Abstract
Introduction:
Lung cancer survivors are at high risk of a second primary lung cancer (SPLC). However, SPLC risk factors have not been established and the impact of tobacco smoking remains controversial. We examined risk factors for SPLC across multiple epidemiologic cohorts and assessed the impact of smoking cessation on reducing SPLC risk.
Methods:
We analyzed data from 7,059 participants in the Multiethnic Cohort (MEC) diagnosed with an initial primary lung cancer (IPLC) between 1993 and 2017. Cause-specific proportional hazards models estimated SPLC risk. We conducted validation studies using the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO, N=3,423 IPLC cases) and European Prospective Investigation into Cancer and Nutrition (EPIC, N=4,731 IPLC cases) cohorts and pooled the SPLC risk estimates using random effects meta-analysis.
Results:
Overall, 163 (2.3%) MEC cases developed a SPLC. Smoking pack-years (HR 1.18 per 10 pack-years; P<0.001) and smoking intensity (HR 1.30 per 10 cigarettes per day (CPD); P<0.001) were significantly associated with increased SPLC risk. Individuals who met the 2013 U.S. Preventive Services Task Force’s (USPSTF) screening criteria at IPLC diagnosis also had an increased SPLC risk (HR 1.92; P<0.001). Validation studies with PLCO and EPIC showed consistent results. Meta-analysis yielded pooled HRs of 1.16 per 10 pack-years (Pmeta<0.001), 1.25 per 10 CPD (Pmeta<0.001), and 1.99 (Pmeta<0.001) for meeting the USPSTF criteria. In MEC, smoking cessation after IPLC diagnosis was associated with an 83% reduction in SPLC risk (HR 0.17; P<0.001).
Conclusions:
Tobacco smoking is a risk factor for SPLC. Smoking cessation after IPLC diagnosis may reduce the risk of SPLC. Additional strategies for SPLC surveillance and screening are warranted.
Keywords: second primary lung cancer, tobacco smoking, smoking cessation, surveillance, screening
INTRODUCTION
Lung cancer has the second-highest cancer incidence among women and men and continues to lead cancer-related mortality in the United States. Thus, lung cancer remains a significant public health problem. However, the last decade saw a 26% improvement in 5-year survival rates1, indicating that the number of lung cancer survivors is increasing. Multiple studies have demonstrated that survivors of an initial primary lung cancer (IPLC) are at high risk of developing a second primary lung cancer (SPLC)2–5, with incidence rates after IPLC surgical resection ranging from 1–2% per patient-year2. Development of a SPLC can complicate a patient’s clinical assessment and may require further aggressive intervention, adding to an already heavy burden for lung cancer survivors.
In 2013, the United States Preventive Services Task Force (USPSTF) established national guidelines for IPLC screening based on age (55 to 80 years) and smoking history (≥30 pack-years, cessation ≤15 years)6—known risk factors for IPLC—which are currently under revision7. However, evidence-based guidelines for SPLC surveillance and screening are lacking8, in large part due to an absence of established risk factors for SPLC9. Prior studies have sought to identify SPLC risk factors, but these have been limited to single institutions or population-based registries comprised of selected patient populations without validation in independent cohorts10–13. Furthermore, these studies have utilized different methodological designs and/or statistical approaches for identifying SPLC risk factors and, accordingly, have reached conflicting conclusions. For example, while tobacco smoking is an established risk factor for IPLC, the association between smoking and SPLC risk has been controversial, with some studies reporting a positive association5,10 and others showing non-significant relationships11,12. Our group previously utilized a risk stratification approach to distinguish between IPLC patients at high versus low risk of SPLC using the Surveillance, Epidemiology, and End Results (SEER) database13. However, this cohort did not contain data on potentially important risk factors such as tobacco smoking.
In the present study, we leveraged data from the Multiethnic Cohort (MEC) to identify risk factors for SPLC among IPLC cases with a focus on tobacco smoking. We validated these findings with two additional cohorts: the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial (PLCO) and the European Prospective Investigation into Cancer and Nutrition (EPIC). Finally, in a subset analysis in MEC, we assessed the impact of smoking cessation on reducing SPLC risk.
METHODS
MEC: Participants and Study Design (Discovery Cohort)
MEC is a population-based cohort that prospectively follows >215,000 California and Hawaii residents aged 45 to 75 years at enrollment (1993–1996)14. In this study, we included all participants who were diagnosed with an incident IPLC between 1993 and 2017 and who had non-missing tobacco smoking data (see Supplementary Methods for details). Demographic and behavioral data were analyzed as self-reported in the baseline questionnaire; smoking-related variables were assigned values from the questionnaire nearest in time and prior to IPLC diagnosis: either the baseline (1993–1996) or 10-year follow-up questionnaire (2003–2008), if available (N=1,872; Supplementary Methods). Smoking-related variables included: smoking status, pack-years, intensity (i.e., cigarettes per day [CPD]), and quit years (i.e., years since cessation). Smoking intensity reflected the average lifetime consumption of cigarettes. Incident IPLC and SPLC were identified through linkage to SEER registries together with IPLC age at diagnosis, stage, histology, and therapies. SPLC was defined according to Martini and Melamed criteria (Supplementary Methods)15. Deaths were ascertained via linkage to the National Death Index and death certificate files14.
As primary analyses, we evaluated potential risk factors for SPLC focusing on smoking-related variables and clinical variables that have not previously been examined in the literature (i.e., body mass index [BMI], personal history of cancer, family history of lung cancer)10–13. We also estimated the SPLC risk associated with meeting the 2013 USPSTF lung cancer screening criteria at IPLC diagnosis, as a composite smoking and age measure of individuals at particularly high risk of IPLC. We additionally conducted a set of confirmatory secondary analyses to evaluate the associations between SPLC and factors that were examined in prior studies (i.e., sex, race/ethnicity, education, IPLC therapies)10,12,13.
Given the focus on the smoking-SPLC association, we conducted a subset analysis examining the impact of smoking cessation on SPLC risk using the longitudinal measurements of smoking at baseline and 10-year follow-up. Smoking cessation was defined as the change in smoking status from “current” at baseline to “former” at follow-up (versus “current” at baseline and “current” at follow-up). Participants included those who were current smoking at baseline, had 10-year follow-up smoking data, and were diagnosed with their IPLC before 10-year follow-up (N=156; Supplementary Figure 1). We evaluated the association between smoking cessation after IPLC diagnosis and SPLC after 10-year follow-up.
MEC: Statistical Analyses
Cause-specific proportional hazards models evaluated the associations between candidate risk factors and SPLC, accounting for the competing risk of death from all causes16. The cause-specific function was selected because it can estimate the effects of factors on SPLC risk with sustained power in multiple competing risk scenarios while minimizing type-I error17. All models evaluated SPLC risk from the date of IPLC diagnosis. The proportional hazards assumption was confirmed for all models. In light of prior data indicating that IPLC age at diagnosis, stage, and histology are relevant predictors for SPLC13, we adjusted for these as covariates in all analyses. To account for multiple testing, we implemented a Bonferroni threshold for the primary analyses (P=0.05/9=0.005). For all other analyses, statistical significance was defined at a two-sided P<0.05. Cumulative incidences were calculated using Gray’s method18. We handled missing data by performing multiple imputation19,20 and assessed the robustness of the findings using complete cases (Supplementary Methods).
