Abstract
Standard-of-care infliximab dosing regimens were developed prior to the routine use of therapeutic drug monitoring and identification of target concentrations. Not surprisingly, subtherapeutic infliximab concentrations in pediatric Crohn’s disease (CD) are common. The primary aim was to conduct a real-world pharmacokinetic (PK) evaluation to discover blood biomarkers of rapid clearance, identify exposure targets, and a secondary aim to translate PK modeling to the clinic. In a multicenter observational study, 671 peak and trough infliximab concentrations from 78 patients with CD were analyzed with a drug-tolerant assay (Esoterix; LabCorp, Calabasas, CA). Individual area under the curve (AUC) estimates were generated as a measure of drug exposure over time. Population PK modeling (nonlinear mixed-effect modeling) identified serum albumin, antibody to infliximab, erythrocyte sedimentation rate (ESR), and neutrophil CD64 as biomarkers for drug clearance. Week 14 and week 52 biochemical remitters (fecal calprotectin < 250 μg/g) had higher infliximab exposure (AUC) throughout induction. The optimal infliximab AUC target during induction for week 14 biochemical remission was 79,348 μg*h/mL (area under the receiver operating characteristic curve (AUROC) 0.77, [0.63–0.90], 85.7% sensitive, and 64.3% specific) with those exceeding the AUC target more likely to achieve a surgery-free week 52 biochemical remission (OR 4.3, [1.2–14.6]). Pretreatment predictors for subtherapeutic week 14 AUC included neutrophil CD64 > 6 (OR 4.5, [1.4–17.8]), ESR > 30 mm/h (OR 3.8, [1.4–11]), age < 10 years old (OR 4.2, [1.2–20]), and weight < 30 kg (OR 6.6, [2.1–25]). We created a decision-support PK dashboard with an iterative process and embedded the modeling program within the electronic health record. Model-informed precision dosing guided by real-world PKs is now available at the bedside in real-time.
Crohn’s disease (CD) is characterized by a remitting and relapsing course that without optimization of effective therapies may lead to irreversible intestinal damage.1,2 Inhibition of tumor necrosis factor-α (TNF) with monoclonal antibodies (infliximab and adalimumab) has transformed pediatric CD management strategies with evidence that early anti-TNF use (“top-down”) reverses severe growth failure, reduces corticosteroid exposures, improves quality of life, decreases hospitalization rates, and results in a reduction in penetrating (B3) complications.3–5
Despite a high clinical response rate during induction in clinical trials, use of as-labeled (5 mg/kg) infliximab dosing regimens in children with inflammatory bowel disease (IBD) have been associated with a high rate of subtherapeutic trough concentrations during induction,6 and modest rates of clinical remission (51–55.8%) and endoscopic healing (39%) at 1 year that combine to contribute to a durability rate of < 50% (at 5 years) in pediatric CD.3,7–9
Given the high rate of subtherapeutic drug levels in pediatric CD, enhancing infliximab durability may be best addressed by optimizing drug exposure during induction as the prospective PANTS study found a subtherapeutic drug concentration at week 14 was the only independent factor associated with both week 14 primary nonresponse and week 54 nonremission.10 Not only were higher postinduction infliximab concentrations (> 7 μg/mL) associated with lower week 14 fecal calprotectin (fCal), but were also protective against immunogenicity (odds ratio (OR) 0.43, 95% confidence interval (CI) 0.3–0.61).10
An additional limitation to effective dose optimization strategies in pediatric CD is prior infliximab pharmacokinetic (PK) and pharmacodynamic analyses have been primarily evaluated in pediatric and adult clinical trial participants11 who received as-labeled infliximab regimens in combination with immunomodulators that alter infliximab clearance (CL).12 Identification of patient-specific predictors of rapid drug CL and infliximab exposure targets from a real-world cohort could be useful to inform PK modeling software programs as more precision dosing tools (such as dashboards) become available.
The primary aim was to identify the cumulative exposure (area under the curve (AUC)) targets during induction and maintenance that were associated with biochemical and deep remission. The secondary aim was to integrate the PK parameters into a user-friendly, decision support dashboard embedded within the electronic health record (EHR) to simulate individual precision dosing regimens at the bedside in real-time.
METHODS
Study design and patient recruitment
The Targeting the Inflammatory Signature to Personalize Biologics in Pediatric IBD (REFINE) study is a multicenter, observational study of anti-TNF naïve children and young adults (< 22 years old) enrolled prior to starting infliximab and prospectively monitored for treatment outcomes with longitudinal biospecimens (blood and stool) collected for up to 2 years. Infliximab dosing regimens were at the discretion of the treating physicians at Cincinnati Children’s Hospital Medical Center (CCHMC), Connecticut Children’s Medical Center, Medical College of Wisconsin, and Nationwide Children’s Hospital between August 2014 and October 2019. The REFINE study was approved by the Institutional Review Board at all four participating medical centers.
Response measures
Infliximab exposure (AUC) targets were assessed for biochemical response (50% reduction in baseline fCal), biochemical remission (fCal < 250 μg/g), and deep remission (combination of biochemical and clinical remission). Clinical remission was evaluated by the weighted pediatric CD activity index (< 12.5).13 Patients discontinuing infliximab for lack of efficacy were classified as nonremitters and their PK data censored at the last infusion. As infliximab dosing regimens were selected by the treating physician, dose intensifications were not considered a treatment failure.
Biologic assays
Infliximab and antibody to infliximab (ATI) concentrations were measured with a drug-tolerant electrochemiluminescence immunoassay (ECLIA; Esoterix, LabCorp specialty lab, Calabasas, CA). With serial dilutions, the infliximab assay had an upper detection limit of 600 μg/mL, a lower detection limit of 0.4 μg/mL, and an interassay coefficient of variation (CV) of 6.07–8.51%. The lower limit of ATI detection is 22 ng/mL. We defined any ATI event > 200 ng/mL as clinically significant based on the high specificity of this cutoff point for loss of response to infliximab in an IBD cohort study with ATI level < 200 ng/mL also found to be associated with higher rates of mucosal healing following dose optimization.14 An enzyme-linked immunosorbent assay with an interassay precision CV of 6.6–14.5% was used for fCal (Buhlmann, Switzerland). Neutrophil surface expression of Fcγ receptor I (CD64) is determined by a ratio of the mean fluorescence intensity of granulocyte CD64 expression to the lymphocyte CD64 mean fluorescence intensity by quantitative flow cytometry (FACSCantos; BD Biosciences, San Jose, CA) and reported as the neutrophil CD64 activity ratio (nCD64; further detailed in Figure S1). Cell populations are determined by forward and side scatter characteristics along with cell specific receptors; CD163 (monocytes) and CD45 (lymphocytes).
Population pharmacokinetic model development
Infliximab peak concentrations (collected 30–60 minutes following an infusion) and trough concentrations (Ctrough) were used to develop the population PK model. A two-compartment model (base model) was used for parameterization to estimate key PK parameters including CL, central volume of distribution (V1), intercompartmental clearance (Q), and peripheral volume of distribution (V2).15 We applied a fixed Q of 0.0095 L/h/65 kg based on a previously published model.11 Population PK analysis and simulations were conducted by nonlinear mixed-effects modeling (NONMEM) software (version 7.2.0; ICON Development Solutions, Ellicott City, MD).
