidence has indicated that exposure to environmental factors [8], especially in utero [9], may contribute to their development.
Hypertensive disorders during pregnancy (HDP) complicate 5–10% of all pregnancies [10]. HDP may affect not only maternal health and pregnancy outcomes [11], but also predispose the offspring to neurobehavioral disorders later in life through impacting developing fetal brain due to poor vascularization of the placenta [12, 13]. Previous studies have reported that maternal HDP is associated with increased risks of ADHD [14], ASD [15, 16], cerebral palsy [17], and emotional and behavioral problems in offspring [18–21]. However, HDP is a group of conditions that include chronic hypertension, gestational hypertension, and pre-eclampsia/eclampsia (de novo or superimposed on chronic hypertension) [22]. Studies so far have mainly focused on pre-eclampsia [16, 17, 19, 21], evidence on other types of HDP is lacking [15, 20]. One recent population-based study found that maternal HDP was associated with increased risks of mental disorders in offspring, but the timing of pre-eclampsia and unmeasured familiar (genetic or environmental) risk factors were not taken into consideration [23]. As such, there is a need to evaluate whether unmeasured familial factors exert an influence on the associations between maternal HDP and neurodevelopmental disorders in offspring.
Taking advantage of national Danish and Swedish registers, we aimed to investigate the associations between specific types of maternal HDP and offspring neurodevelopmental disorders using population-based cohort and sibling designs, accounting for unmeasured familial confounding. We further examined whether the timing of onset and severity of HDP would affect these associations [13, 16, 17].
Methods
Design and population
We conducted a nationwide cohort study using data from several registers in Denmark [24–30] and Sweden [31–34]. The description of the registers is provided in Table S1. In both countries, all live births have a unique personal identification number that permits an accurate linkage of individual-level data. We identified all singleton live births in Denmark from 1978 to 2012 (n = 2,098,944) and in Sweden from 1987 to 2010 (n = 2,402,666). After excluding children who had extreme gestational age (< 154 or > 315 days) (n = 461 in Denmark and n = 122 in Sweden), without information on sex (n = 86 in Denmark and n = 14 in Sweden), and/or with chromosomal abnormalities (n = 7782 in Denmark and n = 4101 in Sweden), the final analysis included 2,090,615 children in Denmark and 2,398,429 children in Sweden. We followed them from birth until the date of the first diagnosis of a specific neurodevelopmental disorder, emigration, death, 18-year birthday, or end of follow-up (December 31, 2016 in Denmark and December 31, 2014 in Sweden), whichever came first.
In both Denmark and Sweden, prenatal care is standardized and free of charge, which includes visits every fourth week up to 24 gestational weeks, then every second week to 36 weeks, and weekly thereafter. Information on the maternal medical and obstetric history is collected with an interview during the first antenatal visit (around 12 gestational weeks) [30, 33]. After delivery, the responsible doctor would record each women’s diseases and complications during pregnancy and delivery, according to the appropriate ICD codes. The Medical Birth Registries (MBR) in both countries contain detailed information on maternal disease history and offspring birth outcomes. As in other studies [35–37], we have used MBR supplemented by National Patient Register (NPR) to extract information on maternal HDP, which has been considered highly reliable [29, 33].
The health care system in both countries is government-funded and ensures equal and free of charge access to hospital care [28, 30, 31, 34]. The doctors register all diagnoses associated with each hospital contact, and the reporting is mandatory by Danish and Swedish legislation, ensuring nationwide and almost complete coverage. We have used NPR supplemented by Psychiatric Central Research Registry to extract data on the diagnosis of maternal HDP (chronic hypertension, pre-eclampsia, gestational hypertension) and other disorders, as well as child neurodevelopmental disorders [5, 38].
Assessment of exposures
Information on maternal HDP was obtained from the combination of the Medical Birth Register (MBR) and the Danish National Patient Register (DNPR) in Denmark [28, 29], and the MBR and the Swedish National Patient Register (SNPR) in Sweden [33, 34]. Maternal HDP was categorized into three subtypes of chronic hypertension, gestational hypertension, and pre-eclampsia. We grouped eclampsia and the HELLP syndrome with pre-eclampsia because these conditions are rare as shown in previous studies [35, 36]. If women had more than one diagnosis of hypertensive disorders, we categorized them according to the following hierarchy: chronic hypertension, pre-eclampsia, and gestational hypertension [37].
Pre-eclampsia was divided into early-onset and late-onset pre-eclampsia according to the current consensus of disease definition of early-onset pre-eclampsia (early-onset pre-eclampsia: < 34 completed gestational weeks; late-onset pre-eclampsia: ≥ 34 completed gestational weeks) [39]. In Sweden, information on the timing of onset of pre-eclampsia is not available, pre-eclampsia was considered as early-onset when delivery occurred before 34 completed gestational weeks [36]. Pre-eclampsia was further categorized into severe pre-eclampsia (including eclampsia and the HELLP syndrome) and moderate pre-eclampsia [35–37]. Detailed description of the method used to identify HDP is provided in Table S2.
Ascertainment of outcomes
Information on diagnosis of ADHD, ASD and intellectual disability (ID) was obtained from the DNPR and the Danish Psychiatric Central Research Register in Denmark [25, 28], and from the SNPR in Sweden [34]. The International Statistical Classification of Diseases and Related Health Problems (ICD) codes are available in Table S3 [38, 40, 41]. When investigating the specific neurodevelopmental disorders, we defined the date of onset as the first day of each specific diagnosis, irrespective of other neurodevelopmental disorders diagnoses, if existed.
Covariates
Based on previous research [13, 16, 18, 21], the following variables were considered as potential confounders: sex of the child (male, female), calendar period of birth (a 5-year interval during 1978–2012 in Denmark and a 4-year interval during 1987–2010 in Sweden), parity (1, 2, ≥ 3), maternal age at birth (≤ 25, 26–30, 31–35, ≥ 36 years), maternal country of origin (Denmark/Sweden, other countries), maternal education level (0–9, 10–14, ≥ 15 years), maternal cohabitation status at birth (yes, no), and maternal psychiatric disorder before the childbirth (yes, no).
Statistical analysis
Cox proportional hazards regression model with the child’s age as the time scale was used to estimate the hazard ratio (HR) with 95% confidence intervals (CI) for the association of maternal HDP with the risk of offspring neurodevelopmental disorders, taking the timing of onset and severity of HDP into account. In the final model, adjustments were made for sex, calendar year of birth, parity, maternal age at birth, maternal education level, maternal cohabitation, and maternal psychiatric disorders before the childbirth. We used the robust sandwich estimator for standard errors to account for the clustering of individuals within nuclear families bound by the same biological mother. Kaplan–Meier curves were used to illustrate the probability of the neurodevelopmental disorders diagnoses in exposed and unexposed groups.
To account for unmeasured familial confounding, we performed sibling comparison analysis in which only sibling pairs discordant for both maternal HDP and offspring neurodevelopmental disorders contributed to the effect estimate [42]. As chronic hypertension would not change between pregnancies and the number of gestational hypertension is small, the sibling comparison model was appropriate for pre-eclampsia that could vary among siblings born to the same mother in the analysis.
