Abstract
Brown and beige adipocytes are mitochondria-enriched cells capable of dissipating energy in the form of heat. These thermogenic fat cells were originally considered to function solely in heat generation through the action of the mitochondrial protein uncoupling protein 1 (UCP1). In recent years, significant advances have been made in our understanding of the ontogeny, bioenergetics and physiological functions of thermogenic fat. Distinct subtypes of thermogenic adipocytes have been identified with unique developmental origins, which have been increasingly dissected in cellular and molecular detail. Moreover, several UCP1-independent thermogenic mechanisms have been described, expanding the role of these cells in energy homeostasis. Recent studies have also delineated roles for these cells beyond the regulation of thermogenesis, including as dynamic secretory cells and as a metabolic sink. This Review presents our current understanding of thermogenic adipocytes with an emphasis on their development, biological functions and roles in systemic physiology.
Adipose tissue was once considered to be merely a passive lipid-storing repository. However, it is now clear that adipose tissue is a metabolically active organ with key roles in the regulation of whole-body energy homeostasis, including food intake, glucose handling, insulin sensitivity, thermogenesis as well as regulation of immune responses1. The main parenchymal cells of adipose tissue are adipocytes or fat cells. Historically, mammals were recognized to possess two types of functionally and morphologically distinct fat cells — white and brown adipocytes. In brief, white adipocytes contain a large unilocular lipid droplet and few mitochondria, whereas brown adipocytes possess numerous small multilocular lipid droplets and high amounts of mitochondria, which are also cristae-dense, indicating their high potential for energy-generating processes (FIG. 1a). The thermogenic activity of brown adipocytes is potently activated by cold, which promotes the uptake of energy substrates (such as fat and sugar) and promotes cell metabolism, prominently including mitochondrial metabolism. In addition, as described in adult mice, some adipocyte progenitors and white adipocytes residing in white adipose tissue (WAT) are capable of ‘beiging’ or ‘browning’, terms referring to the process by which thermogenic brown-like adipocytes, also known as beige (or brite) adipocytes, emerge in response to stimuli including cold exposure, catecholamines, exercise, thiazolidinediones and injury2,3. These beige adipocytes are also thermogenic and share many morphological and biochemical characteristics with brown adipocytes, including multilocular lipid droplets and cristae-dense mitochondria (FIG. 1a). Mice contain several adipose tissue depots where brown adipocytes reside, known as brown adipose tissue (BAT), which are established prenatally and maintained in adult animals. In humans, ‘classical’ brown adipocytes reside in developmentally committed interscapular BAT depots and are restricted to the period of infancy4–7 (FIG. 1b; Supplementary Box 1).
Fig. 1 |. characteristics and anatomical distribution of thermogenic adipocytes.
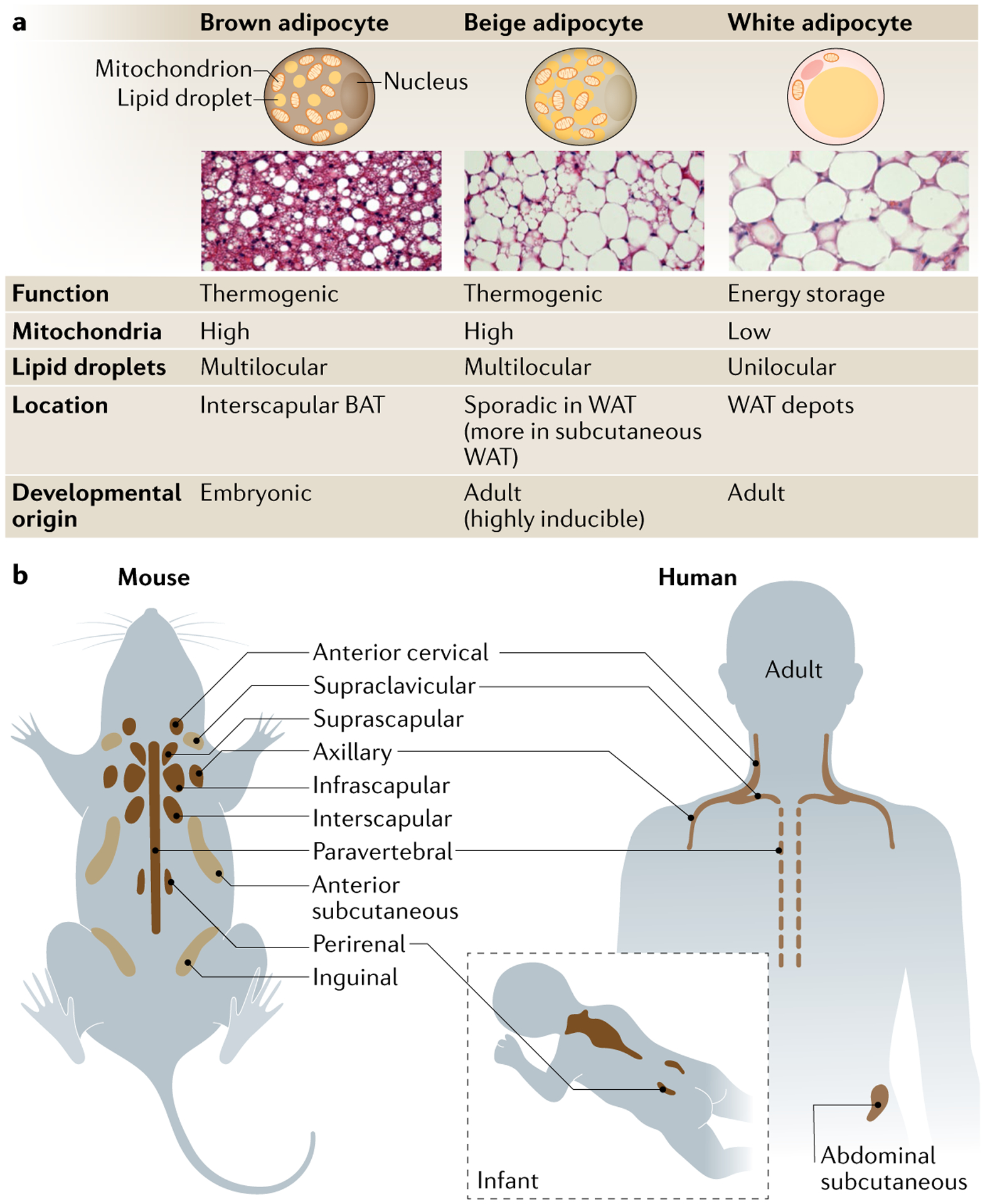
a | Mammals possess several types of adipocytes with distinct metabolism, morphology, location and developmental timing. Brown adipocytes are a constitutive, embryonic (dermomyotome)-origin cell type and cluster in designated depots, such as interscapular brown adipose tissue (BAT) depots of mice and infants. On the other hand, beige adipocytes are a recruitable and non-dermomyotome-derived cell type often seen in white adipose tissue (WAT) depots in the postnatal stage. As beige adipocytes are generally surrounded by white adipocytes, beige adipocytes are thought of as intermediate in colour between white and brown adipocytes. Regardless, both brown and beige adipocytes dissipate energy in the form of heat, whereas white adipocytes are responsible for energy storage. The abundance of mitochondria is the major determinant of fat colour (brown/beige and white). Brown and beige adipocytes contain substantially higher amounts of cristae-dense mitochondria with high levels of iron, which give the tissue containing these cells its brown colour. By contrast, white adipocytes possess relatively few mitochondria. These cells also differ in the morphology of their lipid droplets. Brown and beige adipocytes have numerous small lipid droplets (multilocular lipid droplets), whereas white adipocytes contain a large lipid droplet (unilocular lipid droplet) for lipid storage. b | Adult mice possess several depots of BAT190.The interscapular BAT is the largest BAT depot in mice, and its development is completed prenatally. In addition to these depots, certain signals (cold, adrenergic signalling) can promote emergence of beige adipocytes in anterior subcutaneous and inguinal WAT in mice. Suprascapular fat depot has also been characterized as beige fat. Of note, beige adipocyte biogenesis is regulated distinctly from brown adipocytes studied in the interscapular BAT. Thermogenic fat cells in mice have also been identified in perivascular adipose tissue surrounding the thoracic aorta and in epicardial adipose tissue surrounding the coronary arteries191,192 (not shown). It remains unclear whether these cells are brown or beige adipocytes, or another thermogenic adipocyte altogether. Humans also possess BAT depots in, at least, six anatomic regions, including cervical, supraclavicular, axillary, mediastinal, paraspinal and abdominal174. The interscapular BAT depot is most prominent in infants and gradually declines with age. In adults, some of the BAT depots are anatomically analogous to those in mice.
Cristae.
Folds in the inner membrane of a mitochondrion where active electron transport takes place.
Catecholamines.
Monoamine neurotransmitters that mediate cold-induced thermogenesis.
Thiazolidinediones.
(Also known as glitazones). Synthetic ligands of PPArγ typically used in the treatment of type 2 diabetes. They also increase thermogenesis and have been shown to promote recruitment of beige thermogenic adipocytes.
Functionally, thermogenic adipose tissue is known to have a central role in non-shivering thermogenesis, a heat-generating process that prevents hypothermia without muscle shivering and that is activated by cold exposure; these mechanisms are also known to support maintenance of energy balance by dissipating excess energy as heat. However, it is important to note that the biological significance of brown and beige fat in systemic energy homeostasis extends far beyond just enhanced energy expenditure, including their roles in regulating glucose and lipid metabolism and insulin sensitivity as well as overall adipose tissue homeostasis. Hence, regulation of brown/beige adipocyte amount and function is emerging as a strategy to regulate energy metabolism in humans, with a promise of developing approaches that support management of obesity and diabetes. Here, we review the current standing of the field with an emphasis on the cellular and functional heterogeneity of thermogenic adipocytes, and their roles in physiology.
Thermogenic fat biogenesis
Both brown and beige adipocytes are similar in terms of their morphological characteristics, and both are capable of thermogenesis. As discussed in the next section, several mechanisms are involved in this process, but the best understood process involves the role of uncoupling protein 1 (UCP1), which is also a marker for brown adipocytes and many, if not all, beige adipocytes. Nevertheless, despite these similarities, brown and beige adipocytes are considered distinct cell types because of differences in their developmental origin and anatomical location (FIG. 1b), and also in regulation and function8. Furthermore, recent studies have identified developmentally distinct subtypes of beige adipocytes that likely serve unique biological functions, suggesting cellular heterogeneity of thermogenic fat.
Thermogenic adipocyte precursors and their development.
Brown adipocytes develop prenatally, such that their thermogenic function is fully active at birth. This is important because BAT is essential for non-shivering thermogenesis in newborn animals. Developmentally, embryonic brown adipocytes in mice arise from precursors in the dermomyotome and express somite markers, such as engrailed 1 (En1), myogenic factor 5 (Myf5), paired-box protein 3 (Pax3), Pax7 and mesenchyme homeobox 1 (Meox1)9–13. Pulse-chase lineage tracing studies suggest that brown adipocyte fate is determined by mid-gestation9,14. A recent study generated functional human brown adipocytes from pluripotent stem cells via a paraxial mesoderm state with the expression of MYF5 and PAX3 (REF.15), suggesting that the developmental origin of brown adipocytes is evolutionally conserved in mice and humans.
Dermomyotome.
Epithelial tissue present during development that combines a dermatome (giving rise to the epidermis) and a myotome (giving rise to skeletal muscle) before they separate in embryogenesis.
By contrast, beige adipocytes develop postnatally and derived from non-dermomyotome lineage. In mice, UCP1+ adipocytes initially emerge in retroperitoneal WAT at 10–30 days of postnatal age and subsequently disappear or become dormant16. Functional beige adipocytes in adult mice appear in an inducible manner — in response to defined beiging signals, such as cold and β3-adrenergic signalling. Accumulating evidence suggests the presence of beige adipocyte progenitor cell (APC) pools that are distinct from embryonic brown APCs. For example, beige adipocytes emerging during postnatal development in inguinal WAT of mice are derived from Myf5-negative precursors10. In addition, cells with a unique gene expression signature — expressing Acta2 (encoding smooth muscle actin (αSMA)), Sm22, Pax3, Cd81 and Pdgfra — were identified in the stromal vascular fraction of WAT as precursors of beige adipocytes following cold exposure12,17–20. Chronic cold exposure up to 2 weeks also stimulates beige adipogenesis from progenitors expressing Myh11 (REF.21) or Pdgfrb22. The extent to which these Myh11 or Pdgfrb-expressing cells are progenitors distinct from αSMA-expressing cells is unknown.
Retroperitoneal WAT.
White adipose tissue (WAT) in the area between the posterior portion of the parietal peritoneum and the posterior abdominal wall muscles.
β3-Adrenergic signalling.
Signalling potently stimulated by cold that is mediated by catecholamines that bind to β3-adrenergic receptors (β3-Ars), G protein-coupled receptors that activate adenylate cyclase to produce a second messenger cAMP.
Inguinal WAT.
Subcutaneous adipose tissue located at the juncture of the lower portion of the anterior abdominal wall and legs. inguinal white adipose tissue (WAT) contains high levels of beige adipocytes.
Until recently, it was unclear what distinguishes these beige APCs from other APCs, with both expressing common cell surface protein markers such as PDGFRα and stem cell antigen 1 (SCA1)17,23–26. New data from single-cell RNA sequencing identified a subpopulation of APCs marked by a cell surface protein CD81, which exhibited a smooth muscle gene signature (including expression of Acta2 and Sm22), resided in the stroma of adipose tissues and gave rise to beige adipocytes following cold exposure. These data indicate that CD81 is a specific cell surface marker of beige APCs20. It was further shown that CD81 mediates integrin signalling in these cells and that its expression is required to prevent diet-induced obesity and insulin resistance, suggesting that CD81 functions as a key sensor of external inputs (hormonal signals, see also below) and controls beige APC biogenesis and whole-body energy homeostasis. Previous studies also identified the transcription factor EBF2 as a specific marker for beige adipogenesis in adult WAT in response to cold14. Similarly, human beige fat biogenesis in subcutaneous WAT is induced by chronic activation of β3-adrenergic receptor (β3-AR) signalling27,28. Human beige APCs have been shown to reside within the walls of adipose tissue capillaries and to differentiate into thermogenic beige adipocytes in response to pro-angiogenic factors29. Furthermore, stromal cells positive for PDGFRα and lacking expression of WAT APC marker CD34 were isolated from human abdominal subcutaneous WAT and shown to undergo differentiation to beige adipocyte in cell culture30. More recently, human beige adipocytes were derived from adipose-derived stromal cells in serum-free medium31. Of note, the transcriptional profile of beige preadipocytes is already distinct from that of white preadipocytes prior to differentiation5,6. Hence, beige fat fate appears to be determined at the progenitor cell stage. Nevertheless, adipocyte progenitors likely retain a certain degree of plasticity to enable them to respond to environmental stressors (for example, prolonged cold) because the majority of, if not all, adipocytes in the subcutaneous WAT can transform to beige adipocytes after prolonged cold exposure1. More data from in vivo lineage tracing will be needed to further clarify these findings, and to firmly establish the developmental origin and regulation of beige APCs.
Insulin resistance.
Insulin acts on the insulin receptor on the plasma membrane of target organs and triggers insulin signalling to stimulate anabolic reactions. insulin action is impaired under insulin resistance conditions, which can eventually lead to type 2 diabetes.
De novo biogenesis and white-to-beige conversion in thermogenic fat establishment.
As discussed above, there is evidence that thermogenic adipocytes arise via designated precursors, and lineage tracing experiments using the Adipo-Chaser (inducible adiponectin–Cre lineage tracing) system lend support to the notion that beige adipocytes predominantly arise through differentiation of precursors (that is, de novo biogenesis)32. However, pioneering studies of thermogenic fat in rodents indicated that beige adipocytes — induced by cold exposure or the β3-AR agonist CL316,243 — arise directly from existing mature white adipocytes in a process of direct white-to-beige conversion or transdifferentiation33,34. Although these observations appear to be in conflict, a recent study demonstrated that both direct conversion and de novo biogenesis occur in mice, with the contribution from each pathway influenced by experimental conditions, such as the temperature before cold exposure (ambient temperature or thermoneutrality), external cues (exposure to cold or β3-AR agonist) and reporter system35. For example, when mice are raised under ambient temperature (20–23 °C) and subsequently exposed to cold (6 °C), approximately 50% of beige adipocytes (scored as UCP1+ multilocular adipocytes) are derived via de novo adipogenesis, with the other half coming from existing adipocytes. However, when mice at thermoneutrality (30 °C) are exposed to 6 °C, a large proportion (88%) of newly recruited beige adipocytes arise from de novo biogenesis, which involves active progenitor proliferation (FIG. 2). Earlier work detected nearly no signs of proliferation in beige adipocytes in inguinal WAT17,36; however, a recent study showed that a small subset of PDGFRα+/SCA1+ stromal cells in subcutaneous WAT, marked by CD81, become highly proliferative upon cold exposure and give rise to beige adipocytes20. It is notable that beige APC proliferation is significantly reduced with ageing, when de novo beige fat biogenesis is attenuated20,37,38; hence, age is also an important factor in considering the mechanism of thermogenic fat emergence.