Population parameters and their distributions were estimated using the first-order conditional estimation method with interaction. Model selection was based on good modeling practices and goodness-of-fit indicators, including visual inspection of diagnostic scatter plots, comparisons of the minimum objective function value (OFV), and evaluation of the estimates of population parameters. The full model was created with a stepwise covariate model building approach with covariate inclusion based on a drop in NONMEM OFV > 3.84 (P < 0.05) and model reduction using backward elimination based on a drop in OFV > 10.83 (P < 0.001). The OFV is a goodness-of-fit statistic, a lower value of which indicates a better model fit based on the likelihood ratio test when comparing between two nested models.
Model validation
Population predicted concentrations were based on the covariate values and PK parameter estimates in the final model. The individual predicted concentrations were generated based on individual empirical Bayes parameter estimates. Goodness-of-fit curves were generated to evaluate the precision of the modeling parameters. The final PK model was evaluated by nonparametric bootstrap and visual predicted checks (VPCs). The original dataset was resampled with 500 replicate data sets with the estimated means and 95% CIs of parameter estimates compared with the final model estimates. For VPCs, 1,000 simulations were carried out to visually examine if the simulated concentrations using the final model were representative of the distribution of the observed concentrations.
Dashboard development
The PK dashboard (RoadMAB) user-interface was developed with an iterative approach in collaboration with Pomiet (a human factors engineering firm, Dayton, OH) for product design and layout, Medimatics (a PK software firm, Maastricht, The Netherlands) to incorporate the software needed to conduct the PK Bayesian modeling, and a combination of Insight (Cincinnati, OH) and CCHMC Information Services personnel for digital innovation, development, and data integration within the EHR. The primary focus for this collaborative team was to create and deploy an expert system for model-informed precision dosing that was operational within the Epic (Verona, WI) EHR.
Statistical analysis
Continuous variables are represented as means with SDs or medians with 25–75% interquartile range (IQR) depending on data distribution. Infliximab exposure was defined as the AUC (μg*h/mL) of the predicted individual infliximab concentration-time profile and calculated by NONMEM software. Median (IQR) Ctrough (μg/mL at weeks 2, 6, 14, and 52) and infliximab exposure (AUC, weeks 2, 6, 14, 26, and 52) were compared between biochemical remitters/nonremitters and deep remitters/nonremitters using either the Mann–Whitney test (nonparametric) or the Student’s t-test (parametric) based on the data distribution for each. Similarly, AUC, Ctrough and CL were compared among the fCal quartiles by Kruskal–Wallis test with Dunn’s post-test or analysis of variance with Tukey’s multiple comparison test. The AUC cutoff point (target) was identified for treatment outcomes using the Youden’s J statistic from the receiver operating characteristic (ROC) curve. As any interruption in the infusion regimen would reduce total AUC, patients who discontinued infliximab or had surgery during the study had their respective AUC calculation censored at the time of the corresponding event for these analyses. For each cutoff point, we also identified the area under the ROC curve (AUROC) and 95% CI with sensitivity, specificity, positive predictive value, and the negative predictive value calculated. The AUROC between predictors for biochemical remission were compared using the DeLong test. Univariate logistic regression was used to determine pretreatment predictors for subtherapeutic exposure during induction with multicollinearity assessed to test the association between predictors. Multivariate logistic regression with backward stepwise variable selection and Akaike information criterion was used to identify the predictors (univariate P < 0.05) independently associated with subtherapeutic AUC. Statistical analyses were performed using GraphPad PRISM version 8.0.1 (GraphPad, San Diego, CA), and R (Core Team, 2020, version 4.0.1).
RESULTS
Patient demographics and outcomes
Seventy-eight anti-TNF naïve CD subjects were included in the real-world population PK model (Table 1) with 82.1% of the cohort receiving as-labeled dosing during induction and 96.2% on infliximab monotherapy. At 1 year, 88.5% (69/78) remained on infliximab, 10.3% (8/78) had surgery, and 68% (53/78) developed antibody to infliximab (ATI, ≥ 22 ng/mL). The mean (SD) time to ATI was 166 (77) days with the ATI concentration < 200 ng/mL in the majority (73.6%) of the 53 patients who were ATI positive.
Table 1.
Clinical characteristics and baseline laboratory results
| Patients in PK analysis, n | |
| Female, n | 28 (35.9%) |
| White race, n | 72 (92.3%) |
| Weight, kg (median, IQR) | 41 (28–56.8) |
| < 30 kg, n | 23 (30%) |
| Age at first infusion, years (mean, SD) | 13 (3.7) |
| < 10 years old, n | 15 (19.2%) |
| Disease duration, days (median, IQR) | 49 (18–382) |
| Infliximab dose, mg/kg (median, IQR) | 6.1 (5.2–7.1) |
| Crohn’s location | |
| Ileal only: colon only: ileocolonic | 9:7:62 |
| Crohn’s behavior | |
| Inflammatory | 66 |
| Stricturing | 7 |
| Penetrating | 4 |
| Both stricturing/penetrating | 1 |
| Perianal fistula, n | 12 (15.4%) |
| wPCDAI (median, IQR) | 38 (20–65) |
| Erythrocyte sedimentation rate, mm/h (median, IQR) | 15 (8–38) |
| CRP, mg/dL (median, IQR) | 1.0 (0.29–2.4) |
| Serum albumin, g/dL (mean, SD) | 3.3 (0.6) |
| Neutrophil CD64 activity ratio, MFI (median, IQR) | 6.5 (6–7.1) |
| Fecal calprotectin, μg/g (median, IQR) | 1611 (994–2501) |
IQR, interquartile range; MFI, mean fluorescence intensity; PK, pharmacokinetic; wPCDAI, weighted pediatric CD activity index.
Real-world population PK model
The PK model was developed utilizing 528 Ctrough and 143 peak concentrations. We first utilized a base two-compartment model using patient weight as a covariate on Q and V2, as reported by Fasanmade et al.11 The mean infliximab CL estimate in the cohort was 0.0138 L/h/65 kg and the median infliximab terminal half-life was 18.8 days.
With our stepwise covariate analysis, we found that patient weight, serum albumin, ATI concentration, erythrocyte sedimentation rate (ESR), and nCD64 were significantly associated with infliximab CL (Table 2). In comparison to the base model, the final model (with all 5 covariates) predicted individual PK profiles that more accurately mirrored the observed infliximab concentrations (Figure 1). The magnitude of the contribution of each covariate in the prediction of infliximab CL and V1 is demonstrated in Figure S2. The inclusion of all 5 covariates resulted in a 30.7% reduction in between-subject variability of CL from the base model, and a 11.6% reduction from a model including weight and albumin. The impact of each individual covariate (by intensity) on the predicted Ctrough for a 65 kg patient receiving a 5 mg/kg maintenance regimen is shown in Figure 2.
Table 2.