We further evaluated the smoking-related variables in a subgroup analysis of early-stage IPLC cases to reduce potential non-causal effects from the competing risk of death17 and to make the results comparable to prior data10–12. Additional sensitivity analyses examined the smoking-SPLC associations among ever smokers, within major IPLC histological subtypes (i.e., adenocarcinoma, squamous cell carcinoma), and among advanced-stage IPLC cases. For continuous smoking variables (i.e., pack-years, CPD), we evaluated their potential non-linear effects using natural cubic splines21. All analyses were performed using R version 4.0.2 (Vienna, Austria).
PLCO and EPIC: Validation Cohorts
We validated the SPLC associations using two additional epidemiologic cohorts: PLCO (N=3,423 IPLC cases with 110 SPLC) and EPIC (N=4,731 IPLC cases with 16 SPLC). Detailed information on these cohorts is included in the Supplementary Methods. As with the discovery cohort, cause-specific proportional hazards models evaluated the SPLC associations in PLCO and EPIC. To pool the SPLC associations across the three cohorts (discovery and validation), we used random effects meta-analysis22. Statistical significance was defined at a two-sided P<0.05. Smoking cessation was not evaluated in these cohorts because longitudinal smoking data were unavailable for this collaborative study.
RESULTS
Multiethnic Cohort: Participant Characteristics
Among 7,059 IPLC cases in MEC, the mean age at diagnosis was 74.3 years and a slight majority were male (55.9%) (Table 1). Most IPLC cases were former or current smoking (87.8%), but prospective SPLC cases had particularly high mean smoking pack-years (31.2) and mean CPD (17.6). SPLC cases also had high frequencies of local IPLC stage (57.1%), adenocarcinoma IPLC (58.3%), prior IPLC surgery (73.6%), and meeting the USPSTF screening criteria (41.7%).
Table 1.
Multiethnic Cohort Participant Characteristics.
| Characteristic | Overall N=7,059 | Outcome | ||
|---|---|---|---|---|
| SPLC N=163 | Deceased N=5,646 | Censored N=1,250 | ||
| Age at IPLC diagnosis (years), mean (SD) | 74.3 (8.3) | 72.2 (8.1) | 74.0 (8.2) | 76.2 (8.7) |
| BMI (kg/m2)a, mean (SD) | 25.9 (4.7) | 26.1 (4.6) | 25.9 (4.7) | 26.2 (4.8) |
| Sex, N (%) | ||||
| Male | 3,949 (55.9) | 84 (51.5) | 3,297 (58.4) | 568 (45.4) |
| Female | 3,110 (44.1) | 79 (48.5) | 2,349 (41.6) | 682 (54.6) |
| Race/ethnicity, N (%) | ||||
| African American | 1,798 (25.5) | 37 (22.7) | 1,477 (26.2) | 284 (22.7) |
| Japanese American | 1,603 (22.7) | 37 (22.7) | 1,289 (22.8) | 277 (22.2) |
| Latino | 998 (14.1) | 20 (12.3) | 723 (12.8) | 255 (20.4) |
| Native Hawaiian | 573 (8.1) | 14 (8.6) | 481 (8.5) | 78 (6.2) |
| White | 1,714 (24.3) | 47 (28.8) | 1,382 (24.5) | 285 (22.8) |
| Other | 373 (5.3) | 8 (4.9) | 294 (5.2) | 71 (5.7) |
| Education, N (%) | ||||
| High school or less | 3,592 (50.9) | 71 (43.6) | 2,974 (52.7) | 547 (43.8) |
| Some college or graduate | 2,811 (39.8) | 77 (47.2) | 2,170 (38.4) | 564 (45.1) |
| Postgraduate | 626 (8.9) | 15 (9.2) | 481 (8.5) | 130 (10.4) |
| Unknown | 30 (0.4) | 0 (0.0) | 21 (0.4) | 9 (0.7) |
| Personal history of cancer, N (%) | ||||
| Yes | 1,824 (25.8) | 52 (31.9) | 1,429 (25.3) | 343 (27.4) |
| No | 5,235 (74.2) | 111 (68.1) | 4,217 (74.7) | 907 (72.6) |
| Family history of lung cancer, N (%) | ||||
| Yes | 622 (8.8) | 14 (8.6) | 483 (8.6) | 125 (10.0) |
| No | 6,437 (91.2) | 149 (91.4) | 5,163 (91.4) | 1,125 (90.0) |
| Smoking status, N (%) | ||||
| Never | 863 (12.2) | 19 (11.7) | 607 (10.8) | 237 (19.0) |
| Former | 3,243 (45.9) | 72 (44.2) | 2,603 (46.1) | 568 (45.4) |
| Current | 2,953 (41.8) | 72 (44.2) | 2,436 (43.1) | 445 (35.6) |
| Smoking pack-yearsb, mean (SD) | 27.0 (20.0) | 31.2 (21.3) | 28.0 (20.0) | 22.1 (19.5) |
| Cigarettes per dayb, mean (SD) | 15.5 (10.0) | 17.6 (10.8) | 16.0 (9.9) | 13.3 (10.2) |
| Smoking quit yearsb,c, median (IQR) | 0.5 (0–13) | 0.3 (0–11) | 0.5 (0–13) | 4.0 (0–18) |
| IPLC stage, N (%) | ||||
| Local | 1,223 (17.3) | 93 (57.1) | 702 (12.4) | 428 (34.2) |
| Regional | 1,583 (22.4) | 51 (31.3) | 1,185 (21.0) | 347 (27.8) |
| Distant | 3,811 (54.0) | 17 (10.4) | 3,378 (59.8) | 416 (33.3) |
| Unknown | 442 (6.3) | 2 (1.2) | 381 (6.7) | 59 (4.7) |
| IPLC histology, N (%) | ||||
| Adenocarcinoma | 2,832 (40.1) | 95 (58.3) | 2,070 (36.7) | 667 (53.4) |
| Squamous cell carcinoma | 1,401 (19.8) | 36 (22.1) | 1,120 (19.8) | 245 (19.6) |
| Large cell carcinoma | 225 (3.2) | 9 (5.5) | 189 (3.3) | 27 (2.2) |
| Small cell lung carcinoma | 731 (10.4) | 3 (1.8) | 651 (11.5) | 77 (6.2) |
| Otherd | 1,870 (26.5) | 20 (12.3) | 1,616 (28.6) | 234 (18.7) |
| IPLC surgery, N (%) | ||||
| Yes | 1,512 (21.4) | 120 (73.6) | 830 (14.7) | 562 (45.0) |
| No | 4,942 (70.0) | 34 (20.9) | 4,264 (75.5) | 644 (51.5) |
| Unknown | 605 (8.6) | 9 (5.5) | 552 (9.8) | 44 (3.5) |
| IPLC radiotherapy, N (%) | ||||
| Yes | 2,375 (33.6) | 26 (16.0) | 2,050 (36.3) | 299 (23.9) |
| No | 4,496 (63.7) | 137 (84.0) | 3,443 (61.0) | 916 (73.3) |
| Unknown | 188 (2.7) | 0 (0.0) | 153 (2.7) | 35 (2.8) |
| IPLC chemotherapy, N (%) | ||||
| Yes | 2,299 (32.6) | 23 (14.1) | 1,906 (33.8) | 370 (29.6) |
| No | 4,426 (62.7) | 139 (85.3) | 3,454 (61.2) | 833 (66.6) |
| Unknown | 334 (4.7) | 1 (0.6) | 286 (5.1) | 47 (3.8) |
| Met the USPSTF criteria, N (%) | ||||
| Yes | 2,058 (29.2) | 68 (41.7) | 1,755 (31.1) | 235 (18.8) |
| No | 5,001 (70.8) | 95 (58.3) | 3,891 (68.9) | 1,015 (81.2) |
| Follow-up (months), median (IQR) | 10.0 (3–33) | 46.0 (15–78) | 7.0 (2–17) | 59.0 (35–111) |
Demographic variables were collected from the baseline Multiethnic Cohort questionnaire except smoking-related variables (see below). Age at IPLC diagnosis, IPLC stage, IPLC histology, and IPLC therapies were obtained through linkage to SEER registries. The 2013 USPSTF lung cancer screening eligibility was calculated using age at IPLC diagnosis and smoking-related variables from the MEC questionnaires. Percentages may not sum to 100% due to rounding. Abbreviations: N sample size, SPLC second primary lung cancer, IPLC initial primary lung cancer, SD standard deviation, BMI body mass index, kg kilogram, m meter, IQR interquartile range, USPSTF United States Preventive Services Task Force.
