Abstract
Petrochemical-based plastics have not only contaminated all parts of the globe but are also causing potentially irreversible damage to our ecosystem, due to their non-biodegradability. As bioplastics are limited in number, there is an urgent need to design and develop more biodegradable alternatives to mitigate the plastic menace. In this regard, we report aquaplastic, a new class of microbial biofilm-based biodegradable bioplastic that is water-processable, robust, templatable and coatable. Herein, Escherichia coli was genetically engineered to produce protein-based hydrogels, which are cast and dried under ambient conditions to produce aquaplastic that can withstand strong acid/base and organic solvents. In addition, aquaplastic can be healed and welded to form three-dimensional architectures using water. The combination of straightforward microbial fabrication, water-processability, and biodegradability make aquaplastic a unique material worthy of further exploration for packaging and coating applications.
Introduction
Living cells have a remarkable capability to make complex molecules, materials and minerals from abundantly available benign components under ambient conditions. With advances in biomanufacturing and synthetic biology, living cells are being engineered to manufacture a wide range of chemicals, drugs, and fuels1. This microbially-based biomanufacturing strategy extends into polymers, which are an attractive way to make biodegradable bioplastics. In the last few years, the concept of living factories has extended to include not just molecular biosynthesis, but biologically directed materials fabrication, leading to the emergence of a new field entitled engineered living materials (ELMs)2–4. To date, ELMs that can bind to synthetic surfaces, catalyze reactions, sequester chemicals and be used for many other applications have been demonstrated5–12. Herein, we present the first report of engineered microbial biofilms to produce a new class of bioplastic, which we have termed aquaplastic, as an alternative composition and approach to biodegradable bioplastics fabrication.
Plastic pollution is a worsening environmental problem that stems from the inherent non-degradability of most conventional plastics, leading to their accumulation in landfills, oceans, and waterways13. Several approaches have been proposed to address this global challenge, including the development of next-generation recycling technologies and biodegradable bioplastics. A niche approach involves the use of water-soluble or water-dispersible plastics in applications where water resistance is not a main requirement, for example in primary packaging. Polyvinyl alcohol (PVA) is the most prevalent water-soluble plastic that is used in a wide range of applications related to packaging and coatings14. Although bulk materials composed of PVA can dissolve in water, the polymer is synthesized from petrochemically derived ethylene by free-radical polymerization. Moreover, PVA has been consistently found to have limited biodegradation (several months to years) in natural-solid matrices such as soil and compost, as well as in waterbodies that lack PVA degrading microbes14. Plastics that can be produced from biological components in water offer greener fabrication methodologies than those obtained from synthetic and non-biodegradable components in non-aqueous media. Moreover, microbially-derived biodegradable bioplastics are attractive alternatives to conventional plastics as they offer a more holistically sustainable life cycle.
Here, we demonstrate aquaplastic as a new water-processable bioplastic that is fabricated directly from bacterial culture with minimal purification and can easily be processed from a hydrogel state to form bulk materials (Fig. 1a–c). The mechanical properties of aquaplastic are comparable to petrochemical plastics and other bioplastics. Additionally, aquaplastic possesses unique water-processable characteristics that we term aqua-molding, aqua-welding and aqua-healing.
Figure 1. Fabrication of aquaplastic directly from engineered microbial biofilms.
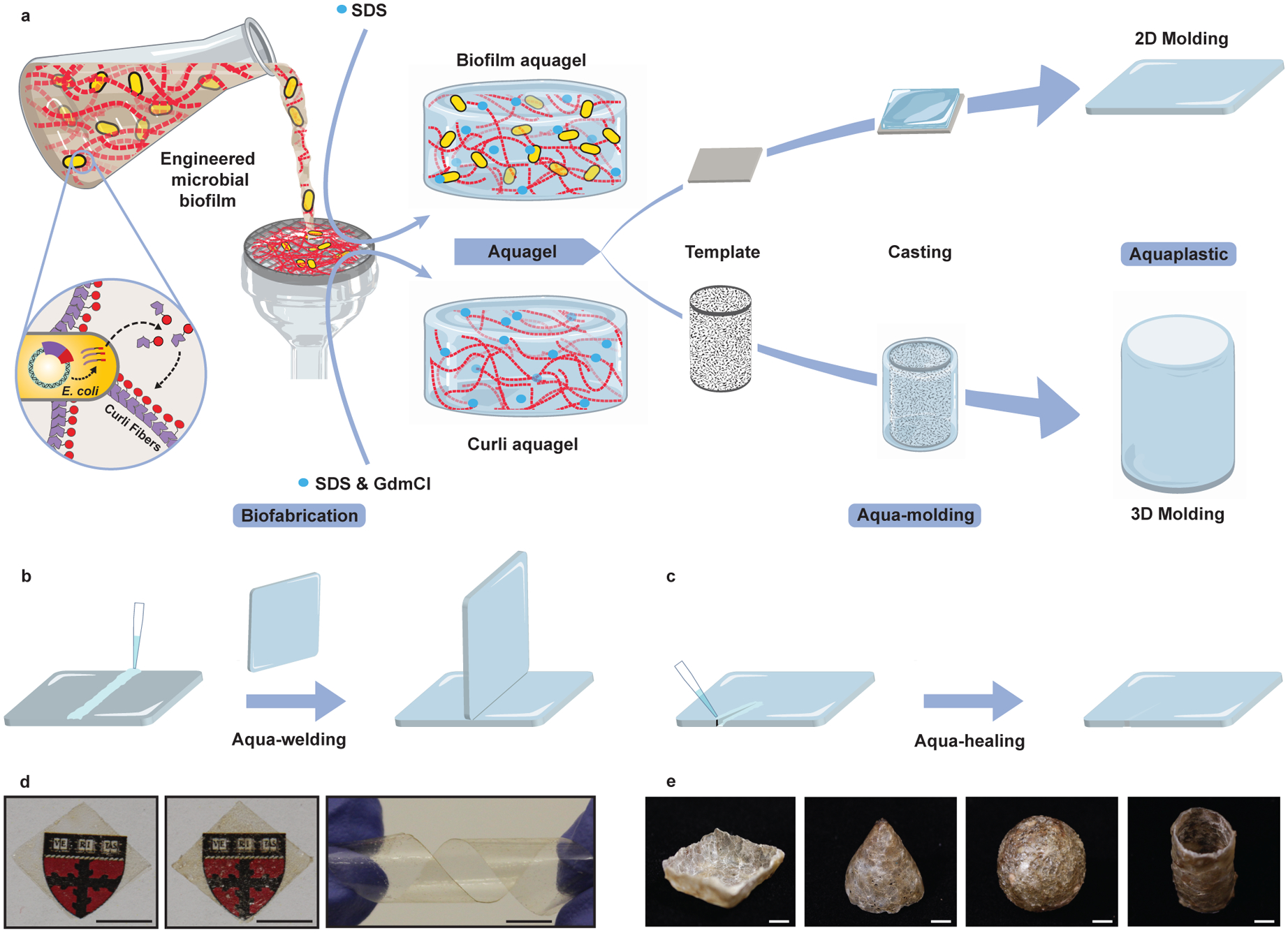
a–c. Schematics of aquaplastic fabrication from genetically engineered bacteria programmed to produce a functional curli fiber-based aquagel that can be molded into 2D and 3D architectures (a), and aqua-welding (b) and aqua-healing (c) of aquaplastics. SDS: sodium dodecyl sulfate; GdmCl: guanidinium chloride. d. Images of biofilm aquaplastic (left), curli aquaplastic (middle), and the flexibility of biofilm aquaplastic (right). Scale bar, 0.5 cm. e. Images of 3D-molded aquaplastic: cone, bowl, sphere and cylinder. Scale bar, 1 cm.