Fig. 2 |. Beige fat biogenesis.
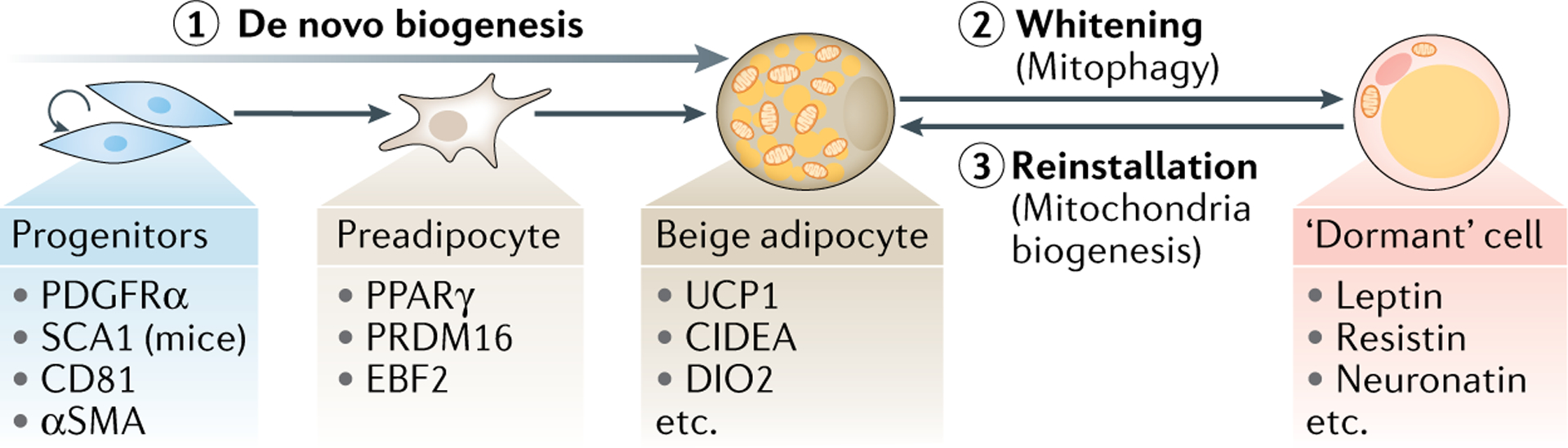
Emergence of beige adipocytes in white adipose tissue (beiging) is an inducible process stimulated by external beiging stimuli (for example, cold, adrenergic ligands). This can involve either de novo differentiation engaging progenitor cells (path 1) or reinstallation of the thermogenic phenotype by dormant cells (path 3). Key molecular markers for the different cell types associated with these transitions are indicated. De novo differentiation of beige adipocytes involves designated progenitors, which undergo cell proliferation, fate commitment to preadipocytes and differentiation into adipocytes. Progenitors and preadipocytes contain small amounts of mitochondria relative to differentiated adipocytes. When external beiging stimuli are withdrawn, mitochondria-enriched beige adipocytes transform into dormant adipocytes that resemble white adipocytes (whitening or beige-to-white fat conversion) (path 2). The whitening of beige fat is initiated by active mitochondrial clearance through selective autophagy (mitophagy), and does not involve de-differentiation/re-differentiation of adipocytes. Many of those dormant adipocytes can reinstall the thermogenic programme in response to re-exposition to beiging stimuli (path 3). This process involves active mitochondrial biogenesis. SCA1, stem cell antigen 1; UCP1, uncoupling protein 1.
Another intriguing observation is that following the withdrawal of the external beiging stimulus, the recruited beige adipocytes transform into dormant adipocytes exhibiting white fat signatures (unilocular lipid droplets, low mitochondria, non-thermogenic) — a process referred to as ‘whitening’ or beige-to-white fat conversion39,40 (FIG. 2). This beige-to-white fat conversion does not involve de-differentiation to progenitors. This is supported by data from single cell-resolution morphological tracing and transcriptional studies, which demonstrated that as cells acquire unilocular lipid droplets after removing beiging stimuli, they gradually lose mitochondria and diminish expression of thermogenic genes40. In turn, these ‘whitened’ dormant adipocytes can reinstall the thermogenic programme after another cold stimulus (FIG. 2). Following the second pulse of cold, approximately half of the beige adipocytes in inguinal WAT are derived from such dormant cells39. Accordingly, beige adipocyte biogenesis from existing adipocytes occurs through reinstallation of the thermogenic programme in whitened/dormant adipocytes. Mechanistically, the beige-to-white fat conversion is triggered by mitochondrial clearance via mitophagy40,41 and is negatively regulated by the zinc-finger protein ZFP423, deletion of which leads to white-to-beige conversion42. Furthermore, beige-to-white fat conversion is accompanied by chromatin architecture changes generating a chromatin state resembling that of white adipocytes42. Curiously, brown adipocytes in interscapular BAT also acquire unilocular lipid droplets upon warming, but they maintain their chromatin architecture42.
Mitophagy.
A selective mechanism to degrade defective mitochondria by autophagy.
In sum, both the de novo biogenesis and direct conversion mechanisms contribute to the establishment of thermogenic fat. The emerging picture of a stimulated origin of thermogenic fat is that stimuli in early life trigger initial beige APC proliferation and de novo beige fat biogenesis. These beige adipocytes lose their thermogenic features upon withdrawal of stimuli, with the majority of beige adipocytes acquiring white adipocyte characteristics and becoming dormant. As these ‘whitened’ adipocytes can rapidly reinstall the thermogenic programme, the initial pool of beige APCs and their dormant progeny can be considered major determinants of beiging capacity in adults. Having said this, the contribution of the different mechanisms — de novo biogenesis of beige adipocytes from designated progenitors, the maintenance of active beige adipocytes and reinstallation from dormancy — may vary depending on the location within WAT and the genetic background. Addressing these differences in the origin of beige adipocytes will be exciting areas of future research.
Regulatory pathways for thermogenic fat biogenesis.
The most well-known thermogenic stimulus is cold. First of all, cold exposure activates differentiated brown and beige adipocytes. This occurs via catecholamines that are released from sympathetic nerve terminals upon cold exposure and trigger intracellular signalling, including cAMP signalling via stimulation of β-AR. Adenosine, originating from breakdown of ATP and released from sympathetic nerves or by autocrine/paracrine efflux from brown adipocytes, also activates thermogenesis in brown adipocytes and promotes beige fat biogenesis in the subcutaneous WAT of mice via the A2A receptor43. In addition, recent studies identified various compounds — fibroblast growth factor 21 (FGF21), natriuretic peptides and thiazolidinediones, to name a few — that mediate cold-induced thermogenic fat biogenesis44–46, and several transcription regulators and epigenetic regulators have been implicated in controlling this biogenetic programme (reviewed elsewhere47,48). Thermogenesis could also be regulated on a transgenerational level, as cold exposure of male mice was shown to lead to epigenetic changes in their sperm, resulting in offspring with enhanced thermogenic fat function49.
Natriuretic peptides.
Peptide hormones that induce sodium excretion by the kidney, including atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), C-type natriuretic peptide (CNP) and dendroaspis natriuretic peptide (DNP).
Although we know that various stimuli can promote beige fat biogenesis, the understanding of stimulus-induced activation and proliferation of beige APCs remains incomplete. For example, β3-AR agonists stimulate beige fat biogenesis in WAT; however, β3-AR is only expressed in differentiated adipocytes but not progenitors. Pharmacological inhibition of β1-AR impairs cold-induced beige fat biogenesis50 and norepinephrine-stimulated DNA synthesis in cultured brown preadipocytes51; thus, it is likely that norepinephrine from sympathetic nerves acts on beige APC growth, at least in part, via β1-AR but not via β3-AR. Nevertheless, in stimulus-induced beige fat emergence, β3-AR agonists are expected to primarily act on existing, ‘whitened’ adipocytes to trigger reinstallation of their thermogenic programme, whereas progenitor growth may be stimulated by factors released from differentiated adipocytes (such as fatty acids, lipids, secretory peptides) following β3-AR activation (FIG. 3a). Indeed, cold promotes de novo beige adipocyte differentiation from stromal progenitor cells positive for αSMA, whereas selective β3-AR agonists largely induce the formation of beige adipocytes converted from existing white adipocytes, but not from αSMA-expressing progenitors in the stroma35,50. In addition to this non-cell autonomous communication between fat cells, there is a growing body of evidence indicating a non-cell autonomous role of immune cells in regulating thermogenic fat function (BOX 1). The immune cell-derived factors act in a paracrine manner and regulate the differentiation and function of thermogenic adipocytes.
Fig. 3 |. Regulation and heterogeneity of thermogenic fat.
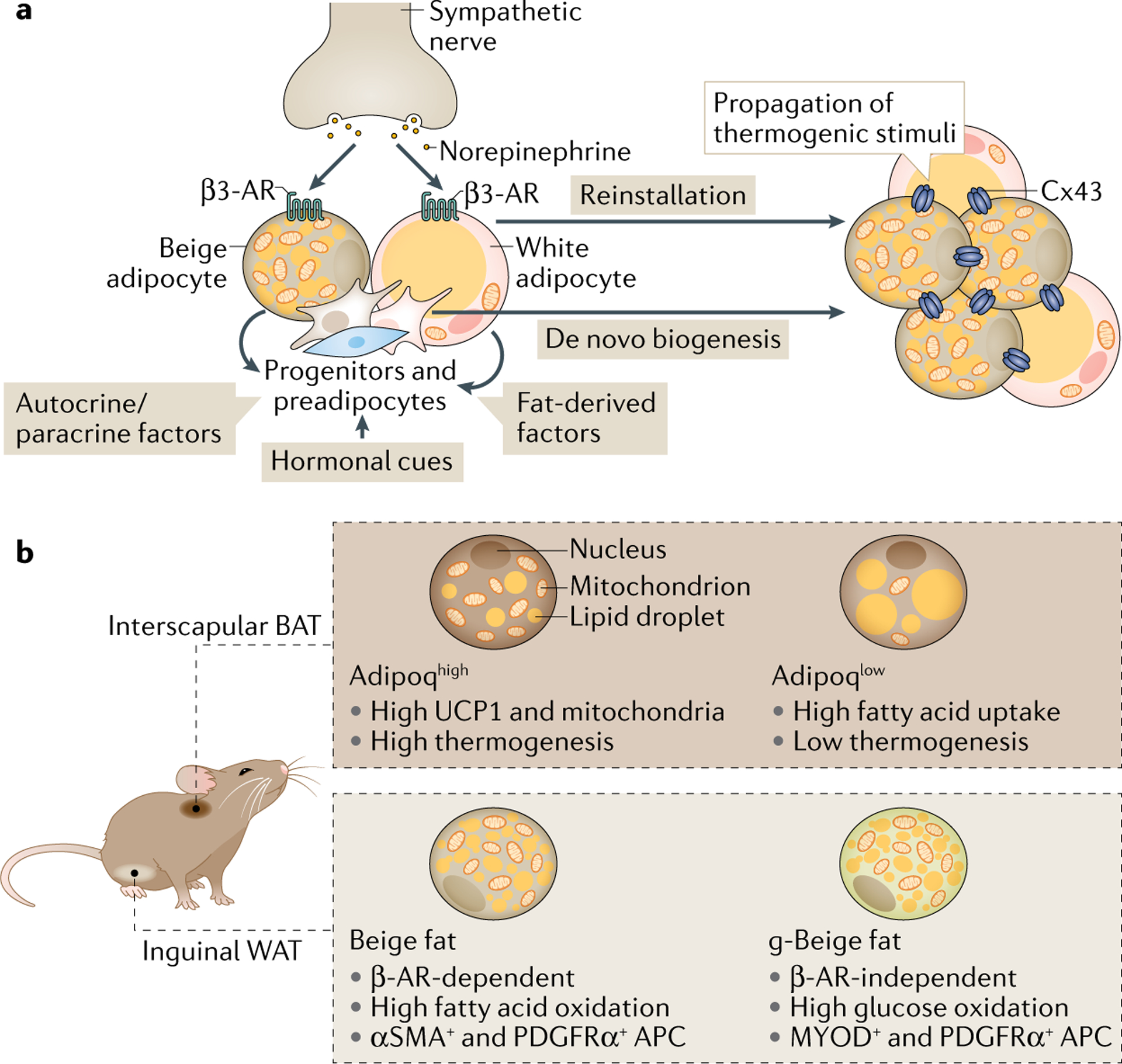
a | Norepinephrine, released from sympathetic nerve terminals, binds to β3-adrenergic receptor (β3-AR) in differentiated adipocytes. This triggers reinstallation of the thermogenic programme in dormant cells and release of fatty acids and paracrine/autocrine factors (for example, fibroblast growth factor 21 (FGF21)) from adipocytes that stimulate de novo differentiation of beige adipocytes from progenitor cells. Hormonal cues, including IL-6, bone morphogenetic proteins (BMPs), FGF21 and atrial natriuretic peptide (ANP), are shown to promote beige adipocyte biogenesis, although the extent to which each beiging hormone promotes de novo differentiation or white to beige conversion needs further investigation. The beiging hormones have been summarized elsewhere48. Even in regions that are not densely innervated, and hence not exposed to β3-adrenergic signals, connection of cells via the gap junction channel formed by connexin 43 (Cx43) has been shown to propagate intracellular thermogenic signals to promote thermogenic fat emergence. b | Recent advances with single-cell analysis revealed that thermogenic fat is heterogeneous, harbouring adipocyte populations with different molecular and functional characteristics. For example, the interscapular brown adipose tissue (BAT) of mice contains two functionally distinct subtypes of brown adipocytes with high or low expression of adiponectin (Adipoqhigh and Adipoqlow). In inguinal white adipose tissue (WAT) of mice, induction of beige fat is generally under the control of β-AR signalling. However, a β-AR signalling-independent population of beige adipocytes has been identified in inguinal WAT that is driven by signalling downstream of the nicotinic acetylcholine receptor subunit CHRNA2. Notably, these adipocytes primarily use glucose as their metabolic fuel, in contrast to fatty acids that are the main fuel for thermogenic adipocytes, and hence have been termed g-beige adipocytes. They are also known to be derived from a distinct population of adipocyte progenitor cells (APCs) that, instead of being positive for smooth muscle actin (αSMA), show expression of PDGFRα and MYOD, the latter of which which does not typically associate with either white or beige adipocyte lineage. UCP1, uncoupling protein 1.
Box 1 |. The role of immune cells in the regulation of thermogenic fat function.
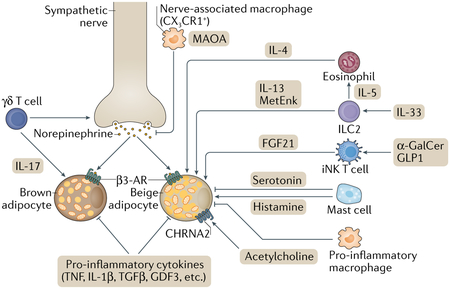
Besides signalling from the central nervous system and systemic hormonal cues, recent studies have illuminated the importance of immune cells in regulating thermogenic adipocytes194 (see the figure). In general, pro-inflammatory cues (for example, TNF, IL-1β), induced by obesogenic diets, inhibit thermogenesis in adipose tissues195–197, whereas inhibition of pro-inflammatory responses by genetic ablation of IκB kinase-ε (IKKε) or interferon regulatory factor 3 (IRF3), a transcriptional mediator of pro-inflammatory signalling, stimulates adipose tissue thermogenesis198–200. Similarly, obesity-induced pro-inflammatory signals, such as transforming growth factor-β (TGFβ) and growth differentiation factor 3 (GDF3), repress brown and beige fat thermogenesis, whereas inhibition of these pathways stimulates brown/beige fat biogenesis and ameliorates diet-induced obesity and glucose intolerance201–203. Also, IL-10, a type 2 cytokine with anti-inflammatory properties, is released from multiple immune cells (for example, macrophages, dendritic cells, B cells and T cells) and represses the thermogenic gene programme via IL-10Rα in adipocytes204,205.
