Abstract
Dopamine, serotonin and endocannabinoids are key neuromodulators involved in many aspects of motivated behavior, including reward processing, reinforcement learning and behavioral flexibility. Among the longstanding views about possible relationships between these neuromodulators, is the idea of dopamine and serotonin acting as opponents. This view has been challenged by emerging evidence that serotonin support reward seeking via activation of dopamine neurons in the ventral tegmental area. Adding an extra layer of complexity to these interactions, the endocannabinoid system is uniquely placed to influence dopaminergic and serotonergic neurotransmisison. In this review, we discuss how these three neuromodulatory systems interact at cellular and circuit levels. Technological advances that facilitate precise identification and control of genetically targeted neuronal populations will help achieve a better understanding of the complex relationship between these essential systems, and the potential relevance for motivated behavior.
Keywords: neuromodulation, motivation, circuits, reward, addiction
Circuit determinants of interactions between dopamine, serotonin and the endocannabinoid system
The neuromodulators dopamine (DA), serotonin (5-hydroxytryptamine; 5-HT) and endocannabinoids (ECs) are involved in adaptive behaviors including movement and motivation [1-4], and all three have been implicated in multiple psychiatric conditions [5-7]. DA and 5-HT are produced in small nuclei of tightly clustered neurons – predominantly the ventral tegmental area (VTA) and substantia nigra pars compacta (SNc) for DA and the dorsal raphe nucleus (DRN) and median raphe nucleus (MRN) for 5-HT [8]. These neurons project extensively throughout the entire mammalian brain [9]. Thus, DA and 5-HT mainly exert their biological function after they are released by neuromodulatory afferent pathways to target brain regions. Whilst ECs have similarly profound modulatory effects, they are synthesized and released on-demand locally within brain circuits, where they primarily act via retrograde signaling [10].
The concerted activity and interaction between neuromodulatory afferent pathways and locally produced signals is key in shaping the activity of pre- and postsynaptic circuit elements to implement brain computations. This is particularly true for the interaction between the dopaminergic, serotonergic and EC systems (Figure 1-3). Indeed, DA and 5-HT neurons can release ECs to regulate glutamatergic- and GABAergic-impinging afferents. The activity and release of these three neuromodulators influences excitability and plasticity in many neural systems and their signals are transduced by networks of diverse receptor types.
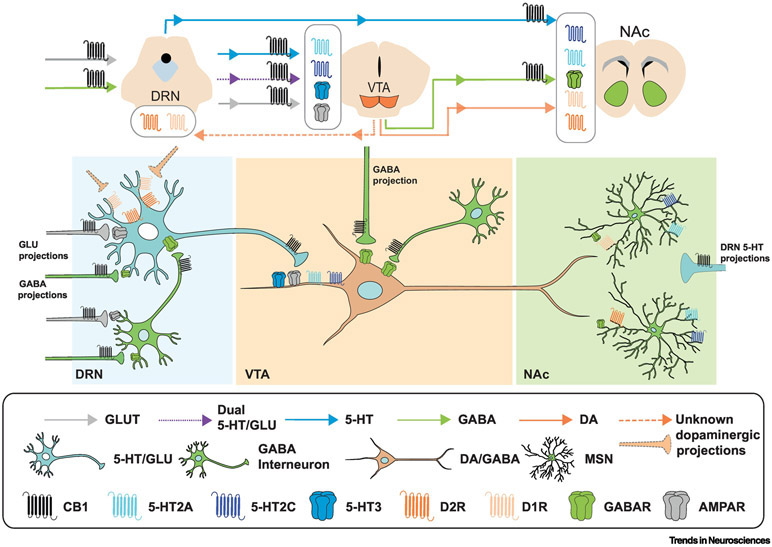
Figure 1, Key Figure. Interactions between the dopaminergic, serotonergic and endocannabinoid systems, key projections and sites of interaction.
Reciprocal modulation of the VTA and DRN by several key neurotransmitters is shown, as well as projections to the NAc. Axons of GABA and glutamate neurons projecting to the DRN (left) express CB1 receptors. These inputs to the DRN can either inhibit or excite DRN neurons. Key cell types, pathways and receptors expressed are shown (see key). DRN projections to the VTA (see top diagram) include glutamatergic (grey), serotonergic (blue) and dual serotonin-glutamate releasing neurons (dashed purple) whereas DRN projections to the NAc are primarily serotonergic; these signals are transduced by multiple 5-HT receptors as well as AMPA receptors (within the VTA). In addition to reward-related dopaminergic inputs to the NAc, the VTA sends reciprocal projections to the DRN; some of these may be dopaminergic (orange) and are transduced by both dopamine D1 and D2 receptors. Importantly, there are multiple cell types in both the DRN and VTA, and it is not fully understood which neurons project specifically to which cell types. In addition, synaptic contacts within the DRN and VTA (not shown in the figure) mediate local interactions . Additionally, populations of DA neurons and GABAergic interneurons within the DRN and VTA, respectively, contribute to the modulation of these circuits. Abbreviations: GLU – Glutmate; MSN – medium spiny neuron
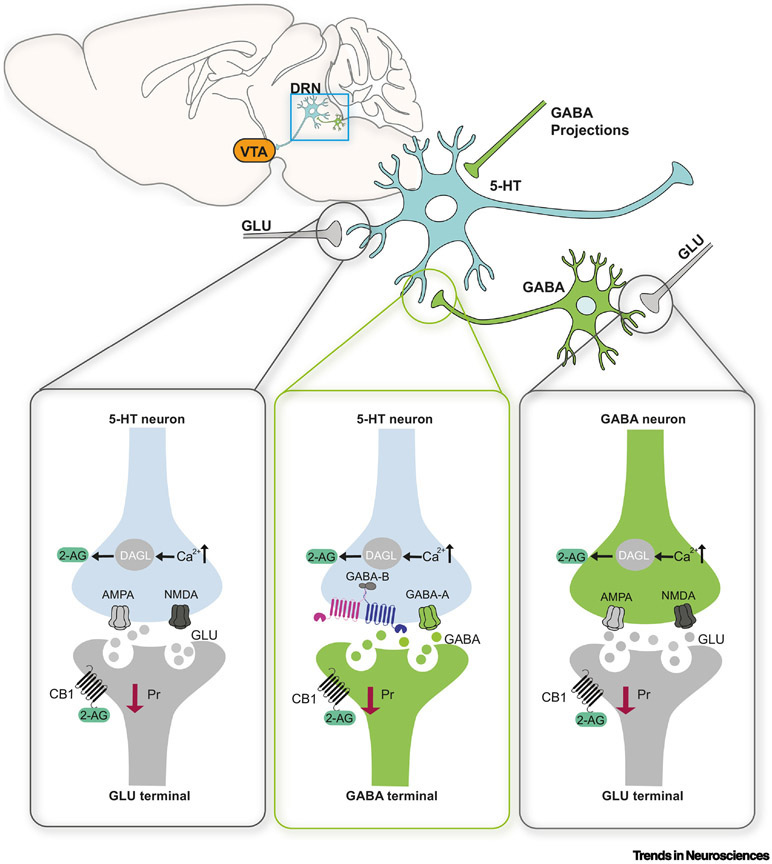
Figure 3. Indirect and polysynaptic modulation of 5-HT neurons by CB1 receptors.
Potential sites of cannabinoid receptor modulation of DRN 5-HT neurons. Left: Cortical glutamate afferents to the DRN express CB1 receptors. Activation of these receptor by ECs (such as 2-AG) would result in reduced excitatory drive onto DRN 5-HT neurons, reducing activity. Middle: GABAergic interneurons within the DRN as well as incoming GABAergic projections, which exert an inhibitory effect on the activity of DRN 5-HT, also express CB1 receptors that, when activated, lead to disinhibition. Right: Cortical afferents synapse on GABA interneurons; activation of CB1 receptors on these cortical axons would reduce excitatory drive onto GABA neurons, leading to reduced GABA release probability onto 5-HT cells disinhibiting them and promoting their activity. The timing of activation of these multiple CB1 receptors in different neural cell types is not fully characterized. The net effect of these often competing and opposite modulatory sites, and the mechanisms by which the activation of one group of CB1 receptors expressed in excitatory neurons (for example) predominates over others in inhibitory cell types is yet to be determined.