Results
Fabrication of aquaplastic.
Aquaplastic is composed of recombinantly produced biofilm matrix proteins. Biofilms are communities of microbial cells embedded in an extracellular matrix that provides them adhesion capabilities, and protection from the surrounding environment15. We leverage the curli system of E. coli, which is the primary proteinaceous component of its biofilm matrix, formed by the secretion and extracellular self-assembly of CsgA protein monomers into a fibrous mesh16. In previous work, we have demonstrated that a refactored curli expression system can serve as a platform for the biological fabrication of numerous functional materials2,7,8,11,17. In one specific example, fusions of the curli fiber monomer, CsgA, to a human cytokine, trefoil factor 2 (TFF2), enabled us to harvest hydrogels simply by filtering bacterial cultures, using sodium dodecyl sulfate (SDS) as a gelator (Supplementary Table 1 and Supplementary Figure 1)18,19. In this work, we denote these hydrogels as either biofilm aquagels, which contain viable bacterial cells, or curli aquagels, which are inert gels from which the cells have been removed. Here we show that both types of gels can be cast onto surfaces or molds to create plastic films, which we call biofilm aquaplastic and curli aquaplastic, respectively.
Unlike traditional thermoplastics and thermoset plastics, which are melted at high temperatures to form semi-solids that are then molded into desired shapes, aquaplastics are made by casting the aquagels on a template with the desired shape and drying them under ambient conditions to mold their form. When the biofilm/curli aquagels are cast on a flexible or soft flat surface, they form plastic films that can be easily peeled off to form free-standing structures (Fig. 1d, Supplementary Figure 1). For the biofilm aquaplastics, these films were flexible enough to be twisted repeatedly into helical shapes without wrinkling or cracking (Supplementary Figure 2). Because of the remarkable robustness of the constituent curli fibers, the biofilm aquaplastics can also be molded into three-dimensional architectures, like cones, bowls, tubes, and hollow spheres by casting them on a sacrificial polystyrene foam mold, followed by selective dissolution of the mold (Fig. 1e, Supplementary Figure 3).
Physical and chemical properties of aquaplastic.
Thermogravimetric analysis (TGA) showed that both the biofilm aquaplastic and curli aquaplastic start to degrade above 200 °C (Fig. 2a, Supplementary Figure 4). Differential scanning calorimetric (DSC) analysis of aquaplastics revealed glass transitions for biofilm aquaplastic and curli aquaplastic at 150 °C and 145 °C, respectively, during the cooling cycles (Fig. 2b, Supplementary Figure 5). Wide-angle X-ray scattering analysis (WAXS) on both biofilm aquaplastic and curli aquaplastic revealed the characteristic signatures of cross-β secondary structure of CsgA with d-spacing values of 0.98 nm and 0.46 nm (Fig. 2c, Supplementary Figure 6), corresponding to the inter- and intra-β-sheet distances, respectively, and indicating that the curli amyloid structure is maintained through the aquaplastic processing steps20. The mechanical properties of the aquaplastics were first investigated by nanoindentation, which showed Young’s modulus values, E, of 2.2 ± 0.7 GPa (biofilm aquaplastic) and 4.3 ± 0.9 GPa (curli aquaplastic), respectively (Fig. 2d–g)21. The corresponding hardness, H, values at maximum load were found to be 167 ± 109 MPa and 252 ± 140 MPa, while the yield strength, σy, values (estimated using the relation σy = H/3) were 55 ± 36 MPa and 84 ± 47 MPa, respectively22. Tensile tests performed on aquaplastic films clearly showed the plastic deformation of the material (Fig. 2h). The biofilm aquaplastic exhibited E of 1 ± 0.2 GPa (Fig. 2i), ultimate tensile strength, σ, of 18 ± 5 MPa (Fig. 2j) and tensile toughness, UT, of 102 ± 57 MJm−3 (Supplementary Figure 7). On the other hand, the curli aquaplastic was found to have E, σ, and UT of 1.2 ± 0.2 GPa, 29 ± 5 MPa and 261 ± 77 MJm−3, respectively. In Supplementary Table 2, we compare the mechanical properties of biofilm aquaplastic and curli aquaplastic with that of petrochemical plastics and other bioplastics.
Figure 2. Physical and chemical properties of aquaplastics.
