Abstract
Introduction.
Patients with a prior history of cancer (PHC) are at increased risk of second primary malignancy, of which lung cancer is the most common. We compared the performance metrics of positive screening rates and cancer detection rates among those with versus without PHC.
Methods.
We conducted a secondary analysis of 26,366 National Lung Screening Trial participants screened with LDCT between August 2002 and September 2007. We examined absolute rates and age-adjusted relative risks (RRs) of positive screening rates based on retrospective Lung-RADS application, invasive diagnostic procedure rate, complication rate, and cancer detection rate in those with versus without PHC using a binary logistic regression model using Firth’s penalized likelihood. We also compared histology, stage, and treatment in those with versus without PHC.
Results.
4.1% (n=1,071) of patients had PHC. Age-adjusted rates of positive findings were similar in those with versus without PHC (Baseline: PHC=13.7% versus no PHC=13.3%, RR (95%CI)=1.04 (0.88-1.24); Subsequent: PHC=5.6% versus no PHC=5.5%, RR (95%CI)=1.02 (0.84-1.23)). Age-adjusted cancer detection rates were higher in those with versus without PHC on baseline (PHC=1.9% versus no PHC=0.8%, RR (95%CI)=2.51 (1.67-3.81)) but not on subsequent screenings (PHC=0.6% versus no PHC=0.4%, RR (95%CI)=1.37 (0.99-1.93)). There were no differences in cancer stage, histology, or treatment by PHC status.
Conclusions.
Patients with PHC may benefit from lung cancer screening, and with their providers, should be made aware of the possibility of higher cancer detection, invasive procedures, and complication rates on baseline lung cancer screening, but not on subsequent LDCT screening examinations.
Keywords: lung cancer, screening, prior history of cancer, low dose computed tomography
Introduction
Improved cancer care in the United States has resulted in a growing population of cancer survivors, with approximately 19 million in 2019 and over 22 million expected by 2030.1 Cancer survivors have a 14% higher risk of developing a second primary malignancy (SPM).2 In a population-based cohort of cancer survivors, 1 in 12 developed a SPM,3 with the most commonly diagnosed SPM being lung cancer, representing 18% of all SPM.3 The highest standardized incidence ratios (SIR) of observed-to-expected cases of lung cancer as a SPM have been reported in patients with prior smoking-related cancers, including lung (SIR 4.9), head and neck (H&N) (SIR 4.0), and bladder (SIR 1.97) cancer.4 Also, patients with prior hematologic malignancy or breast cancer treated with radiation have higher SIRs for SPM of lung cancer, 1.4 and 2.5 respectively.4
Early detection of lung cancer in high-risk individuals using low dose computed tomography (LDCT) versus chest radiography screening reduced lung cancer mortality by 20% in the National Lung Screening Trial (NLST).5 Annual lung cancer screening (LCS) is recommended in patients ages 55 to 80 years with a 30 pack-year smoking history who currently or formerly (quit ≥ 15 years) smoked,6 with no specific guidelines among those with a prior history of cancer (PHC). Extending LCS to individuals with PHC after 4 years of surveillance without recurrence has been recommended by some organizations.7
While the lung cancer detection rate (CDR) in patients with PHC may be higher due to increased risk, the clinical heterogeneity of patients with PHC, including variability in the type of prior cancer, underlying comorbidities, and prior cancer treatments, may impact positive screening rates, diagnostic and invasive procedures rates, and complication rates. Extending LCS to individuals with a PHC is complicated because the benefit of LCS in this group of patients is unknown and the risk for developing lung cancer must be balanced against potential complications related to work-up of abnormal findings, the diagnosis, and treatment of lung cancer, as well as recurrence or dying from the prior cancer.8 Approximately 4.1% of participants enrolled in the NLST had a PHC.9 We sought to compare the outcome of annual LCS with LDCT among those with PHC and those without PHC and to evaluate differences in positive screening rates, CDR, downstream procedures, complications, cancer characteristics and treatment.
Materials and Methods
Study Population and Data Source
We used publicly available NLST data,9 details of which have been described elsewhere.10 Briefly, the NLST enrolled individuals ages 55-74 years free of lung cancer symptoms with a 30 pack-year or greater smoking history, currently smoking, or who used to smoke (quit ≥15 years before enrollment). NLST participants were randomized to either three annual LDCTs or chest radiographs which occurred from August 2002 through September 2007.5 Median follow-up duration was 6.5 years. For the current study, all participants who underwent at least one LDCT were included.
Main Exposure – Personal History of Cancer
Participants were categorized as having a PHC if they self-reported yes to having ever been diagnosed with any of the following cancer types before randomization: bladder, breast, cervical, colorectal, esophageal, renal, H&N, lung, pancreas, gastric, and thyroid. While patients with a prior history of lung cancer were intended to be excluded from the NLST, 20 patients with prior lung cancer were enrolled. Prior history of prostate cancer was not asked. Patients with a PHC reported the age at prior cancer diagnosis and this information was used to calculate the number of years between prior cancer diagnosis and the first LDCT screening exam. Among participants reporting more than one prior cancer, earliest cancer occurrence was used. While the NLST documentation states that patients diagnosed with or treated for any cancer other than nonmelanoma skin cancer or carcinoma in situ (with the exception of transitional cell carcinoma in situ or bladder carcinoma in situ) in the 5 years prior to eligibility assessment were excluded, the NLST dataset included 134 patients with PHC diagnosed up to 5 years before the first LDCT lung cancer screening exam. Since individuals with a past history of prostate cancer were not documented and individuals with lung cancer were intended to be excluded, the cohort of patients with a PHC in the NLST favors sampling of women over men.