Although DA and 5-HT are involved in related functions within motivation and reward processing and interact with each other [11], until recently, the anatomical basis of these interactions was largely unknown. The development of rabies virus-based tracing methods [9,12] has made it possible to identify monosynaptic inputs to defined brain areas. This approach has allowed researchers to uncover substantial connections between 5-HT neurons of the DRN (DRN 5-HT) and DA neurons of the VTA [13]. DRN 5-HT neurons also heavily innervate other dopaminergic areas including the SNc and terminal regions such as the nucleus accumbens (NAc) and dorsal striatum [8,9]. In addition, a plethora of 5-HT receptors are expressed throughout the dopaminergic system, where they transduce 5-HT signals and modulate DA release in a variety of ways (discussed in the following sections). Furthermore, recent evidence indicates that 5-HT and DA neurons co-reside in the DRN [14,15] providing a possible means for direct functional cross-talk between these two neuronal subpopulations, although the specifics of this cross-talk remain to be investigated.
As a third player, the EC system modulates dopaminergic and serotonergic neurotransmission through multiple direct and indirect mechanisms [10,16,17](Figure 2-3). ECs exert their effects mainly through presynaptic CB1 receptors, which act via Gi/o proteins to limit neurotransmitter release. These receptors influence dopaminergic responses to reward and reinforcement, and modulate drug-evoked DA efflux in the NAc [18-20]. ECs also modulate 5-HT release [21] and 5-HT receptor expression [22], and cannabinoid and 5-HT systems have overlapping roles in diverse functions including appetite, body temperature as well as sleep and arousal [16]. Because CB1 receptors play a pivotal role in synaptic plasticity, their modulation of DA and 5-HT neurotransmission is crucial in flexible and adaptive behavior [23,24].
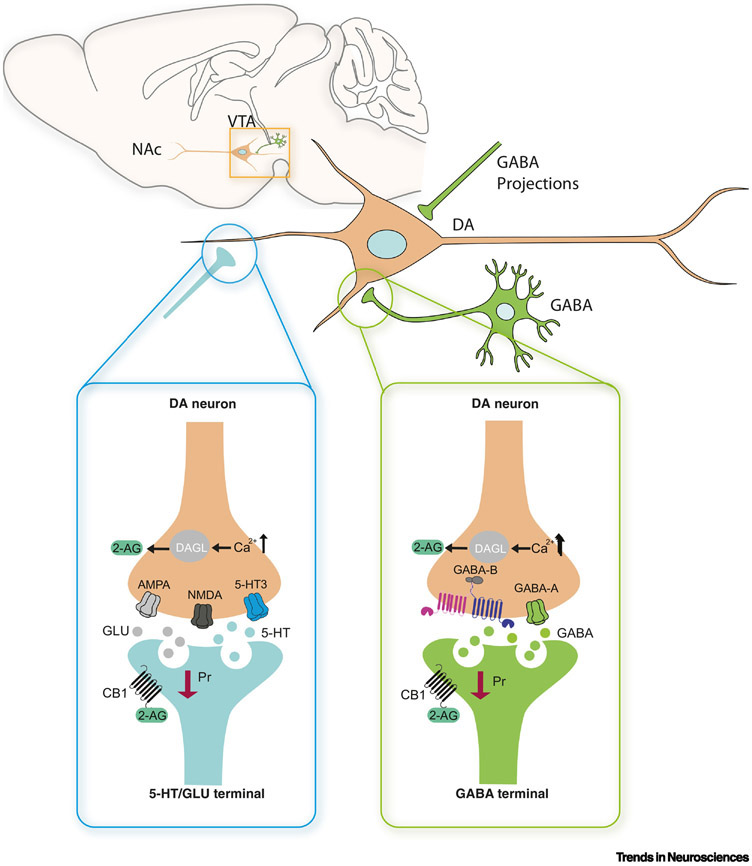
Figure 2. Modulation of VTA neurons by DRN and GABAergic inputs, as well as local interneurons.
The schematic illustrates a VTA dopamine neuron (orange) projecting to the NAc, and receiving synaptic inputs from a 5-HT/GLU DRN neuron (cyan) and a local GABAergic neuron (green). Left inset: DRN to VTA projections modulate dopamine release and are regulated by CB1 receptors. Axonal terminals of DRN 5-HT neurons within the VTA can co-release 5-HT and glutamate (GLU); this signal is transduced by 5-HT receptors (specifically 5-HT3) and AMPA and NMDA receptors on dopaminergic neurons (DA neurons) [51]. Excitation of VTA DA neurons by DRN terminals results in raised intra-cellular calcium (Ca2+) and leads to the production of ECs (eg. 2-AG). CB1 receptors on these 5-HT/glutamate terminals within the VTA are activated by 2-AG. CB1 activation in turn decreases the probablity of neurotransmitter/neuromodulator release (Pr), dampening the drive onto DA neurons.
Right inset: GABAergic projections to VTA dopamine neurons are regulated by CB1 receptors, resulting in disinhibition of dopamine release. DA neurons within the VTA are normally inhibited by GABA, but regulation via the EC system provides a disinhibitory mechanism. That is, when DA neurons fire in high frequency bursts, intracellular Ca2+ levels increase. As a result, 2-AG is synthesized and released into the extrasynaptic space. 2-AG retrogradely activates CB1 receptors on GABAergic terminals and GABA release is suppressed. The resulting disinhibition of the dopamine neuron increases dopamine activity and consequent terminal release.
In this review, we highlight key interactions between the dopaminergic and serotonergic systems, with particular emphasis on how DRN inputs to the VTA can influence motivated behaviors by ultimately altering terminal DA release in the NAc. We discuss recent evidence of substantial heterogeneity within dopaminergic and serotonergic brain nuclei, and how this complicates the investigation of the interaction between DA and 5-HT signals. In addition, we consider how the expression of 5-HT receptors in DA neurons (particularly 5-HT2A and 5-HT2C) profoundly influences dopaminergic function. Finally, we consider how ECs provide an additional level of modulation of the dopaminergic and serotonergic systems, and its potential relevance for motivated behavior.
Anatomy and function of the dopaminergic and serotonergic systems
Brief introduction to the dopaminergic system
The dopaminergic system comprises the mesolimbic (VTA to NAc, amygdala and hippocampus), mesocortical (VTA to prefrontal cortex) and nigrostriatal (SNc to striatum) projections [25] as well as the tuberoinfundibular system.
Phasic DA increases in the NAc have been often associated with the reinforcing effects of drugs of abuse and natural rewards [26,27]. The activity of the mesolimbic DA system, however, is not restricted to reward processing, and has been implicated also in responses to salience, uncertainty and novelty, as well as response to aversive stimuli [28-31]. In the context of reward processing, mesolimbic DA release has been theorized to encode a reward prediction error – the difference between expected and received rewards [32-34]. In this framework, DA increases become associated with cues that signal positive reward prediction error and thereby facilitate learning to maximize positive outcomes.
DA plays a role in the control of motivation as well as in behavioral activation – the invigoration needed to perform rewarding behaviors and to persist in ongoing behaviors [ 35, 36, 37, 38]. In fact, dopaminergic outputs in the NAc and dorsal striatum are involved in setting the sensitivity to effort: DA has been reported to promote effort to obtain future rewards [39] and is necessary for performing tasks that require effort [40]. Some have argued that slow, tonic changes in DA release play a role in gating movement [39,41], but this interpretation is complicated by the fact that phasic DA signaling is similarly implicated in action initiation [42]. SNc DA neurons increase their firing before movement initiation, and this correlates with response vigor [43]. Furthermore, the timing of these rapid signals is disrupted in mouse models of Parkinson’s disease [44].
Dysfunctions in dopaminergic circuitry are also central in psychiatric conditions including addiction, where aberrant incentive salience is attributed to drug-associated cues, making them powerful reinforcers [45,46], or in schizophrenia, where DA D2 receptor antagonism is a feature of many of the current pharmacological treatments [47]. Thus, dopamine is an important mediator of reward learning in health and disease.
Brief introduction to the serotonergic system
5-HT neurons originating in the DRN, the median raphe (MR) and the caudal raphe nuclei (raphe magnus, pallidus, and obscurus) project to almost all areas of the brain [8,9], including innervation of DA cell bodies in the midbrain [9]. 5-HT exerts its effects through an array of (mainly) G-protein coupled receptors. There are at least 16 different classes of 5-HT receptors, expressed on presynaptic and postsynaptic circuit elements, which are coupled to multiple signaling cascades [48]. Of the different classes, 5-HT1 receptors hyperpolarize host cells whereas 5-HT2 and 5-HT4, 5-HT5, 5-HT6 and 5-HT7 receptors depolarize neurons through different intracellular signaling pathways [49]. 5-HT3 receptors are the only class of 5-HT ligand-gated cation channels and there are many binding sites for these receptors in dopaminergic areas such as the SNc and the VTA as well as dopaminoceptive areas such as the NAc and PFC [49-51].