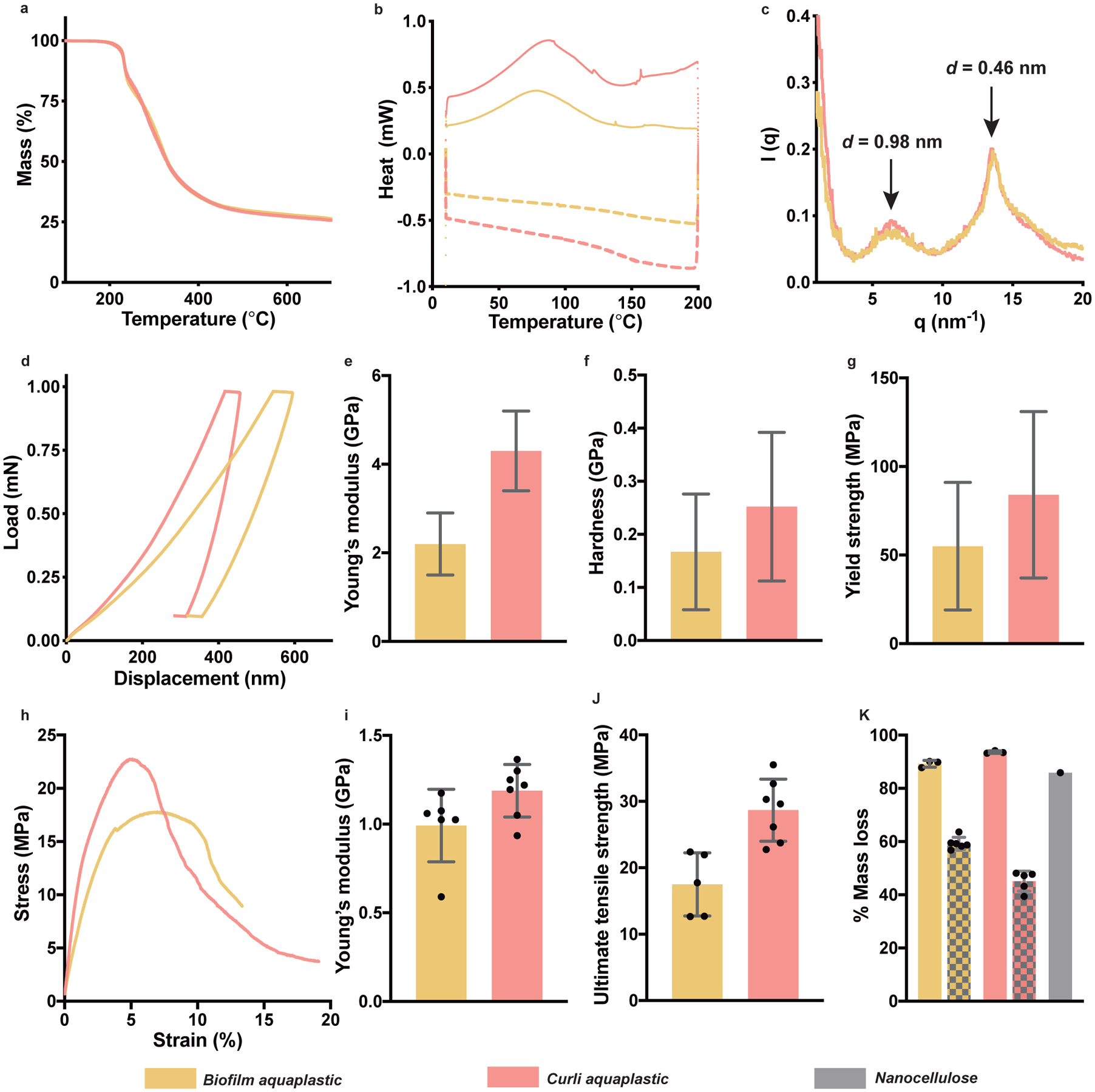
a. Thermogravimetric analysis (TGA) thermograms. b. Differential Scanning Calorimetry (DSC) thermograms. Heating cycle: solid line and cooling cycle: dashed line. c. Wide-angle X-ray scattering (WAXS) intensity profile of biofilm aquaplastic and curli aquaplastic. d-g. Mechanical properties of biofilm aquaplastic and curli aquaplastic obtained by nanoindentation tests: load–displacement curves under a maximum indentation load of 1 mN in a single indentation cycle (d), Young’s modulus (e), hardness (f), and yield strength (g). n=128 for biofilm aquaplastic and n=82 for curli aquaplastic. h-j. Mechanical properties of biofilm aquaplastic and curli aquaplastic obtained by tensile tests: stress-strain curves (h), Young’s modulus (i), and ultimate tensile strength (j). k. Biodegradation analysis of biofilm aquaplastic and curli aquaplastic, compared to nanocellulose, based on mass loss after exposure to a wastewater-derived microbial consortium. The patterned bars correspond to mass loss of biofilm aquaplastic and curli aquaplastic due to dissolution of water-soluble sub-components in water. The bar graphs represent mean values, and the error bars are standard deviation.
The biodegradability of the aquaplastic was investigated under aerobic conditions by incubating them for 45 days in the presence of a mixed culture of microorganisms obtained from the primary effluent of a wastewater treatment plant23. Microorganisms in the mixed culture media metabolize samples into biogas and water-soluble species (i.e., ions or simple protein/sugars) resulting in mass loss for each sample. The aquaplastics were compared to nanocellulose, which is known to undergo rapid and complete biodegradation. Both biofilm aquaplastic and curli aquaplastic were found to lose 94% and 89% of their initial masses, respectively, over the course of 45 days, while the positive control, nanocellulose lost 85% mass over the same time span (Fig. 2k). As negative controls, biofilm aquaplastic and curli aquaplastic were incubated in milliQ water without the degrading microorganisms, wherein we obtained nearly 59% and 45% mass loss, respectively, that could be attributed to dissolution of SDS and other water-soluble cellular components. Indeed, square sheets of biofilm aquaplastic and curli aquaplastic, when immersed in water, swell to 324% and 225% in terms of their lateral dimensions, respectively (Supplementary Figure 8). After several water washes, both aquaplastic films shrink back to their original dimensions, leaving behind transparent and water-insoluble films of curli nanofibers. Therefore, the mass losses observed in the biodegradation experiments (Fig. 2k) reflect a composite of dissolution and biodegradation of the remaining protein-enriched material.
Although the aquaplastics are biodegradable, their constituent curli fibers are also known to be resistant to disassembly in the presence of solvents, harsh pH values and detergents24. We found that both aquaplastics were stable in non-polar solvents like n-hexane, even after 24 hours of incubation (Fig. 3a, Supplementary Figures 9 and 10). Aquaplastics were also stable in chloroform, which is known to dissolve coatings, rubber, and many plastics such as polystyrene and polyvinyl chloride (Fig. 3a, Supplementary Figure 9). The weight of aquaplastics did not change appreciably after incubation in the organic solvents (Supplementary Figure 9). Surprisingly, both aquaplastics remained intact and unchanged after 24 h in 18 M sodium hydroxide (Fig. 3a, Supplementary Figure 11). On the other hand, upon incubation in concentrated sulfuric acid (98%), both aquaplastics formed gelatinous films with double the original film dimensions within a few minutes (Fig. 3a, Supplementary Figure 11). The biofilm aquaplastic disintegrated into smaller fragments, while the curli aquaplastic lost ~45% of its mass in concentrated sulfuric acid (Supplementary Figure 9). Similar exposures to concentrated nitric acid (70%) and concentrated hydrochloric acid (35%), led to a slight yellow coloration and degradation of both aquaplastics within a couple hours (Supplementary Figures 11 and 12). Thus, it might be that the Na+ and sulfate groups of SDS in association with the curli fibers and other components of aquaplastic, provides a protective barrier in acidic and basic conditions. Aquaplastic films incubated in sodium hydroxide had salts precipitated on their surface, which we could not remove completely, and accurate weight values could not be determined for mass loss analysis.
Figure 3. Chemically resistant aquaplastic and their templated surface structure properties.