Parameter estimates of the final population PK model
| Parameters | Final estimate (RSE%) | 95% CIs |
|---|---|---|
| CL, L/h/65 kg | 0.0138 (5.7) | 0.012–0.015 |
| Weight for CL | 0.594 (10.2) | 0.475–0.713 |
| ALB for CL | −1.07 (9.6) | −1.272 to −0.868 |
| ESR for CL | 0.101 (27.7) | 0.046–0.156 |
| nCD64 for CL | 0.168 (16) | 0.115–0.221 |
| ATI for CL | 0.134 (9.6) | 0.109–0.159 |
| V1, L/65kg | 2.97 (4.5) | 2.709–3.231 |
| Weight for V1 | 0.55 (20.2) | 0.332–0.768 |
| Q, L/h/65 kg | 0.0095 FIXED | – |
| Weight for Q | 1 FIXED | – |
| V2, L/65 kg | 2.84 (3.6) | 2.642–3.038 |
| Weight for V2 | 0.586 FIXED | – |
| Interindividual variability (%CV) | Shrinkage (%) | |
| ω (CL) | 25.1 (9) | 6.3 |
| Residual error (SD) | Shrinkage (%) | |
| σadd | 0.435 (1.5) | 5.2 |
The covariate analysis for CL included age, body weight, body mass index, sex, race, clinical activity wPCDAI, and laboratory measurements including ESR, serum ALB, CRP, nCD64, hemoglobin, platelet count, fecal calprotectin, and ATI concentrations. The equation for the final model is CLind = CLpop × WT/65)0.594 × (ALB/3.5)−1.07 × (ESR/9)0.101 × (nCD64/4.6)0.168 × (ATI/22)0.134. V1ind = V1pop × (WT/65) 0.55 . Qind = Qpop × (WT/65). V2ind = V2pop × (WT/65)0.586. Residual error is additive error for log transformed concentrations.
ALB, albumin; ATI, antibody to infliximab; CI, confidence interval; CL, clearance; CV, coefficient of variation; ESR, erythrocyte sedimentation rate; Ind, individual; nCD64, neutrophil CD64 activity ratio; pop, population; PK, pharmacokinetic; Q, inter-compartmental clearance; RSE, relative standard error; V1, central compartment volume of distribution; V2, peripheral compartment volume of distribution; wPCDAI, weighted pediatric CD activity index.
Figure 1.
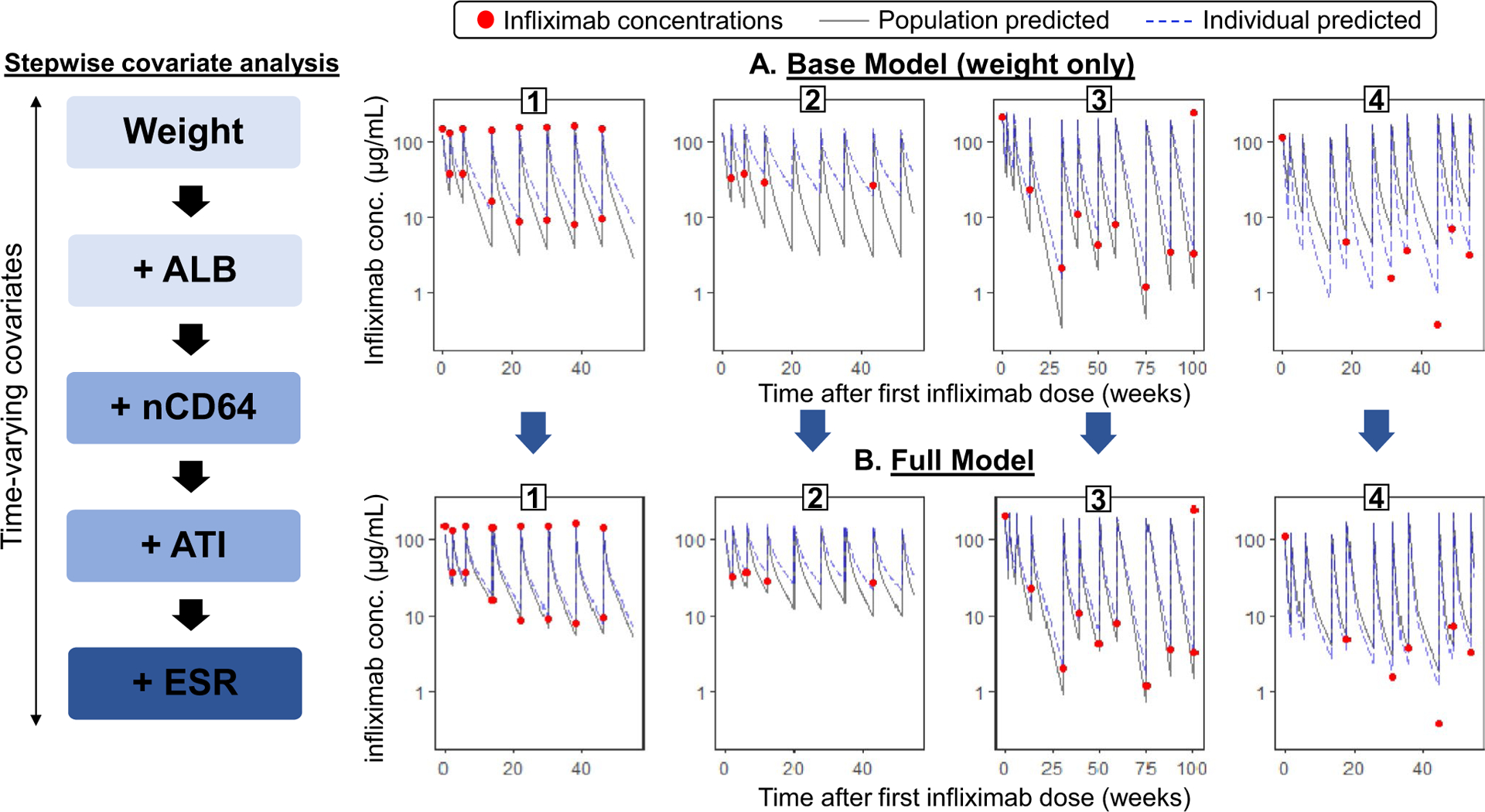
Comparison of model accuracy between the (a) base pharmacokinetic (PK) model and the (b) full model that includes weight, serum albumin (ALB), neutrophil CD64 activity ratio (nCD64), antibody to infliximab (ATI), and erythrocyte sedimentation rate (ESR) as covariates of drug clearance. With the full model, the improved predicted accuracy is depicted as the observed drug concentrations (dots) and individual predicted curves (dashed-lines) are in closer proximity to the population predicted PK curve (solid line) when compared with the base model for each representative patient.1–4
Figure 2.
