Abstract
Extracellular vesicles (EVs) are membrane-delimited particles secreted by nearly all cell types. EVs mediate critical physiological functions and pathophysiological processes in the central nervous system (CNS). As carriers of diverse bioactive cargo (e.g., proteins, lipids and nucleic acids) that can be modified in response to external stimuli, EVs have emerged as pathological mediators after neurotrauma, such as spinal cord injury (SCI). Here, we discuss the roles of endogenous EVs within the CNS as well as crosstalk with peripheral EVs in relation to neurotrauma, with particular focus on SCI. We then summarize the status of EV-based therapeutic advances in preclinical animal models for these conditions. Finally, we discuss new bioengineering strategies that are poised to enhance CNS-specific therapeutic capabilities of EVs.
Keywords: Exosomes, Neural Regeneration, Neurotrauma
Extracellular Vesicles in Spinal Cord Injury Pathobiology and Therapy
Traumatic injuries to the central nervous system (CNS), such as spinal cord injury (SCI) and traumatic brain injury (TBI), constitute a significant portion of the global injury burden, with 0.935 million new cases and an incidence rate of 17,730 new SCI cases each year according to the National SCI Statistical Center [1,2]. Historical advances in symptom management have reduced mortality rates, with an estimated 363,000 SCI survivors currently living in the United States. However, there is no effective treatment to counteract the long-term functional deficits following SCI. In part, this reflects an incomplete understanding of the complex pathophysiology after SCI as well as a lack of effective tools that safely regulate known therapeutic targets.
One emerging therapeutic option is the use of extracellular vesicles (EVs; see Glossary) that comprise the secretome of nearly all cell types, including those of the CNS. EVs can be detected in all body fluids including blood, urine, and cerebrospinal fluid (CSF) and play significant roles in regulation of neuro-glia communication as well as neural plasticity and the immune response during development, adulthood, and pathology based on their payload of diverse biological cargo [3–9]. Additionally, EVs have emerged as alternatives to cell-based therapies due to their potential for improved safety and therapeutic efficacy across diverse regenerative applications [10]. Yet, the pathophysiological roles of EVs in the CNS are still largely unknown, hampering rational design of novel EV-based treatment approaches.
This review summarizes current knowledge of the role of CNS EVs after neurotrauma, with an emphasis on SCI. It further discusses how this knowledge can be applied in combination with biotechnological advances to inform development of therapeutic EVs for SCI treatment. Additionally, the emerging role of EV crosstalk between the CNS and peripheral systems is considered throughout. It should be noted that the literature specific to EV-based interventions for SCI is relatively nascent compared to other treatment modalities, such as gene- or cell-therapy. As pathophysiological mechanisms of SCI considerably overlap with that of TBI, stroke, and some neurodegenerative diseases (e.g. inflammation, apoptosis, metabolic dysfunction, etc.), relevant studies from broader applications have also been included in this discussion.
CNS Injury Involves Multicellular EV-Mediated Crosstalk
Neurotrauma in the brain and spinal cord share many features that are often categorized into primary and secondary phases. Primary injury is defined by the acute damage to neural tissue resulting from mechanical forces that leads to largely irreversible cell loss [11]. The ensuing secondary injury phase is characterized by a delayed biochemical sequelae encompassing metabolic and inflammatory dysfunction that may contribute to further tissue damage over time [11]. Secondary injury mechanisms in particular represent critical therapeutic targets after SCI, with investigational strategies aimed to promote angiogenesis, axonal sparing, and regeneration/remyelination, while limiting autophagy dysfunction, inflammation, and glial scarring [12–14]. Recent studies suggest that EVs participate in the progression of secondary injury by transporting parent cell-specific signaling cargo (e.g. signal lipids, genetic information, cytokines, receptors, etc.) that alter the function of recipient cells within the CNS and beyond [15]. EV bioactivity and biological cargo are related to the phenotype of the EV parent cell and can vary depending on stimulus and the surrounding microenvironment.
Inflammation Alters EV Cargo, Thereby Affecting Neuro-Glia Communication
While current understanding of physiological EV communication in the CNS is limited (see also Box 1), emerging evidence indicates that EV-mediated functions are likely altered in association with many pathological features of neurotrauma. Modifications in EV cargo, such as microRNAs (miRNAs), may significantly disrupt homeostatic balance between neurons and surrounding glia, as indicated by in vitro studies (Figure 1). For example, upon ATP or proinflammatory (e.g. IL-1β, TNFα, IFNγ, or LPS) stimulation, microglia release EVs enriched with proinflammatory cytokines (e.g. IL-1β) and miRNAs (e.g. miR-146a-5p, miR-155) [3,16,17]. Transfer of miR-146a-5p from microglia to neurons leads to downregulation of key pre- and post-synaptic proteins (e.g., synaptotagmin1, neuroligin1), thus reducing synaptic density and strength [17]. EVs from LPS-stimulated microglia also carry the enzyme glutaminase that may contribute to neurotoxicity in vitro through excessive glutamate production [18]. Microglial EVs released under these stimuli may also contribute as mediators of inflammation by activating surrounding microglia and astrocytes [16,19].
Box 1: EVs play roles in regulating physiological processes in the CNS.
During physiological conditions, neuronal depolarization and glutamate release stimulate EV transfer from multiple cell types mediating neuro-glia communication [3,109]. Neuronal EVs act as vehicles for anterograde/retrograde transfer of biomolecules such as L1 cell adhesion molecule and AMPA-type receptor subunits, which indicates their potential contribution to regulating synaptic transmission and plasticity mechanisms [109]. Neuronal EVs enriched with miR-124a are trafficked selectively to astrocytes, upregulating excitatory amino acid transporter 2 (EAAT2/GLT-1) expression as a means for maintaining glutamate homeostasis [108,110]. In fact, selective transfer of EVs between specific producer/acceptor cells appears to be an important feature in CNS physiology. EVs containing proteolipid protein (PLP) isolated from a mouse oligodendrocyte precursor cell (OPC) line in vitro selectively accumulated in unstimulated (MHC-class-II-negative) microglia via macropinocytosis, and not in astrocytes or neurons [111]. In the same study, activation of microglia through interferon gamma (IFNγ) or lipopolysaccharide (LPS) stimulation significantly decreased macropinocytosis, indicating a potential role of unstimulated microglia in internalizing and degrading OPC-derived EVs during physiological conditions [111].
Astrocyte-derived EVs under physiological conditions can carry synapsin I, molecular chaperones and matrix metalloproteinases (involved in extracellular matrix remodeling) that regulate neuronal survival and neurite outgrowth [22]. Microglial EVs modulate synaptic activity and neurotransmission by inducing sphingolipid metabolism [3]. Oligodendrocyte (OL)-derived EVs can carry catalase, superoxide dismutase (SOD), heat shock proteins, glycolytic enzymes, and various myelin-related proteins (e.g. proteolipid protein, myelin oligodendrocyte glycoprotein, etc.) that regulate myelination, neuronal activity (e.g. action potential firing rate and gene expression) and exert neuroprotection from oxygen/glucose deprivation [109,112]. NPSCs reside in neurogenic niches throughout the brain (e.g. subgranular zone, dentate gyrus and subventricular zone; SVZ) as well as in the spinal cord central canal (CC). EVs from SVZ-derived neural progenitor/stem cells (NPSCs) have been reported to carry miRNAs (e.g. miR-9, Let-7, miR-26, and miR-181), mRNA, proteins (e.g. IFNγ) and active enzymes (e.g. asparaginase-like protein 1; Asrgl1) related to NPSC function such as proliferation, migration and differentiation [113,114].
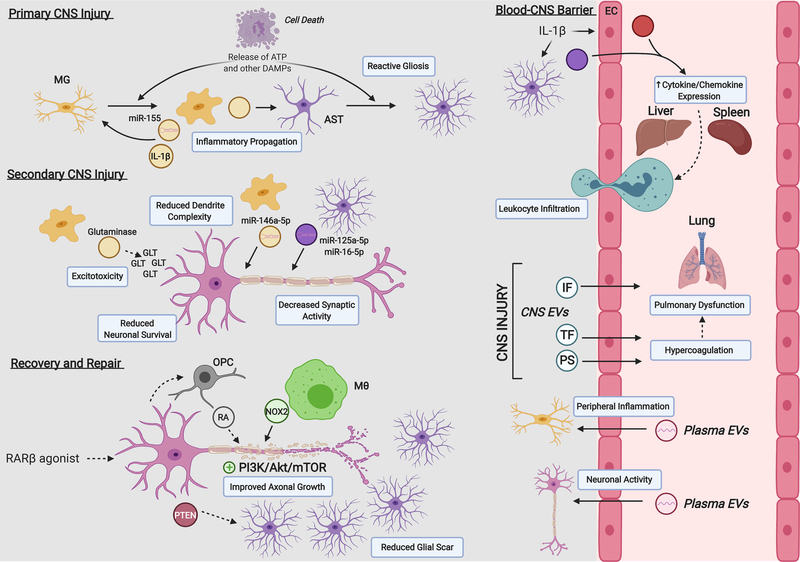
Figure 1: EVs contribute to local and systemic crosstalk after neurotrauma, including SCI.