Participant Characteristics
Participant characteristics included age at time of randomization, race, ethnicity, gender, body mass index (BMI), highest educational level, marital status, first-degree family history of lung cancer, smoking status (current versus former), pack-years, and comorbid conditions ascertained at enrollment through participant completion of a questionnaire. Conditions included adult-diagnosed asthma, asbestosis, bronchiectasis, chronic bronchitis, chronic obstructive pulmonary disease (COPD), diabetes, emphysema, pulmonary fibrosis, heart disease or heart attack, hypertension, pneumonia, or tuberculosis.
Outcomes and Measures
Each participant could contribute up to three LDCT screening exams. Since Lung CT Screening Reporting & Data System (Lung-RADS) was developed after the NLST, we retrospectively applied Lung-RADS v1.0 assessments to the LDCT screening exams.11 Screening exams with Lung-RADS of 1 (negative) or 2 (benign appearance) were classified as negative and those with Lung-RADS of 3 (probably benign) or 4 (suspicious) were classified as positive.11 Participants with at least one follow-up procedure in the 365 days following a Lung-RADS positive screening exam, were classified as having diagnostic work-up (eTable 1). In participants with invasive procedures linked to a Lung-RADS positive screening examination, complications were assessed and categorized based on severity into major, intermediate, and minor.12 We do not include follow-up among patients with negative Lung-RADS so that our findings are directly translatable to current LCS clinical care which is based on Lung-RADS.
Lung cancers diagnosed in the 365 days after the screening exam were included. Positive screening rates and CDRs were calculated per 100 screening examinations performed. CDR was calculated as the number of true-positive exams within one year of the screening LDCT divided by the total number of screening exams per 100. Lung cancer stage was evaluated using the American Joint Committee on Cancer staging system version 6, (version at the time of NLST), and collapsed into early (stage I and II) and late (stage III and IV).13 Histology was categorized into non-small cell lung cancer (NSCLC) with subtypes of bronchioloalveolar carcinoma (terminology used at the time of the NLST), adenocarcinoma, squamous cell, large cell, and other NSCLC; small cell lung cancer (SCLC), carcinoid, and unknown. Receipt of lung cancer treatments included surgery, radiation, chemotherapy, other, and none.
Statistical Analysis
Sociodemographic and clinical characteristics of NLST LDCT participants with and without a PHC were compared using t-tests, chi-square, Cochran-Armitage, Wilcoxon, and Kruskal-Wallis tests, where appropriate. The 5-year risk of lung cancer diagnosis and 5-year risk of lung cancer death per 1,000 people was calculated using the Lung Cancer Risk Models for Screenings risk calculator, which is based on the following covariates: age, education, gender, race and ethnicity, BMI, years smoked, cigarettes per day, years quit, family history of lung cancer, prior history of lung cancer, and comorbidities.14,15 Since the risk calculator requires complete covariate information, for participants with missing covariate information the risk estimates were based on 10 samples imputed using Markov Chain Monte Carlo (MCMC) and a maximum of 1,000 iterations, with the risk score taken as the median of the estimates from the 10 imputed samples. Missing race, ethnicity, education, and marital status covariate information did not exceed 2% for any of the covariates. We compared risk of lung cancer diagnosis and death between patients with and without PHC using a Kruskal-Wallis test.
Incidence rates (IR) of the prior cancer type were calculated. For participants with PHC, the time between the first LDCT screening exam and the participant’s reported age at prior cancer diagnoses was used to determine the person-years contribution. For participants with no PHC, age at randomization represented person-years at risk and was included in the denominator. For participants reporting more than one prior cancer the youngest reported age was used. The unadjusted and age-adjusted positive screening rates, invasive procedure rates, complication rates, and CDRs among those with versus without a PHC were estimated using a binary logistic regression model using Firth’s penalized likelihood. Odds ratio estimates from the logistic regression models were used to estimate relative risks. The screen was the unit of analysis and models were adjusted for age. Cancer characteristics and treatment in those with and without a PHC were compared with chi-square tests.
Risk of lung cancer diagnosis and risk of lung cancer death analyses were conducted in R Studio version 1.2.5033 using the lcrisks package for Lung Cancer Risk Models for Screening.16,17 All other analyses were conducted using SAS version 9.4.
Results
Overall, 26,366 individuals with at least one LDCT were studied: 4.1% (n=1,071) had PHC and 95.9% (n=25,295) had no PHC (Table 1). Compared to those with no PHC, NLST participants with documented PHC were older, more likely to be female, more likely to be underweight or normal weight, more likely to have an educational level of high school education or less, and less likely to be married. NLST participants with documented PHC were more likely to have adult-diagnosed asthma, chronic bronchitis, COPD, emphysema, hypertension, and pneumonia than those with no PHC. Risk of lung cancer diagnosis and death was significantly higher for NLST participants with a PHC, compared to participants without a PHC.