We performed several sensitivity analyses. First, we tested whether the associations varied by the sex of the child, parity, preterm birth, and maternal factors, including maternal age and psychiatric disorders. Second, in order to assess potential effects of neonatal complications, we performed the analyses after excluding children with preterm birth (< 37 gestational weeks), low birth weight (< 2500 g), and low Apgar score at 5 min (< 7) [43]. Third, we performed mediation analyses to examine whether gestational age mediated the observed association by calculating direct and indirect effects in the STATA modulate PARAMED. Fourth, given the change in ICD revisions (ICD-10 was adopted in 1994 in Denmark and in 1997 in Sweden) [28, 31] and the offspring neurodevelopmental disorders identification strategy (all outpatient diagnosis were available since 1995 in Denmark and all specialized outpatient care were available since 2001 in Sweden) [25, 31], we restricted the analysis to offspring born after 1995 in Denmark and 2001 in Sweden. Fifth, we applied multiple imputation procedure by chained equations to impute ten replications to handle missing values on covariates (i.e., maternal education level and cohabitation status). Sixth, to address concerns about the validity of early neurodevelopmental diagnoses, we performed analysis in subsamples excluding offspring with diagnoses before the age of 3 years. Seventh, we additionally included superimposed pre-eclampsia as a separate group of exposure. Eighth, we also used the Swedish approach (pre-eclampsia was considered as early-onset when delivery occurred before 34 completed gestational weeks) of defining early-onset pre-eclampsia in the Danish participants to check whether the approaches for the definition of early-onset pre-eclampsia in the two countries is comparable. Ninth, we performed analyses excluding individuals with chronic hypertension to check whether the overall estimation of the association between maternal HDP and offspring outcomes will be affected. Tenth, we further categorized chronic hypertension into two subgroups: chronic hypertension from primary diagnosis or chronic hypertension from secondary diagnosis. Eleventh, we additionally adjusted the Charlson Comorbidity Index (CCI) scores (0 [low] referring to no recorded underlying diseases implemented in the CCI; 1–2 [moderate]; and > 2 [high]) [44–47]. Lastly, to assess whether maternal pre-pregnancy body mass index (BMI) confounded the associations, the analyses were restricted to offspring born after 2004 when maternal BMI became available. Maternal pre-pregnancy BMI was classified into underweight (< 18.5 kg/m2), normal weight (18.5–24.9 kg/m2), overweight (25.0–29.9 kg/m2), and obese (≥ 30 kg/m2). All statistical analyses were performed using Stata, version 15.1 (StataCorp).
Results
Descriptive statistics stratified by maternal HDP
During the study period, we identified 184 033 (4.1%) individuals born to mothers with HDP (chronic hypertension: 0.6%, gestational hypertension: 0.8%, and pre-eclampsia: 2.7%). In Denmark, 89 240 (4.3%) were born to mothers with HDP (chronic hypertension: 0.7%, gestational hypertension: 0.8% and pre-eclampsia: 2.8%) and in Sweden, 94 793 (4.0%) were born to mothers with HDP (chronic hypertension: 0.5%, gestational hypertension: 0.9% and pre-eclampsia: 2.6%). Compared with unexposed offspring, exposed offspring were more likely to be born preterm or had lower Apgar scores at 5 min. Mothers of exposed offspring were more likely to be older, to have a higher parity, or more comorbid psychiatric disorders (Table 1).
Table 1.
Characteristics of the study population at baseline according to maternal hypertensive disorders during pregnancy
| Denmark | Sweden | |||
|---|---|---|---|---|
| No | Yes | No | Yes | |
| Sex | ||||
| Boys | 1,026,939 (51.3) | 46,386 (51.9) | 1,183,231 (51.4) | 49,580 (52.3) |
| Girls | 974,436 (48.7) | 42,854 (48.1) | 1,120,405 (48.6) | 45,213 (47.7) |
| Preterm birth (< 37 gestational weeks) | ||||
| No | 1,839,865 (91.9) | 77,381 (86.7) | 2,199,179 (95.5) | 77,495 (81.8) |
| Yes | 84,043 (4.2) | 11,684 (13.1) | 101,826 (4.4) | 17,173 (18.1) |
| Missing | 77,467 (3.9) | 175 (0.2) | 2631 (0.1) | 128 (0.1) |
| Apgar score at 5 min | ||||
| 10 | 1,836,432 (91.8) | 78,005 (87.4) | 1,913,306 (83.0) | 70,164 (74.0) |
| ≤ 9 | 132,835 (6.6) | 10,387 (11.6) | 368,200 (16.0) | 23,603 (24.9) |
| Missing | 32,108 (1.6) | 848 (1.0) | 22,130 (1.0) | 1026 (1.1) |
| Parity | ||||
| 1 | 878,604 (43.9) | 52,237 (58.5) | 969,936 (42.1) | 56,816 (59.9) |
| 2 | 753,614 (37.6) | 24,265 (27.2) | 841,565 (36.5) | 23,021 (24.3) |
| ≥ 3 | 369,157 (18.5) | 12,738 (14.3) | 492,135 (21.4) | 14,956 (15.8) |
| Maternal age (years) | ||||
| ≤ 24 | 421,987 (21.0) | 19,248 (21.6) | 441,908 (19.2) | 18,262 (19.3) |
| 25–29 | 742,193 (37.1) | 31,224 (35.0) | 785,823 (34.1) | 29,802 (31.4) |
| 30–34 | 587,804 (29.4) | 24,475 (27.4) | 711,839 (30.9) | 27,693 (29.2) |
| ≥ 35 | 249,391 (12.5) | 14,293 (16.0) | 364,066 (15.8) | 19,036 (20.1) |
| Maternal education (years) | ||||
| ≤ 9 | 558,202 (27.9) | 25,158 (28.2) | 799,080 (34.7) | 33,214 (35.0) |
| 10–15 | 873,455 (43.6) | 40,410 (45.3) | 1,121,677 (48.7) | 48,668 (51.3) |
| ≥ 15 | 557,226 (26.9) | 22,744 (25.5) | 324,747 (14.1) | 11,440 (12.1) |
| Missing | 32,492 (1.6) | 928 (1.0) | 57,132 (2.5) | 1471 (1.6) |
| Maternal psychiatric disorders | ||||
| No | 1,897,588 (94.8) | 84,006 (94.1) | 2,219,248 (96.3) | 90,934 (95.9) |
| Yes | 103,787 (5.2) | 5234 (5.9) | 84,388 (3.7) | 3859 (4.1) |
| Maternal cohabitation at birth | ||||
| Yes | 1,123,132 (56.1) | 46,211 (51.8) | 1,232,307 (53.5) | 56,301 (59.4) |
| No | 876,533 (43.8) | 43,004 (48.2) | 1,062,179 (46.1) | 38,291 (40.4) |
| Missing | 1710 (0.1) | 25 (< 0.1) | 9150 (0.4) | 201 (0.2) |
| Attention-deficit/hyperactivity disorders | ||||
| Yes | 31,629 (1.6) | 1629 (1.8) | 58,252 (2.5) | 2966 (3.1) |
| No | 1,969,746 (98.4) | 87,611 (98.2) | 2,245,384 (97.5) | 91,827 (96.9) |
| Autism spectrum disorders | ||||
| Yes | 22,536 (1.1) | 1306 (1.5) | 27,654 (1.2) | 1571 (1.7) |
| No | 1,978,838 (98.9) | 87,934 (98.5) | 2,275,982 (98.8) | 93,222 (98.3) |
| Intellectual disability | ||||
| Yes | 10,305 (0.5) | 620 (0.7) | 16,364 (0.7) | 1042 (1.1) |
| No | 1,991,040 (99.5) | 88,620 (99.3) | 2,287,272 (99.3) | 93,751 (98.9) |
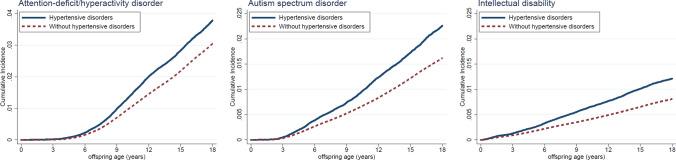
Compared with unexposed offspring, exposed offspring had a higher risk of developing neurodevelopmental disorders (Fig. 1). Among offspring exposed to maternal HDP, the estimates of cumulative incidences by 18 years of age were 3.78% (95% CI 3.67–3.89%) for ADHD, 2.26% (95% CI 2.18–2.35%) for ASD, and 1.21% (95% CI 1.15–1.27%) for ID, which are higher than those among unexposed offspring (3.05% [95% CI 3.03–3.07%] for ADHD, 1.62% [95% CI 1.61–1.63%] for ASD, and 0.81% [95% CI 0.80–0.82%] for ID, respectively). The cumulative incidences for the neurodevelopmental disorders by countries showed similar patterns (Figure S1–2).