Intercellular interactions and EV-associated cargos contribute to the injury response within the CNS and beyond. Primary injury leads to the release of intracellular ATP and other danger associated molecular patterns (DAMPs) that activate surrounding microglia (MG) and astrocytes (AST). Reactive MG independently activate other MG and AST through the transfer of pro-inflammatory molecules within EVs. Both reactive MG and AST can transfer EV-associated microRNAs to surrounding neurons that impact their overall synaptic function and survival in the secondary injury phase. Glutaminase-carrying EVs from MG may also contribute to glutamate (GLT) excitotoxic neuronal cell death. AST and endothelial cells (EC) under pro-inflammatory conditions (e.g. IL-1β stimulation) release EVs into blood circulation that can trigger peripheral organ cytokine/chemokine release regulating leukocyte infiltration into the CNS. CNS-derived EVs of unknown cellular origin may carry inflammasome (IF) components and pro-coagulant factors (e.g. TF, PS) after injury that can contribute to secondary pulmonary dysfunction. Under conditions of peripheral inflammation or neuronal activity, MG and neurons can receive functional RNA from hematopoietic cell-derived plasma EVs. Local EV release may also contribute to recovery and repair mechanisms relevant to SCI. Neuronal RARβ stimulation leads to the release of PTEN in neuronal EVs that promote intrinsic axonal growth (increased PI3K/Akt/mTOR signaling) and reduced AST proliferation; RA-containing EVs from oligodendrocyte precursor cells (OPCs) also contribute to axonal growth under this paradigm. NOX2-containing EVs from macrophages (Mθ) taken up at injured axons and transferred to the neuronal soma promote axon growth as well. Abbreviations: IL-1β, interleukin-1β; NOX2, NAPDH-oxidase 2; PS, phosphatidylserine; PTEN, phosphatase and tensin homolog; RA, retinoic acid; RARβ, retinoic acid receptor β; TF, tissue factor.
In response to similar pro-inflammatory stimuli, astrocytes undergo a phenotypic transformation known as reactive astrogliosis, which includes an increased rate of EV release relative to baseline [20,21]. EVs derived from reactive astrocytes are enriched with small GTPases (e.g. profilin-1, fascin actin-bundling protein-1 and destrin), proteins (e.g. IL-1β, human immunodeficiency virus 1 protein Nef), and miRNAs that largely inhibit neuronal function by decreasing neurite outgrowth and spike firing rates and may also lead to neuronal apoptosis [20–23]. In response to TNFα or IL-1β stimulation, astrocyte EVs are enriched with miR-125a-5p and miR-16–5p that downregulate expression of the neurotrophin receptor NTRK3 in neurons, leading to reduced dendrite complexity and synaptic burst activity [23]. While current understanding of EV-mediated neuro-glia communication largely derives from in vitro systems, emerging evidence supports a role for EVs from activated glia in mediating secondary injury after SCI. Future work involving isolating cell-specific EVs in vivo will help define the complex and dynamic factors that dictate their cargo and associated bioactivity after SCI.
EVs Contribute to Axonal Regeneration and Myelination
Repair of white matter damage remains a major hurdle in SCI treatment. The limited capacity of axon regeneration in the adult mammalian CNS is well established and results from a low intrinsic capacity for neurons to regenerate and formation of the glial scar [24]. Recent studies have demonstrated that EVs can serve as both positive and negative cues for axonal regeneration [25–28]. For example, the C-terminal fragment of Nogo-A, a myelin-associated inhibitor (MAI) protein localized in the oligodendrocyte membrane, can be released into EVs after cleavage by BACE1 and inhibit axon growth by binding to the NgR1 receptor on neurons [25]. Moreover, EVs containing Nogo-A have greater potency for axonal inhibition than purified Nogo-A alone [25]. A diffusible means for modulation of axonal growth through EVs is particularly relevant after SCI, where normal contact-mediated interactions between neurons and oligodendrocytes are mechanically disrupted. Whether Nogo-A containing EVs may contribute to sustained axonal regrowth failure after SCI requires further investigation.
In contrast, endogenous EV release may also promote axonal regeneration. For example, systemic treatment with an agonist for retinoic acid receptor β (RARβ) was shown to improve axonal regeneration through EV release in a rat SCI model (Figure 1) [26–28]. Following treatment, neuronal EVs transferred PTEN into astrocytes, which had a dual benefit: extrusion of PTEN removed inhibition of the PI3K/Akt/mTOR pathway within neurons and thus improved intrinsic growth capacity, while the transfer of PTEN into astrocytes reduced their proliferation and led to a reorganization of the glial scar into a more permissive environment for axonal growth [26]. RARβ agonist treatment also promotes neuronal interaction with oligodendrocyte precursor cells (OPCs), leading to upregulation of retinoic acid (RA) synthesis in the latter [27]. OPCs release RA in association with EVs, which can be taken up by nearby axons and serve as a positive signal for axonal outgrowth [27]. When OPCs transition into a mature myelinating oligodendrocyte (OL) phenotype, EV secretion is reduced and may coincide with myelination of regenerated axons [28]. Interestingly, bone marrow derived macrophages (BMDMs) also stimulate intrinsic axonal growth capacity mechanistically through modulation of PTEN. After peripheral nerve transection, BMDMs recruited to the injury site release EVs containing NADPH oxidase-2 (NOX2), which are taken up by the remaining axon and retrogradely transported back to the soma [29]. Reactive oxygen species produced by NOX2 oxidizes PTEN, leading to its inactivation and promotion of PI3K/Akt/mTOR signaling [29]. While BMDMs are present in the CNS after SCI, it is unknown whether a similar mechanism occurs endogenously to promote axonal regeneration within the CNS and, if so, whether there is a limitation on this capacity compared to peripheral nerves.
Bidirectional Blood-Borne EV Crosstalk between CNS and Peripheral Systems
In addition to local communication, EVs transported in circulation play a role in long-distance signaling and may contribute to overall SCI progression. While the relative contribution of CNS-specific EVs in the bloodstream is unknown, plasma EVs bearing neuronal markers (L1CAM, Glur2), as well as astrocytic (GLT-1, GLAST) and microglial (CD45, CD11b, P2yr12) markers are a focus of recent clinical studies that examine specific populations for diagnostic and prognostic monitoring after neurotrauma [30–32]. Beyond biomarker potential, emerging preclinical data suggest that circulating EVs play an active role in CNS injury progression (Figure 1). It is well-established that CNS inflammation and injury robustly activate the systemic immune system, leading to peripheral leukocyte infiltration. This effect is largely coordinated by cytokine/chemokine production in the liver, but the factors that modulate the hepatic acute phase response (APR) were largely unknown until recent evidence implicated EV release from the CNS [8][21]. In a GFAP-GFP transgenic mouse model, astrocyte EVs rapidly mobilized to the liver, lung, and spleen within two hours after striatal IL-1β injection. The associated cytokine responses in these peripheral organs were attenuated by local co-administration of a sphingomyelinase inhibitor to prevent CNS EV release and could be recovered by intravenous (IV) injection of EVs from IL-1β stimulated astrocytes [21]. Bioinformatics analysis based on the miRNA and protein content in stimulated astrocyte EVs identified specific modulation of the PPARα/NFκB pathway in recipient organs, which was further validated in vivo [21]. In addition to astrocytes, CNS endothelial cells may also contribute to circulating EVs that regulate the APR [33,34]. IV administration of EVs from LPS-stimulated endothelial cells after acute mouse TBI enhanced the magnitude of the liver APR, which subsequently expanded lesion volume size [34]. Thus far, no studies (to our knowledge) have characterized the role of EVs during APR specifically after SCI. Future work in this area will allow rational design of EV-based therapeutics that tune APR to limit its detrimental effects.
EVs have also been implicated in the development of various systemic inflammatory complications after neurotrauma, including pulmonary dysfunction [35,36]. Proteins related to the inflammasome complex increase locally after neurotrauma and are present in EVs isolated from CSF from human SCI patients and from blood in human TBI patients [35–37]. EVs released from the injured CNS may also carry pro-coagulant molecules (e.g. tissue factor, phosphatidylserine) and can associate with other members of the coagulation cascade [38–40]. Together, these effects can disrupt endothelial cell barrier function leading to vascular edema and fibrin deposition in the lung [38,40].