Table 1.
Demographic and clinical characteristics of NLST participants who underwent low dose computed tomography by prior history of cancer (PHC) status
| Characteristic | All N=26,366 |
PHC N=1,071 |
No PHC N=25,295 |
p-value a |
|---|---|---|---|---|
| Age, years | ||||
| Median (IQR) | 60 (57-65) | 62 (58-66) | 60 (57-65) | <.001 |
| Gender, No. (%) | ||||
| Male | 15,583 (59.1) | 289 (27.0) | 15,294 (60.5) | <.001 |
| Female | 10,783 (40.9) | 782 (73.0) | 10,001 (39.5) | |
| Race, No. (%) | ||||
| White | 24,045 (91.2) | 1,007 (94.0) | 23,038 (91.1) | 0.12 |
| Black | 1,171 (4.4) | 36 (3.4) | 1,135 (4.5) | |
| Asian | 547 (2.1) | 14 (1.3) | 533 (2.1) | |
| American Indian/Alaska Native | 90 (0.3) | 2 (0.2) | 88 (0.4) | |
| Native Hawaiian/Pacific Islander | 86 (0.3) | 3 (0.3) | 83 (0.3) | |
| More than one race | 326 (1.2) | 8 (0.8) | 318 (1.3) | |
| Missing | 100 (0.4) | 1 (0.1) | 100 (0.4) | |
| Ethnicity, No. (%) | ||||
| Hispanic or Latino | 466 (1.8) | 16 (1.5) | 450 (1.8) | 0.58 |
| Non-Hispanic nor Latino | 25,793 (97.8) | 1,052 (98.2) | 24,741 (97.8) | |
| Missing | 107 (0.4) | 3 (0.3) | 104 (0.4) | |
| BMI, No. (%) | ||||
| Underweight | 226 (0.9) | 12 (1.1) | 214 (0.9) | 0.004 |
| Normal | 7,427 (28.2) | 354 (33.1) | 7,073 (28.0) | |
| Overweight | 11,146 (42.3) | 413 (38.6) | 10,733 (42.4) | |
| Obese | 7,475 (28.4) | 289 (27.0) | 7,186 (28.4) | |
| Missing | 92 (0.4) | 3 (0.3) | 89 (0.4) | |
| Median (IQR) | 27 (24-31) | 26 (24-30) | 27 (24-31) | |
| Educational Level, No. (%) | ||||
| ≤8th grade | 358 (1.4) | 13 (1.2) | 345 (1.4) | <0.001 |
| 9-11th grade | 1,258 (4.8) | 65 (6.1) | 1,193 (4.7) | |
| HS / GED | 6,199 (23.5) | 279 (26.1) | 5,920 (23.4) | |
| Post HS training | 3,699 (14.0) | 147 (13.7) | 3,552 (14.0) | |
| Assoc. degree/some college | 6,127 (23.2) | 268 (25.0) | 5,859 (23.2) | |
| Bachelor’s degree | 4,455 (16.9) | 157 (14.7) | 4,298 (17.0) | |
| Graduate school | 3,737 (14.2) | 121 (11.3) | 3,616 (14.3) | |
| Missing | 533 (2.0) | 21 (2.0) | 512 (2.0) | |
| Marital Status, No. (%) | ||||
| Married or living as married | 17,652 (66.9) | 586 (54.7) | 17,066 (67.5) | <.001 |
| Divorced | 5,107 (19.4) | 263 (24.6) | 4,844 (19.2) | |
| Widowed | 1,961 (7.4) | 144 (13.5) | 1,817 (7.2) | |
| Separated | 334 (1.3) | 16 (1.5) | 318 (1.3) | |
| Never married | 1,236 (4.7) | 62 (5.8) | 1,174 (4.6) | |
| Missing | 76 (0.3) | 0 | 76 (0.3) | |
| Family History of Lung Cancer, b No. (%) | ||||
| Yes | 5,760 (21.8) | 232 (21.7) | 5,528 (21.9) | 0.97 |
| No | 20, 606 (78.2) | 839 (78.3) | 19,767 (78.2) | |
| Smoking Status, No. (%) | ||||
| Former | 13,686 (51.9) | 578 (54.0) | 13,108 (51.8) | 0.18 |
| Current | 12,680 (48.1) | 493 (46.0) | 12,187 (48.2) | |
| Pack-years | ||||
| Median (IQR) | 48 (39-66) | 49 (40-66) | 48 (39-66) | 0.80 |
| Comorbidities, c No. (%) | ||||
| Median No. comorbidities (IQR) | 1 (0-2) | 1 (0-2) | 1 (0-2) | <.001 |
| Asthma (adult) | 1,630 (6.2) | 104 (9.7) | 1,526 (6.0) | <.001 |
| Asbestosis | 274 (1.0) | 9 (0.8) | 265 (1.1) | 0.50 |
| Bronchiectasis | 844 (3.2) | 35 (3.3) | 809 (3.2) | 0.94 |
| COPD | 4,602 (17.5) | 258 (24.1) | 4,344 (17.2) | <.001 |
| Diabetes | 2,555 (9.7) | 106 (9.9) | 2,449 (9.7) | 0.76 |
| Fibrosis | 69 (0.3) | 4 (0.4) | 65 (0.3) | 0.48 |
| Heart disease or heart attack | 3,410 (12.9) | 152 (14.2) | 3,258 (12.9) | 0.11 |
| Hypertension | 9,268 (35.2) | 407 (38.0) | 8,861 (35.0) | 0.04 |
| Pneumonia | 5,863 (22.2) | 324 (30.3) | 5,539 (21.9) | <.001 |
| Tuberculosis | 276 (1.1) | 13 (1.2) | 263 (1.0) | 0.61 |
| Risk of lung cancer diagnosis (5-year risk per 1,000) d | ||||
| Median (IQR) | 11.7 (7.5-19.4) | 13.3 (8.4-21.7) | 11.7 (7.5-19.3) | <.001 |
| Risk of lung cancer death (5-year risk per 1,000) e | ||||
| Median (IQR) | 6.2 (3.7-10.9) | 6.8 (4.1-12.3) | 6.2 (3.7-10.9) | <.001 |
Chi-square, Cochran-Armitage, Wilcoxon, and Kruskal-Wallis test statistics for differences by PHC status where appropriate, missing values not included.