Recent studies have also revealed more complex interactions between adipose tissue-resident immune cells and thermogenic adipocytes. For example, a subpopulation of macrophages (CX3 CR1+ macrophages) reside along sympathetic nerve bundles in brown adipose tissue (BAT) and suppress norepinephrine-induced BAT thermogenesis by reducing the local concentration of norepinephrine by active clearance via monoamine oxidase A (MAOA)206,207. Intriguingly, GDF3 increases MAOA expression in adipose tissue-resident macrophages, resulting in the degradation of norepinephrine and other catecholamines, thereby contributing to impaired lipolysis in adipose tissues — the first step in the generation of energy substrates in thermogenic adipocytes208. Furthermore, inflammatory macrophages directly interact with adipocytes through α4 integrin and VCAM1, which blunt beige fat biogenesis in white adipose tissue (WAT)209. Some immune cells, on the other hand, stimulate brown/beige fat thermogenesis. For instance, BAT-resident γδ T cells play a key role in promoting sympathetic innervation by secreting IL-17A that acts on its receptor IL-17RC in adipocytes210. group 2 innate lymphoid cells (ILC2s) in WAT also regulate beige fat biogenesis. ILC2s, activated in response to IL-33, positively regulate beige fat biogenesis by secreting methionine-enkephalin (MetEnk)211. Also, IL-33-elicited ILC2s release IL-13 and trigger the secretion of IL-4 from eosinophils, both of which act on PDGFRα+ progenitors to promote beige adipogenesis212; however, PDGFRα+–CD81+ beige progenitors do not express IL-4 receptor, and, thus, this model requires further investigation. Mast cells also play a role in regulating beige fat biogenesis. Genetic or pharmacological inactivation of mast cells in mice promoted beige adipocyte biogenesis, and inhibition of beige adipocytes by mast cells was shown to be due to the secretion of serotonin that curbs the proliferation of PDGFRa+ progenitors213. However, mast cells in human WAT were shown to promote beige adipocyte biogenesis by releasing histamine and IL-4 (REF.214). The discrepancy between the above studies awaits future investigation. Lastly, a recent study demonstrated that invariant natural killer T cells (iNKT cells) residing in adipose tissue are activated in response to α-galactosylceramide (α-GalCer) or liraglutide, and that activated iNKT cells stimulate beige fat biogenesis by releasing fibroblast growth factor 21 (FGF21)215, an endocrine hormone that increases energy expenditure. β3-AR, β3-adrenergic receptor.
γδ T cells.
A subset of T cells that express a distinctive set (γ and δ-chains) of T cell receptor (TCR) on their surface, distinct from that of conventional T cells (αβ T cells).
Group 2 innate lymphoid cells.
(ILC2S). A subset of innate lymphoid cells that produce type 2 cytokines, such as iL-5 and iL-13.
Methionine-enkephalin.
(MetEnk). An endogenous opioid peptide that acts on δ-opioid receptor and μ-opioid receptor to a lesser extent.
Mast cells.
Immune cells that release histamine and other substances during inflammatory and allergic reactions.
Invariant natural killer T cells.
(iNKT cells). Specialized T cells that recognize lipid antigens.
α-Galactosylceramide.
(α-GalCer). A synthetic glycolipid that stimulates invariant natural killer T cells.
Liraglutide.
A glucagon-like peptide 1 receptor (GLP1R) agonist that acts as an incretin mimetic and increases insulin secretion.
The recent identification of a progenitor population marked by PDGFRα/SCA1 and CD81 provides new insights into the molecular regulation of beige APC growth. CD81 forms a complex with αVβ1 and αVβ5 integrins in beige APCs, and the CD81–integrin complex is required for triggering signalling by focal-adhesion kinase (FAK) to promote progenitor cell proliferation in response to irisin, a myokine that stimulates beige fat biogenesis in response to exercise20. Several hormones, such as angiopoietin-like 4 (ANGPTL4), are known to stimulate integrin–FAK signalling via β1 and β5 integrins, and promote beige fat biogenesis52,53. Hence, it is conceivable that the CD81–integrin complex controls beige APC growth in response to hormonal cues besides irisin. Additional autocrine factors regulating the adipogenic niche are discussed below (see subsection Secretory function). In addition, upon induction, beige adipocytes establish larger cell clusters within WAT, even in regions that are not densely innervated and, hence, are not expected to robustly respond to adrenergic signalling. In this case, connection of cells via the gap junction channel connexin 43 (Cx43) has been shown to propagate intracellular thermogenic signals (such as cAMP)54 (FIG. 3a). Together, various neuronal and hormonal factors control beige fat biogenesis via acting on progenitor cells (de novo biogenesis) and on existing adipocytes (maintenance, reinstallation).
Myokine.
A secretory molecule released from skeletal muscle. examples include iL-6 and irisin, both of which are known to activate thermogenic fat biogenesis.
Cellular heterogeneity in thermogenic fat niches.
As highlighted in the discussion above, thermogenic fat biogenesis is a multifaceted process, involving various cell types, signalling molecules and cellular regulators as well as multiple means of intercellular communication. This picture is further complicated by evidence suggesting the existence of multiple progenitors and adipocyte populations that go beyond the simple distinction of brown and beige adipocytes (FIG. 3b). A recent example is the identification of a unique subtype of beige adipocytes, which arise from myogenic PDGFRα+ progenitors residing near the lymph node of inguinal WAT depots in a manner dependent on GA-binding protein-α (GABPα). This beige adipocyte subpopulation is characterized by high glucose catabolism (using mostly glucose as metabolic fuel instead of fatty acids), and hence has been termed g-beige fat55. Notably, g-beige fat is induced by non-adrenergic signalling, occurring through signalling downstream of the nicotinic acetylcholine receptor subunit CHRNA2 in response to acetylcholine produced by multiple immune cells residing in inguinal WAT56. This adrenergic-independent signalling pathway through a unique subset of thermogenic adipocytes has a compensatory role in energy metabolism — such as in response to a high-fat diet — particularly under conditions in which canonical β-adrenergic signalling is impaired55,56. A recent study also reported that perinatal development of beige adipocytes in inguinal WAT of mice is regulated independently of sympathetic nerve signalling by the transcription factor B cell lymphoma 6 (BCL-6)57.
Another example of thermogenic cell heterogeneity is in the interscapular BAT depots in mice, which contain two subtypes of brown adipocytes expressing high or low levels of adiponectin (Adipoqhigh and Adipoqlow)58. Adipoqlow brown adipocytes express lower levels of thermogenic genes and contain larger lipid droplets and lower mitochondrial content relative to Adipoqhigh cells (FIG. 3b). Adipoqlow brown adipocytes also express endothelial markers. Intriguingly, the ratio of these subtypes in BAT is adaptable and can be shifted without induction of cell death. The number of Adipoqhigh brown adipocytes is higher when mice are exposed to cold and declines gradually in aged mice. In addition, clonal analyses of adipocytes from a single WAT depot in mice identified multiple adipocyte populations that display differences in cellular metabolism and responses to the inflammatory cytokine TNF, insulin and growth hormone, although the biological role of each population in whole-body metabolism needs further investigation59. Similarly, clonal analyses of human adipose tissue identified multiple subtypes of adipocytes with distinct molecular and metabolic characteristics60,61. Furthermore, single-nucleus RNA-sequencing on BAT of mice and thermogenic fat depots in humans identified a low-abundance population of adipocytes that act on neighbouring brown adipocytes and negatively impact their thermogenic activity by increasing the local concentration of the short-chain fatty acid acetate62. These studies provide new insights into diverse subpopulations of adipocytes with distinct molecular and metabolic features within a single adipose depot. Given these distinct molecular profiles of preadipocytes and differentiated adipocytes, it is likely that the cellular lineages of these subpopulations are determined at the precursor stage, but future studies are needed to delineate the adipocyte precursor heterogeneity.
Adiponectin.
A fat-selective secretory protein hormone (adipokine) that is involved in regulating glucose and lipid homeostasis. in general, adiponectin levels are positively correlated with metabolic health.
Recent single-cell RNA-sequencing analyses have also identified diverse stromal populations within adipose tissues, such as ancillary/niche cells that influence adipocyte differentiation. For example, the subcutaneous stromal fraction of WAT contains functionally distinct SCA1+ cells, called ‘adipogenesis-regulatory’ cells (CD142+ABCG1+SCA1+), that suppress adipogenic capacity of neighbouring preadipocytes63. Mouse epididymal WAT also contains distinct subpopulations of PDGFRβ+ perivascular cells, termed fibro-inflammatory progenitors, that limit adipogenic capacity and, instead, activate a pro-inflammatory gene programme64. Furthermore, subcutaneous WAT of mice and humans contains multiple cell types, such as highly proliferative progenitors expressing dipeptidyl peptidase 4 (DPP4), committed preadipocytes expressing intercellular adhesion molecule 1 (ICAM1) and other distinct progenitors expressing CD142 and CLEC11a65. An important next step will be to determine how each stromal population contributes to the regulation and maintenance of adipose tissue homeostasis and energy metabolism.
Ancillary/niche cells.
Supporting cells releasing paracrine factors.
Epididymal WAT.
Visceral adipose tissue attached to the epididymis. epididymal white adipose tissue (WAT) is known to have lower beiging propensity relative to inguinal WAT of mice.
Mechanisms of thermogenesis
As the name implies, the key function of thermogenic adipocytes is to engage in the process of thermogenesis. This activity is potently induced by cold. The most well-established non-shivering thermogenic mechanism (that is, independent of muscle activity), substantiated by decades of research, is centred on mitochondrial metabolism, whereby during mitochondrial respiration the electron transfer through the respiratory chain is uncoupled from generating ATP (uncoupled respiration) by the activity of UCP1. The activation of this pathway is dependent on the transcription co-regulatory protein PRDM16, which complexes with numerous other proteins such as EHMT1 (REFS66–70).
Respiratory chain.
(Also known as electron transport chain). Multiple protein complexes that transfer electrons from electron donors, such as NADH, to electron acceptors, thereby generating a proton (H+) gradient across the mitochondrial inner membrane.
The long-standing notion has been that UCP1 is the only ‘thermogenin’ in mammals. However, as is often the case for essential pathways such as thermoregulation and energy homeostasis, we now realize that the UCP1-mediated programme is not solely responsible for non-shivering thermogenesis (one notable example here is the fact that some mammalian species, such as pigs, lack the functional UCP1 gene yet can still defend their body temperature)71,72. Indeed, recent discoveries have highlighted several UCP1-independent pathways of non-shivering thermogenesis in mammals.
UCP1-dependent thermogenesis.
UCP1 was isolated in 1976 (REFS73,74), and subsequently its sequence was determined75,76. Genetic deletion of UCP1 in mice causes severe hypothermia following acute cold exposure77, whereas genetic deletion of its homologues UCP2 or UCP3 does not recapitulate the hypothermic phenotype78,79. These studies led to the notion that UCP1 was a key mediator of adaptive thermogenesis.
In contrast to the well-established role of mitochondria in oxidative phosphorylation to generate ATP, brown fat mitochondria generate heat by uncoupling electron transport from ATP synthesis. UCP1 dissipates the proton (H+) gradient across the inner mitochondrial membrane, thereby producing heat, while alleviating the inhibition of respiration that results from an elevation of the ATP/ADP ratio (FIG. 4a). The role of UCP1 in this respiratory uncoupling process has been demonstrated by a series of elegant studies80,81. First, purine nucleotides (ATP, ADP, GTP, GDP) were shown to potently inhibit mitochondrial uncoupling activity by directly binding to UCP1, and this critical feature was used to experimentally isolate UCP1 from brown fat mitochondria80. Another key regulator of UCP1 activity is long-chain fatty acids. Direct binding of long-chain fatty acids (longer than C8) to UCP1 can overcome the inhibitory effect of purine nucleotides and trigger mitochondrial proton conductance82–84, although the structural mechanisms of the competition between long-chain fatty acids and purine nucleotides on UCP1 remain unclear. UCP1 functions as a fatty acid anion/H+ symporter, whereby direct binding of the negatively charged long-chain free fatty acid to UCP1 triggers H+ influx into the matrix80.
Fig. 4 |. Thermogenic mechanisms in adipocytes.
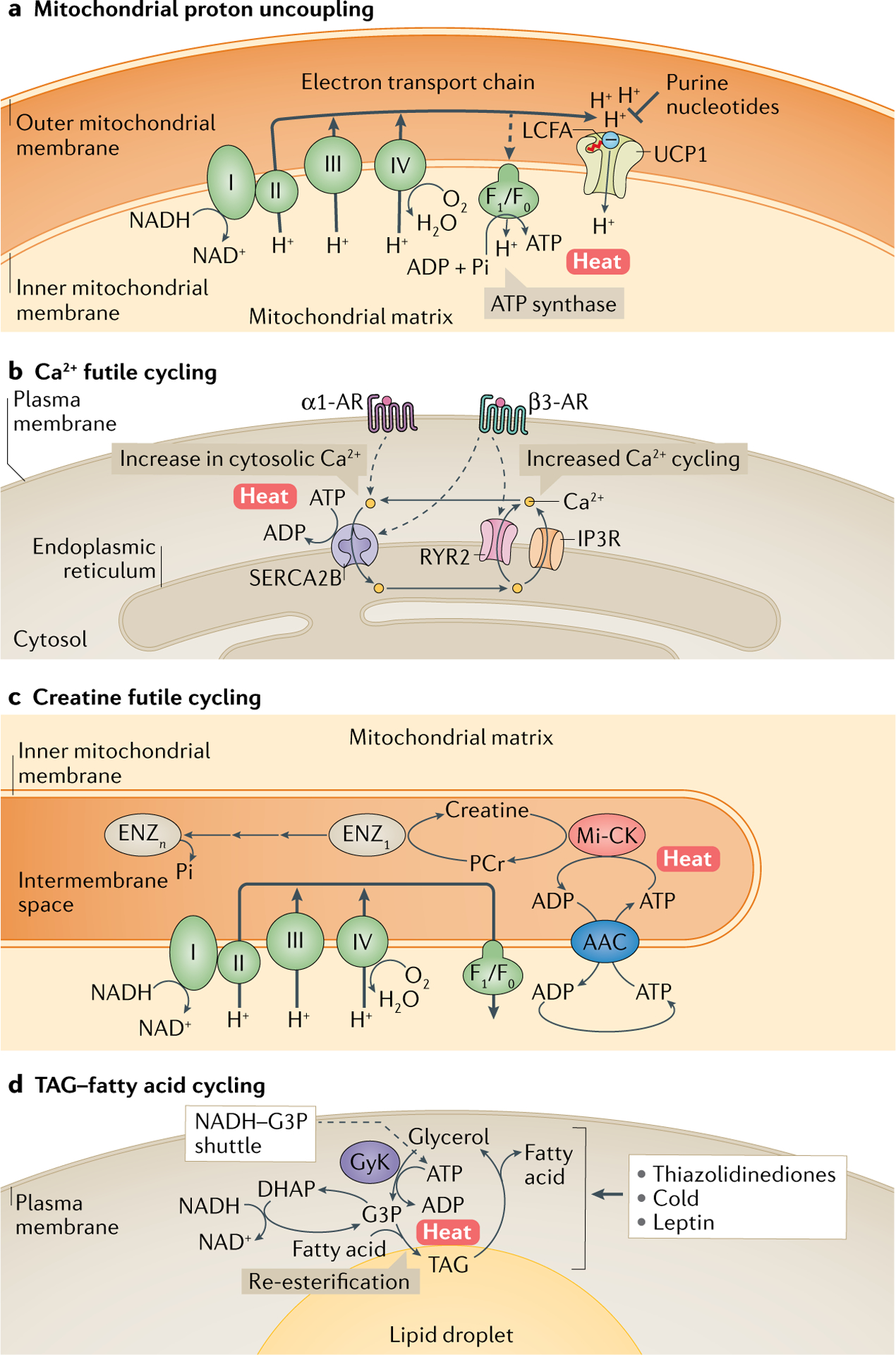
a | Mitochondrial respiration uncoupling via uncoupling protein 1 (UCP1). UCP1 is located in the mitochondrial inner membrane and uncouples the proton (H+) gradient from ATP synthesis. UCP1 activity is inhibited by purine nucleotides, whereas long-chain free fatty acids (LCFAs; marked with a red tail), which are negatively charged at the carboxyl end, bind to UCP1 and trigger the transfer of H+ into the matrix as a fatty acid anion/H+ symporter. b | One of the mechanisms of UCP1-independent thermogenesis in beige adipocytes is futile Ca2+ cycling in and out of the endoplasmic reticulum. This involves Ca2+ uptake into the endoplasmic reticulum by sarcoplasmic/endoplasmic reticulum calcium ATPase 2B (SERCA2B) and its release by ryanodine receptor 2 (RYR2) and inositol trisphosphate receptor (IP3R), which is coupled to ATP hydrolysis by SERCA2B and heat generation. Activation of α1-adrenergic receptor (α1-AR) and β3-AR, in response to norepinephrine, triggers cellular Ca2+ flux and its futile cycling. Under certain conditions, such as increased cytosolic Ca2+ levels (for example, enhanced Ca2+release from RYR2 or IP3R), ATP abundance (high ATP/ADP ratio) or reduced binding affinity of SERCA2 to Ca2+ (often regulated by micropeptides), ATP hydrolysis by SERCA2 is uncoupled from Ca2+ uptake, thereby being highly exothermic. c | Another UCP1-independent thermogenic mechanism is creatine substrate cycling, which involves ATP-dependent phosphorylation of creatine by mitochondria-localized creatine kinase (Mi-CK) to phosphocreatine (PCr) and PCr dephosphorylation by unknown diphosphatases (Enz1–n). d | Lipolysis of triglycerides (TAGs) generates glycerol and fatty acids, which can be re-esterified back to TAG (TAG–fatty acid cycling). This process involves ATP-dependent conversion of glycerol to glycerol 3-phosphate (G3P) by glycerol kinase (GyK). G3P is also a key component of the NADH–G3P shuttle, which involves interconversion of G3P and dihydroxyacetone phosphate (DHAP) and allows for rapid ATP synthesis in the mitochondria. This cycle is promoted by thiazolidinediones, which activate GyK, as well as by cold exposure and satiety hormone leptin, which promote lipolysis. AAC, ADP/ATP carrier.