Just as important but less well examined are the effects of circulating EVs directly on CNS cells in vivo. CNS cells receive functional RNA via EVs from hematopoietic cell sources, which is further enhanced under LPS-induced peripheral inflammation [41]. Additional studies using this same model suggest that microglia are an important recipient cell type for plasma EVs (specifically from hematopoietic cells) and further highlight the importance of immune-related crosstalk between the CNS and peripheral systems [42,43]. Interestingly, neuronal activity also drives the uptake of hematopoietic cell-derived EVs into neurons under various paradigms including kainite injection (to induce seizure-like activity), optogenetic stimulation, and behavioral exploration of novel objects [43]. These studies are among the earliest to demonstrate the functional transfer of EV cargo from blood cells to the CNS and suggest that circulating EVs may impact brain function under a variety of normal physiological situations. The functional significance of this blood-to-brain signaling in SCI needs to be elucidated further.
Spinal Cord Injury Alters Circulating EV Count and miRNA Cargo
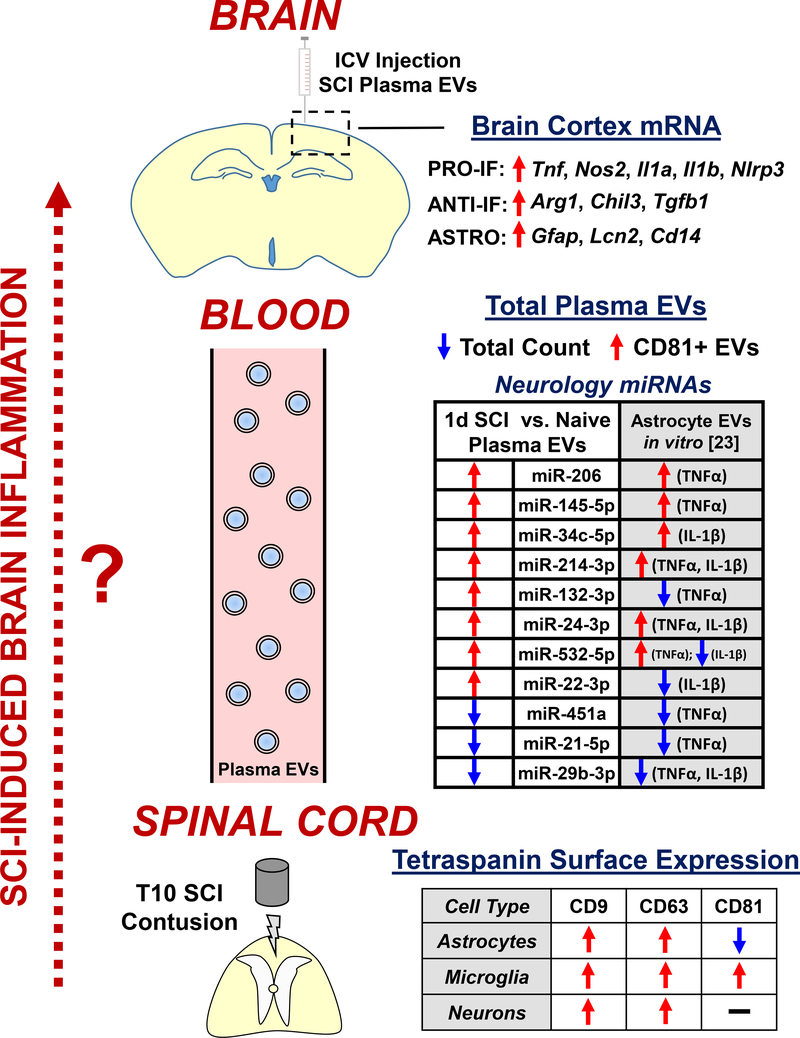
While a few studies have analyzed alterations in circulating EVs after rodent TBI [34,35], little data is currently available from animal models of SCI. Two studies published in 2020 presented RNA-sequencing data of EV-associated miRNAs in serum at 1 and 7 days post-injury in female rats [44,45]. Adding to these findings, our group recently described dynamic and multifaceted changes in plasma EVs after thoracic contusion SCI in adult male mice (Figure 2) [46]. Using a combination of techniques to measure EV count, size, and cargo, we reported a significant increase in plasma tetraspanin CD81+ EVs after SCI in conjunction with an overall reduction in total plasma EV count [46]. At the injury site, there was a robust decrease in CD81 surface expression on astrocytes specifically, suggesting these cells may shed CD81+ EVs into circulation. Furthermore, SCI modified CNS-related miRNA content in the total plasma EV population, where changes in miRNA profiles were similar to those previously described in astrocyte EVs after IL-1β or TNFα stimulation in vitro (Figure 2) [23,46].
Figure 2: Alterations in circulating EVs and miRNAs after SCI may contribute to remote brain inflammation.
Schematic diagram that summarizes key findings from [46] analyzing SCI-induced changes in circulating plasma EVs. Thoracic contusion SCI in male mice resulted in multifaceted changes in total plasma EVs at 1d post-injury including a decrease in the overall count, an increase in tetraspanin CD81+ EVs, and modifications in CNS-related miRNA cargo associated with proinflammatory stimulation of astrocytes (comparison with in vitro data from [23]). Tetraspanin protein expression primarily increased in cells at the injury site except for a unique decrease in surface CD81 on astrocytes, which may be the source of increased plasma CD81+ EVs. ICV injection of total plasma EVs from 1d SCI animals induced robust inflammatory gene expression in the brain cortex, including increased reactive astrocyte genes. Future investigation (dotted red arrow, left) is required to further test the hypothesis that EVs released by cells directly from the injury site travel through the blood circulation to seed brain inflammation, which may contribute to long-term neurodegeneration and associated cognitive deficits and depressive-like behavior after SCI. Abbreviations: ANTI-IF, anti-inflammatory; ASTRO, astrocyte; ICV, intracerebroventricular; PRO-IF, pro-inflammatory; miRNA, microRNA; T10, thoracic segment 10; TNFα; tumor necrosis factor α.
Cognitive impairment and affective symptoms are reported clinically in nearly half of SCI patients [47,48], but the mechanisms underlying this brain dysfunction have not been well explored. Studies from our group [49,50] and others [51] report a link between neurodegeneration and neuroinflammation in the brain after rodent SCI, leading to cognitive and depressive-like behavioral deficits. Based on prior evidence of astrocyte EVs regulating the APR [21], the possibility that circulating EVs after SCI could promote inflammation in recipient target organs – specifically the brain – was assessed by intracerebroventricular (ICV) injection of plasma EVs from either SCI or control (uninjured) mice into healthy mice (Figure 2) [46]. At 24 hours post-injection, increased expression of several key inflammatory genes, including markers related to astrocyte reactivity [52], was observed in associated with injection of SCI plasma EVs [46]. Furthermore, flow cytometry analysis demonstrated increased intracellular IL-1β and IL-1α levels in brain astrocytes with this same paradigm [46]. These data, in conjunction with prior studies using neuroinflammation models, suggest a critical role for astrocytes in both the production of and response to plasma EVs after SCI.
Potential for EV-based Therapeutics for SCI
The emerging evidence for roles of EVs in regulating SCI biology can inform not only a better understanding of pathophysiology but also the development of a variety of new treatment strategies and in particular, provides impetus for investigation of CNS cell-derived EVs as potential SCI therapeutics. Further, many of the reparative and functional benefits of stem cell transplantation in various CNS disease models can now be linked to the combined autocrine/paracrine bioactivity of its secretome [53]. As a key component of the cellular secretome, EVs have emerged as a viable candidate for cell-free therapies due to their potential for therapeutic bioactivity, inherent biocompatibility, and potential for cell targeting while mitigating concerns related to immunogenicity and uncontrolled proliferation/differentiation of cellular transplants [54].
Cell Source is a Critical Determinant of EV Bioactivity
EV producer cell phenotype partly determines the array of cytosolic proteins (i.e. molecular chaperones, metabolic enzymes, ribosomal proteins, etc.), cell-surface proteins, and nucleic acids available to be packaged within EVs during their biogenesis (Figure 3) [55]. Although the multifunctional properties of EVs and their mechanisms of action relative to producer cell types are not well understood, the diversity of EV-associated cargo suggests that pleiotropic, complementary, and/or synergistic effects could be critical to their therapeutic potential [10]. In particular, small ncRNAs (<200 nucleotides) have attracted substantial attention, as these molecules represent a significant proportion of EV cargo and EV-mediated horizontal transfer of ncRNA appears to be an important mechanism for regulating CNS development, homeostasis, aging, and pathology [56,57]. The EV content of one critical class of small ncRNAs, miRNAs, varies widely with producer cell type (Box 2). This highlights the possibility of cell source as an important design criterion when developing EV-based therapeutic strategies for SCI.
