Aortic dimension is the most commonly used criterion of the severity of thoracic aortic aneurysms and can be determined by several approaches in mice. In situ imaging has been used to measure aortic diameters. However, a potential caveat of aortic measurements using this mode is the absence of arterial blood pressure that can lead to under-estimation of aortic dimeters, particularly in mice with a flaccid aortic wall. The present study developed an in situ imaging approach to overcome this shortcoming, and demonstrated authentic dimensions of thoracic aortic aneurysms in mice.
β-aminopropionitrile (BAPN, 0.5% wt/vol in drinking water) was administered to induce thoracic aortic aneurysms in C57BL/6J mice (male, 4-week-old, n=30). Ultrasound imaging (Vevo 2100, MS550) was performed after 4, 8, and 12 weeks of BAPN administration, and aortic diameters were measured at the most dilated area at end-diastole. During BAPN administration, 16 mice died and ultrasonography detected a wide range of luminal dilatations of the thoracic aorta from mild to severe in 10 mice (Figure A, B). In these 10 mice, 7 mice were randomly selected and in situ imaging was performed. The right atrial appendage was excised and saline (8 ml) was perfused through the left ventricle. Perivascular tissues of the thoracic aorta were removed gently and a black plastic sheet was inserted behind the aorta to enhance contrast of the aortic wall. Optimal cutting temperature (OCT) compound (150 μl) was then introduced into the left ventricle in 30 seconds using an insulin syringe to maintain aortic patency. Two dimensional images and cine loops were recorded from pre-injection to 50 seconds post-OCT injection using a dissection microscope with a high-resolution camera (#SMZ800, #DS-Ri1, Nikon). Imaging procedure was completed within 10–15 minutes in each mouse. To determine the aortic patency during imaging, aortic diameters were compared between ultrasound and in situ images. Several mice exhibited considerably smaller aortic diameters in images acquired prior to