Oxidative phosphorylation.
A metabolic process in which cells use series of enzymes to oxidize glucose, fatty acids and other metabolites to produce ATP.
The source of fatty acids for UCP1 uncoupling was initially thought to derive from de novo lipolysis within brown adipocytes — cold or β3-AR agonists induce lipolysis in brown adipocytes, generating fatty acids that then could bind UCP1 and also be used for β-oxidation. However, BAT-specific deletion of critical regulators of lipolysis, such as adipose triglyceride lipase (ATGL) or the ATGL-activating protein comparative gene identification 58 (CGI58), does not attenuate BAT thermogenesis in vivo in mice. Instead, fat-specific deletion — encompassing both white and brown fat — of ATGL or CGI58 is associated with a defect in BAT thermogenesis85,86. Thus, fatty acids derived from WAT lipolysis, rather than de novo lipolysis in thermogenic adipocytes, are required for activating UCP1. Lipolysis-derived fatty acids are taken up by thermogenic adipocytes through the plasma membrane transporter CD36, which is required for cold-induced thermogenesis87,88. Of note, acylcarnitines are another important substrate for β-oxidation to fuel BAT thermogenesis. WAT lipolysis promotes acylcarnitine production in the liver, which is then actively taken up and utilized by BAT89.
Acylcarnitine.
A metabolite derived from carnitine and acyl-coenzyme A (acyl-CoA). generation of acylcarnitine allows the transport of fatty acids into the mitochondrial matrix for oxidation.
Recent studies have also described regulation of UCP1 via post-translational modifications. Cys253 of UCP1 is sulfenylated upon cold exposure through the action of mitochondrial reactive oxygen species90. This sulfenylation site is located on the mitochondrial matrix side and proximal to a purine nucleotide binding site. Mutation of Cys253 reduces norepinephrine-stimulated uncoupled respiration, suggesting a functional requirement for UCP1 sulfenylation for its activity. UCP1 is also succinylated at Lys56 and Lys151 located on the mitochondrial matrix side, whereas succinylation is reversed by the action of the mitochondrial sirtuin Sirt5 (REF.91). Mutation of these sites to an acyl-mimetic residue glutamic acid significantly decreases protein stability, suggesting that succinylation of UCP1 negatively impacts uncoupling activity.
Sulfenylation.
A post-translational protein modification involving the addition of a sulfenyl group to cysteine residues.
Succinylation.
A post-translational modification in which a succinyl group is added to proteins at lysine residues.
Sirtuin.
A protein with NAD-dependent deacetylase activity that plays key roles in cellular homeostasis, including ageing, transcription, stress response, inflammation and apoptosis.
UCP1-independent mechanisms.
UCP1 is undoubtedly a key driver of thermogenesis. Nonetheless, studies in the last few years identified several UCP1-independent thermogenic pathways that have been shown to participate in the regulation of whole-body energy homeostasis.
The basis of UCP1-independent thermogenesis is provided by futile metabolic cycling mechanisms, whereby one ATP-consuming reaction occurs simultaneously with an inverse energetic reaction. These futile cycles have no overall effect other than consumption of ATP and dissipation of energy, which occurs in the form of heat. It is worth noting that beige adipocytes possess high ATP synthesis capacity relative to brown adipocytes. Thus, UCP1-null mice, if they possess enough beige fat — which can be accomplished by gradual acclimation to cold conditions — can use the ATP-dependent futile cycles in the beige fat to maintain thermogenesis92,93.
One of the UCP1-independent futile mechanisms is Ca2+ cycling thermogenesis (FIG. 4b). Intracellular Ca2+ levels are tightly balanced between uptake into the endoplasmic reticulum (or sarcoplasmic reticulum in the muscle) by sarcoplasmic/endoplasmic reticulum calcium ATPase (SERCA) and release through ryanodine receptors (RYRs) or inositol trisphosphate receptor (IP3R). Under ADP-limiting conditions (high ATP/ADP ratio) and when the cytosolic Ca2+ concentration is high, ATP hydrolysis by SERCA is uncoupled from Ca2+ pumping into the endoplasmic reticulum/sarcoplasmic reticulum lumen, resulting in constant ATP consumption and energy dissipation by SERCA that becomes exothermic (estimated as 14–16 kcal/mol of Ca2+ in skeletal muscle, although the value depends on the tissues)94. This becomes the dominant thermogenic mechanism in malignant hyperthermia, a condition in which uncontrolled Ca2+ release from the sarcoplasmic reticulum/endoplasmic reticulum into the cytoplasm is triggered by genetic mutations in RYR1 located in the sarcoplasmic reticulum membrane or by anaesthetic drugs. Also, sarcolipin (Sln), a micropeptide that binds SERCA1 in the skeletal muscle, uncouples ATP hydrolysis from Ca2+ pumping into the sarcoplasmic reticulum and promotes non-shivering thermogenesis95. Although these mechanisms have been mostly studied in skeletal muscle, beige adipocytes express the SERCA isoform, SERCA2B, and there is evidence that its activity is an important UCP1-independent thermogenic mechanism93. This pathway was shown to be stimulated by cold exposure followed by activation of the α1-AR, which triggers intracellular Ca2+ influx, as well as by activation of β3-AR signalling, which triggers Ca2+ release from the endoplasmic reticulum by promoting RYR2 activity93. A recent study also demonstrated that fat-selective activation of intracellular Ca2+ cycling by a wireless, implantable optogenetic device triggered UCP1-independent Ca2+ cycling-dependent thermogenesis in mice, and this increased whole-body energy expenditure sufficiently to prevent diet-induced obesity96. More recently, 5′ AMP-activated protein kinase (AMPK) activation, in response to a low-protein/high-carbohydrate diet, was shown to stimulate Ca2+ cycling thermogenesis in subcutaneous WAT of mice via the SERCA pathway97.
5′ AMP-activated protein kinase.
(AMPK). A heterotrimeric enzyme complex that is activated in response to low cellular ATP, including low glucose and hypoxia, and stimulates glucose and fatty acid catabolism and autophagy.
A second example of futile, thermogenic metabolite cycling is creatine cycling involving its phosphorylation and de-phosphorylation (FIG. 4c). Under ADP-limiting conditions, phosphorylation of creatine by mitochondrial creatine kinase (Mi-CK) occurs simultaneously with hydrolysis of phosphocreatine (PCr), thereby creating creatine-driven futile substrate cycling98. Fat-specific deletion of glycine amidinotransferase (Gatm), the rate-limiting enzyme in creatine biosynthesis, reduces whole-body energy expenditure and causes diet-induced obesity, even in the presence of UCP1 (REF.99). Furthermore, deletion of the cell surface creatine transporter SLC6A8 reduces energy expenditure in mice100. These studies suggest that under ADP-limited conditions, creatine cycling is an important thermogenic pathway regardless of UCP1 expression. Nevertheless, how this cycle is induced and regulated in response to thermogenic stimuli remains unexplored.
A third thermogenic futile cycle is based on conversion between triacylglycerol (TAG) and fatty acids (FIG. 4d). TAG hydrolysis is catalysed by a series of lipases, including ATGL, hormone-sensitive lipase (HSL) and monoacylglycerol lipase (MGL), to generate glycerol and free fatty acids (FFAs), which are released and utilized by the liver and muscle in a fasted state. However, these fatty acids can also be re-esterified back to TAG, resulting in futile TAG–fatty acid cycling in adipocytes. Notably, this futile pathway is induced by beiging cues. For example, thiazolidinediones powerfully stimulate glycerol incorporation into TAG by activating glycerol kinase (GyK) expression, which phosphorylates glycerol to glycerol 3-phosphate (G3P) — a primary backbone for TAGs101. Also, cold exposure promotes fatty acid generation via lipolysis and their re-esterification in mouse WAT102. In addition, leptin treatment activates TAG–fatty acid cycling, which is estimated to account for 14% of leptin-stimulated energy expenditure103. Of note, an ancillary mechanism to this pathway is the activity of the NADH–G3P shuttle, a mechanism that transfers reducing equivalents (H+, e−) from cytoplasm to mitochondria. G3P, as generated by TAG–fatty acid cycling, can be cyclically reduced and oxidized by cytoplasmic and mitochondrial G3P dehydrogenases, respectively, resulting in the transfer of reducing equivalents — as NADH — to complex III of the mitochondrial respiratory chain. Dihydroxyacetone-3-phosphate (DHAP) serves as an acceptor of NADH generated in the cytoplasm. This allows for rapid ATP generation aerobically, but less efficiently than that from glycolysis because the reducing equivalents from G3P enter the respiratory chain further down, at the level of complex III, and thus only two ATPs per atom of oxygen are generated, instead of three ATPs when reducing equivalents are transferred into complex I. As mitochondrial G3P dehydrogenase is highly expressed in BAT, this pathway is suggested to contribute to energy dissipation in thermogenic fat104. In line with this, whole-body deficiency of mitochondrial G3P dehydrogenase in mice was associated with a modest but significant reduction in energy expenditure and reduced norepinephrine-stimulated thermogenesis in BAT105. Curiously, however, when both UCP1 and mitochondrial G3P dehydrogenase were deleted in mice, they exhibited higher energy expenditure than controls, accompanied by increased beige fat biogenesis and respiration in inguinal WAT106. Hence, combined inactivation of UCP1 and mitochondrial G3P dehydrogenase appears to induce alternative thermogenic pathways that overcome the loss of the above thermogenic pathways; it remains unclear whether UCP1 and mitochondrial G3P dehydrogenase loss activate UCP1-independent thermogenesis via Ca2+ cycling or creatine cycling, or whether other compensatory mechanisms are at play.
Leptin.
An adipocyte-derived hormone that regulates food intake and energy expenditure.
Of interest, mitochondrial uncoupling can be induced in a UCP1-independent fashion. One of the described mechanisms involves mitochondrial ATP/ADP symporters of the SLC25 family, SLC25A4 and SLC25A5 (also known as ANT1 and ANT2), which can mediate proton influx into the mitochondrial matrix in a manner stimulated by N-acyl amino acids (for example, C18:1-Phe or Leu)107. These metabolites are generated by peptidase M20 domain containing 1 (PM20D1) — an enzyme that is secreted from brown and beige fat and catalyses the condensation of fatty acids and amino acids into N-acyl amino acids. Hence, N-acyl amino acids function as endogenous mitochondrial uncouplers107. Accordingly, different pathways and mechanisms that interfere with mitochondrial respiratory efficiency could contribute to thermogenesis.
N-Acyl amino acids.
Lipids that contain a fatty-acid tail covalently conjugated to an amino acid head group.
Metabolic roles beyond thermogenesis
The functions of brown and beige fat are far more complex than merely as thermogenic cells, with multiple roles in metabolic homeostasis108. Here, we summarize our recent understanding of the non-thermogenic mechanisms of brown and beige fat with an emphasis on the metabolic sink function, secretion of polypeptides and metabolites, and their contribution to adipose tissue homeostasis (FIG. 5).
Fig. 5 |. The multifaceted roles of brown and beige fat.
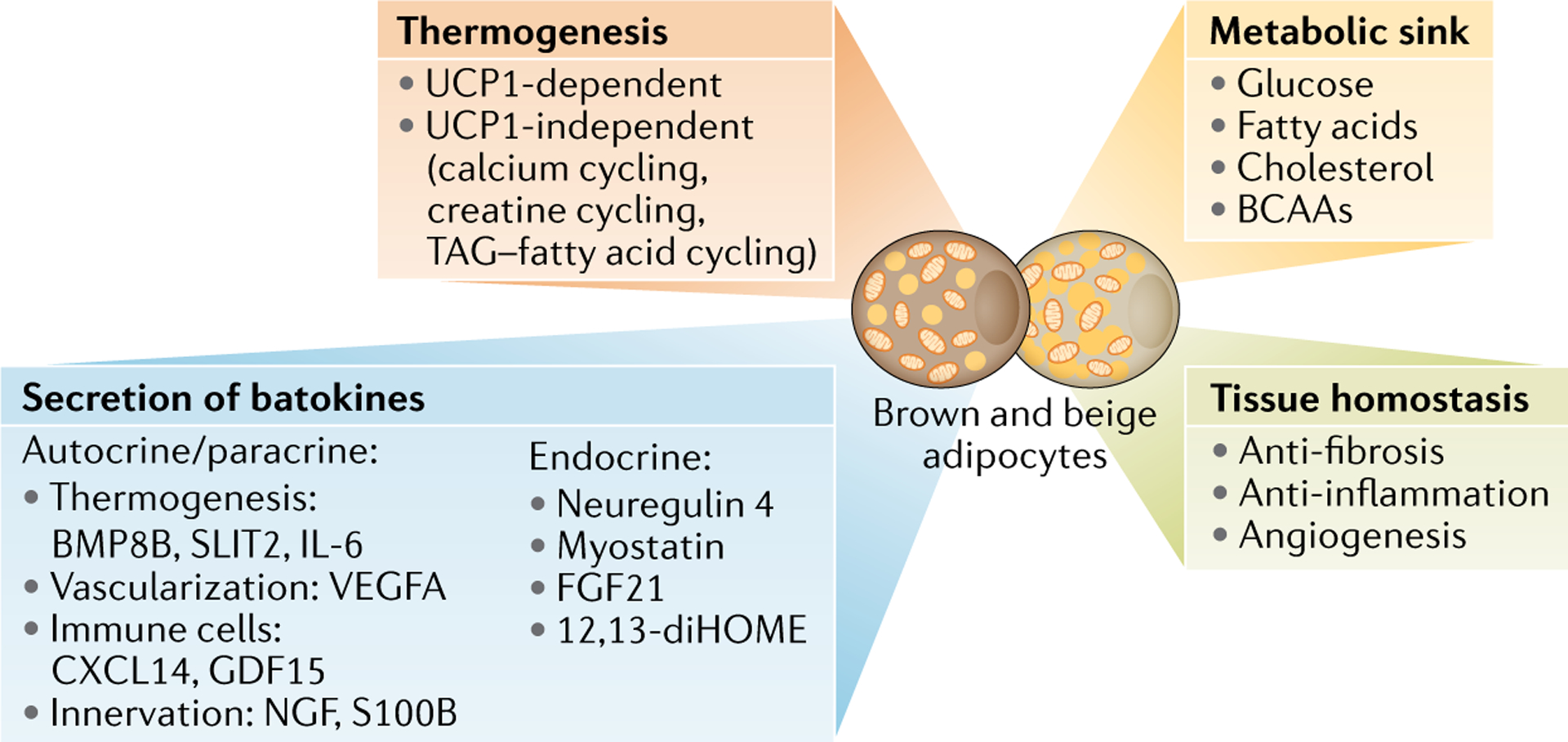
One of the biological roles of brown and beige fat is thermogenesis that involves uncoupling protein 1 (UCP1)-dependent and UCP1-independent mechanisms (FIG. 4). Besides thermogenesis, brown and beige fat function as a metabolic sink for glucose, fatty acids, cholesterol and branched-chain amino acids (BCAAs), thereby controlling metabolite clearance in the circulation. At the tissue level, enhanced beige fat biogenesis is coupled with reduced fibrosis, reduced inflammation and increased angiogenesis within adipose tissues (supporting tissue homeostasis). Also, brown and beige fat secrete various molecules (also known as batokines, as listed in the figure) that mediate communication with central and peripheral organs (endocrine) and cell to cell communication within adipose tissues (autocrine/paracrine). FGF21, fibroblast growth factor 21.
Roles in glucose metabolism.
BAT actively takes up glucose, which is an important substrate for fuelling thermogenesis, particularly in response to cold exposure. Glucose uptake in brown fat is stimulated by both insulin and catecholamines and contributes significantly to whole-body glucose disposal109. This section focuses on the broad role of brown fat in glucose metabolism, particularly in animal models; the importance of these mechanisms in human physiology is discussed in the next section.