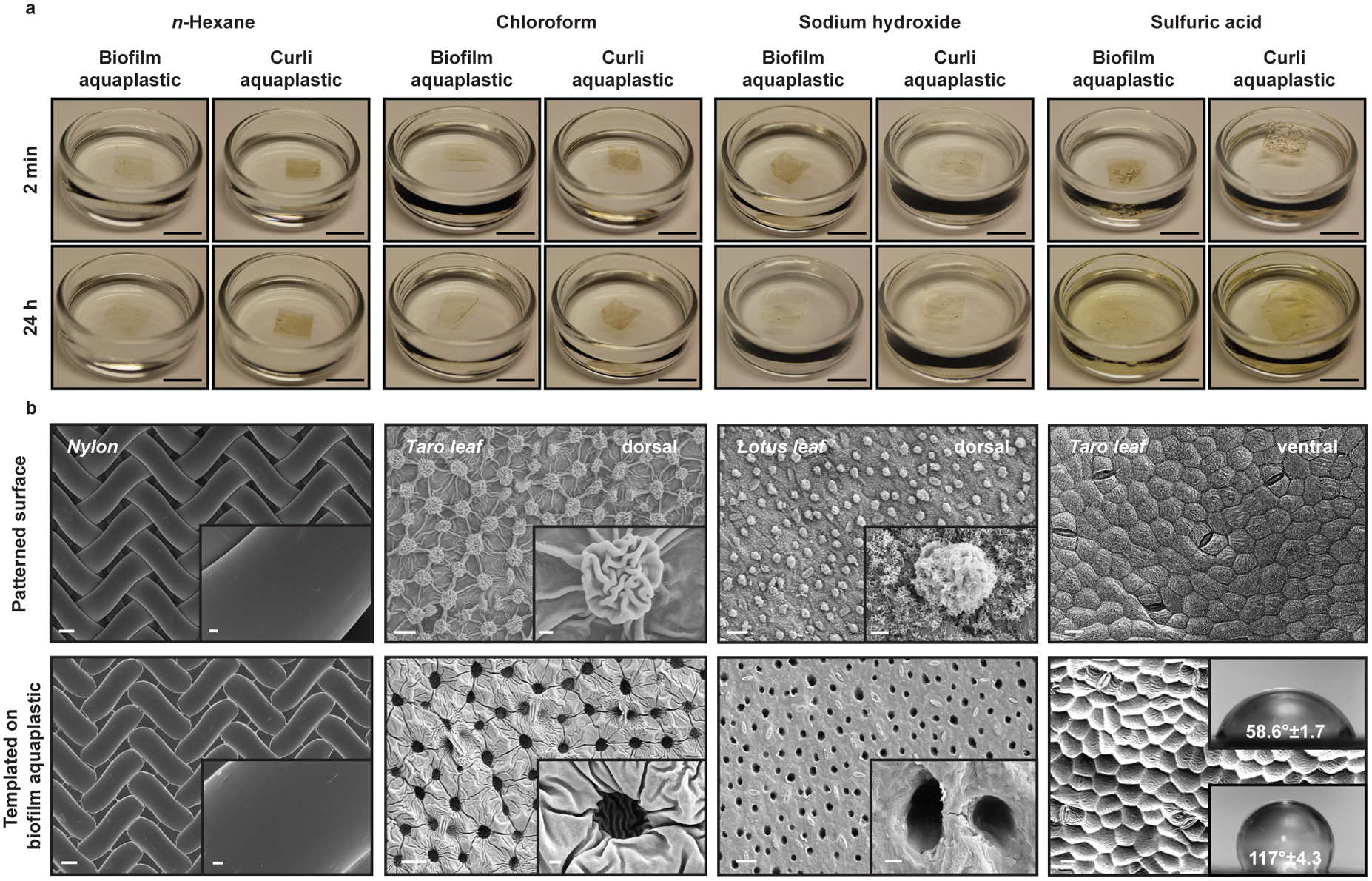
a. Images showing the stability of a 1 cm2 sample of aquaplastic in organic solvents (n-hexane, chloroform), strong acid (98% sulfuric acid) and strong base (18M sodium hydroxide) after 2 minutes or 24 hours of exposure. Scale bar, 1 cm. b. FESEM images show the patterns transferred (bottom row) on biofilm aquaplastic by templating biofilm aquagel on various surfaces (top row) by ambient drying. Scale bar, 20 μm. Insets in the first three columns show higher magnification of surface features. Scale bar, 2 μm. Insets in the last column are water contact angles for non-templated (top) and templated aquaplastic surfaces (bottom), showing increased hydrophobicity.
Templated and coatable aquaplastic.
The chemical resistance of aquaplastics and the known adhesive properties of curli fibers further inspired us to fabricate protective coatings, which are currently made with the non-biodegradable plastics9. Unlike traditional plastic powder-based coating strategies25, aquaplastic coatings were formed by casting aquagels onto various surfaces followed by air-drying under ambient conditions. Aquaplastics could be applied easily to create strongly adhered coatings on convoluted surfaces, like leather or plywood, or on flat surfaces, such as mobile phone touch screens, aluminum automobile exterior body parts, and copper wire (Extended Data Figure 1). The results not only demonstrate the versatility of aquaplastics to conform to different shapes (1D wires and 2D surfaces) but also their ability to firmly adhere to materials with a range of roughnesses and compositions (e.g. biomaterials, electronics, metals).
Another feature of aquaplastic fabrication is the ability to template patterns onto its surface during the drying process. For aquaplastics of either type, drying of the gel precursors on nylon meshes composed of 30–35 μm width fibers, led to transfer of the mesh pattern onto the dried film with high fidelity (Fig. 3b and Supplementary Figure 13). More irregular patterns could also be templated onto aquaplastics this way. Leaves of Colocasia esculenta (i.e. taro), are known to be hydrophobic on their dorsal side due in part to their surface nanotopography. After casting aquaplastics on the leaf and peeling them off to form the self-standing films, we observed pockets formed on the film surface by the 10–15 μm mushroom-like structures on the leaf surface (Fig. 3b), along with other features down to ~1 μm. We tested the size resolution limits of this templating by casting aquaplastics on the dorsal surface of a Nelumbo nucifera (i.e. lotus) leaf. In addition to the characteristic papillae structures (pillars with 5–10 μm diameters) from the leaf surface, the aquaplastics successfully captured leaf surface features as small as ~30 nm, originating from waxy crystalline aggregates (Fig. 3b, Supplementary Figure 13 and 14). This templating capability could also be leveraged to alter surface wettability, with aquaplastics cast on the ventral surface of the hydrophobic taro leaf being imparted with hydrophobic characteristics (contact angle of 117° ± 4.3°), which result from the leaf nanotopography, compared to non-templated surfaces (contact angle of 58.6° ± 1.7°) (Fig. 3b)26.
Water-processable aquaplastic.
Although the tendency of aquaplastics to rehydrate upon exposure to water hinders their direct comparison to petrochemical plastics for certain applications27,28, it does lead to unusual and possibly beneficial material performance characteristics. We exploited the water-responsiveness of the aquaplastics to demonstrate their ability to self-heal. Scratches made on the surface of a biofilm aquaplastic could be removed completely by spraying a few microliters of water onto the scratch and allowing it to air dry for 2 minutes (Fig. 4a). Full-thickness cuts in the biofilm aquaplastic could be healed in a similar manner. Curli aquaplastic could also heal but slightly less efficiently, showing “scars” after the aqua-healing process, when analyzed by FESEM (Supplementary Figure 15). Using a similar protocol, independently fabricated aquaplastic films could be “aqua-welded” together using only water to trigger their adhesive properties. Rectangular aquaplastic films could be attached to one another in perpendicular and parallel (overlapping) orientations just by spraying water at the interface and allowing for 2 min of drying (Fig. 4b and Supplementary Figure 16). Tensile tests on laterally aqua-welded films exhibited E of 605 ± 206 MPa indicating that aquaplastic can be welded effectively with water (Fig. 4c,d). This strategy could be leveraged to create self-standing three-dimensional structures, such as a miniature house with a 4 cm × 4 cm footprint, composed of ten aqua-welded panels (Fig. 4e). Notably, the aqua-welded house was strong enough to support its own weight for at least 18 months after its original construction.