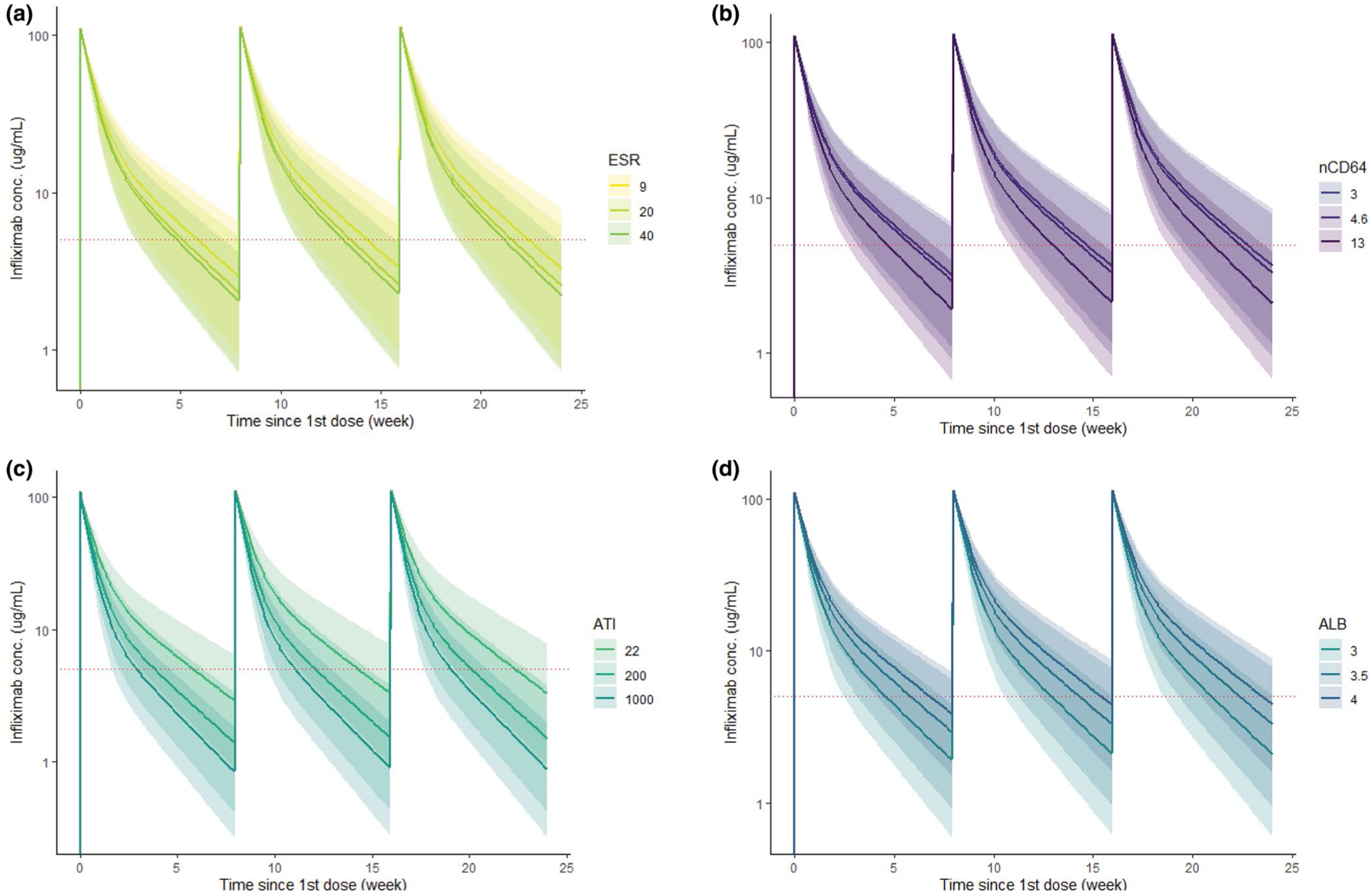
The predicted pharmacokinetic (PK) profile by the magnitude of each covariate with as-labeled (5 mg/kg) infliximab. The model default value for (a) ESR is 9 mm/h, (b) nCD64 is 4.6, (c) ATI is 22 ng/mL (undetectable level) and (d) ALB is 3.5 mg/dL. The respective colored line indicates the median PK profile for each covariate intensity, the shaded ribbon indicates the correspondent 5–95% prediction interval and the dashed horizontal line represents an infliximab concentration of 5 μg/mL. ALB, albumin; ATI, antibody to infliximab; ESR, and erythrocyte sedimentation rate; nCD64, neutrophil CD64.
Model validation
Goodness-of-fit curves show the final model adequately predicted the observed infliximab levels (Figure S3). All model parameter estimates were within the 95% CI and deviated < 10% from the median value obtained by the bootstrap analysis (data not shown) further confirming the stability of the model. Finally, the predictive performance of the population PK model was evaluated by VPC plots during the induction and maintenance phases (Figure S4).
Infliximab exposure-outcome analysis
In a subanalysis, we evaluated infliximab AUC and Ctrough among biochemical/deep remitters and nonremitters. Week 14 biochemical response was 44.8% (26/58) wheres 24.1% (14/58) achieved biochemical remission. At week 52, biochemical and deep remission was achieved in 38.8% (19/49) and 24.5% (12/49), respectively.
The median induction AUCwk0–wk14 for week 14 biochemical remitters was 106,695 μg*h/mL (81,851–126,103) compared with a median of 66,711 μg*h/mL (48,899–102,973) in nonremitters (P = 0.002) with similar differences in drug exposure between remitters and nonremitters throughout induction (Figure 3a,b). We found the optimal AUCwk0–wk14 during induction for week 14 biochemical remitters was 79,348 μg*h/mL with a sensitivity of 85.7% and specificity of 64.3% (AUROC 0.77, 95% CI 0.63–0.90; Figure 3c). Patients exceeding the AUC target of 79,348 μg*h/mL (47.4% of the cohort) were more likely to achieve a surgery-free week 52 biochemical remission (OR 4.3, 95% CI 1.2–14.6). Week 14 deep remission was achieved in 15.5% (9/58) with an AUCwk0–wk14 cutoff point of 96,307 μg*h/mL (AUROC 0.74, 95% CI 0.56–0.91) associated with week 14 deep remission. Week 52 biochemical remitters had a higher AUCwk0–wk14 and AUCwk0–wk26 than nonremitters (Figure 3d–f).
Figure 3.