Adipocytes express the insulin receptor and take up glucose via the insulin-stimulated glucose transporter type 4 (GLUT4) pathway, functioning as important sinks of glucose, thereby regulating systemic blood glucose levels. Selective deletion of the insulin receptor in UCP1-positive adipocytes has been reported to result in an age-dependent loss of interscapular BAT and systemic glucose intolerance in mice110. Unlike white fat cells that solely rely on GLUT4 for glucose uptake, brown adipocytes can also take up glucose via the GLUT1 transporter in an adrenergic-dependent and insulin-independent manner111. This mechanism involves the activity of nutrient sensor mTORC2 downstream of β3-AR signalling112. The relative roles and modes of regulation of GLUT1 versus GLUT4-dependent glucose uptake in thermogenic fat in vivo will require further investigation.
Several in vivo studies have confirmed an important role for thermogenic fat in systemic glucose homeostasis. Genetic ablation of UCP1+ cells in mice resulted in obesity, hyperglycaemia and hyperinsulinemia113, although it was unclear whether the effects on glucose metabolism were direct or secondary to excess adiposity associated with UCP1 deletion. In support of a more direct role of thermogenic fat in glucose regulation, transplantation of BAT depots to the visceral cavity of mice improved insulin-stimulated glucose uptake into endogenous white and brown fat and resulted in improved glucose and insulin tolerance114. Moreover, treatment of rats with the β3-AR agonist CL316,243 enhanced basal and insulin-stimulated glucose uptake in the absence of any decrease in body weight115. More recently, increasing beige fat biogenesis in mice by transgenic overexpression of PRDM16, the key transcriptional regulator of beige adipocytes, improved glucose and insulin tolerance68, whereas adipocyte-specific deletion of PRDM16 resulted in systemic insulin resistance accompanied by beige fat loss69. It is notable that the metabolic effects of beige fat on enhanced glucose tolerance is UCP1-independent, as adipose-specific PRDM16 transgenic animals lacking UCP1 still display improved glucose tolerance93.
In general, activation of thermogenesis in brown and beige fat, whether via UCP1-dependent or UCP1-independent pathways, is associated with improved glucose and insulin tolerance, particularly in the context of dietary obesity that is known to result in impaired thermogenic fat function, including insulin resistance in BAT116. Moreover, enhancing or ablating thermogenic fat function also appears to modulate glucose homeostasis in other tissues such as the liver and, perhaps, skeletal muscle. The underlying mechanisms through which thermogenic fat results in increased glucose clearance and improved insulin sensitivity are an area of active investigation. Some possibilities include their contribution to increased energy expenditure, anti-inflammatory function, reduced adipose tissue fibrosis and endocrine effects on other tissues such as the liver and skeletal muscle (FIG. 5).
Roles in lipid metabolism.
Although the activation of thermogenic fat is associated with lower blood glucose levels and improved insulin sensitivity, the FFAs constitute a preferred energetic substrate for most thermogenic adipocytes, establishing thermogenic adipocytes as important sinks for FFAs. This fuel preference raises the question of whether thermogenic fat may exert effects on systemic lipid metabolism. Indeed, increasing thermogenic fat activity in mice by short-term cold exposure results in accelerated clearance of triglyceride-rich lipoproteins from the blood, with uptake by BAT depots playing a dominant role117. Clearance of triglyceride-rich lipoproteins by BAT is dependent on lipoprotein lipase and the lipid transporter CD36, and is sufficient to lower triglycerides in the Apoa5 knockout model of severe hyperlipidaemia. A subsequent study found that cold exposure or pharmacologic activation of thermogenic fat with the β3-AR agonist CL316,243 improved dyslipidaemia in hyperlipidaemic mice and, importantly, attenuated the development of atherosclerosis118. In addition to clearing atherogenic lipoproteins from the blood, stimulation of thermogenesis also increases high-density lipoprotein (HDL) cholesterol levels in hyperlipidaemic mice, promoting HDL cholesterol turnover and reverse cholesterol transport, which may further contribute to the atheroprotective roles of BAT119. Interestingly, statin drugs, the most widely used agents for treating hypercholesterolaemia, happen to suppress the activity of thermogenic fat via effects on the mevalonate pathway intermediate, geranylgeranyl pyrophosphate120.
Triglyceride-rich lipoproteins.
Lipoproteins that transport triglycerides and cholesterol; these include very low-density lipoprotein (VLDL) and chylomicrons.
Reverse cholesterol transport.
A process in which cholesterol from peripheral organs is returned to the liver via the circulation.
Mevalonate pathway.
A metabolic pathway for the synthesis of sterols and isoprenoids.
The mechanisms through which thermogenic adipocytes regulate lipid metabolism are likely multifaceted and complex. As an illustrative example, one study demonstrated that thermogenic adipocytes engage in inter-organ communication to regulate cholesterol metabolism. In this case, cold promotes the conversion of cholesterol to bile acids in the liver, which are subsequently directed for faecal excretion. In the gut, bile acids modulate the microbiome, which feeds back to promote thermogenesis of adipocytes, whereby gut microbiome-derived endocrine factors and/or metabolites act together with higher plasma bile acids to sustain thermogenic fat function121. Additionally, thermogenic fat secretes phospholipid transfer protein (PLTP), a key enzyme controlling the size and composition of HDL, which promotes faecal lipid excretion and reduces phospholipids and ceramides in the circulation122.
Roles in amino acid metabolism.
Besides fatty acids and glucose, thermogenic adipocytes are a significant metabolic sink for branched-chain amino acids (BCAAs; for example, Val, Leu and Ile)123,124. Notably, cold acclimation profoundly enhances systemic BCAA clearance preferentially in human individuals with active thermogenic fat124. Consistent with observations in humans, cold exposure significantly reduced plasma Val, Leu and Ile in mice, but this effect was absent in mice with ablated thermogenic adipocytes. This BCAA sink function is an important aspect of thermogenic fat function in glucose homeostasis, because defective BCAA oxidation leads to diet-induced weight gain, glucose intolerance and insulin resistance in mice124. In line with this, increased circulating BCAA levels, owing to reduced BCAA clearance, are linked to obesity, insulin resistance and type 2 diabetes in humans125–132.
Of note, BCAAs are more than an energetic fuel in thermogenic adipocytes. Carbon from Leu and Ile catabolism generates monomethyl branched-chain fatty acids (mmBCFAs), which contribute to de novo lipogenesis in differentiated adipocytes133,134, and BAT is the organ that most actively generates mmBCFAs upon cold exposure135. In humans, mmBCFA levels in adipose tissues were significantly lower in individuals with obesity as compared to lean individuals, and mmBCFA levels were increased following weight loss after bariatric surgery. Furthermore, the adipose tissue mmBCFA content positively correlates with skeletal muscle insulin sensitivity136. Although the causal link between mmBCFAs and metabolic traits remains unestablished, the role of thermogenic fat as a BCAA sink and the main site of mmBCFA synthesis provides new insights into mechanisms by which activation of brown and beige fat improves systemic glucose homeostasis and insulin sensitivity.
Bariatric surgery.
A surgical procedure that promotes weight loss. These procedures include the Roux-en-Y gastric bypass, sleeve gastrectomy, adjustable gastric band and biliopancreatic diversion with duodenal switch.
Secretory function.
Multiple lines of evidence suggest that some of the physiological effects of thermogenic fat are mediated by secreted polypeptides or small molecule metabolites, collectively referred to as batokines. First, the broad number of physiological systems influenced by thermogenic fat in mice and humans suggest that these tissues contribute to metabolic homeostasis by more than thermogenesis alone108. Second, the discrepancy in phenotypes between mice with an ablation of brown fat113 versus mice with a genetic deletion of UCP1 (REF.77) could be consistent with an endocrine role for these tissues, beyond heat generation. Third, the fact that mice with increased beige fat biogenesis induced by fat-selective PRDM16 transgenic expression retain most of their metabolic benefits even in the absence of UCP1 (REF.93) not only points to the existence of alternative thermogenic mechanisms but could also point towards the role of PRDM16 in regulating the secretory function of these cells. Fourth, transplantation studies in mice have shown that even small amounts of brown fat can have rather prominent effects on systemic metabolism114,137. Finally, transcriptomic, proteomic and metabolomic analyses have identified many molecules potentially secreted by thermogenic fat122,138–140.
In recent years, an increasing number of studies have characterized physiologically relevant polypeptides and metabolites secreted by thermogenic fat. An overview of all of these discoveries is beyond the scope of this Review, but has recently been comprehensively reviewed by others141. In accord with the different roles of white and brown fat in energy storage versus energy dissipation, these two cell types also have distinct secretory properties. To date, most studies have focused on polypeptides, and their roles can be broadly divided into local (autocrine/paracrine) and distant (endocrine) actions. Locally acting factors include proteins that regulate thermogenesis (BMP8B142, SLIT2 (REF.143), IL-6 (REF.144)), vascularization (VEGFA)145,146, tissue immune cell activity (CXCL14 (REF.147), GDF15 (REF.148)) and adipose tissue innervation (NGF1 (REF.149), S100B150). Batokines with endocrine function include neuregulin 4 (NRG4), which negatively regulates de novo lipogenesis in the liver151; myostatin, which regulates skeletal muscle function152; FGF21, which regulates cardiac remodelling and beige fat biogenesis45,153; and PLTP, which regulates glucose and lipid metabolism in the liver122.
Thermogenic fat likely secretes an even larger number of small molecules, although their identification and characterization has lagged behind that of secreted polypeptides. Using global lipidomic analysis, the oxylipin 12,13-diHOME was identified as a circulating metabolite that is increased following cold exposure and facilitates fatty acid transport into BAT154. 12,13-diHOME is also induced by exercise and promotes skeletal muscle fatty acid uptake155. In addition to polypeptides and metabolites, thermogenic fat secretes extracellular vesicles that contain miRNAs that exert local or distant effects156,157.
Oxylipin.
An oxygenated lipid derived from polyunsaturated fatty acid.
Collectively, these studies have identified several hundred candidate batokines from mice and humans, the roles of most of which have yet to be identified. A future challenge for the field will be to define the full secretome of thermogenic fat and to characterize the target and mode of action of each of these molecules.
Anti-fibrotic activity.
Adipose tissue contains high levels of extracellular matrix (ECM) proteins, such as collagens, that play an essential role in tissue remodelling and expansion (key events associated with increases in adiposity). However, pathologically excessive ECM proteins in adipose tissue are closely linked to increased infiltration of pro-inflammatory immune cells and the development of adipose tissue fibrosis, which leads to chronic tissue inflammation that has been linked to adipose tissue dysfunction, including the impairment of adipogenesis and thermogenesis158 (BOX 1). ECM accumulation in subcutaneous WAT is strongly associated with insulin resistance and type 2 diabetes159–163. In fact, ECM accumulation is not merely a molecular hallmark of adipose tissue dysfunction but also a causal and exacerbating factor in the pathogenesis of type 2 diabetes. For instance, genetic loss of collagen VI, a major ECM protein in adipose tissues that accumulates in obesity, prevents WAT fibrosis and inflammation, thereby allowing healthy tissue expansion and improved insulin sensitivity in mice164.
Notably, enhanced beige fat biogenesis is inversely correlated with the development of adipose tissue fibrosis in subcutaneous WAT. Cold acclimation or fat-specific PRDM16 overexpression prevents the accumulation of ECM proteins and adipose tissue fibrosis165,166. The reduced ECM is noted even in Ucp1-null mice, suggesting that the suppressive effects of beige fat on adipose tissue fibrosis are UCP1-independent165. Recent studies have identified two complementary mechanisms. First, beige adipocytes secrete β-hydroxybutyrate (BHB) that acts on precursor cells to repress fibrosis via the PRDM16-driven transcriptional pathway166. Second, PRDM16 forms a complex with a cold-activated transcription factor GTF2IRD1 to repress pro-fibrosis gene expression in adipose tissues. Significantly, dietary supplementation of BHB or fat-selective overexpression of GTF2IRD1 potently represses adipose tissue fibrosis and improves glucose tolerance and insulin sensitivity in mice165,166. Thus, anti-fibrotic action of thermogenic fat also impacts systemic glucose homeostasis.
β-Hydroxybutyrate.
(BHB). A major form of ketone bodies that is generated through fatty acid oxidation or leucine oxidation.
Thermogenic fat in human physiology
Until relatively recently, thermogenic fat was thought to only be relevant in small mammals and human infants as a means to protect against hypothermia. However, findings over the past two decades have overturned this view and confirmed that thermogenic adipocytes are present and functional in adult humans. Importantly, their prevalence and activity are positively associated with improved metabolic health in humans. Investigators have been actively searching for pharmacologic agents that recapitulate the metabolic benefits of cold-activated thermogenic fat (BOX 2), with an aim of therapeutic support for the management of obesity, diabetes and other metabolic disorders.
Box 2 |. Pharmacologic targeting of thermogenic fat in humans.
Although, as established in mouse models and in human studies, the activation of brown fat by cold is associated with metabolic benefits, cold exposure is uncomfortable, making it unlikely to be adopted as a therapeutic approach in humans. Investigators have been working intensively to identify pharmacologic agents that can recapitulate the benefits of cold-activated thermogenic fat. Although typical substances that mimic or modify the actions of endogenous catecholamines (sympathomimetics, also known as adrenergic drugs and adrenergic amines) such as ephedrine do not appear to activate brown fat in humans216, thermogenic adipocytes express high levels of the β3-adrenergic receptor (β3-AR) and treatment with the β3-AR agonist mirabegron is capable of activating brown fat217. Mirabegron is clinically approved for overactive bladder, and administration of this drug promoted robust 18F-fluorodeoxyglucose (FDG) uptake in brown fat depots and increased the resting metabolic rate, although it also led to increased heart rate and systolic blood pressure. In a recent study of individuals with obesity who are insulin resistant, mirabegron improved glucose tolerance and insulin sensitivity27. Interestingly, in this study there was no measurable increase in active brown adipose tissue (BAT) volume (as assessed by FDG positron emission tomography combined with computed tomography (FDG-PET/CT)), but there was an increase in uncoupling protein 1 (UCP1) expression in the subcutaneous white adipose tissue (WAT), which would be consistent with the activation of beige fat27. In a contemporaneous study in young healthy women, 4 weeks of mirabegron treatment increased the BAT activity and resting energy expenditure with associated increases in glucose tolerance as well as high-density lipoprotein (HDL) and adiponectin levels218.
Although mirabegron has been the most intensively studied pharmacological agonist in humans, a recent study suggests that β2-AR might be involved in activating thermogenic fat in humans, although the mechanisms involved here are unclear219. In addition, other approaches, beyond the use of adrenergic agonists, hold promise. Based on preclinical data showing that bile acids activate brown/beige fat via binding to the cell surface receptor TGR5, a small study in young healthy females showed that 2 days of oral chenodeoxycholic acid was sufficient to increase BAT activity220. In another small study in young healthy men, three oral doses of the steroid prednisolone, a synthetic corticosteroid that promotes the expression of the bile acid transporter, resulted in an increase in glucose uptake in BAT221. Interestingly, glucocorticoids appear to decrease UCP1 expression in murine brown adipocytes, highlighting a potentially important difference between mice and humans.
Chenodeoxycholic acid.
A primary bile acid synthesized in the liver.
Dietary factors also have the potential to be repurposed as therapeutics activating thermogenic fat. Administration of capsaicin and capsinoids, which are responsible for the hot sensation of chili peppers, resulted in a significant increase in energy expenditure in young healthy individuals with active BAT222 and in mice223. Other dietary factors including curcumin224 and cinnemaldehyde225 have been shown to activate thermogenic fat in animal models. Future studies will be needed to determine whether these and other dietary ingredients and natural products activate thermogenic fat in humans and to dissect underlying mechanisms, physiological consequences and their applicability as therapeutics.
Identification of thermogenic fat in adult humans.
Although humans, particularly early in life, have been long known to possess BAT in the interscapular and perirenal regions167, the first suggestion of the presence of functional thermogenic fat depots in adults came from clinical studies in radiology. 18F-fluorodeoxyglucose positron emission tomography combined with computed tomography (FDG-PET/CT) scans are commonly used to diagnose and stage cancers. By measuring uptake of radiolabelled glucose and overlaying PET data with whole-body computed tomography scans, clinicians can identify suspected malignancies and metastases. In reading these scans, it became apparent that many patients had bilateral, symmetric regions of increased glucose uptake in the neck region, which on computed tomography scan had the density of adipose tissue. These tissues were originally referred to as upper supraclavicular area fat, although their physiological significance was unclear168,169.
18F-fluorodeoxyglucose positron emission tomography combined with computed tomography.
(FDG-PET/CT). An imaging-based technique that measures the uptake of a radioactive glucose analogue.
A series of landmark papers published in 2009 confirmed that upper supraclavicular area fat was actually a human BAT equivalent. The activity, as measured by radiolabelled glucose uptake, was inducible by brief exposure to cold, and biopsy specimens showed that these tissues had the morphological and molecular characteristics of thermogenic fat170–173. Moreover, retrospective analysis of a large cohort of FDG-PET scans found that the prevalence of human BAT depots was higher in women than in men and inversely related to ambient temperature, age and body mass index. Furthermore, the presence of these depots was associated with lower circulating glucose levels173. These studies provided the foundation for a growing field of research on thermogenic fat in humans. In addition to supraclavicular human BAT depots, thermogenic fat has also been detected in cervical, axillary, mediastinal, paraspinal and abdominal depots174.