Family history includes mother, father, brother, sister or biological child with a lung cancer diagnosis.
For comorbidities, number shown represents those who reported yes ever diagnosed prior to trial. COPD includes chronic bronchitis, COPD, and Emphysema; # comorbidities: a count of specific co-morbid conditions reported as ever diagnosed prior to trial: Asthma (adult), Asbestosis, Bronchiectasis, Chronic bronchitis, COPD, Diabetes, Emphysema, Fibrosis of the lung, Heart disease or heart attack, Hypertension, Pneumonia, Tuberculosis.
Among 1,000 people in the US with this risk-factor profile, this is the number who will be diagnosed with lung cancer if they do not attend screening (LCRAT). Based on taking the median of 10 imputed samples for those with missing covariate information.
Among 1,000 people in the US with this risk-factor profile, this is the number who will die from lung cancer if they do not attend screening.
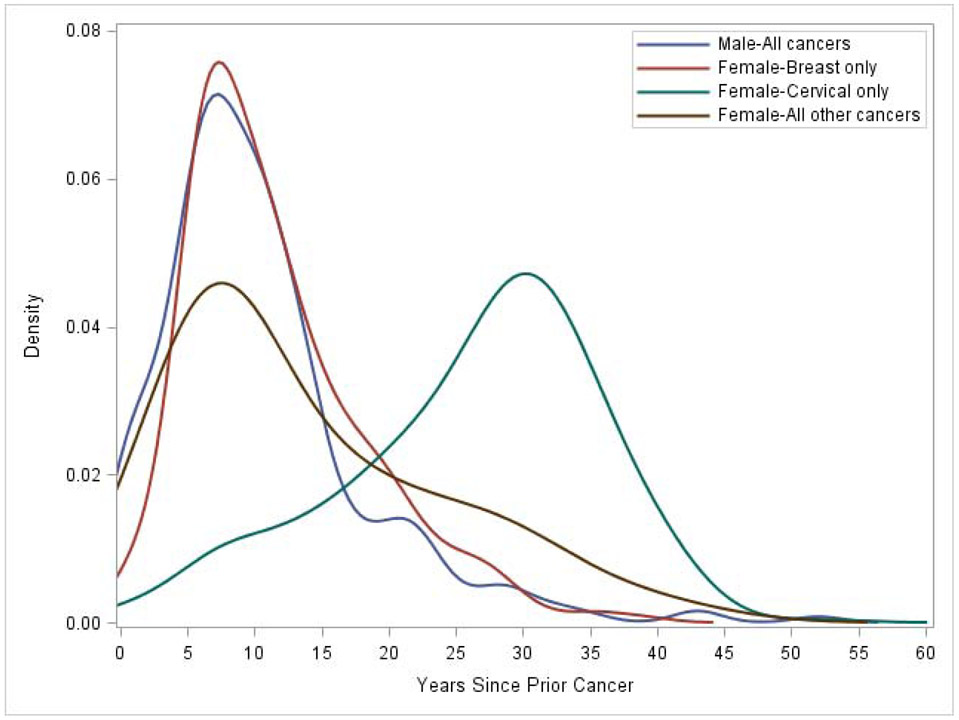
The incidence rate of any prior cancer per 100,000 person-years was 121.3/100,000 person-years for females and 30.2/100,000 person-years for males. The lower cancer incidence rate in men may be explained by the fact that a prior history of prostate cancer was not asked, and direct comparisons of total cancer numbers between men and women cannot be made (Table 2). For females, incidence rates per 100,000 person-years were highest for prior breast cancer (53.0/100,000 person-years) and cervical cancer (55.7/100,000 person-years). In males, incidence rates per 100,000 person-years were highest for prior bladder cancer (10.1/100,000 person-years), colorectal cancer (7.2/100,000 person-years), and H&N cancer (6.9/100,000 person-years). The time between prior cancer diagnosis and the first LCS LDCT differed for females with cervical cancer versus females with other non-cervical cancer types and males (Figure 1). The median time to diagnosis for males was 9 years (IQR=6-13), for females with breast cancer was 10 years (IQR=7-15), for females with non-breast or non-cervical cancer was 10 years (IQR=6-20.5), and for females with cervical cancer was 28 years (IQR=20-33). Nearly 23.9% (n=69/289) of men and 8.3% (n=65/782) of women underwent LDCT LCS within 5 years of their prior cancer diagnosis.