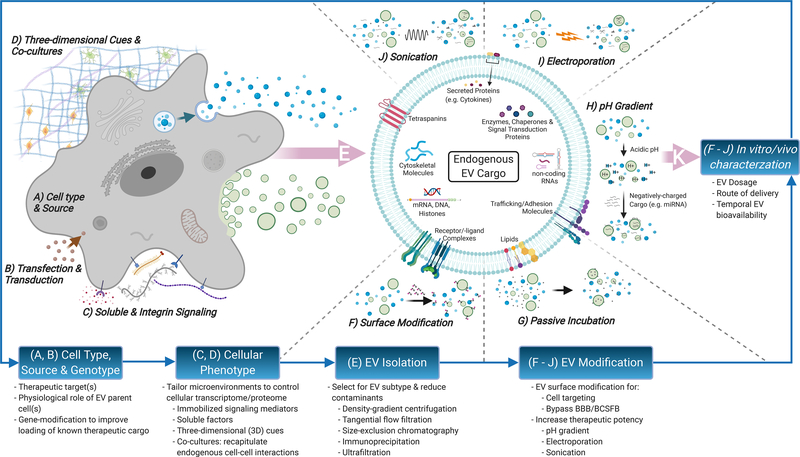
Figure 3: Strategies for enhancing CNS-specific regenerative potential of EV-based SCI therapeutics.
Engineering approaches to probe for populations of therapeutic EVs begin with selection of therapeutic target(s) for SCI (e.g. neurogenesis, axonal sprouting, remyelination, etc.) matched with appropriate EV producer cell types hypothesized to play direct/indirect physiological roles (A). EV producer cells may be genetically modified to further enhance EV loading of known biological mediators (B). Control over cellular phenotype through immobilized signaling mediators (e.g. extracellular proteins) or soluble factors (e.g. cytokines, hormones, etc.) can modify cargo and bioactivity of secreted EV populations (C). Additionally, biophysical cues such as three-dimensional culture and incorporation of specific cell-cell interactions using co-cultures (e.g. neuronal/oligodendroglial co-cultures to target remyelination) can further tune loading of EV-associated cargo (D). EVs are highly heterogeneous in size, membrane composition, cargo, and bioactivity. Many of these parameters are closely linked to the EVs’ biogenesis pathways, which include: i) release of intraluminal vesicles within multivesicular bodies (MVBs) upon fusion with the plasma membrane, ii) direct budding of the plasma membrane and, iii) formation of apoptotic bodies. A significant hurdle in current EV isolation techniques is the inability to robustly differentiate between these EV subtypes. Thus, isolated EV populations will inevitably contain particles originating from multiple biogenesis pathways (E). Overall functional cargo makeup on EV surface (F) and/or EV interior (G-J) can also be modified to varying degrees after EV biogenesis and isolation. Lastly, in vitro/vivo characterization of biomanufactured EVs can inform further modifications to optimize for therapeutic efficacy and potency (K). Principal considerations for in vitro characterization include cell-based assays that probe for relevant EV bioactivity (e.g. neuroprotection, neuroinflammation). Considerations for in vivo characterization include selection of SCI injury model, EV dosage, route of delivery to the CNS and temporal bioavailability of delivered EVs over an appropriate therapeutic time window.
Box 2: CNS EV-associated miRNA cargo is specific to cell type and microenvironment.
Cellular content of miRNAs varies based on cell type, and the CNS is no exception. For example, miR-383 is highly enriched in neurons relative to glial cells [115] whereas miR-219 and miR-338 (crucial in regulating myelination) are thought to be specific to oligodendrocyte-lineage cells under physiological conditions [116]. Additionally, miRNA content for the same cell-type may depend on its location in the CNS. In particular, miR-326 is strongly expressed in microglia within the brainstem yet nearly absent in spinal microglia [115]. Further, heterogeneity in primary adult NPSCs populations from distinct stem cell niches within the CNS is well documented and may also be a key feature in determining the miRNA cargo function of their EVs [117]. Critically, pathological progression after neurotrauma appears to be a factor that differentially affects spatiotemporal miRNA expression across CNS cell types [115]. Furthermore, cargo loading into EVs comprises both active (selective) and passive mechanisms that are yet to be fully elucidated [118], and there is strong evidence that suggests the cellular transcriptome/proteome does not always correlate directly with EV cargo makeup [89]. Uncovering the complex relationships and interplay that determine EV bioactivity secreted by CNS cell types as a function of pathological progression after neurotrauma remains largely in its infancy. Only a limited number of studies have gone beyond identifying predicted mRNA targets for CNS cell-specific miRNAs towards characterizing/validating their bioactivity and potential means of intercellular trafficking.
The role of cell source in EV therapeutics has also been examined via direct comparison. Mesenchymal stem/stromal cell (MSC) transplants have anti-inflammatory and pro-angiogenic effects in a wide array of regenerative applications [58–60] and thus have been extensively studied as a potential source for therapeutic EVs for SCI and other types of neurotrauma [61–64]. However, EVs from MSCs and other non-CNS types to date have generally been unable to induce key neuroregenerative processes relevant to SCI therapy, including neurogenesis, adaptive neural plasticity, and remyelination and regeneration of injured axons over long-distances. Notably, EVs from isogenic human pluripotent stem cell (hPSC)-derived NPSCs and MSCs were assessed in a rodent model of ischemic stroke, with the NPSC-derived EVs inducing improved cognitive and behavioral outcomes with significantly reduced lesion volumes relative to MSC EVs [65].
Further, EVs isolated from CNS cell types have shown the potential to recapitulate the regenerative properties of their parent cells in several animal models of CNS pathology. In a rodent model of radiation-induced brain injury, neuroprotective effects after administration of EVs from human neural progenitor/stem cells (NPSCs) were comparable to cranial transplantation of the cells themselves [66]. Additional studies also showed improved functional recovery and neuroprotection after transplants of both exogenous NPSCs [67] and NPSC-derived EVs in experimental SCI and TBI [68–70]. Other studies include a rodent model of focal TBI in which IV administration of NPSC EVs led to decreased glial scarring and cortical lesion volume that were correlated with motor function recovery [68]. IV administration of NPSC EVs also showed efficacy in a clinically relevant porcine model of ischemic stroke by decreasing cerebral lesion volume, edema, and recovery time while improving fine-motor coordination [71]. Schwann cell (SC)-derived EVs also improved therapeutic outcome in a rodent model of peripheral neuropathy by increasing axonal sprouting and remyelination of sciatic nerves [72].
Only a limited number of studies have characterized administration of EVs derived from resident CNS cell types in animal models of SCI (Table 1). The available data indicates that intrathecal or IV administration of NPSC-derived EVs after SCI can modulate autophagy, neuroprotection, angiogenesis, and inflammation, and that some of these changes were correlated with functional improvements [70,73,74]. NPSC-derived EVs are linked with suppressing the formation of NLRP3 inflammasome complexes, which corroborates a separate study that shows NPSC-EVs are likely enriched with 14–3-3 family of proteins, known to regulate autophagy and inflammasome activation [73,74]. Another report suggests that NPSC EV-mediated angiogenesis after SCI in mice likely occurs through transfer of EV-associated proteins from the vascular endothelial growth factor (VEGF) family to spinal cord microvascular endothelial cells (SMEC) [75]. Further, EVs derived from primary cortical neurons reduced neuroinflammation after SCI in mice by suppressing activation of pro-inflammatory microglia and neurotoxic astrocytes via transfer of EV-associated miR-124–3p [76]. Although these initial studies indicate that EVs from CNS-resident cells can improve functional deficits after SCI, overall it is still unclear how their therapeutic potency and mechanism of action in SCI contrasts with EVs from non-CNS cells (with MSCs being the most widely studied thus far).
Table 1.
Therapeutic outcomes and key bioactive components identified in EVs derived from CNS cell types in animal models of SCI
| Cell Source | EV Parent Cell Micro-Environment | Route of Delivery | EV Dosage | Effects | Highlighted EV-associated cargo | Refs |
|---|---|---|---|---|---|---|
| Primary rodent neural stem cells | Tail-vein injection | 200μg (PC) | • ↑ motor function • ↓ lesion volume and neuroinflammation • ↑ autophagy and neuroprotection |
14-3-3t family of proteins | [70,74] | |
| Primary rodent neurons | Tail-vein injection | 200μg (PC) | • ↑ motor function • ↓ lesion volume • ↓ activation of proinflammatory microglia and neurotoxic astrocytes |
miR-124-3p | [76] | |
| Primary rodent neural stem cells | Tail-vein injection | 5 doses of 200μg (PC) each over 12 days post-SCI | • ↑ motor function • ↓ lesion volume • ↑ angiogenesis |
VEGF-A | [75] | |
| Primary rodent neural stem cells | Intrathecal | 10μg (PC) | • ↑ motor function • ↓ lesion volume • ↑ neuroprotection • ↓ expression of inflammasome proteins in the CNS |
[73] | ||
| Primary rodent neural stem cells | + Insulin-like growth factor-1 | Tail-vein injection | 100μg (PC) | • ↑ motor function • ↓ neuroinflammation • ↑ neuroprotection and axonal connectivity of neural fasciculus |
miR-219a-2-3p | [81] |
Key: PC – EV dosing determined via analysis of total protein content; ↑ – increased/upregulated; ↓ – decreased/downregulated
Enhanced EV Potency Through Engineering Producer Cell Microenvironments
In addition to elucidating the relationship between cellular phenotype and EV therapeutic potential in SCI, optimal cell culture parameters and microenvironment on tuning the bioactivity of EVs is another important topic for study (Figure 3). Various culture parameters such as cell seeding density, cell age/passage, and EV collection frequency can lead to significant changes in EV bioactivity and their production rates across multiple cell types [77]. For example, EVs isolated from microglia differentially regulated myelination under proinflammatory or pro-regenerative conditions in an in vivo model of lysolecithin-induced demyelinated lesions [78]. Further, oxygen- and glucose-deprived astrocytes secreted EVs that exerted neuroprotection in vitro, likely due to increased EV-associated miR-92b-3p content compared to unstressed astrocytic EVs [79,80]. Similarly, NPSCs primed with insulin-like growth factor-1 produced EVs enriched with miR-219a-2–3p, associated with OPC maturation, which was linked with attenuated neuroinflammation, apoptosis, and improved axonal regeneration after SCI [81]. NPSCs primed with ethanol increased EV-associated miR-140–3p and miR-140–5p content, which alters NPSC proliferation and differentiation dynamics in vitro [82]. Under proinflammatory conditions, RNA and protein content of NPSC-derived EVs can change significantly, with one study showing that enrichment of EV membrane-bound IFN-γ and interferon gamma receptor 1 (Ifngr1) complexes can induce Ifngr1 and Stat1 signal transduction (key regulators of cell proliferation and neuroinflammation) in target cells [83].