Fig. 1.
Cumulative risk for neurodevelopmental disorders in children after exposure to maternal hypotensive disorders during pregnancy
Population-wide association and comparison of timing and severity of maternal HDP
At the whole population level, maternal HDP was associated with increased risks of ADHD (HR, 1.24; 95% CI 1.20–1.28), ASD (HR, 1.29; 95% CI 1.24–1.34), and ID (HR, 1.58; 95% CI 1.50–1.66). When exploring the risk by types of HDP, we found the overall estimation was mostly driven by maternal pre-eclampsia. The HRs in offspring exposed to maternal pre-eclampsia were 1.27 (95% CI 1.23–1.32) for ADHD, 1.36 (95% CI 1.30–1.42) for ASD, and 1.70 (95% CI 1.60–1.81) for ID, respectively (Tables 2, 3, 4).
Table 2.
Associations between maternal hypertensive disorders and attention-deficit/hyperactivity disorders in offspring
| Attention-deficit/hyperactivity disorders | ||||||
|---|---|---|---|---|---|---|
| Combined | Denmark | Sweden | ||||
| Cases (No.) | aHRa (95% CI) | Cases (No.) | aHRa (95% CI) | Cases (No.) | aHRa (95% CI) | |
| Maternal hypertensive disorders | ||||||
| No hypertensive disorders | 89,881 | 1.00 (ref) | 31,629 | 1.00 (ref) | 58,252 | 1.00 (ref) |
| Hypertensive disorders | 4595 | 1.24 (1.20–1.28) | 1629 | 1.16 (1.10–1.22) | 2966 | 1.23 (1.18–1.27) |
| Chronic hypertension | 584 | 1.23 (1.13–1.34) | 261 | 1.04 (0.91–1.17) | 323 | 1.36 (1.22–1.52) |
| Gestational hypertension | 829 | 1.13 (1.05–1.21) | 259 | 1.06 (0.94–1.20) | 570 | 1.14 (1.05–1.24) |
| Pre-eclampsia | 3182 | 1.27 (1.23–1.32) | 1109 | 1.22 (1.15–1.30) | 2073 | 1.23 (1.18–1.29) |
| By timing of pre-eclampsia | ||||||
| Early-onset | 506 | 1.77 (1.62–1.94) | 230 | 1.51 (1.32–1.72) | 276 | 1.94 (1.71–2.19) |
| Late-onset | 2676 | 1.21 (1.16–1.26) | 879 | 1.17 (1.09–1.25) | 1797 | 1.16 (1.11–1.22) |
| By severity of pre-eclampsia | ||||||
| Moderate | 2223 | 1.22 (1.17–1.27) | 822 | 1.15 (1.07–1.24) | 1401 | 1.18 (1.12–1.25) |
| Severe | 959 | 1.41 (1.32–1.51) | 287 | 1.48 (1.31–1.66) | 672 | 1.35 (1.24–1.46) |
| By timing # severity | ||||||
| Early-onset # Severe pre-eclampsia | 329 | 1.93 (1.73–2.16) | 114 | 1.84 (1.53–2.22) | 215 | 1.88 (1.64–2.16) |
| Early-onset # Moderate pre-eclampsia | 177 | 1.54 (1.32–1.79) | 116 | 1.28 (1.06–1.54) | 61 | 2.16 (1.67–2.80) |
| Late-onset # Severe pre-eclampsia | 630 | 1.24 (1.14–1.34) | 173 | 1.30 (1.12–1.52) | 457 | 1.19 (1.08–1.30) |
| Late-onset # Moderate pre-eclampsia | 2046 | 1.20 (1.15–1.25) | 706 | 1.13 (1.05–1.22) | 1340 | 1.16 (1.10–1.22) |
aHR adjusted hazard ratio, CI confidence interval, No. number
aAdjusted for sex, calendar year, parity, maternal age, maternal education, maternal cohabitation at birth, maternal history of psychiatric disorders before childbirth
Table 3.
Associations between maternal hypertensive disorders and autism spectrum disorders in offspring
| Autism spectrum disorders | ||||||
|---|---|---|---|---|---|---|
| Combined | Denmark | Sweden | ||||
| Cases (No.) | aHRa (95% CI) | Cases (No.) | aHRa (95% CI) | Cases (No.) | aHRa (95% CI) | |
| Maternal hypertensive disorders | ||||||
| No hypertensive disorders | 50,190 | 1.00 (ref) | 22,536 | 1.00 (ref) | 27,654 | 1.00 (ref) |
| Hypertensive disorders | 2877 | 1.29 (1.24–1.34) | 1306 | 1.23 (1.16–1.30) | 1571 | 1.30 (1.24–1.37) |
| Chronic hypertension | 388 | 1.18 (1.07–1.31) | 236 | 1.19 (1.05–1.36) | 152 | 1.12 (0.95–1.32) |
| Gestational hypertension | 508 | 1.15 (1.05–1.26) | 207 | 1.07 (0.93–1.23) | 301 | 1.20 (1.06–1.35) |
| Pre-eclampsia | 1981 | 1.36 (1.30–1.42) | 863 | 1.29 (1.20–1.38) | 1118 | 1.36 (1.28–1.45) |
| By timing of pre-eclampsia | ||||||
| Early-onset | 313 | 1.74 (1.55–1.95) | 163 | 1.42 (1.21–1.66) | 150 | 2.13 (1.81–2.52) |
| Late-onset | 1668 | 1.30 (1.24–1.37) | 700 | 1.26 (1.17–1.36) | 968 | 1.29 (1.21–1.38) |
| By severity of pre-eclampsia | ||||||
| Moderate | 1413 | 1.33 (1.26–1.40) | 665 | 1.28 (1.18–1.39) | 748 | 1.30 (1.21–1.41) |
| Severe | 568 | 1.44 (1.33–1.57) | 198 | 1.31 (1.14–1.51) | 370 | 1.50 (1.35–1.67) |
| By timing # severity | ||||||
| Early-onset # severe pre-eclampsia | 189 | 1.86 (1.61–2.15) | 68 | 1.44 (1.13–1.83) | 121 | 2.16 (1.79–2.59) |
| Early-onset # moderate pre-eclampsia | 124 | 1.58 (1.33–1.89) | 95 | 1.40 (1.14–1.72) | 29 | 2.05 (1.40–2.99) |
| Late-onset # severe pre-eclampsia | 379 | 1.30 (1.17–1.44) | 130 | 1.25 (1.05–1.49) | 249 | 1.31 (1.15–1.49) |
| Late-onset # moderate pre-eclampsia | 1289 | 1.31 (1.23–1.38) | 570 | 1.26 (1.16–1.38) | 719 | 1.29 (1.19–1.39) |
aHR adjusted hazard ratio, CI confidence interval, No. number
aAdjusted for sex, calendar year, parity, maternal age, maternal education, maternal cohabitation at birth, maternal history of psychiatric disorders before childbirth
Table 4.