Despite considerable literature on the functions of 5-HT [52], a consensus on the primary roles of 5-HT neurons still remains elusive [53] and manipulations of the 5-HT system have produced equivocal results [54]. Among the physiological and behavioral processes modulated by 5-HT are sleep/wakefulness and behavioral inhibition [55]. A growing literature has also implicated the 5-HT system in reward processing [56,57] and temporal discounting [58]. 5-HT is also an important neurotransmitter in psychiatric conditions and is the main pharmacological target in depression and anxiety disorders [59,60].
Serotonin and dopamine, friends or foes?
5-HT and dopamine as opponent or synergistic neuromodulators
Historically, 5-HT and DA have often been thought to act in opposition. Contrary to DA neurons, which facilitate action and respond preferentially to rewards, 5-HT neurons increase their activity in response to aversive stimuli [55,61] and promote behavioral inhibition [62]. 5-HT neurons are implicated in animal models of patience [63] and 5-HT-mediated response inhibition may support waiting for delayed rewards and to avoid punishment. However, there are considerable interactions between these two key modulators. DA and 5-HT can, in fact, encode strikingly similar information to promote adaptive, flexible behavior in complex, changing environments.
Recent data suggest that, like DA neurons, 5-HT cells encode differences between expected and received rewards to promote behavioral flexibility. In mice, fiber photometry recordings during reversal learning have shown that DA encodes a ‘signed’ error: activity increases with an unexpectedly greater reward and decreases with an unexpectedly worse outcome (i.e. reward omission). Conversely, DRN 5-HT neurons display an ‘unsigned’ prediction error, or a surprise signal when contingencies are switched, increasing activity to all errors regardless of direction [64]. Importantly, these responses have different kinetics, with the dopaminergic response being initially fast and decaying rapidly, whereas the 5-HT signal is slower to rise and slower to dissipate (i.e. the 5-HT signal is slower to adapt to expectancy changes)[55]. These different temporal dynamics may cause a shift in the relative availability of DA and 5-HT to shape behavioral flexibility: in the case of prediction errors, toward DA for positive and toward 5-HT for negative [64].
On a circuit and synaptic level, the temporal, combinatorial logic of DA and 5-HT might be expressed through the regulation of synaptic plasticity rules in an integrated, timing-dependent manner. That is, DA and 5-HT may operate relative to coordinated presynaptic-postsynaptic neuronal activity to enable long-term synaptic plasticity (timing-dependent synaptic plasticity rules). At least in the mouse dorsal striatum, DA and 5-HT, through the DA D1 and 5-HT4 receptor subtypes, act as two complementary neuromodulatory systems controlling synaptic plasticity at two segregated glutamatergic inputs (cortical and thalamic synapses) by signaling in different functional microdomains (dendritic spine and dendritic shaft) [14]. This might allow for greater selectivity in tuning the activity of striatal neurons to support reinforcement and reward as well as behavioral flexibility.
Several questions remain to be addressed, such as whether there is a hierarchical organization of DA and 5-HT signals in determining net synaptic output, or whether rules of synaptic neuromodulation are input-specific. Furthemore, the EC system modulates the release of DA and 5-HT through several synaptic mechanisms. How or under what circumstances the EC system may facilitate the interaction between DA and 5-HT remains unclear.
Dysfunction in integrated synaptic neuromodulation may represent a cellular basis of maladaptive behaviors that characterize neurological and neuropsychiatric conditions. Indeed, maladaptive timing-dependent neuromodulation (i.e., altered temporal dynamics of neuromodulator release) could lead to mis-timed release of distinct neuromodulators at synapses. This might result in aberrant assignment of salience responses to environmental events and internal representations during synaptic information processing.
Heterogeneity within the DRN and VTA
Considerable heterogeneity in cell types, physiological responses, presynaptic inputs and projection targets exists within both serotonergic and dopaminergic brain nuclei, in particular within the DRN and VTA.
The often-equivocal results in attributing specific functions to 5-HT neurons in electrophysiological studies may arise, in part, from the difficulty in accurately identifying these neurons within a heterogeneous area such as the DRN. Following recent advances in optogenetics, it is now possible to identify and activate 5-HT terminals within specific targets with greater accuracy [51,65]. In mice, electrophysiological recordings from optogenetically-tagged 5-HT neurons reveal higher tonic activity in DRN 5-HT neurons in response to rewards than to punishments [66]. In fact, tonic 5-HT release can encode information about the average reward rate – something that is typically thought to be tracked by DA [41]. Mouse DRN 5-HT neurons are molecularly, physiologically and anatomically heterogeneous and have been categorized by their gene expression patterns into 5 subtypes [67]. These subtypes vary in their expression of key neurotransmitter genes such as Gad1 and Slc6a1 (GABA-related genes) and Slcl7a8 (vesicular glutamate transporter genes). Within the DRN – based on differential gene expression, anatomical location and connectivity – recent work has defined as many as 18 different functional clusters of neurons [67,68]. Besides 5-HT neurons, the DRN contains many other cell types including GABA, glutamate, DA and dual 5-HT-glutamate releasing neurons [51,66,69-71]. The projection targets of these neurons throughout the brain, and especially within the VTA, are not fully characterized and this considerable heterogeneity affords a vast array of potential modulatory influences.
In terms of 5-HT and DA interactions, DRN DA neurons are an understudied population. However, there is evidence that these neurons may be important in wakefulness as they increase their activity in response to salient stimuli, which might promote vigilance [72]. In rodents, DRN DA neurons have also been implicated in negative affective states related to social isolation [15]. A complete dissection of DRN DA neurons is lacking, however their proximity within the DRN to 5-HT neurons makes them well-placed to influence the circuits discussed here. Cross-talk between DA and 5-HT neurons within the raphe may represent an important site of interaction.
The VTA also has considerable molecular, physiological and functional heterogeneity [73,74]. Traditionally electrophysiological studies have identified DA neurons through defined physiological criteria, such as action potential width and ionic currents. However, the reliability of these criteria has been the subject of intense debate [75]. The observed variability in DA neuron phenotypes may arise because recordings are often performed in different subregions of the VTA. Retrograde tracing methods that map inputs and outputs of VTA neurons [12,76] have revealed distinct topographical organization of DA neurons depending upon their projection targets [73,77,78]. Neurons in the medial and posterior VTA regions project to the NAc medial shell and core and prefrontal cortex (PFC), whereas those from more lateral posterior and anterior regions preferentially project to the accumbens lateral shell [73]. Subpopulations show different physiological properties [73], and this diversity may mediate different behavioral responses [28]. Like the DRN, the VTA also contains a multitude of cell types beyond DA neurons including neurons that release GABA [79], those that co-release DA and GABA [80] , and those that co-release DA and glutamate [81,82]. The percentages of each of these cell types is not fully characterized and the functions of combinatorial neurons warrants further study. The development of new tools, such as methods for combinatorial targeting of promoters, will undoubtedly enhance our understanding of how these various cell types contribute to motivated behaviors and help to identify novel therapeutic opportunities [78].
Serotoninergic projections can potentiate dopamine release and support reinforcement
Despite the longstanding view that 5-HT and DA act as antagonistic neuromodulators, there is now evidence of synergistic interactions between them in reward-related behaviors [11,55,83]. In vivo electrophysiological and fiber photometry recordings have demonstrated that DRN 5-HT neurons are activated by reward [58] and reward-predictive cues [66,84] similarly to dopamine neurons. In addition, optogenetic activation of DRN 5-HT neurons promotes instrumental behaviors to obtain reward in optical self-stimulation paradigms [84,85], and these effects are specific to DRN to VTA projections. Conversely, projections to the lateral hypothalamus, amygdala, nucleus accumbens or ventral pallidum do not sustain sufficient levels of optical self-stimulation. Nevertheless, some conflicting findings report a lack of reinforcing effects of 5-HT in the VTA [86,87]. These discrepancies may be due to the heterogeneity of DRN neurons and differences in the projection sites within the VTA (as DRN neurons synapse onto both dopaminergic and non-dopaminergic cells).