Although FDG-PET/CT remains the gold standard for the detection and quantification of thermogenic fat in humans, numerous other modalities have been employed, including magnetic resonance imaging (MRI), MRI spectroscopy, ultrasound, infrared imaging and measurement of local skin temperature. In addition, the Brown Adipose Reporting Criteria in Imaging Studies (BARCIST) provide a consistent framework for imaging and measuring brown fat175. One limitation to larger studies of thermogenic fat has been the absence of a reliable non-invasive test or biomarker that would allow large patient cohorts to be screened. In that regard, circulating levels of miR-92a have been inversely correlated with thermogenic fat activity in humans156. The suitability of miR-92a or other circulating biomarkers will require further study in independent larger cohorts.
Although they remain poorly characterized in vivo, similar to mice, adult humans possess beige adipocytes4–6,176, and their biogenesis is stimulated by chronic cold exposure or the β3-AR agonist mirabegron27,28. Notably, the presence of beige adipocytes was detected in adult human BAT depots and in subcutaneous WAT27,28. Thus, BAT of adult humans is heterogeneous, with the presence of mixed thermogenic adipocyte populations (Supplementary Box 1).
Roles for thermogenic fat in human physiology and pathophysiology.
A series of small interventional studies have highlighted the fact that the glucose uptake activity of human BAT — which is robustly promoted by cold — mediates effects on systemic metabolism. One of the earliest such studies started by exposing young healthy individuals to 19 °C for 2 h to identify individuals with inducible metabolic activity in the BAT depots assessed through FDG-PET scans177. The individuals who showed low BAT activity were then repeatedly exposed to 17 °C for 2 h each day for 6 weeks, which promoted BAT activity and cold-induced thermogenesis. These individuals demonstrated a significant reduction in total fat mass with a concomitant induction of glucose uptake in BAT177. A separate study specifically studied the metabolic effects of the induction of glucose uptake activity in BAT of individuals with type 2 diabetes. Individuals were studied at baseline or after 10 days of exposure to 14–15 °C for 2 h each day. Cold acclimation was associated with a significant increase in glucose uptake in the cold-activated BAT and this was associated with increased systemic insulin sensitivity178. Similar effects were documented in a small study comparing men with active BAT versus those who did not show BAT activity, as assessed by FDG-PET/CT, after 5–8 h of cold exposure179. Speaking to the potential public health relevance of these studies, another group studied healthy individuals who slept in a climate-controlled research unit for 4 months. Nightly temperatures were set at 24 °C for the first month, 19 °C for the second month, 24 °C for the third month and 27 °C for the fourth month. FDG-PET scans were performed at the end of each month, along with measurement of select circulating hormones. This study revealed an induction of BAT activity after the second month, which then returned to baseline after housing at 24 °C during the third month180. Furthermore, the month of cold nightly temperatures resulted in a significant increase in insulin sensitivity, along with increased serum adiponectin and decreased serum leptin levels. Cold exposure can also activate BAT and increase energy expenditure in individuals with obesity, who are most at risk of insulin resistance and type 2 diabetes181. A separate study also found that bariatric surgery was associated with the activation of BAT182.
Broader physiological effects of human thermogenic fat.
As highlighted in this Review, the published literature — from both animal models and humans — supports clear effects of thermogenic fat activity on energy expenditure, glucose homeostasis and cholesterol metabolism. A recent study suggests that thermogenic fat may also protect against the development of atherosclerosis183. In this study, a small group of individuals underwent baseline FDG-PET/CT imaging to measure cold-inducible BAT activity, and 5 years later, ultrasound studies were performed to measure markers of atherosclerosis and vascular dysfunction, which inversely correlated with BAT activity. Another recent study explored the cardio-metabolic effects of thermogenic fat by harnessing information available in electronic health records. By categorizing BAT activity from more than 130,000 FDG-PET/CT scans from more than 50,000 patients over nearly a decade, a propensity score-matched cohort was assembled comprising individuals with and without brown fat activity who were matched for age, sex, body mass index and outdoor temperature at the time of the scan. This study found that BAT activity was associated with significantly lower incidence of type 2 diabetes, dyslipidaemia, coronary artery disease, cerebrovascular disease, congestive heart failure and hypertension184. These findings were supported by improved glucose, triglyceride and HDL levels in individuals showing BAT activity. Moreover, the benefits of BAT activity were particularly prominent in individuals that were overweight or obese, supporting the potential for thermogenic fat to offset the deleterious effects of excess adiposity. Based on this evidence, there is strong interest to harness thermogenic fat for therapeutic benefit. Importantly, however, the full spectrum of physiologic effects mediated by this tissue as well as mechanisms through which thermogenic adipocytes contribute to metabolic fitness remain uncertain.
Conclusions and perspective
Thermogenic adipocytes have garnered considerable attention in recent years, owing to their varied contributions to whole-body energy homeostasis, glucose metabolism and lipid metabolism that could be harnessed to improve metabolic fitness in humans. Nevertheless, our knowledge of fat cell populations is still incomplete. Initially, thermogenic fat was categorized into brown adipocytes (that is, developmentally fixed dermomyotome-derived thermogenic adipocytes as seen in early life) or beige adipocytes (that is, ‘recruitable’ non-dermomyotome-derived thermogenic adipocytes present in postnatal stages). However, recent advances in cellular lineage determination and single-cell transcriptomics suggest that, in fact, adipocytes are much more diverse, with the existence of multiple subtypes that originate from distinct progenitors and are uniquely regulated by external and hormonal cues. Examples include g-beige adipocytes that originate from a unique subset of PDGFRα+ progenitors55 and cold-induced (but not β3-AR agonist-induced) Acta2-derived beige adipocytes50. A future goal will be to determine the lineage hierarchy within adipose tissues and to address the following questions: how many subtypes of adipocytes do mice and humans have; do they have unique biological or pathological functions; and how plastic is cellular fate in the adipocyte lineage? Advances in single-cell analysis technology make it now possible to address these questions. Importantly, single-cell technology will be able to address the quantitative dynamics in cellular composition within a metabolic organ in response to internal and external cues. One example is the finding that caloric restriction attenuates age-related changes in cellular composition, such as reduced pro-inflammatory cells, and modulates the transcriptional programme in adipose tissues and other organs185.
Studies from mice and humans indicate that the activity of BAT depots correlates with a lower prevalence of type 2 diabetes, dyslipidaemia and cardiovascular diseases. This raises the possibility of using BAT activity or prevalence as a means to stratify patients for therapeutic and/or nutritional interventions (FIG. 6). Uncovering the precise mechanisms by which BAT prevalence and activity alters metabolism and clinical phenotypes will be a prerequisite for incorporating the BAT status into precision medicine-based recommendations for lifestyle modification, risk factor management and therapeutic intervention.
Fig. 6 |. Harnessing thermogenic fat activity for precision medicine.
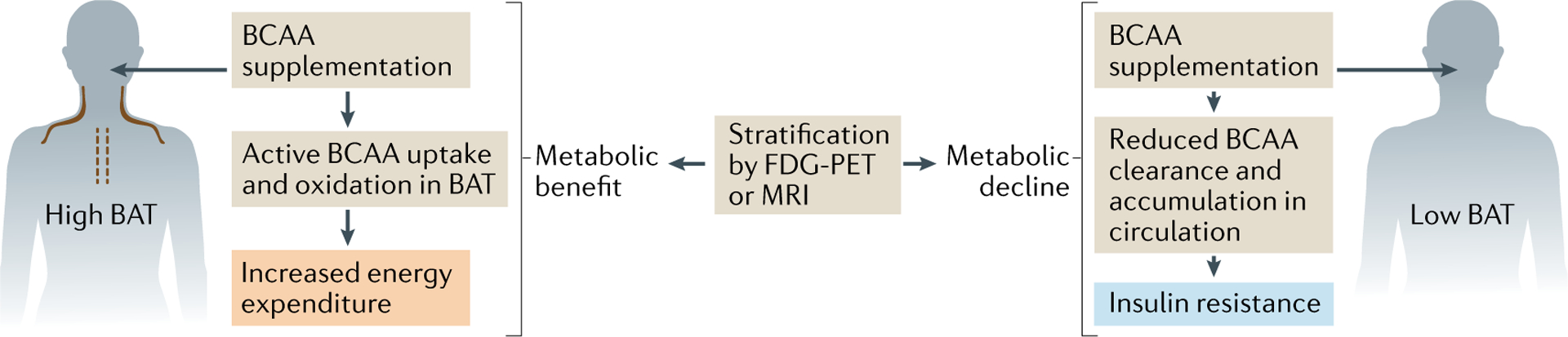
Nutritional supplementation with branched-chain amino acids (BCAAs) has differential outcomes in humans depending on their metabolic state: BCAAs are known to enhance energy expenditure, and, paradoxically, higher circulating levels of BCAAs are found in individuals with obesity and/or individuals with diabetes who exhibit lower energy expenditure125,128,193. BCAAs are actively imported into brown fat mitochondria and stimulate thermogenesis, and thus BCAA supplementation enhances energy expenditure for those individuals who are capable of oxidizing BCAAs in their brown adipose tissue (BAT) depots124. By contrast, impaired BAT activity as a BCAA sink, as often seen in obesity and ageing, reduces BCAA clearance, thereby increasing circulating BCAA levels, leading to the overflow of BCAAs into skeletal muscle and resulting in insulin resistance123. Hence, stratification of human individuals based on their BAT activity (using 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) or magnetic resonance imaging (MRI)) can be explored as a component of precision medicine to tailor therapeutic or dietary interventions to the patient’s metabolic state.
It is currently unknown to what extent brown fat volume and activity are determined by genetics as opposed to environmental stimuli. In mouse models, beige fat abundance is strongly dependent on the genetic background, with obesity-resistant inbred strains such as A/J possessing significantly more beige fat than obesity-prone strains such as C57Bl6/J. In humans, however, comparatively little is known about the genetic underpinnings of thermogenic fat function. Polymorphisms in UCP1 (−3826 A/G substitution in the UCP1 enhancer) and the β3-AR (Trp to Arg substitution at position 64 of the protein) have been linked to accelerated age-related decline in BAT activity186. However, it is not clear whether additional genetic variants might contribute to thermogenic fat development and/or function. Several lines of evidence raise the possibility that such genetic determinants may exist. For example, a small case–control study found that Dutch individuals with South Asian ancestry had significantly lower brown fat volume than matched white individuals187, raising the notion that some of these differences may have a genetic basis. Intriguingly, a Dutch man in his 50s known as the ‘Ice Man’ who holds multiple records for endurance cold exposure had shown brown fat activity comparable with that of much younger men. Although this may simply be due to his environmental exposures, he has a monozygotic twin brother who is not exposed to extreme cold, and he too had brown fat activity similar to that of younger men188. Although this may be an isolated example, it suggests the possibility that genetic variants may exist that result in enhanced thermogenic fat function in humans. Unlike obesity, however, there is no straightforward, non-invasive way to screen large populations for increased BAT activity. With the completion of larger retrospective and prospective analyses of FDG-PET/CT scans, the potential exists to identify other individuals with exceptionally high brown fat activity who can be studied genetically. There is also the possibility that recent genetic studies of individuals who are thin but otherwise healthy may identify variants in genes that contribute to elevated BAT function and increased energy expenditure189. Ultimately, the functional relevance and mechanistic basis of any genetic variant will require experiments in cell lines and animal models. Such studies may provide new biomedically relevant insights into the biology of thermogenic fat, thereby facilitating clinical approaches aimed at modulating the activity of thermogenic adipocytes.
Supplementary Material
Acknowledgements
The authors apologize for being unable to cite large numbers of important contributions to the field due to space limitations. This work was supported by the American Diabetes Association Pathway Program (1-17-ACE-17) to P.C., and by the National Institutes of Health (NIH) (DK097441, DK125281, DK126160, DK127575, DK125283) and the Edward Mallinckrodt, Jr. Foundation to S.K.
Footnotes
Competing interests
The authors declare no competing interests.
Supplementary information
The online version contains supplementary material available at https://doi.org/10.1038/s41580-021-00350-0.
References
- 1.Cinti S Obesity, Type 2 Diabetes and the Adipose Organ: A Pictorial Atlas from Research to Clinical Applications 1st edn (Springer, 2017). [Google Scholar]
- 2.Wu J, Cohen P & Spiegelman BM Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev. 27, 234–250 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harms M & Seale P Brown and beige fat: development, function and therapeutic potential. Nat. Med 19, 1252–1263 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Lidell ME et al. Evidence for two types of brown adipose tissue in humans. Nat. Med 19, 631–634 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Wu J et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shinoda K et al. Genetic and functional characterization of clonally derived adult human brown adipocytes. Nat. Med 21, 389–394 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cypess AM et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat. Med 19, 635–639 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikeda K, Maretich P & Kajimura S The common and distinct features of brown and beige adipocytes. Trends Endocrinol. Metab 29, 191–200 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lepper C & Fan CM Inducible lineage tracing of Pax7-descendant cells reveals embryonic origin of adult satellite cells. Genesis 48, 424–436 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seale P et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 454, 961–967 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atit R et al. β-Catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev. Biol 296, 164–176 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Sanchez-Gurmaches J & Guertin DA Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat. Commun 5, 4099 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sebo ZL, Jeffery E, Holtrup B & Rodeheffer MS A mesodermal fate map for adipose tissue. Development 145, dev166801 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang W et al. Ebf2 is a selective marker of brown and beige adipogenic precursor cells. Proc. Natl Acad. Sci. USA 111, 14466–14471 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L et al. Generation of functional brown adipocytes from human pluripotent stem cells via progression through a paraxial mesoderm state. Cell Stem Cell 27, 784–797.e11 (2020). [DOI] [PubMed] [Google Scholar]; This study generates human brown adipocytes from pluripotent stem cells by a serum-free directed differentiation strategy.
- 16.Xue B et al. Genetic variability affects the development of brown adipocytes in white fat but not in interscapular brown fat. J. Lipid Res 48, 41–51 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Lee YH, Petkova AP, Mottillo EP & Granneman JG In vivo identification of bipotential adipocyte progenitors recruited by β-adrenoceptor activation and high-fat feeding. Cell Metab 15, 480–491 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berry DC, Jiang Y & Graff JM Mouse strains to study cold-inducible beige progenitors and beige adipocyte formation and function. Nat. Commun 7, 10184 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu W et al. A heterogeneous lineage origin underlies the phenotypic and molecular differences of white and beige adipocytes. J. Cell Sci 126, 3527–3532 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oguri Y et al. CD81 controls beige fat progenitor cell growth and energy balance via FAK signaling. Cell 182, 563–577.e20 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Long JZ et al. A smooth muscle-like origin for beige adipocytes. Cell Metab 19, 810–820 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vishvanath L et al. Pdgfrβ+ mural preadipocytes contribute to adipocyte hyperplasia induced by high-fat-diet feeding and prolonged cold exposure in adult mice. Cell Metab 23, 350–359 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulz TJ et al. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc. Natl Acad. Sci. USA 108, 143–148 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodeheffer MS, Birsoy K & Friedman JM Identification of white adipocyte progenitor cells in vivo. Cell 135, 240–249 (2008). [DOI] [PubMed] [Google Scholar]
- 25.Berry R & Rodeheffer MS Characterization of the adipocyte cellular lineage in vivo. Nat. Cell Biol 15, 302–308 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cattaneo P et al. Parallel lineage-tracing studies establish fibroblasts as the prevailing in vivo adipocyte progenitor. Cell Rep 30, 571–582.e2 (2020). [DOI] [PubMed] [Google Scholar]
- 27.Finlin BS et al. The β3-adrenergic receptor agonist mirabegron improves glucose homeostasis in obese humans. J. Clin. Invest 130, 2319–2331 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reports that chronic activation of the β3-AR by mirabegron improves insulin sensitivity and activates beige fat in humans with obesity.
- 28.Finlin BS et al. Human adipose beiging in response to cold and mirabegron. JCI Insight 3, e121510 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Min SY et al. Human ‘brite/beige’ adipocytes develop from capillary networks, and their implantation improves metabolic homeostasis in mice. Nat. Med 22, 312–318 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raajendiran A et al. Identification of metabolically distinct adipocyte progenitor cells in human adipose tissues. Cell Rep 27, 1528–1540.e7 (2019). [DOI] [PubMed] [Google Scholar]
- 31.Singh AM et al. Human beige adipocytes for drug discovery and cell therapy in metabolic diseases. Nat. Commun 11, 2758 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang QA, Tao C, Gupta RK & Scherer PE Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat. Med 19, 1338–1344 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Himms-Hagen J et al. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am. J. Physiol. Cell Physiol 279, C670–C681 (2000). [DOI] [PubMed] [Google Scholar]
- 34.Barbatelli G et al. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am. J. Physiol 298, E1244–E1253 (2010). [DOI] [PubMed] [Google Scholar]
- 35.Shao M et al. Cellular origins of beige fat cells revisited. Diabetes 68, 1874–1885 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reports the quantitative contribution of beige adipocyte biogenesis via de novo differentiation versus reinstallation of existing adipocytes in vivo.