Associations between maternal hypertensive disorders and intellectual disability in offspring
| Intellectual disability | ||||||
|---|---|---|---|---|---|---|
| Combined | Denmark | Sweden | ||||
| Cases (No.) | aHRa (95% CI) | Cases (No.) | aHRa (95% CI) | Cases (No.) | aHRa (95% CI) | |
| Maternal hypertensive disorders | ||||||
| No hypertensive disorders | 26,669 | 1.00 (ref) | 10,305 | 1.00 (ref) | 16,364 | 1.00 (ref) |
| Hypertensive disorders | 1662 | 1.58 (1.50–1.66) | 620 | 1.41 (1.30–1.54) | 1042 | 1.64 (1.53–1.75) |
| Chronic hypertension | 178 | 1.41 (1.21–1.64) | 73 | 1.18 (0.93–1.49) | 105 | 1.60 (1.31–1.96) |
| Gestational hypertension | 285 | 1.28 (1.13–1.45) | 111 | 1.32 (1.08–1.60) | 174 | 1.21 (1.03–1.43) |
| Pre-eclampsia | 1199 | 1.70 (1.60–1.81) | 436 | 1.49 (1.35–1.64) | 763 | 1.77 (1.64–1.92) |
| By timing of pre-eclampsia | ||||||
| Early-onset | 284 | 3.69 (3.27–4.17) | 116 | 2.74 (2.26–3.30) | 168 | 4.54 (3.86–5.33) |
| Late-onset | 915 | 1.46 (1.36–1.56) | 320 | 1.27 (1.13–1.43) | 595 | 1.51 (1.39–1.65) |
| By severity of pre-eclampsia | ||||||
| Moderate | 796 | 1.51 (1.40–1.63) | 308 | 1.30 (1.16–1.47) | 488 | 1.60 (1.46–1.76) |
| Severe | 403 | 2.27 (2.05–2.52) | 128 | 2.25 (1.88–2.70) | 275 | 2.19 (1.93–2.49) |
| By timing # severity | ||||||
| Early-onset # severe pre-eclampsia | 183 | 3.99 (3.42–4.65) | 68 | 3.89 (3.04–4.99) | 115 | 3.74 (3.06–4.56) |
| Early-onset # moderate pre-eclampsia | 101 | 3.28 (2.69–4.00) | 48 | 1.95 (1.46–2.60) | 53 | 7.72 (5.86–10.16) |
| Late-onset # severe pre-eclampsia | 220 | 1.69 (1.47–1.94) | 60 | 1.53 (1.18–1.99) | 160 | 1.71 (1.45–2.02) |
| Late-onset # moderate pre-eclampsia | 695 | 1.39 (1.29–1.51) | 260 | 1.23 (1.08–1.39) | 435 | 1.45 (1.31–1.60) |
aHR adjusted hazard ratio, CI confidence interval, No. number
aAdjusted for sex, calendar year, parity, maternal age, maternal education, maternal cohabitation at birth, maternal history of psychiatric disorders before childbirth
Children born to mothers with early-onset pre-eclampsia had particularly high risks of ADHD (HR, 1.77; 95% CI 1.62–1.94), ASD (HR, 1.74; 95% CI 1.55–1.95), and ID (HR, 3.69; 95% CI 3.27–4.17). We also observed increased risks among offspring exposed to severe pre-eclampsia, the risk estimates were 1.41 (95% CI 1.32–1.51) for ADHD, 1.44 (95% CI 1.33–1.57) for ASD, and 2.27 (95% CI 2.05–2.52) for ID. It is worth noting that 57.9% of early-onset pre-eclampsia was also diagnosed with severe pre-eclampsia. Therefore, we further examined the associations according to both timing of onset and severity of pre-eclampsia. The association between early-onset pre-eclampsia and the outcomes was slightly affected by severity of pre-eclampsia. For ADHD, the HR was 1.93 (95% CI 1.73–2.16) for early-onset and severe pre-eclampsia, and 1.54 (95% CI 1.32–1.79) for early-onset and moderate pre-eclampsia. On the other hand, the association between late-onset pre-eclampsia and the outcomes was similar across the severity of pre-eclampsia. For ADHD, the HR was 1.24 (95% CI 1.14–1.34) for late-onset and severe pre-eclampsia, and 1.20 (95% CI 1.15–1.25) for late-onset and moderate pre-eclampsia. The same pattern of results was found for ASD and ID (Tables 2, 3, 4)).
Sibling comparisons
For the sibling comparison analyses, we identified a subsample of 1,481,847 families with at least 2 siblings. Of these families, 12,490 (0.84%) included sibling discordant for pre-eclampsia and ADHD, 6804 (0.46%) included sibling discordant for pre-eclampsia and ASD, and 4313 (0.29%) included sibling discordant for pre-eclampsia and ID. In the sibling comparison models, maternal pre-eclampsia was associated with ADHD (HR, 1.19; 95% CI 1.11–1.28), ASD (HR, 1.36; 95% CI 1.24–1.49), and ID (HR, 1.43; 95% CI 1.27–1.61) (Table 5).
Table 5.
Associations between maternal pre-eclampsia and neurodevelopmental disorders in offspring using sibling analyses
| Neurodevelopmental disorders | Combined | Denmark | Sweden | |||
|---|---|---|---|---|---|---|
| No. of cases | Adjusteda HR (95% CI) | No. of cases | Adjusteda HR (95% CI) | No. of cases | Adjusteda HR (95% CI) | |
| For ADHD | ||||||
| No pre-eclampsia | 2170 | 1.00 (ref) | 851 | 1.00 (ref) | 1319 | 1.00 (ref) |
| Maternal pre-eclampsia | 1746 | 1.19 (1.11–1.28) | 633 | 1.16 (1.02–1.33) | 1113 | 1.20 (1.08–1.33) |
| By timing of onset | ||||||
| Early-onset | 233 | 1.54 (1.34–1.77) | 129 | 1.40 (1.14–1.72) | 104 | 1.80 (1.44–2.26) |
| Late-onset | 1513 | 1.15 (1.07–1.24) | 504 | 1.12 (0.97–1.28) | 1009 | 1.16 (1.05–1.29) |
| By severity | ||||||
| Moderate | 1270 | 1.16 (1.08–1.25) | 483 | 1.12 (0.98–1.29) | 787 | 1.18 (1.06–1.31) |
| Severe | 476 | 1.29 (1.16–1.43) | 150 | 1.32 (1.09–1.60) | 326 | 1.26 (1.09–1.46) |
| For ASD | ||||||
| No pre-eclampsia | 1172 | 540 | 632 | 1.00 (ref) | ||
| Maternal pre-eclampsia | 1076 | 1.36 (1.24–1.49) | 494 | 1.40 (1.19–1.65) | 582 | 1.29 (1.11–1.49) |
| By timing of onset | ||||||
| Early-onset | 134 | 1.61 (1.34–1.94) | 77 | 1.41 (1.19–1.66) | 57 | 2.27 (1.68–3.06) |
| Late-onset | 942 | 1.33 (1.21–1.46) | 417 | 1.38 (1.05–1.80) | 525 | 1.23 (1.06–1.43) |
| By severity | ||||||
| Moderate | 809 | 1.32 (1.20–1.46) | 395 | 1.37 (1.16–1.62) | 414 | 1.25 (1.07–1.46) |
| Severe | 267 | 1.49 (1.30–1.72) | 99 | 1.52 (1.20–1.93) | 168 | 1.41 (1.15–1.73) |
| For ID | ||||||
| No pre-eclampsia | 720 | 1.00 (ref) | 322 | 1.00 (ref) | 398 | 1.00 (ref) |
| Maternal pre-eclampsia | 653 | 1.43 (1.27–1.61) | 254 | 1.29 (1.04–1.60) | 399 | 1.60 (1.33–1.93) |
| By timing of onset | ||||||
| Early-onset | 150 | 2.50 (2.07–3.02) | 65 | 2.13 (1.60–2.85) | 85 | 3.26 (2.47–4.31) |
| Late-onset | 503 | 1.27 (1.12–1.44) | 189 | 1.13 (0.89–1.43) | 314 | 1.42 (1.17–1.72) |
| By severity | ||||||
| Moderate | 439 | 1.31 (1.15–1.49) | 185 | 1.20 (0.95–1.51) | 254 | 1.45 (1.19–1.78) |
| Severe | 214 | 1.80 (1.52–2.12) | 69 | 1.59 (1.18–2.15) | 145 | 1.98 (1.57–2.51) |
HR hazard ratio, CI confidence interval, ADHD attention-deficit/hyperactivity disorder, ASD autism spectrum disorder, ID intellectual disability
aAdjusted for sex, calendar year parity, maternal age, maternal education, maternal cohabitation at birth, maternal history of psychiatric disorders before childbirth
Sensitivity analyses
Stratification by sex of the offspring, parity, Apgar score at 5 min, maternal age and maternal psychiatric disorders did not indicate any significant differences in the studied associations (Tables S4–8). Analyses excluding offspring with adverse birth outcomes such as preterm birth, low birth weight and low Apgar score yielded the similar estimates (Table S9). The proportions of the total effect mediated by gestational age for the associations between maternal HDP and ADHD, ASD, and ID, were 30.7%, 19.2% and 34.9%, respectively (Table S10). Children exposed to superimposed pre-eclampsia had higher risks of ADHD (HR, 1.56; 95% 1.33–1.82), ASD (HR, 1.48; 95% CI 1.21–1.80), and ID (HR, 1.85; 95% CI 1.38–2.48) (Table S11). When applying the Swedish approach of defining early-onset pre-eclampsia in the Danish participants, the estimates for early-onset pre-eclampsia were similar to those in the Swedish cohort (Table S12). Similar associations were observed in the analyses (1) restricted to offspring born after 1995 in Denmark and 2001 in Sweden (Table S13) when specialized outpatient diagnoses were added, or (2) excluding neurodevelopmental diagnosis before the age of three (Table S14), or (3) using the multiple imputation method (Table S15), or (4) additionally adjusting for maternal pre-pregnancy BMI (Table S16), or (5) excluding individuals with chronic hypertension (Table S17), or (6) categorizing chronic hypertension into two subgroups: primary diagnosis or secondary diagnosis (Table S18), or (7) additionally adjusting for CCI scores (Table S19).