The past decade has seen significant developments in in situ hybridization methods to assess cell-type-specific receptor expression, anatomical tracing methods and genetic and viral tools to manipulate specific neurons. One of the dividends from such tools has been the discovery of dual 5-HT-glutamate (VGluT3 expressing) neurons capable of co-release [84,88]. DRN neurons that innervate the VTA have been identified as 46% glutamatergic, 13% serotonergic and 14% dual 5-HT-glutamatergic [89]. Within the VTA, around 80% of DA neurons are also positive for the 5-HT3A receptor [51], an important node in transducing a rapid 5-HT signal. 5-HT3A receptors, in addition to the 5-HT2 receptors allow for subsecond 5-HT influence on accumbal DA release [51]. This requires 5-HT3A and AMPA receptor activation of DA neurons in the VTA (Figure 2). Furthermore, in mice, this specific activation of DRN 5HT-glutamate neurons promotes conditioned place preference [51] which is reversed by depletion of either glutamate or 5-HT [84]. Identified 5-HT projections from the DRN to the VTA support reward-related learning by enhancing DA release in the NAc (Figure 1). The existence of specific cell types in the DRN and their corresponding projection targets has highlighted discrete sub-populations of DRN to VTA neurons involved in reward.
5-TH2A and 5HT2C receptors differentially modulate dopamine release
5-HT receptors influence DA signaling through multiple direct and indirect mechanisms. A number of 5-HT receptors facilitate DA release – including 5-HT1A, 5-HT1B and several 5-HT3 and 5-HT4 receptors [48]. However, the 5-HT2A and 5-HT2C receptors appear to have a key influence on dopaminergic activity and transmission [90,91]. Both receptors are expressed throughout the mesolimbic and mesocortical systems, in DA and GABA neurons within the VTA, in medium spiny neurons in the NAc and within both glutamatergic and GABAergic neurons of the PFC [92].
5-HT2A and 5-HT2C receptors both couple to Gαq/11, where they activate the downstream effectors phospholipase-C (PLC) and phospholipase-A2 (PLA2) [93]. 5-HT2C preferentially activates the PLC pathway whereas 5-HT2A favors PLA2 activation [94,95]. PLC induces hydrolysis of membrane phospholipids and the production of inositol-1-4-5-triphosphate (IP3) and diacylglycerol to increase intracellular calcium [96], whereas PLA2 results in arachidonic acid release which modulates synaptic transmission and ion channel activity [97].
Activation of 5-HT2A and 5-HT2C receptors has opposite effects on dopaminergic neurotransmission. 5-HT2A agonists enhance the release of DA in the striatum, an effect that is blocked by specific antagonists [98]. However, 5-HT2A antagonists alone have no effect on DA neuron activity or release [90]. These receptors do not tonically modulate DA release, but may alter dopamine release and its effects on target areas under stimulated or activated states [98]. Conversely, the 5-HT2C receptor does provide tonic and phasic modulation of DA release. Indeed, systemic 5-HT2C agonists inhibit basal firing rates of DA neurons and curtail terminal release. Furthermore, 5-HT2C receptor antagonists actively increase firing in VTA DA neurons and enhance NAc DA efflux [91]. Thus, the expression of these different receptors provides multiple ways for serotonin to influence dopamine release.
Experiments in mice and rats have shown that antagonism of 5-HT2C or activation of 5-HT2A enhances hyperlocomotion induced by cocaine, amphetamine and other drugs of abuse, while activation of 5-HT2C or antagonism of 5-HT2A blocks stimulant-induced locomotor effects [99-101]. These behavioral effects are accompanied by facilitation or attenuation of drug-induced DA release in terminals to NAc [49]. These effects on DA release appear to be specific to the mesolimbic pathway. Indeed, intra-VTA blockade of 5-HT2C receptors increases drug-evoked DA release in the NAc and agonism decreases it. At the same time, intra-SNc administration does not affect DA neuron activity or striatal DA release [49]. 5-HT receptor modulation of DA release could have implications for therapeutic targets for conditions where dopaminergic neurotransmission is crucially altered such as addiction, depression and Parkinson’s disease. 5-HT2A and 5-HT2C receptors co-exist in many areas and it is not yet clear how activity is biased towards one or the other, or how both tonic and phasic serotonin release differentially affect these receptors in these areas. However, it is clear that 5-HT2 receptor expression modulates DA release and impacts DA-related behaviors in a state-dependent manner.
Dopaminergic modulation of DRN 5-HT neurons via dopamine D2 receptors
In addition to DRN to VTA projections, there are also substantial connections in the reciprocal direction – from the VTA to DRN [8], and DA within the DRN modulates the activity of 5-HT neurons, in particular through DA D2 receptors. Single-cell RNA sequencing data within the DRN show a population of neurons expressing both PET1 and DRD2 [70]. Those DRD2s expressed in DRN 5-HT neurons are functional: in whole-cell electrophysiology experiments in brain slices from mouse and rat, application of either DA or the DA agonist quinpirole modulates neuronal activity in 5-HT neurons expressing these receptors [102-105]. However, the net effect of D2R activation on DRN5-HT cell firing is debated and needs further investigation. Additionally, DRD2s form heteromers with 5-HT1A receptors, where they may play a role in negative feedback for 5-HT release [102]. DRD2-expressing 5-HT cells are preferentially localized at the most dorsal and lateral extents of the DRN [70], which places them anatomically close to DRN DA neurons. This anatomical proximity may hint at possible interactions and cross-talk between 5-HT and DA signaling, both within the DRN and from inputs originating in the VTA.
DRD2-expressing 5-HT neurons make up less than 5% of all PET1-expressing cells in the mouse DRN [70], however they have a profound effect on 5-HT activity in behaviorally-relevant circuits. For example, chronic silencing of these specific neurons facilitates active coping in the forced swim test [106] and DRD2 knockout mice show aberrant activation of 5-HT neurons within the DRN and a consequent stress vulnerability [102].
Cannabinoid receptor modulation of serotonin and dopamine systems
The endogenous cannabinoid system
The EC system consists of several G-protein coupled receptors (GPCRs), lipid molecules that are receptor ligands and their biosynthetic and metabolic enzymes. EC signaling is mainly transduced by the CB1 receptor, which is abundantly expressed throughout the mammalian brain [107]. CB1 receptors are coupled to Gi/o proteins, which upon activation, promote inhibition of adenylyl cyclase; CB1 receptors inhibit the activity of neurons in which they are expressed [108]. The two main ECs are 2-arachidonoylglycerol (2-AG) and N-arachidonoylethanolamine (anandamide). These are synthesized from 2-arachidonoyl-containing phospholipid diacylglycerol lipase (DAGL) and N-arachidonoyl phosphatidyl ethanol (NAPE), respectively, and are broken down by monoacylglycerol lipase (MGL) and fatty acid amidohydrolase (FAAH) [10,109]. A key feature of the EC system is that the synthetic enzymes for 2-AG and anandamide are activated during neuronal activity. Following the increases in intracellular calcium that accompany sustained neuronal activity, the ligands are synthesized and released de novo. They then retrogradely activate CB1 receptors on presynaptic terminals [110]. This de novo synthesis and retrograde action make ECs uniquely-placed modulators of neuronal activity, providing negative feedback to filter and select afferent inputs to given neurons.
Cannabinoid receptor modulation of dopaminergic neurotransmission
DA neurons are equipped with the molecular machinery required for EC synthesis, and concordantly, EC signaling is increasingly implicated in normal reward processing and learning and in disorders of motivation, such as addiction. ECs, via their actions at CB1 receptors, modulate dopaminergic responses to rewards and cues. Systemic blockade of CB1 receptors reduces NAc DA release in response to a variety of primary and secondary reinforcers, and attenuates drug-evoked dopamine release in rats [19,111,112]. However, CB1 receptors are not found on DA neurons within the VTA, so this modulation is indirect.
Under standard conditions, DA neurons within the VTA receive substantial inhibitory GABA inputs [113]. Removing GABA input using selective optogenetic inhibition reduces tonic inhibition, which evokes DA release in the NAc and drives appetitive behavior, such as reward seeking [114,115]. This disinhibition is a possible mechanism for how ECs modulate DA release (Figure 2). In fact, ECs binding at presynaptic CB1 receptors results in the suppression of GABA-mediated inhibition [116].
There are several other influences on terminal DA release that can occur independently of cell body firing, such as that of incoming cortical glutamate afferents and cholinergic interneurons [117,118]. For instance, in mice, cholinergic interneurons (CINs) stimulate DA release within the NAc independently of VTA firing [117]. This CIN-driven DA release is significantly decreased by systemic CB1 receptor agonists in optogenetic experiments; the effect is prevented by CB1 receptor blockade [119]. This is recapitulated by raising tissue levels of the EC 2AG and prevented by 2AG synthesis blockade [119]. Importantly, cholinergic interneurons in the NAc also do not express CB1 receptors, and thus these effects have a different site of action. A proposed site of action are cortical glutamate afferents which synapse onto CINs in the NAc, as these afferents do express CB1 receptors abundantly. Mechanistically, activation of prefrontal cortical glutamate afferents stimulates CINs, which enhances dopamine release via nicotinic acetylcholine receptors (nAChRs) [10,120]. Thus, CB1 receptors expressed in multiple neuron types (glutamatergic, GABAergic) have the ability to modulate dopamine release at multiple sites.