BMI was unknown in 80 (1.1%) participants: 0 (0.0%) SPLC, 72 (1.3%) deceased, and 8 (0.6%) censored.
Smoking-related variables are from the questionnaire closest in time and prior to the date of IPLC: at baseline or at 10-year follow-up, if available (N=1,872).
Smoking quit years were assessed only among ever smokers (N=6,196).
Other histologies are listed in the Supplementary Methods and include histologies such as adenosquamous, lung neuroendocrine tumors, carcinoma not otherwise specified, and others.
Multiethnic Cohort: SPLC Incidence and Risk Factors
Median follow-up in the cohort was 10.0 months overall and 57.0 months among SPLC or censored cases (Table 1). Overall, 163 (2.3%) IPLC cases developed a SPLC (Supplementary Figure 2), with a median time from IPLC diagnosis to SPLC diagnosis of 3.8 years (Supplementary Figure 3). Smoking pack-years (hazard ratio [HR] 1.18 per 10 pack-years, 95% confidence interval [CI] 1.09–1.27; P<0.001) and smoking intensity (HR 1.30 per 10 CPD, 95% CI 1.12–1.51; P<0.001) were significantly associated with an increased risk of SPLC after adjusting for IPLC age at diagnosis, stage, and histology (Table 2, Figure 1A). Participants who met the USPSTF criteria had a nearly two-fold increased risk of SPLC (HR 1.92, 95% CI 1.39–2.64; P<0.001). Current smoking status and quit years trended towards having significant associations with SPLC but did not meet the Bonferroni threshold. Other primary variables (e.g., BMI) were not associated with SPLC. Sensitivity analyses using complete cases provided consistent results (Supplementary Table 1).
Table 2.
Associations Between Second Primary Lung Cancer and Participant Characteristics in the Multiethnic Cohort.
| Characteristic | Hazard Ratio (95% CI) | P |
|---|---|---|
| Primary Analyses | ||
| Smoking status | ||
| Never | Reference | |
| Former | 1.32 (0.79–2.20) | 0.284 |
| Current | 1.80 (1.07–3.03) | 0.028 |
| Smoking per 10 pack-years | 1.18 (1.09–1.27) | <0.001 |
| Smoking per 10 cigarettes per day | 1.30 (1.12–1.51) | <0.001 |
| Smoking per 1 quit yeara | 0.97 (0.95–0.99) | 0.010 |
| Met the USPSTF criteria | ||
| No | Reference | |
| Yes | 1.92 (1.39–2.64) | <0.001 |
| BMI (per 1 kg/m2) | 1.02 (0.99–1.05) | 0.267 |
| Personal history of cancer | ||
| No | Reference | |
| Yes | 1.30 (0.93–1.82) | 0.120 |
| Family history of lung cancer | ||
| No | Reference | |
| Yes | 0.82 (0.47–1.42) | 0.472 |
| Secondary Analyses | ||
| Sex | ||
| Female | Reference | |
| Male | 1.24 (0.91–1.70) | 0.179 |
| Race/ethnicity | ||
| White | Reference | |
| African American | 1.00 (0.65–1.55) | 0.989 |
| Japanese American | 0.80 (0.52–1.24) | 0.324 |
| Latino | 0.89 (0.53–1.51) | 0.679 |
| Native Hawaiian | 1.09 (0.60–2.00) | 0.769 |
| Other | 0.71 (0.33–1.50) | 0.370 |
| Education | ||
| High school or less | Reference | |
| Some college or graduate | 1.12 (0.81–1.55) | 0.501 |
| Postgraduate | 0.93 (0.53–1.63) | 0.799 |
| IPLC surgery | ||
| No | Reference | |
| Yes | 1.89 (1.16–3.07) | 0.010 |
| IPLC radiotherapy | ||
| No | Reference | |
| Yes | 0.64 (0.41–0.98) | 0.041 |
| IPLC chemotherapy | ||
| No | Reference | |
| Yes | 0.55 (0.35–0.88) | 0.013 |
| Covariates | ||
| Age at IPLC diagnosis (per 1 year) | 1.00 (0.98–1.02) | 0.841 |
| IPLC stage | ||
| Local/regional | Reference | |
| Distant | 0.33 (0.20–0.56) | <0.001 |
| Expanded IPLC stage | ||
| Local | Reference | |
| Regional | 0.68 (0.48–0.96) | 0.028 |
| Distant | 0.28 (0.16–0.47) | <0.001 |
| IPLC histology | ||
| Squamous cell carcinoma | Reference | |
| Adenocarcinoma | 1.11 (0.76–1.64) | 0.584 |
| Large cell carcinoma | 1.66 (0.80–3.46) | 0.173 |
| Small cell carcinoma | 0.43 (0.13–1.40) | 0.162 |
| Other | 0.65 (0.38–1.13) | 0.130 |
All cause-specific proportional hazards models accounted for the competing risk of death. Variables in the primary and secondary analyses were evaluated in individual cause-specific proportional hazards regression models adjusting for age at IPLC diagnosis, IPLC histology, and IPLC stage. Among covariates, age at IPLC diagnosis was adjusted for IPLC histology and stage, IPLC histology was adjusted for IPLC age and stage, and IPLC stage was adjusted for IPLC age and histology.
Abbreviations: CI confidence interval, USPSTF United States Preventive Services Task Force, BMI body mass index, kg kilogram, m meter, IPLC initial primary lung cancer.
Smoking quit years were assessed only among ever smokers (N=6,196).