Discussion
In this large population-based cohort study, we found that all the three types of maternal HDP, including chronic hypertension, gestational hypertension and pre-eclampsia, were associated with 1.2- to 1.6-fold risks of neurodevelopmental disorders in offspring, while the overall associations were mostly driven by pre-eclampsia. The strength of associations varied significantly according to the timing of onset or severity of pre-eclampsia and our findings further indicated that the timing of onset of pre-eclampsia was probably more important than the severity. The consistent results from sibling comparison analysis indicated that the observed associations are unlikely confounded by the shared genetic and environmental factors. Moreover, these results remained unchanged when taking into account of a number of important factors, such as maternal socioeconomic status, birth order, maternal history of psychiatric disorders, and adverse birth outcomes.
Comparison with other studies and interpretation of results
To our knowledge, this is the largest population-based and sibling comparison study to date to investigate the link between maternal HPD and the risk of neurodevelopmental disorders in offspring. Our findings of increased risk of neurodevelopmental disorders related to maternal HDP are inconsistent with several previous studies which reported null or even a protective association between maternal pre-eclampsia and neurodevelopmental disorders in offspring [20, 48, 49]. For example, a case–control study in Australia, including 465 cases matched with 1313 controls, found no association between pre-eclampsia and ASD [20]; the study used un-validated data on maternal pre-eclampsia. A Japanese study found that maternal pre-eclampsia reduced the risk of autistic disorder in offspring admitted to the neonatal intensive care unit, which is subject to selection bias [48].
Our findings are in line with those in several other previous observational studies, demonstrating increased risks of neurodevelopmental disorders in offspring born to mothers with pre-eclampsia [13, 16–19, 50]. A systematic review showed a 1.2-fold risk of ADHD and a 1.3-fold risk of ASD in offspring of mothers with pre-eclampsia [50]. In a registry study in the US, maternal pre-eclampsia was associated with a 1.8-fold increased risk of ASD in offspring, without adjusting for maternal psychiatric disorders [16]. Another registry study in Finland suggested that maternal pre-eclampsia was associated with 20%, 30% and 50% higher risks of ADHD, ASD and ID, respectively [17]. However, this study was only conducted in term births which may lead to collider bias. The Childhood Autism Risks from Genetics and Environmental cohort in US found that offspring exposed to pre-eclampsia had a 2.3-fold risk of ASD and a 1.4-fold risk for developmental delay [13]. Consistent with our results, the risks increased with greater pre-eclampsia severity, but the number of severe pre-eclampsia was very low (n = 11) [13].
We are only aware of one recent birth cohort study (n = 4743) reporting that maternal severe pre-eclampsia and mild pre-eclampsia were associated with 2.0-fold and 1.4-fold risk of overall mental disorders in offspring, respectively [23]. However, data on timing of pre-eclampsia and specific neurodevelopmental disorders were not reported [23]. It is important to note that early-onset pre-eclampsia was associated with almost twofold to fourfold risk of neurodevelopmental disorders in offspring, and these associations were less affected by severity of pre-eclampsia. This suggests that the timing of onset may play a more important role in the pathophysiology than the severity of the disease [12, 51, 52]. Moreover, our study further supports the previous findings by showing that the observed associations were not modified by maternal socioeconomic status, parity, and maternal history of psychiatric disorders.
Most previous studies have not distinguished between gestational hypertension and pre-eclampsia [14, 48, 53, 54]. One birth cohort study found no association between gestational hypertension (n = 263) and mental disorders in children, with a relatively small number of gestational hypertension cases [23]. Similar to our study, other prospective birth cohort studies found that gestational hypertension with or without pre-eclampsia was associated with increased risks of emotional and behavioral problems [55], and impaired cognitive development [56, 57].
There is currently limited evidence on the association between chronic hypertension and neurodevelopmental disorders in offspring. A registry study in Norway showed that there was no association between maternal chronic hypertension and offspring ADHD [58]. However, the prevalence of chronic hypertension in this Norwegian study was only 0.3%, which is substantially lower than that observed in other Nordic countries [59, 60]. As mentioned by the authors, the diagnostic validity of chronic hypertension has not been formally validated in the Medical Birth Registry of Norway. Thus, the potentially under-diagnosed maternal chronic hypertension would most likely attenuate the associations. However, our findings suggest that maternal chronic hypertension may play a role in the development of neurodevelopmental disorders, especially for intellectual disability. Nevertheless, our finding that maternal chronic hypertension was associated with increased risks of neurodevelopmental disorders needs further investigation, in particular given the fact that there existed great challenges in identifying all cases with chronic hypertension because many mild cases might be managed only in primary care and would not be registered in the NPR or MBR [61, 62].
The underlying mechanism linking maternal HDP and neurodevelopmental disorders remains to be elucidated. The fetal programming theory hypothesizes that adverse fetal environment or insult can affect brain development, which leads to a range of neurodevelopmental disorders [8]. It is possible that hypertension during pregnancy may involve a decrease in oxygen and nutrient supply to the fetus as a result of utero-placental underperfusion and hypoxia [12, 51]. Depleted oxygen and nutrition supply to the fetus may impair fetal brain development and thus contribute to greater risk of emotional and behavioral disorders later in life [63]. In addition, early-onset pre-eclampsia is a disorder of defect placentation, whereas other types of HDP are more a metabolic disorder related to maternal risk factors [22]. Early-onset pre-eclampsia is associated with severe placental insufficiency, which contribute to increased susceptibility to neurodevelopmental disorders in offspring [8]. Previous studies have suggested that the association between pre-eclampsia and mental disorders in children might partly be mediated by preterm birth and fetal growth restriction[23, 43]. With the large sample size and long follow-up, our study had sufficient power to examine the mediation effects on the associations of maternal HDP with specific mental disorders. For the first time, our study confirmed that a substantial portion of the association with ADHD, ASD, and ID was mediated via shortened gestational age.