Table 2.
Type of prior cancer and incidence rates among NLST participants with a prior history of cancer undergoing low dose computed tomography, stratified by gender
| Overall | Female | Male | ||||
|---|---|---|---|---|---|---|
| Type of Prior Cancer |
N | Incidence rate per 100,000 person-years |
N | Incidence rate per 100,000 person-years |
N | Incidence rate per 100,000 person-years |
| All | 1,071 | 66.9 | 782 | 121.3 | 289 | 30.2 c |
| Bladder a | 119 | 7.4 | 22 | 3.4 | 97 | 10.1 |
| Breast | 346 | 21.6 | 342 | 53.0 | 4 | 0.4 |
| Cervical | 359 | 55.7 | 359 | 55.7 | n/a | n/a |
| Colorectal | 108 | 6.7 | 39 | 6.0 | 69 | 7.2 |
| Esophageal | 12 | 0.8 | 3 | 0.5 | 9 | 0.9 |
| Head and Neck b | 79 | 4.9 | 13 | 2.0 | 66 | 6.9 |
| Kidney | 35 | 2.2 | 9 | 1.4 | 26 | 2.7 |
| Lung | 10 | 0.6 | 1 | 0.2 | 9 | 0.9 |
| Pancreas | 4 | 0.2 | 0 | - | 4 | 0.4 |
| Stomach | 11 | 0.7 | 3 | 0.5 | 8 | 0.8 |
| Thyroid | 34 | 2.1 | 19 | 2.9 | 15 | 1.6 |
Bladder includes transitional cell
Head and neck cancers include oral, larynx, nasal, pharynx (n=1 have both larynx and oral, counted once)
A history of prostate cancer was not obtained which may explain the lower cancer incidence rate in men.
Figure 1.
Time in years from prior cancer diagnosis to first low dose computed tomography screening exam by gender and female specific cancer type, NLST
Retrospective application of Lung-RADS to the NLST LDCT cohort resulted in similar age-adjusted positive screening rates for baseline and subsequent LDCT screening exams by PHC status (Baseline: 13.7% in PHC versus 13.3% in no PHC, RR=1.04, 95%CI: 0.88-1.24; Subsequent: 5.6% PHC versus 5.5% no PHC, RR=1.02, 95%CI: 0.84-1.23) (Table 3). The age-adjusted invasive procedure rate was higher in those with versus without PHC on baseline (3.4% versus 1.8%, respectively; RR=1.98, 95%CI: 1.44-2.74) but not on subsequent screening rounds (1.3% versus 1.1%, respectively; RR=1.24, 95%CI: 0.86-1.79). Similarly, the age-adjusted complication rate was higher for those with versus without PHC on baseline with no difference on subsequent screenings. The age-adjusted CDR was higher among those with versus without PHC on baseline (1.9% versus 0.8%, respectively; RR=2.51, 95%CI: 1.67-3.81) and was similar on subsequent screening rounds.
Table 3.
Unadjusted and adjusted rates and relative risks of positive screening rates, invasive procedures, complications, and cancer detection by screening round, NLST
| Baseline Screening | Subsequent Screening | |||||
|---|---|---|---|---|---|---|
| PHC Rate/100 (95% CI) |
No PHC Rate/100 (95% CI) |
Relative Risk (95% CI) |
PHC Rate/100 (95% CI) |
No PHC Rate/100 (95% CI) |
Relative Risk (95% CI) |
|
| Unadjusted Rates | ||||||
| Lung-RADS Positive a | 14.9 (12.9-17.1) |
13.5 (13.1-14.0) |
1.12 (0.94-1.33) |
6.0 (5.0-7.1) |
5.6 (5.4-5.8) |
1.07 (0.88-1.29) |
| Invasive Procedure b | 3.9 (2.9-5.3) |
1.9 (1.7-2.0) |
2.17 (1.58-2.99) |
1.5 (1.1-2.2) |
1.2 (1.1-1.3) |
1.34 (0.93-1.95) |
| Complication c | 1.4 (0.9-2.3) |
0.6 (0.5-0.7) |
2.54 (1.50-4.30) |
0.5 (0.2-0.9) |
0.6 (0.5-0.6) |
0.87 (0.46-1.67) |
| Cancer Detection d | 2.3 (1.6-3.4) |
0.8 (0.7-1.0) |
2.85 (1.88-4.32) |
0.6 (0.5-0.9) |
0.4 (0.4-0.5) |
1.52 (1.13-2.10) |
| Age Adjusted Rates | ||||||
| Lung-RADS Positive a | 13.7 (11.9-15.8) |
13.3 (12.8-13.7) |
1.04 (0.88-1.24) |
5.6 (4.7-6.7) |
5.5 (5.3-5.8) |
1.02 (0.84-1.23) |
| Invasive Procedure b | 3.4 (2.5-4.6) |
1.8 (1.6-1.9) |
1.98 (1.44-2.74) |
1.3 (0.9-1.9) |
1.1 (1.0-1.2) |
1.24 (0.86-1.79) |
| Complication c | 1.1 (0.6-1.8) |
0.5 (0.4-0.6) |
2.21 (1.30-3.76) |
0.4 (0.2-0.7) |
0.5 (0.45-0.6) |
0.78 (0.41-1.50) |
| Cancer Detection d | 1.9 (1.2-2.8) |
0.8 (0.7-0.9) |
2.51 (1.67-3.81) |
0.6 (0.4-0.8) |
0.4 (0.4-0.5) |
1.37 (0.99-1.93) |
The number of participants with a positive lung screening exam based on Lung-RADS of 3, 4A, 4B, or 4X per 100 screening examinations.