- 36.Lee YH, Petkova AP, Konkar AA & Granneman JG Cellular origins of cold-induced brown adipocytes in adult mice. FASEB J 29, 286–299 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tajima K et al. Mitochondrial lipoylation integrates age-associated decline in brown fat thermogenesis. Nat. Metab 1, 886–898 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Berry DC et al. Cellular aging contributes to failure of cold-induced beige adipocyte formation in old mice and humans. Cell Metab 25, 481 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Rosenwald M, Perdikari A, Rulicke T & Wolfrum C Bi-directional interconversion of brite and white adipocytes. Nat. Cell Biol 15, 659–667 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Altshuler-Keylin S et al. Beige adipocyte maintenance is regulated by autophagy-induced mitochondrial clearance. Cell Metab 24, 402–419 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu X et al. Mitophagy controls beige adipocyte maintenance through a Parkin-dependent and UCP1-independent mechanism. Sci. Signal 11, eaap8526 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roh HC et al. Warming induces significant reprogramming of beige, but not brown, adipocyte cellular identity. Cell Metab 27, 1121–1137.e5 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gnad T et al. Adenosine activates brown adipose tissue and recruits beige adipocytes via A2A receptors. Nature 516, 395–399 (2014). [DOI] [PubMed] [Google Scholar]
- 44.Bordicchia M et al. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J. Clin. Invest 122, 1022–1036 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fisher FM et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev 26, 271–281 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohno H, Shinoda K, Spiegelman BM & Kajimura S PPARγ agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab 15, 395–404 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Inagaki T, Sakai J & Kajimura S Transcriptional and epigenetic control of brown and beige adipose cell fate and function. Nat. Rev. Mol. Cell Biol 17, 480–495 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sidossis L & Kajimura S Brown and beige fat in humans: thermogenic adipocytes that control energy and glucose homeostasis. J. Clin. Invest 125, 478–486 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun W et al. Cold-induced epigenetic programming of the sperm enhances brown adipose tissue activity in the offspring. Nat. Med 24, 1372–1383 (2018). [DOI] [PubMed] [Google Scholar]
- 50.Jiang Y, Berry DC & Graff JM Distinct cellular and molecular mechanisms for β3 adrenergic receptor-induced beige adipocyte formation. eLife 6, e30329 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bronnikov G, Houstek J & Nedergaard J β-Adrenergic, cAMP-mediated stimulation of proliferation of brown fat cells in primary culture. Mediation via β1 but not via β3 adrenoceptors. J. Biol. Chem 267, 2006–2013 (1992). [PubMed] [Google Scholar]
- 52.McQueen AE et al. The C-terminal fibrinogen-like domain of angiopoietin-like 4 stimulates adipose tissue lipolysis and promotes energy expenditure. J. Biol. Chem 292, 16122–16134 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goh YY et al. Angiopoietin-like 4 interacts with integrins β1 and β5 to modulate keratinocyte migration. Am. J. Pathol 177, 2791–2803 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu Y et al. Connexin 43 mediates white adipose tissue beiging by facilitating the propagation of sympathetic neuronal signals. Cell Metab 24, 420–433 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identifies the role of the gap junction in beige fat biogenesis via propagation of the sympathetically derived cAMP signal to neighbouring adipocytes.
- 55.Chen Y et al. Thermal stress induces glycolytic beige fat formation via a myogenic state. Nature 565, 180–185 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jun H et al. Adrenergic-independent signaling via CHRNA2 regulates beige fat activation. Dev. Cell 54, 106–116.e5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu Y, Kinnebrew MA, Kutyavin VI & Chawla A Distinct signaling and transcriptional pathways regulate peri-weaning development and cold-induced recruitment of beige adipocytes. Proc. Natl Acad. Sci. USA 117, 6883–6889 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song A et al. Low- and high-thermogenic brown adipocyte subpopulations coexist in murine adipose tissue. J. Clin. Invest 130, 247–257 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee KY et al. Developmental and functional heterogeneity of white adipocytes within a single fat depot. EMBO J 38, e99291 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Min SY et al. Diverse repertoire of human adipocyte subtypes develops from transcriptionally distinct mesenchymal progenitor cells. Proc. Natl Acad. Sci. USA 116, 17970–17979 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reports diverse adipocyte progenitors in human adipose tissue that give rise to beige adipocytes.
- 61.Xue R et al. Clonal analyses and gene profiling identify genetic biomarkers of the thermogenic potential of human brown and white preadipocytes. Nat. Med 21, 760–768 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun W et al. Single-nucleus RNA-seq reveals a new type of brown adipocyte regulating thermogenesis. Nature 587, 98–102 (2020).33116305 [Google Scholar]; This study employs single-nucleus RNA-sequencing to characterize adipocyte heterogeneity in mice and humans, and identifies a subpopulation of adipocytes that uses acetate to regulate the thermogenic capacity of neighbouring adipocytes.
- 63.Schwalie PC et al. A stromal cell population that inhibits adipogenesis in mammalian fat depots. Nature 559, 103–108 (2018). [DOI] [PubMed] [Google Scholar]; This study, by single-cell RNA-sequencing analysis, identifies distinct subpopulations of adipose precursor cells, including adipogenesis-regulatory cells, in mouse adipose tissue.
- 64.Hepler C et al. Identification of functionally distinct fibro-inflammatory and adipogenic stromal subpopulations in visceral adipose tissue of adult mice. eLife 7, e39636 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reveals the functional heterogeneity of visceral WAT perivascular cells and identifies fibro-inflammatory progenitors.
- 65.Merrick D et al. Identification of a mesenchymal progenitor cell hierarchy in adipose tissue. Science 364, eaav2501 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study employs single-cell RNA sequencing to identify mesenchymal progenitor cells that give rise to adipocytes in mice and humans.
- 66.Seale P et al. Transcriptional control of brown fat determination by PRDM16. Cell Metab 6, 38–54 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kajimura S et al. Regulation of the brown and white fat gene programs through a PRDM16/CtBP transcriptional complex. Genes Dev 22, 1397–1409 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seale P et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J. Clin. Invest 121, 96–105 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cohen P et al. Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell 156, 304–316 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ohno H, Shinoda K, Ohyama K, Sharp LZ & Kajimura S EHMT1 controls brown adipose cell fate and thermogenesis through the PRDM16 complex. Nature 504, 163–167 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Berg F, Gustafson U & Andersson L The uncoupling protein 1 gene (UCP1) is disrupted in the pig lineage: a genetic explanation for poor thermoregulation in piglets. PLoS Genet 2, e129 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gaudry MJ et al. Inactivation of thermogenic UCP1 as a historical contingency in multiple placental mammal clades. Sci. Adv 3, e1602878 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ricquier D & Kader JC Mitochondrial protein alteration in active brown fat: a sodium dodecyl sulfate-polyacrylamide gel electrophoretic study. Biochem. Biophys. Res. Commun 73, 577–583 (1976). [DOI] [PubMed] [Google Scholar]
- 74.Nicholls DG Hamster brown-adipose-tissue mitochondria. Purine nucleotide control of the ion conductance of the inner membrane, the nature of the nucleotide binding site. Eur. J. Biochem 62, 223–228 (1976). [DOI] [PubMed] [Google Scholar]
- 75.Aquila H, Link TA & Klingenberg M The uncoupling protein from brown fat mitochondria is related to the mitochondrial ADP/ATP carrier. Analysis of sequence homologies and of folding of the protein in the membrane. EMBO J 4, 2369–2376 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bouillaud F, Ricquier D, Thibault J & Weissenbach J Molecular approach to thermogenesis in brown adipose tissue: cDNA cloning of the mitochondrial uncoupling protein. Proc. Natl Acad. Sci. USA 82, 445–448 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Enerback S et al. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 387, 90–94 (1997). [DOI] [PubMed] [Google Scholar]
- 78.Arsenijevic D et al. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat. Genet 26, 435–439 (2000). [DOI] [PubMed] [Google Scholar]
- 79.Gong DW et al. Lack of obesity and normal response to fasting and thyroid hormone in mice lacking uncoupling protein-3. J. Biol. Chem 275, 16251–16257 (2000). [DOI] [PubMed] [Google Scholar]
- 80.Klingenberg M UCP1 — a sophisticated energy valve. Biochimie 134, 19–27 (2017). [DOI] [PubMed] [Google Scholar]
- 81.Ricquier D UCP1, the mitochondrial uncoupling protein of brown adipocyte: a personal contribution and a historical perspective. Biochimie 134, 3–8 (2017). [DOI] [PubMed] [Google Scholar]
- 82.Winkler E & Klingenberg M Effect of fatty acids on H+ transport activity of the reconstituted uncoupling protein. J. Biol. Chem 269, 2508–2515 (1994). [PubMed] [Google Scholar]
- 83.Jezek P, Orosz DE, Modriansky M & Garlid KD Transport of anions and protons by the mitochondrial uncoupling protein and its regulation by nucleotides and fatty acids. A new look at old hypotheses. J. Biol. Chem 269, 26184–26190 (1994). [PubMed] [Google Scholar]
- 84.Urbankova E, Voltchenko A, Pohl P, Jezek P & Pohl EE Transport kinetics of uncoupling proteins. Analysis of UCP1 reconstituted in planar lipid bilayers. J. Biol. Chem 278, 32497–32500 (2003). [DOI] [PubMed] [Google Scholar]
- 85.Schreiber R et al. Cold-induced thermogenesis depends on ATGL-mediated lipolysis in cardiac muscle, but not brown adipose tissue. Cell Metab 26, 753–763.e7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shin H et al. Lipolysis in brown adipocytes is not essential for cold-induced thermogenesis in mice. Cell Metab 26, 764–777.e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Anderson CM et al. Dependence of brown adipose tissue function on CD36-mediated coenzyme Q uptake. Cell Rep 10, 505–515 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Putri M et al. CD36 is indispensable for thermogenesis under conditions of fasting and cold stress. Biochem. Biophys. Commun 457, 520–525 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Simcox J et al. Global analysis of plasma lipids identifies liver-derived acylcarnitines as a fuel source for brown fat thermogenesis. Cell Metab 26, 509–522.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identifies a mechanism whereby FFAs from adipose tissue promote acylcarnitine production in the liver, which provides fuel for cold-induced thermogenesis.
- 90.Chouchani ET et al. Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature 532, 112–116 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang G et al. Regulation of UCP1 and mitochondrial metabolism in brown adipose tissue by reversible succinylation. Mol. Cell 74, 844–857.e7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ukropec J, Anunciado RP, Ravussin Y, Hulver MW & Kozak LP UCP1-independent thermogenesis in white adipose tissue of cold-acclimated Ucp1−/− mice. J. Biol. Chem 281, 31894–31908 (2006). [DOI] [PubMed] [Google Scholar]
- 93.Ikeda K et al. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat. Med 23, 1454–1465 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper provides direct evidence of a UCP1-independent mechanism in beige fat that controls thermogenesis and glucose homeostasis.
- 94.de Meis L Uncoupled ATPase activity and heat production by the sarcoplasmic reticulum Ca2+-ATPase. Regulation by ADP. J. Biol. Chem 276, 25078–25087 (2001). [DOI] [PubMed] [Google Scholar]
- 95.Bal NC et al. Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat. Med 18, 1575–1579 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tajima K et al. Wireless optogenetics protects against obesity via stimulation of non-canonical fat thermogenesis. Nat. Commun 11, 1730 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aquilano K et al. Low-protein/high-carbohydrate diet induces AMPK-dependent canonical and non-canonical thermogenesis in subcutaneous adipose tissue. Redox Biol 36, 101633 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kazak L et al. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell 163, 643–655 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identifies a UCP1-independent thermogenic mechanism that involves creatine futile cycling.
- 99.Kazak L et al. Genetic depletion of adipocyte creatine metabolism inhibits diet-induced thermogenesis and drives obesity. Cell Metab 26, 660–671.e3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kazak L et al. Ablation of adipocyte creatine transport impairs thermogenesis and causes diet-induced obesity. Nat. Metab 1, 360–370 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Guan HP et al. A futile metabolic cycle activated in adipocytes by antidiabetic agents. Nat. Med 8, 1122–1128 (2002). [DOI] [PubMed] [Google Scholar]
- 102.Flachs P et al. Induction of lipogenesis in white fat during cold exposure in mice: link to lean phenotype. Int. J. Obes 41, 372–380 (2017). [DOI] [PubMed] [Google Scholar]
- 103.Reidy SP & Weber JM Accelerated substrate cycling: a new energy-wasting role for leptin in vivo. Am. J. Physiol 282, E312–E317 (2002). [DOI] [PubMed] [Google Scholar]
- 104.Silva JE Thermogenic mechanisms and their hormonal regulation. Physiol. Rev 86, 435–464 (2006). [DOI] [PubMed] [Google Scholar]
- 105.DosSantos RA, Alfadda A, Eto K, Kadowaki T & Silva JE Evidence for a compensated thermogenic defect in transgenic mice lacking the mitochondrial glycerol-3-phosphate dehydrogenase gene. Endocrinology 144, 5469–5479 (2003). [DOI] [PubMed] [Google Scholar]
- 106.Anunciado-Koza R, Ukropec J, Koza RA & Kozak LP Inactivation of UCP1 and the glycerol phosphate cycle synergistically increases energy expenditure to resist diet-induced obesity. J. Biol. Chem 283, 27688–27697 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Long JZ et al. The secreted enzyme pm20d1 regulates lipidated amino acid uncouplers of mitochondria. Cell 166, 424–435 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kajimura S, Spiegelman BM & Seale P Brown and beige fat: physiological roles beyond heat generation. Cell Metab 22, 546–559 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cooney GJ, Caterson ID & Newsholme EA The effect of insulin and noradrenaline on the uptake of 2-[1–14C]deoxyglucose in vivo by brown adipose tissue and other glucose-utilising tissues of the mouse. FEBS Lett 188, 257–261 (1985). [DOI] [PubMed] [Google Scholar]
- 110.Guerra C et al. Brown adipose tissue-specific insulin receptor knockout shows diabetic phenotype without insulin resistance. J. Clin. Invest 108, 1205–1213 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dallner OS, Chernogubova E, Brolinson KA & Bengtsson T β3-Adrenergic receptors stimulate glucose uptake in brown adipocytes by two mechanisms independently of glucose transporter 4 translocation. Endocrinology 147, 5730–5739 (2006). [DOI] [PubMed] [Google Scholar]
- 112.Olsen JM et al. Glucose uptake in brown fat cells is dependent on mTOR complex 2-promoted GLUT1 translocation. J. Cell Biol 207, 365–374 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lowell BB et al. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature 366, 740–742 (1993). [DOI] [PubMed] [Google Scholar]
- 114.Stanford KI et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J. Clin. Invest 123, 215–223 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.de Souza CJ, Hirshman MF & Horton ES CL-316,243, a β3-specific adrenoceptor agonist, enhances insulin-stimulated glucose disposal in nonobese rats. Diabetes 46, 1257–1263 (1997). [DOI] [PubMed] [Google Scholar]
- 116.Roberts-Toler C, O’Neill BT & Cypess AM Diet-induced obesity causes insulin resistance in mouse brown adipose tissue. Obesity 23, 1765–1770 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bartelt A et al. Brown adipose tissue activity controls triglyceride clearance. Nat. Med 17, 200–205 (2011). [DOI] [PubMed] [Google Scholar]
- 118.Berbee JF et al. Brown fat activation reduces hypercholesterolaemia and protects from atherosclerosis development. Nat. Commun 6, 6356 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bartelt A et al. Thermogenic adipocytes promote HDL turnover and reverse cholesterol transport. Nat. Commun 8, 15010 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reports a possible atheroprotective role of thermogenic fat via increased cholesterol flux through HDL.
- 120.Balaz M et al. Inhibition of mevalonate pathway prevents adipocyte browning in mice and men by affecting protein prenylation. Cell Metab 29, 901–916.e8 (2019). [DOI] [PubMed] [Google Scholar]
- 121.Worthmann A et al. Cold-induced conversion of cholesterol to bile acids in mice shapes the gut microbiome and promotes adaptive thermogenesis. Nat. Med 23, 839–849 (2017). [DOI] [PubMed] [Google Scholar]
- 122.Sponton CH et al. The regulation of glucose and lipid homeostasis via PLTP as a mediator of BAT–liver communication. EMBO Rep 21, e49828 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Neinast MD et al. Quantitative analysis of the whole-body metabolic fate of branched-chain amino acids. Cell Metab 29, 417–429.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yoneshiro T et al. BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature 572, 614–619 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reports the role of thermogenic fat in BCAA metabolism and identified the first mitochondrial BCAA transporter.