Strengths and Limitations
Our study has several strengths. First, the large sample up to four million mother–child pairs made it possible to examine all forms of HDP, and to stratify pre-eclampsia by timing of onset or severity, which are not commonly ascertained in other studies [13, 14, 17]. Second, information on HDP and neurodevelopmental disorders were virtually prospectively collected, which can reduce the possibility of recall bias [28, 32]. Third, the diagnoses recorded for HDP and neurodevelopmental disorders have been demonstrated to have high validity [27, 32, 38]. Fourth, the conclusions were based on population-based and sibling comparison studies that are less susceptible to both measured and unmeasured familial confounding factors.
Some limitations need to be noted. First, as the data from the primary care was not available, it is likely that some mild cases could not be identified through the registers, for example, chronic hypertension is often managed in primary care particularly [62]. However, this misclassification of mild cases as healthy controls would probably attenuate the association [64]. In addition, the estimates from our supplementary analyses, in which only hospitalized cases were included or additional adjustment for comorbid diseases was performed, were similar to those from primary analyses. Second, neurodevelopmental disorders were ascertained from NPR diagnoses, while specialized outpatient data which contain substantial proportion of neurodevelopmental disorders records were only available since 1995 in Denmark and since 2001 in Sweden [31, 38]. Thus, we might have missed the diagnoses for children who were born earlier in our cohort. Nevertheless, analyses restricted to years when both outpatient and inpatient registers were in use did not essentially change our estimates. In addition, a number of studies have been conducted to examine the validity of register-based diagnoses of neurodevelopmental disorders in both Denmark and Sweden, all showing high quality [25, 65–67]. Third, even though sibling analysis was used to address unmeasured stable familiar confounding, such as maternal chronic disease, this design could not account for familiar unshared factors that varied across pregnancies. Thus, residual confounding could still be a source of bias to consider. One potential confounder is maternal pre-pregnancy BMI [68]. However, we obtained similar results when additionally adjusting for pre-pregnancy BMI in women with available data (Table S16).
Conclusion and implications
Our findings suggest that all types of maternal HDP, especially early-onset pre-eclampsia, are associated with increased risks of neurodevelopmental disorders (ADHD, ASD, and ID) in offspring. From a public health perspective, these findings highlight the importance of better management of maternal HDP and earlier screening and detection for neurodevelopmental disorders in their offspring.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- HR
Hazard ratio
- CI
Confidence interval
- DNPR
Danish National Patient Register
- DPCR
Danish Psychiatric Central Register
- SNPR
Swedish National Patient Register
- MBR
Medical Birth Registry
- ICD-8
International Classification of Disease, Eighth Edition
- ICD-10
International Classification of Disease, Tenth Edition
- ADHD
Attention-deficit/hyperactivity disorder
- ASD
Autism spectrum disorder
- ID
Intellectual disability
- HDP
Hypertensive disorders during pregnancy
Author contributions
HW performed the literature review, conducted data analyses and drafted the manuscript. KL, YY and JL have full access to all of the data in this study and take responsibility for the integrity of the data and the accuracy of the data analysis. MG, JZ and FL contributed to the interpretation of the data and critical revision of the paper for important intellectual content. YY and JL developed the study conception, directed the analytic strategy of the study and supervised the drafting of the manuscript. All authors approved the final manuscript as submitted and agreed to be accountable for all aspects of the work. The corresponding authors attest that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding
This work was supported by grants from the Danish Council for Independent Research and Independent Research Fund Denmark (DFF-6110-00019B and 9039-00010B), the National Natural Science Foundation of China (Nos. 81703237, 81761128035, 81930095, 81701334, 81703249, and 82073570), the Novo Nordisk Foundation (NNF18OC0052029), the Nordic Cancer Union (R217-A13234-18-S65, R275-A15770), Karen Elise Jensens Fond (2016), the Swedish Council for Working Life and Social Research (No. 2015-00837), the Karolinska Institutet’s Research Foundation (No. 2018-01547), the Swedish Heart and Lung Foundation (No. 20180306), Shanghai Municipal Commission of Health and Family Planning (Nos. GWV-10.1-XK07, 2017ZZ02026, 2018BR33, 2017EKHWYX-02), Shanghai Shenkang Hospital Development Center (No. 16CR2025B), Shanghai Clinical Key Subject Construction Project (shslczdzk02902), Shanghai Committee of Science and Technology (Nos. 17XD1403200, 19410713500, 18DZ2313505), Shanghai Municipal Science and Technology Major Project (No. 2018SHZDZX01), Key Scientific and Technological Projects of Guangdong Province (2018B030335001), Collaborative Innovation Program of Shanghai Municipal Health Commission (2020CXJQ01), Xinhua Hospital of Shanghai Jiao Tong University School of Medicine (2018YJRC03). The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Availability of data and materials
No additional data available.
Code availability
The codes used in this study are available upon request.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The study was approved by the Danish Data Protection Agency (No 2013-41-2569) and Ethics Review Board in Stockholm. The requirement for individual informed consent was not applied for a register-based study in Denmark and Sweden.
Consent to participate
Consent forms from participants are waived since the study is based on anonymous registry data.
Consent to publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yongfu Yu, Email: yu@fudan.edu.cn.
Jiong Li, Email: jl@clin.au.dk.
References
- 1.Thapar A, Cooper M, Rutter M. Neurodevelopmental disorders. Lancet Psychiatry. 2017;4(4):339–346. doi: 10.1016/S2215-0366(16)30376-5. [DOI] [PubMed] [Google Scholar]
- 2.Dalsgaard S, McGrath J, Ostergaard SD, et al. Association of mental disorder in childhood and adolescence with subsequent educational achievement. JAMA Psychiat. 2020;77(8):797–805. doi: 10.1001/jamapsychiatry.2020.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu Y, Sjölander A, Cederlöf M, et al. Association between medication use and performance on higher education entrance tests in individuals with attention-deficit/hyperactivity disorder. JAMA Psychiat. 2017;74(8):815–822. doi: 10.1001/jamapsychiatry.2017.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hedley D, Uljarević M, Foley KR, Richdale A, Trollor J. Risk and protective factors underlying depression and suicidal ideation in autism spectrum disorder. Depress Anxiety. 2018;35(7):648–657. doi: 10.1002/da.22759. [DOI] [PubMed] [Google Scholar]