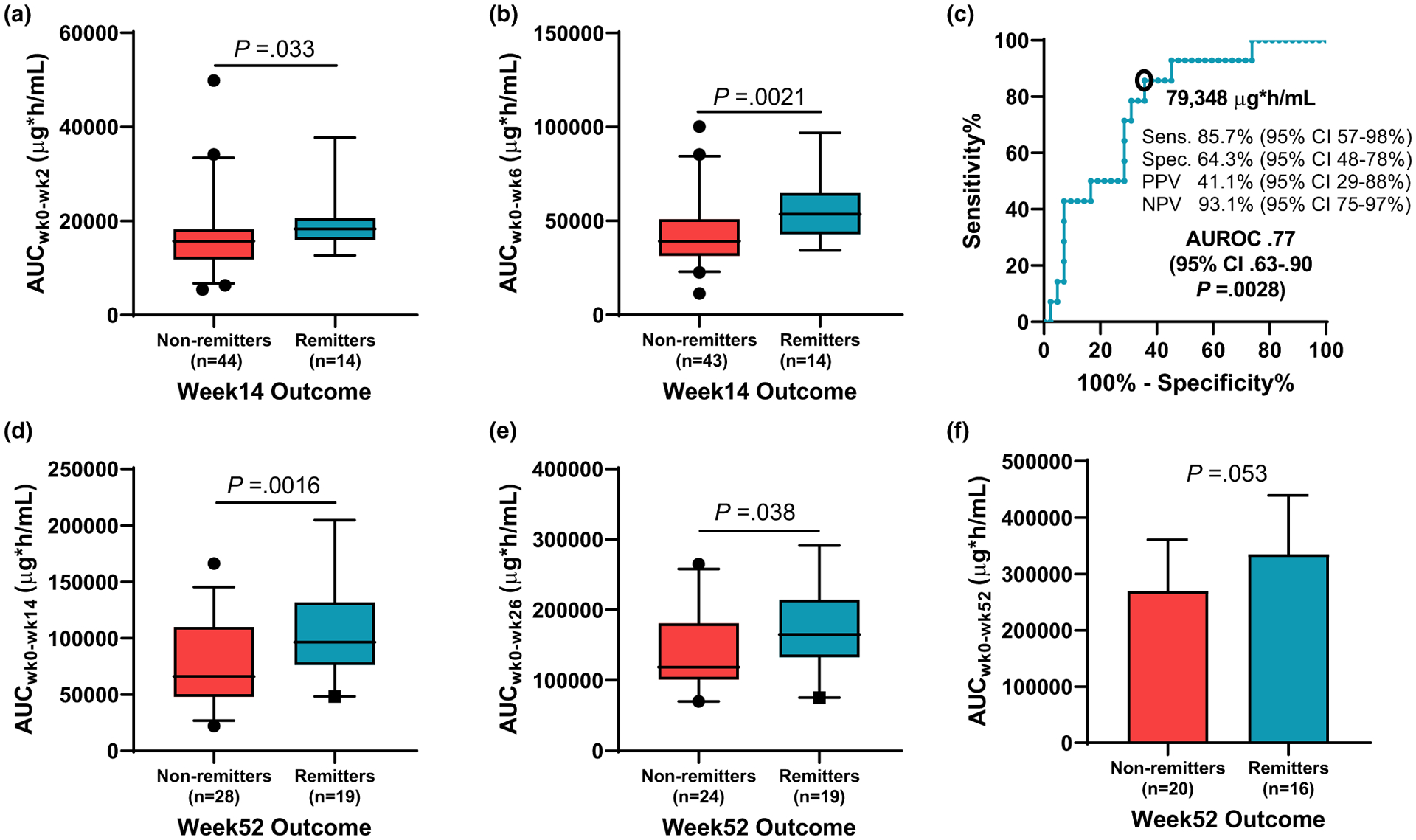
Infliximab area under the curve (AUC) at (a) week 2 and (b) week 6 was compared for week 14 biochemical outcomes. (c) The week 14 AUC cutoff point associated with week 14 biochemical remission was determined with the Youden index from the receiver operating characteristic curve. Infliximab AUC at (d) week 14 (e) week 26, and (f) week 52 was compared for week 52 biochemical outcomes. AUROC, area under the receiver operating characteristic curve; CI, confidence interval; NPV, negative predictive value; PPV, positive predictive value; Sens., sensitivity; Spec., specificity.
There was a strong correlation between week 14 infliximab Ctrough and infliximab AUCwk0–wk14 (Spearman r = 0.89, P < 0.001) with a more modest correlation between week 52 Ctrough and AUCwk0–wk52 (Spearman r = 0.63, P < 0.001). Compared with nonremitters (median 2.7 μg/mL, IQR 1.6–8), week 14 biochemical remitters median infliximab Ctrough was 13 μg/mL (IQR 6.4–15, P < 0.001). We found the optimal Ctrough prior to the first maintenance dose for week 14 biochemical remitters was 5.1 μg/mL with a sensitivity of 92.3% and specificity of 67.8% (AUROC 0.85, 95% CI 0.73–0.97), whereas the optimal week 14 Ctrough associated with week 14 deep remission was 13 μg/mL (AUROC 0.81, 95% CI 0.64–0.97, 62.5% sensitivity, and 87.8% specificity). Although numerically higher, there was no statistical difference between the AUROC for either predictor (week 14 Ctrough or week 14 AUC, P = 0.38) for biochemical remission. Finally, the median week 52 Ctrough was 9.2 μg/mL (IQR 7–15) and 8.6 μg/mL (IQR 6.4–14.5) for week 52 biochemical remitters and deep remitters compared with a median of 4.4 μg/mL (IQR 3.1–7, P = 0.003) and 4.8 μg/mL (IQR 3.3–10.6, P = .038) for biochemical nonremitters and deep nonremitters, respectively.
Fecal calprotectin and infliximab pharmacokinetics
Incremental elevations in week 14 fCal (across quartiles) were associated with reductions in week 14 AUC (P = 0.012) and week 14 Ctrough (P < 0.001) along with a more rapid infliximab CL (P = 0.014; Figure 4).
Figure 4.
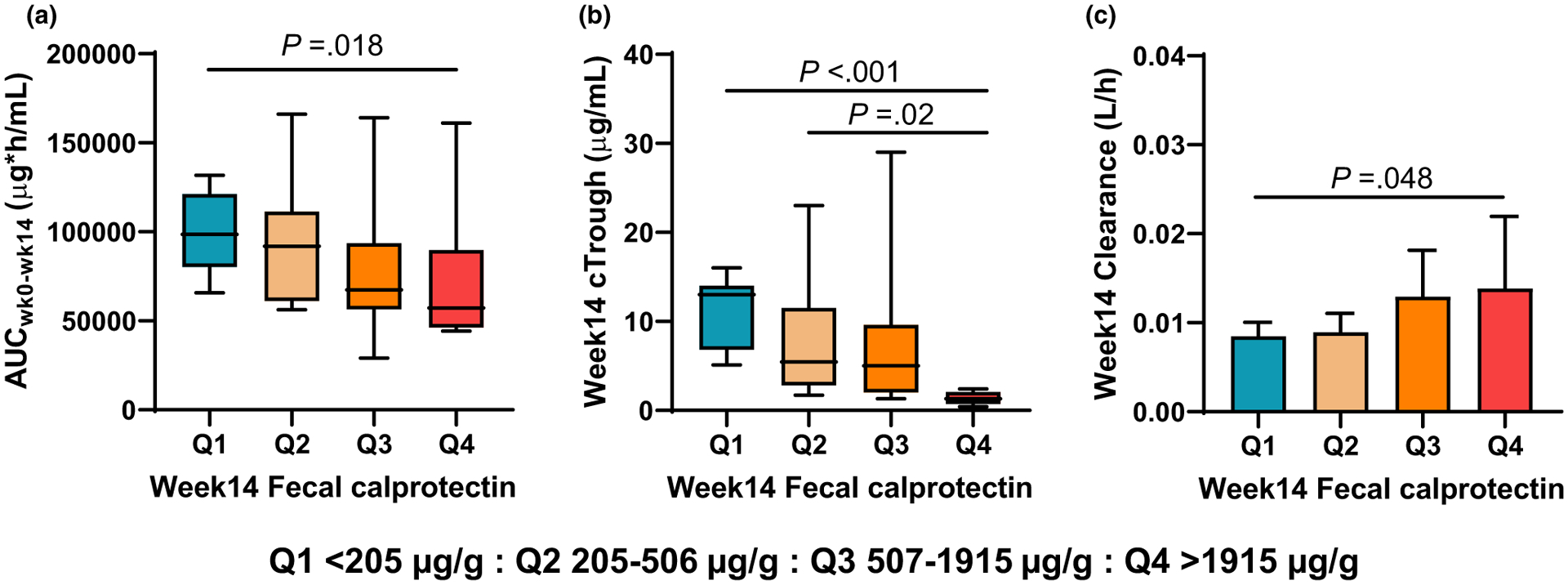
Week 14 fecal calprotectin by quartiles and individual measures of infliximab pharmacokinetics including (a) total induction AUC, (b) week 14 Ctrough, and (c) week 14 drug clearance. Post-tests to analyze between group comparisons were also performed for each measure with the associated Pvalues shown in each panel. AUC, area under the curve; Ctrough, trough plasma concentration.