Figure 1. Forest Plots of Associations Between Smoking-Related Factors and Second Primary Lung Cancer in the Multiethnic Cohort.
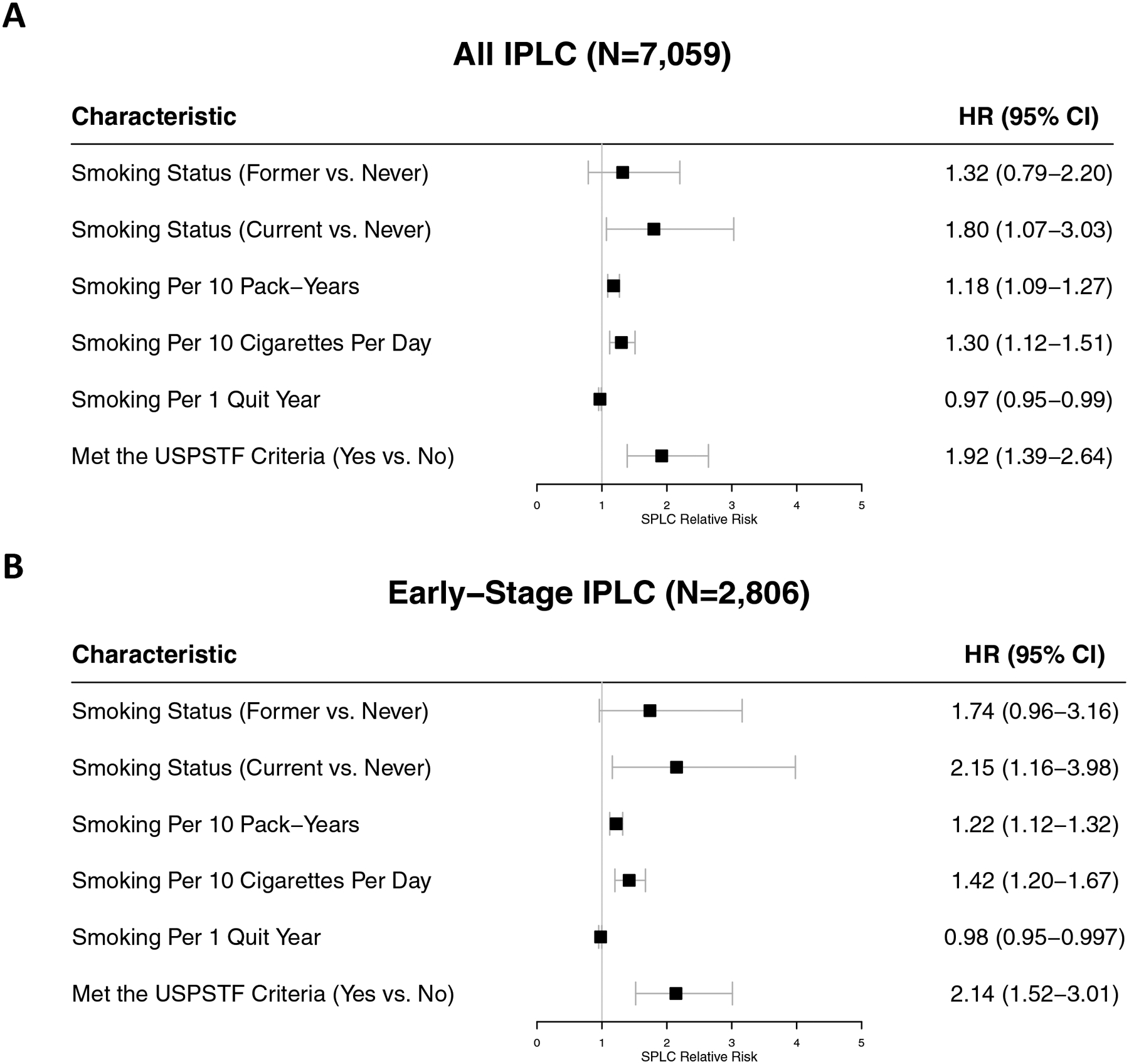
The smoking-SPLC associations were evaluated among (A) all IPLC cases (N=7,059) and (B) early-stage (I-III) IPLC cases (N=2,806). Smoking-related data were collected from the baseline questionnaire or 10-year follow-up questionnaire prior to IPLC diagnosis, if available. Meeting the 2013 USPSTF criteria was determined at IPLC diagnosis. All variables were evaluated in individual cause-specific proportional hazards models accounting for the competing risk of death. Models for all-stage IPLC cases adjusted for age at IPLC diagnosis, IPLC histology, and IPLC stage; models for early-stage IPLC cases adjusted for age at IPLC diagnosis and IPLC histology. Abbreviations: SPLC second primary lung cancer, IPLC initial primary lung cancer, N sample size, USPSTF United States Preventive Services Task Force, HR hazard ratio, CI confidence interval, vs. versus.
In the secondary analyses, only the treatment variables demonstrated significant associations with SPLC, including IPLC surgery which was associated with an increased risk of SPLC (HR 1.89, 95% CI 1.16–3.07; P=0.010; Table 2). Other secondary variables such as sex, race/ethnicity, and education did not show significant associations with SPLC, consistent with previous assessments13. Distant IPLC stage was associated with a reduced risk of SPLC compared to local or regional IPLC (HR 0.33, 95% CI 0.20–0.56; P<0.001), reflecting higher mortality before developing SPLC.
The subgroup analysis of early-stage IPLC cases revealed that the point estimates and statistical significance for almost all smoking-related variables were heightened (Figure 1B). Among ever smokers, the SPLC risk estimates were consistent (Supplementary Figure 4A). Stratified by IPLC histology, the effects of smoking remained largely consistent, although the significance was reduced among those with squamous IPLCs (N=1,401), possibly due to a smaller sample size (Supplementary Figure 4B–C). Among advanced-stage IPLC cases, none of the smoking variables had a significant association with SPLC, except for smoking intensity which had an inverted point estimate (HR 0.57 per 10 CPD, 95% CI 0.33–0.98; Supplementary Figure 4D) compared to that in all-stage and early-stage IPLC cases; this inverse association potentially reflects a higher competing risk of mortality—and, hence, a reduced SPLC incidence—among advanced-stage IPLC cases.
Categorization of smoking pack-years and CPD revealed that the highest levels (≥30 pack-years and ≥30 CPD) conferred the greatest risk of SPLC (Figure 2). In applying smoothing splines, we confirmed that the linear models offered the best fit for both smoking pack-years and smoking intensity (Supplementary Figure 5).
Figure 2. Sensitivity Analyses for Associations Between Categorical (A) Smoking Pack-Years and (B) Cigarettes Per Day and Second Primary Lung Cancer in the Multiethnic Cohort.