- 5.Sun S, Kuja-Halkola R, Faraone SV, et al. Association of psychiatric comorbidity with the risk of premature death among children and adults with attention-deficit/hyperactivity disorder. JAMA Psychiat. 2019;76(11):1141–1149. doi: 10.1001/jamapsychiatry.2019.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gyllenberg D, Marttila M, Sund R, et al. Temporal changes in the incidence of treated psychiatric and neurodevelopmental disorders during adolescence: an analysis of two national Finnish birth cohorts. Lancet Psychiatry. 2018;5(3):227–236. doi: 10.1016/S2215-0366(18)30038-5. [DOI] [PubMed] [Google Scholar]
- 7.Martin J, Taylor MJ, Lichtenstein P. Assessing the evidence for shared genetic risks across psychiatric disorders and traits. Psychol Med. 2018;48(11):1759–1774. doi: 10.1017/S0033291717003440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Donnell KJ, Meaney MJ. Fetal origins of mental health: the developmental origins of health and disease hypothesis. Am J Psychiatry. 2017;174(4):319–328. doi: 10.1176/appi.ajp.2016.16020138. [DOI] [PubMed] [Google Scholar]
- 9.MacKinnon N, Kingsbury M, Mahedy L, Evans J, Colman I. The association between prenatal stress and externalizing symptoms in childhood: evidence from the avon longitudinal study of parents and children. Biol Psychiatry. 2018;83(2):100–108. doi: 10.1016/j.biopsych.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Umesawa M, Kobashi G. Epidemiology of hypertensive disorders in pregnancy: prevalence, risk factors, predictors and prognosis. Hypertens Res. 2017;40(3):213–220. doi: 10.1038/hr.2016.126. [DOI] [PubMed] [Google Scholar]
- 11.Cruz MO, Gao W, Hibbard JU. Obstetrical and perinatal outcomes among women with gestational hypertension, mild preeclampsia, and mild chronic hypertension. Am J Obstet Gynecol. 2011;205(3):260-e1. doi: 10.1016/j.ajog.2011.06.033. [DOI] [PubMed] [Google Scholar]
- 12.Myatt L, Webster RP. Vascular biology of preeclampsia. J Thromb Haemost. 2009;7(3):375–384. doi: 10.1111/j.1538-7836.2008.03259.x. [DOI] [PubMed] [Google Scholar]
- 13.Walker CK, Krakowiak P, Baker A, Hansen RL, Ozonoff S, Hertz-Picciotto I. Preeclampsia, placental insufficiency, and autism spectrum disorder or developmental delay. JAMA Pediatr. 2015;169(2):154–162. doi: 10.1001/jamapediatrics.2014.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Böhm S, Curran EA, Kenny LC, O’Keeffe GW, Murray D, Khashan AS. The effect of hypertensive disorders of pregnancy on the risk of ADHD in the offspring. J Atten Disord. 2019;23(7):692–701. doi: 10.1177/1087054717690230. [DOI] [PubMed] [Google Scholar]
- 15.Krakowiak P, Walker CK, Bremer AA, et al. Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics. 2012;129(5):e1121–e1128. doi: 10.1542/peds.2011-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mann JR, McDermott S, Bao H, Hardin J, Gregg A. Pre-eclampsia, birth weight, and autism spectrum disorders. J Autism Dev Disord. 2010;40(5):548–554. doi: 10.1007/s10803-009-0903-4. [DOI] [PubMed] [Google Scholar]
- 17.Sun BZ, Moster D, Harmon QE, Wilcox AJ. Association of preeclampsia in term births with neurodevelopmental disorders in offspring. JAMA Psychiat. 2020;77(8):823–829. doi: 10.1001/jamapsychiatry.2020.0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dachew BA, Scott JG, Betts K, Mamun A, Alati R. Hypertensive disorders of pregnancy and the risk of offspring depression in childhood: findings from the avon longitudinal study of parents and children. Dev Psychopathol. 2019;32:1–7. doi: 10.1017/S0954579419000944. [DOI] [PubMed] [Google Scholar]
- 19.Maher GM, O'Keeffe GW, Dalman C, et al. Association between preeclampsia and autism spectrum disorder: a population-based study. J Child Psychol Psychiatry. 2020;61(2):131–139. doi: 10.1111/jcpp.13127. [DOI] [PubMed] [Google Scholar]
- 20.Glasson EJ, Bower C, Petterson B, de Klerk N, Chaney G, Hallmayer JF. Perinatal factors and the development of autism: a population study. Arch Gen Psychiatry. 2004;61(6):618–627. doi: 10.1001/archpsyc.61.6.618. [DOI] [PubMed] [Google Scholar]
- 21.Dachew BA, Mamun A, Maravilla JC, Alati R. Pre-eclampsia and the risk of autism-spectrum disorder in offspring: meta-analysis. Br J Psychiatry. 2018;212(3):142–147. doi: 10.1192/bjp.2017.27. [DOI] [PubMed] [Google Scholar]
- 22.Brown MA, Magee LA, Kenny LC, et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. 2018;72(1):24–43. doi: 10.1161/HYPERTENSIONAHA.117.10803. [DOI] [PubMed] [Google Scholar]
- 23.Lahti-Pulkkinen M, Girchenko P, Tuovinen S, et al. Maternal hypertensive pregnancy disorders and mental disorders in children. Hypertension. 2020;75(6):1429–1438. doi: 10.1161/HYPERTENSIONAHA.119.14140. [DOI] [PubMed] [Google Scholar]
- 24.Petersson F, Baadsgaard M, Thygesen LC. Danish registers on personal labour market affiliation. Scand J Public Health. 2011;39(7_suppl):95–98. doi: 10.1177/1403494811408483. [DOI] [PubMed] [Google Scholar]
- 25.Mors O, Perto GP, Mortensen PB. The Danish psychiatric central research register. Scand J Public Health. 2011;39(7_suppl):54–57. doi: 10.1177/1403494810395825. [DOI] [PubMed] [Google Scholar]
- 26.Wallach Kildemoes H, Toft Sørensen H, Hallas J. The Danish national prescription registry. Scand J Public Health. 2011;39(7_suppl):38–41. doi: 10.1177/1403494810394717. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt M, Schmidt SAJ, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449. doi: 10.2147/CLEP.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lynge E, Sandegaard JL, Rebolj M. The Danish national patient register. Scand J Public Health. 2011;39(7_suppl):30–33. doi: 10.1177/1403494811401482. [DOI] [PubMed] [Google Scholar]
- 29.Bliddal M, Broe A, Pottegard A, Olsen J, Langhoff-Roos J. The Danish medical birth register. Eur J Epidemiol. 2018;33(1):27–36. doi: 10.1007/s10654-018-0356-1. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt M, Pedersen L, Sørensen HT. The Danish civil registration system as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541–549. doi: 10.1007/s10654-014-9930-3. [DOI] [PubMed] [Google Scholar]
- 31.Ludvigsson JF, Almqvist C, Bonamy AKE, et al. Registers of the Swedish total population and their use in medical research. Eur J Epidemiol. 2016;31(2):125–136. doi: 10.1007/s10654-016-0117-y. [DOI] [PubMed] [Google Scholar]
- 32.Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11(1):450. doi: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swedish National Board of Health and Welfare. The Swedish Medical Birth Register: a summary of content and quality. https://www.socialstyrelsen.se/Lists/Artikelkatalog/Attachments/8306/2009-125-15_200912515_rev2.pdf. Accessed 14 Aug 2020.
- 34.Swedish National Board of Health and Welfare. Quality and content in the Swedish Patient Register. http://www.socialstyrelsen.se/Lists/Artikelkatalog. Accessed Aug 15 2020.