Invasive procedure rate calculated as the number of participants with an invasive procedure per 100 screening examinations.
Complication rate calculated as the number of participants with a complication from work-up per 100 screening examinations.
Cancer detection rate calculated as the number of true-positive exams within one-year of the screening LDCT per 100 screening examinations.
A total of 550 lung cancers were diagnosed in the one-year after a Lung-RADS positive LDCT (Table 4). Of these, 40 had a PHC, and 510 had no PHC. In both groups, the majority of lung cancers were adenocarcinoma with no differences in histology among those with versus without PHC. Early-stage lung cancers accounted for 62.5% of those with PHC and 67.8% of those with no PHC. Over 90% of participants with early-stage lung cancer underwent surgery, which was similar by PHC status. Among participants with late-stage disease, no treatment differences by PHC were observed.
Table 4.
Cancer characteristics and treatments, NLST
| PHC No. (%) |
No PHC No. (%) |
p-valuea | |
|---|---|---|---|
| Lung cancers diagnosed within 1-year of positive LDCT screening, No. | 40 | 510 | n/a |
| Histology, No. (%) | |||
| All non-small cell lung cancer (NSCLC) | 36 (90.0) | 462 (90.6) | 0.44 |
| Bronchioloalveolar carcinoma (BAC) | 2 (5.0) | 12 (2.4) | |
| Adenocarcinoma | 20 (50.0) | 260 (51.0) | |
| Squamous cell | 5 (12.5) | 119 (23.3) | |
| Large cell | 3 (7.5) | 30 (5.9) | |
| Other NSCLC | 6 (15.0) | 41 (8.0) | |
| Small cell lung cancer | 3 (7.5) | 38 (7.5) | |
| Carcinoid | 0 (0) | 6 (1.2) | |
| Unknown | 1 (2.5) | 4 (0.8) | |
| Lung Cancer Stage, No. (%) | |||
| Early | 25 (62.5) | 346 (67.8) | 0.43 |
| Late | 15 (37.5) | 153 (30.0) | |
| Not staged b | 0 (0) | 11 (2.2) | |
| Treatment by Stage | |||
| Early Stage, No. (%) | N=25 | N=346 | |
| Any surgery | 23 (92.0) | 329 (95.1) | 0.17 |
| Radiation only | 1 (4.0) | 2 (0.6) | |
| Radiation and chemotherapy | 0 (0) | 5 (1.5) | |
| Otherc | 1 (4.0) | 3 (0.9) | |
| None | 0 (0) | 7 (2.0) | |
| Late Stage, No. (%) | N=15 | N=153 | |
| Any surgery | 6 (40.0) | 53 (34.6) | 0.85 |
| Chemotherapy only | 3 (20.0) | 30 (19.6) | |
| Radiation and chemotherapy | 5 (33.3) | 50 (32.7) | |
| Otherd | 1 (6.7) | 8 (5.2) | |
| None | 0 (0) | 12 (7.8) |
Chi-square test statistic for differences by PHC status
Non-staged includes occult carcinoma, carcinoid, and those with missing or unavailable TNM
Other includes immunotherapy, radiofrequency ablation, brachytherapy, and all other treatments
In addition to ‘other’ this group includes those with radiation + chemotherapy + other and those with radiation only
Discussion
In this retrospective analysis of LCS performance in NLST participants, our results highlight similar rates of positive screening LDCT but higher rates of cancer detection, invasive procedures, and complications in those with versus without PHC at baseline screening. Since individuals with PHC had higher 5-year risk of developing lung cancer at the time of enrollment in the compared sample with versus without PHC, we expected both the rate of positive LDCT and cancer detection to be higher at the baseline screening examination. While this was the case for cancer detection, the positive LDCT rates did not differ.
Several small studies have reported on LCS in patients with PHC. In a single-institution study, initial evaluation of 139 patients with PHC (43% breast, 19% H&N, and 12% lung) with retrospective application of Lung-RADS criteria resulted in positive screening rates of 16% and 13% on baseline and subsequent exams, respectively.18 Expansion of this single-institution study to 543 patients with PHC who underwent LCS reported positive screening rates of 22% at baseline and 9-22% on subsequent screens,19 which are higher than those we report (13.7% on baseline and 5.6% on subsequent). The unadjusted CDR on baseline screenings among the 543 patients with PHC from the O’Dwyer study was 3.9% compared with 2.3% in our analysis of the NLST data. These differences in rates of positive LDCT and baseline cancer detection may be attributed to differences in the underlying characteristics of the populations being screened or differences in evaluation of LDCT images. Larger studies from diverse populations of patients with PHC are needed. Specifically, patients with PHC who have an increased risk of developing lung cancer (prior smoking-related lung, H&N, and bladder cancer) may appropriately have higher positive screening rates because more lung cancers are detected. In the NLST, the most common types of prior cancers for women were breast and cervical while for men, since a history of prostate cancer was not obtained, the most common were bladder, colorectal, and H&N. In a single-institution study of 543 patients, the most common types of prior cancers included 38% breast, 19% H&N, 16% lung, and 12% prostate.19 The likelihood of developing lung cancer after a prior malignancy likely varies based on the type of prior cancer and if the prior malignancy is smoking-related.