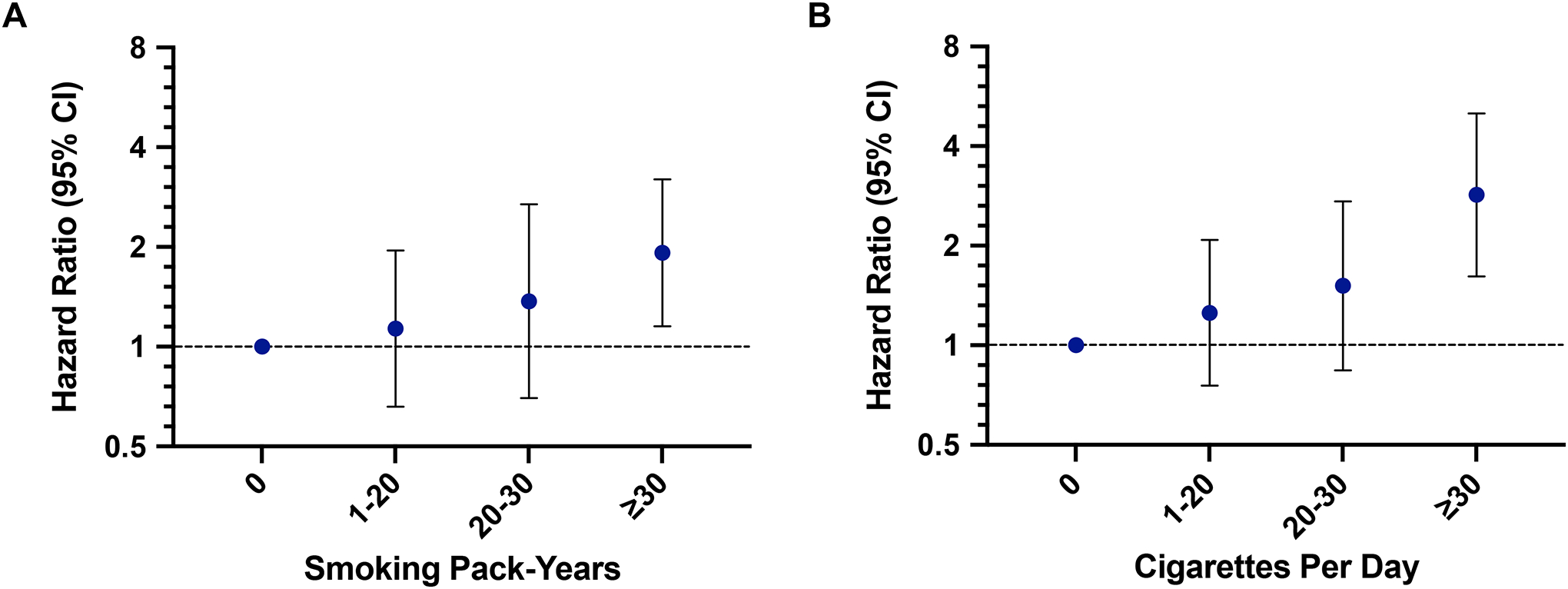
All risk estimates were generated from cause-specific proportional hazards models adjusting for age at IPLC diagnosis, IPLC histology, and IPLC stage and accounting for the competing risk of death. Abbreviation: CI confidence interval.
PLCO and EPIC: SPLC Validation Cohorts and Meta-Analysis
The validation PLCO cohort included 3,423 IPLC cases and EPIC included 4,731 IPLC cases (Supplementary Tables 2–3). In PLCO, smoking pack-years (HR 1.10 per 10 pack-years, 95% CI 1.04–1.15; P<0.001), smoking intensity (HR 1.20 per 10 CPD, 95% CI 1.06–1.36; P=0.004), and meeting the USPSTF criteria (HR 2.09, 95% CI 1.35–3.24; P=0.001) were significantly associated with an increased risk of SPLC after adjusting for IPLC age, stage, and histology (Figure 3, Supplementary Table 4). In EPIC, smoking pack-years (HR 1.37 per 10 pack-years, 95% CI 1.11–1.68; P=0.003) was significantly associated with an increased risk of SPLC after adjusting for IPLC age and histology, whereas smoking intensity (HR 1.52 per 10 CPD, 95% CI 0.96–2.41; P=0.074) and meeting the USPSTF criteria (HR 2.34, 95% CI 0.80–6.85; P=0.120) both trended towards statistical significance in the same directions of the HRs in MEC.
Figure 3. Meta-Analyses of Associations Between Smoking-Related Factors and Second Primary Lung Cancer Across MEC, PLCO, and EPIC.
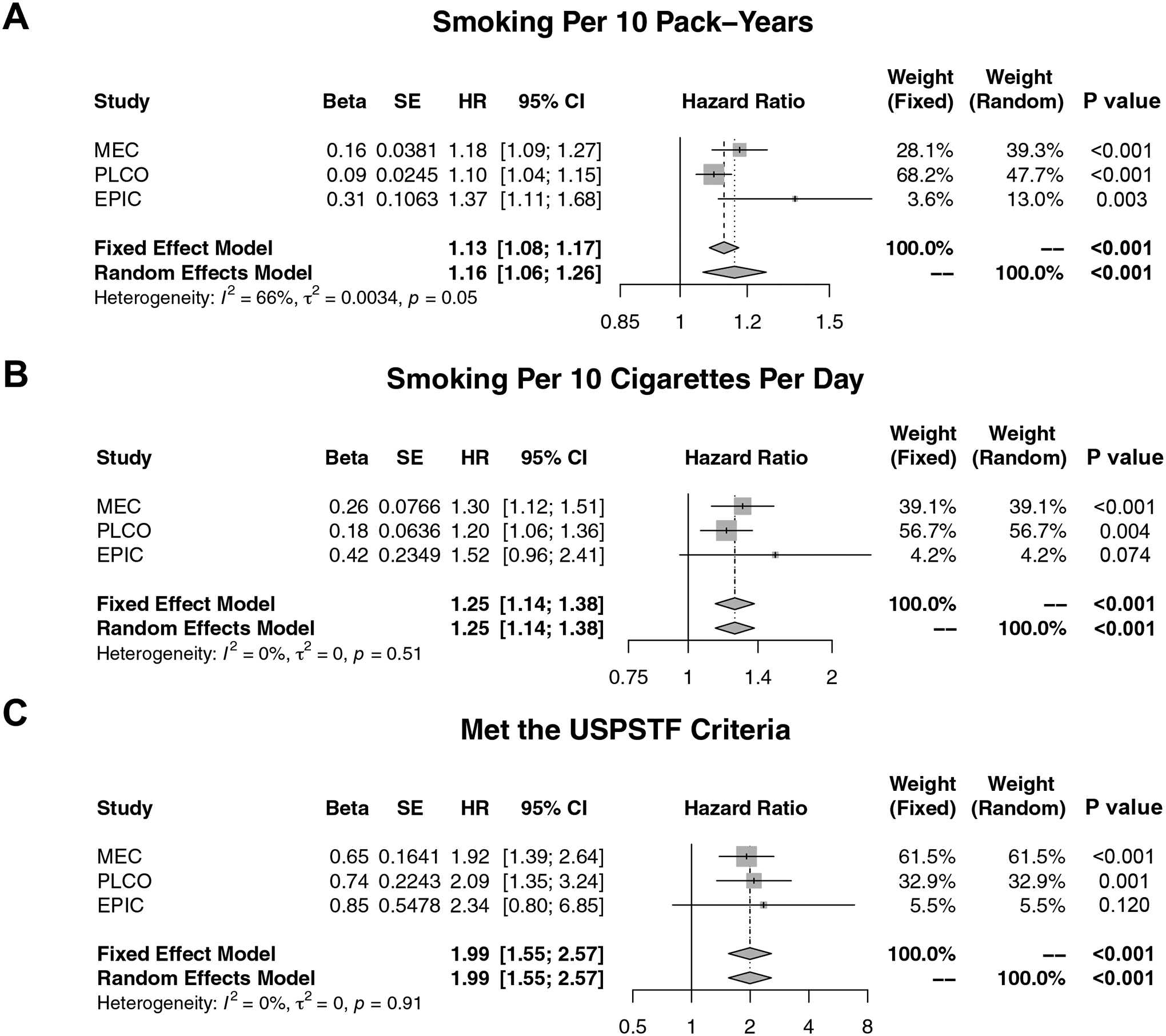
Cause-specific proportional hazards models accounting for the competing risk of death from all causes were used to evaluate the risk of second primary lung cancer by (A) smoking per 10 pack-years, (B) smoking per 10 cigarettes per day, and (C) meeting the 2013 USPSTF screening criteria, adjusting for IPLC age at diagnosis, stage, and histology in MEC and PLCO and by IPLC age at diagnosis and histology in EPIC. Abbreviations: MEC Multiethnic Cohort; PLCO Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial; EPIC European Prospective Investigation into Cancer and Nutrition; USPSTF United States Preventive Services Task Force; IPLC initial primary lung cancer; SE standard error; HR hazard ratio; CI confidence interval.