Ongoing biomaterials-based development of artificial extracellular microenvironments as in vitro models for neurotrauma, neurodegenerative diseases, and neurodevelopmental disorders may inform future engineering of optimal EV production environments for SCI [84]. To this end, three-dimensional (3D) bioprinting provides increased control over spatial distribution of materials (e.g. bioactive components, cells, drug depots, etc.) using “bioinks” (e.g. designer hydrogels and biocompatible plastics) to construct spatially complex architectures at milli/micro-meter resolution with high reproducibility [85]. Fully recapturing in vivo EV production microenvironments in artificial constructs is currently not feasible, but implementing existing knowledge from biomolecular frameworks into biomaterials design may aid in generating therapeutic EVs with potent CNS-specific regenerative capacity. For example, one study reported that EVs derived from dental pulp stem cells cultured in 3D had neuroprotective effects, while EVs from the same cell type in two-dimensional (2D) culture did not [86]. Although EVs derived from CNS cell types have scarcely been scrutinized with respect to one or more key biophysical cues, studies using other cell types have shown that, in principal, it is possible to improve therapeutic potency and biomanufacturing yield of collected EVs using 3D bioprinted perfusion bioreactors [87,88]. A potential design limitation to note about 3D cultures is the difficulty in isolating EVs secreted within hydrogel-based scaffolds due to potential EV-hydrogel interactions [87]. It remains to be seen whether rational leveraging of EV producer cell phenotype along with EV production microenvironment can: 1) yield EV populations with potent CNS-specific therapeutic capacity and, 2) enable identification of EV-associated bioactive components with relevance for SCI.
Enhanced EV Potency Through Loading of Therapeutic Cargo
The endogenous roles of EVs include protection of nucleic acids and other bioactive components from degradation while ensuring delivery to target recipient cell cytosol without eliciting an immunogenic response. These properties make EVs a promising delivery vehicle for RNA-based therapeutics such as antisense oligonucleotides, small interfering RNAs (siRNAs), miRNAs, mRNAs, and others [89]. However, average endogenous ncRNA loading can be as low as one bioactive copy of a given miRNA per EV, which may not potently modulate gene expression in target cells [90,91]. Thus, even after identifying populations of therapeutic EV populations and their bioactive components, clinical translation may still be hampered by low potency that necessitates high or repeated EV doses to achieve a therapeutic effect.
Several strategies to manipulate EV parent cells have been proposed to control cargo loading into EVs during biogenesis (Figure 3A–D) [89,92]. In the context of neurotrauma, EVs derived from gene-modified MSCs that overexpress miRNAs associated with neurogenesis and neurite outgrowth (e.g. miR-124 & miR-133b) led to functional improvements in animal models of SCI and TBI [93,94]. Similarly, a separate study generated EVs enriched with miR-219a-5p (specific to OL-lineage cells that regulate OPC proliferation and maturation) using a gene-modified HEK293T cell line [95]. These studies highlight the possibility of loading CNS-specific miRNA cargo into non-resident CNS cell types using genetic modification and subsequent overexpression [93]. As for gene-modified CNS cell types, neurons overexpressing miR-21–5p induced neuroprotective effects in vitro [96] while EVs from microglia overexpressing miR-124–3p exerted neuroprotection and improved functional outcome in a rodent model of TBI [97]. However, several challenges remain for optimizing strategies for producer cell loading of EVs with desired cargo. Simple cytosolic overexpression of ncRNA via gene modification does not always improve endogenous bioactive cargo loading in EVs, and plasmid modifications, such as inclusion of targeting moieties, may be necessary to direct ncRNA cargo loading to EVs specifically [98]. Even after successful enrichment of desired cargo into EVs, the process of stable gene overexpression itself can produce changes in parent cell phenotype that may alter the overall cargo/bioactivity of secreted EV populations, potentially causing undesired off-target effects. Additionally, endogenous enrichment of individual ncRNA cargo within EVs may not provide control over other factors in intercellular EV transfer, such as uptake and subsequent processing by acceptor cells [89,99].
Several studies have also explored means for modifying EV cargo after biogenesis and isolation (Figure 3F–J). A straightforward method albeit with limited efficacy for small molecule drugs is passive loading via co-incubation of EVs with desired cargo, where the presence of detergents may facilitate EV encapsulation [100]. Lipid- or cholesterol functionalization of desired cargo to target association with EV membranes is another option that has been effective in delivering functional siRNA [101]. Several studies have also exploited the high surface concentration of phosphatidylserine (PS) on EVs to decorate bifunctional fusion proteins that contain the C1C2 domain of lactadherin that binds to PS with high affinity [102]. However, it is important to note that surface modification is unlikely to shield EV membrane-associated functional cargo from degradation by endogenous factors relative to cargo encapsulated inside the EV itself. Other strategies for active internal cargo loading use techniques that transiently permeabilize EV membranes to allow encapsulation of desired cargo. Electroporation can successfully load functionally active small ncRNA into EVs [103]. Sonication is another demonstrated method for active EV loading of various types of functional protein cargo including siRNA [104]. Some potential disadvantages for the electroporation and sonication-based loading strategies include loss, aggregation or degradation of EV-associated cargo due to the energy added and disruption of EV membrane integrity. More recently, our group has used a pH gradient across EV membranes as an effective means for loading functional miRNA, siRNA, and single-stranded DNA without compromising EV cargo/bioactivity [105]. However, all post-isolation EV modification strategies have scalability concerns that could potentially limit the large-scale biomanufacturing that would be necessary to create a translational therapy for SCI patients.
Concluding Remarks and Future Perspectives
How CNS-specific regenerative processes after SCI are regulated by EV transfer between specific producer and acceptor cells is not well understood. Furthermore, the extent to which crosstalk between circulating EVs and CNS resident cells contributes to pathophysiological mechanisms after SCI and other forms of neurotrauma still remains to be established. A significant technical hurdle is the difficulty in identifying cell-specific EVs from in vivo tissue samples within the CNS, where neurons, astrocytes, microglia, and other resident cells contribute to a heterogeneous pool of EVs. EV-associated cargo can include cell-specific biomarkers, but the relative packaging of these molecules into EVs has not been well examined (see Outstanding Questions) [106,107]. Emerging strategies that fluorescently tag EV subtypes in situ during biogenesis in a cell-type-specific manner represent an important step towards addressing some of these unanswered questions [108].
Outstanding Questions.
Much of our understanding of local EV cell-to-cell communication is inferred from in vitro work as technical challenges remain to visualize and monitor EV trafficking in vivo. Can cell-specific EVs and their cargo be identified from this complex environment? How do these parameters change over time after SCI? And how might they be similar to or differ from other forms of neurotrauma, such as TBI?
How do EVs in blood circulation impact the progression of SCI? Are there specific peripheral cell types (e.g. BMDMs, red blood cells) that communicate preferentially with CNS cells after injury? What are the critical cargoes, including proteins, lipids, and nucleic acids, that contribute to this type of signaling?
Do cells with direct functional involvement in CNS repair (e.g. neurogenesis, axonal sprouting, remyelination, etc.) secrete EVs that specifically enhance CNS-specific regenerative responses after SCI? For example, EVs isolated from NPSCs appear to regulate neuroinflammation and mediate neuroprotection after SCI. What are the putative EV-associated cargo(s) that mediate these CNS-specific regenerative processes?
Can emerging technologies such as 3D bioprinting and control over cell-cell communication (e.g. co-cultures) be used to generate biomimetic EV production environments that endow EVs with enhanced CNS-specific regenerative potency? Can these technologies also assist in moving towards scalable biomanufacturing of therapeutic EVs for SCI treatment?