- 35.Kristensen JH, Basit S, Wohlfahrt J, Damholt MB, Boyd HA. Pre-eclampsia and risk of later kidney disease: nationwide cohort study. BMJ. 2019;365:l1516. doi: 10.1136/bmj.l1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khashan AS, Evans M, Kublickas M, et al. Preeclampsia and risk of end stage kidney disease: a Swedish nationwide cohort study. PLoS Med. 2019;16(7):e1002875. doi: 10.1371/journal.pmed.1002875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arendt LH, Henriksen TB, Lindhard MS, Parner ET, Olsen J, Ramlau-Hansen CH. Hypertensive disorders of pregnancy and genital anomalies in boys: a Danish nationwide cohort study. Epidemiology. 2018;29(5):739–748. doi: 10.1097/EDE.0000000000000878. [DOI] [PubMed] [Google Scholar]
- 38.Pedersen CB, Mors O, Bertelsen A, et al. A comprehensive nationwide study of the incidence rate and lifetime risk for treated mental disorders. JAMA Psychiat. 2014;71(5):573–581. doi: 10.1001/jamapsychiatry.2014.16. [DOI] [PubMed] [Google Scholar]
- 39.Nelson DB, Ziadie MS, McIntire DD, Rogers BB, Leveno KJ. Placental pathology suggesting that preeclampsia is more than one disease. Am J Obstet Gynecol. 2014;210(1):66-e1. doi: 10.1016/j.ajog.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 40.Berni TR, Morgan CL, Berni ER, Rees DA. Polycystic ovary syndrome is associated with adverse mental health and neurodevelopmental outcomes. J Clin Endocrinol Metab. 2018;103(6):2116–2125. doi: 10.1210/jc.2017-02667. [DOI] [PubMed] [Google Scholar]
- 41.Cesta CE, Månsson M, Palm C, Lichtenstein P, Iliadou AN, Landén M. Polycystic ovary syndrome and psychiatric disorders: co-morbidity and heritability in a nationwide Swedish cohort. Psychoneuroendocrinology. 2016;73:196–203. doi: 10.1016/j.psyneuen.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 42.Cnattingius S, Kramer MS, Norman M, Ludvigsson JF, Fang F, Lu D. Keep it in the family: comparing perinatal risks in small-for-gestational-age infants based on population vs within-sibling designs. Int J Epidemiol. 2019;48(1):297–306. doi: 10.1093/ije/dyy196. [DOI] [PubMed] [Google Scholar]
- 43.Backes CH, Markham K, Moorehead P, Cordero L, Nankervis CA, Giannone PJ. Maternal preeclampsia and neonatal outcomes. J Pregnancy. 2011;2011:214365. doi: 10.1155/2011/214365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ludvigsson JF, Appelros P, Askling J, et al. Adaptation of the Charlson comorbidity index for register-based research in Sweden. Clin Epidemiol. 2021;13:21–41. doi: 10.2147/clep.S282475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Christensen S, Johansen MB, Christiansen CF, Jensen R, Lemeshow S. Comparison of Charlson comorbidity index with SAPS and APACHE scores for prediction of mortality following intensive care. Clin Epidemiol. 2011;3:203–211. doi: 10.2147/clep.S20247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomsen RW, Hundborg HH, Lervang HH, Johnsen SP, Sørensen HT, Schønheyder HC. Diabetes and outcome of community-acquired pneumococcal bacteremia: a 10-year population-based cohort study. Diabetes Care. 2004;27(1):70–76. doi: 10.2337/diacare.27.1.70. [DOI] [PubMed] [Google Scholar]
- 47.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 48.Matsuishi T, Yamashita Y, Ohtani Y, et al. Brief report: incidence of and risk factors for autistic disorder in neonatal intensive care unit survivors. J Autism Dev Disord. 1999;29(2):161–166. doi: 10.1023/a:1023048812202. [DOI] [PubMed] [Google Scholar]
- 49.Langridge AT, Glasson EJ, Nassar N, et al. Maternal conditions and perinatal characteristics associated with autism spectrum disorder and intellectual disability. PLoS ONE. 2013;8(1):e50963. doi: 10.1371/journal.pone.0050963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maher GM, O'Keeffe GW, Kearney PM, et al. Association of hypertensive disorders of pregnancy with risk of neurodevelopmental disorders in offspring: a systematic review and meta-analysis. JAMA Psychiat. 2018;75(8):809–819. doi: 10.1001/jamapsychiatry.2018.0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pinheiro T, Brunetto S, Ramos J, Bernardi J, Goldani M. Hypertensive disorders during pregnancy and health outcomes in the offspring: a systematic review. J Dev Orig Health Dis. 2016;7(4):391–407. doi: 10.1017/S2040174416000209. [DOI] [PubMed] [Google Scholar]
- 52.Tranquilli AL, Brown MA, Zeeman GG, Dekker G, Sibai BM. The definition of severe and early-onset preeclampsia. Statements from the International Society for the Study of Hypertension in Pregnancy (ISSHP) Pregnancy Hypertens Int J Women's Cardiovasc Health. 2013;3(1):44–47. doi: 10.1016/j.preghy.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 53.Polo-Kantola P, Lampi KM, Hinkka-Yli-Salomaki S, Gissler M, Brown AS, Sourander A. Obstetric risk factors and autism spectrum disorders in Finland. J Pediatr. 2014;164(2):358–365. doi: 10.1016/j.jpeds.2013.09.044. [DOI] [PubMed] [Google Scholar]
- 54.Robinson M, Mattes E, Oddy WH, et al. Hypertensive diseases of pregnancy and the development of behavioral problems in childhood and adolescence: the Western Australian Pregnancy Cohort Study. J Pediatr. 2009;154(2):218–224. doi: 10.1016/j.jpeds.2008.07.061. [DOI] [PubMed] [Google Scholar]
- 55.Tearne JE, Allen KL, Herbison CE, et al. The association between prenatal environment and children's mental health trajectories from 2 to 14 years. Eur Child Adolesc Psychiatry. 2015;24(9):1015–1024. doi: 10.1007/s00787-014-0651-7. [DOI] [PubMed] [Google Scholar]
- 56.Heikura U, Hartikainen AL, Nordström T, Pouta A, Taanila A, Järvelin MR. Maternal hypertensive disorders during pregnancy and mild cognitive limitations in the offspring. Paediatr Perinat Epidemiol. 2013;27(2):188–198. doi: 10.1111/ppe.12028. [DOI] [PubMed] [Google Scholar]
- 57.Whitehouse AJ, Robinson M, Newnham JP, Pennell CE. Do hypertensive diseases of pregnancy disrupt neurocognitive development in offspring? Paediatr Perinat Epidemiol. 2012;26(2):101–108. doi: 10.1111/j.1365-3016.2011.01257.x. [DOI] [PubMed] [Google Scholar]
- 58.Instanes JT, Halmøy A, Engeland A, Haavik J, Furu K, Klungsøyr K. Attention-deficit/hyperactivity disorder in offspring of mothers with inflammatory and immune system diseases. Biol Psychiatry. 2017;81(5):452–459. doi: 10.1016/j.biopsych.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 59.Ros HS, Cnattingius S, Lipworth L. Comparison of risk factors for preeclampsia and gestational hypertension in a population-based cohort study. Am J Epidemiol. 1998;147(11):1062–1070. doi: 10.1093/oxfordjournals.aje.a009400. [DOI] [PubMed] [Google Scholar]
- 60.László KD, Liu XQ, Svensson T, et al. Psychosocial stress related to the loss of a close relative the year before or during pregnancy and risk of preeclampsia. Hypertension. 2013;62(1):183–189. doi: 10.1161/HYPERTENSIONAHA.111.00550. [DOI] [PubMed] [Google Scholar]
- 61.Seely EW, Ecker J. Chronic hypertension in pregnancy. Circulation. 2014;129(11):1254–1261. doi: 10.1161/circulationaha.113.003904. [DOI] [PubMed] [Google Scholar]
- 62.Brunström M. Hypertension, the Swedish patient register, and selection bias. JAMA Intern Med. 2016;176(6):862–863. doi: 10.1001/jamainternmed.2016.1556. [DOI] [PubMed] [Google Scholar]
- 63.Schlotz W, Phillips DI. Fetal origins of mental health: evidence and mechanisms. Brain Behav Immun. 2009;23(7):905–916. doi: 10.1016/j.bbi.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 64.Jurek AM, Greenland S, Maldonado G, Church TR. Proper interpretation of non-differential misclassification effects: expectations vs observations. Int J Epidemiol. 2005;34(3):680–687. doi: 10.1093/ije/dyi060. [DOI] [PubMed] [Google Scholar]
- 65.Lauritsen MB, Jørgensen M, Madsen KM, et al. Validity of childhood autism in the Danish Psychiatric Central Register: findings from a cohort sample born 1990–1999. J Autism Dev Disord. 2010;40(2):139–148. doi: 10.1007/s10803-009-0818-0. [DOI] [PubMed] [Google Scholar]
- 66.Mohr-Jensen C, Koch SV, Lauritsen MB, Steinhausen H-C. The validity and reliability of the diagnosis of hyperkinetic disorders in the Danish Psychiatric Central Research Registry. Eur Psychiatry. 2016;35:16–24. doi: 10.1016/j.eurpsy.2016.01.2427. [DOI] [PubMed] [Google Scholar]
- 67.Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11(1):1–16. doi: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wolf M, Kettyle E, Sandler L, Ecker JL, Roberts J, Thadhani R. Obesity and preeclampsia: the potential role of inflammation. Obstet Gynecol. 2001;98(5):757–762. doi: 10.1016/s0029-7844(01)01551-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No additional data available.
The codes used in this study are available upon request.