When pooling the SPLC associations across all three cohorts through random effects meta-analysis, smoking pack-years (HR 1.16 per 10 pack-years, 95% CI 1.06–1.26; Pmeta<0.001), smoking intensity (HR 1.25 per 10 CPD, 95% CI 1.14–1.38; Pmeta<0.001), and meeting the USPSTF criteria (HR 1.99, 95% CI 1.55–2.57; Pmeta<0.001) were all significantly associated with an increased risk of SPLC (Figure 3). In contrast, the other primary and secondary variables did not exhibit significant associations with SPLC, with the exception of the IPLC therapies (Supplementary Figure 6).
Multiethnic Cohort: Smoking Cessation Analysis
To follow up on the smoking-SPLC association, we evaluated the effect of smoking cessation after IPLC diagnosis on SPLC risk in the MEC subset of 156 participants (Supplementary Figure 1). Of these, 125 (80.1%) participants who were current smoking at baseline reported having quit smoking at the 10-year follow-up (Supplementary Table 5). Overall, 15 (9.6%) IPLC cases developed a SPLC after the 10-year follow-up (Figure 4). When adjusting for age at IPLC diagnosis solely (to support model convergence in the small sample size), smoking cessation was associated with an 83% reduction in SPLC risk (HR 0.17, 95% CI 0.06–0.47; P<0.001; Supplementary Table 6).
Figure 4. Smoking Cessation and Risk of Second Primary Lung Cancer in the Multiethnic Cohort (N=156).
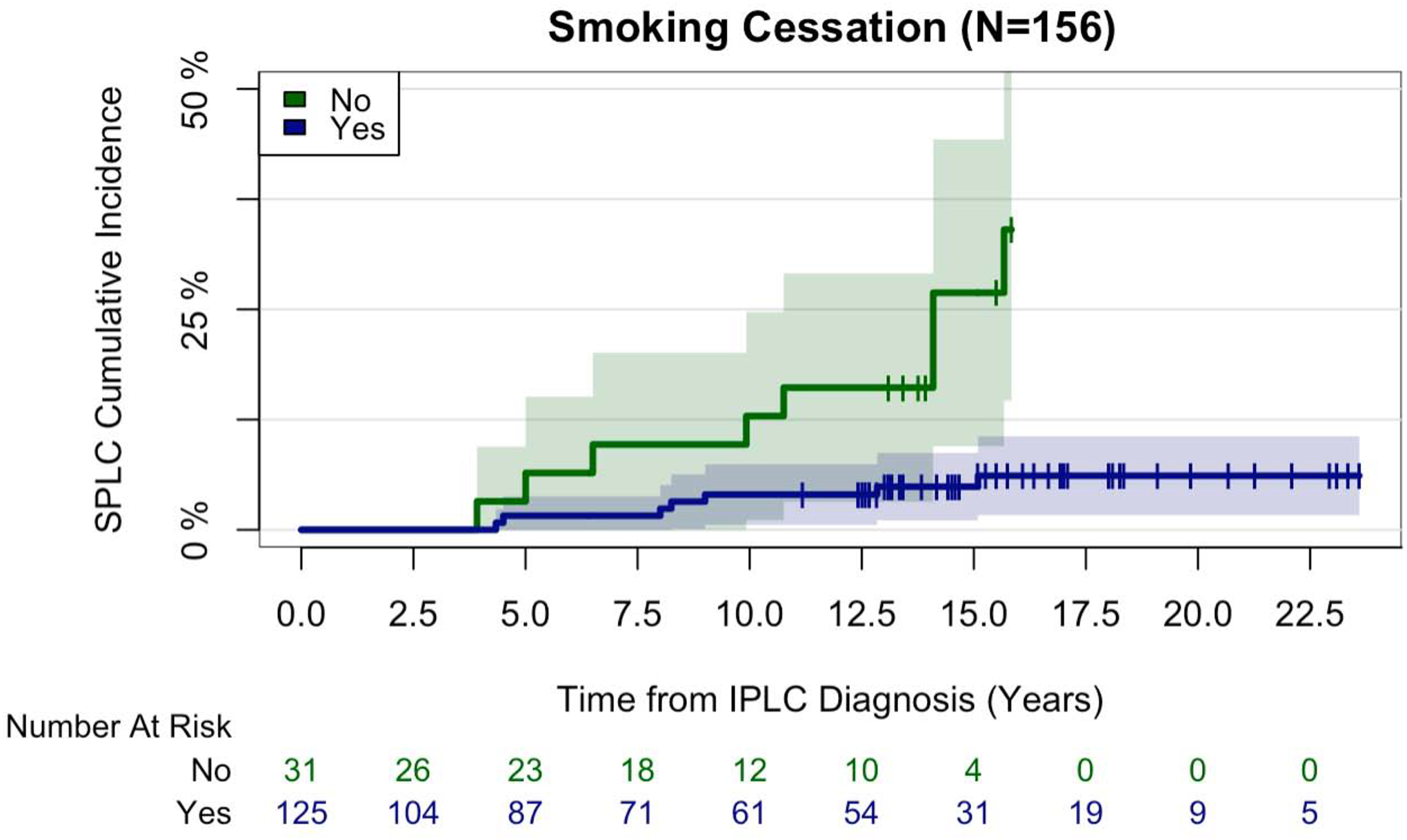
Participants in this subset analysis were current smoking at baseline, had 10-year follow-up smoking data, and were diagnosed with an initial primary lung cancer before follow-up. Participants who reported that they were “former” smoking at 10-year follow-up were classified as undergoing smoking cessation (“yes”), whereas those who reported that they were “current” smoking at 10-year follow-up were classified as not undergoing smoking cessation (“no”). Participants were followed for the development of SPLC after 10-year follow-up. This cumulative incidence plot was generated using Gray’s method, accounting for the competing risk of death from all causes. Abbreviations: N sample size, SPLC second primary lung cancer, IPLC initial primary lung cancer.