Figure 4. Aqua-healing and aqua-welding of aquaplastic.
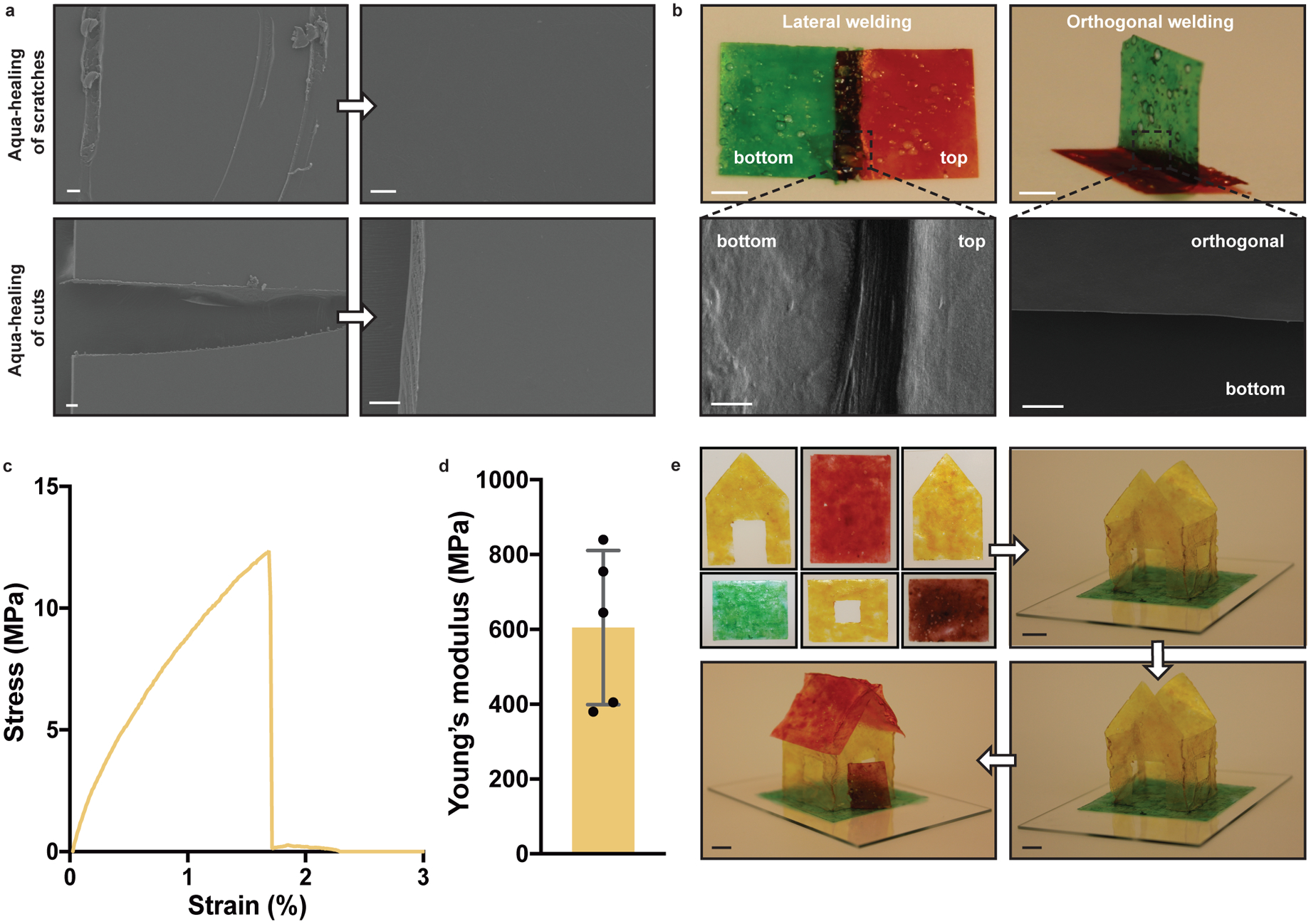
a. Scratches and cuts of biofilm aquaplastic were aqua-healed by adding water at the site of abrasion. Scale bars, 20 μm. b. Aqua-welding of two biofilm aquaplastic panels in parallel and perpendicular orientations. Scale bars for optical images, 5 mm and for FESEM images, 20 μm. c. Stress-strain curve of aqua-welded biofilm aquaplastic. d. Young’s modulus obtained by tensile tests of aqua-welded biofilm aquaplastic. (n=5; bar graph represents mean; error bars represent standard deviation) e. A three-dimensional architecture, aquaplastic house, constructed by aqua-welding two-dimensional panels. Scale bars, 1 cm.
Discussion
None of the properties described for the aquaplastics above apply to the wild-type CsgA protein, despite its ability to self-assemble into fibrous structures. We found that both the fused TFF2 (or other protein) domains and the presence of SDS in the washes was necessary to arrive at a hydrogel, which, when dried, exhibited moldable properties. Our preliminary data support the conclusion that both of these factors contribute to the formation of a hydrated network of curli fiber polymers that adopts a plastic state upon drying, whereas in the absence of either feature (CsgA fusion or SDS-induced gelation), the protocol led to brittle films that crack upon drying (Supplementary Figure 17). These observations are also consistent with SDS’s known role as a gelator, though this is the first report of its role as a plasticizer.
In general, solvent-cast biopolymer films often form amorphous (soft) or crystalline (rigid) structures upon drying, hindering their utility as plastics that can hold a three-dimensional shape29–33. However, several proteins (e.g. silk, suckerein), polysaccharides (e.g. chitin, starch) and their blends have been shown to form bioplastics34–36. Aquaplastic technology provides an opportunity to fabricate three-dimensional structures without having to do rigorous purification or material processing. It combines the features of moldability into arbitrary 3D shapes, complete water processability, ambient fabrication, microbial production, and genetic programmability. These properties result in a biomaterial fabrication platform with considerably more versatility in material properties compared to other microbially-derived bioplastics, like polylactides and polyhydroxyalkanoates. Ongoing work will focus on increasing the yield and production scale of the curli fiber-based bioplastics (currently at 100 mg of aquaplastic per liter of bacterial culture) and exploring the use of engineered curli fibers with various fused domains in conjunction with other materials to form composite materials with an even wider range of properties relevant for plastics. By employing optimized chassis with advanced pathways in bioreactors, the production yields can be further increased by 1–2 orders of magnitude. These efforts, in addition to advances in biomanufacturing technology and synthetic biology will provide opportunities for the production of biodegradable plastics from sustainably derived molecular components and energy sources.