Pretreatment predictors of subtherapeutic exposure during induction
Univariate logistic regression identified nCD64 > 6 (OR 4.5, 95% CI 1.4–17.8), ESR > 30 mm/h (OR 3.8, 95% CI 1.4–11), age < 10 years old (OR 4.2, 95% CI 1.2–20), and weight < 30 kg (OR 6.6, 95% CI 2.1–25) as pretreatment predictors for subtherapeutic end of induction AUC (< 79,348 μg*h/mL; Table S1). Multivariate regression with backward stepwise variable selection identified weight < 30 kg and ESR > 30 mm/h as independent pre-infliximab predictors for a subtherapeutic AUC during induction.
Practical application of infliximab pharmacokinetics
Throughout the study, our team followed a structured design and implementation process with software engineers, application designers, gastroenterologists, and pharmacologists to create an EHR-integrated PK dashboard (RoadMAB) that provides clinicians with access to model-informed precision dosing at the bedside. With multiple rounds of discovery interviews and design feedback sessions with CCHMC clinical faculty (gastroenterologists and pharmacologists), an interactive prototype was developed for further user testing. Guided by the insights collected from the user testing sessions, the final RoadMAB user interface display was incorporated and fully integrated within Epic by Insight and CCHMC IS.
User selection of the RoadMAB tab within Epic calls on the PK software program to access past drug administration records (date, time, and dose) and laboratory results to conduct Bayesian PK modeling16 and display previous dosing intervals (weeks and days) along with the predicted PK for the next two future infliximab administrations (Figure 5). In addition to the predicted PK curves for the individual patient (informed by the observed Ctrough concentrations), the user can modify the target range for Ctrough and view past laboratory results. Instantaneously, the dashboard will propose up to three dosing regimens to achieve the user-selected Ctrough target (the model defaults to target the mean concentration within the selected target range in μg/mL; Figure S5). These dosing recommendations are generated for (1) the current dose and interval, (2) by a dashboard automated (optimized) process, and/or (3) for the user selected dose and interval. Enhanced features of the PK dashboard include user selection of any published infliximab PK model to conduct the Bayesian estimations, provide corrections (residual error) for up to four commercially available drug assays and a “new start wizard” (not shown) for model-informed precision dosing during induction.
Figure 5.
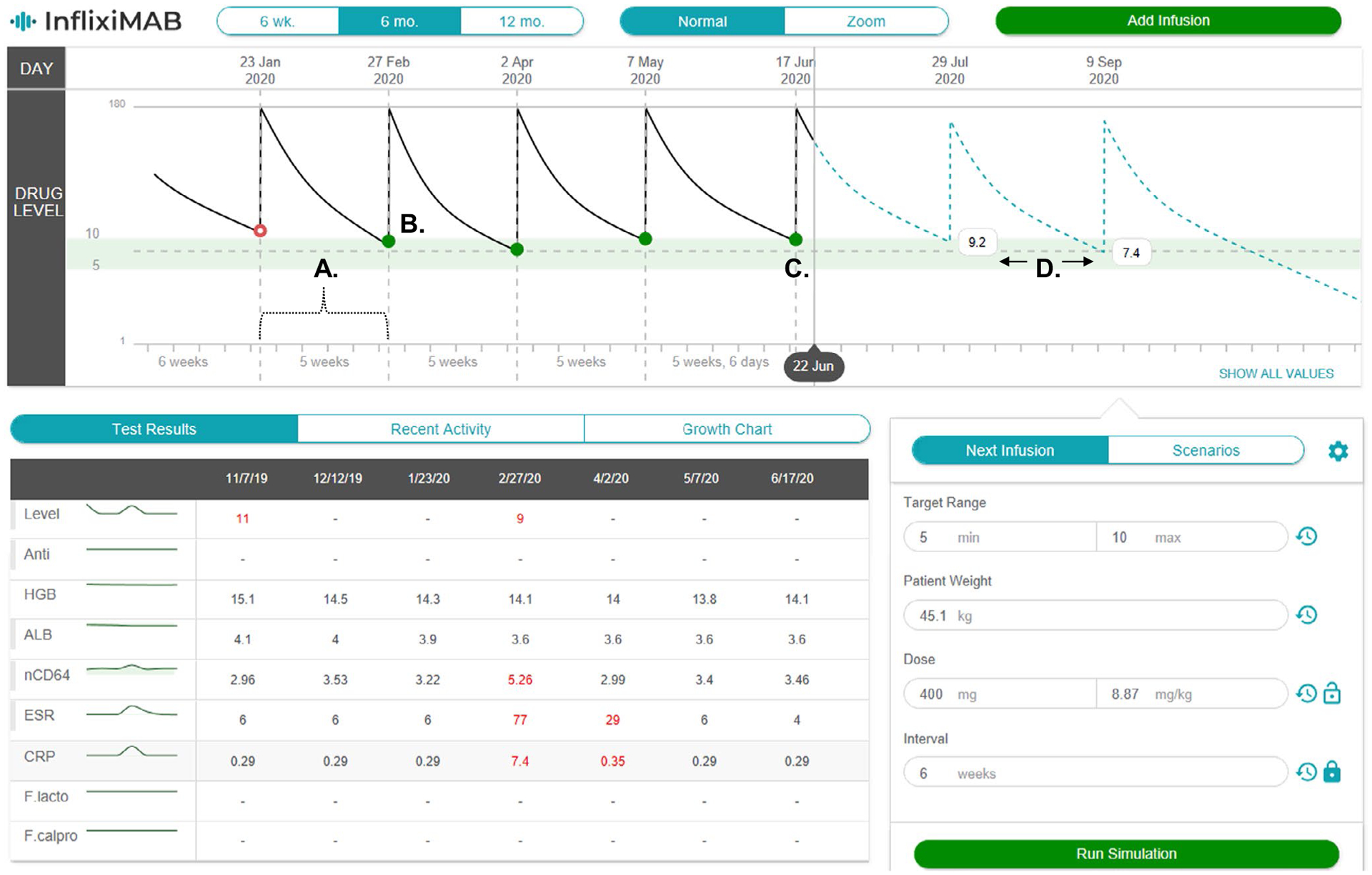
The infliximab pharmacokinetic (PK) dashboard is shown as a screen shot from the electronic health record. The patient’s previous infusion (a) intervals (weeks and days), (b) measured (actual) trough concentrations, (c) the model-predicted trough concentration from the last infusion, and (d) model-predicted trough concentrations and PK curves (blue dashed-line) for the next two infusions are shown. The dashboard is equipped with an adjustable target range (green-shaded) for trough concentrations and a display of previous laboratory results in the lower left panel. ALB, albumin; ESR, and erythrocyte sedimentation rate; nCD64, neutrophil CD64.