introduction of OCT compared to diameters measured using ultrasound (−0.8 to −0.4 mm), and OCT injection inflated the aortic dimensions (Figure A, B). Bland-Altman plots revealed that the bias between in situ and ultrasound diameters was closer to 0 in post- than that in pre-OCT images (Figure C). The range of limits of agreement was smaller in post-OCT (−0.4 to 0.3 mm) than in pre-OCT (−1.0 to 0.5 mm). Furthermore, a significant positive correlation between ultrasound and in situ measurements was only observed in post-, but not pre-, OCT images (Figure D). Therefore, in situ imaging using OCT injection provided more accurate aortic measurements in mice with thoracic aortic aneurysms.
Figure. Ultrasound and in situ imaging of mouse thoracic aortas.
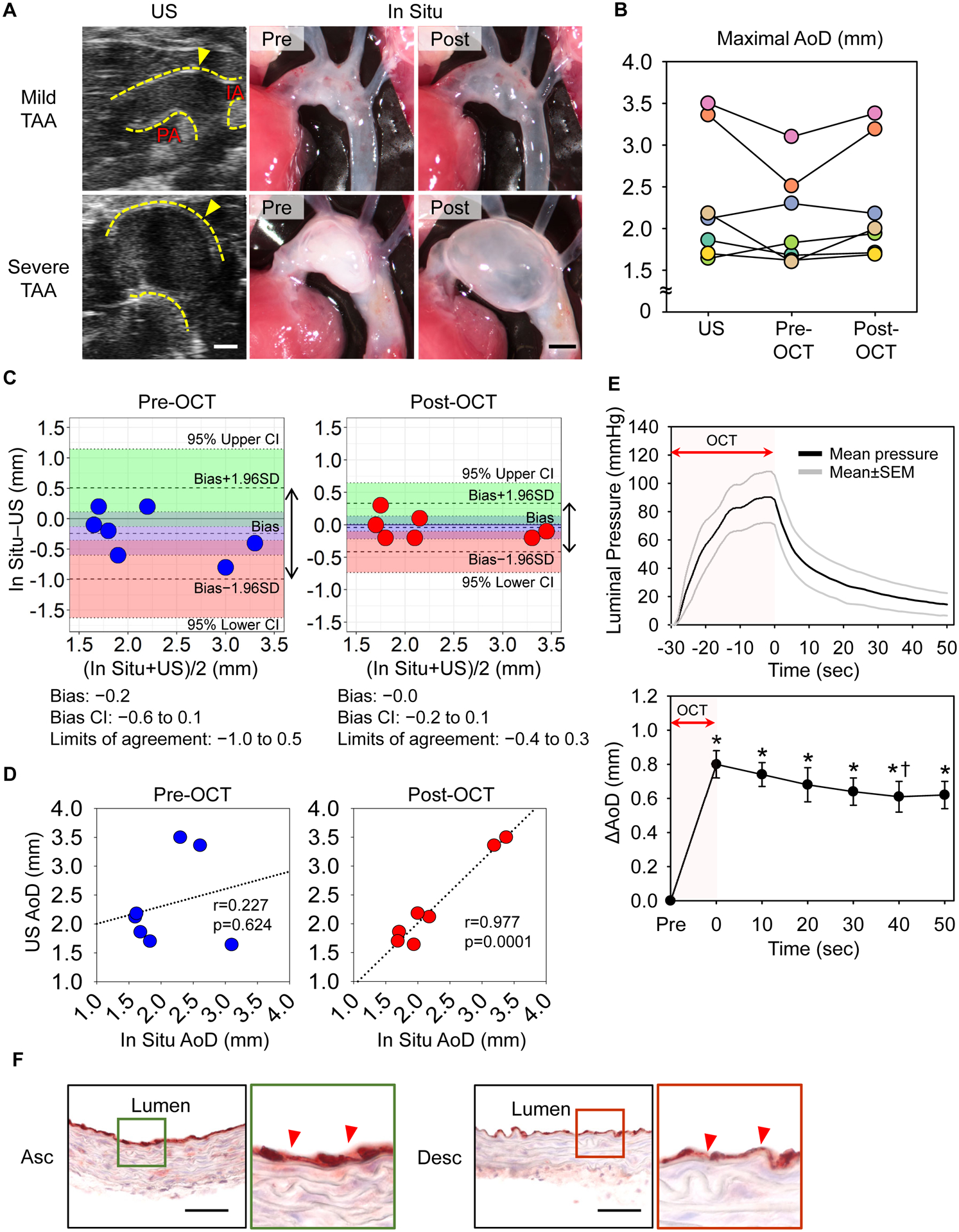
(A) Representative ultrasound and in situ images of thoracic aortas in BAPN-administered mice. Post-OCT images were captured 50 seconds after completion of OCT injection. TAA indicates thoracic aortic aneurysm; Yellow dotted lines, aortic wall; IA, innominate artery; PA, pulmonary artery. Scale bar, 1 mm. n=7. (B) Aortic diameters measured at the most dilated area of ultrasound and in situ images with/without OCT injection. n=7. (C) Bland-Altman plots reveal the low variation of aortic measurements with OCT injection. Double arrows indicate the limits of agreement. Light green and red area; 95% lower and upper limit agreement confidence intervals (CIs). (D) Correlations of aortic measurements between ultrasound and in situ pre- or post-OCT images. (E) The upper panel exhibits luminal pressures during OCT injection in normal aortas (n=5) and the lower panel shows sequential transition of aortic diameters after OCT injection in aneurysmal tissues (n=3). * p<0.05 vs Pre, † p<0.05 vs 0 sec by One-Way repeated measures ANOVA followed by t-test with Bonferroni correction. (F) Immunostaining for CD31 of normal aortas with OCT injection. n=5. Red triangles indicate endothelial cells; L, lumen; Asc, ascending aorta; Desc, descending; scale bar, 50 μm.