Methods
Cell strains and plasmids
Trefoil factor 2 (TFF2) was fused to the C-terminus of CsgA with an intervening 36 amino acid flexible linker. Gene encoding the trefoil factor (cTFF2) were synthesized (Integrated DNA Technologies) and cloned by overlap extension into pET21d vector. The plasmids also contained genes encoding for other proteins necessary for curli biosynthesis, including csgC, csgE, csgF, and csgG. All experiments used cell strain PQN4, an E. coli strain derived from LSR10 (MC4100, ΔcsgA, λ(DE3), CamR).
Preparation of biofilm aquagel
Bacterial cultures were incubated at 37 °C to express engineered curli fibers for 48 h that were concentrated using vacuum filtration through a 47 mm (dia.) polycarbonate membrane with 11 μm pores (EMD Millipore). The concentrated biofilm (engineered fibers) was washed with 25 mL of sterile DI water on the filter membrane. Next, the biofilm was incubated with 5 mL of gelator/plasticizer that is 5% (m/v) sodium dodecyl sulfate (SDS) in water for 5 min, followed by vacuum filtration of the liquid, which was then washed with 25 mL of DI water. Biofilm aquagel that was formed on the filter membrane was collected and stored at 4 °C.
Preparation of curli aquagel
Functional curli fibers were expressed in bacterial cultures for 48 h, which was then treated with guanidinium chloride (GdmCl) to a final concentration of 0.8 M and kept at 4 °C for 1 h. Treated cultures were concentrated by filtration, as described for the biofilm aquagel. Next, the curli fibers were treated with 5 mL of 8 M GdmCl for 5 min, followed by vacuum filtration of the liquid with 25 mL of sterile DI water wash to remove bacteria, cellular proteins and other components. The remaining biomass was treated with 5 mL of an aqueous solution (2 μM MgCl2) of nuclease (Benzonase, Sigma-Aldrich, 1.5 U/mL) for 10 minutes to remove DNA/RNA bound to curli fibers. Finally, curli fibers were incubated with 5 mL of gelator/plasticizer, 5% (m/v) SDS in water for 5 minutes followed by vacuum filtration of the liquid and 25 mL of DI water wash. The curli aquagel was collected and stored at 4 °C.
Fabrication of aquaplastic
2D aquaplastic sheets: Aquagel (biofilm aquagel/curli aquagel) was casted on a flat flexible substrate (plastic wrap, aluminum foil), which upon overnight drying under ambient condition forms aquaplastic (biofilm aquaplastic/curli aquaplastic). Completely dried aquaplastic was carefully peeled off from surface and stored in ambient conditions.
3D aquaplastic architectures: Various shapes of polystyrene molds (cone, bowl, cylinder and spherical) were casted with biofilm aquagels/curli aquagels and left to dry overnight at ambient conditions. The polystyrene molds were selectively removed by dissolving in chloroform to obtain 3D aquaplastic.
Thermal Gravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC)
TGA experiments were performed using a Mettler Toledo TGA/DSC 3+ STARe System. Samples (2.6 – 4.4 mg dry weight) were run at 4 °C min−1 under N2 purging at 50 mL min−1 in alumina crucibles. DSC measurements were done using a Mettler Toledo DSC 1 STARe System. Measurements were run under N2 purging at 30 mL min−1 and at 4 °C min−1 with 1.8 – 4.9 mg sample. Each measurement consisted of a heat – cool – heat cycle in the range of 10 – 200 °C and was performed in perforated aluminum crucibles. Three experiments were performed for each sample condition and a representative curve is reported.
Wide-Angle X-ray Scattering (WAXS)
Wide-angle X-ray scattering (WAXS) experiments were performed using a Rigaku MicroMax-002+ equipped with a microfocused beam (40 W, 45 kV, 0.88 mA) with the λCuKα = 0.15418 nm radiation collimated by three pinhole collimators (0.4, 0.3, and 0.8 mm) in order to obtain direct information on the scattering patterns. The WAXS intensity was collected by a two-dimensional Fujifilm BAS-MS 2025 imaging plate system (15.2 × 15.2 cm2, 50 μm resolution). An effective scattering vector range of 1 nm−1 < q < 25 nm−1 was obtained, where q is the scattering wave vector defined as q = 4π sin θ/λCuKα with a scattering angle of 2θ. Three experiments were performed for each sample condition and a representative curve is reported.
Nanoindentation
Biofilm aquagels and curli aquagels were casted on glass cover slip to obtain a topographical thickness of about 50–100 μm. Nanoindentation studies were performed on the samples using the Agilent Technologies G200 Nanoindenter. The machine continuously monitors the load, P, and the depth of the penetration, h, of the indenter with the resolutions of 1 nN and 0.2 nm, respectively. A Berkovich diamond tip indenter with the tip radius of ~100 nm is used for the indentation. A peak load, Pmax of 1 mN with the loading and unloading rates of 0.2 mN s−1 and a hold time (at Pmax) of 5 s is employed. A minimum of 80 indentations are performed in each case and the average is reported. The P-h curves were analyzed using the Oliver-Pharr method to extract the elastic modulus (E), and the hardness (H) of the samples. The yield strength, σy was estimated using the relation σy = H/3. n=128 for biofilm aquaplastic and n=82 for curli aquaplastic.
Tensile Tests
Tensile measurements were made using a DHR-3 rheometer (TA Instruments, New Castle, DE, USA), configured with tensile grips. Testing was performed under ambient lab conditions. Film specimens were clamped to an initial gauge length of 6 mm. A constant linear deformation of 1 μm per second was applied (1% strain per minute). The surfaces of the grips were modified with tape at the contact area, to avoid damage to the film specimens during loading. Biofilm aquaplastic and curli aquaplastic films of 3cm × 1cm were utilized for tensile tests. For aquawelding, two biofilm aquaplastic films of 1.5cm × 1cm were used and 0.5cm × 1cm of the contact area was aquawelded with water. A minimum of 5 tests for biofilm aquaplastic and curli aquaplastic were obtained and the average is reported.
Biodegradation Assay
Aerobic biodegradability was determined by measuring mass loss after exposure to an aerobic mixed culture of microorganisms obtained from the primary effluent of a wastewater treatment plant (Back River Wastewater Treatment Plant, Baltimore, MD). Bacterial media was made in MilliQ water, and consisted of 10% v/v primary effluent, 200 mg/L sodium acetate trihydrate as a carbon source, and 10% v/v of salt stock (7.18 mM K2HPO4, 2.79 mM KH2PO4, 0.757 mM (NH4)2SO4, 0.0406 mM MgSO4*7H2O) as well as trace elements necessary for bacterial growth. Samples were run alongside a nanocellulose positive control, which is known to readily biodegrade in mixed cultures of microorganisms37. Approximately 50 mg of each sample was added to 36 mL of blank media (i.e., no cells added yet) in a 50 mL conical vial. n=3 for biofilm aquaplastic and curli aquaplastic. Samples were pelletized via centrifuge (4300 rpm, 5 min.) to minimize dispersion in the supernatant, and then 4 mL of primary effluent bacteria was introduced to each vial. Samples were shaken (for aeration) at 125 rpm and 28° C to encourage aerobic bacterial growth. After 45 days of exposure, bacterial supernatant was carefully removed via pipette to avoid disturbing the pellet in the bottom of the vials. This was crucial to avoid the pelletization of biomass and natural organic matter (NOM) into the residual bioplastic samples after biodegradation. At this point, the large majority of NOM and biomass from the vial was removed, allowing for washing of the pellet with 3×30 mL milliQ water, re-centrifuging between each wash without adding artificial mass to the pellet. After washing, the pellets were recovered and dried before massing. Samples were dried first on a hot plate, and then in a vacuum oven. Biodegradation was reported as the percentage of mass lost after 45 days of biodegradation compared to the initial sample mass. Freeze-dried cellulose nanofibrils (CNFs) derived from wood pulp were purchased from the University of Maine Process Development Center. CNFs were milled to a powder before biodegradation with a FlackTek Speed Mixer (DAC 150) using 2 mm yttrium-stabilized zirconium milling beads.