Cannabinoid receptor modulation of serotonergic neurotransmission
Much like DA neurons, 5-HT neurons within the DRN can synthesize, release and break down ECs. They express CB1 receptors and display phasic release of ECs in an activity-dependent manner [16,121]. Systemic CB1 receptor agonists increase levels of 5-HT and its metabolite 5-HIAA [122]. However, the effects of systemic CB1 receptor activation are region-specific; with reduced 5-HT release in the medial PFC and hippocampus [123] but increases in the NAc [21]. This is probably due to indirect effects at GABA neurons within the NAc and highlights how ECs can have different effects depending on which neuronal populations they are acting [124].
Little is known about how ECs influence the 5-HT system. The precise location of CB1 receptors and how these regulate 5-HT activity is not fully characterized; however, there are several potential mechanisms (Figure 3). One potential site of regulation could be axon terminals of GABAergic inputs or local interneurons within the DRN [17,125]. Another is glutamatergic afferents from the PFC to the DRN, which also express CB1 receptors [126,127]. In DRN brain slices, CB1 receptor agonists suppress glutamatergic inputs onto 5HT cells via presynaptic CB1 receptors [128,129]. Alternatively, glutamate projections may synapse onto local GABAergic interneurons as intermediaries. Consistent with this idea, CB1 receptor antagonists inhibit the firing rate of DRN 5-HT cells through GABA neurotransmission in vitro [130].
The presence of CB1 receptors in GABAergic and glutamatergic neurons that interact with the 5-HT system provides complex, indirect and polysynaptic modulation of 5-HT. In addition, transcriptomic clustering in single-cell transcription studies shows that in the DRN, CNR1 (the gene for CB1 receptors) is also expressed in PET1 positive (5-HT) neurons [68,131]. This subset of neurons highly expresses VGluT3-related transcripts, suggesting that 5-HT neurons that can package and release glutamate [51] also express CB1 receptors at the level of their terminal fields [131] and may constitute a site of direct cannabinoid modulation. Many questions remain with regards to how ECs act in these circuits, and how they may impact motivated behavior, via multiple direct and indirect points of interaction (see Outstanding Questions).
Outstanding Questions.
Similarly to VTA DA neurons, 5-HT neurons of the DRN play a role in reward-guided behaviors, and this may involve interactions with the dopaminergic system. How the nature of the temporal coding of DRN 5-HT neurons affects modulation of dopamine circuits is not fully characterized; do tonic and phasic patterns produce different effects? In areas where multiple 5-HT receptor subtypes are co-expressed (e.g., 5-HT2A and 5-HT2C in the VTA) do the temporal dynamics of 5-HT release bias activation of one over another?
To date, it has been difficult to identify 5-HT-specific modulation of DRN target regions, including the VTA, because 5-HT can be co-released with glutamate. 5-HT and co-released glutamate may encode parallel signals that operate along different time scales. What are the behavioral implications of the dual signaling capacity of 5-HT and co-released glutamate within the dopaminergic circuits?
The DRN contains anatomically defined and functionally distinct serotonergic sub-systems that show biased input and output connectivity, axonal collateralization patterns, and behavioral functions. Do certain 5-HT neurons synapse preferentially onto specific subpopulations of DA neurons? What are the consequences for the behaviors they modulate?
DA neurons in the VTA can synthetize and release ECs. ECs, via their actions at CB1 receptors, modulate dopaminergic responses to rewards and cues. Does CB1 receptor activation curtail 5-HT and/or glutamate release from DRN terminals in the VTA to shape terminal DA release in reward-related behaviors? How does exposure to cannabis during development and adolescence influence the functional maturation of dopamine and 5-HT systems and their interactions, ultimately affecting motivation and reward processing?
Concluding remarks and future perspectives
DA and 5-HT were often thought to encode opposite valences (reward vs punishment) as well as modulate opposite behavioral responses (action initiation vs inhibition) [55]. However, it is becoming increasingly clear that these two neuromodulators closely interact to shape adaptive, flexible responses to environmental stimuli. Despite evidence that 5-HT neurons have a role in behavioral inhibition and responses to punishment, there is clear evidence that DRN projections to the VTA also support reinforcement and promote DA release in the NAc [51]. Heterogeneity in serotonergic and dopaminergic nuclei, notably the presence of combinatorial neurons and functional sub-regions with target-specific responses, may be key to understanding the contribution of DA, 5-HT and their interactions for motivation.
DA and 5-HT also interact at the level of receptors. There is an opposing modulation of dopaminergic signaling by 5-HT2A and 5-HT2C receptors, with stimulatory and inhibitory effects on DA release, as well as on drug-evoked hyperlocomotion [92]. Furthermore, DA and 5-HT are modulated by ECs through CB1 receptors, and this modulation may provide fine-tuning of both systems. The development of novel genetic, pharmacological and viral approaches will be essential to unpack the complexities of these neuromodulatory systems, and resolve subpopulations within regions such as the DRN and VTA based on their inputs and outputs and on their physiological and molecular signatures [9,74]. Genetically-encoded neurotransmitter sensors for DA, 5-HT and ECs [132] combined with recording methods such as fiber photometry will allow real-time monitoring of these modulators in vivo during motivated behavior. While significant progress has been made, research is only beginning to uncover how the complex heterogeneity of the DA and 5-HT systems influence motivated behaviors, and how their interactions with ECs orchestrate complex behaviors in health and disease.
Highlights.
The interaction between the dopaminergic, serotonergic and endocannabinoid systems in anatomically defined circuits underlies complex aspects of reward-guided behavior.
The development of virus-based tracing methods has made it possible to uncover substantial reciprocal connectivity between specific cell types in subcortical brain nuclei, ultimately delineating the circuit and synaptic determinants of the interaction between dopaminergic, serotonergic and endocannabinoid systems.
Temporal differences in the activity and release of dopamine and serotonin may explain how seemingly opposite actions can result in adaptive, motivated behavior. By modulating the release of dopamine and serotonin through multiple direct and indirect mechanisms, the endocannabinoid system provides an additional level of complexity.
The complexity of co-releasing neurons with anatomical, physiological and functional heterogeneity is a major challenge in understanding the contribution of these systems to motivation.
Acknowledgments
We thank Drs. Yann Pelloux and Leonardo Bontempi for their critical reading of the manuscript and for helping with figure preparation. K.Z.P and J.F.C were supported by DA045639 from the National Instituted of Health (NIH); R.T was supported by the Fondazione Istituto Italiano di Tecnologia and by the IdEx Visiting Scholars - University of Bordeaux program.
Glossary
- Reversal learning
A behavioral paradigm to assess cognitive or behavioral flexibility in rodents. The animal is trained to respond to a lever paired with a cue for a given reward, at some point the contingencies (e.g., which levers / cues result in reward) are switched and the animal must alter their behavior to continue to receive rewards. The rodent must actively inhibit reward responding and disengage to perform the task successfully. Reveral learning paradigms can also be used to measure constructs such as impulsivity and compulsivity.
- Optogenetically tagged
A method for verifying and optically controlling certain populations of neurons using genetically encoded opsins. For example, expressing channelrhodopsin-2 (ChR2) using a Cre dependent adenovirus containing ChR2 injected into a target region in mice expressing Cre recombinase under the control of a specific promotor, to identify neurons that respond to light activation and verify their identity.
- Reward prediction error
The difference between expected (or predicted) and received rewards. The representation of reward prediction errors is seen as fundamental in learning within reward contexts. Dopamine is thought to encode such errors, with increases in activity in response to rewards that exceed those expected by predictive cues.
- Aberrant incentive salience
A term often described in addiction literature to define a process whereby stimuli associated with drug rewards (or other natural rewards) become imbued with importance (or salience). They capture attention and increase the likelihood of drug seeking and taking.
- Fiber photometry
A neural recording method for various indicators. These include genetically encoded calcium indicators (GCaMPs) which can be used to measure fluctuations in calcium, as well as newer neurotransmitter sensors (such as GRAB sensors) which report neurotransmitter release. These sensors are expressed in target brain areas typically using adeno-associated viruses. Fiber optic implants are used to excite these fluorophores and to record fluorescent changes from activity of a given sensor.