DISCUSSION
In this study, we showed that tobacco smoking is a significant risk factor for SPLC among IPLC cases based on three epidemiologic cohorts. We also demonstrated in a landmark analysis that smoking cessation after IPLC diagnosis is associated with a substantial reduction in the risk of SPLC. To the best of our knowledge, this is the first population-based study to leverage multiple epidemiologic cohorts in identifying and validating risk factors for SPLC, including tobacco smoking. We evaluated several candidate SPLC risk factors that have not been previously examined (e.g., personal history of cancer, family history of lung cancer) and assessed detailed smoking exposures through multiple measures, including smoking status, pack-years, intensity, and years since cessation.
Importantly, these data support tobacco smoking as a modifiable risk factor for SPLC, with smoking cessation after IPLC diagnosis associated with a significant reduction in SPLC risk. Although this effect was observed in a small subset analysis, it lends further support to smoking cessation efforts in patients even after an IPLC diagnosis and/or treatment. This finding further supports the relationship between tobacco smoking and SPLC23, though additional validation of the precise effect is required.
The smoking-SPLC associations in the literature have been inconsistent, possibly due to a lack of appropriate statistical methods, small sample sizes, or heterogeneity in the smoking exposures examined across studies. The smoking-SPLC relationship was first detailed by Boyle, et al. who reported that smoking per 10 pack-years was associated with an 8% increased risk of SPLC in ever-smoking patients after definitive surgery10. This is in contrast to two other studies which failed to find a smoking-SPLC association11,12. Notably, the latter two studies applied a Fine and Gray subdistribution model which may be underpowered to detect a smoking-SPLC effect17,24, as smoking is associated with both SPLC (the event of interest) and the competing risk of mortality25. In this analytical setting, a cause-specific hazard model may more accurately estimate the effect of smoking on SPLC risk and is the preferred approach for “causal” (i.e., risk-centered) analyses17,26. Accordingly, when we applied a Fine and Gray model to the MEC data, we found that the smoking effects, while still present, were attenuated (data not shown).
In stratifying the MEC population by IPLC stage at diagnosis, we found that the smoking effects were especially prominent among participants with an early-stage (I-III) IPLC. However, in participants who were diagnosed with an advanced IPLC, most of the smoking-related variables did not have a significant association with SPLC, except for smoking intensity which had an inverted point estimate compared to that in all-stage and early-stage IPLC cases. This inverse association is likely due to a higher competing risk of mortality and, hence, a reduced SPLC incidence among advanced-stage IPLC cases. As described above, smoking is not only associated with an increased risk of SPLC but also with an increased risk of mortality25,27,28, and among participants with advanced IPLCs, the competing risk of mortality predominates. However, a definitive conclusion among these participants is precluded given the relatively few number of SPLC events. Regardless, smoking cessation efforts targeted to this population—while perhaps having less impact on the risk of SPLC—could potentially reduce the risk of smoking-associated mortality and should still be explored28.
Interestingly, the 2013 USPSTF criteria, a composite measure of smoking and age that identifies individuals at high risk of IPLC, were a significant indicator of a two-fold increased risk of SPLC. This effect was largely driven by the risk conferred from a heavy smoking history, as observed in the categorical smoking plots. Although the smoking-SPLC associations were significant, the magnitude and significance of the smoking effects on SPLC are smaller than those reported for IPLC29–32. This attenuation is partly because the comparison of SPLC cases to non-SPLC cases among lung cancer patients likely represents a comparison of heavier and lighter smoking histories, whereas the comparison of IPLC cases to non-cases more likely represents a comparison of ever-smoking and never-smoking histories. Furthermore, to develop a SPLC, an individual must first survive the IPLC long enough to develop another lung cancer, which itself can carry a long latency period33. Thus, while there is a biological rationale for tobacco smoke carcinogens inducing SPLC oncogenesis through similar DNA-damaging mechanisms as with IPLC34, it appears that the effect of tobacco smoking, while present, is modulated somewhat with SPLC.
BMI, personal history of cancer, and family history of lung cancer—while protective or risk factors for IPLC35–38—had negligible associations with SPLC, as did demographics including sex, race/ethnicity, and education. This study confirmed IPLC age at diagnosis, histology, and stage as relevant predictors of SPLC, which were previously identified in a large, population-based cohort13. IPLC surgery and chemotherapy showed significant associations with SPLC as risk and protective factors, respectively, in the meta-analyses. However, these effects were driven largely by MEC and were not observed in PLCO. It is likely that these effects are not directly associated with the therapies themselves but perhaps mediated through mortality, as IPLC surgeries are typically performed in the early-stage setting and chemotherapies reserved for more advanced disease. Thus, these treatment effects should be interpreted with caution.
Despite its strengths, this study has several limitations. MEC includes a diverse population of subjects but it enrolled lower rates of individuals with a current smoking status compared to national surveys at the time of enrollment14, though these rates were comparable to the general U.S. population in 201839. Furthermore, validation of the smoking-related findings in two independent cohorts—which consisted of distinct study populations from various geographic regions—demonstrated consistent results. EPIC, while a sizeable cohort, contained few SPLC events and lacked data on certain secondary variables. It is possible that SPLC cases were not fully captured due to different cohort surveillance strategies between Europe and the United States. Nonetheless, these deficiencies were accounted for appropriately in the meta-analyses using random effects models. Demographic and environmental data were collected through self-reported questionnaires in all cohorts; thus, misclassification is possible, but the consistency of the SPLC associations strengthens these findings. The smoking cessation analysis consisted of a small subset of MEC participants and requires further validation in independent cohorts, which is currently underway. Lastly, one relevant question concerns the relationship between tobacco smoking and overall survival in patients with IPLC, specifically regarding the impact of SPLC on survival and how tobacco smoking contributes to survival differences. Future directions should aim to elucidate these relationships, which are beyond the scope of the present study.
In summary, tobacco smoking is a risk factor for SPLC among IPLC patients in multiple large epidemiologic cohorts with long-term follow-up. Smoking cessation after an IPLC diagnosis may reduce the risk of SPLC. Comprehensive risk models for SPLC that incorporate relevant risk factors, including tobacco smoking, are needed to identify IPLC cases at high risk of SPLC and guide the development of evidence-based SPLC surveillance and screening strategies.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by the National Institutes of Health and Stanford University. The funders had no role in the design and conduct of the study; management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Funding:
This work was supported by the National Institutes of Health (1R37CA226081, U01 CA164973) and a Stanford Medical Scholars Research Grant.
Disclosures:
Dr. Kurian reports research funding to the institution from Myriad Genetics, outside the submitted work. Dr. Wakelee reports personal consulting fees from Janssen, Daiichi Sankyo, Helsinn, Mirati, AstraZeneca, Blueprint; Grants to institution for clinical trial conduct from ACEA Biosciences, Arrys Therapeutics, AztraZeneca/MedImmune, BMS, Celgene, Clovis Oncology, Exelixis, Lilly, Pfizer, Pharmacyclics, all outside the submitted work. All remaining authors report no other disclosures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Publisher's Disclaimer: Disclaimer: Where authors are identified as personnel of the International Agency for Research on Cancer / World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer / World Health Organization.