Field Emission Scanning Electron Microscope (FESEM)
FESEM samples were prepared by sputtering a 10–20 nm layer of Pt/Pd/Au. Images were acquired using a Zeiss Ultra55/Supra55VP FESEM equipped with a field emission gun operating at 5–10 kV.
Chemical Resistance of aquaplastic
Biofilm aquaplastic and curli aquaplastic (1 cm2) were fully immersed in the organic solvent (n-hexane, chloroform) and strong acid (98% sulfuric acid) and strong base (18 M sodium hydroxide) for 24 h. The optical images of aquaplastic were taken after 2 min and 24 h of immersion. After 24 h incubation, the aquaplastic immersed in n-hexane, chloroform, 18 M sodium hydroxide and 98% sulfuric acid were removed and air dried for 24 h. Weight of aquaplastic before and after the treatment was noted. After the 24 h incubation, the biofilm aquaplastic disintegrated into smaller fragments in 98% sulfuric acid, while both biofilm aquaplastic and curli aquaplastic had salts precipitated on their surfaces in 18 M sodium hydroxide, which we could not remove completely, and the accurate weight of aquaplastic films could not be determined. n = 3 for biofilm aquaplastic and curli aquaplastic.
Coating of aquaplastic
Curli aquagel was casted on various surfaces (cow leather, plywood, mobile phone touch screen, automobile exterior aluminum body part and copper wire), which upon drying in ambient condition resulted in aquaplastic coated surfaces. All surfaces in their coated and uncoated states were imaged by FESEM. n ≥ 3 for biofilm aquaplastic and curli aquaplastic.
Templating aquaplastic
Biofilm aquagel / curli aquagel was casted on various patterned surfaces viz. nylon, taro leaf (Colocasia esculenta) and lotus leaf (Nelumbo nucifera). Upon overnight drying in ambient condition, the aquaplastic was peeled off from the surface and imaged using FESEM. n ≥ 3 for biofilm aquaplastic and curli aquaplastic.
Rehydration of aquaplastic
Biofilm aquaplastic and curli aquaplastic (1 cm2) were incubated in MilliQ water and within 2 minutes the aquaplastic swells to its maximum capacity. After 2 min, the excess water was wicked off. The aquaplastic films retain their swollen dimensions, if dried after rehydrating. The weight and lateral dimensions of aquaplastic were recorded before and after rehydration. n=3 for biofilm aquaplastic and curli aquaplastic.
Aquahealing
Surface scratches and cuts were made to biofilm aquaplastic and curli aquaplastic. Water (~10 μL) was added at the place of abrasion and upon air drying, the aquaplastic was healed. n ≥ 3 for biofilm aquaplastic and curli aquaplastic.
Aquawelding
Water was employed as a glue to weld two pieces of biofilm aquaplastic or curli aquaplastic. 10–20 μL of water line spanning 1 cm was found to be enough to weld two aquaplastics of 1 cm2 dimensions. n ≥ 3 for biofilm aquaplastic and curli aquaplastic.
Water-resistant aquaplastic (aquaplastic washed)
Both biofilm aquaplastic and curli aquaplastic, when washed with excess water removes all the water-soluble components such as SDS and other cellular components, giving rise to a transparent water-insoluble film. Both biofilm aquaplastic and curli aquaplastic swell initially but with excessive water wash they ultimately shrink to original lateral dimensions. The water washed biofilm aquaplastic and curli aquaplastic samples exhibit resistance to water and do not wet. n ≥ 5 for biofilm aquaplastic and curli aquaplastic.
Statistics and Reproducibility
All experiments presented in this manuscript have been repeated at least 3 times (n≥3) on distinct samples, which has been clearly specified in figure legends or the relevant methods sections. In all cases, we have presented the mean and the standard deviation. GraphPad PRISM 8 software was used for plotting and analyzing data. For micrographs and optical images, we have presented the representative images.
Data and materials availability
The authors declare that all relevant data supporting the findings of this study and the plasmids and strains used are available within the article and its supplementary files or from the corresponding authors on request. Source data are provided in the Source Data files.
Extended Data
Extended Data Fig. 1. Coating of curli aquaplastic on various surfaces.

Optical images of a. Cow leather, b. Plywood c. Mobile phone touch screen, d. Aluminum automobile exterior body part and e. Copper wire coated with curli aquaplastic. FESEM images of coated and uncoated surfaces are shown below.
Supplementary Material
Acknowledgments:
Work was performed in part at the Center for Nanoscale Systems at Harvard. Funding: Work in the N.S.J. laboratory is supported by the National Institutes of Health (1R01DK110770-01A1, N.S.J.), the National Science Foundation (DMR 2004875, N.S.J.) and the Wyss Institute for Biologically Inspired Engineering at Harvard University. Parts of the schematics were adopted from BioRender.com
Footnotes
Competing interests: A.M.D.-T., N.-M.D.C and N.S.J. are inventors on patent application WO2017201428A8 submitted by Harvard University.