We found that compared to patients with no PHC, patients with PHC had higher rates of invasive procedures and complications on baseline but not subsequent screens. A plausible explanation for the higher rates of invasive procedures in patients with PHC may be a heightened concern for the possibility of cancer, not only for a second primary lung cancer but also for a metastatic lesion from a prior cancer. We also found individuals with PHC had a higher mean number of comorbidities and were more likely to have chronic bronchitis, COPD, and emphysema; another possible explanation for increased rates of invasive procedures. A study reporting patient outcomes following LDCT in the NLST found that patients with COPD were more likely to undergo invasive procedures and to have serious complications following procedures.20
LCS in individuals with PHC is complicated by the fact that cancer survivors represent a heterogeneous group of individuals with variability in underlying comorbidities (for example, patients with bladder cancer may be older and have more comorbidities than patients with prior breast cancer), prior cancer treatments, and experiences that may influence the patients’ ability to undergo treatment for a SPM which may confound the improved survival benefits of LCS. While patient age and smoking history form the cornerstones of LCS eligibility criteria, incorporating additional lung cancer risk factors such as PHC may tilt the benefit to harm ratio for some high-risk populations.21 Moreover, consideration of the type of prior cancer and prior cancer treatment warrants additional evaluation. Significant comorbidities may impact the benefit from screening, particularly in those who are at increased risk of death from competing respiratory and cardiovascular causes, and who may be at higher risk of complications from diagnostic procedures and may be unable to undergo curative treatment.22
Studies reporting on the impact of PHC on lung cancer outcomes have yielded conflicting results. An early study of 2,991 patients with resected stage I NSCLC showed that in 192 patients with PHC, lung cancer survival was worse (HR 1.44, 95%CI: 1.16-1.78).23 On the other hand, several studies have determined that a PHC does not adversely impact survival in early, locally advanced, or advanced-stage lung cancer.24,25 A retrospective study of 821,323 patients diagnosed with NSCLC between 2004–2014 evaluated the impact of PHC (found in 179,517; 21.9%) on lung cancer outcomes and found that PHC had a variable impact with decreased survival in patients with stage I NSCLC who had undergone surgery (HR 1.28, 95%CI: 1.25-1.30, p<0.001) and better survival in patients with PHC and advanced NSCLC.26 We found that NLST participants with and without PHC who were diagnosed with lung cancer had similar distribution of disease stage, tumor histology, and treatment for both early and late-stage lung cancer. There is a need to generate more evidence in this area. A future international collaborative study on lung cancer screening among those with a PHC should focus on collecting harmonized, detailed data on previous cancer type, histology, stage, treatment and response to evaluate the risk and benefits and inform screening in this population.
While a strength of our study is that it represents the largest examination of LCS in patients with versus without PHC, there are several limitations. The number of NLST participants with PHC was relatively small, which meant it was not feasible to examine specific types of prior cancer separately. Since Lung-RADS was developed after the publication of the NLST results, we retrospectively applied the Lung-RADS criteria to evaluate the NLST results in a setting more similar to how LCS is being conducted in clinical practice. Use of Lung-RADS may differ from the retrospective application used in this study. In addition, NLST participants were healthier than the general population eligible for LCS so these findings may not be generalizable to real-world settings.27
Conclusions
In conclusion, the rates of positive LCS in NLST participants with versus without a PHC were similar. However, cancer detection, invasive procedure and complication rates were higher on baseline screening examinations in those with PHC; which may be due to underlying comorbidities, prior cancer treatments, and heightened concern for recurrence of prior cancer. Our findings suggest that PHC should not be an impediment to LCS in those individuals who are at high risk for lung cancer but are currently healthy enough to potentially benefit from screening. Awareness of the possibility of higher lung cancer detection, invasive procedures, and complication rates on baseline LDCT, but not on subsequent screening in individuals with PHC, is important to guide patient selection and risk-stratification to optimize LCS. To better understand the impact of LCS in cancer survivors at increased risk for developing lung cancer, ongoing research should consider exploring how prior cancer experiences and comorbidities may influence LCS outcomes.
Supplementary Material
Acknowledgements
The authors thank the National Cancer Institute for access to NCI’s data collected by the National Lung Screening Trial (NLST). The statements contained herein are solely those of the authors and do not represent or imply concurrence or endorsement by NCI.
Funding/Support: This study was supported by NIH/NCI under grant numbers R01CA212014 and R01CA251686.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: Dr. Rivera served as an Advisory Board Member for Biodesix and bioAffinities Tech, and served on an advisory research panel for Johnson and Johnson. No other disclosures.