REFERENCES
- 1.American Lung Association. State of Lung Cancer. 2019. [Google Scholar]
- 2.Johnson BE. Second lung cancers in patients after treatment for an initial lung cancer. J Natl Cancer Inst 1998;90:1335–1345. [DOI] [PubMed] [Google Scholar]
- 3.Thakur MK, Ruterbusch JJ, Schwartz AG, et al. Risk of Second Lung Cancer in Patients with Previously Treated Lung Cancer: Analysis of Surveillance, Epidemiology, and End Results (SEER) Data. J Thorac Oncol 2018;13:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Surapaneni R, Singh P, Rajagopalan K, et al. Stage I lung cancer survivorship: risk of second malignancies and need for individualized care plan. J Thorac Oncol 2012;7:1252–1256. [DOI] [PubMed] [Google Scholar]
- 5.Rice D, Kim HW, Sabichi A, et al. The risk of second primary tumors after resection of stage I nonsmall cell lung cancer. Ann Thorac Surg 2003;76:1001–1007; discussion 1007–1008. [DOI] [PubMed] [Google Scholar]
- 6.Moyer VA, Force USPST. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2014;160:330–338. [DOI] [PubMed] [Google Scholar]
- 7.U.S Preventive Services Task Force. Draft Recommendation Statement- Lung Cancer: Screening.
- 8.Wood DE. National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines for Lung Cancer Screening. Thorac Surg Clin 2015;25:185–197. [DOI] [PubMed] [Google Scholar]
- 9.Wozniak AJ, Schwartz AG. The risk of second primary lung cancer: an unsolved dilemma. Transl Lung Cancer Res 2018;7:S54–S56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyle JM, Tandberg DJ, Chino JP, et al. Smoking history predicts for increased risk of second primary lung cancer: a comprehensive analysis. Cancer 2015;121:598–604. [DOI] [PubMed] [Google Scholar]
- 11.Ripley RT, McMillan RR, Sima CS, et al. Second primary lung cancers: smokers versus nonsmokers after resection of stage I lung adenocarcinoma. Ann Thorac Surg 2014;98:968–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leroy T, Monnet E, Guerzider S, et al. Let us not underestimate the long-term risk of SPLC after surgical resection of NSCLC. Lung Cancer 2019;137:23–30. [DOI] [PubMed] [Google Scholar]
- 13.Han SS, Rivera GA, Tammemagi MC, et al. Risk Stratification for Second Primary Lung Cancer. J Clin Oncol 2017;35:2893–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolonel LN, Henderson BE, Hankin JH, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol 2000;151:346–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martini N, Melamed MR. Multiple primary lung cancers. J Thorac Cardiovasc Surg 1975;70:606–612. [PubMed] [Google Scholar]
- 16.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol 2009;170:244–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varadhan R, Weiss CO, Segal JB, et al. Evaluating health outcomes in the presence of competing risks: a review of statistical methods and clinical applications. Med Care 2010;48:S96–105. [DOI] [PubMed] [Google Scholar]
- 18.Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Ann Statist 1988;16:1141–1154. [Google Scholar]
- 19.Graham JW, Hofer SM. Multiple imputation in multivariate research. In: Little TD, Schnabel KU, Baumert J, eds. Modeling longitudinal and multilevel data: Practical issues, applied approaches, and specific examples. Mahwah, NJ: Lawrence Erlbaum; 2000:201–218. [Google Scholar]
- 20.Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wahba G Spline Models for Observational Data. Philadelphia: SIAM; 1990. [Google Scholar]
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 23.United States. Public Health Service. Office of the Surgeon General. The Health Consequences of Smoking: A Report of the Surgeon General. Atlanta, GA: U.S. Dept. of Health and Human Services, Public Health Service; 2004. [Google Scholar]
- 24.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 25.United States. Public Health Service. Office of the Surgeon General. The Health Consequences of Smoking--50 Years of Progress: A Report of the Surgeon General. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service, Office of the Surgeon General; 2014. [Google Scholar]
- 26.Freidlin B, Korn EL. Testing treatment effects in the presence of competing risks. Stat Med 2005;24:1703–1712. [DOI] [PubMed] [Google Scholar]
- 27.Tammemagi CM, Neslund-Dudas C, Simoff M, et al. Smoking and lung cancer survival: the role of comorbidity and treatment. Chest 2004;125:27–37. [DOI] [PubMed] [Google Scholar]
- 28.Fares AF, Jiang M, Yang P, et al. Smoking cessation (SC) and lung cancer (LC) outcomes: A survival bene. American Society of Clinical Oncology 2020 Annual Meeting. 2020. [Google Scholar]
- 29.Alberg AJ, Samet JM. Epidemiology of lung cancer. Chest 2003;123:21S–49S. [DOI] [PubMed] [Google Scholar]
- 30.Remen T, Pintos J, Abrahamowicz M, et al. Risk of lung cancer in relation to various metrics of smoking history: a case-control study in Montreal. BMC Cancer 2018;18:1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pesch B, Kendzia B, Gustavsson P, et al. Cigarette smoking and lung cancer--relative risk estimates for the major histological types from a pooled analysis of case-control studies. Int J Cancer 2012;131:1210–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Keeffe LM, Taylor G, Huxley RR, et al. Smoking as a risk factor for lung cancer in women and men: a systematic review and meta-analysis. BMJ Open 2018;8:e021611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Bruin EC, McGranahan N, Mitter R, et al. Spatial and temporal diversity in genomic instability processes defines lung cancer evolution. Science 2014;346:251–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.United States. Public Health Service. Office of the Surgeon General. How Tobacco Smoke Causes Disease: The Biology and Behavioral Basis for Smoking-Attributable Disease: A Report of the Surgeon General. Rockville, MD: U.S. Dept. of Health and Human Services, Public Health Service; 2010. [Google Scholar]
- 35.Duan P, Hu C, Quan C, et al. Body mass index and risk of lung cancer: Systematic review and dose-response meta-analysis. Sci Rep 2015;5:16938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ang L, Chan CPY, Yau WP, et al. Association between family history of lung cancer and lung cancer risk: a systematic review and meta-analysis. Lung Cancer 2020;148:129–137. [DOI] [PubMed] [Google Scholar]
- 37.Cassidy A, Myles JP, van Tongeren M, et al. The LLP risk model: an individual risk prediction model for lung cancer. Br J Cancer 2008;98:270–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mery CM, Pappas AN, Bueno R, et al. Relationship between a history of antecedent cancer and the probability of malignancy for a solitary pulmonary nodule. Chest 2004;125:2175–2181. [DOI] [PubMed] [Google Scholar]
- 39.Creamer MR, Wang TW, Babb S, et al. Tobacco Product Use and Cessation Indicators Among Adults - United States, 2018. MMWR Morb Mortal Wkly Rep 2019;68:1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