DISCUSSION
In this real-world PK study, we report a novel induction AUC target and identified four laboratory biomarkers of infliximab CL. In clinical practice, these additional biomarkers will not only identify those at risk for subtherapeutic exposure, but also improve the predictive performance of PK modeling to guide dose optimization strategies. Finally, we designed and embedded an interactive, decision-support PK dashboard within the EHR to streamline precision dosing at the bedside in real-time.
There is a critical need for a real-world PK investigations as infliximab monotherapy is favored over combination therapy in pediatric IBD practices in North America with an alarming rate of subtherapeutic infliximab levels with as-labeled dosing.6,17 Additionally, although the PK parameters are comparable to previously published PK models in CD,11,18 our study has notable improvements in study design and model prediction. Specifically, we prospectively enrolled patients with CD across four medical centers and collected longitudinal blood samples to conduct a more thorough PK analysis for patients receiving infliximab monotherapy. Moreover, with the inclusion of ESR, ATI, and nCD64 as continuous variables, the prediction accuracy improved with less unexplained variability in comparison to the Bauman et al. model.18
In the Fasanmade et al. study, the variability reported was between-subject variability in CL (25.2% CV), V1 (16.3% CV), V2 (34.9% CV), interoccasional variability in CL (21.9% CV), and residual error that is comprised of both proportional (27.5% CV) and additive error (0.244).11 In our analysis, we used time-varying covariates and, therefore, did not identify interoccasional variability. We have reduced between subject variability to only CL (25.1% CV). The between-subject variability of the other PK parameters was estimated to be close to the boundary and thus fixed at zero. Although it is difficult to directly compare the variabilities between the two models, the PK parameter estimates reported here are more clinically relevant for pediatric patients in real-world practice as precision dosing may rely on the prediction accuracy of the model before drug concentrations are measured. This is especially key during the induction phase when drug concentrations may not be available and the current model can be informed by the blood biomarkers (ESR, nCD64, and serum albumin) to suggest an adequate dose to minimize the probability of a subtherapeutic drug concentration and immunogenicity.
Use of a drug-tolerant assay to detect ATI in this study permitted the evaluation of drug CL as a continuous, binary, or ordinal measure. Among them, including ATI as a continuous measure in the current model, was superior in reducing between-subject variability (more robust) than the previously published PK model by our center.18 The monotonic relationship of ATI and CL indicates that ATI defined as a binary or ordinal covariate may not timely reflect the change in CL.
CRP and ESR remain the most commonly utilized markers to detect inflammation in pediatric CD, however, both have limitations in accuracy.19,20 Although CRP has been identified as a predictor of infliximab response and infliximab CL in a univariate analysis,21 CRP did not meet the strict inclusion criteria (OFV) in our stepwise model development and has not been included in other infliximab PK models following the same rigorous analysis.11,18,21 In our analysis, the difference in significance between ESR and CRP could have been related to the number of missing values for CRP (27.4%) compared with ESR (13%) and the high rate of normal CRP values (45.6%). We recognize that a practical limitation of our study is the inclusion of nCD64 as a covariate of CL as nCD64 testing is not widely available. Although nCD64 is not required for predictions, including nCD64 in the PK model, significantly improved the model performance following rigorous analysis (drop in OFV of 24.616, P < 0.001) and attributable to its reliability as a biomarker of CD-related intestinal inflammation.22 In fact, significant increases in nCD64 (> 12.2, the 95th percentile of measured nCD64 values) resulted in an ~ 18% increase in drug CL.
Although we acknowledge the limitation of developing AUC targets without evaluating mucosal healing with repeat colonoscopy, the induction AUC target of 79,348 μg*h/mL was identified for patients with a fCal < 250 μg/g, a strict criterion considering the median pre-infliximab fCal was 1611 μg/g (IQR 994–2501). As a surrogate to endoscopic remission, we also identified an induction AUC target of 96,307 μg*h/mL for deep remission. As it is not standard practice to perform colonoscopy following induction, it is possible the cutoff point estimates that were established for AUC and CL in this cohort would be different if endoscopic healing was used as the dependent variable. Additionally, in a post hoc analysis of the TAILORIX clinical trial, fCal correlated with endoscopic outcomes, and was a responsive pharmacodynamic biomarker after infliximab dose escalation.23 Because repeat endoscopy is not standard-of-care during induction therapy, improvements in AUC and fCal between infusions appear to be feasible surrogate markers for mucosal healing. Last, agreement between the enzyme-linked immunosorbent assay and ECLIA based methods have been shown for both infliximab concentrations and ATI,24 whereas there is a paucity of data directly comparing between other laboratory techniques for ATI detection. As we found, ATI as a continuous measure was more informative for model prediction, our study further highlights the need to cross-validate and standardize the units of measure of ATI detection between all commercially available assays (including the homogenous mobility shift assay) as our current model is limited to ATI quantification with the ECLIA method.
Our team created the PK dashboard to provide clinicians with access to complex Bayesian modeling for precision dosing. The PK dashboard has been integrated within the EHR at CCHMC (Epic) and the application programming interface has been constructed to integrate with other EHRs at any medical center. The advantage of using PK software to optimize infusion regimens is to combine the rigorous PK analyses conducted during model development with the individual patient data to seamlessly produce data-informed simulations in real-time. The PK dashboard directly applies the established model as part of a Bayesian estimator, which updates individual PK parameters when concentration results become available (at any time point, either induction or maintenance). Bayesian estimators have been validated and successfully used for many different drugs, including infliximab.25 Although NONMEM can also generate post hoc Bayesian estimates, the goal of our study was not to compare the dashboard with NONMEM (although the results are expected to be similar as shown in a previous study),26 but to utilize NONMEM to generate the population estimates of PK parameters and identify significant covariates. With more recent studies finding improved outcomes with proactive therapeutic drug monitoring in pediatric IBD,27,28 it is anticipated that more frequent use of therapeutic drug monitoring will continue for patients receiving any biologic to ensure therapeutic exposure and a timely assessment of primary nonresponse.
In conclusion, there are limited biologic therapies to manage pediatric patients with CD and previous studies have shown an alarming incidence of subtherapeutic infliximab Ctrough and ATI with as-labeled dosing regimens in the pediatric CD population.6,10 In this study, we identified laboratory biomarkers of infliximab CL and exposure cutoff points associated with favorable outcomes and have shown that individual dosing predictions are enhanced with our PK model. It will be critical for future studies to evaluate whether receiving dashboard enhanced dosing regimens not only have superior drug exposure, but also evaluate the effect precision dosing has on ATI development and rates of endoscopic and transmural healing.
Supplementary Material
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Previous studies have found a high incidence of subtherapeutic infliximab trough concentrations and development of antibodies to infliximab in children with Crohn’s disease receiving standard (as-labeled) dosing regimens.
WHAT QUESTION DID THIS STUDY ADDRESS?