- Conditioned place preference (CPP)
Behavioral paradigm to assess motivation and reinforcing properties of substances or events in rodents. Typically, animals are introduced to a two-chamber apparatus and allowed to explore both its sides. Following this initial habituation, one side is paired with the administration of a drug (or electrical stimulation in the case of intracranial stimulation studies). Preference for reinforcer paired side is measured as time spent in that side compared to the non-reinforced side [133]. Rodents readily show CPP for many drugs of abuse, sucrose and intra-cranial stimulation of the mesolimbic pathway.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sagheddu C et al. (2015) Endocannabinoid Signaling in Motivation, Reward, and Addiction: Influences on Mesocorticolimbic Dopamine Function. Int. Rev. Neurobiol 125, 257–302 [DOI] [PubMed] [Google Scholar]
- 2.Liu Z et al. (2020) Reward Contributions to Serotonergic Functions. [DOI] [PubMed] [Google Scholar]
- 3.Fischer AG and Ullsperger M (2017) An Update on the Role of Serotonin and its Interplay with Dopamine for Reward. Front. Hum. Neurosci 11, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coddington LT and Dudman JT (2019) Learning from Action: Reconsidering Movement Signaling in Midbrain Dopamine Neuron Activity. Neuron 104, 63–77 [DOI] [PubMed] [Google Scholar]
- 5.Desfossés J et al. (2010) Endocannabinoids and Schizophrenia. Pharmaceuticals 3, 3101–3126 [Google Scholar]
- 6.Carhart-Harris RL (2018) Serotonin, psychedelics and psychiatry. World Psychiatry 17, 358–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wise RA and Robble MA (2020) Dopamine and Addiction. Annu. Rev. Psychol 71, 79–106 [DOI] [PubMed] [Google Scholar]
- 8.Ogawa SK et al. (2014) Organization of Monosynaptic Inputs to the Serotonin and Dopamine Neuromodulatory Systems. Cell Rep. 8, 1105–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogawa SK and Watabe-Uchida M (2018) Organization of dopamine and serotonin system: Anatomical and functional mapping of monosynaptic inputs using rabies virus. Pharmacol. Biochem. Behav 174, 9–22 [DOI] [PubMed] [Google Scholar]
- 10.Peters KZ et al. (2020) A Brain on Cannabinoids: The Role of Dopamine Release in Reward Seeking and Addiction. Cold Spring Harb. Perspect. Med DOI: 10.1101/cshperspect.a039305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boureau Y-L and Dayan P (2011) Opponency Revisited: Competition and Cooperation Between Dopamine and Serotonin. Neuropsychopharmacology 36, 74–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watabe-Uchida M et al. (2012) Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron 74, 858–873 [DOI] [PubMed] [Google Scholar]
- 13.Beier KT et al. (2015) Circuit Architecture of VTA Dopamine Neurons Revealed by Systematic Input-Output Mapping. Cell 162, 622–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taylor NE et al. (2019) The Role of Glutamatergic and Dopaminergic Neurons in the Periaqueductal Gray/Dorsal Raphe: Separating Analgesia and Anxiety. eNeuro DOI: 10.1523/ENEURO.0018-18.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matthews GA et al. (2016) Dorsal Raphe Dopamine Neurons Represent the Experience of Social Isolation. Cell 164, 617–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haj-Dahmane S and Shen R-Y (2011) Modulation of the Serotonin System by Endocannabinoid Signaling. Neuropharmacology 61, 414–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mendiguren A et al. (2018) Regulation of noradrenergic and serotonergic systems by cannabinoids: relevance to cannabinoid-induced effects. Life Sci. 192, 115–127 [DOI] [PubMed] [Google Scholar]
- 18.Covey D et al. (2017) Endocannabinoid modulation of dopamine neurotransmission. Neuropharmacology 124, 52–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Covey DP et al. (2016) Amphetamine elevates nucleus accumbens dopamine via an action potential-dependent mechanism that is modulated by endocannabinoids. Eur. J. Neurosci 43, 1661–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wenzel JM and Cheer JF (2018) Endocannabinoid Regulation of Reward and Reinforcement through Interaction with Dopamine and Endogenous Opioid Signaling. Neuropsychopharmacology 43, 103–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tao R and Ma Z (2012) Neural Circuit in the Dorsal Raphe Nucleus Responsible for Cannabinoid-Mediated Increases in 5-HT Efflux in the Nucleus Accumbens of the Rat Brain. ISRN Pharmacol. 2012, 276902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aso E et al. (2009) Lack of CB1 receptor activity impairs serotonergic negative feedback. J. Neurochem 109, 935–944 [DOI] [PubMed] [Google Scholar]
- 23.Cavaccini A et al. (2018) Serotonergic Signaling Controls Input-Specific Synaptic Plasticity at Striatal Circuits. Neuron 98, 801–816.e7 [DOI] [PubMed] [Google Scholar]
- 24.Mathur BN and Lovinger DM (2012) Endocannabinoid–Dopamine Interactions in Striatal Synaptic Plasticity. Front. Pharmacol 3, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hillarp NA et al. (1966) Demonstration and mapping of central neurons containing dopamine, noradrenaline, and 5-hydroxytryptamine and their reactions to psychopharmaca. Pharmacol. Rev 18, 727–741 [PubMed] [Google Scholar]
- 26.Phillips PEM et al. (2003) Subsecond dopamine release promotes cocaine seeking. 422, 6. [DOI] [PubMed] [Google Scholar]
- 27.Roitman MF et al. (2004) Dopamine Operates as a Subsecond Modulator of Food Seeking. J. Neurosci 24, 1265–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bromberg-Martin ES et al. (2010) Dopamine in motivational control: rewarding, aversive, and alerting. Neuron 68, 815–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schultz W (2007) Multiple Dopamine Functions at Different Time Courses. Annu. Rev. Neurosci 30, 259–288 [DOI] [PubMed] [Google Scholar]
- 30.Ungless MA et al. (2010) Effects of stress and aversion on dopamine neurons: implications for addiction. Neurosci. Biobehav. Rev 35, 151–156 [DOI] [PubMed] [Google Scholar]
- 31.Young AMJ (2004) Increased extracellular dopamine in nucleus accumbens in response to unconditioned and conditioned aversive stimuli: studies using 1 min microdialysis in rats. J. Neurosci. Methods 138, 57–63 [DOI] [PubMed] [Google Scholar]
- 32.Matsumoto M and Hikosaka O (2009) Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature 459, 837–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schultz W (2016) Dopamine reward prediction error coding. Dialogues Clin. Neurosci 18, 23–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schultz W et al. (1997) A neural substrate of prediction and reward. Science 275, 1593–1599 [DOI] [PubMed] [Google Scholar]
- 35.Walton ME and Bouret S (2019) What Is the Relationship between Dopamine and Effort? Trends Neurosci. 42, 79–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dauer W and Przedborski S (2003) Parkinson’s disease: mechanisms and models. Neuron 39, 889–909 [DOI] [PubMed] [Google Scholar]
- 37.Mamelak M (2018) Parkinson’s Disease, the Dopaminergic Neuron and Gammahydroxybutyrate. Neurol. Ther 7, 5–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salamone JD (2009) Dopamine, Behavioral Economics, and Effort. Front. Behav. Neurosci 3, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salamone JD and Correa M (2012) The mysterious motivational functions of mesolimbic dopamine. Neuron 76, 470–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nicola SM (2010) The flexible approach hypothesis: unification of effort and cue-responding hypotheses for the role of nucleus accumbens dopamine in the activation of reward-seeking behavior. J. Neurosci. Off. J. Soc. Neurosci 30, 16585–16600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niv Y et al. (2007) Tonic dopamine: opportunity costs and the control of response vigor. Psychopharmacology (Berl.) 191, 507–520 [DOI] [PubMed] [Google Scholar]
- 42.Howe MW and Dombeck DA (2016) Rapid signalling in distinct dopaminergic axons during locomotion and reward. Nature 535, 505–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.da Silva JA et al. (2018) Dopamine neuron activity before action initiation gates and invigorates future movements. Nature 554, 244–248 [DOI] [PubMed] [Google Scholar]
- 44.Dodson PD et al. (2016) Representation of spontaneous movement by dopaminergic neurons is cell-type selective and disrupted in parkinsonism. Proc. Natl. Acad. Sci. U. S. A 113, E2180–2188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Berridge KC and Robinson TE (1998) What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res. Brain Res. Rev 28, 309–369 [DOI] [PubMed] [Google Scholar]