Even after EV populations with therapeutic effects are identified, a low therapeutic potency requiring high/repeated EV dosages may be a critical hurdle, as demonstrated across other applications. Once EV-associated cargo linked to CNS repair are identified, can these mediators be efficiently loaded in manufactured EVs (during or after EV biogenesis) to further improve their therapeutic potency?
Along with an improved understanding of EV-mediated signaling after SCI, we believe that selection of biologically-relevant EV parent cell(s) and production microenvironments (incorporating key biophysical and biochemical cues) will aid in the discovery of EV populations that target CNS-specific regenerative processes after SCI (e.g. axonal sparing/regeneration, remyelination, autophagy, glial scar formation, etc.). Profiling of EV-associated cargo under these circumstances can be used to identify and validate any critical bioactive components that may mediate therapeutic benefit. Finally, emerging technologies such as 3D bioprinting and new approaches to therapeutic cargo loading into EVs may enable the reproducible and scalable biomanufacturing of EVs with high therapeutic potency required for clinical translation (see Outstanding Questions).
Highlights.
EVs have functional roles in mediating neuro-glia and CNS-periphery communication after neurotrauma by regulating neuronal function, axonal regeneration and remyelination, metabolic activity, and the inflammatory milieu.
Understanding of EV signaling after SCI is limited, but recent studies have identified differences in circulating EV count and miR cargo that may contribute to remote inflammatory changes.
The therapeutic potential of EVs for SCI treatment depends on source cell phenotype and is linked to the repertoire of EV-associated cargo.
New bioengineering approaches such as 3D printing demonstrate improved control over the EV production microenvironment and cellular phenotype that can accelerate the development of therapeutic EVs with high potency for applications in neurotrauma, including SCI, in a reproducible and scalable fashion.
Acknowledgments
S.M.J. was supported by RF1 NS110637 from the National Institute on Aging (NIA), R01 GM130923 from the National Institute of General Medical Sciences (NIGMS), R01s HL141611 and HL141922 from the National Heart, Lung, and Blood Institute (NHLBI), and award 1750542 from the National Science Foundation (NSF). J.W. was supported by RF1 NS110637 from NIA, R01 NS110825, R01 NS094527, R01 NS110635, and R01 NS110567 from the National Institute of Neurological Disorders and Stroke (NINDS), and R01 NR013601 from the National Institute of Nursing Research (NINR). D.D. was supported by 2020-MSCRFF-5368 from the Maryland Stem Cell Research Foundation. Illustrations in this manuscript were partially created with BioRender.com.
Glossary
- Extracellular vesicles (EVs)
A general term for any non-nucleated particle released from cells with a double-leaflet membrane. EVs originate via various biogenesis pathways, including budding of the plasma membrane or release from multivesicular bodies
- Tetraspanin
Membrane proteins (e.g. CD9, CD63, CD81) with four transmembrane domains that are highly enriched in certain EV subtypes
- Neural plasticity
Innate capacity of the developing and adult CNS to undergo functional and biochemical reorganization within individual neurons (e.g. synaptic plasticity) as well as across large-scale neural networks in response to experience, injury, and disease
- Autophagy
Greek for “self-eating,” autophagy is a regulated process for removal and degradation of damaged cytosolic components, misfolded/aggregated proteins, and invasive microbes during physiological and pathological conditions
- Glial scarring
Reactive astrocytes and microglia encapsulate the lesion site after neurotrauma, creating a lesion core enriched with extracellular matrix proteins that are inhibitory for axonal growth and remyelination. The glial scar may also be neuroprotective by further confining inflammatory pathology to the lesion core
- Neural progenitor/stem cells (NPSCs)
Multipotent progenitor cells of the CNS that retain the ability to differentiate and generate most, if not all, glial or neuronal cell types. These cells can be found anatomically in specific neurogenic niches in the brain and spinal cord
- Inflammasome
Multiprotein molecular complex involved in innate immune defense that leads to caspase-1 activation and downstream production of pro-inflammatory cytokines such as IL-1β and IL-18. Activation can also result in pyroptosis, a caspase-1 dependent form of cell death
- Acute phase response (APR)
Systemic inflammatory and metabolic response rapidly (minutes to hours) initiated to enhance body defenses to injury, trauma, or infection. If not resolved, long-term activation of the APR may lead to further secondary tissue damage
- Glutamate homeostasis
Primarily glial cell-mediated control over non-synaptic extracellular glutamate through direct uptake and cystine/glutamate exchange to regulate synaptic efficiency and plasticity
- Oligodendrocyte precursor cell (OPC)
Progenitor cells that are abundant in the adult CNS and have the capacity to generate mature oligodendrocytes, contributing to adaptive myelination and remyelination following injury/disease
- Macropinocytosis
Non-specific, endocytic sampling of the external environment through projections of the cellular membrane that engulf and internalize extracellular fluid and particles
- Sphingomyelinase
Cellular enzyme that hydrolyzes sphingomyelin to ceramide, which contributes to the biogenesis of certain EV subtypes
- PI3K/Akt/mTOR pathway
A critical intracellular signaling pathway that regulates cell growth, metabolism, and survival. Negatively regulated by the phosphatase and tensin homolog (PTEN) protein
Footnotes
Declaration of interests
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Dwyer-Lindgren L et al. (2016) US County-Level Trends in Mortality Rates for Major Causes of Death, 1980–2014. JAMA 316, 2385–2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National SCI Statistical Center, Facts and Figures at a Glance.. (2019), University of Alabama; at Birmingham [Google Scholar]
- 3.Holm MM et al. (2018) Extracellular Vesicles: Multimodal Envoys in Neural Maintenance and Repair. Trends in Neurosciences 41, 360–372 [DOI] [PubMed] [Google Scholar]
- 4.Caruso Bavisotto C et al. (2019) Extracellular Vesicle-Mediated Cell–Cell Communication in the Nervous System: Focus on Neurological Diseases. Int J Mol Sci 20, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ramirez SH et al. (2018) Extracellular vesicles: mediators and biomarkers of pathology along CNS barriers. Fluids Barriers CNS 15, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saeedi S et al. (2019) The emerging role of exosomes in mental disorders. Translational Psychiatry 9, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi M et al. (2019) New windows into the brain: Central nervous system-derived extracellular vesicles in blood. Progress in Neurobiology 175, 96–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yates AG et al. (2019) Systemic Immune Response to Traumatic CNS Injuries—Are Extracellular Vesicles the Missing Link? Front. Immunol 10, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciregia F et al. (2017) Extracellular Vesicles in Brain Tumors and Neurodegenerative Diseases. Front. Mol. Neurosci 10, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Branscome H et al. (2020) Use of Stem Cell Extracellular Vesicles as a “Holistic” Approach to CNS Repair. Front Cell Dev Biol 8, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahuja CS et al. (2017) Traumatic spinal cord injury. Nature Reviews Disease Primers 3, 1–21 [DOI] [PubMed] [Google Scholar]
- 12.Wu J and Lipinski MM (2019) Autophagy in Neurotrauma: Good, Bad, or Dysregulated. Cells 8, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venkatesh K et al. (2019) Spinal cord injury: pathophysiology, treatment strategies, associated challenges, and future implications. Cell Tissue Res 377, 125–151 [DOI] [PubMed] [Google Scholar]
- 14.Orr MB and Gensel JC (2018) Spinal Cord Injury Scarring and Inflammation: Therapies Targeting Glial and Inflammatory Responses. Neurotherapeutics 15, 541–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rufino-Ramos D et al. (2017) Extracellular vesicles: Novel promising delivery systems for therapy of brain diseases. Journal of Controlled Release 262, 247–258 [DOI] [PubMed] [Google Scholar]
- 16.Kumar A et al. (2017) Microglial-derived microparticles mediate neuroinflammation after traumatic brain injury. J Neuroinflammation 14, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prada I et al. (2018) Glia-to-neuron transfer of miRNAs via extracellular vesicles: a new mechanism underlying inflammation-induced synaptic alterations. Acta Neuropathol 135, 529–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu B et al. (2015) Glutaminase-containing microvesicles from HIV-1-infected macrophages and immune-activated microglia induce neurotoxicity. Molecular Neurodegeneration 10, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drago F et al. (2017) ATP Modifies the Proteome of Extracellular Vesicles Released by Microglia and Influences Their Action on Astrocytes. Front Pharmacol 8, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.You Y et al. (2020) Activated human astrocyte-derived extracellular vesicles modulate neuronal uptake, differentiation and firing. Journal of Extracellular Vesicles 9, 1706801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dickens AM et al. (2017) Astrocyte-shed extracellular vesicles regulate the peripheral leukocyte response to inflammatory brain lesions. Sci Signal 10, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaudhuri AD et al. (2020) Stimulus-dependent modifications in astrocyte-derived extracellular vesicle cargo regulate neuronal excitability. Glia 68, 128–144 [DOI] [PubMed] [Google Scholar]