- 125.Newgard CB et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 9, 311–326 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Huffman KM et al. Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care 32, 1678–1683 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pietilainen KH et al. Global transcript profiles of fat in monozygotic twins discordant for BMI: pathways behind acquired obesity. PLoS Med 5, e51 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang TJ et al. Metabolite profiles and the risk of developing diabetes. Nat. Med 17, 448–453 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Newgard CB Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab 15, 606–614 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu J et al. Metabolomics based markers predict type 2 diabetes in a 14-year follow-up study. Metabolomics 13, 104 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Guasch-Ferre M et al. Metabolomics in prediabetes and diabetes: a systematic review and meta-analysis. Diabetes Care 39, 833–846 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Felig P, Marliss E & Cahill GF Jr. Plasma amino acid levels and insulin secretion in obesity. N. Engl. J. Med 281, 811–816 (1969). [DOI] [PubMed] [Google Scholar]
- 133.Crown SB, Marze N & Antoniewicz MR Catabolism of branched chain amino acids contributes significantly to synthesis of odd-chain and even-chain fatty acids in 3T3-L1 adipocytes. PloS ONE 10, e0145850 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Green CR et al. Branched-chain amino acid catabolism fuels adipocyte differentiation and lipogenesis. Nat. Chem. Biol 12, 15–21 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wallace M et al. Enzyme promiscuity drives branched-chain fatty acid synthesis in adipose tissues. Nat. Chem. Biol 14, 1021–1031 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Su X et al. Adipose tissue monomethyl branched-chain fatty acids and insulin sensitivity: effects of obesity and weight loss. Obesity 23, 329–334 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gunawardana SC & Piston DW Reversal of type 1 diabetes in mice by brown adipose tissue transplant. Diabetes 61, 674–682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ali Khan A et al. Comparative secretome analyses of primary murine white and brown adipocytes reveal novel adipokines. Mol. Cell Proteom 17, 2358–2370 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Villarroya J, Cereijo R, Giralt M & Villarroya F Secretory proteome of brown adipocytes in response to camp-mediated thermogenic activation. Front. Physiol 10, 67 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Deshmukh AS et al. Proteomics-based comparative mapping of the secretomes of human brown and white adipocytes reveals EPDR1 as a novel batokine. Cell Metab 30, 963–975.e7 (2019). [DOI] [PubMed] [Google Scholar]
- 141.Villarroya J et al. New insights into the secretory functions of brown adipose tissue. J. Endocrinol 243, R19–R27 (2019). [DOI] [PubMed] [Google Scholar]
- 142.Whittle AJ et al. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell 149, 871–885 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Svensson KJ et al. A secreted slit2 fragment regulates adipose tissue thermogenesis and metabolic function. Cell Metab 23, 454–466 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kristof E et al. Interleukin-6 released from differentiating human beige adipocytes improves browning. Exp. Cell Res 377, 47–55 (2019). [DOI] [PubMed] [Google Scholar]
- 145.Sun K et al. Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proc. Natl Acad. Sci. USA 109, 5874–5879 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mahdaviani K, Chess D, Wu Y, Shirihai O & Aprahamian TR Autocrine effect of vascular endothelial growth factor-A is essential for mitochondrial function in brown adipocytes. Metabolism 65, 26–35 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cereijo R et al. CXCL14, a brown adipokine that mediates brown-fat-to-macrophage communication in thermogenic adaptation. Cell Metab 28, 750–763.e6 (2018). [DOI] [PubMed] [Google Scholar]
- 148.Campderros L et al. Brown adipocytes secrete GDF15 in response to thermogenic activation. Obesity 27, 1606–1616 (2019). [DOI] [PubMed] [Google Scholar]
- 149.Nisoli E, Tonello C, Benarese M, Liberini P & Carruba MO Expression of nerve growth factor in brown adipose tissue: implications for thermogenesis and obesity. Endocrinology 137, 495–503 (1996). [DOI] [PubMed] [Google Scholar]
- 150.Zeng X et al. Innervation of thermogenic adipose tissue via a calsyntenin 3β-S100b axis. Nature 569, 229–235 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wang GX et al. The brown fat-enriched secreted factor Nrg4 preserves metabolic homeostasis through attenuation of hepatic lipogenesis. Nat. Med 20, 1436–1443 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kong X et al. Brown adipose tissue controls skeletal muscle function via the secretion of myostatin. Cell Metab 28, 631–643.e3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ruan CC et al. A2A receptor activation attenuates hypertensive cardiac remodeling via promoting brown adipose tissue-derived FGF21. Cell Metab 28, 476–489.e5 (2018). [DOI] [PubMed] [Google Scholar]
- 154.Lynes MD et al. The cold-induced lipokine 12,13-diHOME promotes fatty acid transport into brown adipose tissue. Nat. Med 23, 631–637 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reports a cold-inducible batokine, 12,13-diHOME, that stimulates fatty acid uptake in brown fat.
- 155.Stanford KI et al. 12,13-DiHOME: an exercise-induced lipokine that increases skeletal muscle fatty acid uptake. Cell Metab 27, 1111–1120.e3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chen Y et al. Exosomal microRNA miR-92a concentration in serum reflects human brown fat activity. Nat. Commun 7, 11420 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Thomou T et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 542, 450–455 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sun K, Tordjman J, Clement K & Scherer PE Fibrosis and adipose tissue dysfunction. Cell Metab 18, 470–477 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lackey DE et al. Contributions of adipose tissue architectural and tensile properties toward defining healthy and unhealthy obesity. Am. J. Physiol 306, E233–E246 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Muir LA et al. Adipose tissue fibrosis, hypertrophy, and hyperplasia: correlations with diabetes in human obesity. Obesity 24, 597–605 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Divoux A et al. Fibrosis in human adipose tissue: composition, distribution, and link with lipid metabolism and fat mass loss. Diabetes 59, 2817–2825 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Reggio S et al. Increased basement membrane components in adipose tissue during obesity: links with TGFβ and metabolic phenotypes. J. Clin. Endocrinol. Metab 101, 2578–2587 (2016). [DOI] [PubMed] [Google Scholar]
- 163.Henegar C et al. Adipose tissue transcriptomic signature highlights the pathological relevance of extracellular matrix in human obesity. Genome Biol 9, R14 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Khan T et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol. Cell. Biol 29, 1575–1591 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hasegawa Y et al. Repression of adipose tissue fibrosis through a PRDM16–GTF2IRD1 complex improves systemic glucose homeostasis. Cell Metab 27, 180–194.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang W et al. A PRDM16-driven metabolic signal from adipocytes regulates precursor cell fate. Cell Metab 30, 174–189.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Heaton JM The distribution of brown adipose tissue in the human. J. Anat 112, 35–39 (1972). [PMC free article] [PubMed] [Google Scholar]
- 168.Hany TF et al. Brown adipose tissue: a factor to consider in symmetrical tracer uptake in the neck and upper chest region. Eur. J. Nucl. Med. Mol. Imaging 29, 1393–1398 (2002). [DOI] [PubMed] [Google Scholar]
- 169.Cohade C, Osman M, Pannu HK & Wahl RL Uptake in supraclavicular area fat (“USA-Fat”): description on 18F-FDG PET/CT. J. Nucl. Med 44, 170–176 (2003). [PubMed] [Google Scholar]
- 170.van Marken Lichtenbelt WD et al. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med 360, 1500–1508 (2009). [DOI] [PubMed] [Google Scholar]
- 171.Virtanen KA et al. Functional brown adipose tissue in healthy adults. N. Engl. J. Med 360, 1518–1525 (2009). [DOI] [PubMed] [Google Scholar]
- 172.Saito M et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58, 1526–1531 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cypess AM et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med 360, 1509–1517 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Leitner BP et al. Mapping of human brown adipose tissue in lean and obese young men. Proc. Natl Acad. Sci.USA 114, 8649–8654 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study maps brown fat in six distinct anatomical depots in young men, comparing lean individuals and individuals with obesity.
- 175.Chen KY et al. Brown adipose Reporting Criteria in Imaging STudies (BARCIST 1.0): recommendations for standardized FDG-PET/CT experiments in humans. Cell Metab 24, 210–222 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Sharp LZ et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PloS ONE 7, e49452 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yoneshiro T et al. Recruited brown adipose tissue as an antiobesity agent in humans. J. Clin. Invest 123, 3404–3408 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hanssen MJ et al. Short-term cold acclimation improves insulin sensitivity in patients with type 2 diabetes mellitus. Nat. Med 21, 863–865 (2015). [DOI] [PubMed] [Google Scholar]
- 179.Chondronikola M et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes 63, 4089–4099 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lee P et al. Temperature-acclimated brown adipose tissue modulates insulin sensitivity in humans. Diabetes 63, 3686–3698 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Hanssen MJ et al. Short-term cold acclimation recruits brown adipose tissue in obese humans. Diabetes 65, 1179–1189 (2016). [DOI] [PubMed] [Google Scholar]; This study shows that short-term cold exposure can lead to the recruitment of brown fat in humans with obesity.
- 182.Vijgen GH et al. Increase in brown adipose tissue activity after weight loss in morbidly obese subjects. J. Clin. Endocrinol. Metab 97, E1229–E1233 (2012). [DOI] [PubMed] [Google Scholar]
- 183.Raiko J, Orava J, Savisto N & Virtanen KA High brown fat activity correlates with cardiovascular risk factor levels cross-sectionally and subclinical atherosclerosis at 5-year follow-up. Arterioscler. Thromb. Vasc. Biol 40, 1289–1295 (2020). [DOI] [PubMed] [Google Scholar]; This study finds that the presence of cold-induced brown fat activity correlates with lower cardiovascular risk factors and decreased carotid intima-media thickness and higher carotid elasticity on 5-year follow-up.
- 184.Becher T et al. Brown adipose tissue is associated with cardiometabolic health. Nat. Med 27, 58–65 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study finds that brown fat in humans is associated with protection from cardio-metabolic diseases, particularly in individuals that are overweight and obese.
- 185.Ma S et al. Caloric restriction reprograms the single-cell transcriptional landscape of rattus norvegicus aging. Cell 180, 984–1001.e22 (2020). [DOI] [PubMed] [Google Scholar]
- 186.Yoneshiro T et al. Impact of UCP1 and β3AR gene polymorphisms on age-related changes in brown adipose tissue and adiposity in humans. Int. J. Obes 37, 993–998 (2013). [DOI] [PubMed] [Google Scholar]
- 187.Bakker LE et al. Brown adipose tissue volume in healthy lean South Asian adults compared with white Caucasians: a prospective, case-controlled observational study. Lancet Diabetes Endocrinol 2, 210–217 (2014). [DOI] [PubMed] [Google Scholar]
- 188.Vosselman MJ, Vijgen GH, Kingma BR, Brans B & van Marken Lichtenbelt WD Frequent extreme cold exposure and brown fat and cold-induced thermogenesis: a study in a monozygotic twin. PloS ONE 9, e101653 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Riveros-McKay F et al. Genetic architecture of human thinness compared to severe obesity. PLoS Genet 15, e1007603 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Zhang F et al. An adipose tissue atlas: an image-guided identification of human-like BAT and beige depots in rodents. Cell Metab 27, 252–262.e3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Fitzgibbons TP et al. Similarity of mouse perivascular and brown adipose tissues and their resistance to diet-induced inflammation. Am. J. Physiol. Heart Circ. Physiol 301, H1425–H1437 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Sacks HS et al. Uncoupling protein-1 and related messenger ribonucleic acids in human epicardial and other adipose tissues: epicardial fat functioning as brown fat. J. Clin. Endocrinol. Metab 94, 3611–3615 (2009). [DOI] [PubMed] [Google Scholar]
- 193.Lynch CJ & Adams SH Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol 10, 723–736 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Villarroya F, Cereijo R, Villarroya J, Gavalda-Navarro A & Giralt M Toward an understanding of how immune cells control brown and beige adipobiology. Cell Metab 27, 954–961 (2018). [DOI] [PubMed] [Google Scholar]
- 195.Sakamoto T et al. Macrophage infiltration into obese adipose tissues suppresses the induction of UCP1 level in mice. Am. J. Physiol 310, E676–E687 (2016). [DOI] [PubMed] [Google Scholar]
- 196.Goto T et al. Proinflammatory cytokine interleukin-1β suppresses cold-induced thermogenesis in adipocytes. Cytokine 77, 107–114 (2016). [DOI] [PubMed] [Google Scholar]
- 197.Valladares A, Roncero C, Benito M & Porras A TNF-α inhibits UCP-1 expression in brown adipocytes via ERKs. Opposite effect of p38MAPK. FEBS Lett 493, 6–11 (2001). [DOI] [PubMed] [Google Scholar]
- 198.Chiang SH et al. The protein kinase IKKε regulates energy balance in obese mice. Cell 138, 961–975 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Mowers J et al. Inflammation produces catecholamine resistance in obesity via activation of PDE3B by the protein kinases IKKε and TBK1. eLife 2, e01119 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kumari M et al. IRF3 promotes adipose inflammation and insulin resistance and represses browning. J. Clin. Invest 126, 2839–2854 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yadav H et al. Protection from obesity and diabetes by blockade of TGF-β/Smad3 signaling. Cell Metab 14, 67–79 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Koncarevic A et al. A novel therapeutic approach to treating obesity through modulation of TGFβ signaling. Endocrinology 153, 3133–3146 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Guo T et al. Adipocyte ALK7 links nutrient overload to catecholamine resistance in obesity. eLife 3, e03245 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Rajbhandari P et al. Single cell analysis reveals immune cell-adipocyte crosstalk regulating the transcription of thermogenic adipocytes. eLife 8, e49501 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Rajbhandari P et al. IL-10 signaling remodels adipose chromatin architecture to limit thermogenesis and energy expenditure. Cell 172, 218–233 e217 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study characterizes adipocytes and stromal cells identifying crosstalk between immune cells and thermogenic adipocytes.
- 206.Wolf Y et al. Brown-adipose-tissue macrophages control tissue innervation and homeostatic energy expenditure. Nat. Immunol 18, 665–674 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Pirzgalska RM et al. Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat. Med 23, 1309–1318 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Camell CD et al. Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis during ageing. Nature 550, 119–123 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chung KJ et al. A self-sustained loop of inflammation-driven inhibition of beige adipogenesis in obesity. Nat. Immunol 18, 654–664 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hu B et al. γδ T cells and adipocyte IL-17RC control fat innervation and thermogenesis. Nature 578, 610–614 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Brestoff JR et al. Group 2 innate lymphoid cells promote beiging of white adipose tissue and limit obesity. Nature 519, 242–246 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lee MW et al. Activated type 2 innate lymphoid cells regulate beige fat biogenesis. Cell 160, 74–87 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zhang X et al. Functional inactivation of mast cells enhances subcutaneous adipose tissue browning in mice. Cell Rep 28, 792–803.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Finlin BS et al. Mast cells promote seasonal white adipose beiging in humans. Diabetes 66, 1237–1246 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lynch L et al. iNKT cells induce FGF21 for thermogenesis and are required for maximal weight loss in GLP1 therapy. Cell Metab 24, 510–519 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Cypess AM et al. Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proc. Natl Acad. Sci. USA 109, 10001–10005 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Cypess AM et al. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab 21, 33–38 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.O’Mara AE et al. Chronic mirabegron treatment increases human brown fat, HDL cholesterol, and insulin sensitivity. J. Clin. Invest 130, 2209–2219 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study shows that chronic treatment with mirabegron increases human brown fat activity, which is associated with increased HDL and improved insulin sensitivity.
- 219.Blondin DP et al. Human brown adipocyte thermogenesis is driven by β2-AR stimulation. Cell Metab 32, 287–300.e7 (2020). [DOI] [PubMed] [Google Scholar]
- 220.Broeders EP et al. The bile acid chenodeoxycholic acid increases human brown adipose tissue activity. Cell Metab 22, 418–426 (2015). [DOI] [PubMed] [Google Scholar]
- 221.Ramage LE et al. Glucocorticoids acutely increase brown adipose tissue activity in humans, revealing species-specific differences in UCP-1 regulation. Cell Metab 24, 130–141 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Yoneshiro T, Aita S, Kawai Y, Iwanaga T & Saito M Nonpungent capsaicin analogs (capsinoids) increase energy expenditure through the activation of brown adipose tissue in humans. Am. J. Clin. Nutr 95, 845–850 (2012). [DOI] [PubMed] [Google Scholar]
- 223.Ohyama K et al. A synergistic antiobesity effect by a combination of capsinoids and cold temperature through promoting beige adipocyte biogenesis. Diabetes 65, 1410–1423 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wang S et al. Curcumin promotes browning of white adipose tissue in a norepinephrine-dependent way. Biochem. Biophys. Res. Commun 466, 247–253 (2015). [DOI] [PubMed] [Google Scholar]
- 225.Jiang J et al. Cinnamaldehyde induces fat cell-autonomous thermogenesis and metabolic reprogramming. Metabolism 77, 58–64 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