- 46.Berridge KC and Robinson TE (2016) Liking, Wanting and the Incentive-Sensitization Theory of Addiction. Am. Psychol 71, 670–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seeman P (2010) Dopamine D2 receptors as treatment targets in schizophrenia. Clin. Schizophr. Relat. Psychoses 4, 56–73 [DOI] [PubMed] [Google Scholar]
- 48.Digiovanni G et al. (2008) Serotonin–dopamine interaction: electrophysiological evidence. In Progress in Brain Research 172pp. 45–71, Elsevier; [DOI] [PubMed] [Google Scholar]
- 49.Dimatteo V et al. (2008) Serotonin control of central dopaminergic function: focus on in vivo microdialysis studies. In Progress in Brain Research 172pp. 7–44, Elsevier; [DOI] [PubMed] [Google Scholar]
- 50.Barnes NM and Sharp T (1999) A review of central 5-HT receptors and their function. Neuropharmacology 38, 1083–1152 [DOI] [PubMed] [Google Scholar]
- 51.Wang H-L et al. (2019) Dorsal Raphe Dual Serotonin-Glutamate Neurons Drive Reward by Establishing Excitatory Synapses on VTA Mesoaccumbens Dopamine Neurons. Cell Rep. 26, 1128–1142.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muller C and Cunningham K (2020) Handbook of the behavioral neurobiology of serotonin., Academic Press. [Google Scholar]
- 53.Dayan P and Huys Q (2015) Serotonin’s many meanings elude simple theories. eLife 4, e07390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Urban DJ et al. (2016) Elucidation of The Behavioral Program and Neuronal Network Encoded by Dorsal Raphe Serotonergic Neurons. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol 41, 1404–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cools R et al. (2011) Serotonin and Dopamine: Unifying Affective, Activational, and Decision Functions. Neuropsychopharmacology 36, 98–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakamura K (2013) The role of the dorsal raphe nucleus in reward-seeking behavior. Front. Integr. Neurosci 7, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seymour B et al. (2012) Serotonin Selectively Modulates Reward Value in Human Decision-Making. J. Neurosci 32, 5833–5842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miyazaki K et al. (2011) Activation of Dorsal Raphe Serotonin Neurons Underlies Waiting for Delayed Rewards. J. Neurosci 31, 469–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Belmaker RH and Agam G (2008) Major depressive disorder. N. Engl. J. Med 358, 55–68 [DOI] [PubMed] [Google Scholar]
- 60.Ravindran LN and Stein MB (2010) The pharmacologic treatment of anxiety disorders: a review of progress. J. Clin. Psychiatry 71, 839–854 [DOI] [PubMed] [Google Scholar]
- 61.Takase LF et al. (2004) Inescapable shock activates serotonergic neurons in all raphe nuclei of rat. Behav. Brain Res 153, 233–239 [DOI] [PubMed] [Google Scholar]
- 62.Amo R et al. (2014) The habenulo-raphe serotonergic circuit encodes an aversive expectation value essential for adaptive active avoidance of danger. Neuron 84, 1034–1048 [DOI] [PubMed] [Google Scholar]
- 63.Miyazaki KW et al. (2014) Optogenetic activation of dorsal raphe serotonin neurons enhances patience for future rewards. Curr. Biol. CB 24, 2033–2040 [DOI] [PubMed] [Google Scholar]
- 64.Matias S et al. (2017) Activity patterns of serotonin neurons underlying cognitive flexibility. eLife 6, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marcinkiewcz CA et al. (2016) Serotonin engages an anxiety and fear-promoting circuit in the extended amygdala. Nature 537, 97–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cohen J et al. (2015) Serotonergic neurons signal reward and punishment on multiple timescales. eLife 4, e06346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang KW et al. (2019) Molecular and anatomical organization of the dorsal raphe nucleus. eLife 8, e46464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Okaty BW et al. (2020) A single-cell transcriptomic and anatomic atlas of mouse dorsal raphe Pet1 neurons. eLife 9, e55523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fernandez SP et al. (2016) Multiscale single-cell analysis reveals unique phenotypes of raphe 5-HT neurons projecting to the forebrain. Brain Struct. Funct 221, 4007–4025 [DOI] [PubMed] [Google Scholar]
- 70.Okaty BW et al. (2019) Embracing diversity in the 5-HT neuronal system. Nat. Rev. Neurosci DOI: 10.1038/s41583-019-0151-3 [DOI] [PubMed] [Google Scholar]
- 71.Waselus M et al. (2011) Collateralized dorsal raphe nucleus projections: a mechanism for the integration of diverse functions during stress. J. Chem. Neuroanat 41, 266–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cho JR et al. (2017) Dorsal Raphe Dopamine Neurons Modulate Arousal and Promote Wakefulness by Salient Stimuli. Neuron 94, 1205–1219.e8 [DOI] [PubMed] [Google Scholar]
- 73.Lammel S et al. (2014) Reward and aversion in a heterogeneous midbrain dopamine system. Neuropharmacology 76, 351–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Poulin J-F et al. (2020) Classification of Midbrain Dopamine Neurons Using Single-Cell Gene Expression Profiling Approaches. Trends Neurosci. 0, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ungless MA and Grace AA (2012) Are you or aren’t you? Challenges associated with physiologically identifying dopamine neurons. Trends Neurosci. 35, 422–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tye KM and Deisseroth K (2012) Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat. Rev. Neurosci 13, 251–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beckley JT et al. (2013) Medial prefrontal cortex inversely regulates toluene-induced changes in markers of synaptic plasticity of mesolimbic dopamine neurons. J. Neurosci. Off. J. Soc. Neurosci 33, 804–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morales M and Margolis EB (2017) Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nat. Rev. Neurosci 18, 73–85 [DOI] [PubMed] [Google Scholar]
- 79.Margolis EB et al. (2012) Identification of rat ventral tegmental area GABAergic neurons. PloS One 7, e42365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tritsch NX et al. (2012) Dopaminergic neurons inhibit striatal output through non-canonical release of GABA. Nature 490, 262–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hnasko TS et al. (2012) Ventral tegmental area glutamate neurons: electrophysiological properties and projections. J. Neurosci. Off. J. Soc. Neurosci 32, 15076–15085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stuber GD et al. (2010) Dopaminergic Terminals in the Nucleus Accumbens But Not the Dorsal Striatum Corelease Glutamate. J. Neurosci 30, 8229–8233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hayes DJ and Greenshaw AJ (2011) 5-HT receptors and reward-related behaviour: a review. Neurosci. Biobehav. Rev 35, 1419–1449 [DOI] [PubMed] [Google Scholar]
- 84.Liu Z et al. (2014) Dorsal Raphe Neurons Signal Reward through 5-HT and Glutamate. Neuron 81, 1360–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li Y et al. (2016) Serotonin neurons in the dorsal raphe nucleus encode reward signals. Nat. Commun 7, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fonseca MS et al. (2015) Activation of dorsal raphe serotonergic neurons promotes waiting but is not reinforcing. Curr. Biol. CB 25, 306–315 [DOI] [PubMed] [Google Scholar]
- 87.McDevitt RA et al. (2014) Serotonergic versus non-serotonergic dorsal raphe projection neurons: differential participation in reward circuitry. Cell Rep. 8, 1857–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hioki H et al. (2010) Vesicular glutamate transporter 3-expressing nonserotonergic projection neurons constitute a subregion in the rat midbrain raphe nuclei. J. Comp. Neurol 518, 668–686 [DOI] [PubMed] [Google Scholar]
- 89.Qi J et al. (2014) A glutamatergic reward input from the dorsal raphe to ventral tegmental area dopamine neurons. Nat. Commun 5, 5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bubar M and Cunningham K (2008) Prospects for serotonin 5-HT2R pharmacotherapy in psychostimulant abuse. In Progress in Brain Research 172pp. 319–346, Elsevier; [DOI] [PubMed] [Google Scholar]