References:
- 1.Khalil AS & Collins JJ Synthetic biology: applications come of age. Nat Rev Genet 11, 367–379, doi: 10.1038/nrg2775 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen PQ, Courchesne ND, Duraj-Thatte A, Praveschotinunt P & Joshi NS Engineered Living Materials: Prospects and Challenges for Using Biological Systems to Direct the Assembly of Smart Materials. Adv Mater 30, e1704847, doi: 10.1002/adma.201704847 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen AY, Zhong C & Lu TK Engineering living functional materials. ACS Synth Biol 4, 8–11, doi: 10.1021/sb500113b (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilbert C & Ellis T Biological Engineered Living Materials: Growing Functional Materials with Genetically Programmable Properties. ACS Synth Biol 8, 1–15, doi: 10.1021/acssynbio.8b00423 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Duraj-Thatte AM et al. Genetically Programmable Self-Regenerating Bacterial Hydrogels. Adv Mater 31, e1901826, doi: 10.1002/adma.201901826 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Botyanszki Z, Tay PK, Nguyen PQ, Nussbaumer MG & Joshi NS Engineered catalytic biofilms: Site-specific enzyme immobilization onto E. coli curli nanofibers. Biotechnol Bioeng 112, 2016–2024, doi: 10.1002/bit.25638 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Nguyen PQ, Botyanszki Z, Tay PK & Joshi NS Programmable biofilm-based materials from engineered curli nanofibres. Nat Commun 5, 4945, doi: 10.1038/ncomms5945 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Chen AY et al. Synthesis and patterning of tunable multiscale materials with engineered cells. Nat Mater 13, 515–523, doi: 10.1038/nmat3912 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhong C et al. Strong underwater adhesives made by self-assembling multi-protein nanofibres. Nat Nanotechnol 9, 858–866, doi: 10.1038/nnano.2014.199 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez LM, Mukhitov N & Voigt CA Resilient living materials built by printing bacterial spores. Nat Chem Biol 16, 126–133, doi: 10.1038/s41589-019-0412-5 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Pei Kun R. Tay, M.-B. A a. J. NS Repurposing bacterial extracellular matrix for selective and differential abstraction of rare earth elements. Green Chemistry 20, 3512–3520 (2018). [Google Scholar]
- 12.Liu X et al. Stretchable living materials and devices with hydrogel-elastomer hybrids hosting programmed cells. Proc Natl Acad Sci U S A 114, 2200–2205, doi: 10.1073/pnas.1618307114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geyer R, Jambeck JR & Law KL Production, use, and fate of all plastics ever made. Sci Adv 3, e1700782, doi: 10.1126/sciadv.1700782 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emo Chiellini AC, D’Antone Salvatore, Roberto Solaro. Biodegradation of poly (vinyl alcohol) based materials. Progress in Polymer Science 28, 963–1014 (2003). [Google Scholar]
- 15.Flemming HC & Wingender J The biofilm matrix. Nat Rev Microbiol 8, 623–633, doi: 10.1038/nrmicro2415 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Barnhart MM & Chapman MR Curli biogenesis and function. Annu Rev Microbiol 60, 131–147, doi: 10.1146/annurev.micro.60.080805.142106 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nussbaumer MG et al. Bootstrapped Biocatalysis: Biofilm-Derived Materials as Reversibly Functionalizable Multienzyme Surfaces. ChemCatChem 9, 4328–4333, doi: 10.1002/cctc.201701221 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duraj-Thatte AM, Praveschotinunt P, Nash TR, Ward FR & Joshi NS Modulating bacterial and gut mucosal interactions with engineered biofilm matrix proteins. Sci Rep 8, 3475, doi: 10.1038/s41598-018-21834-8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu X et al. Sodium dodecyl sulfate-induced rapid gelation of silk fibroin. Acta Biomater 8, 2185–2192, doi: 10.1016/j.actbio.2012.03.007 (2012). [DOI] [PubMed] [Google Scholar]
- 20.Wei G et al. Self-assembling peptide and protein amyloids: from structure to tailored function in nanotechnology. Chem Soc Rev 46, 4661–4708, doi: 10.1039/c6cs00542j (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adamcik J et al. Measurement of intrinsic properties of amyloid fibrils by the peak force QNM method. Nanoscale 4, 4426–4429, doi: 10.1039/c2nr30768e (2012). [DOI] [PubMed] [Google Scholar]
- 22.Avinash MB, Raut D, Mishra MK, Ramamurty U & Govindaraju T Bioinspired Reductionistic Peptide Engineering for Exceptional Mechanical Properties. Sci Rep 5, 16070, doi: 10.1038/srep16070 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Phan DC, Goodwin DG Jr., Frank BP, Bouwer EJ & Fairbrother DH Biodegradability of carbon nanotube/polymer nanocomposites under aerobic mixed culture conditions. Sci Total Environ 639, 804–814, doi: 10.1016/j.scitotenv.2018.05.137 (2018). [DOI] [PubMed] [Google Scholar]
- 24.Knowles TP & Mezzenga R Amyloid Fibrils as Building Blocks for Natural and Artificial Functional Materials. Adv Mater 28, 6546–6561, doi: 10.1002/adma.201505961 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Zhongyan Du SW, Jihu Wang, Changle Yin, Dayang Yu & Jian Luo. The Review of Powder Coatings. Chemistry and Material Science 4, 54–59, doi: 10.4236/msce.2016.43007 (2016). [DOI] [Google Scholar]
- 26.Avinash MB, Verheggen E, Schmuck C & Govindaraju T Self-cleaning functional molecular materials. Angew Chem Int Ed Engl 51, 10324–10328, doi: 10.1002/anie.201204608 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Albertsson AC & Hakkarainen M Designed to degrade. Science 358, 872–873, doi: 10.1126/science.aap8115 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Abdullaha Zainab Waheed, Y. D a., Ian Jeffery Daviesa, Salim Barbhuiyab. PVA, PVA Blends, and Their Nanocomposites for Biodegradable Packaging Application. Polymer-Plastics Technology and Engineering 56, 1307–1344 (2017). [Google Scholar]
- 29.Amsden JJ et al. Rapid nanoimprinting of silk fibroin films for biophotonic applications. Adv Mater 22, 1746–1749, doi: 10.1002/adma.200903166 (2010). [DOI] [PubMed] [Google Scholar]
- 30.Kim DH et al. Dissolvable films of silk fibroin for ultrathin conformal bio-integrated electronics. Nat Mater 9, 511–517, doi: 10.1038/nmat2745 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perry H,Gopinath A, Kaplan DL., Dal Negro, L. & Omenetto, F.. Nano‐ and Micropatterning of Optically Transparent, Mechanically Robust, Biocompatible Silk Fibroin Films. Advanced Materials 20, 3070–3072 (2008). [Google Scholar]
- 32.Brenckle M, Partlow B, Tao H, Applegate MB., Reeves A, Paquette M, Marelli B, Kaplan D & Omenetto F Methods and Applications of Multilayer Silk Fibroin Laminates Based on Spatially Controlled Welding in Protein Films. Advanced Functional Materials 26, 44–50 (2015). [Google Scholar]
- 33.Moreau D, Chauvet C, Etienne F, Rannou FP & Corte L Hydrogel films and coatings by swelling-induced gelation. Proc Natl Acad Sci U S A 113, 13295–13300, doi: 10.1073/pnas.1609603113 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fernandez JG & Ingber DE Manufacturing of Large-Scale Functional Objects Using Biodegradable Chitosan Bioplastic. Macromolecular Materials and Engineering 299, 932–938, doi: 10.1002/mame.201300426 (2014). [DOI] [Google Scholar]
- 35.Guo C et al. Thermoplastic moulding of regenerated silk. Nat Mater 19, 102–108, doi: 10.1038/s41563-019-0560-8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Latza V et al. Multi-scale thermal stability of a hard thermoplastic protein-based material. Nat Commun 6, 8313, doi: 10.1038/ncomms9313 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beguin P & Aubert JP The biological degradation of cellulose. FEMS Microbiol Rev 13, 25–58, doi: 10.1111/j.1574-6976.1994.tb00033.x (1994). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