- 23.Chaudhuri AD et al. (2018) TNFα and IL-1β modify the miRNA cargo of astrocyte shed extracellular vesicles to regulate neurotrophic signaling in neurons. Cell Death Dis 9, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silver J et al. (2015) Central Nervous System Regenerative Failure: Role of Oligodendrocytes, Astrocytes, and Microglia. Cold Spring Harb Perspect Biol 7, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sekine Y et al. (2020) A proteolytic C-terminal fragment of Nogo-A (reticulon-4A) is released in exosomes and potently inhibits axon regeneration. J. Biol. Chem 295, 2175–2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goncalves MB et al. (2015) Neuronal RARβ Signaling Modulates PTEN Activity Directly in Neurons and via Exosome Transfer in Astrocytes to Prevent Glial Scar Formation and Induce Spinal Cord Regeneration. J. Neurosci 35, 15731–15745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goncalves MB et al. (2018) Retinoic acid synthesis by NG2 expressing cells promotes a permissive environment for axonal outgrowth. Neurobiol. Dis 111, 70–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goncalves MB et al. (2019) Regulation of Myelination by Exosome Associated Retinoic Acid Release from NG2-Positive Cells. J. Neurosci 39, 3013–3027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hervera A et al. (2018) Reactive oxygen species regulate axonal regeneration through the release of exosomal NADPH oxidase 2 complexes into injured axons. Nat. Cell Biol 20, 307–319 [DOI] [PubMed] [Google Scholar]
- 30.Ko J et al. (2019) Multi-Dimensional Mapping of Brain-Derived Extracellular Vesicle MicroRNA Biomarker for Traumatic Brain Injury Diagnostics. J. Neurotrauma DOI: 10.1089/neu.2018.6220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goetzl EJ et al. (2019) Neuron-Derived Exosome Proteins May Contribute to Progression From Repetitive Mild Traumatic Brain Injuries to Chronic Traumatic Encephalopathy. Front Neurosci 13, 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goetzl EJ et al. (2020) Traumatic brain injury increases plasma astrocyte-derived exosome levels of neurotoxic complement proteins. FASEB J. 34, 3359–3366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Couch Y et al. (2017) Circulating endothelial cell-derived extracellular vesicles mediate the acute phase response and sickness behaviour associated with CNS inflammation. Sci Rep 7, 9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hazelton I et al. (2018) Exacerbation of Acute Traumatic Brain Injury by Circulating Extracellular Vesicles. J. Neurotrauma 35, 639–651 [DOI] [PubMed] [Google Scholar]
- 35.Kerr NA et al. (2018) Traumatic Brain Injury-Induced Acute Lung Injury: Evidence for Activation and Inhibition of a Neural-Respiratory-Inflammasome Axis. J. Neurotrauma 35, 2067–2076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kerr NA et al. (2019) Human Lung Cell Pyroptosis Following Traumatic Brain Injury. Cells 8, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Rivero Vaccari JP et al. (2016) Exosome-mediated inflammasome signaling after central nervous system injury. J. Neurochem. 136 Suppl 1, 39–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yasui H et al. (2016) Early coagulation events induce acute lung injury in a rat model of blunt traumatic brain injury. Am. J. Physiol. Lung Cell Mol. Physiol 311, L74–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tian Y et al. (2015) Brain-derived microparticles induce systemic coagulation in a murine model of traumatic brain injury. Blood 125, 2151–2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu Y et al. (2018) von Willebrand factor enhances microvesicle-induced vascular leakage and coagulopathy in mice with traumatic brain injury. Blood 132, 1075–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ridder K et al. (2014) Extracellular Vesicle-Mediated Transfer of Genetic Information between the Hematopoietic System and the Brain in Response to Inflammation. PLOS Biology 12, e1001874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li JJ et al. (2018) In vivo evidence for the contribution of peripheral circulating inflammatory exosomes to neuroinflammation. J Neuroinflammation 15, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kur I-M et al. (2020) Neuronal activity triggers uptake of hematopoietic extracellular vesicles in vivo. PLOS Biology 18, e3000643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ding S-Q et al. (2019) Identification of serum exosomal microRNAs in acute spinal cord injured rats. Exp Biol Med (Maywood) 244, 1149–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ding S-Q et al. (2020) Serum exosomal microRNA transcriptome profiling in subacute spinal cord injured rats. Genomics 112, 2092–2105 [DOI] [PubMed] [Google Scholar]
- 46.Khan N et al. (2020) Spinal cord injury alters microRNA and CD81+ exosome levels in plasma extracellular nanoparticles with neuroinflammatory potential. Brain, Behavior, and Immunity DOI: 10.1016/j.bbi.2020.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shin JC et al. (2012) Depression and Quality of Life in Patients within the First 6 Months after the Spinal Cord Injury. Ann Rehabil Med 36, 119–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sachdeva R et al. (2018) Cognitive function after spinal cord injury: A systematic review. Neurology 91, 611–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu J et al. (2014) Spinal cord injury causes brain inflammation associated with cognitive and affective changes: role of cell cycle pathways. J. Neurosci 34, 10989–11006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu J et al. (2014) Isolated spinal cord contusion in rats induces chronic brain neuroinflammation, neurodegeneration, and cognitive impairment. Involvement of cell cycle activation. Cell Cycle 13, 2446–2458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Luedtke K et al. (2014) Assessment of depression in a rodent model of spinal cord injury. J. Neurotrauma 31, 1107–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liddelow SA et al. (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Veneruso V et al. (2019) Stem cell paracrine effect and delivery strategies for spinal cord injury regeneration. Journal of Controlled Release 300, 141–153 [DOI] [PubMed] [Google Scholar]
- 54.Zhang B et al. (2016) Focus on Extracellular Vesicles: Therapeutic Potential of Stem Cell-Derived Extracellular Vesicles. Int J Mol Sci 17, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shao H et al. (2018) New Technologies for Analysis of Extracellular Vesicles. Chem Rev 118, 1917–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cho KHT et al. (2019) Emerging Roles of miRNAs in Brain Development and Perinatal Brain Injury. Front. Physiol 10, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.D’Anca M et al. (2019) Exosome Determinants of Physiological Aging and Age-Related Neurodegenerative Diseases. Front. Aging Neurosci 11, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li H et al. (2019), Immunomodulatory Functions of Mesenchymal Stem Cells in Tissue Engineering., Stem Cells International. [Online]. Available: https://www.hindawi.com/journals/sci/2019/9671206/abs/. [Accessed: 15-May-2019] [DOI] [PMC free article] [PubMed]
- 59.Nourian Dehkordi A et al. (2019) Skin tissue engineering: wound healing based on stem-cell-based therapeutic strategies. Stem Cell Research & Therapy 10, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Arzouni AA et al. (2018) Using Mesenchymal Stromal Cells in Islet Transplantation. STEM CELLS Translational Medicine 7, 559–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Keshtkar S et al. (2018) Mesenchymal stem cell-derived extracellular vesicles: novel frontiers in regenerative medicine. Stem Cell Research & Therapy 9, 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Börger V et al. (2017) Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles and Their Potential as Novel Immunomodulatory Therapeutic Agents. Int J Mol Sci 18, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Galieva LR et al. (2019) Therapeutic Potential of Extracellular Vesicles for the Treatment of Nerve Disorders. Front. Neurosci 13, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Branscome H et al. (2019) Stem Cell Extracellular Vesicles and their Potential to Contribute to the Repair of Damaged CNS Cells. J Neuroimmune Pharmacol DOI: 10.1007/s11481-019-09865-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Webb RL et al. (2018) Human Neural Stem Cell Extracellular Vesicles Improve Tissue and Functional Recovery in the Murine Thromboembolic Stroke Model. Transl Stroke Res 9, 530–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smith SM et al. (2020) Functional equivalence of stem cell and stem cell-derived extracellular vesicle transplantation to repair the irradiated brain. Stem Cells Transl Med 9, 93–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Acharya MM et al. (2015) Human neural stem cell transplantation provides long-term restoration of neuronal plasticity in the irradiated hippocampus. Cell Transplant 24, 691–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sun MK et al. (2020) Extracellular Vesicles Mediate Neuroprotection and Functional Recovery after Traumatic Brain Injury. J. Neurotrauma DOI: 10.1089/neu.2019.6443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Baulch JE et al. (2016) Cranial grafting of stem cell-derived microvesicles improves cognition and reduces neuropathology in the irradiated brain. Proc Natl Acad Sci U S A 113, 4836–4841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rong Y et al. (2019) Neural stem cell-derived small extracellular vesicles attenuate apoptosis and neuroinflammation after traumatic spinal cord injury by activating autophagy. Cell Death & Disease 10, 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Webb RL et al. (2018) Human Neural Stem Cell Extracellular Vesicles Improve Recovery in a Porcine Model of Ischemic Stroke. Stroke 49, 1248–1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang L et al. (2020) Exosomes Derived From Schwann Cells Ameliorate Peripheral Neuropathy in Type 2 Diabetic Mice. Diabetes 69, 749–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mohammed I et al. (2020) Subventricular zone-derived extracellular vesicles promote functional recovery in rat model of spinal cord injury by inhibition of NLRP3 inflammasome complex formation. Metab Brain Dis DOI: 10.1007/s11011-020-00563-w [DOI] [PubMed] [Google Scholar]