- 91.Cunningham KA and Anastasio NC (2014) Serotonin at the nexus of impulsivity and cue reactivity in cocaine addiction. Neuropharmacology 76 Pt B, 460–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Howell LL and Cunningham KA (2015) Serotonin 5-HT2 Receptor Interactions with Dopamine Function: Implications for Therapeutics in Cocaine Use Disorder. Pharmacol. Rev 67, 176–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Felder CC et al. (1990) Serotonin stimulates phospholipase A2 and the release of arachidonic acid in hippocampal neurons by a type 2 serotonin receptor that is independent of inositolphospholipid hydrolysis. Proc. Natl. Acad. Sci. U. S. A 87, 2187–2191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Berg K et al. (2008) Physiological and therapeutic relevance of constitutive activity of 5-HT2A and 5-HT2C receptors for the treatment of depression. In Progress in Brain Research 172pp. 287–305, Elsevier; [DOI] [PubMed] [Google Scholar]
- 95.Teitler M et al. (2002) Constitutive activity of G-protein coupled receptors: emphasis on serotonin receptors. Curr. Top. Med. Chem 2, 529–538 [DOI] [PubMed] [Google Scholar]
- 96.Hoyer D et al. (2002) Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol. Biochem. Behav 71, 533–554 [DOI] [PubMed] [Google Scholar]
- 97.Katsuki H and Okuda S (1995) Arachidonic acid as a neurotoxic and neurotrophic substance. Prog. Neurobiol 46, 607–636 [DOI] [PubMed] [Google Scholar]
- 98.Navailles S and De Deurwaerdère P (2011) Presynaptic control of serotonin on striatal dopamine function. Psychopharmacology (Berl.) 213, 213–242 [DOI] [PubMed] [Google Scholar]
- 99.Auclair A et al. (2004) 5-HT2A and alpha1b-adrenergic receptors entirely mediate dopamine release, locomotor response and behavioural sensitization to opiates and psychostimulants. Eur. J. Neurosci 20, 3073–3084 [DOI] [PubMed] [Google Scholar]
- 100.Fletcher PJ et al. (2006) The effects of the 5-HT(2C) receptor antagonist SB242084 on locomotor activity induced by selective, or mixed, indirect serotonergic and dopaminergic agonists. Psychopharmacology (Berl.) 187, 515–525 [DOI] [PubMed] [Google Scholar]
- 101.Fletcher PJ and Higgins GA (2011) Serotonin and Reward-Related Behavior: Focus on 5-HT2C Receptors. In 5-HT2C Receptors in the Pathophysiology of CNS Disease (Di Giovanni G et al. , eds), pp. 293–324, Humana Press [Google Scholar]
- 102.Shioda N et al. (2019) Dopamine D2L Receptor Deficiency Causes Stress Vulnerability through 5-HT1A Receptor Dysfunction in Serotonergic Neurons. J. Neurosci 39, 7551–7563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Aman TK et al. (2006) D2-like dopamine receptors depolarize dorsal raphe serotonin neurons through the activation of nonselective cationic conductance. J. Pharmacol. Exp. Ther DOI: 10.1124/jpet.106.111690 [DOI] [PubMed] [Google Scholar]
- 104.Haj-Dahmane S (2001) D2-like dopamine receptor activation excites rat dorsal raphe 5-HT neurons in vitro. Eur. J. Neurosci 14, 125–134 [DOI] [PubMed] [Google Scholar]
- 105.Niederkofler V et al. (2015) Functional Interplay between Dopaminergic and Serotonergic Neuronal Systems during Development and Adulthood. ACS Chem. Neurosci 6, 1055–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Niederkofler V et al. (2016) Identification of Serotonergic Neuronal Modules that Affect Aggressive Behavior. Cell Rep. 17, 1934–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mechoulam R and Parker LA (2013) The Endocannabinoid System and the Brain. Annu. Rev. Psychol 64, 21–47 [DOI] [PubMed] [Google Scholar]
- 108.Howlett AC et al. (2002) International Union of Pharmacology. XXVII. Classification of Cannabinoid Receptors. Pharmacol. Rev 54, 161–202 [DOI] [PubMed] [Google Scholar]
- 109.Lu H-C and Mackie K (2016) An introduction to the endogenous cannabinoid system. Biol. Psychiatry 79, 516–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Alger BE and Kim J (2011) Supply and demand for endocannabinoids. Trends Neurosci. 34, 304–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Oleson EB et al. (2012) Endocannabinoids Shape Accumbal Encoding of Cue-Motivated Behavior via CB1 Receptor Activation in the Ventral Tegmentum. Neuron 73, 360–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang H et al. (2015) Cocaine-Induced Endocannabinoid Mobilization in the Ventral Tegmental Area. Cell Rep. 12, 1997–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Paladini CA and Tepper JM (2016) Chapter 17 - Neurophysiology of Substantia Nigra Dopamine Neurons: Modulation by GABA and Glutamate. In Handbook of Behavioral Neuroscience 24 (Steiner H and Tseng KY, eds), pp. 335–360, Elsevier [Google Scholar]
- 114.Nieh EH et al. (2016) Inhibitory Input from the Lateral Hypothalamus to the Ventral Tegmental Area Disinhibits Dopamine Neurons and Promotes Behavioral Activation. Neuron 90, 1286–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Stamatakis AM et al. (2013) A unique population of ventral tegmental area neurons inhibits the lateral habenula to promote reward. Neuron 80, 1039–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wilson RI and Nicoll RA (2001) Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 410, 588–592 [DOI] [PubMed] [Google Scholar]
- 117.Cachope R et al. (2012) Selective Activation of Cholinergic Interneurons Enhances Accumbal Phasic Dopamine Release: Setting the Tone for Reward Processing. Cell Rep. 2, 33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Threlfell S et al. (2012) Striatal dopamine release is triggered by synchronized activity in cholinergic interneurons. Neuron 75, 58–64 [DOI] [PubMed] [Google Scholar]
- 119.Mateo Y et al. (2017) Endocannabinoid Actions on Cortical Terminals Orchestrate Local Modulation of Dopamine Release in the Nucleus Accumbens. Neuron 96, 1112–1126.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kosillo P et al. (2016) Cortical Control of Striatal Dopamine Transmission via Striatal Cholinergic Interneurons. Cereb. Cortex N. Y. N 1991 26, 4160–4169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Egertová M and Elphick MR (2000) Localisation of cannabinoid receptors in the rat brain using antibodies to the intracellular C-terminal tail of CB. J. Comp. Neurol 422, 159–171 [DOI] [PubMed] [Google Scholar]
- 122.Segawa T et al. (1976) Mechanism for the increase of brain 5-hydroxy-tryptamine and 5-hydroxyindoleacetic acid following delta9-tetrahydrocannabinol administration to rats. Jpn. J. Pharmacol 26, 377–379 [DOI] [PubMed] [Google Scholar]
- 123.Egashira N et al. (2002) Involvement of 5-hydroxytryptamine neuronal system in Delta(9)-tetrahydrocannabinol-induced impairment of spatial memory. Eur. J. Pharmacol 445, 221–229 [DOI] [PubMed] [Google Scholar]
- 124.Haj-Dahmane S and Shen R-Y (2013) Endocannabinoid Signaling and the Regulation of the Serotonin System. In Endocannabinoid Regulation of Monoamines in Psychiatric and Neurological Disorders (Van Bockstaele EJ, ed), pp. 239–254, Springer [Google Scholar]
- 125.Jolas T and Aghajanian GK (1997) Opioids suppress spontaneous and NMDA-induced inhibitory postsynaptic currents in the dorsal raphe nucleus of the rat in vitro. Brain Res. 755, 229–245 [DOI] [PubMed] [Google Scholar]
- 126.Hermann H et al. (2002) Coexpression of the cannabinoid receptor type 1 with dopamine and serotonin receptors in distinct neuronal subpopulations of the adult mouse forebrain. Neuroscience 109, 451–460 [DOI] [PubMed] [Google Scholar]
- 127.Marsicano G and Lutz B (1999) Expression of the cannabinoid receptor CB1 in distinct neuronal subpopulations in the adult mouse forebrain. Eur. J. Neurosci 11, 4213–4225 [DOI] [PubMed] [Google Scholar]
- 128.Haj-Dahmane S and Shen R-Y (2009) Endocannabinoids suppress excitatory synaptic transmission to dorsal raphe serotonin neurons through the activation of presynaptic CB1 receptors. J. Pharmacol. Exp. Ther 331, 186–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Haj-Dahmane S and Shen R-Y (2011) Modulation of the serotonin system by endocannabinoid signaling. Neuropharmacology 61, 414–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mendiguren A and Pineda J (2009) Effect of the CB1 receptor antagonists rimonabant and AM251 on the firing rate of dorsal raphe nucleus neurons in rat brain slices. Br. J. Pharmacol 158, 1579–1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ren J et al. (2019) Single-cell transcriptomes and whole-brain projections of serotonin neurons in the mouse dorsal and median raphe nuclei. eLife 8, e49424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sun F et al. (2018) A Genetically Encoded Fluorescent Sensor Enables Rapid and Specific Detection of Dopamine in Flies, Fish, and Mice. Cell 174, 481–496.e19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Prus AJ et al. (2009) Conditioned Place Preference. In Methods of Behavior Analysis in Neuroscience (2nd edn) (Buccafusco JJ, ed), CRC Press/Taylor & Francis [Google Scholar]