OCT was injected using a small gauge needle (28G) that provided a slow flow of this viscous material. We measured luminal pressures during OCT injection using a telemetry system (#TA11PA-C10, Data Sciences International) in C57BL/6J male mice (n=5). Luminal pressures were 90±18 mmHg at the peak and decreased to less than 50 mmHg after at 10 seconds (Figure E). Then, aortic diameters were measured during OCT injection in mice with severe aortic dilatation (n=3). Aortic diameters were the largest immediately following OCT injection, with a subsequent modest reduction that was plateaued after at 40 seconds (Figure E). Importantly, maximal external diameters measured with in situ images were comparable to luminal diameters at mid systole in ultrasound images (ultrasound: 3.0±0.4; in situ: 3.0±0.4 mm, p=0.70). Endothelial cells were also examined by immunostaining for CD31 to determine whether OCT injection led to loss of endothelial cells. Aortic tissues were harvested from C57BL/6J male mice after OCT injection (n=5). CD31 positive cells were detected throughout the intima of both ascending and descending thoracic aortas (Figure F). These results demonstrated that OCT injection did not cause aortic over-expansion due to pressure overload and overt aortic tissue damage.
High frequency ultrasound is a powerful tool to evaluate aortic diameters in mice, and have been used by many studies.1, 2 However, the availability of ultrasound systems is restricted by its expense. Also, ultrasonography requires appropriate training for reliable imaging and authentic measurements. In addition, some mouse models of thoracic aortic aneurysms involve the descending aorta which is not readily imaged by ultrasonography. The present study demonstrated in situ image with OCT injection exhibited comparable aortic measurements to ultrasonography. Furthermore, in situ images can measure aortic dimensions directly in any regions of the thoracic aorta. Thus, in situ imaging with OCT injection can be considered as not only an optimal alternative approach but also a validation mode to ultrasound aortic measurements. The combination of ultrasound and in situ measurements would provide more robust evaluation of the severity of thoracic aortic aneurysms in mice.
Aortic diameters can be measured by ex vivo approach; however, aortic patency is often not maintained during ex vivo imaging of aneurysmal aortas, which may lead to underestimation of aortic dimensions. Therefore, formalin or latex perfusion are frequently utilized to recapitulate aortic morphology ex vivo.3–5 However, these procedures need a perfusion system and are time-consuming. OCT is a nonhazardous reagent and can be readily applied. In addition, OCT did not cause discernable tissue damage. Therefore, OCT injection is a simple and effective procedure for in situ aortic measurements. Notable, in situ images measure aortic diameters in a lateral (right-left) axis. Imaging planes should be optimized individually, especially mice with significant aortic dilatation on the anterior or posterior wall.
In conclusion, OCT injection is an optimal approach for maintaining aortic patency in situ, which provides more reliable aortic measurements in mice.
Sources of Funding
The authors’ research work was supported by the NHLBI of the NIH (R01HL133723, R01HL142973) and the AHA (18SFRN33960163).
Footnotes
Methods and Data Availability
Detailed methods and results are available in bioRxiv (https://doi.org/10.1101/2020.12.24.424013). All experiments were approved by the University of Kentucky Institutional Animal Care and Use Committee in accordance with the guidelines of the National Institutes of Health. Analyses were performed using 3 – 7 mice.
Disclosure
None
References
- 1.Sawada H, Chen JZ, Wright BC, Moorleghen JJ, Lu HS, Daugherty A. Ultrasound imaging of the thoracic and abdominal aorta in mice to determine aneurysm dimensions. J Vis Exp. 2019: 10.3791/59013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sawada H, Franklin MK, Moorleghen JJ, Howatt DA, Kukida M, Lu HS, Daugherty A. Ultrasound monitoring of descending aortic aneurysms and dissections in mice. Arterioscler Thromb Vasc Biol. 2020;40:2557–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiao Y, Li G, Korneva A, Caulk AW, Qin L, Bersi MR, Li Q, Li W, Mecham RP, Humphrey JD, Tellides G. Deficient circumferential growth is the primary determinant of aortic obstruction attributable to partial elastin deficiency. Arterioscler Thromb Vasc Biol. 2017;37:930–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gage GJ, Kipke DR, Shain W. Whole animal perfusion fixation for rodents. J Vis Exp. 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, Podowski M, Neptune ER, Halushka MK, Bedja D, Gabrielson K, Rifkin DB, Carta L, Ramirez F, Huso DL, Dietz HC. Losartan, an at1 antagonist, prevents aortic aneurysm in a mouse model of marfan syndrome. Science. 2006;312:117–121 [DOI] [PMC free article] [PubMed] [Google Scholar]