References
- 1.Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363–385. [DOI] [PubMed] [Google Scholar]
- 2.Curtis RE. New malignancies among cancer survivors: SEER cancer registries, 1973–2000. In: Bethesda, Maryland: National Cancer Institute, NIH Publ. No. 05-5302; 2006. [Google Scholar]
- 3.Donin N, Filson C, Drakaki A, et al. Risk of second primary malignancies among cancer survivors in the United States, 1992 through 2008. Cancer. 2016;122(19):3075–3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu GX, Nelson RA, Kim JY, Raz DJ. Non-Small Cell Lung Cancer as a Second Primary Among Patients With Previous Malignancy: Who Is at Risk? Clin Lung Cancer. 2017;18(5):543–550.e543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moyer VA. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330–338. [DOI] [PubMed] [Google Scholar]
- 7.Jaklitsch MT, Jacobson FL, Austin JHM, et al. The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. The Journal of Thoracic and Cardiovascular Surgery. 2012; 144(1):33–38. [DOI] [PubMed] [Google Scholar]
- 8.Rivera MP, Tanner NT, Silvestri GA, et al. Incorporating Coexisting Chronic Illness into Decisions about Patient Selection for Lung Cancer Screening. An Official American Thoracic Society Research Statement. Am J Respir Crit Care Med. 2018;198(2):e3–e13. [DOI] [PubMed] [Google Scholar]
- 9.NLST Trial Summary. National Cancer Institute: Cancer Data Access System. https://cdas.cancer.gov/learn/nlst/trial-summary/. Published 2017. Accessed September 4, 2020.
- 10.Aberle DR, Berg CD, Black WC, et al. The National Lung Screening Trial: overview and study design. Radiology. 2011;258(1):243–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pinsky PF, Gierada DS, Black W, et al. Performance of Lung-RADS in the National Lung Screening Trial: a retrospective assessment. Ann Intern Med. 2015;162(7):485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening [Supplement Material: NLST Medical Complications]. N Engl J Med. 2011;365(5):395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cancer Staging Manual. American Joint Committee on Cancer. https://cancerstaging.org/references-tools/deskreferences/Pages/default.aspx. Published 2020. Accessed September 4, 2020. [Google Scholar]
- 14.Katki HA, Kovalchik SA, Berg CD, Cheung LC, Chaturvedi AK. Development and Validation of Risk Models to Select Ever-Smokers for CT Lung Cancer Screening. Jama. 2016;315(21):2300–2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung LC, Berg CD, Castle PE, Katki HA, Chaturvedi AK. Life-Gained-Based Versus Risk-Based Selection of Smokers for Lung Cancer Screening. Ann Intern Med. 2019;171(9):623–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lung Cancer Risk Models for Screening (R package: lcrisks) National Cancer Institute: Division of Cancer Epidemiology & Genetics. https://dceg.cancer.gov/tools/risk-assessment/lcrisks. Published 2020. Accessed September 4, 2020.
- 17.R & Python: Professional Data Science is Done in Code. R Studio. https://rstudio.com/. Published 2020. Accessed September 4, 2020. [Google Scholar]
- 18.Halpenny DF, Cunningham JD, Long NM, Sosa RE, Ginsberg MS. Patients with a Previous History of Malignancy Undergoing Lung Cancer Screening: Clinical Characteristics and Radiologic Findings. J Thorac Oncol. 2016;11(9):1447–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Dwyer E, Halpenny DF, Ginsberg MS. Lung cancer screening in patients with previous malignancy: Is this cohort at increased risk for malignancy? European Radiology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iaccarino JM, Silvestri GA, Wiener RS. Patient-Level Trajectories and Outcomes After Low-Dose CT Screening in the National Lung Screening Trial. Chest. 2019; 156(5):965–971. [DOI] [PubMed] [Google Scholar]
- 21.Ten Haaf K, Jeon J, Tammemägi MC, et al. Risk prediction models for selection of lung cancer screening candidates: A retrospective validation study. PLoS Med. 2017;14(4):e1002277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Young RP, Duan F, Greco E, Chiles C, Aberle D, Gamble GD. Lung Cancer Screening and the Effects of Competing Causes of Death in the ACRIN-NLST Sub-Study. American Thoracic Society International Conference; 2017. [Google Scholar]
- 23.López-Encuentra A, Gómez de la Cámara A, Rami-Porta R, Duque-Medina JL, de Nicolás JL, Sayas J. Previous tumour as a prognostic factor in stage I non-small cell lung cancer. Thorax. 2007;62(5):386–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laccetti AL, Pruitt SL, Xuan L, Halm EA, Gerber DE. Prior cancer does not adversely affect survival in locally advanced lung cancer: A national SEER-medicare analysis. Lung Cancer. 2016;98:106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pruitt SL, Laccetti AL, Xuan L, Halm EA, Gerber DE. Revisiting a longstanding clinical trial exclusion criterion: impact of prior cancer in early-stage lung cancer. Br J Cancer. 2017; 116(6):717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Monsalve AF, Hoag JR, Resio BJ, et al. Variable impact of prior cancer history on the survival of lung cancer patients. Lung Cancer. 2019;127:130–137. [DOI] [PubMed] [Google Scholar]
- 27.Howard DH, Richards TB, Bach PB, Kegler MC, Berg CJ. Comorbidities, smoking status, and life expectancy among individuals eligible for lung cancer screening. Cancer. 2015;121(24):4341–4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.