We conducted a real-world pharmacokinetic (PK) analysis of infliximab as prior PK studies have focused on clinical trial participants who received as-labeled dosing in combination with immunomodulators that are known to alter infliximab clearance. Our primary aim was to identify patient-specific predictors of rapid drug clearance and infliximab exposure targets to inform a precision dosing dashboard.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
We identified blood biomarkers of rapid infliximab clearance, drug exposure targets associated with favorable outcomes and created a PK dashboard for infliximab to guide precision dosing.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
The PK dashboard has been embedded within the electronic health record and seamlessly perform precision dosing with Bayesian PK calculations to minimize subtherapeutic concentrations.
FUNDING
This work was supported by the National Institutes of Health (K23DK105229 (P.M.), R03DK118314 (P.M.), 5T32HD69054 (Y.X.), and T32DK007727 (R.C.)), the Crohn’s and Colitis Foundation (P.M.), and the Academic Research Committee at Cincinnati Children’s Research Foundation (P.M.).
Footnotes
Publisher's Disclaimer: DISCLAIMER
Publisher's Disclaimer: As an Associate Editor of Clinical Pharmacology & Therapeutics, Alexander A. Vinks was not involved in the review or decision process for this paper.
SUPPORTING INFORMATION
Supplementary information accompanies this paper on the Clinical Pharmacology & Therapeutics website (www.cpt-journal.com).
CONFLICT OF INTEREST
N.P. is president of Medimatics, a company that provides consulting services on medical information systems. All other authors declared no competing interests for this work.
References
- 1.Cosnes J, Gower-Rousseau C, Seksik P & Cortot A Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 140, 1785–1794 (2011). [DOI] [PubMed] [Google Scholar]
- 2.Pariente B et al. Development of the Crohn’s disease digestive damage score, the Lemann score. Inflamm. Bowel Dis 17, 1415–1422 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hyams J et al. Induction and maintenance infliximab therapy for the treatment of moderate-to-severe Crohn’s disease in children. Gastroenterology 132, 863–873 (2007), quiz 1165–1166. [DOI] [PubMed] [Google Scholar]
- 4.Casellas F et al. Restoration of quality of life of patients with inflammatory bowel disease after one year with antiTNFalpha treatment. J. Crohns Colitis 6, 881–886 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Kugathasan S et al. Prediction of complicated disease course for children newly diagnosed with Crohn’s disease: a multicentre inception cohort study. Lancet 389, 1710–1718 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarkston K, Tsai YT, Jackson K, Rosen MJ, Denson LA & Minar P Development of infliximab target concentrations during induction in pediatric Crohn disease patients. J. Pediatr. Gastroenterol. Nutr 69, 68–74 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Church PC et al. Infliximab maintains durable response and facilitates catch-up growth in luminal pediatric Crohn’s disease. Inflamm. Bowel Dis 20, 1177–1186 (2014). [DOI] [PubMed] [Google Scholar]
- 8.D’Arcangelo G et al. Predictors of long-term clinical and endoscopic remission in children with Crohn disease treated with infliximab. J. Pediatr. Gastroenterol. Nutr 68, 841–846 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Grossi V et al. Concomitant use of immunomodulators affects the durability of infliximab therapy in children with Crohn’s disease. Clin. Gastroenterol. Hepatol 13, 1748–1756 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Kennedy NA et al. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn’s disease: a prospective, multicentre, cohort study. Lancet Gastroenterol. Hepatol 4, 341–353 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Fasanmade AA, Adedokun OJ, Blank M, Zhou H & Davis HM Pharmacokinetic properties of infliximab in children and adults with Crohn’s disease: a retrospective analysis of data from 2 phase III clinical trials. Clin. Ther 33, 946–964 (2011). [DOI] [PubMed] [Google Scholar]
- 12.Colombel JF et al. Combination therapy with infliximab and azathioprine improves infliximab pharmacokinetic features and efficacy: a post hoc analysis. Clin. Gastroenterol. Hepatol 17, 1525–1532.e1 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Turner D et al. Mathematical weighting of the pediatric Crohn’s disease activity index (PCDAI) and comparison with its other short versions. Inflamm. Bowel Dis 18, 55–62 (2012). [DOI] [PubMed] [Google Scholar]
- 14.Paul S et al. Therapeutic drug monitoring of infliximab and mucosal healing in inflammatory bowel disease: a prospective study. Inflamm. Bowel Dis 19, 2568–2576 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Sherwin CM, Kiang TK, Spigarelli MG & Ensom MH Fundamentals of population pharmacokinetic modelling: validation methods. Clin. Pharmacokinet 51, 573–590 (2012). [DOI] [PubMed] [Google Scholar]
- 16.Neely M & Jelliffe R Practical, individualized dosing: 21st century therapeutics and the clinical pharmacometrician. J. Clin. Pharmacol 50, 842–847 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Church PC, Hyams J, Ruemmele F, de Ridder L, Turner D & Griffiths AM The continental divide: anti-TNF use in pediatric IBD is different in north America compared to other parts of the world. Can. J. Gastroenterol. Hepatol 2018, 3190548 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bauman LE et al. Improved population pharmacokinetic model for predicting optimized infliximab exposure in pediatric inflammatory bowel disease. Inflamm. Bowel Dis 26, 429–439 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vermeire S, Van Assche G & Rutgeerts P Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut 55, 426–431 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alper A, Zhang L & Pashankar DS Correlation of erythrocyte sedimentation rate and C-reactive protein with pediatric inflammatory bowel disease activity. J. Pediatr. Gastroenterol. Nutr 65, e25–e27 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Fasanmade AA et al. Population pharmacokinetic analysis of infliximab in patients with ulcerative colitis. Eur. J. Clin. Pharmacol 65, 1211–1228 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minar P, Jackson K, Tsai YT, Sucharew H, Rosen MJ & Denson LA Validation of neutrophil CD64 blood biomarkers to detect mucosal inflammation in pediatric Crohn’s disease. Inflamm. Bowel Dis 24, 198–208 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dreesen E et al. Monitoring a combination of calprotectin and infliximab identifies patients with mucosal healing of Crohn’s disease. Clin. Gastroenterol. Hepatol 18, 637–646.e11 (2020). [DOI] [PubMed] [Google Scholar]
- 24.Marini JC et al. Comparisons of serum infliximab and antibodies-to-infliximab tests used in inflammatory bowel disease clinical trials of Remicade®. AAPS J. 19, 161–171 (2017). [DOI] [PubMed] [Google Scholar]
- 25.Mould DR, D’Haens G & Upton RN Clinical decision support tools: the evolution of a revolution. Clin. Pharmacol. Ther 99, 405–418 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Eser A et al. Prediction of individual serum infliximab concentrations in inflammatory bowel disease by a Bayesian dashboard system. J. Clin. Pharmacol 58, 790–802 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Assa A et al. Proactive monitoring of adalimumab trough concentration associated with increased clinical remission in children with Crohn’s disease compared with reactive monitoring. Gastroenterology 157, 985–996.e2 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Fernandes SR et al. Proactive infliximab drug monitoring is superior to conventional management in inflammatory bowel disease. Inflamm. Bowel Dis 26, 263–270 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