- 74.Rong Y et al. (2019) Neural stem cell small extracellular vesicle-based delivery of 14–3-3t reduces apoptosis and neuroinflammation following traumatic spinal cord injury by enhancing autophagy by targeting Beclin-1. Aging (Albany NY) 11, 7723–7745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhong D et al. (2020) Neural stem cell-derived exosomes facilitate spinal cord functional recovery after injury by promoting angiogenesis. Exp Biol Med (Maywood) 245, 54–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiang D et al. (2020) Neuron-derived exosomes-transmitted miR-124–3p protect traumatically injured spinal cord by suppressing the activation of neurotoxic microglia and astrocytes. Journal of Nanobiotechnology 18, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Patel DB et al. (2017) Impact of cell culture parameters on production and vascularization bioactivity of mesenchymal stem cell‐derived extracellular vesicles. Bioeng Transl Med 2, 170–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lombardi M et al. (2019) Detrimental and protective action of microglial extracellular vesicles on myelin lesions: astrocyte involvement in remyelination failure. Acta Neuropathol 138, 987–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu L et al. (2019) Exosome-shuttled miR-92b-3p from ischemic preconditioned astrocytes protects neurons against oxygen and glucose deprivation. Brain Research 1717, 66–73 [DOI] [PubMed] [Google Scholar]
- 80.Guitart K et al. (2016) Improvement of neuronal cell survival by astrocyte-derived exosomes under hypoxic and ischemic conditions depends on prion protein. Glia 64, 896–910 [DOI] [PubMed] [Google Scholar]
- 81.Ma K et al. (2019) Insulin-like growth factor-1 enhances neuroprotective effects of neural stem cell exosomes after spinal cord injury via an miR-219a-2–3p/YY1 mechanism. Aging (Albany NY) 11, 12278–12294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tseng AM et al. (2019) Ethanol Exposure Increases miR-140 in Extracellular Vesicles: Implications for Fetal Neural Stem Cell Proliferation and Maturation. Alcoholism: Clinical and Experimental Research 43, 1414–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cossetti C et al. (2014) Extracellular vesicles from neural stem cells transfer IFN-γ via Ifngr1 to activate Stat1 signaling in target cells. Mol. Cell 56, 193–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yildirimer L et al. (2019) Engineering three-dimensional microenvironments towards in vitro disease models of the central nervous system. Biofabrication 11, 032003. [DOI] [PubMed] [Google Scholar]
- 85.Cui H et al. (2017) 3D Bioprinting for Organ Regeneration. Advanced Healthcare Materials 6, 1601118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jarmalavičiūtė A et al. (2015) Exosomes from dental pulp stem cells rescue human dopaminergic neurons from 6-hydroxy-dopamine-induced apoptosis. Cytotherapy 17, 932–939 [DOI] [PubMed] [Google Scholar]
- 87.Patel DB et al. (2018) Towards rationally designed biomanufacturing of therapeutic extracellular vesicles: impact of the bioproduction microenvironment. Biotechnology Advances 36, 2051–2059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Patel DB et al. (2018) Enhanced extracellular vesicle production and ethanol-mediated vascularization bioactivity via a 3D-printed scaffold-perfusion bioreactor system. Acta Biomaterialia DOI: 10.1016/j.actbio.2018.11.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.O’Brien K et al. (2020) RNA delivery by extracellular vesicles in mammalian cells and its applications. Nature Reviews Molecular Cell Biology DOI: 10.1038/s41580-020-0251-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chevillet JR et al. (2014) Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc Natl Acad Sci U S A 111, 14888–14893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Martellucci S et al. (2020) Extracellular Vesicles: New Endogenous Shuttles for miRNAs in Cancer Diagnosis and Therapy? IJMS 21, 6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang Z et al. (2020) Large-scale generation of functional mRNA-encapsulating exosomes via cellular nanoporation. Nature Biomedical Engineering 4, 69–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang Y et al. (2019) MiR-124 Enriched Exosomes Promoted the M2 Polarization of Microglia and Enhanced Hippocampus Neurogenesis After Traumatic Brain Injury by Inhibiting TLR4 Pathway. Neurochem Res 44, 811–828 [DOI] [PubMed] [Google Scholar]
- 94.Li D et al. (2018) Exosomes Derived From miR-133b-Modified Mesenchymal Stem Cells Promote Recovery After Spinal Cord Injury. Front Neurosci 12, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Osorio-Querejeta I et al. (2020) MiR-219a-5p Enriched Extracellular Vesicles Induce OPC Differentiation and EAE Improvement More Efficiently Than Liposomes and Polymeric Nanoparticles. Pharmaceutics 12, 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li D et al. (2019) Exosomes from MiR-21–5p-Increased Neurons Play a Role in Neuroprotection by Suppressing Rab11a-Mediated Neuronal Autophagy In Vitro After Traumatic Brain Injury. Med Sci Monit 25, 1871–1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ge X et al. (2020) Increased Microglial Exosomal miR-124–3p Alleviates Neurodegeneration and Improves Cognitive Outcome after rmTBI. Molecular Therapy 28, 503–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sutaria DS et al. (2017) Low active loading of cargo into engineered extracellular vesicles results in inefficient miRNA mimic delivery. J Extracell Vesicles 6, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.de Jong OG et al. (2019) Drug Delivery with Extracellular Vesicles: From Imagination to Innovation. Acc. Chem. Res 52, 1761–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fuhrmann G et al. (2015) Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. Journal of Controlled Release 205, 35–44 [DOI] [PubMed] [Google Scholar]
- 101.O’Loughlin AJ et al. (2017) Functional Delivery of Lipid-Conjugated siRNA by Extracellular Vesicles. Mol Ther 25, 1580–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Longatti A et al. (2018) High affinity single-chain variable fragments are specific and versatile targeting motifs for extracellular vesicles. Nanoscale 10, 14230–14244 [DOI] [PubMed] [Google Scholar]
- 103.Kooijmans SAA et al. (2013) Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. J Control Release 172, 229–238 [DOI] [PubMed] [Google Scholar]
- 104.Lamichhane TN et al. (2016) Oncogene Knockdown via Active Loading of Small RNAs into Extracellular Vesicles by Sonication. Cellular and Molecular Bioengineering 9, 315–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jeyaram A et al. (2020) Enhanced Loading of Functional miRNA Cargo via pH Gradient Modification of Extracellular Vesicles. Molecular Therapy 28, 975–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Willis CM et al. (2020) Astrocyte Support for Oligodendrocyte Differentiation can be Conveyed via Extracellular Vesicles but Diminishes with Age. Scientific Reports 10, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yu Z et al. (2020) Reduced oligodendrocyte exosome secretion in multiple system atrophy involves SNARE dysfunction. Brain 143, 1780–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Men Y et al. (2019) Exosome reporter mice reveal the involvement of exosomes in mediating neuron to astroglia communication in the CNS. Nature Communications 10, 1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Budnik V et al. (2016) Extracellular vesicles round off communication in the nervous system. Nat Rev Neurosci 17, 160–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Morel L et al. (2013) Neuronal exosomal miRNA-dependent translational regulation of astroglial glutamate transporter GLT1. J. Biol. Chem 288, 7105–7116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fitzner D et al. (2011) Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J Cell Sci 124, 447–458 [DOI] [PubMed] [Google Scholar]
- 112.Frühbeis C et al. (2013) Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 11, e1001604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Morton MC et al. (2018) Neonatal Subventricular Zone Neural Stem Cells Release Extracellular Vesicles that Act as a Microglial Morphogen. Cell Rep 23, 78–89 [DOI] [PubMed] [Google Scholar]
- 114.Iraci N et al. (2017) Extracellular vesicles are independent metabolic units with asparaginase activity. Nat. Chem. Biol 13, 951–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pomper N et al. (2020) CNS microRNA profiles: a database for cell type enriched microRNA expression across the mouse central nervous system. Scientific Reports 10, 4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nguyen L et al. (2020) Effects of miR-219/miR-338 on microglia and astrocyte behaviors and astrocyte-oligodendrocyte precursor cell interactions. Neural Regen Res 15, 739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Obernier K and Alvarez-Buylla A (2019) Neural stem cells: origin, heterogeneity and regulation in the adult mammalian brain. Development 146, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yunsun Kim S et al. (2018) None of us is the same as all of us: resolving the heterogeneity of extracellular vesicles using single-vesicle, nanoscale characterization with resonance enhanced atomic force microscope infrared spectroscopy (AFM-IR). Nanoscale Horizons 3, 430–438 [DOI] [PubMed] [Google Scholar]